Abstract
The gram-negative bacterium Legionella pneumophila grows in both natural and man-made water systems and in the mammalian lung as a facultative intracellular parasite. The PilD prepilin peptidase of L. pneumophila promotes type IV pilus biogenesis and type II protein secretion. Whereas pili enhance adherence, Legionella type II secretion is critical for intracellular growth and virulence. Previously, we observed that pilD transcript levels are greater in legionellae grown at 30 versus 37°C. Using a new pilD::lacZ fusion strain, we now show that pilD transcriptional initiation increases progressively as L. pneumophila is grown at 30, 25, and 17°C. Legionella pilD mutants also had a dramatically reduced ability to grow in broth and to form colonies on agar at the lower temperatures. Whereas strains specifically lacking type IV pili were not defective for low-temperature growth, mutations in type II secretion (lsp) genes greatly impaired the capacity of L. pneumophila to form colonies at 25, 17, and 12°C. Indeed, the lsp mutants were completely unable to grow at 12°C. The growth defect of the pilD and lsp mutants was complemented by reintroduction of the corresponding intact gene. Interestingly, the lsp mutants displayed improved growth at 25°C when plated next to a streak of wild-type but not mutant bacteria, implying that a secreted, diffusible factor promotes low-temperature growth. Mutants lacking either the known secreted acid phosphatases, lipases, phospholipase C, lysophospholipase A, or protease grew normally at 25°C, suggesting the existence of a critical, yet-to-be-defined exoprotein(s). In summary, these data document, for the first time, that L. pneumophila replicates at temperatures below 20°C and that a bacterial type II protein secretion system facilitates growth at low temperatures.
The genus Legionella was recognized in 1977, after the isolation of L. pneumophila from patients that had succumbed to a form of pneumonia now known as Legionnaires' disease (29). The genus currently includes 49 species of gram-negative bacteria that inhabit aquatic environments (24, 62). Human infection occurs after the inhalation of contaminated water droplets originating from aerosol-generating devices (13, 24), and disease follows from bacterial invasion of lung macrophages (16, 80). L. pneumophila is now a common cause of community- and hospital-acquired pneumonia (13, 24).
Shortly after its discovery, L. pneumophila was detected in virtually all of the 267 freshwater habitats (i.e., lakes, ponds, rivers, creeks, swamps, and wet soil) examined in the United States (27, 28). The ubiquity of L. pneumophila in freshwater has been confirmed throughout the world (10, 14, 20, 39, 44, 58, 83, 84) and, in recent years, the organism has been found in marine and estuarine environments (34, 58, 59, 63). In artificial water systems, L. pneumophila is similarly widespread, existing in some areas within the plumbing systems of 60% of large and small public buildings, as well as in private residences (1, 45). The broad distribution of L. pneumophila is partly due to the organism's capacity to survive at 4 to 63°C (7, 27, 28, 34, 39, 89). However, since the bacterium grows best in the laboratory at 32 to 37°C (11, 42, 43, 91), there are few data on its physiology at lower temperatures (52). In its aquatic habitats, L. pneumophila exists planktonically, sessile within biofilms, as an intracellular parasite of protozoa, and as a viable but nonculturable entity (7, 18, 23, 33, 38, 50, 54, 63, 64). The definition of the bacterial factors that promote intracellular infection and low-temperature growth is key to fully understanding the natural history of legionellosis (16, 24, 80).
In previous studies (47, 48), our investigators determined that the PilD prepilin peptidase is required for the formation of L. pneumophila pili, surface appendages that enhance attachment to macrophages, amoebae, and biofilms (76; C. E. Lucas, E. Brown, T. S. Forster, R. Murga, R. M. Donlan, N. P. Cianciotto, Y. Abu Kwaik, and B. S. Fields, Abstr. Am. Soc. Microbiol. 102nd Annu. Meet., abstr. Q-258, p. 422, 2002). In later work (69), our group determined that PilD also promotes L. pneumophila type II secretion, a form of protein export that others and we have shown to be critical for intracellular growth (31, 68, 70). In the gram negatives, PilD promotes pilus biogenesis and protein secretion by cleaving and then methylating distinct sets of pilin-like proteins (pseudopilins) that form either the type IV pilus scaffold or the type II secretion apparatus (2, 12, 56, 57, 66, 73, 79). Using a murine model of pneumonia, our investigators recently showed that PilD and type II protein secretion, but not type IV pili, play major roles in Legionnaires' disease (70). Secreted activities associated with the L. pneumophila type II system include protease, acid phosphatase, RNase, lipase, phospholipase A, phospholipase C, and lysophospholipase activities (4-6, 25, 26, 31, 69, 70). Importantly, the Legionella type II secretion pathway (Lsp) remains the only type II system linked to intracellular infection (70, 71, 74).
In contrast to the progress made toward elucidating the components of the type II secretion apparatus, there are few data on the regulation of its genes, although quorum sensing has been linked to xcp expression in Pseudomonas aeruginosa (15, 73, 74). While demonstrating that L. pneumophila piliation is temperature regulated, our laboratory observed an increase in the level of pilD-containing transcripts when wild-type legionellae were grown at 30 versus 37°C (48). Thus, we began this study by confirming that pilD transcription increases at temperatures below 37°C. In the process, we have uncovered a novel role for type II protein secretion in bacterial growth at low temperatures.
MATERIALS AND METHODS
Bacterial strains and media.
L. pneumophila serogroup 1 strain 130b (ATCC BAA-74) served as the wild-type strain in this study. Previously described mutants of strain 130b that contain kanamycin resistance (Kmr) or gentamicin resistance (Gmr) cassette insertions into genes associated with type II protein secretion, type IV piliation, or type IV secretion were also examined (Table 1). Another 130b derivative, NU236, that was studied contains both a promoterless lacZ and a Kmr cassette inserted into an iron-regulated gene (frg) (37). Legionellae were cultured at 37°C on buffered charcoal yeast extract (BCYE) agar or in buffered yeast extract (BYE) broth (21). A pilD mutant of P. aeruginosa (i.e., PAK-2B18) containing either vector alone or the complementing pilD gene was previously described and was cultured on BCYE agar (77). Escherichia coli strain NovaBlue (Novagen, Madison, Wis.), host for recombinant plasmids, was grown on Luria-Bertani agar (8). Antibiotics were added to the media at the following final concentrations (in micrograms per milliliter): ampicillin, 100; chloramphenicol, 6 for L. pneumophila and 30 for E. coli; gentamicin, 2.5; and kanamycin, 25 for L. pneumophila and 50 for E. coli.
TABLE 1.
