Abstract
The cytotoxicity of Bordetella bronchiseptica to infected cells is known to be dependent on a B. bronchiseptica type III secretion system. Although BopB, BopN, BopD, and Bsp22 have been identified as type III secreted proteins, these proteins remain to be characterized. In this study, in order to clarify the function of BopD during Bordetella infection, a BopD mutant was generated. Although secretion of BopD into the culture supernatant was completely abolished by the bopD mutation, the secretion of other type III secreted proteins was not affected by this mutation. It has been reported that severe cytotoxicity, including cell detachment from the substrata, and release of lactate dehydrogenase (LDH) into the supernatant are induced in L2 cells by wild-type B. bronchiseptica infection, and these phenotypes are dependent on the type III secretion system. In contrast, neither cell detachment nor LDH release was induced in L2 cells infected with the BopD mutant. Furthermore, the hemolytic activity of the BopD mutant was greatly impaired compared with that of the wild-type strain. On the basis of the results of coimmunoprecipitation assays with anti-BopB antibodies, we conclude that BopD has the ability to associate with BopB. Finally, we show that the BopD-BopB complex is responsible for the pore formation in the host plasma membrane that functions as the conduit for the transition of effector proteins into host cells.
Three major pathogenic species in the genus Bordetella are known. Bordetella pertussis and B. parapertussis are the causative agents of whooping cough in humans, and B. bronchiseptica infects the respiratory tracts of a broad range of mammals (17, 26). Numerous virulence factors have been identified in Bordetella spp., including toxins such as pertussis toxin (expressed only in B. pertussis) (25, 35, 48), adenylate cyclase toxin (24), and dermonecrotic toxin (46) and adhesins such as filamentous hemagglutinin (12, 36), pertactin (37), and fimbriae (31). The expression of these virulence factors is coordinately regulated by the Bordetella virulence gene (bvg) locus (5, 49), which encodes the BvgA/BvgS two-component regulatory system (43). Under growth conditions of 37°C in the relative absence of MgSO4 or nicotinic acid, the BvgAS phosphorelay is activated and bordetellae grow under Bvg+ phase conditions (8). The Bvg+ phase is characterized by the expression of various virulence factors, such as toxins and adhesins, and is necessary for respiratory tract colonization in rabbits and rats (1, 10). The Bvg− phase is avirulent and is characterized by loss of expression of both toxin and adhesin genes and by induction of genes that are not expressed under Bvg+ phase conditions. In B. bronchiseptica, Bvg− phase loci include genes and operons that encode the motility apparatus (2, 3). It has been shown that the type III secretion system in bordetellae is activated in the Bvg+ phase (51).
Wild-type B. bronchiseptica, but not the type III secretion mutant, was shown to induce cytotoxicity against L2 cells that were established from rat lung epithelial cells (51). It has been demonstrated that the type III secretion mutant of B. bronchiseptica was unable to colonize over the long term in the tracheas of immunocompetent mice, and this mutant was unable to induce cell death in J774 macrophages (50). A recent study reported that the cell death induced by infection with B. bronchiseptica was due to necrosis, because it was independent of the activation of caspases and was accompanied by the release of lactate dehydrogenase (LDH) (44). Thus, the type III secretion system was shown to be important for bordetellae in attaining pathogenicity.
A large number of gram-negative pathogenic bacteria secrete virulence factors via the type III secretion system during infection of host cells. These pathogens secrete and translocate functional protein molecules referred to as effectors into the extracellular milieu, the plasma membrane, and/or the cytoplasm of eukaryotic cells via this secretion system. In yersiniae, several effectors and their functions have been reported. YopE, YopT, and YpkA/YopO act on Rho family GTPases (4, 6, 53), and YopH is a protein tyrosine phosphatase that inhibits the phagocytic activity of macrophages (52). On the other hand, this machinery also secretes the proteins that localize and form pores on the host plasma membrane, which enables the translocation of effectors into the host cytoplasm. Both YopB and YopD have been reported as pore-forming factors in yersiniae (18, 32). Recently, we have identified a Bordetella type III secreted protein, BopB, that functions as the pore-forming factor in B. bronchiseptica (23). In addition, three type III secreted proteins, i.e., BopN, BopD, and Bsp22, have been reported in B. bronchiseptica (50). However, the detailed functions of these secreted proteins remain to be determined.
In the present study, we focused on the role of BopD in Bordetella infection. Our data demonstrate for the first time that BopD is one of the critical virulence factors in B. bronchiseptica.
MATERIALS AND METHODS
Bacterial strains, cell culture, and media.
The wild-type strain used in this study was B. bronchiseptica S798 (23). Both the BopB and type III (disrupted bscN gene) mutants were derived from S798 (23). Bordetella strains were cultured in Stainer-Scholte liquid medium with a starting optical density at 600 nm of 0.2, and the inoculum was prepared from colonies grown on Bordet-Gengou agar as described previously (10, 11, 28). For the infection assay, B. bronchiseptica strains cultured for 18 h at 37°C with vigorous shaking were used. Escherichia coli DH10B, MC1061, and SM10λpir were used as hosts for the construction of various plasmids. E. coli M15 harboring pREP4 was used for the purification of a histidine-tagged recombinant protein. L2 cells (ATCC CCL-149) were maintained in F-12K (GIBCO) with 10% fetal calf serum, and HeLa cells (ATCC CCL-2) were maintained in minimal essential medium (Sigma) with 10% fetal calf serum.
Plasmids.
In construction of the BopD mutant, pDONR201 (Invitrogen) and pABB-CRS2 (41) were used as the cloning and positive suicide vectors, respectively. pQE-30 (Qiagen) was used for purification of histidine-tagged BopD (His-BopD). An expression vector in bordetellae, pABB415, was constructed as follows. pRK415 (22), which includes two NotI sites in its sequence, was digested with NotI, and the cohesive ends of the resulting two linear fragments were treated with T4 DNA polymerase. The fragments were then ligated to obtain pRK415 with the NotI site deleted, which was designated pRK415ΔNotI. On the other hand, pDEST14 (Invitrogen) was digested with BamHI and SalI and the cohesive ends of the resulting linear fragment were treated with T4 DNA polymerase. The fragment was then self-ligated to obtain pDEST14 with the ccdB gene deleted, which was designated pDEST14B/S. A 1.2-kbp fragment encoding chloramphenicol acetyltransferase encompassed by attR1 and attR2 sites was amplified by PCR with the primers Xh-attR1 (5′-CCGCTCGAGACAAGTTTGTACAAAAAAGCT-3′) and Hin-attR2 (5′-CCCAAGCTTTTTCGGGCTTTGTTAGCAGCC-3′) with circular pDEST14B/S as the template. This fragment was inserted into the TA cloning site of pCR2.1-TOPO (Invitrogen) in the opposite direction of lacZα, and the resulting plasmid was designated pABB-TPCmR. To obtain pABB415, a 1.2-kbp fragment encoding chloramphenicol acetyltransferase encompassed by attR1 and attR2 sites was obtained by digestion of pABB-TPCmR with BamHI and XbaI and then inserted into the BamHI and XbaI sites of pRK415ΔNotI.
