Abstract
RNA polymerase sigma factor σF initiates the prespore-specific program of gene expression during Bacillus subtilis sporulation. σF governs transcription of spoIIIG, encoding the late prespore-specific regulator σG. However, transcription of spoIIIG is delayed relative to other genes under the control of σF, and after synthesis, σG is initially kept in an inactive form. Activation of σG requires the complete engulfment of the prespore by the mother cell and expression of the spoIIIA and spoIIIJ loci. We screened for random mutations in spoIIIG that bypassed the requirement for spoIIIA for the activation of σG. We found a mutation (spoIIIGE156K) that resulted in an amino acid substitution at position 156, which is adjacent to the position of a mutation (E155K) previously shown to prevent interaction of SpoIIAB with σG. Comparative modelling techniques and in vivo studies suggested that the spoIIIGE156K mutation interferes with the interaction of SpoIIAB with σG. The σGE156K isoform restored σG-directed gene expression to spoIIIA mutant cells. However, expression of sspE-lacZ in the spoIIIA spoIIIGE156K double mutant was delayed relative to completion of the engulfment process and was not confined to the prespore. Rather, β-galactosidase accumulated throughout the entire cell at late times in development. This suggests that the activity of σGE156K is still regulated in the prespore of a spoIIIA mutant, but not by SpoIIAB. In agreement with this suggestion, we also found that expression of spoIIIGE156K from the promoter for the early prespore-specific gene spoIIQ still resulted in sspE-lacZ induction at the normal time during sporulation, coincidently with completion of the engulfment process. In contrast, transcription of spoIIIGE156K, but not of the wild-type spoIIIG gene, from the mother cell-specific spoIID promoter permitted the rapid induction of sspE-lacZ expression. Together, the results suggest that SpoIIAB is either redundant or has no role in the regulation of σG in the prespore.
Gene expression in the prespore and mother cell chambers of sporulating Bacillus subtilis is controlled by RNA polymerase sigma subunits whose activity is restricted to a specific cell type (22, 31, 37, 46). The activation of the sporulation-specific sigma factors is tightly coupled to the completion of key morphological intermediates in the process and also relies on signaling pathways that operate between the two cell types and that keep the prespore and mother cell lines of gene expression in close register (22, 31, 37, 46). Soon after the asymmetric division of the sporangial cell, an event that creates the prespore and the much larger mother cell, the first compartment-specific sigma factor σF becomes active in the prespore (22, 31, 37, 46). σF triggers the activation of σE in the mother cell, which together with σF drive the migration of the septal membranes around the prespore. This process is termed engulfment and results in the formation of a protoplast isolated from the external medium, fully encircled by the mother cell cytoplasm (22, 31, 37, 46). After engulfment, σF is replaced by σG, which controls late stages of development in this compartment and which also triggers the activation of the late mother cell-specific regulator σK (22, 31, 37, 46). The activities of both σG and σK are required for the assembly of the protective layers that encase the mature spore (22, 31, 37, 46).
Synthesis of σF occurs in the predivisional cell, but its activation is restricted to the prespore by the action of three regulatory proteins, SpoIIAA, SpoIIAB, and SpoIIE. SpoIIAB is an anti-sigma factor that binds to σF as a dimer, preventing its association with RNA polymerase, whereas SpoIIAA is an anti-anti-sigma factor that in an unphosphorylated state interacts with SpoIIAB and releases σF from the SpoIIAB-σF complex (1, 2; reviewed in references 31 and 37). SpoIIE is a septum-bound phosphatase that is also produced in the predivisional cell that promotes the preferential dephosphorylation of SpoIIAA-P in the prespore (reviewed in references 31 and 37).
The transcriptional activity of σF can be divided into an early phase and a late phase. Transcription of the spoIIIG gene (encoding σG) is induced as part of the late phase, towards the end of the engulfment process (29). After synthesis, σG does not become active until the engulfment process is complete (29). Once activated and since σG efficiently recognizes its own promoter, its cellular levels increase rapidly, allowing for the deployment of the σG regulon (17, 47). Because of this autoregulation, both the late transcription of spoIIIG and the negative regulation of σG appear to ensure that its transcriptional activity is effectively coupled to completion of the engulfment process and does not occur prematurely or ectopically (31, 37, 45). The tight coupling of σG activation to the conclusion of the engulfment sequence may serve to ensure that biogenesis of the spore integuments is not initiated during movement of the engulfment membranes (31, 37, 45, 46).
Conclusion of the engulfment process is not sufficient for the activation of σG, which further requires expression of several genes, including the eight cistrons of the spoIIIA operon and the spoIIIJ gene (6, 19, 34). σG accumulates in spoIIIA or spoIIIJ mutant cells but is unable to activate transcription from its target promoters (19, 41). The spoIIIA operon encodes several putative membrane proteins and is expressed in the mother cell under the direction of σE (15). The spoIIIJ gene is expressed during vegetative growth and encodes a membrane protein translocase of the YidC/Oxap1 family (6, 27, 48). Despite the fact that its product may accumulate in both the prespore and the mother cell (6, 27), expression of spoIIIJ in the prespore is sufficient for the activation of σG and sporulation (41).
Two negative regulators of σG are known, the anti-sigma factor SpoIIAB and the LonA protease (3, 8, 19, 21, 35, 40). Expression of spoIIIG prior to the asymmetric division of the sporangial cell blocks sporulation, a phenotype that can be suppressed by a multicopy allele of spoIIAB (21), and certain point mutations in spoIIAB result in expression of σG-dependent genes under conditions that do not support efficient sporulation (8, 35). Moreover, SpoIIAB binds to σG in vitro under conditions that also promote binding of SpoIIAB to σF (19), and the structure of a dimer of Bacillus stearothermophilus SpoIIAB in complex with σF shows that most of the residues involved in the interaction are conserved in σG, but not in other sigma factors (2).
While it seems clear that SpoIIAB can regulate σG under nonsporulation conditions or in the predivisional cell at the onset of sporulation, the evidence for a role in the control of σG in the prespore is less clear (3, 8, 19, 21, 35). On the one hand, SpoIIAB seems to disappear from the prespore coincidently with the first manifestations of σG activity, but it persists in the prespore of a spoIIIA mutant (21). In addition, production of a form of σG (σGE155K) that is not efficiently bound by SpoIIAB in vitro allows expression of the σG-controlled sspE gene in spoIIIA or spoIIIJ mutants, suggesting that the expression of both loci is required to antagonize the inhibitory action of SpoIIAB upon σG (19, 41). However, expression of sspE in spoIIIGE155K cells bearing mutations in either spoIIIA or spoIIIJ does not occur prematurely, suggesting that the activity of σGE155K is still regulated in the double mutants (19, 41). Also, there seems to be very little, if any, free SpoIIAB in the prespore (28), and the anti-sigma factor would have to be able to negatively regulate σG at a time when SpoIIAB itself is antagonized by the anti-anti-sigma SpoIIAA in order to release active σF (reviewed in references 31 and 37). Since the interaction of SpoIIAB with σG appears to be very similar to the interaction of SpoIIAB with σF (7), it seems unlikely that at least prior to completion of the engulfment process, SpoIIAB decisively contributes to the regulation of σG. Mutations in lonA, coding for the ATP-dependent LonA protease, also result in σG activity under nonsporulation conditions, and result in some expression of sspE-lacZ in cells of a spoIIIA mutant during sporulation (40).
Here we have analyzed the role SpoIIAB plays in the regulation of σG in sporulating cells. We screened for mutations in spoIIIG that allowed expression of the σG-controlled sspE-lacZ fusion in a spoIIIA background and found a single mutation that converted a glutamate at position 156 of σG to a lysine. However, we found that expression of sspE-lacZ in a spoIIIGE156K spoIIIA double mutant was delayed relative to the completion of the engulfment process and was not confined to the prespore. Rather, β-galactosidase accumulated throughout the whole cell at late times of sporulation. We also forced the early expression of spoIIIGE156K in the prespore from the spoIIQ promoter and found no premature induction of sspE-lacZ expression. In contrast, expression of spoIIIGE156K in the mother cell readily results in sspE-lacZ expression. The results suggest that the activity of σG is regulated in the prespore compartment by a SpoIIAB-independent mechanism and that SpoIIAB is either redundant or plays only a minor role.
MATERIALS AND METHODS
Bacterial strains and general methods.
The B. subtilis strains used in this work (listed in Table 1) are congenic derivatives of the Spo+ strain MB24 (trpC2 metC3) (14). Luria-Bertani (LB) medium was used for the maintenance of Escherichia coli DH5α (Bethesda Research Laboratories) and B. subtilis. Sporulation was induced in Difco sporulation medium (DSM) and assessed as described previously (13). All other general methods were performed as described previously (13).
TABLE 1.
B. subtilis strains used in this study
| Strain | Relevant genotypea | Origin |
|---|---|---|
| MB24 | trpC2 metC3 | Laboratory stock (14) |
| AH62 | trpC2 metC3 spoIIIA::Tn917ΩHU24 | Laboratory stock (19) |
| AH1043 | trpC2 metC3 ΔamyE::spoIIIG-gusA | This work |
| AH1842 | trpC2 metC3 ΔamyE::spoIIIG | This work |
| AH1843 | trpC2 metC3 ΔspoIIIG::cat ΔamyE::spoIIIG | This work |
| AH1870 | trpC2 metC3 ΔspoIIIG::cat | This work |
| AH2321 | trpC2 metC3 ΔsspE::sspE-lacZ | This work |
| AH2452 | trpC2 metC3 ΔspoIIIG ΔsspE::sspE-lacZ | This work |
| AH2456 | trpC2 metC3 ΔspoIIIG ΔsspE::sspE-lacZ spoIIIA::Tn917ΩHU24 | This work |
| AH2460 | trpC2 metC3 ΔspoIIIG ΔsspE::sspE-lacZ ΔamyE::PspoIID-spoIIIG | This work |
| AH2461 | trpC2 metC3 ΔspoIIIG ΔsspE::sspE-lacZ ΔamyE::PspoIID-spoIIIGE156K | This work |
| AH2462 | trpC2 metC3 ΔspoIIIG ΔsspE::sspE-lacZ ΔlonA::cat | This work |
| AH2463 | trpC2 metC3 ΔspoIIIG ΔsspE::sspE-lacZ ΔlonA::cat ΔamyE::PspoIID-spoIIIG | This work |
| AH2464 | trpC2 metC3 ΔlonA::cat | Laboratory stock (40) |
| AH2465 | trpC2 metC3 ΔspoIIIG ΔsspE::sspE-lacZ ΔlonA::cat ΔamyE::PspoIID-spoIIIGE156K | This work |
| AH2490 | trpC2 metC3 ΔspoIIIG ΔsspE::sspE-lacZ ΔamyE::PxylA-spoIIIG | This work |
| AH2491 | trpC2 metC3 ΔspoIIIG ΔsspE::sspE-lacZ ΔamyE::PxylA-spoIIIGE156K | This work |
| AH2492 | trpC2 metC3 ΔspoIIIG ΔsspE::sspE-lacZ ΔamyE::PxylA-spoIIIG ΔthrC::Pspac-spoIIAB | This work |
| AH2493 | trpC2 metC3 ΔspoIIIG ΔsspE::sspE-lacZ ΔamyE::PxylA-spoIIIGE156K ΔthrC::Pspac-spoIIAB | This work |
| AH3786 | trpC2 metC3 ΔspoIIIG ΔsspE::sspE-lacZ ΔamyE::spoIIIG | This work |
| AH3787 | trpC2 metC3 ΔspoIIIG ΔsspE::sspE-lacZ ΔamyE::spoIIIGE156K | This work |
| AH3788 | trpC2 metC3 ΔspoIIIG ΔsspE::sspE-lacZ ΔamyE::PspoIIQ-spoIIIG | This work |
| AH3789 | trpC2 metC3 ΔspoIIIG ΔsspE::sspE-lacZ ΔamyE::PspoIIQ-spoIIIGE156K | This work |
| AH3790 | trpC2 metC3 ΔspoIIIG ΔsspE::sspE-lacZ spoIIIA::Tn917ΩHU24 ΔamyE::spoIIIG | This work |
| AH3791 | trpC2 metC3 ΔspoIIIG ΔsspE::sspE-lacZ spoIIIA::Tn917ΩHU24 ΔamyE::spoIIIGE156K | This work |
| AH3795 | trpC2 ΔspoIIIG | This work |
| ZB307 | Prototrophic | Laboratory stock (52) |
For simplicity, the spoIIIA::Tn917ΩHU24, ΔsspE::sspE-lacZ, ΔamyE::PspoIID-spoIIIG, ΔamyE::PspoIID-spoIIGE156K, ΔamyE::PspoIIQ-spoIIIG, and ΔamyE::PspoIIQ-spoIIIGE156K alleles are abbreviated spoIIIA::Tn917, sspE-lacZ, PspoIID-spoIIIG, PspoIID-spoIIIGE156K, PspoIIQ-spoIIIG, and PspoIIQ-spoIIIG, respectively. Note that the spoIIIGE156K allele under the control of its normal promoter or other promoters is always used at the amyE locus. Fusions of PxylA to spoIIIG alleles at amyE or of Pspac to spoIIAB at thrC are abbreviated PxylA-spoIIIG, PxylA-spoIIIGE156K, or Pspac-spoIIAB, respectively.