L. pneumophila strains and their efficiency of plating (EOP) at room temperature
| Strain | Mutation(s) | Deficiency | Reference | EOP at 25°Ca |
|---|---|---|---|---|
| 130b | Wild type | None | 22 | 61 ± 33 |
| NU286 | pilD::lacZ Kmr | Prepilin peptidase | This study | 0.032 ± 0.009 |
| NU243 | pilD::Kmr | Prepilin peptidase | 47 | 0.036 ± 0.036 |
| NU272 | pilD::Gmr | Prepilin peptidase | 70 | 0.003 ± 0.003 |
| NU258 | lspDE::Kmr | Type II secretin and ATPase | 69 | 0.028 ± 0.021 |
| NU275 | lspF::Kmr | Type II membrane protein | 70 | 0.010 ± 0.002 |
| BS100 | pilEL::Kmr | Type IV pilus pilin | 76 | 52 ± 12 |
| NU279 | pilQ::Gmr | Type IV pilus secretin | 70 | 37 ± 5 |
| NU283 | lspDE::Kmr | Type II secretin and ATPase | 70 | 0.0005 ± 0.0001 |
| pilQ::Gmr | Type IV pilus secretin | |||
| GG105 | dotA::Kmr | Type IV secretion | 92 | 57 ± 21 |
| GQ262 | dotDCB::Kmr | Type IV secretion | 92 | 39 ± 27 |
| GN142 | icmGCD::Kmr | Type IV secretion | 92 | 68 ± 7 |
| NU291 | pilD::Gmr | Prepilin peptidase | This study | 0.006 ± 0.006 |
| icmGCD::Kmr | Type IV secretion | |||
| AA200 | proA::Kmr | ProA metalloprotease | 53 | 73 ± 42 |
| NU254 | map::Kmr | Major acid phosphatase | 4 | 74 ± 26 |
| NU267 | lipA::Kmr | LipA and LipB lipases | 6 | 109 ± 5 |
| lipB::Gmr | ||||
| NU268 | plcA::Kmr | PlcA phospholipase C | 6 | 87 ± 18 |
| NU270 | plaA::Kmr | PlaA lysophospholipase A | 26 | 80 ± 9 |
Calculated as follows: 100 × (CFU formed at 25°C in 7 to 10 days/CFU formed at 37°C in 3 days). The results are the means and standard deviations of at least two independent experiments.
Construction of an L. pneumophila strain containing a pilD::lacZ fusion.
In order to monitor pilD transcription in L. pneumophila, we sought to place a promoterless lacZ gene under the control of the pilD promoter in wild-type strain 130b. Toward that end, the lacZ-containing, BamHI fragment of pMC1871 (Pharmacia Biotech, Piscataway, N.J.) was inserted into the BamHI site of pVK3 (85), yielding pVK6, and then the BamHI/BclI fragment of pVK6 was replaced with the BamHI/BclI fragment of pRS551 (75), resulting in pVK10 and a removable, promoterless lacZ gene that contains its own translational signal. Next, the SalI/XbaI fragment of pVK10 that contains lacZ and a downstream Kmr gene was isolated, Klenow treated, and ligated into the NarI site of pilD in pML219 (48), creating pMS10. Finally, an L. pneumophila strain (i.e., NU286) containing the pilD::lacZ fusion was isolated upon natural transformation (26) of strain 130b with pMS10. The genotype of the strain was verified by PCR and Southern hybridization analysis (data not shown). Genomic DNA was isolated from L. pneumophila as previously described (22).
Construction of an L. pneumophila strain lacking both type II and type IV secretion.
For the construction of a strain lacking both PilD and Dot/Icm, pGD::Gm (70) containing pilD::Gmr was introduced by transformation into icmGCD::Kmr mutant GN142 (92), and kanamycin-resistant, gentamicin-resistant clones were isolated. The genotype of the double mutant (i.e., NU291) was verified by PCR, and its phenotype was verified by plating on egg yolk agar (5, 26, 70) (data not shown).
Extracellular growth determinations.
In order to compare the extracellular growth of L. pneumophila strains at different temperatures, equal numbers of CFU of wild-type and mutant bacteria taken from log-phase BYE cultures grown at 37°C were inoculated into 40 ml of BYE broth (in a 125-ml flask) and then incubated with shaking (225 rpm) at 37, 30, 25, and 17°C. The extent of bacterial growth was assessed by measuring the optical density of the cultures at 660 nm. Additionally, bacteria taken from 3-day old, 37°C BCYE plates were resuspended in water, diluted, and plated for isolated colonies on BCYE agar incubated in air at 37, 25, 17, 12, and 4°C. The efficiency of plating at 25, 17, 12, or 4°C was determined by dividing the number of CFU obtained at the low temperature on various days by the number of CFU obtained at 37°C. Plates incubated at 37°C were counted on day 3, whereas those at 25, 17, and 12°C were scored on days 7 to 10, 20 to 21, and 70, respectively. The low-temperature growth of P. aeruginosa strains was similarly tested on BCYE agar. To begin to determine if secreted factors facilitate low-temperature growth, a heavy streak of wild-type legionellae taken from BCYE agar that had been incubated at 37°C was added to one side of a BCYE plate unto which had been previously spread ca. 105 CFU of a type II secretion mutant, also derived from a fresh 37°C plate. The plates were then incubated at 25°C in air and observed after 7 to 10 days for accelerated growth of the secretion mutant at the low temperature. Control plates received either no added streak or a streak of the secretion mutant. A parallel set of plates was stored at 37°C and analyzed after 3 days of incubation.
Enzymatic assays.
Filter-sterilized supernatants from late-exponential-phase cultures grown in BYE broth at 37, 25, and 17°C were tested for enzymatic activities as previously described. Tartrate-sensitive and tartrate-resistant acid phosphatase activities were monitored by the release of p-nitrophenol (p-NP) from p-NP phosphate in 200 mM sodium acetate (pH 5.5) in the absence or presence of 5 mM tartrate (4). To measure alkaline phosphatase activity, the hydrolysis of p-NP phosphate was performed in 100 mM Tris, pH 10 (4). Lipolytic activities were determined by p-NP palmitate hydrolysis (5, 6), and protease activity was ascertained by azocasein hydrolysis (5). To quantitate lacZ expression in L. pneumophila gene fusion strains, β-galactosidase activity was measured in 0.1-ml aliquots taken from BYE broth cultures (see above) as previously described (37), and enzyme levels were reported in standard Miller units (8).
RESULTS
Effect of low-temperature growth on pilD expression.