Generation of the BopD mutant and pBopD plasmid.
The sequence data were obtained from the B. bronchiseptica Sequencing Group at the Sanger Institute (ftp://ftp.sanger.ac.uk/pub/pathogens/bb/) (34). To construct the BopD mutant, the 2.9-kbp DNA fragment encoding BopD and its flanking regions was amplified by PCR with the primers B1-bopD (5′-AAAAAGCAGGCTGTCCAATACGCAACAGATGG-3′) and B2-bopD (5′-AGAAAGCTGGGTTGAAGGACCTGGGTGGCTAC-3′) with B. bronchiseptica S798 genomic DNA as the template. The resulting PCR product was cloned into pDONR201 to obtain pDONR-bopD by means of adapter PCR and site-specific recombination techniques with the Gateway cloning system (Invitrogen). Inverse PCR was then carried out with the primers R1-bopD (5′-GGAATTCAGAGCCGGGCGAAGTCGGAG-3′) and R2-bopD (5′-GGAATTCACCGCCCGTAGCGTGGCCCG-3′) with circular pDONR-bopD as the template. The underlined portions are the EcoRI sites. The resulting PCR products were digested with EcoRI and self-ligated to obtain pDONR-ΔbopD, which contained the EcoRI site in addition to an 882-bp in-frame deletion from 30 bp downstream of the 5′ end of the bopD gene to 30 bp upstream of the 3′ end of the gene. pDONR-ΔbopD was mixed with a positive suicide vector, pABB-CRS2, to obtain pABB-ΔbopD with the Gateway cloning system. pABB-ΔbopD was then introduced into E. coli SM10λpir and transconjugated into the B. bronchiseptica S798 wild-type strain (streptomycin resistant) as described previously (13). The resulting mutant strain was designated ΔBopD.
For complementation of the bopD defect in ΔBopD, pBopD was constructed as follows. A 1.0-kbp fragment encoding BopD was amplified by PCR with the primers B1-bopD-comp (5′-AAAAAGCAGGCTACTGAAACGGCGCCATGTCC-3′) and B2-bopD-comp (5′-AGAAAGCTGGGTTGAAGGTTGGAGCCGGACAC-3′) with B. bronchiseptica S798 genomic DNA as the template. The resulting fragment was cloned once into pDONR201 and then transferred to pABB415 with the Gateway cloning system. The resulting plasmid was designated pBopD.
Preparation of proteins from culture supernatant and whole bacterial cells.
The proteins released into the bacterial culture supernatants and the whole bacterial cell lysates were prepared by trichloroacetic acid precipitation. The culture supernatants were filtered, the bacterial pellets were resuspended in distilled water, and then trichloroacetic acid was added to the respective samples at a final concentration of 10%. After incubation on ice for 15 min, the samples were centrifuged for 5 min and the resulting precipitated proteins were dissolved in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer.
Antibodies.
To purify His-BopD, a DNA fragment encoding BopD was amplified by PCR with the primers 5-His-bopD (5′-CGGGATCCATGTCTGTTTCTCCGACTTC-3′) and 3-His-bopD (5′-GCGTCGACTCAGATATTGCGGGCCACGCTAC-3′) with B. bronchiseptica S798 genomic DNA as the template. The resulting PCR product was inserted into the BamHI and SalI sites of pQE-30 (Qiagen) to construct the plasmid pQE-BopD. His-BopD was expressed in E. coli M15 harboring both pREP4 and pQE-BopD and then purified in accordance with the manufacture's protocol. Purified His-BopD was inoculated into rabbits as an immunogen, and the resulting antisera were incubated with His-BopD immobilized on an Affi-Gel column (Bio-Rad) to obtain specific immunoglobulin (Ig) fractions. The anti-BopB and anti-BcrH2 antibodies used in this study have been described previously (23).
Infection assays.
L2 or HeLa cells seeded on coverslips were infected with bacteria at a multiplicity of infection (MOI) of 200 and then centrifuged for 5 min and incubated for 20 min at 37°C under an atmosphere of 5% CO2. The cells were then washed with phosphate-buffered saline (PBS) and fixed in methanol. Fixed cells were stained with Giemsa's solution. For the terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) assay, 4% paraformaldehyde was used as the reagent for fixation and the MOI and incubation time were changed to 100 and 1 h, respectively. The TUNEL assay was performed by using an in situ cell death detection kit (Roche). To examine the release of LDH from B. bronchiseptica-infected cells, 7.5 × 104 L2 cells seeded on 24-well plates were infected at an MOI of 200 and then centrifuged for 5 min and incubated for 30 min at 37°C under an atmosphere of 5% CO2. The amounts of LDH were measured spectrophotometrically with a CytoTox 96 nonradioactive cytotoxicity assay kit (Promega).
Hemolysis assay.
Measurement of type III-dependent hemolytic activity was carried out as described previously (23). Briefly, bacterial pellets from overnight cultures and rabbit red blood cells (RBC) were washed with PBS and adjusted to 5 × 1010 bacteria/ml and 109 cells/ml with PBS, respectively. Fifty-microliter aliquots of each suspension were mixed together on a 96-well plate and centrifuged for 5 min for close contact; the combined suspension was then incubated at 37°C for 30 min in a CO2 incubator. The bacterium-RBC suspensions were gently resuspended with an additional 100 μl of PBS, and then the plate was centrifuged. The supernatants were transferred to a new plate, where the optical density at 492 nm was measured. To assess the effects of dextran 6000, polyethylene glycol (PEG) 1500, PEG 1000, raffinose, and sucrose on hemolytic activity, these reagents were added to the PBS used for washing or suspending buffer at a final concentration of 30 mM.
Immunoprecipitation.
The bacterial pellet resulting from an overnight culture of 1 ml of B. bronchiseptica was resuspended in 1 ml of PBS, and the suspended bacterial cells were disrupted by sonication. The supernatant resulting from centrifugation was used as the bacterial cell fraction. On the other hand, the supernatant fraction was prepared by filtration of the bacterial culture. The anti-BopB antibodies were added to the bacterial cell fractions or supernatant fractions, and the mixture was incubated at 4°C for 12 h. After addition of protein A-Sepharose beads (Sigma) to each fraction, the resulting beads were washed three times with PBS containing 0.1% Triton X-100. The immunocomplexes recovered from the beads were subjected to SDS-PAGE and analyzed by Western blotting with anti-BopD or anti-BopB antibodies.
RESULTS
Construction and secretion profile of a bopD in-frame deletion mutant.