Structure of a complex between B. subtilis SpoIIAB and σG by comparative modelling techniques.
The structure of the SpoIIAB-σF complex from B. stearothermophilus (Protein Data Bank code 1LO0) (2) was used here to derive, by comparative modelling techniques, the SpoIIAB-σF and SpoIIAB-σG complexes from B. subtilis (25, 39). The structure of the SpoIIAB-σF complex from B. stearothermophilus (Protein Data Bank code 1LO0) (2) was used here to derive the SpoIIAB-σF and SpoIIAB-σG complexes from B. subtilis. The structure of SpoIIAB from B. subtilis can be modelled on the basis of SpoIIAB from B. stearothermophilus, because the two sequences present 75% identity and 90% similarity and only five residues at the C terminus cannot be aligned (25, 39). The structure of the SpoIIAB-σF complex from B. stearothermophilus contains information for only part of σF (from residues 104 to 160), and modelling of the sigma factors was restricted to the residues that are homologous to this segment. For B. subtilis σF, this segment presents 84% identity and 88% similarity to σF from B. stearothermophilus, which also suggests that a very good model will be obtained. In contrast, B. subtilis σG shows 30% identity and 63% similarity with σF from B. stearothermophilus. The SpoIIAB-σF from B. stearothermophilus has a bound ADP molecule that was not modelled, because no contacts are made between this region and the sigma factor. Modeller (38) version 6.1 was used for all comparative modelling tasks. Sequences for both proteins in the complex were simultaneously aligned against the X-ray structure of SpoIIAB-σF from B. stearothermophilus, and 20 models were generated using these alignments. The model showing the lowest value of the objective function was chosen and analyzed using PROCHECK (23). In the case of SpoIIAB-σF from B. subtilis, the Ramachandran plot showed 87.4% residues in most favored regions, 9.5% residues in additional allowed regions, 2.5% in generously allowed regions, and 0.6% residues in disallowed regions. The residues in disallowed regions, Leu 103 and Arg 105, are homologous to residues in SpoIIAB-σF from B. stearothermophilus that are also in disallowed regions. This region corresponds to the ADP binding site, and the conformation of these two residues is the most probable one. In the case of SpoIIAB-σG from B. subtilis, we obtained 88.1% residues in most favored regions, 8.5% residues in additional allowed regions, 3.1% in generously allowed regions, and 0.3% is disallowed regions. In this case, only Arg 105 is in a disallowed zone of the Ramachandran plot, and for the reasons stated above, its conformation was considered the most probable.
Construction of an sspE-lacZ fusion.
First, a 500-bp HindIII-to-HincII fragment released from pUC12sspE (10) was inserted between the SmaI and HindIII sites of pBluescript SKII(+) (Stratagene, La Jolla, Calif.) to generate pAH225. Next, NheI- and EcoRI-digested pAH235 was mixed with the lacZ gene released from pPP207 (49) by digestion with SpeI and MscI, and a neomycin resistance (Nmr) determinant was released from pBEST502 (16) with EcoRI and SmaI. Linearization of the resulting plasmid, pMS53, with ScaI permitted integration of the sspE-lacZ fusion into the sspE locus of strain MB24, producing strain AH2321 (Table 1). The ΔsspE::sspE-lacZ allele in strain AH2321 is hereafter abbreviated sspE-lacZ for simplicity (Table 1).
Construction of spoIIIG mutations.
To create an in-frame deletion of the spoIIIG gene, a 1.1-kb DNA fragment was first released from pSP72IIIG (19) by digestion with BglII and SalI and inserted between the BglII and XhoI sites of pLitmus 28 (New England Biolabs, Beverly, Mass.) to yield pAH220, which then served as a PCR template using primers spoIIIG-247R and spoIIIG-559D (Table 2). The PCR template was first treated with DpnI and then with PstI and last, it was autoligated, yielding pMS124. Sequencing confirmed the in-frame deletion of codons 13 to 130 of spoIIIG. Competent cells of strain MB24 were cotransformed with pMS124 and chromosomal DNA from strain ZB307 (52), with selection for methionine prototrophy. Spo− congressants appeared at a frequency of about 3%. One, shown by PCR to carry a deletion of the spoIIIG gene (referred to as ΔspoIIIG) was named AH3795 (Table 1). Strain AH3795 was transformed with ScaI-linearized pMS53 (sspE-lacZ) to produce AH2452. Strain AH2452 was then transformed with chromosomal DNA from strain AH62 (spoIIIA::Tn917ΩHU24, hereafter abbreviated to spoIIIA::Tn917) (Table 1) to create strain AH2456.
TABLE 2.
Primers used in this study
| Primer | Sequence (5′→3′) |
|---|---|
| spoIIIG-247R | TTCATCCTGCAGTCTGCCTAAACAGC |
| spoIIIG-559D | GGAGCCGACTGCAGAAGACATCGC |
| spoIIIG-392D | GGGAAAAAAGATCTCGAGAAATAAAGTCG |
| spec-R | TGTTTGGGAGGATGATTCCACGGTACC |
| spoIIIG-spoIIQ | GTTGCTGAGGTGATGAAACAGTGTCGAGAAATAAAGTCG |
| spoIIIG-spoIID | CGAGCAGGAGGCAGCTGAATGTGTCGAGAAATAAAGTCG |
| spoIIIG-761R | CCCGGAGAGATCTTGAGACACGG |
| spoIIIG-2385R | CCCGGAGTGGATCCTTGAGACACGG |
| spoIIQ-152D | GTTTCAAAGCTGAATTCCAGGCAGCG |
| spoIIQ-500R | TGTTTCATCACCTCAGCAACATTCTG |
| spoIID-1D | CGGAAGAATTCCGCCGTATGAATGG |
| spoIID-500R | ATTCAGCTGCCTCCTGCTCGGG |
| PxylAD | GATCAGCGATATCGAATTCATCCACTCC |
| PxylAR | GTGATTTCCCCCTTAAAAATAAATTC |
| PxylA-spoIIIG | TTTATTTTTAAGGGGGAAATCACGTGTCGAGAAATAAAGTCG |
| spoIIAB-166D | GAACAGCAGGATCCTGACACTGGGGG |
| spoIIAB-665R | CCTCCGCATGCATAACAAATCTCC |
To create an insertion-deletion spoIIIG mutant, a 922-bp DNA fragment containing the spoIIIG gene was first released from pSP72IIIG (19) by partial digestion with EcoRI and HindIII and inserted between the EcoRI and HindIII sites of pLitmus 29 (New England Biolabs) to create pMS33. Next, a chloramphenicol resistance (Cmr) cassette was released from pMS38 (51) by digestion with NsiI and PstI and cloned into PstI-digested pMS33 to yield pMS40. Strain AH1870 (Cmr) in which disruption of the spoIIIG gene by a double-crossover event was verified by PCR resulted from the transformation of strain MB24 with pMS40 (Table 1).
Insertion of an intact copy of the spoIIIG gene at amyE.
A copy of the spoIIIG gene was inserted at amyE in two steps. We first isolated a 427-bp HindIII-to-BamHI fragment from pTK4 encompassing the spoIIGB-spoIIIG intergenic region (20), which was introduced between the HindIII and BamHI sites of the amyE integrational vector pMLK83 (18), to create pAH235. Strain AH1043 (AmyE−/Nmr) (Table 1) resulted from the transformation of strain MB24 with XbaI-linearized pAH235. Then, a spectinomycin resistance (Spr) cassette was released from pAH256 (13) by digestion with SpeI and NcoI and cloned between the same sites of pMS33 (see above), yielding pMS37. Last, a fragment carrying the Spr determinant and the spoIIIG gene was released from pMS37 by digestion with SmaI and EcoRI and cloned between the EcoRI and NruI sites of pDG364 (4). Transformation of AH1043 with the resulting plasmid, pMS45, created AH1842 (Spr/Nms and Spo+) (Table 1), in which the presence of an intact spoIIIG gene at amyE was verified by PCR. Transformation of strain AH1842 with chromosomal DNA from AH1870 (ΔspoIIIG::cat) produced AH1843 (ΔspoIIIG::cat ΔamyE::spoIIIG Spr) (Table 1).
Random mutagenesis of spoIIIG.
Strain AH1843 (Table 1) was mutagenized with N-methyl-N′-nitro-N-nitrosoguanidine essentially as described previously (4). The mutagenesis was effective, as 1% of all colonies obtained from the transformation of strain AH1870 (ΔspoIIIG::cat) (Table 1) with chromosomal DNA from mutagenized AH1843 selecting for Spr (which selects for the spoIIIG copy at amyE) failed to complement the null mutation in the spoIIIG gene. In order to select for σG mutants that would bypass the need of the spoIIIA locus for σG activity, chromosomal DNA from mutagenized AH1843 was used to transform AH2456 (ΔspoIIIG spoIIIA::Tn917 sspE-lacZ [see above]) to Spr. Transformants that showed β-galactosidase activity on DSM plates supplemented with 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) (dark blue colonies) were selected and purified. The linkage between the Lac+ phenotype and the Spr marker was verified by retransforming the screening strain AH2456 with chromosomal DNA from the Lac+ mutants (only mutants showing 100% linkage between the Lac+ and Spr traits or close to 100% linkage were considered). One Lac+ transformant (AH3791) was selected (Table 1), and the spoIIIG gene present at amyE was sequenced after PCR amplification using primers spoIIIG-392D and spec-R. Strain AH3791 was found to harbor a single-nucleotide change (GAA to AAA) at codon 156 of the spoIIIG gene. A control strain, AH3790, with the wild-type allele of spoIIIG at amyE, was constructed by transformation of AH2456 (ΔspoIIIG spoIIIA::Tn917 sspE-lacZ [see above]) with chromosomal DNA from AH1842. Strains AH3786 and AH3787 were constructed by transformation of AH2452 (ΔspoIIIG sspE-lacZ [see above]) with chromosomal DNA from AH1842 and AH3791, respectively.
Fusion of the xylA promoter to the spoIIIG gene.
Initially, the 5′ end of the spoIIIG gene and the xylA promoter region (from positions −256 to −1 relative to the transcriptional start site) were amplified separately, from chromosomal DNA of B. subtilis MB24. Primers PxylA-spoIIIG and spoIIIG-2385R were used for the spoIIIG gene, and primers PxylAD and PxylAR were used for the xylA gene (Table 2). The 370-bp spoIIIG fragment was mixed with the 256-bp xylA fragment, and the resulting fragment of 619 bp was amplified using primers PxylAD and spoIIIG-2385R. The PxylA-spoIIIG fragment was digested with EcoRI and BamHI and ligated to similarly cut pDG364 (4), to yield pMS237. Strains AH3786 and AH3787 (Table 1) were transformed with BamHI-linearized pMS237, selecting for Cmr/Spr cells, to yield strains AH2490 and AH2491, which carry a fusion of the xylose-inducible PxylA promoter to the spoIIIG and spoIIIGE156K alleles at amyE, respectively (abbreviated to PxylA-spoIIIG and PxylA-spoIIIGE156K, respectively) (Table 1).