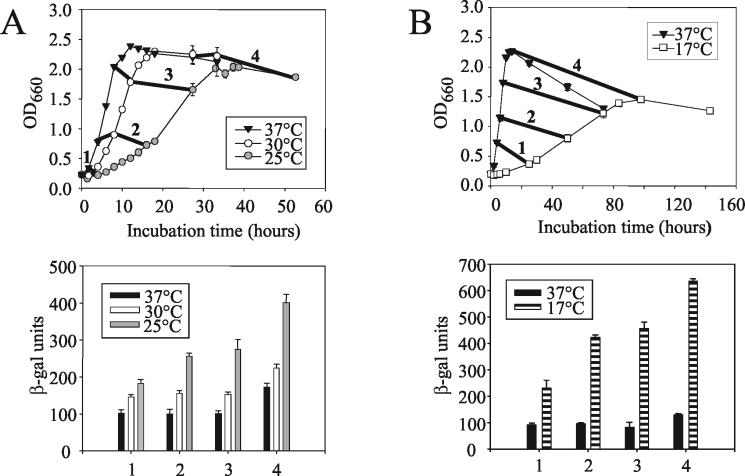
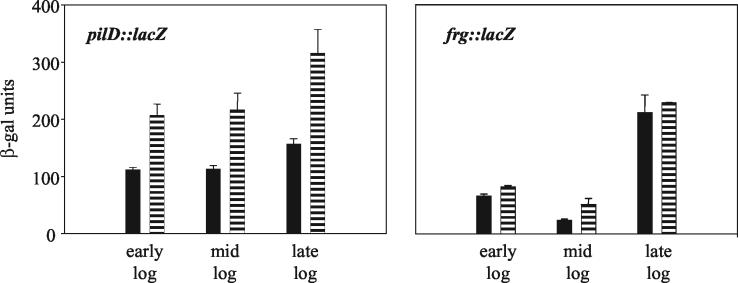
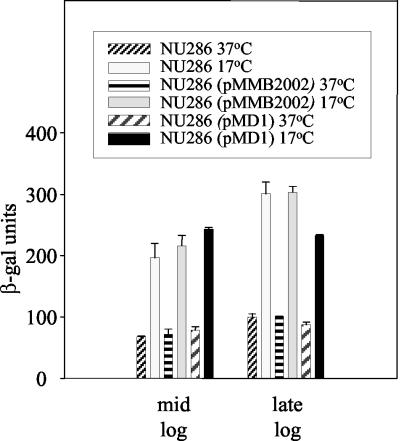
Based upon Northern blotting and RT-PCR analyses, the level of pilD transcripts is higher when L. pneumophila is grown on BCYE agar at 30 versus 37°C (48). To further investigate the effect of temperature on pilD expression, we constructed a derivative of L. pneumophila strain 130b that contains a promoterless lacZ gene inserted into pilD, and then we assayed β-galactosidase levels in legionellae grown at different temperatures. As expected, the pilD::lacZ fusion strain, NU286, had the secretion and infectivity defects of previous pilD mutants (data not shown). To control for growth stage, the pilD::lacZ strain was cultured in BYE broth and enzyme comparisons were made using bacteria taken from the same stage of growth. The fusion strain produced 50% more β-galactosidase at 30 versus 37°C, and when the bacteria were grown at 25°C, the enzyme levels increased by two- to threefold (Fig. 1) (P < 0.05, Student's t test). NU286 cells grown at 17°C had β-galactosidase levels that were often sixfold greater than that produced by 37°C-grown bacteria (Fig. 1) (P < 0.05). In contrast to the results obtained with the pilD::lacZ fusion strain, L. pneumophila strain NU236, which contains lacZ fused to an frg gene (37), did not show elevated β-galactosidase when grown in BYE at 17 versus 37°C (Fig. 2). Thus, the increases in pilD::lacZ expression, which were seen in at least eight trials, were not simply the result of the changes in growth rate that were associated with temperature reduction, nor were they an artifact of using a lacZ reporter gene. To be sure that the increased β-galactosidase levels observed at the low temperature were also not an artifact of assessing pilD::lacZ expression in a PilD-negative strain, we repeated the assay using NU286 containing an intact pilD gene cloned into pMD1. The complemented pilD::lacZ fusion strain expressed the same increase in β-galactosidase activity at 17°C (P < 0.05) as the original NU286, as well as NU286 containing the pMMB2002 vector (Fig. 3). Taken together, the gene fusion data indicate that pilD expression is influenced by temperature, with increases in transcription initiation occurring at low temperatures.
FIG. 1.
Effect of temperature on the growth of and β-galactosidase production by an L. pneumophila strain containing a pilD::lacZ gene fusion. Log-phase NU286 bacteria were inoculated into BYE broth and then incubated, as noted, at 37, 30, and 25°C (A) or 37 and 17°C (B). The growth of the cultures was monitored spectrophotometrically (top panels) and, at four indicated time points (labeled as 1, 2, 3, and 4) when the various cultures had achieved comparable stages of growth, the amount of β-galactosidase was examined (bottom panel). The results presented are the means and standard deviations from four samples and are representative of at least two independent experiments.
FIG. 2.
β-Galactosidase production by pilD::lacZ and frg::lacZ fusion strains grown at 37 and 17°C. Log-phase NU286 (left panel) and NU236 (right panel) bacteria were inoculated into BYE broth and then incubated at 37°C (black bars) and 17°C (lined bars). When the cultures reached early, mid-, and late log phase, the amount of produced β-galactosidase was recorded. The results presented are the means and standard deviations from duplicate samples and are representative of two independent experiments.
FIG. 3.
β-Galactosidase production by the pilD::lacZ fusion in the presence and absence of an intact pilD gene. Log-phase NU286, NU286 containing the vector pMMB2002 (70), and NU286 containing a complementing pilD gene on pMD1 (70) were inoculated into BYE broth and then incubated at 37°C (hatched and lined bars) and 17°C (gray and black bars). When the cultures reached mid-log and late log phase, the amount of produced β-galactosidase was recorded. The results presented are the means and standard deviations from duplicate samples and are representative of two independent experiments.
Role of pilD in L. pneumophila growth at low temperatures.
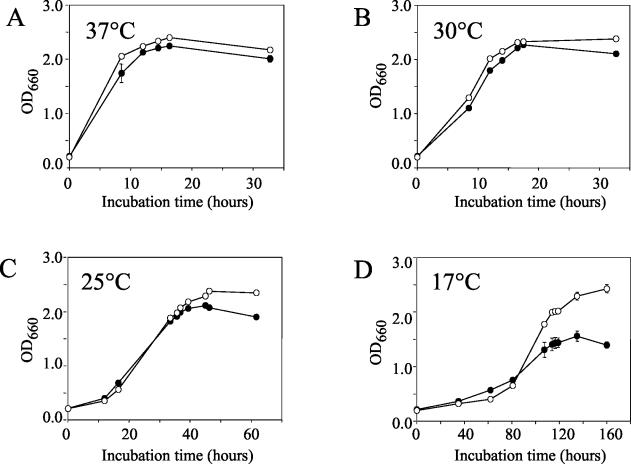
The increases in pilD expression at 17 to 30°C suggested that PilD is critical for survival as temperatures decline and that a pilD mutant would have a growth disadvantage at low temperatures. Hence, wild-type and pilD::lacZ mutant bacteria were incubated in BYE broth at 37, 30, 25, and 17°C, and differences in growth were determined spectrophotometrically (Fig. 4). As seen with our previous pilD mutant NU243 (47), the fusion strain grew as the wild type did at 30 and 37°C (Fig. 4A and B). However, at 25°C and especially at 17°C, the pilD mutant appeared defective for growth (Fig. 4C and D). Indeed, at 17°C, the mutant culture displayed both a reduced growth rate and a lower maximum optical density. As another way of identifying differences in low-temperature growth, we compared wild type and the pilD::lacZ NU286 mutant for their ability to form colonies on BCYE agar incubated at 25°C. After 8 days at room temperature, the wild type exhibited 61% ± 33% (mean ± standard deviation) of the number of CFU that was seen on control plates incubated at 37°C for 3 days (Table 1). In contrast, the number of mutant CFU at 25°C was only 0.032% ± 0.009% of that seen at 37°C (Table 1), indicating that the mutant's efficiency of plating at the lower temperature is ca. 2,000-fold lower than that of wild type (P < 0.001). When the incubation period was prolonged to 14 days, the number of mutant colonies increased but was still 900-fold less than wild type (data not shown). When a swabful of bacteria representing hundreds of mutant colonies that had grown up at room temperature were replated, they still showed poor growth at the lower temperature, indicating that their deficiency in plating was not an artifact of being transferred from an optimal (i.e., 37°C) to suboptimal (i.e., 25°C) growth condition but was a heritable trait. Thus, the L. pneumophila pilD mutant had a greatly reduced ability to grow at 25°C. That three different types of pilD mutant (i.e., NU286, NU243, and NU272) behaved similarly (Table 1) suggested that this phenotype was due to the mutation of pilD and not a spontaneous second-site mutation. Importantly, wild-type and mutant bacteria expressing a plasmid copy of pilD had comparable efficiencies of plating at 25°C; i.e., 41 and 35% of CFU at 37°C, respectively (Table 2), implying that the growth defect was specifically due to loss of PilD. Together, these data indicate, for the first time, that a PilD protein preferentially promotes growth at low temperatures.