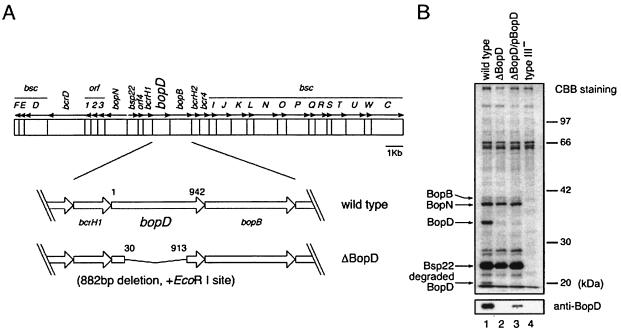
To clarify the function of BopD, a bopD in-frame deletion mutant was constructed as described in Materials and Methods. The mutation resulted in an 882-bp deletion in bopD and produced a truncated BopD protein lacking 294 internal amino acids (Fig. 1A). The culture supernatant sample prepared from the wild-type strain, the BopD mutant, the BopD mutant harboring pBopD (ΔBopD/pBopD), or the type III mutant was subjected to SDS-PAGE and then stained with Coomassie brilliant blue (CBB) (Fig. 1B). In the culture supernatant prepared from the BopD mutant, the band corresponding to BopD, located at about 35 kDa, had disappeared. In contrast, the band intensities of the other type III secreted proteins, such as BopN and Bsp22, were comparable between the wild-type and BopD mutant samples (Fig. 1B). The results of the Western blotting analysis with anti-BopD antibodies revealed that BopD was secreted by the wild-type strain and ΔBopD/pBopD but not by either the BopD or type III mutant (Fig. 1B). To detect the band of BopD secreted by the ΔBopD/pBopD mutant, supernatant samples prepared from the BopD mutant, the ΔBopD/pBopD mutant, and the type III mutant were loaded with 10 times the volume of the sample prepared from the wild-type strain in Western blotting analysis. Furthermore, a band located at 19 kDa (Fig. 1B) was identified as a degraded product of BopD by Western blotting analysis with anti-BopD antibodies and a long duration of exposure of the X-ray film (data not shown). These data suggest that the BopD mutant constructed in this study has a nonpolar mutation that does not exert an effect on the secretion of the other known type III secreted proteins.
FIG. 1.
Construction and secretion profile of a bopD-deficient mutant. (A) Genomic organization of the type III locus in B. bronchiseptica and construction of a bopD-deficient mutant. The sequence data of the type III locus were obtained from the B. bronchiseptica Sequencing Group at the Sanger Institute (ftp://ftp.sanger.ac.uk/pub/pathogens/bb/). The nomenclature of the genes in the type III locus follows that of a former report (14). (B) The proteins secreted by the wild type (lane 1), the bopD-deficient mutant (ΔBopD; lane 2), the BopD mutant harboring pBopD (ΔBopD/pBopD; lane 3), and the type III mutant (type III−; lane 4) were separated by SDS-PAGE, stained with CBB (top), and subjected to Western blotting with anti-BopD antibodies (bottom). Supernatant samples were loaded from an equal number of bacteria in CBB staining, while the samples in lanes 2 to 4 were loaded with 10 times the volume of the sample in lane 1 for Western blotting analysis.
BopD is required to induce morphological changes in L2 cells by B. bronchiseptica infection.
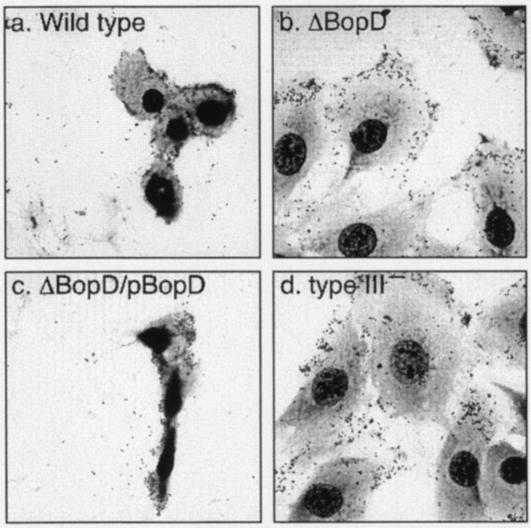
Upon infection of L2 cells with wild-type B. bronchiseptica, the cytoplasm shrinks and the cells round up and detach from the substrata in a type III secretion system-dependent manner (51). To investigate the involvement of BopD in those cytotoxic phenotypes, L2 cells were infected with the wild-type strain, the BopD mutant, the ΔBopD/pBopD mutant, or the type III mutant at an MOI of 200 for 20 min. The resulting infected cells were stained with Giemsa's solution and observed under a light microscope (Fig. 2). As expected, the L2 cells suffered severe cytotoxicity owing to the wild-type infection, and about 90% of the cells were detached from the substrata. In contrast, cytotoxicity against L2 cells was greatly diminished by infection with the BopD or type III mutant. Furthermore, the cytotoxicity was restored in cells infected with the complementary ΔBopD/pBopD mutant strain. To further confirm the adherence efficiency of the BopD mutant, HeLa cells were infected with the wild-type strain or with the BopD mutant, since the rate of detachment of HeLa cells infected with wild-type B. bronchiseptica was lower than that of L2 cells. After Giemsa staining, the bacteria adhering to the HeLa cells were counted under a light microscope. The number of bacteria adhering to each cell was almost equivalent among the wild-type-, ΔBopD-, and type III mutant-infected cells (data not shown). These results demonstrated not only that BopD is an essential factor for inducing cytotoxicity but also that it is not involved in adherence to host cells.
FIG. 2.
Morphological changes induced by B. bronchiseptica infection. L2 cells were infected with the wild type (a) and ΔBopD (b), ΔBopD/pBopD (c), and type III (type III−; d) mutant strains for 20 min. These cells were then visualized by Giemsa staining.
BopD is required for the cell death induced by B. bronchiseptica infection.