Fusion of the spac promoter to the spoIIAB gene.
The spoIIAB gene was PCR amplified from the chromosomal DNA of strain MB24, using primers spoIIAB-166D and spoIIAB-665R (Table 2). The 499-bp spoIIAB fragment was digested with BamHI and SpeI and introduced between the BglII and SpeI sites of pDH88 (12), to yield pMS236. Next, a 2,189-bp EcoRI-to-BamHI fragment released from pMS236 was inserted between the same sites of pDG1664 (9), to generate pMS238. Strains AH2490 and AH2491 (Table 1) were transformed with XhoI-linearized pMS238 selecting for erythromycin resistance (Err), to yield AH2492 and AH2493, respectively, which carry a fusion of the Pspac promoter to spoIIAB inserted at the thrC locus (ΔthrC::Pspac-spoIIAB, abbreviated to Pspac-spoIIAB) (Table 1).
Fusions of spoIIIG to different sporulation promoters.
Fusions of spoIIIG to the spoIIQ and to the spoIID promoters were constructed as follows. Initially, the 5′ end of the spoIIIG gene and its promoter region were amplified separately from chromosomal DNA of a wild-type B. subtilis strain. The following primers were used (Table 2): for spoIIIG, spoIIIG-spoIIQ, and spoIIIG-spoIID, primers spoIIIG-761R and spoIIIG-2385R; for spoIIQ, primers spoIIQ-152D and spoIIQ-500R; for spoIID, primers spoIID-1D and spoIID-500R. The 370-bp spoIIIG fragment was mixed with the 350-bp spoIIQ fragment or with the 500-bp spoIID fragment, and the resulting fragments of 720 and 870 bp were amplified using primers spoIIQ-152D and spoIIIG-761R for PspoIIQ-spoIIIG or primers spoIID-1D and spoIIIG-2385R for PspoIID-spoIIIG. The PspoIIQ-spoIIIG fragment was digested with EcoRI and BglII and ligated to pDG364 (4) digested with EcoRI and BamHI to yield pMS134. The PspoIID-spoIIIG fragment was digested with EcoRI and BamHI and ligated to similarly cut pDG364 (4) to yield pMS162. Strain AH3786 (Table 1) was transformed with pMS134 or pMS162 selecting for Cmr/Spr cells, to yield AH3788 and AH2460, respectively. AH3787 (Table 1) was also transformed with pMS134 and pMS162 to yield the Cmr/Spr strains AH3789 and AH2461, respectively. Fusion of the spoIIIG gene to the spoIIQ promoter and to the spoIID promoter by a double-crossover event at amyE was verified by PCR; the fusion alleles are abbreviated to PspoIIQ-spoIIIG and PspoIID-spoIIIG. Strain AH2462 was constructed by transformation of AH2452 (ΔspoIIIG sspE-lacZ [see above]) with DNA from AH2464 (ΔlonA::cat) (Table 1). Last, AH2462 was transformed with chromosomal DNA from AH2460 or AH2461 to produce AH2463 and AH2465, respectively (Table 1).
Immunoblotting.
B. subtilis whole-cell lysates were prepared and Western blot analysis was performed as described previously (41).
β-Galactosidase assays.
β-Galactosidase activity was assayed with the substrate o-nitrophenol-β-d-galactopyranoside (ONPG), and enzyme activity was expressed in Miller units as described previously (13).
Fluorescence microscopy.
Immunofluorescence microscopy was conducted essentially as described previously (11, 33) except that a non-cross-linking fixative, Histochoice (Amresco, Solon, Ohio) was used. Immunolabeling was performed with rabbit polyclonal antibodies against β-galactosidase (Eppendorf-5 Prime, Inc., Boulder, Colo.) at a 1:1,000 dilution. A secondary antibody conjugated to Alexa Fluor 488 (Molecular Probes, Eugene, Oreg.) was used at a 1:500 dilution. To assess the completion of the engulfment process (morphological stage III of sporulation) (31), samples (0.5 ml) of DSM cultures were collected throughout sporulation and resuspended in the same volume of phosphate-buffered saline (8 mM sodium phosphate [pH 7.5], 150 mM NaCl) supplemented with FM-4-64 (5 μg/ml), 4′,6-diaminodino-2-phenylindole (DAPI) (0.2 μg/ml), and Mitotracker green FM (MTG) (15 μg/ml) (44). Cells were scored as having reached stage III when the membrane-impermeable stain FM4-64 (but not MTG) was excluded from the prespore membrane (44). In immunofluorescence experiments, the pattern of nucleoid staining with DAPI was used as an indication of the sporulation stage: just after formation of the asymmetric septum, the prespore nucleoid appears highly condensed, whereas soon after conclusion of the engulfment process, the mother cell and prespore nucleoids appear equally condensed (11, 43). Samples were observed in a Leica fluorescence microscope (DMRA2) using Leica filters A4, L5, and N3. All samples were observed with a 63x objective lens. Images were acquired with a Cool Snap HQ camera (Roper Scientific, Tucson, Ariz.) and recorded and processed for publication using Adobe Photoshop.
RESULTS
Isolation and characterization of the spoIIIGE156K allele.
To analyze the mechanism by which σG is kept inactive in cells of a spoIIIA mutant, we sought random mutations in spoIIIG at amyE that bypassed the requirement for spoIIIA for expression of the σG-controlled sspE-lacZ fusion (26) (see Materials and Methods). We isolated one Lac+ mutant called AH3791 (Table 1) upon transformation of B. subtilis strain AH2456 (ΔspoIIIG spoIIIA::Tn917 sspE-lacZ) with DNA from strain AH1843 mutagenized by N-methyl-N′-nitro-N-nitrosoguanidine (Table 1). Strain AH3791 harbored a single-nucleotide change (GAA to AAA) at codon 156 of the spoIIIG gene at amyE causing the replacement of a glutamic acid for a lysine. This allele of spoIIIG was designated spoIIIGE156K. Strains bearing a wild-type spoIIIG gene (AH3786) or the spoIIIGE156K allele (AH3787) at amyE in a spoIIIG mutant background (ΔspoIIIG sspE-lacZ) are Spo+ (Table 3). Therefore, the spoIIIGE156K allele fully restored sporulation to a spoIIIG null mutant.
TABLE 3.
Sporulation of six B. subtilis strains
| Strain | Relevant genotype | Sporulationa
|
% Sporulation | |
|---|---|---|---|---|
| Viable cells | Heat-resistant cells | |||
| AH3786 | spoIIIG PspoIIIG-spoIIIG | 6.9 × 108 | 5.0 × 108 | 72.5 |
| AH3787 | spoIIIG PspoIIIG-spoIIIGE156K | 5.0 × 108 | 3.8 × 108 | 76.0 |
| AH3788 | spoIIIG PspoIIQ-spoIIIG | 5.0 × 108 | 4.8 × 108 | 96.0 |
| AH3789 | spoIIIG PspoIIQ-spoIIIGE156K | 1.5 × 108 | 6.0 × 107 | 40.0 |
| AH3790 | spoIIIG spoIIIA PspoIIIG-spoIIIG | 1.4 × 108 | 3.0 × 104 | 0.02 |
| AH3791 | spoIIIG spoIIIA PspoIIIG-spoIIIGE156K | 1.1 × 108 | 1.0 × 104 | 0.01 |
The extent of sporulation was measured 24 h after the onset of the process in liquid sporulation medium as described in Materials and Methods.
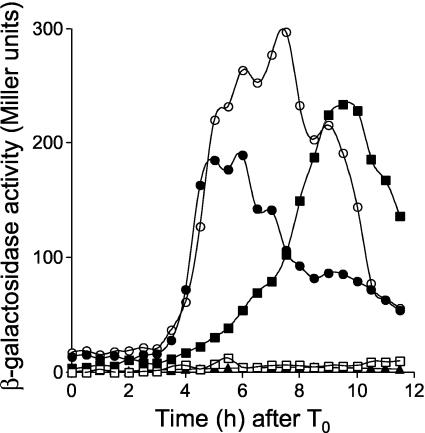
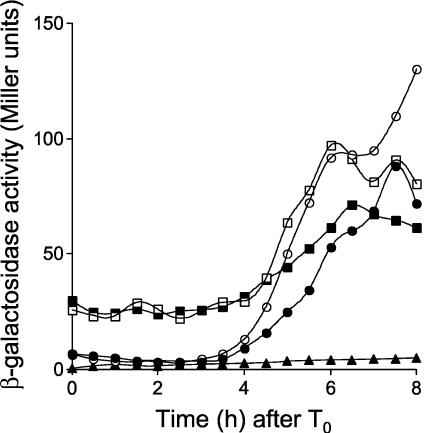
Expression of sspE-lacZ in B. subtilis strains AH3786 and AH3787 was induced around 4 h after the onset of sporulation (Fig. 1), as in a Spo+ strain expressing the wild-type spoIIIG allele at its normal position (26; also data not shown), and reached maximum levels around 6 h after sporulation had started. As expected, expression of sspE-lacZ in strain AH3790 (ΔspoIIIG spoIIIA::Tn917 sspE-lacZ ΔamyE::spoIIIG) was severely impaired (19). However, in the congenic strain AH3791, which bears the spoIIIGE156K allele at amyE, expression of sspE-lacZ was restored (Fig. 1). Nevertheless, while in the spoIIIA+ strain AH3787, expression of sspE-lacZ was strongly induced around 4 h after the onset of sporulation, in the spoIIIA mutant strain AH3791 (spoIIIGE156K), β-galactosidase accumulated at a reduced rate between 4 and 6 h after the onset of sporulation and reached maximum levels only around 10 h after sporulation had started (Fig. 1). Also, even though the spoIIIGE156K allele restores σG activity to spoIIIA cells, strain AH3791 was still unable to sporulate (Table 3). We also found that the spoIIIGE156K allele restored sspE-lacZ expression (but not sporulation) to a ΔspoIIIJ::km mutant (data not shown).
FIG. 1.
The spoIIIGE156K allele bypasses the need for spoIIIA expression for σG activity. Expression of sspE-lacZ was monitored during sporulation in B. subtilis strains AH3786 (ΔspoIIIG sspE-lacZ ΔamyE::spoIIIG) (wild-type spoIIIG in a wild-type background) (closed circles), AH3787 (ΔspoIIIG sspE-lacZ ΔamyE::spoIIIGE156K) (open circles), AH3790 (ΔspoIIIG sspE-lacZ spoIIIA::Tn917 ΔamyE::spoIIIG) (wt) (open squares), and AH3791 (ΔspoIIIG sspE-lacZ spoIIIA::Tn917 ΔamyE::spoIIIGE156K) (closed squares). The complete relevant genotypes of the strains are given in Table 1. Strains were grown in DSM, and samples were taken at the indicated times (in hours) after the onset of sporulation (T0) and assayed for β-galactosidase activity. Endogenous levels of β-galactosidase activity were determined in the wild-type strain MB24 (closed triangles). β-Galactosidase activity is given in Miller units (see Materials and Methods).