FIG. 4.
Effect of temperature on the growth of wild-type and pilD mutant L. pneumophila. Log-phase 130b (○) and NU286 (•) bacteria were inoculated into BYE broth and then incubated at 37°C (A), 30°C (B), 25°C (C), and 17°C (D). The growth of the strains was monitored by recording the optical density of the cultures at the various times. The apparent differences between the wild-type and mutant cultures at 25 and 17°C were statistically significant (P < 0.05; Student's t test). The results presented are the means and standard deviations from duplicate samples and are representative of at least two independent experiments.
TABLE 2.
Complementation analysis of L. pneumophila pilD and lspF mutants
| Straina | Mutation | Plasmid gene | EOP at 25°Cb |
|---|---|---|---|
| 130b(pMMB2002) | Wild type | Cmr vector | 59 ± 9 |
| 130b(pMD1) | Wild type | pilD in pMMB2002 | 41 ± 25 |
| 130b(pMF1) | Wild type | lspF in pMMB2002 | 55 ± 16 |
| NU272(pMMB2002) | pilD::Gmr | Cmr vector | 0.010 ± 0.014 |
| NU272(pMD1) | pilD::Gmr | pilD in pMMB2002 | 35 ± 23 |
| NU275(pMMB2002) | lspF::Kmr | Cmr vector | 0.056 ± 0.071 |
| NU275(pMF1) | lspF::Kmr | lspF in pMMB2002 | 51 ± 6 |
Strains containing the chloramphenicol resistance (Cmr) vector pMMB2002 and the complementing plasmids pMD1 and pMF1 were previously described (70).
Efficiency of plating, calculated as 100 × (CFU formed at 25°C in 7 to 10 days/CFU formed at 37°C in 3 days). The results are the means and standard deviations from two independent experiments.
To begin to determine whether other bacterial PilD proteins promote growth at low temperatures, we compared a pilD mutant of P. aeruginosa and its complemented derivative (77) for their ability to grow on BCYE agar at 37, 25, and 12°C. After 3 days at 25°C, the strain containing an intact pilD gene yielded 90.1% ± 11.8% of the number of CFU obtained at 37°C in 1 day. In contrast to the behavior of the Legionella mutant, the Pseudomonas pilD mutant displayed a normal efficiency of plating at 25°C; i.e., 110% ± 18%. Both PilD+ and PilD− Pseudomonas strains showed a 40 to 46% efficiency of plating when incubated at 12°C for 10 days.
Role of type II protein secretion in L. pneumophila at low-temperature growth.
To begin to understand the role of PilD at low temperatures, we sought to determine whether the defect displayed by the L. pneumophila pilD mutants could be ascribed to the loss of type II protein secretion and/or the loss of type IV pilus biogenesis. In gram negatives, type II secretion is a two-step process (30, 55, 60, 71, 73). Initially, proteins destined for secretion are carried across the inner membrane by the Sec system but, in several cases, they are transported by the twin-arginine translocation system (88). After removal of their signal peptide, the proteins enter the periplasm, where they form disulfide bonds, fold, or oligomerize. The exoproteins then enter the type II secretion apparatus, ultimately exiting the cell through an outer membrane pore (secretin). The secretion apparatus consists of greater than 12 proteins, including inner membranes proteins, an ATP-binding protein, the PilD-dependent pseudopilins that span the periplasm, and the outer membrane secretin (55, 71, 73). In order to address the contribution of type II secretion, we made use of our previously described L. pneumophila lspDE and lspF mutants, which lack the LspD secretin and LspE ATPase, and the inner membrane LspF protein, respectively (69, 70). The type IV pilus apparatus has much similarity to the type II secretion apparatus, possessing, in addition to the pilin subunits, an ATPase, the pseudopilins, and an outer membrane secretin (55, 71). In order to examine the role of the pilus, we employed a pilEL mutant that lacks pilin and a pilQ mutant that lacks the secretin (70, 76). To gauge the role of type II secretion and type IV piliation in L. pneumophila low-temperature growth, we examined the lspDE, lspF, pilEL, and pilQ mutants for their relative growth on BCYE agar at 25°C. Whereas the pilEL and pilQ mutants had an efficiency of plating that was identical to that of wild type, the lsp mutants showed the type of plating defect that was seen with the pilD mutants (Table 1). Indeed, the lspDE mutant and the lspF mutant had efficiencies of plating of 0.028% ± 0.021% and 0.010% ± 0.002%, respectively (Table 1). The growth defect of the lspF mutant was eliminated when the intact lspF gene was reintroduced on a plasmid (Table 2). These data indicate that L. pneumophila type II protein secretion system, but not type IV piliation, is required for optimal bacterial growth at 25°C and that the importance of PilD in low-temperature growth is due mainly, if not completely, to its role in promoting the assembly of the type II secretion apparatus. An L. pneumophila double mutant (i.e., NU283) lacking both lspDE and pilQ was also impaired for low-temperature growth (Table 1). The fact that the double mutant appeared more defective than single lsp mutants suggests that PilQ and/or the pilus apparatus may have a dispensable role in low-temperature growth. Unlike the PilD and Lsp mutants, three previously described L. pneumophila dot/icm mutants (92) grew normally at the lower temperatures (Table 1), indicating that the Legionella type IV secretion system is not required for low-temperature growth. Furthermore, a newly constructed double mutant containing mutations in both pilD and icmGCD (dot FEP) (86) displayed a reduced ability to grow at the lower temperatures that was similar to that seen for mutants lacking just type II secretion (Table 1).
To determine whether type II secretion promotes bacterial growth at temperatures below 25°C, we compared strain 130b and its lspF mutant for their growth on BCYE agar at 17, 12, and 4°C. In the two experiments done at 17°C, wild-type legionellae formed countable colonies in 20 to 21 days, achieving 28% ± 16% of the number of CFU obtained at 37°C. In contrast, the lspF mutant had an efficiency of plating at 17°C that was only 0.0006% ± 0.0004%, a defect that was complemented by reintroduction of lspF on pMF1. At 12°C, strain 130b formed colonies after 46 days, and by day 72 it achieved 0.2% of the number of CFU seen on 37°C plates. Strikingly, the lspF mutant never formed colonies at 12°C. Neither WT nor mutant formed colonies at 4°C. Thus, the L. pneumophila type II protein secretion becomes increasingly more important as temperatures decrease.