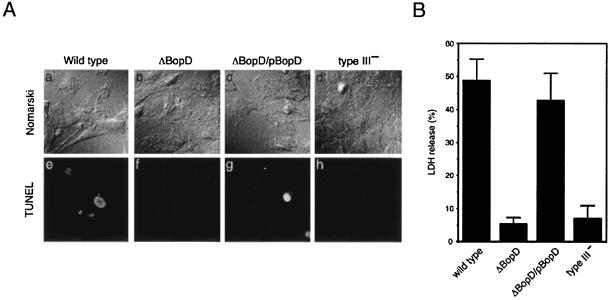
B. bronchiseptica infection has been shown to induce the death of various cells, such as infiltrating polymorphonuclear cells in the lungs of BALB/c mice, L2 cells, and J774 cells (a macrophage-like cell line), and such cell death has been shown to be dependent on functional type III secretion machinery (44, 50). We used the TUNEL method to determine the role of BopD in B. bronchiseptica-induced cell death. To avoid the severe cell detachment induced by the wild-type infection, the MOI in this assay was reduced from 200 to 100. Fluorescent signals in the nuclei indicative of DNA fragmentation were observed in L2 cells infected with the wild-type strain or with the ΔBopD/pBopD strain but not in cells infected with the BopD or type III mutant (Fig. 3A). To further confirm the involvement of BopD in cell death, L2 cells were infected with the wild type or the ΔBopD, ΔBopD/pBopD, or type III mutant strain for 30 min, and the amounts of LDH released into the extracellular milieu by the respective infected cells were measured. The relative amounts of LDH released by cells infected with the wild-type strain and the ΔBopD/pBopD mutant strain were 48.9 and 42.8%, respectively, against the positive control (Fig. 3B). In contrast, the values obtained from cells infected with the BopD and type III mutants were 5.3 and 7.0%, respectively (Fig. 3B). These results suggest that the BopD protein plays a role in B. bronchiseptica-induced cell death.
FIG. 3.
TUNEL and LDH release assays of L2 cells infected with B. bronchiseptica. (A) L2 cells were infected with the wild type (a, e) and ΔBopD (b, f), ΔBopD/pBopD (c, g), and type III (type III−; d, h) mutant strains for 1 h. The cells were then treated with the fluorescent TUNEL reagent and observed under a Nomarski (top) or fluorescence (bottom) microscope. (B) Histogram showing relative amounts of LDH released by L2 cells infected with the B. bronchiseptica strains indicated at the bottom. Results are expressed as the percentage of Triton-lysed L2 cells after subtraction of the value measured in the supernatant of uninfected cells. The error bars represent the standard error of the mean from triplicate experiments.
BopD is required for the type III secretion machinery-dependent hemolytic activity.
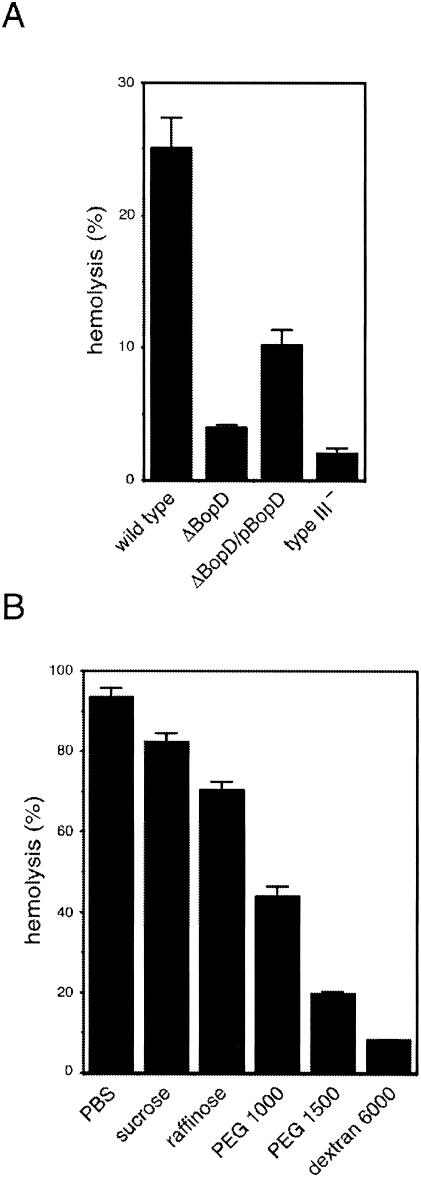
B. bronchiseptica has the ability to disrupt RBC membranes, and this activity is mediated by both adenylate cyclase toxin-hemolysin and type III secretion machinery (23). In a previous report, we established a hemolytic assay that could measure the type III secretion system-mediated hemolytic activity but not the adenylate cyclase toxin-hemolysin-mediated activity (23). In the present study, to test the participation of BopD in type III-dependent hemolysis, the hemolytic activity of the BopD mutant was measured and compared to that of the wild-type strain. The hemolytic activity of the wild-type strain was 25.1% of that of water-lysed RBC (positive control). In contrast, the hemolytic activities of the BopD and type III mutants were 3.9 and 2.0%, respectively (Fig. 4A). By the introduction of pBopD into the BopD mutant, the hemolytic activity was partially restored to 10.2% of that in the positive control (Fig. 4A). To examine the hemolytic ability of type III secreted proteins already secreted into the culture supernatant, filtered supernatant from the wild-type culture was mixed with RBC. However, no hemolytic activity was detected (data not shown). These data indicate that BopD is an essential factor in the type III secretion system-dependent hemolysis in B. bronchiseptica.
FIG. 4.
Type III secretion system-dependent hemolytic activity of B. bronchiseptica. The values in the histogram are the percentages of water-lysed RBC after subtraction of the value measured in uninfected cells (PBS alone). (A) Histogram showing the hemolytic activities of the wild type and ΔBopD, ΔBopD/pBopD, and type III (type III−) mutant B. bronchiseptica strains. (B) The hemolytic activity of the wild type strain was measured in the presence of osmoprotectants (30 mM). The molecular diameters of sucrose, raffinose, PEG 1000, PEG 1500, and dextran 6000 were 0.47, 1.2 to 1.4, 1.8, 2.4, and 3 to 3.5 nm, respectively (7, 16, 21, 30, 40). The error bars represent the standard error of the mean from triplicate experiments.
In previous studies, hemolytic activity was induced by insertion of pores into the RBC membranes, and this activity is inhibited by the addition of osmoprotectants (7, 16, 21, 32, 42). Furthermore, it is possible to determine the minimal diameter of the pores inserted into RBC membranes with protectants of different sizes. The hemolytic activity of the wild-type strain was measured in the presence of sucrose, raffinose, PEG 1000, PEG 1500, or dextran 6000 as a protectant (Fig. 4B). In the presence of sucrose, raffinose, PEG 1000, PEG 1500, or dextran 6000, the hemolytic activity was reduced to 82.3, 70.5, 43.9, 19.7, or 8.2%, respectively. These results suggest that the diameter of the Bop-mediated pores in the RBC membrane is less than that of PEG 1500-mediated pores and larger than that of raffinose-mediated pores.
BopD binds to BopB.