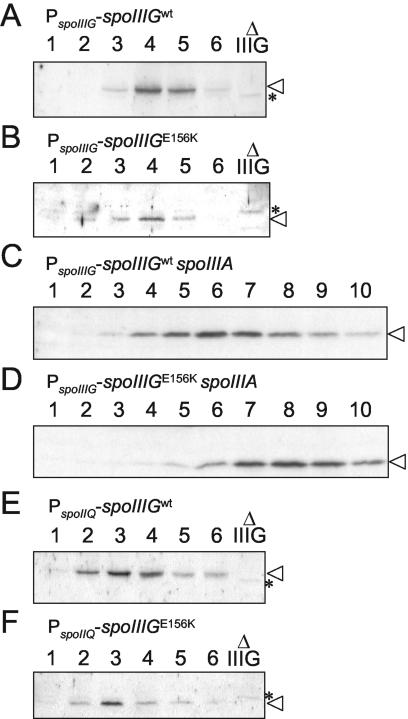
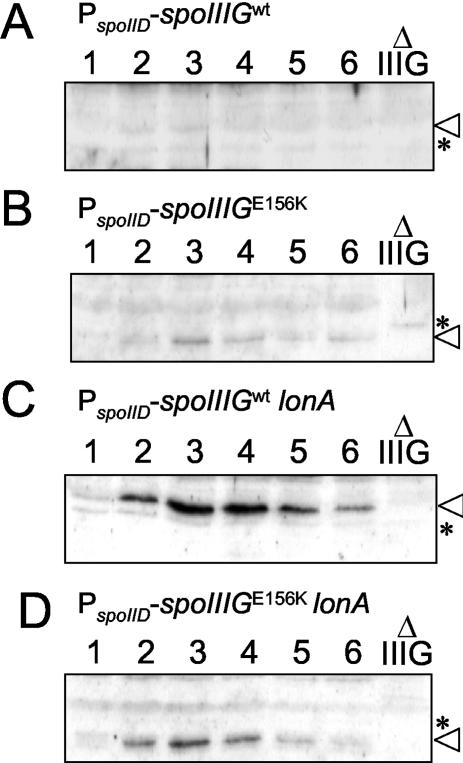
To investigate whether the increased activity of σGE156K in Spo+ cells or in the spoIIIA mutant relative to wild-type σG could be attributed to its increased accumulation, we compared the levels of σG and σGE156K throughout sporulation by immunoblot analysis using a previously described anti-σG antibody (41). We found that in agreement with the timing of sspE-lacZ expression, σG or σGE156K reached peak levels around 4 h after the onset of sporulation in Spo+ cells (Fig. 2A and B). In a spoIIIA background, the accumulation of the wild-type form of σG was delayed, reaching maximum levels around 6 h after the start of sporulation (Fig. 2C). In spoIIIA::Tn917 spoIIIGE156K cells (AH3791), σGE156K is detected only from 5 h on, and its accumulation reaches a maximum around 8 h after the onset of sporulation (Fig. 2D). The late accumulation of σGE156K in the spoIIIA mutant suggests that some σGE156K (but not wild-type σG) escapes inhibition late in sporulation and then amplifies its own synthesis. This late accumulation of σGE156K in the spoIIIA::Tn917 spoIIIGE156K double mutant correlates with the late expression of sspE-lacZ (Fig. 1). Note that under our electrophoretic conditions, the σGE156K form migrates slightly faster than wild-type σG (compare the mobility of σG and σGE156K relative to a background band labeled with an asterisk seen in a sample from a spoIIIG deletion mutant [Fig. 2A and B, for example]). The levels of σGE156K do not appear to be higher than those of wild-type σG in either spoIIIA+ or spoIIIA mutant cells (Fig. 2, compare panels A and B and panels C and D). Thus, the difference in expression of sspE-lacZ in spoIIIGE156K strains AH3787 (spoIIIA+) and AH3791 (spoIIIA mutant) relative to congenic strains expressing a wild-type spoIIIG gene at the amyE locus cannot be explained by an increase in the synthesis or stability of σG. Rather, it may reflect increased activity of the sigma factor. In the case of AH3791 (spoIIIA::Tn917 spoIIIGE156K), the increased activity of σGE156K is manifested only at a late time in development.
FIG. 2.
Immunoblot analysis of σG accumulation during sporulation. σG accumulation in B. subtilis strains AH3786 (ΔspoIIIG sspE-lacZ ΔamyE::spoIIIG) (wild-type spoIIIG in a wild-type background) (A), AH3787 (ΔspoIIIG sspE-lacZ ΔamyE::spoIIIGE156K) (B), AH3790 (ΔspoIIIG sspE-lacZ spoIIIA::Tn917 ΔamyE::spoIIIG) (wt) (C), AH3791 (ΔspoIIIG sspE-lacZ spoIIIA::Tn917 ΔamyE::spoIIIGE156K) (D), AH3788 (ΔspoIIIG sspE-lacZ PspoIIQ-spoIIIG) (wt) (E), and AH3789 (ΔspoIIIG sspE-lacZ PspoIIQ-spoIIIGE156K) (F) during sporulation in DSM was examined by immunoblot analysis. The complete relevant genotypes of strains are given in Table 1. Samples from sporulating cultures were collected 1 h after the onset of sporulation in DSM and at hourly intervals thereafter, as indicated by the numbers above the lanes. Proteins (30 μg) in each sample were subjected to immunoblot analysis using an anti-σG rabbit polyclonal antibody (see Materials and Methods). Lanes Δ IIIG in panels A, B, E, and F contain 30-μg portions of an extract prepared from a spoIIIG deletion mutant (AH3795 [Table 1]) 4 h after the onset of sporulation in DSM (note that the spoIIIG deletion control was not included in panels C and D due to space limitations). The position of σG is indicated by an arrowhead. Other bands represent nonspecific cross-reactive material. One band seen in the spoIIIG deletion mutant is marked with an asterisk for reference.
The E156K mutation is likely to interfere with the interaction between SpoIIAB and σG.
Like spoIIIGE156K, a previously described allele of spoIIIG bearing a glutamate-to-lysine substitution at position 155 (spoIIIGE155K) allows expression of sspE-lacZ in cells with the mutant spoIIIA or spoIIIJ gene (19, 41). The E155K substitution was introduced in σG, because a glutamic acid-to-lysine substitution at an equivalent position of σF (E149K) was found in a genetic screen for σF mutants with reduced affinity for SpoIIAB (5). Moreover, under conditions in vitro that promote binding of SpoIIAB to σF, the anti-sigma factor also binds to σG, but not to σGE155K (19).
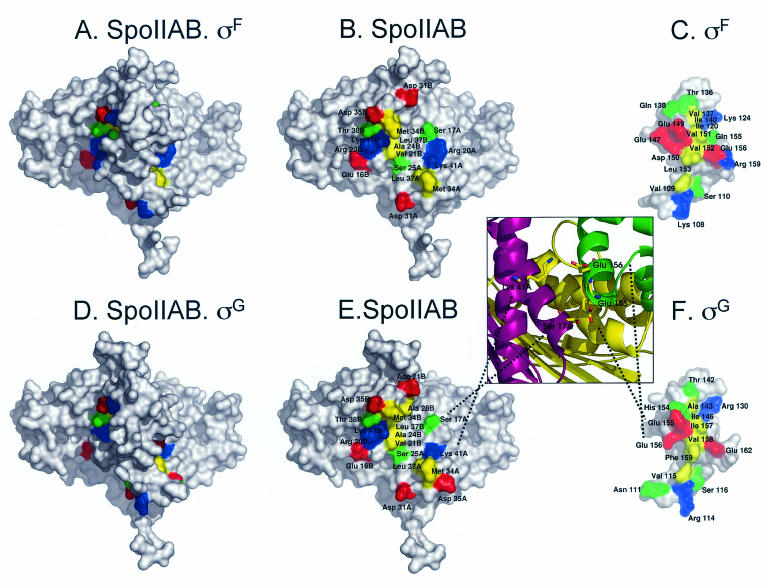
The binding of SpoIIAB to σF or σG can now be described in molecular terms, using the crystal structure (2) of the SpoIIAB-σF complex from B. stearothermophilus and the comparative models for the SpoIIAB-σF and SpoIIAB-σG complexes from B. subtilis derived here. These structures show that in B. stearothermophilus σF, the residue (E147) equivalent to E149 in the σF protein of B. subtilis, as well as three other residues found in genetic screens for mutants resistant to inhibition by SpoIIAB are located within a region that contains 17 amino acids (in B. stearothermophilus) found to interact with SpoIIAB (2, 5) (Fig. 3A to C). Of the amino acids, 15 are either identical or homologous in σG (compare Fig. 3, panels C and F) and 3 are uniquely conserved between σF and σG (2; also data not shown).
FIG. 3.
Structures of the SpoIIAB-σF and SpoIIAB-σG complexes obtained by comparative modelling techniques. Panels A and D depict the whole complex between SpoIIAB and σF or σG, respectively, whereas panels B and E depict only SpoIIAB to display the residues from this protein that contact σF or σG. Panels C and F represent σF or σG, respectively, rotated 180° relative to the complexes shown in panels A and D, to display the contact residues from the σ factors. The positions of all contact residues are labeled. Since SpoIIAB is a dimer, the chain (A or B) of the contact residue is also identified. The structures are rendered using colored molecular surfaces generated using PyMOL (W. L. DeLano, The PyMOL Molecular Graphics System [2002], DeLano Scientific, San Carlos, Calif., http://www.pymol.org). Contact residues between σF or σG and SpoIIAB are colored according to their type; positively charged residues are blue, negatively charged residues are red, polar residues are green, and hydrophobic residues are yellow. The noncontacting surface is white. The insert between panels E and F is an expanded view of the interacting zone of residues E155 and E156 from σG in the SpoIIAB-σG complex. The proteins are represented using cartoons, colored differently to identify them (chains A and B from SpoIIAB are magenta and yellow, respectively, while σG is green).
The nature of the E155K and E156K mutations in σG from B. subtilis can be qualitatively understood by examination of the model for the SpoIIAB-σG complex (Fig. 3D to F). The model shows that residue E155 of σG interacts with S17 in one of the SpoIIAB molecules present in the dimer (Fig. 3D to F and insert), a contact that is also conserved in the SpoIIAB-σF complex (E149) (Fig. 3A to C). The models also predict that residue E156 of σG contacts residue K41 in the same SpoIIAB molecule contacted by residue E155 of σG (Fig. 3, insert), and again, this contact is conserved in the SpoIIAB-σF complex (D150) (Fig. 3A to C). Therefore, both the E155K and E156K substitutions introduce unfavorable interactions expected to destabilize the interaction of SpoIIAB with σG. We infer that the E156K mutation reduces the binding of SpoIIAB to σG in a manner similar to that observed for σGE155K (19). However, the interaction of E155 with a serine residue (S17) from SpoIIAB is likely to be less strong than the interaction of E156 that forms a salt bridge with K41 from SpoIIAB (Fig. 3, insert). Therefore, the effect of the E156K mutation, placing two positively charged residues in close proximity would create packing problems, and appears more disadvantageous for complex formation than the E155K mutation. Since the models for the two complexes show that most of the contacts between SpoIIAB and σF or σG are conserved, our analysis supports the conclusion of Evans et al. that the interaction of SpoIIAB with either σF or σG is very similar (7).
σGE156K is less sensitive to SpoIIAB in vivo.
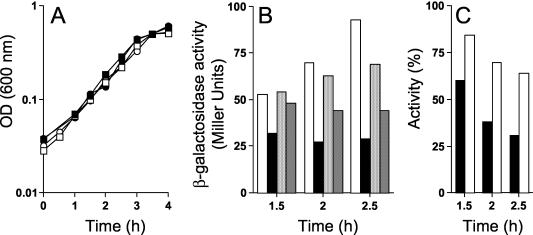
To determine whether the σGE156K form was less sensitive to the inhibitory action of SpoIIAB in vivo, we constructed strains engineered to coexpress spoIIAB and either spoIIIG or spoIIIGE156K during vegetative growth in a medium (LB) that does not support efficient sporulation. Strains AH2492 and AH2493 carry a fusion of the xylose-inducible PxylA promoter to the spoIIIG and spoIIIGE156K genes, respectively, inserted at the amyE locus, as well as an IPTG-inducible Pspac-spoIIAB fusion inserted at the thrC locus; in addition, the two strains carry an sspE-lacZ fusion (Table 1). Preliminary experiments revealed that expression of PxylA-spoIIIG and PxylA-spoIIIGE156K in the absence of xylose resulted in significant expression of sspE-lacZ (data not shown). Therefore, AH2492 and AH2493 were grown in the absence of xylose and in the absence or presence of IPTG (1 mM) to induce SpoIIAB production.