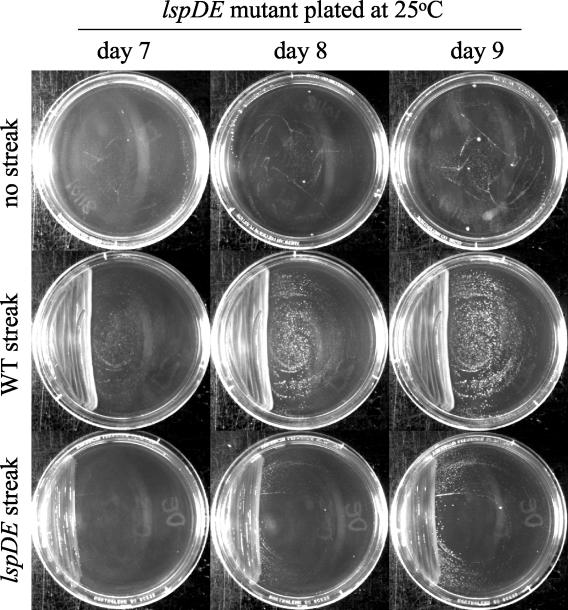
Theoretically, the reduced ability of Lsp mutants to grow at low temperature could be due to the loss of growth-promoting factors that are released into the extracellular milieu and/or a cell-associated defect such as an alteration in outer membrane function. Beginning to distinguish between these possibilities, we observed that the lspDE mutant formed thousands of colonies at 25°C in only 8 to 9 days when plated next to a heavy streak of wild-type bacteria (Fig. 5), implying that a secreted and/or diffusible factor(s) is able to restore growth to the mutant. Such a streak of wild type did not stimulate increased or more rapid colony formation on 25°C plates inoculated with wild type, nor did it enhance mutant growth when the plates were incubated at 37°C (data not shown). Since lspDE and pilD mutant streaks did not similarly enhance growth (Fig. 5 and data not shown), the stimulatory factor appears to be type II dependent. As an initial attempt toward identifying the factor, we tested mutants lacking specific Lsp-dependent activities for their abilities to grow on BCYE agar at 25°C. The map, proA, plaA, lipAlipB, and plcA mutants all grew as wild type did (Table 1), implying that the Map tartrate-sensitive acid phosphatase, ProA (Msp) metalloprotease, PlaA lysophospholipase A, LipA and LipB lipases, and PlcA phospholipase C are not required at low temperatures. In support of this notion, we observed that the level of acid phosphatase, protease, and lipolytic activity toward p-NP palmitate in supernatants did not increase when wild-type bacteria were grown at either 25 or 17°C (data not shown). Taken together, these data suggest that a yet-to-be-defined type II-dependent secreted factor(s) helps promote L. pneumophila growth at low temperatures.
FIG. 5.
The effect of adjacent wild-type bacteria on the low-temperature growth of an L. pneumophila type II secretion mutant. Approximately 105 CFU of lspDE mutant NU258 were plated for CFU on a series of BCYE agar plates. Some plates were then, as indicated, also inoculated in one sector with streaks of wild-type or mutant bacteria. One set of plates (shown here) was incubated at 25°C, and another (data not shown) was stored at 37°C. The picture depicts the 25°C colonial growth of the lspDE mutant on days 7, 8, and 9. The results presented are representative of at least two experiments.
DISCUSSION
Combined with previous Northern blotting and RT-PCR results (48), our gene fusion data indicate that pilD expression is influenced by temperature, with increases in transcriptional initiation occurring at low temperatures. To our knowledge, these data are the first demonstration of pilD regulation in any bacterium and the first information on L. pneumophila gene expression below 30°C. The observed pilD regulation may explain, at least in part, the increased piliation we see when L. pneumophila are grown at 30°C as opposed to 37°C (48). The pilD promoter region has a putative binding site for σ28 factor FliA (48), suggesting that the regulation of pilD has similarity with that of the L. pneumophila flagellin (flaA) gene (36). Indeed, when grown at 30° vs. 37°C, a flaA::lacZ fusion strain shows elevated β-galactosidase akin to what we saw with the pilD::lacZ strain (35). In the course of assessing the impact of temperature on gene expression, we also observed that the level of β-galactosidase produced by the pilD::lacZ fusion strain increased ca. twofold as the culture progressed from log to stationary phase irrespective of temperature (Fig. 1). That pilD mutants show a modest reduction in late stationary phase survival is compatible with this result (47). The onset of stationary phase produces many other changes in L. pneumophila, including increases in flaA expression (32, 35).
We have obtained novel data indicating a role for type II protein secretion in bacterial growth at low temperature. In the process, we have also documented, for the first time, that L. pneumophila is capable of extracellular replication at temperatures less than 20°C.
Previous work on low-temperature growth has focused on “cold-shock” proteins, factors that are induced transiently upon decreases in temperature and reprogram the basic cellular processes of replication, transcription, translation, and protein folding (9, 40, 67, 82, 90). Few studies have characterized those processes that permit long-term growth at low temperature, i.e., cold adaptation (49, 67, 82). Changes in membrane phospholipids, such as increased levels of unsaturated fatty acids, trans isomers of the unsaturated fatty acids, and short-chain fatty acids, are critical for maintaining membrane fluidity at low temperature (17, 46, 72, 82). Indeed, in the only previous study on L. pneumophila physiology at low temperature, Mauchline et al. found increases in the amount of unsaturated fatty acids in bacteria grown at 24°C (52). Newly recognized forms of cold adaptation are increases in trehalose and palmitoylated lipid A within E. coli (41, 87) and solute (e.g., glycine, betaine) uptake in Listeria monocytogenes (3, 49). The ability of wild-type L. pneumophila to stimulate, in trans, low-temperature growth of lsp mutants suggests that low-temperature-induced, type II exoproteins may be another mechanism of cold adaptation. Compatible with this idea, we found, in preliminary experiments, that a number of the proteins present in wild-type culture supernatants change in abundance upon reduction in growth temperature (M. Soderberg and N. P. Cianciotto, unpublished results). Alternatively, the growth stimulation that we observed could be due to a type II-dependent membrane protein which mediates the production of a diffusible factor that enhances growth at the low temperature. Clearly, the further study of the role of Lsp in low-temperature growth will enhance our understanding of L. pneumophila persistence in aquatic environments and therefore its transmission to humans. It will also have implications for other bacteria that possess type II secretion systems, including pathogens, such as Aeromonas hydrophila, Burkholderia spp., P. aeruginosa, and Vibrio spp. that, like L. pneumophila, survive at low environmental temperatures (19, 51, 61, 65, 74, 78, 81).
Acknowledgments
We thank past and present members of the Cianciotto laboratory for their assistance and helpful comments. We acknowledge V. K. Viswanathan for constructing pVK10 and Mark Strom for providing us with P. aeruginosa strains.
This work was supported by NIH grant AI43987 awarded to N.P.C.
REFERENCES
- 1.Alary, M., and J. R. Joly. 1991. Risk factors for contamination of domestic hot water systems by legionellae. Appl. Environ. Microbiol. 57:2360-2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alm, R. A., and J. S. Mattick. 1997. Genes involved in the biogenesis and function of type-4 fimbriae in Pseudomonas aeruginosa. Gene 192:89-98. [DOI] [PubMed] [Google Scholar]
- 3.Angelidis, A. S., L. T. Smith, L. M. Hoffman, and G. M. Smith. 2002. Identification of opuC as a chill-activated and osmotically activated carnitine transporter in Listeria monocytogenes. Appl. Environ. Microbiol. 68:2644-2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aragon, V., S. Kurtz, and N. P. Cianciotto. 2001. Legionella pneumophila major acid phosphatase and its role in intracellular infection. Infect. Immun. 69:177-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aragon, V., S. Kurtz, A. Flieger, B. Neumeister, and N. P. Cianciotto. 2000. Secreted enzymatic activities of wild-type and pilD-deficient Legionella pneumophila. Infect. Immun. 68:1855-1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aragon, V., O. Rossier, and N. P. Cianciotto. 2002. Legionella pneumophila genes that encode lipase and phospholipase C activities. Microbiology 148:2223-2231. [DOI] [PubMed] [Google Scholar]
- 7.Atlas, R. M. 1999. Legionella: from environmental habitats to disease pathology, detection and control. Environ. Microbiol. 1:283-293. [DOI] [PubMed] [Google Scholar]
- 8.Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl (ed.). 1989. Current protocols in molecular biology. Wiley, New York, N.Y.