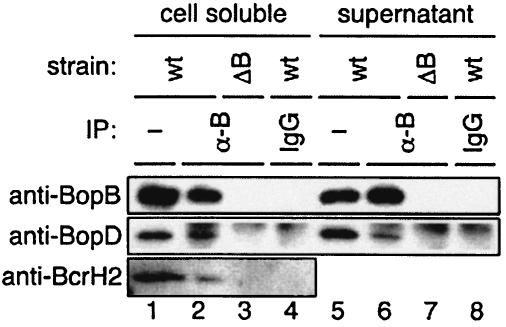
BopD and BopB have 30.3 and 36.1% amino acid sequence similarity to YopD and YopB, respectively, which have been reported as type III secreted proteins in yersiniae. It has been demonstrated that YopD has the ability to bind directly to YopB, and the resulting complex composes a pore on the host plasma membrane to translocate effectors into the cytoplasm of infected cells (33). To investigate the possible association of BopD with BopB, a coimmunoprecipitation assay was carried out with anti-BopB antibodies. The anti-BopB antibody or rabbit IgG was added to the bacterial-cell-soluble or supernatant fraction prepared from the wild-type strain and the BopB mutant, and then the immunocomplexes were recovered by addition of protein A-Sepharose. The resulting samples were subjected to SDS-PAGE and analyzed by Western blotting with anti-BopD antibodies. The BopD signal was detected in the samples precipitated with anti-BopB antibodies from both the wild-type cell-soluble and supernatant fractions (Fig. 5). In contrast, both in the case of protein samples that were precipitated with rabbit IgG from the fractions prepared from the wild-type strain and in the case of those that were precipitated with anti-BopB antibodies from the fractions prepared from the BopB mutant, no BopD signals were detected (Fig. 5). BcrH2, which is a homolog of the Yersinia chaperonin of YopB and YopD, is encoded directly downstream of BopB. To investigate the ability of BcrH2 to bind to the BopB-BopD complex in the cytoplasm, the immunocomplex precipitated with anti-BopB antibodies was analyzed by Western blotting with anti-BcrH2 antibodies. Interestingly, the signal of BcrH2 was detected in the anti-BopB antibody-precipitated sample (Fig. 5). These results strongly suggest that BopD forms a complex with BopB, and a putative type III chaperonin, BcrH2, was also associated with the BopB-BopD complex in the bacterial cytosol.
FIG. 5.
Associations between BopD and BopB. Immunoprecipitation (IP) from the cell-soluble (lanes 2 to 4) and supernatant (lanes 6 to 8) fractions with anti-BopB antibodies (lanes 2, 3, 6, and 7) and rabbit IgG (lanes 4 and 8) was carried out, and the resulting samples were analyzed by Western blotting with anti-BopB (top), anti-BopD (middle), and anti-BcrH2 (bottom) antibodies. The cell-soluble (lane 1) and supernatant (lane 5) fractions used for immunoprecipitation were loaded onto the gel as positive controls. wt, wild type.
DISCUSSION
In this study, we demonstrated that BopD plays a critical role in evoking cytotoxicity in B. bronchiseptica-infected cells and that BopD forms a complex with BopB for pore formation on the host plasma membrane. This conclusion was based on the finding that cytotoxicity, as manifested by the cell death induced by wild-type infection, did not occur upon BopD mutant infection and that BopD specifically associated with BopB in both the bacterial cell and supernatant fractions.
A recent study demonstrated that BCECF [2′,7′-bis-(2-carboxyethyl)-5-(and 6)-carboxyfluorescein, acetoxymethyl ester] can be used to monitor pore formation more directly than the LDH release assay (27). BCECF is a membrane-permeating dye of 623 Da that becomes fluorescent and loses its membrane-permeating ability after cleavage by intracellular esterases. We measured the amounts of BCECF and LDH released from the cells after infection with wild-type B. bronchiseptica for 0, 10, 30, 60 and 120 min as described previously (27). However, there were no significant differences in the release profiles of BCECF and LDH (data not shown). It has been reported that the Yersinia effectors prevent leakage of BCECF from the translocation pore (32). Therefore, the Yersinia ΔHOPEMN strain that does not produce effectors (YopH, YopO, YopP, YopE, YopM, and YopN) was used for the BCECF release method (32). In the genus Bordetella, type III effectors are still unknown. To monitor the direct readout of the pores (BCECF release), not osmotic lysis (LDH release), we need to identify the type III effector proteins in bordetellae and then generate an effector polymutant.
Stockbauer et al. (44) demonstrated that the death of mammalian cells, such as HeLa cells, induced by wild-type B. bronchiseptica infection was necrosis rather than apoptosis. In the present study, the TUNEL assay revealed that DNA fragmentation occurred in the nuclei of B. bronchiseptica-infected L2 (Fig. 3A) and HeLa (data not shown) cells, and this phenotype was also observed in macrophages (50). The TUNEL assay is often used to detect apoptotic cells, but in some cases, a TUNEL-positive signal is seen in response to unconventional apoptotic cells. For example, Salmonella-infected macrophages showed the TUNEL-positive phenotype even though their death was not accompanied by the activation of caspases (19). Although the detailed molecular mechanisms of caspase-independent cell death are unknown, bordetellae and salmonellae may induce such a phenotype during infection.
To investigate whether the hemolytic activity defect of the BopD mutant was reversed by the presence of BopD in the extracellular buffer, purified His-BopD was added to the RBC-BopD mutant suspension in the hemolysis assay. The outcome of this experiment was that the level of hemolysis was almost equal to that of the BopD mutant alone (data not shown). Hemolytic activity was not detected when the RBC were mixed with the wild-type supernatant, even though that solution contained the BopB-BopD complex (data not shown). Moreover, the mixture of the BopB mutant (which secretes BopD at a level equal to that of the wild-type strain but has no hemolytic activity [23]) and the BopD mutant (which secretes BopB at a level equal to that of the wild-type strain [Fig. 1C]) was not able to disrupt the RBC membrane (data not shown). These results suggest that the proper translocation of both BopB and BopD into the host plasma membrane by type III secretion machinery is required for BopB-BopD-mediated pore formation. Interestingly, B. bronchiseptica-induced hemolysis occurred even when the bacterium-RBC suspension was not centrifuged (data not shown). Yersiniae (18, 32) and shigellae (7, 38) also induce hemolysis, but the close contact between bacteria and RBC caused by centrifugation is required to evoke this event. Close-contact-independent hemolysis has been reported in enteropathogenic E. coli (EPEC) (41), suggesting that B. bronchiseptica induces hemolysis by a mechanism similar to that of EPEC.
The partial complementation of hemolytic activity by the ΔBopD/pBopD mutant strain was due to the lower expression of BopD in this strain. In fact, approximately 10 times less BopD was found in the whole-cell lysate prepared from the ΔBopD/pBopD mutant strain than in that from the wild-type strain, as determined by Western blot analysis with anti-BopD antibodies (data not shown). The copy number of bopD in the ΔBopD/pBopD strain should have been higher than that in the wild-type strain. However, the expression level of bopD in the ΔBopD/pBopD strain was lower than that in the wild-type strain. Although the reason for this finding was unclear, the plasmid-derived promoter may be unsuitable for gene expression in bordetellae. Despite the low expression level, the amounts of LDH released were almost identical between the wild-type strain and the ΔBopD/pBopD strain (Fig. 3B). The level of restoration caused by introduction of pBopD into the BopD mutant may depend on the sensitivity of each assay system.