Expression of spoIIIG or spoIIIGE156K in the presence or absence of SpoIIAB did not result in any detectable growth differences between the strains (Fig. 4A). The sspE-lacZ-driven production of β-galactosidase was monitored in the various cultures during the log phase of growth, 1.5, 2, and 2.5 h after inoculation. We found that in the absence of IPTG, the activities of both σG and σGE156K increased during the experiment, even though the activity of σGE156K was always lower than that of the wild-type form (Fig. 4B). In the presence of IPTG to induce spoIIAB expression, the activity of wild-type σG was immediately reduced and remained at low levels (Fig. 4B). In contrast, the activity of σGE156K was reduced slowly (Fig. 4B). Moreover, at all the times tested, the fraction of σGE156K activity remaining after IPTG-induced SpoIIAB synthesis was higher than the fraction of σG activity remaining after SpoIIAB induction (Fig. 4C). These results are consistent with the suggestion that the E156K substitution makes σG less sensitive to the inhibitory action of SpoIIAB.
FIG. 4.
σGE156K is less susceptible to SpoIIAB in vivo than wild-type σG. (A) B. subtilis strains AH2492 (PxylA-spoIIIG Pspac-spoIIAB sspE-lacZ) (circles) and AH2493 (PxylA-spoIIIGE156K Pspac-spoIIAB sspE-lacZ) (squares) were grown in LB medium with 1 mM IPTG (closed symbols) to induce spoIIAB expression or in the absence of inducer (open symbols). OD (600 nm), optical density at 600 nm. (B) Samples were taken at the indicated times and assayed for β-galactosidase production. From left to right, the four bars for each time point show the results for strain AH2492 grown in the absence of IPTG (white bars), AH2492 grown in the presence of IPTG (black bars), AH2493 grown in the absence of IPTG (light grey bars), and AH2493 grown in the presence of IPTG (dark grey bars). (C) Ratio between the activity of σG (black bars) or σGE156K (white bars) in the absence and presence of IPTG (expressed as a percentage).
Activity of σGE156K in a spoIIIA background is delayed relative to completion of the engulfment process.
On the basis of an analogy to the E155K mutation and the results discussed above, we expected that the E156K substitution would also relieve the inhibitory action of SpoIIAB on σG during sporulation. If the interaction of SpoIIAB with σG were reduced, as suggested by our screen, and because σG is autoregulatory (17, 47), we would expect premature expression of sspE-lacZ if SpoIIAB were the primary inhibitor of σG activity in the prespore. In contrast to this expectation, activity of σGE156K was delayed in spoIIIA cells (Fig. 1), which remained unable to sporulate (AH3791) (Table 3).
To determine whether expression of sspE-lacZ was still coupled to the completion of the engulfment process in strain AH3791 (spoIIIA spoIIIGE156K), we used the membrane stains FM4-64 and MTG to monitor completion of the engulfment process (44). We stained samples of the same strains depicted in Fig. 1, and in parallel, we monitored accumulation of β-galactosidase to control for the onset of σG activity. We could not use a fusion of the sspE promoter to the gfp gene for this purpose, because even in the absence of σG, most of the cells showed some prespore decoration, presumably due to the activity of σF (data not shown).
As in the experiment documented in Fig. 1, expression of sspE-lacZ in the Spo+ strains AH3786 (spoIIIG at amyE) and AH3787 (spoIIIGE156K at amyE) commenced around 4 h after the onset of sporulation, when 42 and 31% of the cells, respectively, had completed the engulfment process (Table 4). Activity of β-galactosidase peaked 6 h after the onset of sporulation for strain AH3786, when 48% of the cells showed complete engulfment of the prespore, and 6 to 8 h after the start of sporulation for AH3787, when 51 to 74% of the cells had completed the engulfment process (Table 4). These observations are in agreement with the results of a previous study, suggesting that the activity of σG coincides with the completion of engulfment when sporulation is induced by growth and resuspension in a minimal medium (29). About 20% of AH3791 cells (spoIIIA::Tn917 spoIIIGE156K) showed complete engulfment by 4 h after the onset of sporulation, a fraction that increased to 39% by 6 h (Table 4), and by 8 h after sporulation had started, the number of cells showing clear signs of having completed the engulfment sequence reached a maximum of 54% (Table 4). However, this did not correspond to peak levels of sspE-lacZ expression (Fig. 1). Rather, enzyme production reached a maximum 10 h after the onset of sporulation, when the fraction of cells with clear signs of complete engulfment actually decreased to about 35% (Table 4). The reasons for this decrease may reflect instability of the prespore in cells bearing a spoIIIA mutation (see below). Consistent with this interpretation, 10 h after the onset of sporulation in strains bearing a mutation in spoIIIA, the pattern of fluorescence decoration resulting from MTG staining tended to change from the ellipsoidal contour of the prespore to a more or less indistinct mass of fluorescence, suggesting coalescence of the prespore membranes (data not shown). We note that a spoIIIA mutation per se does not significantly interfere with the timing of engulfment (AH3790) (Table 4). We interpret these observations as indicating that σGE156K becomes active only about 2 h after the completion of engulfment in a spoIIIA mutant.
TABLE 4.
Time of engulfment completion in four B. subtilis strains
| Strain | Genotype | Time (h)a | No. countedb | Sporulating cells/%c | Stage III cells/%d |
|---|---|---|---|---|---|
| AH3786 | spoIIIG wte | 2 | 279 | 91/33 | 0 |
| 4 | 143 | 101/71 | 60/42 | ||
| 6 | 132 | 90/68 | 64/48 | ||
| 8 | 131 | 100/76 | 86/66 | ||
| 10 | 115 | 93/81 | 83/72 | ||
| AH3787 | spoIIIGE156K | 2 | 487 | 153/31 | 0 |
| 4 | 178 | 121/68 | 55/31 | ||
| 6 | 357 | 221/62 | 53/51 | ||
| 8 | 158 | 133/84 | 117/74 | ||
| 10 | 172 | 129/75 | 114/66 | ||
| AH3790 | spoIIIA spoIIIG wt | 2 | 157 | 70/45 | 0 |
| 4 | 172 | 121/70 | 54/31 | ||
| 6 | 167 | 119/71 | 98/59 | ||
| 8 | 102 | 70/69 | 53/52 | ||
| 10 | 152 | 67/44 | 48/32 | ||
| AH3791 | spoIIIA spoIIIGE156K | 2 | 219 | 77/35 | 0 |
| 4 | 260 | 110/42 | 50/20 | ||
| 6 | 135 | 87/64 | 52/39 | ||
| 8 | 111 | 76/68 | 60/54 | ||
| 10 | 153 | 70/46 | 54/35 |
Hours after the onset of sporulation.
The total number of cells counted is indicated.
The number of sporulating cells, estimated on the basis of the pattern of staining by FM4-64 and MTG (see text), is shown before the slash, and the percentage of sporulating cells is shown after the slash.
The number of cells in stage III or above is shown before the slash, and the percentage of cells in stage III or above is shown after the slash.
spoIIIG wt, wild-type spoIIIG.
Activity of σGE156K in a spoIIIA background is not confined to the prespore.
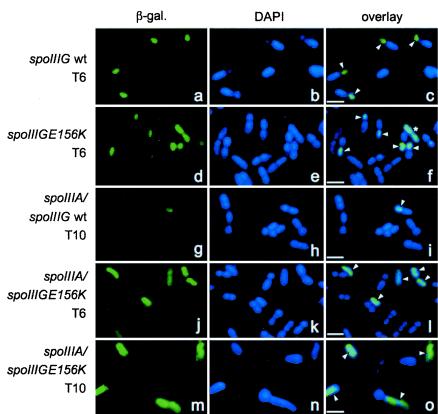
The observation that the activity of σGE156K was delayed relative to the completion of the engulfment process in a spoIIIA mutant led us to examine the location of β-galactosidase produced from the sspE-lacZ fusion in this strain. We used immunofluorescence microscopy to examine samples of the same cultures used in the experiment depicted in Fig. 1 6 and 10 h after the onset of sporulation, as this corresponds to peak levels of sspE-lacZ expression in spoIIIA+ or spoIIIA mutant cells, respectively (Fig. 1). We were unable to collect reasonable phase-contrast images or images of cells in which the membrane had been stained with FM4-64 or MTG after fixation and permeabilization of the cells with lysozyme (also see reference 33). For that reason, the number of sporulating cells was scored on the basis of the analysis of the pattern of nucleoid staining by DAPI (see Materials and Methods), and for each of these cells, the pattern of β-galactosidase localization was recorded (see Materials and Methods) (Table 5).
TABLE 5.
Patterns of β-galactosidase localization
| Strain | Genotype | Time (h)a | No. countedb | Sporulating cells/%c | Localization of β-galactosidased
|
||
|---|---|---|---|---|---|---|---|
| Prespore | MC | Whole cell | |||||
| AH3786 | spoIIIG wt | 6 | 411 | 250/61 | 187 | 0 | 0 |
| 10 | 360 | 358/99 | 9 | 0 | 0 | ||
| AH3787 | spoIIIGE156K | 6 | 446 | 305/68 | 222 | 0 | 9 |
| 10 | 413 | 397/96 | 10 | 0 | 3 | ||
| AH3790 | spoIIIA spoIIIG wt | 6 | 193 | 151/78 | 0 | 0 | 0 |
| 10 | 178 | 107/60 | 1 | 0 | 0 | ||
| AH3791 | spoIIIA spoIIIGE156K | 6 | 431 | 309/75 | 0 | 2 | 12 |
| 10 | 587 | 187/32 | 0 | 1 | 135 | ||
Hours after the onset of sporulation.
The total number of cells counted is indicated.
The number of sporulating cells, estimated on the basis of the pattern of DAPI staining (see text), is shown before the slash, and the percentage of sporulation is shown after the slash.
The number of cells or free spores in prespores, mother cells (MC), and whole cells (cells with no signs of prespore).
In strains AH3786 (ΔspoIIIG sspE-lacZ ΔamyE::spoIIIG) and AH3787 (ΔspoIIIG sspE-lacZ ΔamyE::spoIIIGE156K), production of β-galactosidase was detected only in cells in which the prespore had been completely engulfed by the mother cell (as defined by DAPI staining, which reveals two equal-size mother cell and prespore chromosomes). Production of the enzyme was always confined to the prespore compartment of strain AH3786 (Fig. 5a to c and Table 5), whereas for strain AH3787, a small percentage of cells (around 1 or 2%) 6 or 10 h after the onset of sporulation showed fluorescence throughout the entire cell (Fig. 5f and Table 5). Interestingly, these specimens did not present any distinctive signs of sporulation as judged from the pattern of DAPI staining (Fig. 5d to f). This effect does not seem to be caused by deficient staining of one of the chromosomes, since the fluorescence signal is distributed by what would be the length of the entire sporulating cell (mother cell plus prespore). These specimens may represent vegetative cells or cells in which the normal compartmentalization of σG activity was lost (see below).
FIG. 5.
Immunolocalization patterns of β-galactosidase produced from the sspE-lacZ fusion. The strains of B. subtilis were grown in DSM, and samples were taken at the indicated times after the onset of sporulation, stained with DAPI, and processed for immunofluorescence microscopy as described in Materials and Methods. Typical localization patterns of β-galactosidase (β-gal.) produced from the sspE-lacZ fusion for strains AH3786 (ΔspoIIIG sspE-lacZ ΔamyE::spoIIIG) (wild-type spoIIIG in a wild-type background) (a to c), AH3787 (ΔspoIIIG sspE-lacZ ΔamyE::spoIIIGE156K) (d to f), AH3790 (ΔspoIIIG spoIIIA::Tn917 sspE-lacZ ΔamyE::spoIIIG) (wt) (g to i), and AH3791 (ΔspoIIIG spoIIIA::Tn917 sspE-lacZ ΔamyE::spoIIIGE156K) (j to o) at the indicated times after the onset of sporulation are shown. The complete relevant genotypes of the strains are given in Table 1. The samples were taken 6 or 10 h after the onset of sporulation (T6 and T10, respectively). Arrowheads point to specimens showing sspE-lacZ expression. The specimen labeled with an asterisk in panel f, as well as the specimens in panels l and o, show whole-cell fluorescence (see text). Bars, 2 μm.