- 9.Beckering, C. L., L. Steil, M. H. Weber, U. Volker, and M. A. Marahiel. 2002. Genomewide transcriptional analysis of the cold shock response in Bacillus subtilis. J. Bacteriol. 184:6395-6402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bercovier, H., B. Fattal, and H. Shuval. 1986. Seasonal distribution of legionellae isolated form various types of water in Israel. Isr. J. Med. Sci. 22:644-646. [PubMed] [Google Scholar]
- 11.Berg, J. D., J. C. Hoff, P. V. Roberts, and A. Matin. 1985. Growth of Legionella pneumophila in continuous culture. Appl. Environ. Microbiol. 49:1534-1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bleves, S., R. Voulhoux, G. Michel, A. Lazdunski, J. Tommassen, and A. Filloux. 1998. The secretion apparatus of Pseudomonas aeruginosa: identification of a fifth pseudopilin, XcpX (GspK family). Mol. Microbiol. 27:31-40. [DOI] [PubMed] [Google Scholar]
- 13.Breiman, R. F., and J. C. Butler. 1998. Legionnaires' disease: clinical, epidemiological, and public health perspectives. Semin. Respir. Infect. 13:84-89. [PubMed] [Google Scholar]
- 14.Castellani Pastoris, M., C. Passi, and M. Maroli. 1989. Evidence of Legionella pneumophila in some arthropods and related natural aquatic habitats. FEMS Microbiol. Ecol. 62:259-264. [Google Scholar]
- 15.Chapon-Herve, V., M. Akrim, A. Latifi, P. Williams, A. Lazdunski, and M. Bally. 1997. Regulation of the xcp secretion pathway by multiple quorum-sensing modulons in Pseudomonas aeruginosa. Mol. Microbiol. 24:1169-1178. [DOI] [PubMed] [Google Scholar]
- 16.Cianciotto, N. P. 2001. Pathogenicity of Legionella pneumophila. Int. J. Med. Microbiol. 291:331-343. [DOI] [PubMed] [Google Scholar]
- 17.Cronan, J. E., Jr. 2002. Phospholipid modifications in bacteria. Curr. Opin. Microbiol. 5:202-205. [DOI] [PubMed] [Google Scholar]
- 18.Desai, R., C. Welsh, M. Summy, M. Farone, and A. L. Newsome. 1999. The potential of in situ hybridization and an immunogold assay to identify Legionella associations with other microorganisms. J. Microbiol. Methods 37:155-164. [DOI] [PubMed] [Google Scholar]
- 19.DeShazer, D., P. J. Brett, M. N. Burtnick, and D. E. Woods. 1999. Molecular characterization of genetic loci required for secretion of exoproducts in Burkholderia pseudomallei. J. Bacteriol. 181:4661-4664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dutka, B. J., and P. Ewan. 1983. First isolation of Legionella pneumophila from the Canadian Great Lakes. J. Great Lakes Res. 9:430-432. [Google Scholar]
- 21.Edelstein, P. H. 1981. Improved semiselective medium for isolation of Legionella pneumophila from contaminated clinical and environmental specimens. J. Clin. Microbiol. 14:298-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Engleberg, N. C., D. J. Drutz, and B. I. Eisenstein. 1984. Cloning and expression of Legionella pneumophila antigens in Escherichia coli. Infect. Immun. 44:222-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fields, B. S. 1996. The molecular ecology of legionellae. Trends Microbiol. 4:286-290. [DOI] [PubMed] [Google Scholar]
- 24.Fields, B. S., R. F. Benson, and R. E. Besser. 2002. Legionella and Legionnaires' disease: 25 years of investigation. Clin. Microbiol. Rev. 15:506-526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Flieger, A., S. Gong, M. Faigle, S. Stevanovic, N. P. Cianciotto, and B. Neumeister. 2001. Novel lysophospholipase A secreted by Legionella pneumophila. J. Bacteriol. 183:2121-2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Flieger, A., B. Neumeister, and N. P. Cianciotto. 2002. Characterization of the gene encoding the major secreted lysophospholipase A of Legionella pneumophila and its role in detoxification of lysophosphatidylcholine. Infect. Immun. 70:6094-6106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fliermans, C. B., W. B. Cherry, L. H. Orrison, S. J. Smith, D. L. Tison, and D. H. Pope. 1981. Ecological distribution of Legionella pneumophila. Appl. Environ. Microbiol. 41:9-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fliermans, C. B., W. B. Cherry, L. H. Orrison, and L. Thacker. 1979. Isolation of Legionella pneumophila from non-epidemic-related aquatic habitats. Appl. Environ. Microbiol. 37:1239-1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fraser, D. W., T. R. Tsai, W. Orenstein, W. E. Parkin, H. J. Beecham, R. G. Sharrar, J. Harris, G. F. Mallison, S. M. Martin, J. E. McDade, C. C. Shepard, and P. S. Brachman. 1977. Legionnaires' disease: description of an epidemic of pneumonia. N. Engl. J. Med. 297:1189-1197. [DOI] [PubMed] [Google Scholar]
- 30.Gerard-Vincent, M., V. Robert, G. Ball, S. Bleves, G. P. Michel, A. Lazdunski, and A. Filloux. 2002. Identification of XcpP domains that confer functionality and specificity to the Pseudomonas aeruginosa type II secretion apparatus. Mol. Microbiol. 44:1651-1665. [DOI] [PubMed] [Google Scholar]
- 31.Hales, L. M., and H. A. Shuman. 1999. Legionella pneumophila contains a type II general secretion pathway required for growth in amoebae as well as for secretion of the Msp protease. Infect. Immun. 67:3662-3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hammer, B. K., and M. S. Swanson. 1999. Co-ordination of Legionella pneumophila virulence with entry into stationary phase by ppGpp. Mol. Microbiol. 33:721-731. [DOI] [PubMed] [Google Scholar]
- 33.Hay, J., D. V. Seal, B. Billcliffe, and J. H. Freer. 1995. Non-culturable Legionella pneumophila associated with Acanthamoeba castellanii: detection of the bacterium using DNA amplification and hybridization. J. Appl. Bacteriol. 78:61-65. [DOI] [PubMed] [Google Scholar]
- 34.Heller, R., C. Holler, R. Sussmuth, and K. O. Gundermann. 1998. Effect of salt concentration and temperature on survival of Legionella pneumophila. Lett. Appl. Microbiol. 26:64-68. [DOI] [PubMed] [Google Scholar]
- 35.Heuner, K., B. C. Brand, and J. Hacker. 1999. The expression of the flagellum of Legionella pneumophila is modulated by different environmental factors. FEMS Microbiol. Lett. 175:69-77. [DOI] [PubMed] [Google Scholar]
- 36.Heuner, K., C. Dietrich, C. Skriwan, M. Steinert, and J. Hacker. 2002. Influence of the alternative sigma-28 factor on virulence and flagellum expression of Legionella pneumophila. Infect. Immun. 70:1604-1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hickey, E. K., and N. P. Cianciotto. 1997. An iron- and fur-repressed Legionella pneumophila gene that promotes intracellular infection and encodes a protein with similarity to the Escherichia coli aerobactin synthetases. Infect. Immun. 65:133-143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hussong, D., R. R. Colwell, M. O'Brien, E. Weiss, A. D. Pearson, R. M. Weiner, and W. D. Burge. 1987. Viable Legionella pneumophila not detectable by culture on agar media. Biotechnology 5:947-950. [Google Scholar]