Several reports have noted that the factors responsible for pore formation through the eukaryotic plasma membrane are a prerequisite for the translocation of effectors into host cells (9). For example, YopD and EspB were found to be secreted via the type III secretion system in Yersinia enterocolitica and EPEC, respectively, and both proteins function as one of the pore-forming factors (21, 32, 45). BopD has 30.3 and 26.8% sequence similarity with YopD and EspB, respectively. These pore-forming factors, as well as BopD, have one putative transmembrane domain (as predicted by TMpred; (http://www.ch.embnet.org/software/TMPRED_form.html) in the respective central region. Moreover, according to a coiled-coil prediction result (http://npsa-pbil.ibcp.fr/cgi-bin/npsa_automat.pl?page=/NPSA/npsa_lupas.html), these proteins, including BopD, have a coiled-coil region at their respective C-terminal ends. Although further analyses are needed, these findings suggest that BopD functions as the pore-forming factor in bordetellae. bopD has been found in the genomes of both B. pertussis Tohama I (98.7% identity) and B. parapertussis 12822 (99.7% identity) (34). These findings suggest that BopD and its function may be conserved in the genus Bordetella.
Extensive studies have been conducted in which the complex of YopB, YopD, and LcrV was required for forming the pore on the host plasma membrane during Yersinia infection (15, 18, 20, 32, 33, 39). The diameter of pores composed of YopB-YopD-LcrV was inferred to be about 2 nm by using osmoprotectants in the hemolytic assay (32). The diameter of pores composed of translocator proteins in shigellae, EPEC, or pseudomonads was estimated by using osmoprotectants such as sucrose, raffinose, PEG, and dextran (7, 16, 21). The hemolytic activity of wild-type B. bronchiseptica was greatly reduced by the presence of PEG 1500 (19.7% hemolysis) and was slightly reduced by the presence of raffinose (70.4% hemolysis) (Fig. 4B). These results indicate that the diameter of the pores mediated by the BopD and BopB proteins is 1.5 to 2.5 nm. Although no gene encoding a Yersinia LcrV homolog was found in the genomes of Bordetella spp., we have demonstrated that BopB, which is a homolog of Yersinia YopB, is involved in hemolytic activity (23). In this study, we showed that BopD associated with BopB in the bacterial cytosol and culture supernatant (Fig. 5), suggesting that at least BopB and BopD are required for the pore formation complex in Bordetella spp. In shigellae, the pore-forming factors IpaB and IpaC do not associate in the bacterial cytoplasm. Both proteins have been shown to bind with the specific chaperonin called IpgC to prevent their premature association (29). In contrast, YopB and YopD, which are the pore-forming factors in yersiniae, can form complexes not only in the supernatant but also in the bacterial cytoplasm (33). Our data were consistent with the case of yersiniae. It has been reported that YopB, YopD, and their chaperonin, LcrH/SycD, make a complex in the bacterial cytosol. LcrH/SycD is thought to protect the complex composed of YopB and YopD and facilitate the efficient secretion of YopD and YopB (33, 47). BcrH1 and BcrH2, which were encoded directly upstream of bopD and downstream of bopB, respectively (Fig. 1A), in the Bordetella genome, show 38.9 and 32.7% sequence similarities to Yersinia LcrH. Indeed, BcrH2 was coprecipitated with the BopB-BopD complex in the cell-soluble fraction (Fig. 5). These findings suggest that BcrH1 or BcrH2 might directly bind to BopD or BopB as a chaperonin in the Bordetella cytosol.
Although further experiments are needed to determine the exact associations among BopD and other proteins, our results strongly suggest that BopD is one of the pore-forming factors and functions as the translocator to deliver type III effectors into the host cell cytoplasm.
Acknowledgments
This research was supported by a Grant-in-Aid for Young Scientists (14770123; area B; 2002 to 2003), by a Grant for Scientific Research on Priority Areas (14021109; 2002), and by a grant from the 21st Century COE Program, 2002, from the Ministry of Education, Science, Sports, and Culture of Japan. Support was also received in the form of operating grants from the All Kitasato Project Study 2002 and a Kitasato University Research Grant for Young Researchers 2002, 2003.
We thank Kaori Ishihara for technical assistance.
REFERENCES
- 1.Akerley, B. J., P. A. Cotter, and J. F. Miller. 1995. Ectopic expression of the flagellar regulon alters development of the Bordetella-host interaction. Cell 80:611-620. [DOI] [PubMed] [Google Scholar]
- 2.Akerley, B. J., and J. F. Miller. 1993. Flagellin gene transcription in Bordetella bronchiseptica is regulated by the BvgAS virulence control system. J. Bacteriol. 175:3468-3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Akerley, B. J., D. M. Monack, S. Falkow, and J. F. Miller. 1992. The bvgAS locus negatively controls motility and synthesis of flagella in Bordetella bronchiseptica. J. Bacteriol. 174:980-990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andor, A., K. Trulzsch, M. Essler, A. Roggenkamp, A. Wiedemann, J. Heesemann, and M. Aepfelbacher. 2001. YopE of Yersinia, a GAP for Rho GTPases, selectively modulates Rac-dependent actin structures in endothelial cells. Cell. Microbiol. 3:301-310. [DOI] [PubMed] [Google Scholar]
- 5.Arico, B., J. F. Miller, C. Roy, S. Stibitz, D. Monack, S. Falkow, R. Gross, and R. Rappuoli. 1989. Sequences required for expression of Bordetella pertussis virulence factors share homology with prokaryotic signal transduction proteins. Proc. Natl. Acad. Sci. USA 86:6671-6675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barz, C., T. N. Abahji, K. Trulzsch, and J. Heesemann. 2000. The Yersinia Ser/Thr protein kinase YpkA/YopO directly interacts with the small GTPases RhoA and Rac-1. FEBS Lett. 482:139-143. [DOI] [PubMed] [Google Scholar]