In agreement with the results of sspE-lacZ expression shown in Fig. 1, essentially no β-galactosidase could be detected in cells of AH3790 (ΔspoIIIG spoIIIA::Tn917 sspE-lacZ ΔamyE::spoIIIG) (Fig. 5i shows the only specimen found with signs of fluorescence in the prespore) (Table 5). About 3% of the cells of AH3791 (ΔspoIIIG spoIIIA::Tn917 sspE-lacZ ΔamyE::spoIIIGE156K) at 6 h after the onset of sporulation showed accumulation of β-galactosidase, a percentage that increased to 23% at 10 h (Table 5). Surprisingly, in most of these cells, β-galactosidase was found to localize throughout the entire cell (Fig. 5j to l and Table 5). The percentage of sporulating cells of AH3791 (two visible nucleoids of about the same size at this stage) decreased from 75% at 6 h after the onset of sporulation to about 32% at 10 h (Fig. 5j to l [6 h] and m to o [10 h] and Table 5). Note that the specimens showing whole-cell fluorescence do not show the DAPI staining pattern of a mid to late stage of sporulation and that specimens in which the prespore can be clearly distinguished do not show sspE-lacZ expression (Fig. 5j to o). The percentage of AH3790 cells (wild-type spoIIIG in a spoIIIA background) with two distinct nucleoids also decreased from 6 h (78%) to 10 h after the onset of sporulation (60%) (Table 5) except that in the latter case, the whole-cell pattern of decoration was never found. No decrease in the percentage of sporulating cells was noticed for the spoIIIA+ strains.
The results show that σG156K does not become active exclusively in the prespore of a spoIIIA mutant. The results suggest that spoIIIA may function to antagonize an as yet unknown negative regulator of σG following completion of the engulfment process and that spoIIIA may serve an additional function in sporulation related to the maintenance of compartmentalized gene expression in postengulfment cells. Essentially the same observations, i.e., absence of prespore-specific expression of sspE-lacZ, were made for spoIIIA cells harboring the E155K mutation (19; E. M. Kellner and C. P. Moran, Jr., unpublished results), reinforcing the view that the E155K and E156K substitutions affect the activity of σG similarly.
Early expression of spoIIIGE156K in the prespore does not result in premature activity of σG.
Transcription of the spoIIIG gene by σG is delayed by an unknown mechanism towards the end of the engulfment process relative to the transcription of the first class of σF-dependent genes, which includes the spoIIQ gene (24). We fused the coding region of spoIIIGE156K to the early σF-dependent spoIIQ promoter, reasoning that the fusion allele would bypass both the mechanism that delays transcription of spoIIIG and a possible negative effect of SpoIIAB prior to engulfment. The promoter fusion was introduced at the amyE locus, producing strain AH3789 (ΔspoIIIG sspE-lacZ PspoIIQ-spoIIIGE156K). The spoIIQ promoter was also fused to the coding region of the wild-type spoIIIG gene, and the fusion was inserted at amyE to produce AH3788 (ΔspoIIIG sspE-lacZ PspoIIQ-spoIIIG). Both AH3789 and AH3788 sporulate efficiently (Table 3).
In agreement with unpublished work cited by Stragier and Losick (46), the expression of sspE-lacZ in AH3788 began to increase around 4 h after the onset of sporulation, as in strains bearing the wild-type or spoIIIGE156K allele under the control of its native promoter (AH3786 and AH3787) (Fig. 6). Moreover, induction of sspE-lacZ also occurred around 4 h of sporulation in strain AH3789 (Fig. 6); the spoIIQ promoter drives expression of spoIIIGE156K in AH3789.
FIG. 6.
Early expression of wild-type spoIIIG and spoIIIGE156K genes in the prespore. Expression of sspE-lacZ was monitored during sporulation in B. subtilis strains AH3786 (ΔspoIIIG sspE-lacZ amyE::spoIIIG) (wild-type spoIIIG in a wild-type background) (closed circles), AH3787 (ΔspoIIIG sspE-lacZ ΔamyE::spoIIIGE156K) (open circles), AH3788 (ΔspoIIIG sspE-lacZ PspoIIQ-spoIIIG) (wt) (open squares), and AH3789 (ΔspoIIIG sspE-lacZ PspoIIQ-spoIIIGE156K) (closed squares). The complete relevant genotypes of strains are given in Table 1. Strains were grown in DSM, and samples were taken at the indicated times (in hours) after the onset of sporulation (T0) and assayed for β-galactosidase activity. The endogenous levels of β-galactosidase activity were determined in the wild-type strain MB24 (closed triangles). β-Galactosidase activity is given in Miller units (see Materials and Methods).
When produced from their own promoter, σG and σGE156K were first detected 3 h after the onset of sporulation, and the level of the σG factor increased until 4 h (Fig. 2A and B), whereas utilization of the spoIIQ promoter permitted the accumulation of σG or σGE156K from 2 h on, with maximum levels between 3 and 4 h after the onset of sporulation (Fig. 2E and F). In all the strains included in the experiment of Fig. 6, induction of sspE-lacZ expression 4 h after the onset of sporulation coincided with completion of the engulfment process (as assayed by FM4-64 or MTG staining) (between 31 and 56% [data not shown]). Both strains bearing the wild-type or spoIIIGE156K allele under the control of the spoIIQ promoter presented a high background of σG activity starting at the onset of sporulation. The reason for this behavior is not known, but it could be the result of high levels of expression of spoIIIG or spoIIIGE156K from the strong spoIIQ promoter (24). In any event, we note that expression of spoIIIGE156K does not result in a higher background relative to expression of the wild-type spoIIIG gene (Fig. 6). Moreover, we note that in both AH3788 and AH3789, expression of sspE-lacZ remains constant (Fig. 6), while the cellular levels of σG increase (Fig. 2E and F). It appears that even though σGE156K accumulates starting 2 h after the onset of sporulation, it directs induction of sspE-lacZ expression only around 4 h, when the process of engulfment of the prespore by the mother cell is complete. Together, the results suggest that SpoIIAB may not contribute decisively to the inhibition of σG activity in the prespore. The fact that very little free SpoIIAB seems to accumulate in the prespore and the similarity of the interaction of SpoIIAB with both σF and σG (7, 28; this work) are consistent with this interpretation.
Activity of σG in the mother cell is antagonized by SpoIIAB and LonA.
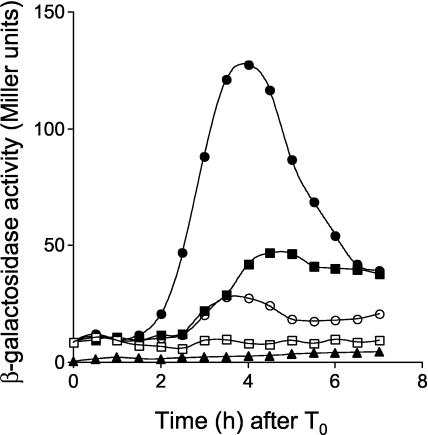
Since σG is autoregulatory and SpoIIAB is present in both the prespore and mother cell compartments, we wanted to determine whether SpoIIAB could have a role in the negative regulation of σG in the mother cell, as previously suggested (3, 8, 19, 21, 35). To investigate this possibility, we fused the coding regions of the wild-type and spoIIIGE156K alleles to the mother cell-specific, σE-dependent spoIID promoter (36). The fusions were transferred to the amyE locus of a strain bearing an in-frame deletion of the spoIIIG gene and an sspE-lacZ fusion (AH2452), yielding strains AH2460 (ΔspoIIIG sspE-lacZ PspoIID-spoIIIG) and AH2461 (ΔspoIIIG sspE-lacZ PspoIID-spoIIIGE156K) (Table 1). No σG activity was detected by monitoring sspE-lacZ-driven β-galactosidase production, when the wild-type spoIIIG allele was expressed in the mother cell (Fig. 7). In contrast, expression of the spoIIIGE156K allele promptly resulted in sspE-lacZ expression, which occurred prior to normal expression of sspE in the prespore, in agreement with the timing of utilization of the spoIID and sspE promoters during sporulation (26, 36) (Fig. 7). This observation supports a role for SpoIIAB in the regulation of σG activity in the mother cell.
FIG. 7.
Role of SpoIIAB in the regulation of σG activity in the mother cell. Expression of sspE-lacZ was monitored during sporulation in B. subtilis strains AH2460 (ΔspoIIIG sspE-lacZ PspoIID-spoIIIG) (wild-type spoIIIG in a wild-type background) (open squares), AH2461 (ΔspoIIIG sspE-lacZ PspoIID-spoIIIGE156K) (closed squares), AH2463 (ΔspoIIIG sspE-lacZ lonA::cat PspoIID-spoIIIG) (wt) (open circles), and AH2465 (ΔspoIIIG sspE-lacZ lonA::cat PspoIID-spoIIIGE156K) (closed circles). The complete relevant genotypes of strains are given in Table 1. Strains were grown in DSM, and samples were taken at the indicated times (in hours) after the onset of sporulation (T0) and assayed for β-galactosidase activity. The endogenous levels of β-galactosidase production were determined in the wild-type strain MB24 (closed triangles). β-Galactosidase activity is given in Miller units (see Materials and Methods).
To determine whether the elevated levels of σG activity observed in strain AH2461 (PspoIID-spoIIIGE156K) relative to strain AH2460 (PspoIID-spoIIIG) correlated with increased accumulation of σG, we conducted immunoblot experiments. We found that the σGE156K protein accumulated starting 2 h after the onset of sporulation, reaching maximum levels around 3 h after sporulation had begun (Fig. 8B), which is in accordance with the temporal pattern of expression of a spoIID-lacZ fusion (36). In contrast, the wild-type form of σG was detected only in trace amounts (Fig. 8A), suggesting that σG is subjected to proteolysis in the mother cell.
FIG. 8.
Immunoblot analysis of σG accumulation produced in the mother cell during sporulation. σG accumulation in B. subtilis strains AH2460 (ΔspoIIIG sspE-lacZ PspoIID-spoIIIG) (wild-type spoIIIG in a wild-type background) (A), AH2461 (ΔspoIIIG sspE-lacZ PspoIID-spoIIIGE156K) (B), AH2463 (ΔspoIIIG sspE-lacZ lonA::cat PspoIID-spoIIIG) (wt) (C), and AH2465 (ΔspoIIIG sspE-lacZ lonA::cat PspoIID-spoIIIGE156K) (D) during sporulation in DSM was examined by immunoblot analysis. Samples from sporulating cultures were collected 1 h after the onset of sporulation in DSM and at hourly intervals thereafter, as indicated by the numbers above the lanes. Proteins (30 μg) in each sample were subjected to immunoblot analysis using an anti-σG rabbit polyclonal antibody (see Materials and Methods). Lanes Δ IIIG contain 30-μg portions of an extract prepared from a culture of a spoIIIG deletion mutant (AH3795 [Table 1]) 4 h after the onset of sporulation. The position of σG is indicated by an arrowhead. Other bands represent nonspecific cross-reactive material. One band, which appears close to σG in the spoIIIG deletion mutant, is marked with an asterisk for reference.
Since the ATP-dependent LonA protease has been implicated in the negative regulation of σG (40, 42), we examined whether a mutation in the lonA gene would also result in increased σG activity in the mother cell. A ΔlonA::cat allele was introduced in strain AH2460, yielding strain AH2463 (ΔspoIIIG sspE-lacZ PspoIID-spoIIIG lonA::cat) (Table 1). Like the spoIIIGE156K allele, the lonA mutation also permitted expression of sspE-lacZ (Fig. 7), and the strain accumulated wild-type σG starting 2 h after the onset of sporulation, with peak levels around 3 h after sporulation had begun (Fig. 8C). Even though the levels of wild-type σG in AH2463 (lonA) appeared higher than the levels of σGE156K in AH2461 (Fig. 8, compare panels B and C), the latter strain showed the highest levels of sspE-lacZ expression (Fig. 7), suggesting that σGE156K is still regulated by LonA.