- 39.Joly, J. R., M. Boissinot, J. Duchaine, M. Duval, J. Rafrafi, D. Ramsay, and R. Letarte. 1984. Ecological distribution of Legionellaceae in the Quebec City area. Can. J. Microbiol. 30:63-67. [DOI] [PubMed] [Google Scholar]
- 40.Kaan, T., G. Homuth, U. Mader, J. Bandow, and T. Schweder. 2002. Genome-wide transcriptional profiling of the Bacillus subtilis cold-shock response. Microbiology 148:3441-3455. [DOI] [PubMed] [Google Scholar]
- 41.Kandror, O., A. DeLeon, and A. L. Goldberg. 2002. Trehalose synthesis is induced upon exposure of Escherichia coli to cold and is essential for viability at low temperatures. Proc. Natl. Acad. Sci. USA 99:9727-9732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kramer, M. H., and T. E. Ford. 1994. Legionellosis: ecological factors of an environmentally “new” disease. Zentbl. Hyg. 195:470-482. [PubMed] [Google Scholar]
- 43.Kusnetsov, J. M., E. Ottoila, and P. J. Martikainen. 1996. Growth, respiration and survival of Legionella pneumophila at high temperatures. J. Appl. Bacteriol. 81:341-347. [DOI] [PubMed] [Google Scholar]
- 44.Lawrence, C., M. Reyrolle, S. Dubrou, F. Forey, B. Decludt, C. Goulvestre, P. Matsiota-Bernard, J. Etienne, and C. Nauciel. 1999. Single clonal origin of a high proportion of Legionella pneumophila serogroup 1 isolates from patients and the environment in the area of Paris, France, over a 10-year period. J. Clin. Microbiol. 37:2652-2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee, J. V., and A. A. West. 1991. Survival and growth of Legionella species in the environment. J. Appl. Bacteriol. Suppl. 70:121S-129S. [PubMed] [Google Scholar]
- 46.Li, J., M. L. Chikindas, R. D. Ludescher, and T. J. Montville. 2002. Temperature- and surfactant-induced membrane modifications that alter Listeria monocytogenes nisin sensitivity by different mechanisms. Appl. Environ. Microbiol. 68:5904-5910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liles, M. R., P. H. Edelstein, and N. P. Cianciotto. 1999. The prepilin peptidase is required for protein secretion by and the virulence of the intracellular pathogen Legionella pneumophila. Mol. Microbiol. 31:959-970. [DOI] [PubMed] [Google Scholar]
- 48.Liles, M. R., V. K. Viswanathan, and N. P. Cianciotto. 1998. Identification and temperature regulation of Legionella pneumophila genes involved in type IV pilus biogenesis and type II protein secretion. Infect. Immun. 66:1776-1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu, S., J. E. Graham, L. Bigelow, P. D. Morse II, and B. J. Wilkinson. 2002. Identification of Listeria monocytogenes genes expressed in response to growth at low temperature. Appl. Environ. Microbiol. 68:1697-1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Marrao, G., A. Verissimo, R. G. Bowker, and M. S. daCosta. 1993. Biofilms as major sources of Legionella spp. in hydrothermal areas and their dispersion into stream water. FEMS Microbiol. Ecol. 12:25-33. [Google Scholar]
- 51.Marsh, J. W., and R. K. Taylor. 1998. Identification of the Vibrio cholerae type 4 prepilin peptidase required for cholera toxin secretion and pilus formation. Mol. Microbiol. 29:1481-1492. [DOI] [PubMed] [Google Scholar]
- 52.Mauchline, W. S., R. Araujo, R. Wait, A. B. Dowsett, P. J. Dennis, and C. W. Keevil. 1992. Physiology and morphology of Legionella pneumophila in continuous culture at low oxygen concentration. J. Gen. Microbiol. 138:2371-2380. [DOI] [PubMed] [Google Scholar]
- 53.Moffat, J. F., P. H. Edelstein, D. P. Regula, Jr., J. D. Cirillo, and L. S. Tompkins. 1994. Effects of an isogenic Zn-metalloprotease-deficient mutant of Legionella pneumophila in a guinea-pig pneumonia model. Mol. Microbiol. 12:693-705. [DOI] [PubMed] [Google Scholar]
- 54.Murga, R., T. S. Forster, E. Brown, J. M. Pruckler, B. S. Fields, and R. M. Donlan. 2001. Role of biofilms in the survival of Legionella pneumophila in a model potable-water system. Microbiology 147:3121-3126. [DOI] [PubMed] [Google Scholar]
- 55.Nunn, D. 1999. Bacterial type II protein export and pilus biogenesis: more than just homologies? Trends Cell Biol. 9:402-408. [DOI] [PubMed] [Google Scholar]
- 56.Nunn, D. N., and S. Lory. 1993. Cleavage, methylation, and localization of the Pseudomonas aeruginosa export proteins XcpT, -U, -V, and -W. J. Bacteriol. 175:4375-4382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nunn, D. N., and S. Lory. 1991. Product of the Pseudomonas aeruginosa gene pilD is a prepilin leader peptidase. Proc. Natl. Acad. Sci. USA 88:3281-3285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ortiz-Roque, C. M., and T. C. Hazen. 1987. Abundance and distribution of Legionellaceae in Puerto Rican waters. Appl. Environ. Microbiol. 53:2231-2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Palmer, C. J., Y. L. Tsai, C. Paszko-Kolva, C. Mayer, and L. R. Sangermano. 1993. Detection of Legionella species in sewage and ocean water by polymerase chain reaction, direct fluorescent antibody, and plate culture methods. Appl. Environ. Microbiol. 59:3618-3624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Palomaki, T., R. Pickersgill, R. Riekki, M. Romantschuk, and H. T. Saarilahti. 2002. A putative three-dimensional targeting motif of polygalacturonase (PehA), a protein secreted through the type II (GSP) pathway in Erwinia carotovora. Mol. Microbiol. 43:585-596. [DOI] [PubMed] [Google Scholar]
- 61.Paranjpye, R. N., J. C. Lara, J. C. Pepe, C. M. Pepe, and M. S. Strom. 1998. The type IV leader peptidase/N-methyltransferase of Vibrio vulnificus controls factors required for adherence to HEp-2 cells and virulence in iron-overloaded mice. Infect. Immun. 66:5659-5668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Park, M. Y., K. S. Ko, H. K. Lee, M. S. Park, and Y. H. Kook. 2003. Legionella busanensis sp. nov., isolated from cooling tower water in Korea. Int. J. Syst. Evol. Microbiol. 53:77-80. [DOI] [PubMed] [Google Scholar]
- 63.Paszko-Kolva, C., M. Shahamat, and R. R. Colwell. 1993. Effect of temperature on survival of Legionella pneumophila in the aquatic environment. Microb. Releases 2:73-79. [PubMed] [Google Scholar]
- 64.Paszko-Kolva, C., M. Shahamat, H. Yamamoto, T. Sawyer, J. Vives-Rego, and R. R. Colwell. 1991. Survival of Legionella pneumophila in the aquatic environment. Microb. Ecol. 22:75-83. [DOI] [PubMed] [Google Scholar]