- 7.Blocker, A., P. Gounon, E. Larquet, K. Niebuhr, V. Cabiaux, C. Parsot, and P. Sansonetti. 1999. The tripartite type III secreton of Shigella flexneri inserts IpaB and IpaC into host membranes. J. Cell Biol. 147:683-693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boucher, P. E., K. Murakami, A. Ishihama, and S. Stibitz. 1997. Nature of DNA binding and RNA polymerase interaction of the Bordetella pertussis BvgA transcriptional activator at the fha promoter. J. Bacteriol. 179:1755-1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buttner, D., and U. Bonas. 2002. Port of entry—the type III secretion translocon. Trends Microbiol. 10:186-192. [DOI] [PubMed] [Google Scholar]
- 10.Cotter, P. A., and J. F. Miller. 1994. BvgAS-mediated signal transduction: analysis of phase-locked regulatory mutants of Bordetella bronchiseptica in a rabbit model. Infect. Immun. 62:3381-3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cotter, P. A., and J. F. Miller. 1997. A mutation in the Bordetella bronchiseptica bvgS gene results in reduced virulence and increased resistance to starvation, and identifies a new class of Bvg-regulated antigens. Mol. Microbiol. 24:671-685. [DOI] [PubMed] [Google Scholar]
- 12.Cotter, P. A., M. H. Yuk, S. Mattoo, B. J. Akerley, J. Boschwitz, D. A. Relman, and J. F. Miller. 1998. Filamentous hemagglutinin of Bordetella bronchiseptica is required for efficient establishment of tracheal colonization. Infect. Immun. 66:5921-5929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Donnenberg, M. S., and J. B. Kaper. 1991. Construction of an eae deletion mutant of enteropathogenic Escherichia coli by using a positive-selection suicide vector. Infect. Immun. 59:4310-4317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fauconnier, A., A. Veithen, P. Gueirard, R. Antoine, L. Wacheul, C. Locht, A. Bollen, and E. Godfroid. 2001. Characterization of the type III secretion locus of Bordetella pertussis. Int. J. Med. Microbiol. 290:693-705. [DOI] [PubMed] [Google Scholar]
- 15.Francis, M. S., and H. Wolf-Watz. 1998. YopD of Yersinia pseudotuberculosis is translocated into the cytosol of HeLa epithelial cells: evidence of a structural domain necessary for translocation. Mol. Microbiol. 29:799-813. [DOI] [PubMed] [Google Scholar]
- 16.Frithz-Lindsten, E., A. Holmstrom, L. Jacobsson, M. Soltani, J. Olsson, R. Rosqvist, and A. Forsberg. 1998. Functional conservation of the effector protein translocators PopB/YopB and PopD/YopD of Pseudomonas aeruginosa and Yersinia pseudotuberculosis. Mol. Microbiol. 29:1155-1165. [DOI] [PubMed] [Google Scholar]
- 17.Goodnow, R. A. 1980. Biology of Bordetella bronchiseptica. Microbiol. Rev. 44:722-738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hakansson, S., K. Schesser, C. Persson, E. E. Galyov, R. Rosqvist, F. Homble, and H. Wolf-Watz. 1996. The YopB protein of Yersinia pseudotuberculosis is essential for the translocation of Yop effector proteins across the target cell plasma membrane and displays a contact-dependent membrane disrupting activity. EMBO J. 15:5812-5823. [PMC free article] [PubMed] [Google Scholar]
- 19.Hernandez, L. D., M. Pypaert, R. A. Flavell, and J. E. Galan. 2003. A Salmonella protein causes macrophage cell death by inducing autophagy. J. Cell Biol. 163:1123-1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holmstrom, A., J. Olsson, P. Cherepanov, E. Maier, R. Nordfelth, J. Pettersson, R. Benz, H. Wolf-Watz, and A. Forsberg. 2001. LcrV is a channel size-determining component of the Yop effector translocon of Yersinia. Mol. Microbiol. 39:620-632. [DOI] [PubMed] [Google Scholar]
- 21.Ide, T., S. Laarmann, L. Greune, H. Schillers, H. Oberleithner, and M. A. Schmidt. 2001. Characterization of translocation pores inserted into plasma membranes by type III-secreted Esp proteins of enteropathogenic Escherichia coli. Cell. Microbiol. 3:669-679. [DOI] [PubMed] [Google Scholar]
- 22.Keen, N. T., S. Tamaki, D. Kobayashi, and D. Trollinger. 1988. Improved broad-host-range plasmids for DNA cloning in gram-negative bacteria. Gene 70:191-197. [DOI] [PubMed] [Google Scholar]
- 23.Kuwae, A., M. Ohishi, M. Watanabe, M. Nagai, and A. Abe. 2003. BopB is a type III secreted protein in Bordetella bronchiseptica and is required for cytotoxicity against cultured mammalian cells. Cell. Microbiol. 5:973-983. [DOI] [PubMed] [Google Scholar]
- 24.Ladant, D., C. Brezin, J. M. Alonso, I. Crenon, and N. Guiso. 1986. Bordetella pertussis adenylate cyclase: purification, characterization, and radioimmunoassay. J. Biol. Chem. 261:16264-16269. [PubMed] [Google Scholar]
- 25.Locht, C., P. A. Barstad, J. E. Coligan, L. Mayer, J. J. Munoz, S. G. Smith, and J. M. Keith. 1986. Molecular cloning of pertussis toxin genes. Nucleic Acids Res. 14:3251-3261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Magyar, T., N. Chanter, A. J. Lax, J. M. Rutter, and G. A. Hall. 1988. The pathogenesis of turbinate atrophy in pigs caused by Bordetella bronchiseptica. Vet. Microbiol. 18:135-146. [DOI] [PubMed] [Google Scholar]
- 27.Marenne, M. N., L. Journet, L. J. Mota, and G. R. Cornelis. 2003. Genetic analysis of the formation of the Ysc-Yop translocation pore in macrophages by Yersinia enterocolitica: role of LcrV, YscF and YopN. Microb. Pathog. 35:243-258. [DOI] [PubMed] [Google Scholar]
- 28.Martinez de Tejada, G., J. F. Miller, and P. A. Cotter. 1996. Comparative analysis of the virulence control systems of Bordetella pertussis and Bordetella bronchiseptica. Mol. Microbiol. 22:895-908. [DOI] [PubMed] [Google Scholar]
- 29.Menard, R., P. Sansonetti, C. Parsot, and T. Vasselon. 1994. Extracellular association and cytoplasmic partitioning of the IpaB and IpaC invasins of S. flexneri. Cell 79:515-525. [DOI] [PubMed] [Google Scholar]
- 30.Merzlyak, P. G., L. N. Yuldasheva, C. G. Rodrigues, C. M. Carneiro, O. V. Krasilnikov, and S. M. Bezrukov. 1999. Polymeric nonelectrolytes to probe pore geometry: application to the α-toxin transmembrane channel. Biophys. J. 77:3023-3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mooi, F. R., W. H. Jansen, H. Brunings, H. Gielen, H. G. van der Heide, H. C. Walvoort, and P. A. Guinee. 1992. Construction and analysis of Bordetella pertussis mutants defective in the production of fimbriae. Microb. Pathog. 12:127-135. [DOI] [PubMed] [Google Scholar]