To test this possibility, we introduced the ΔlonA::cat allele into strain AH2461, yielding strain AH2465 (ΔspoIIIG sspE-lacZ PspoIID-spoIIIGE156K lonA::cat) (Table 1). Strain AH2465 showed higher levels of sspE-lacZ expression than AH2461 (spoIIIGE156K) (Fig. 7). Moreover, σGE156K accumulated at higher levels than in the lonA+ strain AH2461 (Fig. 8B and D). These results show that the σGE156K form is only partially resistant to LonA. Since the levels of σGE156K in a lonA background were lower than the levels of wild-type σG, the σGE156K form may be a substrate for yet another protease. These observations are in agreement with the reduced accumulation relative to wild-type σG in all backgrounds (Fig. 2 and 8). Together, the results suggest that σG does not normally accumulate to significant levels in the mother cell, because of the action of LonA and that in its absence, σG is still negatively regulated by SpoIIAB. Moreover, since the same form of σG (σGE156K) presumed to interact deficiently with the anti-sigma factor SpoIIAB is also partially resistant to LonA (Fig. 8B), the E156K substitution may protect σG from both SpoIIAB and LonA. In any event, the results suggest that both SpoIIAB and LonA contribute to the negative regulation of σG in the mother cell chamber of the sporulating cell.
DISCUSSION
Previous work has shown that σG accumulates but is mostly inactive in spoIIIA or spoIIIJ mutants of B. subtilis (19, 41), both of which show a morphological block just after completion of the engulfment process (6, 30). This has led to the suggestion that both spoIIIA and spoIIIJ act after engulfment to promote the activation of σG in the prespore. Since the activity of σG can be restored to spoIIIA or spoIIIJ mutants by a form of σG that is not efficiently bound by SpoIIAB in vitro, it has also been proposed that the expression of both loci would be required to antagonize the action of SpoIIAB upon completion of the engulfment process (19, 41). In this study, we have screened for random mutations in spoIIIG that could bypass the need for spoIIIA for expression of the σG-controlled sspE gene. We isolated a single allele of spoIIIG (spoIIIGE156K), which codes for lysine at position 156 of σG, instead of glutamate. On the basis of the analysis of a model of the complex between B. subtilis σG and a SpoIIAB dimer, we infer that this allele interferes with the interaction of σG with SpoIIAB by destroying the salt bridge between glutamate 156 of σG and lysine 41 in one of the SpoIIAB molecules (Fig. 3). The previously described E155K substitution (19) prevents the formation of a hydrogen bond between glutamate 155 of σG and serine 17 in SpoIIAB (Fig. 3). This suggests that σGE156K is at least as refractory to SpoIIAB binding as σGE155K. The reduced susceptibility of σGE156K to SpoIIAB compared to the susceptibility of wild-type σG in vegetative cells of B. subtilis is in agreement with the idea that the E156K mutation interferes with SpoIIAB binding (Fig. 4).
In wild-type cells, the prespore-specific activation of σG coincides with the completion of the engulfment process (29; this work). The main finding of this investigation was that expression of sspE-lacZ in cells of the spoIIIA spoIIIGE156K mutant was delayed relative to completion of engulfment and was not confined to the prespore compartment. In strain AH3791 (a spoIIIA spoIIIGE156K double mutant expressing sspE-lacZ), the fraction of cells that had completed engulfment 4 h after the onset of sporulation was half that observed for a wild-type strain, and by 6 h after the onset of sporulation, the proportion of AH3791 cells with fully engulfed prespores (39%) was even closer to that of the wild-type AH3786 (48%). However, in AH3791, the activity of β-galactosidase peaked at 10 h after the onset of sporulation, not at 6 h as in a wild-type strain (Fig. 1). Moreover, the peak of β-galactosidase activity in AH3791 did not coincide with an increase in the frequency of fully engulfed prespores but with a decrease in cells with clear signs of sporulation (Tables 4 and 5; see below).
In addition, β-galactosidase was not confined to the prespore but distributed throughout the entire cell (Fig. 5). The pattern of whole-cell decoration could represent cells that have not entered the sporulation pathway and maintain their vegetative state. Because σG is autoregulatory (17, 47) and because SpoIIAB negatively regulates σG in nonsporulating cells (8, 35), a mutation impairing SpoIIAB binding could result in increased activity of σG. In that case, σG activity should have been detected during growth, early in sporulation, and presumably also in the mother cell in strains bearing the spoIIIGE156K allele. However, this was not the case: no β-galactosidase accumulated during vegetative growth (data not shown), and very few cells of the Spo+ strain AH3787 (which express spoIIIGE156K in a spoIIIA+ background) presented the same pattern of whole-cell decoration (Fig. 5 and Table 5). An alternative explanation is that the whole-cell decoration pattern corresponds to cells that have entered sporulation but that late in development they fail to maintain compartmentalized gene expression because the spoIIIA mutation results in instability of the prespore envelope. We favor this idea for two reasons. First, the instability of the prespore membranes has been reported for certain mutants that do not proceed past the stage of the completion of engulfment (30, 32). Second, it agrees with the observation that in cells bearing a spoIIIA mutation (expressing either the wild-type allele or spoIIIGE156K), the percentage of sporulation seems to decrease from 6 to 10 h after the onset of sporulation, as scored by the staining patterns of the prespore membranes by MTG (Table 4) and of the nucleoid by DAPI (Table 5). Thus, expression of sspE-lacZ in the spoIIIA spoIIIGE156K mutant (AH3791) would occur only upon partial lysis of the prespore at a late time in development, permitting access of some σGE156K to the mother cell, where it would prime its own synthesis. Note that σGE156K accumulates in the mother cell, whereas wild-type σG does not (Fig. 8; see below). The loss compartmentalization of σF activity in spoIIIE insertional mutants indicates that the prespore membranes become permeable to sigma factors at least under certain conditions (50).
Whatever the correct interpretation, the observation that the spoIIIGE156K allele does not restore prespore-specific expression of sspE-lacZ to a spoIIIA mutant has several implications. If mutations that impair binding of SpoIIAB do not permit activation of σG in the prespore of a spoIIIA mutant after completion of the engulfment process, then it seems likely that the activity of σG is negatively regulated by some other factor. It would be difficult to explain that SpoIIAB inhibits σG at a time when SpoIIAB is inactivated to permit expression of the σF regulon (reviewed in references 31 and 37), especially if the interaction of SpoIIAB with σF is similar to that of SpoIIAB with σG (7; this work). Moreover, because uncomplexed SpoIIAB is rapidly proteolyzed, there seems to be very little, if any, free SpoIIAB in the prespore (28). Normally, σG is produced late, just prior to the completion of the engulfment process (29). However, since σG efficiently recognizes its own promoter, even low levels of σG would have to be subjected to negative regulation. It could be that very low levels of SpoIIAB escaping proteolysis would be sufficient to maintain the inactivity of σG. This seems unlikely, because expression of the wild-type or spoIIIGE156K allele from the strong, early σF-dependent spoIIQ promoter does not result in premature induction of sspE-lacZ expression (46) (Fig. 6). Together, these observations suggest that σG156K is kept inactive in the prespore by an as yet unidentified factor, which is antagonized in the postengulfment prespore in a spoIIIA- and spoIIIJ-dependent manner. Since activity of σGE156K is detected in spoIIIA or spoIIIJ mutants only at late times after sporulation, when the prespore membranes show signs of instability, it is possible that the putative prespore inhibitor of σG is unstable and tends to disappear at late times in sporulation. We do not know whether σG is equally unstable, but presumably very little σG is needed to establish the positive-feedback loop leading to its increased accumulation around 8 h after the onset of sporulation (Fig. 2). The results suggest that SpoIIAB is either not involved in the regulation of σG in the prespore or is redundant. Since no mutations that result in deregulated σG activity in the prespore are known, it may be that the putative inhibitor of σG (if other than SpoIIAB) has remained elusive, because it is the product of a small or essential gene or because mutations that make σG resistant to inhibition also impair its activity.
Also worthy of comment is the role of SpoIIAB in the mother cell, together with the LonA protease. We found that the wild-type σG does not accumulate in the mother cell when expressed from the strong σE-dependent spoIID promoter (Fig. 8A). Because a mutation in the lonA gene, encoding the ATP-dependent LonA protease, results in accumulation of σG and expression of sspE-lacZ, LonA appears to promote the degradation of σG in the mother cell (Fig. 7 and 8C). Since expression of spoIIIGE156K from the spoIID promoter results in higher levels of sspE-lacZ expression, we conclude that SpoIIAB is also capable of inhibiting σG in the mother cell (Fig. 7). LonA has been previously implicated in the negative regulation of σG under nutritional conditions that do not allow efficient sporulation (40). Moreover, a mutation in the lonA gene is also able to partially restore σG activity to a spoIIIA mutant during sporulation but only after a delay of 2 h relative to the normal timing of expression of the σG regulon (40). This effect is reminiscent to that observed for the spoIIIGE155K or spoIIIGE156K allele, and it could be restricted to the mother cell, since expression of lonA from a prespore-specific promoter was found to strongly reduce σG activity and sporulation (42). In any event, since the E156K mutation also confers some immunity against LonA (Fig. 8B), at this time we cannot decide whether expression of sspE-lacZ in cells of the spoIIIA::Tn917 spoIIIGE156K double mutant (AH3791) is a consequence of the impaired ability of SpoIIAB to bind to σG, its resistance to the LonA protease, or both.
In conclusion, our results suggest that mutations that make σG resistant to SpoIIAB do not permit expression of the σG-dependent sspE gene in the prespore of a spoIIIA mutant. While our results support earlier findings indicating that SpoIIAB (together with LonA) is important in the mother cell, they suggest that SpoIIAB is not a decisive regulator of σG in the prespore.
ADDENDUM IN PROOF
A recent report shows that compartmentalized gene expression is compromised in spoIIIA or spoIIIJ B. subtilis mutants because of prespore instability (Z. Li, F. Di Donato, and P. J. Piggot, J. Bacteriol. 186:2221-2223, 2004). These results support our interpretation that the activity of σGE156K seen in spoIIIA cells is due to instability of the prespore and loss of compartmentalized gene expression in this mutant background.
Acknowledgments
We thank Gonçalo Real and Patrick Piggot for helpful discussions and comments on the manuscript and Ellen Kellner and Patrick Piggot for sharing information prior to publication and for helpful discussions. We also thank Filipe Vieira for help with some of the plasmid constructions.
This work was supported in part by grants Praxis XXI/PCNA/C/BIO/13201/98 and PRAXIS/BIO/35109/99 from the Fundação para a Ciência e a Tecnologia (F.C.T.) to A.O.H and grant GM54395 from the National Institutes of Health to C. P. Moran, Jr. M.S. is the recipient of a Ph.D. fellowship (PRAXIS XXI/BD 18 251/98) from the F.C.T.
REFERENCES
- 1.Campbell, E. A., and S. A. Darst. 2000. The anti-σ factor SpoIIAB forms a 2:1 complex with σF, contacting multiple conserved regions of the σ factor. J. Mol. Biol. 300:17-28. [DOI] [PubMed] [Google Scholar]
- 2.Campbell, E. A., S. Masuda, J. L. Sun, O. Muzzin, C. A. Olson, S. Wang, and S. A. Darst. 2002. Crystal structure of the Bacillus stearothermophilus anti-σ factor SpoIIAB with the sporulation factor σF. Cell 108:795-807. [DOI] [PubMed] [Google Scholar]
- 3.Coppolechia, R., H. DeGrazia, and C. P. Moran, Jr. 1991. Deletion of spoIIAB blocks endospore formation in Bacillus subtilis at an early stage. J. Bacteriol. 173:6678-6685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cutting, S. M., and P. B. V. Horn. 1990. Genetics analysis, p. 27-74. In C. R. Harwood and S. M. Cutting (ed.), Molecular biological methods for Bacillus. John Wiley & Sons Ltd., Chichester, England.