- 65.Pepe, C. M., M. W. Eklund, and M. S. Strom. 1996. Cloning of an Aeromonas hydrophila type IV pilus biogenesis gene cluster: complementation of pilus assembly functions and characterization of a type IV leader peptidase/N-methyltransferase required for extracellular protein secretion. Mol. Microbiol. 19:857-869. [DOI] [PubMed] [Google Scholar]
- 66.Pepe, J. C., and S. Lory. 1998. Amino acid substitutions in PilD, a bifunctional enzyme of Pseudomonas aeruginosa. J. Biol. Chem. 273:19120-19129. [DOI] [PubMed] [Google Scholar]
- 67.Phadtare, S., J. Alsina, and M. Inouye. 1999. Cold-shock response and cold-shock proteins. Curr. Opin. Microbiol. 2:175-180. [DOI] [PubMed] [Google Scholar]
- 68.Polesky, A. H., J. T. Ross, S. Falkow, and L. S. Tompkins. 2001. Identification of Legionella pneumophila genes important for infection of amoebas by signature-tagged mutagenesis. Infect. Immun. 69:977-987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rossier, O., and N. P. Cianciotto. 2001. Type II protein secretion is a subset of the PilD-dependent processes that facilitate intracellular infection by Legionella pneumophila. Infect. Immun. 69:2092-2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rossier, O., S. Starkenburg, and N. P. Cianciotto. 2004. Legionella pneumophila type II protein secretion promotes virulence in the A/J. mouse model of Legionnaires' disease pneumonia. Infect. Immun. 72:310-3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Russel, M. 1998. Macromolecular assembly and secretion across the bacterial cell envelope: type II protein secretion systems. J. Mol. Biol. 279:485-499. [DOI] [PubMed] [Google Scholar]
- 72.Sakamoto, T., and N. Murata. 2002. Regulation of the desaturation of fatty acids and its role in tolerance to cold and salt stress. Curr. Opin. Microbiol. 5:208-210. [DOI] [PubMed] [Google Scholar]
- 73.Sandkvist, M. 2001. Biology of type II secretion. Mol. Microbiol. 40:271-283. [DOI] [PubMed] [Google Scholar]
- 74.Sandkvist, M. 2001. Type II secretion and pathogenesis. Infect. Immun. 69:3523-3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Simons, R. W., F. Houman, and N. Kleckner. 1987. Improved single and multicopy lac-based cloning vectors for protein and operon fusions. Gene 53:85-96. [DOI] [PubMed] [Google Scholar]
- 76.Stone, B. J., and Y. Abu Kwaik. 1998. Expression of multiple pili by Legionella pneumophila: identification and characterization of a type IV pilin gene and its role in adherence to mammalian and protozoan cells. Infect. Immun. 66:1768-1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Strom, M. S., P. Bergman, and S. Lory. 1993. Identification of active-site cysteines in the conserved domain of PilD, the bifunctional type IV pilin leader peptidase/N-methyltransferase of Pseudomonas aeruginosa. J. Biol. Chem. 268:15788-15794. [PubMed] [Google Scholar]
- 78.Strom, M. S., D. Nunn, and S. Lory. 1991. Multiple roles of the pilus biogenesis protein PilD: involvement of PilD in excretion of enzymes from Pseudomonas aeruginosa. J. Bacteriol. 173:1175-1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Strom, M. S., D. N. Nunn, and S. Lory. 1993. A single bifunctional enzyme, PilD, catalyzes cleavage and N-methylation of proteins belonging to the type IV pilin family. Proc. Natl. Acad. Sci. USA 90:2404-2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Swanson, M. S., and B. K. Hammer. 2000. Legionella pneumophila pathogenesis: a fateful journey from amoebae to macrophages. Annu. Rev. Microbiol. 54:567-613. [DOI] [PubMed] [Google Scholar]
- 81.Tauschek, M., R. J. Gorrell, R. A. Strugnell, and R. M. Robins-Browne. 2002. Identification of a protein secretory pathway for the secretion of heat-labile enterotoxin by an enterotoxigenic strain of Escherichia coli. Proc. Natl. Acad. Sci. USA 99:7066-7071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Thieringer, H. A., P. G. Jones, and M. Inouye. 1998. Cold shock and adaptation. Bioessays 20:49-57. [DOI] [PubMed] [Google Scholar]
- 83.Tobiansky, L., A. Drath, B. Dubery, and H. J. Koornhof. 1986. Seasonality of Legionella isolates from environmental sources. Isr. J. Med. Sci. 22:640-643. [PubMed] [Google Scholar]
- 84.Verissimo, A., G. Marrao, F. Gomes da Silva, and M. S. Da Costa. 1991. Distribution of Legionella spp. in hydrothermal areas in continental Portugal and the island of Sao Miguel, Azores. Appl. Environ. Microbiol. 57:2921-2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Viswanathan, V. K., P. H. Edelstein, C. D. Pope, and N. P. Cianciotto. 2000. The Legionella pneumophila iraAB locus is required for iron assimilation, intracellular infection, and virulence. Infect. Immun. 68:1069-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Vogel, J. P., and R. R. Isberg. 1999. Cell biology of Legionella pneumophila. Curr. Opin. Microbiol. 2:30-34. [DOI] [PubMed] [Google Scholar]
- 87.Vorachek-Warren, M. K., S. M. Carty, S. Lin, R. J. Cotter, and C. R. Raetz. 2002. An Escherichia coli mutant lacking the cold shock-induced palmitoleoyltransferase of lipid A biosynthesis: absence of unsaturated acyl chains and antibiotic hypersensitivity at 12 degrees C. J. Biol. Chem. 277:14186-14193. [DOI] [PubMed] [Google Scholar]
- 88.Voulhoux, R., G. Ball, B. Ize, M. L. Vasil, A. Lazdunski, L. F. Wu, and A. Filloux. 2001. Involvement of the twin-arginine translocation system in protein secretion via the type II pathway. EMBO J. 20:6735-6741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wadowsky, R. M., R. Wolford, A. M. McNamara, and R. B. Yee. 1985. Effect of temperature, pH, and oxygen level on the multiplication of naturally occurring Legionella pneumophila in potable water. Appl. Environ. Microbiol. 49:1197-1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yamada, M., H. Nagamitsu, H. Izu, K. Nakamura, and A. A. Talukder. 2002. Characterization of the ves gene, which is expressed at a low temperature in Escherichia coli. J. Mol. Microbiol. Biotechnol. 4:163-169. [PubMed] [Google Scholar]
- 91.Yee, R. B., and R. M. Wadowsky. 1982. Multiplication of Legionella pneumophila in unsterilized tap water. Appl. Environ. Microbiol. 43:1330-1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zink, S. D., L. Pedersen, N. P. Cianciotto, and Y. Abu Kwaik. 2002. The Dot/Icm type IV secretion system of Legionella pneumophila is essential for the induction of apoptosis in human macrophages. Infect. Immun. 70:1657-1663. [DOI] [PMC free article] [PubMed] [Google Scholar]