- 32.Neyt, C., and G. R. Cornelis. 1999. Insertion of a Yop translocation pore into the macrophage plasma membrane by Yersinia enterocolitica: requirement for translocators YopB and YopD, but not LcrG. Mol. Microbiol. 33:971-981. [DOI] [PubMed] [Google Scholar]
- 33.Neyt, C., and G. R. Cornelis. 1999. Role of SycD, the chaperone of the Yersinia Yop translocators YopB and YopD. Mol. Microbiol. 31:143-156. [DOI] [PubMed] [Google Scholar]
- 34.Parkhill, J., M. Sebaihia, A. Preston, L. D. Murphy, N. Thomson, D. E. Harris, M. T. Holden, C. M. Churcher, S. D. Bentley, K. L. Mungall, A. M. Cerdeno-Tarraga, L. Temple, K. James, B. Harris, M. A. Quail, M. Achtman, R. Atkin, S. Baker, D. Basham, N. Bason, I. Cherevach, T. Chillingworth, M. Collins, A. Cronin, P. Davis, J. Doggett, T. Feltwell, A. Goble, N. Hamlin, H. Hauser, S. Holroyd, K. Jagels, S. Leather, S. Moule, H. Norberczak, S. O'Neil, D. Ormond, C. Price, E. Rabbinowitsch, S. Rutter, M. Sanders, D. Saunders, K. Seeger, S. Sharp, M. Simmonds, J. Skelton, R. Squares, S. Squares, K. Stevens, L. Unwin, S. Whitehead, B. G. Barrell, and D. J. Maskell. 2003. Comparative analysis of the genome sequences of Bordetella pertussis, Bordetella parapertussis and Bordetella bronchiseptica. Nat. Genet. 35:32-40. [DOI] [PubMed] [Google Scholar]
- 35.Pittman, M. 1979. Pertussis toxin: the cause of the harmful effects and prolonged immunity of whooping cough: a hypothesis. Rev. Infect. Dis. 1:401-412. [DOI] [PubMed] [Google Scholar]
- 36.Relman, D. A., M. Domenighini, E. Tuomanen, R. Rappuoli, and S. Falkow. 1989. Filamentous hemagglutinin of Bordetella pertussis: nucleotide sequence and crucial role in adherence. Proc. Natl. Acad. Sci. USA 86:2637-2641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roberts, M., N. F. Fairweather, E. Leininger, D. Pickard, E. L. Hewlett, A. Robinson, C. Hayward, G. Dougan, and I. G. Charles. 1991. Construction and characterization of Bordetella pertussis mutants lacking the vir-regulated P.69 outer membrane protein. Mol. Microbiol. 5:1393-1404. [DOI] [PubMed] [Google Scholar]
- 38.Sansonetti, P. J., A. Ryter, P. Clerc, A. T. Maurelli, and J. Mounier. 1986. Multiplication of Shigella flexneri within HeLa cells: lysis of the phagocytic vacuole and plasmid-mediated contact hemolysis. Infect. Immun. 51:461-469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sarker, M. R., C. Neyt, I. Stainier, and G. R. Cornelis. 1998. The Yersinia Yop virulon: LcrV is required for extrusion of the translocators YopB and YopD. J. Bacteriol. 180:1207-1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Scherrer, R., and P. Gerhardt. 1971. Molecular sieving by the Bacillus megaterium cell wall and protoplast. J. Bacteriol. 107:718-735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sekiya, K., M. Ohishi, T. Ogino, K. Tamano, C. Sasakawa, and A. Abe. 2001. Supermolecular structure of the enteropathogenic Escherichia coli type III secretion system and its direct interaction with the EspA-sheath-like structure. Proc. Natl. Acad. Sci. USA 98:11638-11643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shaw, R. K., S. Daniell, F. Ebel, G. Frankel, and S. Knutton. 2001. EspA filament-mediated protein translocation into red blood cells. Cell. Microbiol. 3:213-222. [DOI] [PubMed] [Google Scholar]
- 43.Stibitz, S., W. Aaronson, D. Monack, and S. Falkow. 1989. Phase variation in Bordetella pertussis by frameshift mutation in a gene for a novel two-component system. Nature 338:266-269. [DOI] [PubMed] [Google Scholar]
- 44.Stockbauer, K. E., A. K. Foreman-Wykert, and J. F. Miller. 2003. Bordetella type III secretion induces caspase 1-independent necrosis. Cell. Microbiol. 5:123-132. [DOI] [PubMed] [Google Scholar]
- 45.Tardy, F., F. Homble, C. Neyt, R. Wattiez, G. R. Cornelis, J. M. Ruysschaert, and V. Cabiaux. 1999. Yersinia enterocolitica type III secretion-translocation system: channel formation by secreted Yops. EMBO J. 18:6793-6799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Walker, K. E., and A. A. Weiss. 1994. Characterization of the dermonecrotic toxin in members of the genus Bordetella. Infect. Immun. 62:3817-3828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wattiau, P., B. Bernier, P. Deslee, T. Michiels, and G. R. Cornelis. 1994. Individual chaperones required for Yop secretion by Yersinia. Proc. Natl. Acad. Sci. USA 91:10493-10497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weiss, A. A., E. L. Hewlett, G. A. Myers, and S. Falkow. 1984. Pertussis toxin and extracytoplasmic adenylate cyclase as virulence factors of Bordetella pertussis. J. Infect. Dis. 150:219-222. [DOI] [PubMed] [Google Scholar]
- 49.Weiss, A. A., E. L. Hewlett, G. A. Myers, and S. Falkow. 1983. Tn5-induced mutations affecting virulence factors of Bordetella pertussis. Infect. Immun. 42:33-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yuk, M. H., E. T. Harvill, P. A. Cotter, and J. F. Miller. 2000. Modulation of host immune responses, induction of apoptosis and inhibition of NF-κB activation by the Bordetella type III secretion system. Mol. Microbiol. 35:991-1004. [DOI] [PubMed] [Google Scholar]
- 51.Yuk, M. H., E. T. Harvill, and J. F. Miller. 1998. The BvgAS virulence control system regulates type III secretion in Bordetella bronchiseptica. Mol. Microbiol. 28:945-959. [DOI] [PubMed] [Google Scholar]
- 52.Zhang, Z. Y., J. C. Clemens, H. L. Schubert, J. A. Stuckey, M. W. Fischer, D. M. Hume, M. A. Saper, and J. E. Dixon. 1992. Expression, purification, and physicochemical characterization of a recombinant Yersinia protein tyrosine phosphatase. J. Biol. Chem. 267:23759-23766. [PubMed] [Google Scholar]
- 53.Zumbihl, R., M. Aepfelbacher, A. Andor, C. A. Jacobi, K. Ruckdeschel, B. Rouot, and J. Heesemann. 1999. The cytotoxin YopT of Yersinia enterocolitica induces modification and cellular redistribution of the small GTP-binding protein RhoA. J. Biol. Chem. 274:29289-29293. [DOI] [PubMed] [Google Scholar]