- 5.Decatur, A., and R. Losick. 1996. Multiple sites of contact between the Bacillus subtilis developmental transcription factor σF and its anti-sigma factor SpoIIAB. Genes Dev. 10:2348-2358. [DOI] [PubMed] [Google Scholar]
- 6.Errington, J., L. Appleby, R. A. Daniel, H. Goodfellow, S. R. Partridge, and M. D. Yudkin. 1992. Structure and function of the spoIIIJ gene of Bacillus subtilis: a vegetatively expressed gene that is essential for sigma G activity at an intermediate stage of sporulation. J. Gen. Microbiol. 138:2609-2618. [DOI] [PubMed] [Google Scholar]
- 7.Evans, L., J. Clarkson, M. D. Yudkin, J. Errington, and A. Feucht. 2003. Analysis of the interaction between the transcription factor σG and the anti-sigma factor SpoIIAB of Bacillus subtilis. J. Bacteriol. 185:4615-4619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Foulger, D., and J. Errington. 1993. Effects of new mutations in the spoIIAB gene of Bacillus subtilis on the regulation of σF and σG activities. J. Gen. Microbiol. 139:3197-3203. [DOI] [PubMed] [Google Scholar]
- 9.Guérout-Fleury, A. M., N. Frandsen, and P. Stragier. 1996. Plasmids for ectopic integration in Bacillus subtilis. Gene 180:57-61. [DOI] [PubMed] [Google Scholar]
- 10.Hackett, R. H., and P. Setlow. 1987. Cloning, nucleotide sequencing, and genetic mapping of the gene for small, acid-soluble spore protein γ of Bacillus subtilis. J. Bacteriol. 169:1985-1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harry, E. J., K. Pogliano, and R. Losick. 1995. Use of immunofluorescence to visualize cell-specific gene expression during sporulation in Bacillus subtilis. J. Bacteriol. 177:3386-3393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Henner, D. J. 1990. Inducible expression of regulatory genes in Bacillus subtilis. Methods Enzymol. 185:223-228. [DOI] [PubMed] [Google Scholar]
- 13.Henriques, A. O., B. W. Beall, and C. P. Moran, Jr. 1997. CotM of Bacillus subtilis, a member of the α-crystallin family of stress proteins, is induced during development and participates in spore outer coat formation. J. Bacteriol. 179:1887-1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Henriques, A. O., B. W. Beall, K. Rowland, and C. P. Moran, Jr. 1995. Characterization of cotJ, a σE-controlled operon affecting the polypeptide composition of the coat of Bacillus subtilis spores. J. Bacteriol. 177:3394-3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Illing, N., and J. Errington. 1991. The spoIIIA operon of Bacillus subtilis defines a new temporal class of mother-cell-specific sporulation genes under the control of the sigma E form of RNA polymerase. Mol. Microbiol. 5:1927-1940. [DOI] [PubMed] [Google Scholar]
- 16.Itaya, M., K. Kondo, and T. Tanaka. 1989. A neomycin resistance gene cassette selectable in a single copy state in the Bacillus subtilis chromosome. Nucleic Acids Res. 17:4410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karmazyn-Campelli, C., C. Bonamy, B. Savelli, and P. Stragier. 1989. Tandem genes encoding σ-factors for consecutive steps of development in Bacillus subtilis. Genes Dev. 3:150-157. [DOI] [PubMed] [Google Scholar]
- 18.Karow, M. L., and P. J. Piggot. 1995. Construction of gusA transcriptional fusion vectors for Bacillus subtilis and their utilization for studies of spore formation. Gene 163:69-74. [DOI] [PubMed] [Google Scholar]
- 19.Kellner, E. M., A. Decatur, and C. P. Moran, Jr. 1996. Two-stage regulation of an anti-sigma factor determines developmental fate during bacterial endospore formation. Mol. Microbiol. 21:913-924. [DOI] [PubMed] [Google Scholar]
- 20.Kenney, T. J., and C. P. Moran, Jr. 1987. Organization and regulation of an operon that encodes a sporulation-essential sigma factor in Bacillus subtilis. J. Bacteriol. 169:3329-3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kirchman, P. A., H. DeGrazia, E. M. Kellner, and C. P. Moran, Jr. 1993. Forespore-specific disappearance of the sigma-factor antagonist SpoIIAB: implications for its role in determination of cell fate in Bacillus subtilis. Mol. Microbiol. 8:663-671. [DOI] [PubMed] [Google Scholar]
- 22.Kroos, L., B. Zhang, H. Ichikawa, and Y.-T. N. Yu. 1999. Control of σ factor activity during Bacillus subtilis sporulation. Mol. Microbiol. 31:1285-1294. [DOI] [PubMed] [Google Scholar]
- 23.Laskowski, A., M. MacArthur, D. Moss, and J. Thorton. 1993. PROCHECK: a program to check the stereochemical quality of protein structures. J. Appl. Crystallogr. 26:283-291. [Google Scholar]
- 24.Londoño-Vallejo, J.-A., C. Fréhel, and P. Stragier. 1997. spoIIQ, a forespore-expressed gene required for engulfment in Bacillus subtilis. Mol. Microbiol. 24:29-39. [DOI] [PubMed] [Google Scholar]
- 25.Marti-Renom, M., A. Stuart, A. Fiser, R. Sanchez, F. Melo, and A. Sali. 2000. Comparative protein structure modeling of genes and genomes. Annu. Rev. Biophys. Biomol. Struct. 29:291-325. [DOI] [PubMed] [Google Scholar]
- 26.Mason, J. M., R. H. Hackett, and P. Setlow. 1988. Regulation of transcription of genes coding for small, acid-soluble proteins of Bacillus subtilis spores: studies using lacZ fusions. J. Bacteriol. 170:239-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murakami, T., K. Haga, M. Takeuchi, and T. Sato. 2002. Analysis of the Bacillus subtilis spoIIIJ gene and its paralogue gene, yqjG. J. Bacteriol. 184:1998-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pan, Q., D. A. Garsin, and R. Losick. 2001. Self-reinforcing activation of a cell-specific transcription factor by proteolysis of an anti-σ factor in B. subtilis. Mol. Cell 8:873-883. [DOI] [PubMed] [Google Scholar]
- 29.Partridge, S., and J. Errington. 1993. The importance of morphological events and intercellular interactions in the regulation of prespore-specific gene expression during sporulation in Bacillus subtilis. Mol. Microbiol. 8:945-955. [DOI] [PubMed] [Google Scholar]
- 30.Piggot, P. J., and J. G. Coote. 1976. Genetic aspects of bacterial endospore formation. Bacteriol. Rev. 40:908-962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Piggot, P. J., and R. Losick. 2001. Sporulation genes and intercompartmental regulation, p. 483-517. In A. L. Sonenshein, J. A. Hoch, and R. Losick (ed.), Bacillus subtilis and its closest relatives from genes to cells. ASM Press, Washington, D.C.
- 32.Piggot, P. J., J. E. Byland, and M. L. Higgins. 1994. Morphogenesis and gene expression during sporulation, p. 113-137. In P. J. Piggot, C. P. Moran, Jr., and P. Youngman (ed.), Regulation of bacterial differentiation. ASM Press, Washington, D.C.
- 33.Pogliano, K., E. Harry, and R. Losick. 1995. Visualization of the subcellular location of sporulation proteins in Bacillus subtilis using immunofluorescence microscopy. Mol. Microbiol. 18:459-470. [DOI] [PubMed] [Google Scholar]
- 34.Rather, P. N., and C. P. Moran, Jr. 1988. Compartment-specific transcription in Bacillus subtilis: identification of the promoter gdh. J. Bacteriol. 170:5086-5092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rather, P. N., R. Coppolechia, H. deGrazia, and C. P. Moran, Jr. 1990. Negative regulator of σG-controlled gene expression in stationary-phase Bacillus subtilis. J. Bacteriol. 172:709-715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rong, S., M. S. Rosenkrantz, and A. L. Sonenshein. 1986. Transcriptional control of the Bacillus subtilis spoIID gene. J. Bacteriol. 165:771-779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rudner, D. Z., and R. Losick. 2001. Morphological coupling in development: lessons from prokaryotes. Dev. Cell 1:733-742. [DOI] [PubMed] [Google Scholar]
- 38.Sali, A., and T. L. Blundell. 1993. Comparative protein modelling by satisfaction of spatial restraints. J. Mol. Biol. 234:779-815. [DOI] [PubMed] [Google Scholar]
- 39.Sánchez, R., and A. Sali. 1997. Advances in comparative protein-structure modelling. Curr. Opin. Struct. Biol. 7:206-214. [DOI] [PubMed] [Google Scholar]
- 40.Schmidt, R., A. L. Decatur, P. N. Rather, C. P. Moran, Jr., and R. Losick. 1994. Bacillus subtilis Lon protease prevents inappropriate transcription of genes under the control of the sporulation transcription factor σG. J. Bacteriol. 176:6528-6537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Serrano, M., L. Côrte, J. Opdyke, C. P. Moran, Jr., and A. O. Henriques. 2003. Expression of spoIIIJ in the prespore is sufficient for the activation of σG and for sporulation in Bacillus subtilis. J. Bacteriol. 185:3905-3917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Serrano, M., S. Hoevel, C. P. Moran, Jr., A. O. Henriques, and U. Völker. 2001. Prespore-specific transcription of the lonB gene encoding a Lon-like ATP-dependent protease during sporulation in Bacillus subtilis. J. Bacteriol. 183:2032-2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Setlow, B., N. Magill, P. Febbroriello, L. Nakhimovsky, D. E. Koppel, and P. Setlow. 1991. Condensation of the forespore nucleoid early in sporulation of Bacillus species. J. Bacteriol. 173:6270-6278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sharp, M. D., and K. Pogliano. 1999. An in vivo membrane fusion assay implicates SpoIIIE in the final stages of engulfment during Bacillus subtilis sporulation. Proc. Natl. Acad. Sci. USA 96:14553-14558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stragier, P. 1992. Establishment of forespore-specific gene expression during sporulation of Bacillus subtilis, p. 297-310. In J. A. Cole, F. Mohan, and C. Dow (ed.), Prokaryotic structure and function. Society for General Microbiology, Cambridge, England.
- 46.Stragier, P., and R. Losick. 1996. Molecular genetics of sporulation in Bacillus subtilis. Annu. Rev. Genet. 30:297-341. [DOI] [PubMed] [Google Scholar]
- 47.Sun, D., R. M. Cabrera-Martinez, and P. Setlow. 1991. Control of transcription of the Bacillus subtilis spoIIIG gene, which codes for the forespore-specific transcription factor σG. J. Bacteriol. 173:2977-2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tjalsma, H., S. Bron, and J. M. van Dijl. 2003. Complementary impact of paralogous Oxa1-like proteins of Bacillus subtilis on posttranslocational stages in protein secretion. J. Biol. Chem. 278:15622-15632. [DOI] [PubMed] [Google Scholar]
- 49.Wu, J.-J., M. G. Howard, and P. J. Piggot. 1989. Regulation of transcription of the Bacillus subtilis spoIIA locus. J. Bacteriol. 171:692-698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wu, L. J., and J. Errington. 1994. Bacillus subtilis SpoIIIE protein required for DNA segregation during asymmetric cell division. Science 264:572-575. [DOI] [PubMed] [Google Scholar]
- 51.Zilhão, R., M. Serrano, R. Isticato, E. Ricca, and A. O. Henriques. 2003. Assembly of Bacillus subtilis spore coat protein CotB. J. Bacteriol. 186:1110-1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zuber, P., and R. Losick. 1987. Role of AbrB in Spo0A- and Spo0B-dependent utilization of a sporulation promoter in Bacillus subtilis. J. Bacteriol. 169:2223-2230. [DOI] [PMC free article] [PubMed] [Google Scholar]