Abstract
Introduction
This retrospective study investigated the clinical value of post-mastectomy radiotherapy (PMRT) in female Chinese breast cancer patients aged 35 years or younger with positive axillary lymph nodes after mastectomy.
Methods
We performed an analysis of clinical pathological data from 221 female Chinese breast cancer patients aged 35 years or younger treated between 1998 and 2007. Patients were diagnosed with positive axillary lymph nodes and underwent mastectomy. PMRT was delivered to 92 patients.
Results
The median follow-up was 61 months. The 5-year locoregional recurrence-free survival (LRFS), distant metastasis-free survival (DMFS), disease-free survival (DFS), and overall survival (OS) were 84.1%, 65.2%, 61.4%, and 77.2%, respectively. Univariate survival analysis (P=0.003) and multivariate analysis (P<0.001) both suggested that PMRT is an independent prognostic factor of LRFS. PMRT positively affected LRFS (P=0.003), but had no significant impact on DMFS (P=0.429), DFS (P=0.146), and OS (P=0.750). PMRT improved LRFS (P=0.001), DFS (P=0.017), and OS (P=0.042) in patients with four or more positive nodes, but no survival benefit was observed in patients with one to three positive nodes (P>0.05).
Conclusion
PMRT can improve survival in breast cancer patients aged 35 years or younger with four or more positive nodes but not in those with one to three positive nodes.
Keywords: breast cancer, radiation therapy, mastectomy, young age, locoregional recurrence
Introduction
Breast cancer is one of the most common malignant tumors in women. The age of onset of breast cancer varies considerably between women in Asian and Western countries. In the Asian region, breast cancer accounts for 10%–25% of cancer diagnoses in patients aged 35 years or younger,1 whereas this percentage is less than 3% in Western countries.2,3 Studies have suggested that, when compared with older patients, breast cancer patients aged 35 years or younger have markedly different biological characteristics, including negative hormone receptor status, high histological grade, high cell proliferation rate, and vascular tumor thrombus. Although some discrepancies exist,4–7 the majority of research has suggested that breast cancer patients aged 35 years or younger have a significantly worse prognosis than older patients.6,7 Similar results have also been observed in Chinese breast cancer patients.8,9
In recent years, rapid progress has been made in combined modality therapy for breast cancer. Radiotherapy is considered one of the most important postoperative adjuvant therapies for breast cancer patients with positive axillary lymph nodes. Studies have shown that radiotherapy may reduce the incidence of distant metastases and improve survival by controlling locoregional recurrence.10–12 However, most studies have not performed age-stratified analysis specifically for very young breast cancer patients (aged 35 years or younger). Therefore, we performed a retrospective analysis to study the value of postoperative adjuvant radiotherapy in Chinese breast cancer patients aged 35 years or younger with positive axillary lymph nodes after mastectomy.
Patients and methods
Patients
We retrospectively analyzed 3,759 breast cancer patients with complete follow-up records who were registered and treated at the Sun Yat-Sen University Cancer Center from January 1998 to December 2007. Patients were included based on the following criteria: female, unilateral breast cancer, aged 35 years or younger, underwent mastectomy and axillary lymph node dissection, classified into pT1–2-N1 and pT1–4-N2–3 stage by postoperative pathological examination according to the 7th edition (2009) of the American Joint Committee on Cancer (AJCC)/Union for International Cancer Control (UICC) staging system, received postoperative adjuvant chemotherapy for at least 4 cycles with no preoperative neoadjuvant chemotherapy, endocrine therapy performed in patients with positive hormone receptor status, at least ipsilateral chest wall and supra- and infra-clavicular lymph drainage regions involved in patients who received post-mastectomy radiotherapy (PMRT), and no history of second primary cancer. The study was performed in accordance with the Declaration of Helsinki and was approved by the Ethics Committee of the Sun Yat-Sen University Cancer Center. Written consent was given by the patients for their information to be stored in the hospital database and used for research.
Radiation therapy
Decisions about PMRT were based on clinical staging and patient preference. Typically, PMRT was offered to patients with four or more positive lymph nodes and/or tumors >5 cm; patients with pT1–2 and one to three positive nodes were considered for PMRT, but the patient made the final decision over whether to proceed. Some patients did not receive PMRT due to economic and social factors. PMRT was administered and included ipsilateral chest wall and supra- and infra-clavicular lymph regions. The total dose of radiotherapy was 46–50 Gy, at 2 Gy each time. The chest wall was treated with 6 MV X-ray with opposed tangential fields or 6–9 Mev electron beam, with the use of tissue compensation membrane of 0.5–1 cm when needed. Single-field irradiation was performed for the supra- and infra-clavicular lymph drainage regions with 6 MV X-ray combined with 12–15 Mev.
Follow-up and study endpoints
Follow-up was scheduled every 3–6 months from the first day after surgery. The primary study endpoint was locoregional recurrence-free survival (LRFS), with secondary endpoints including distant metastasis-free survival (DMFS), disease-free survival (DFS), and overall survival (OS). Locoregional recurrence was defined as pathologically confirmed relapse on the chest wall, supra- and infra-clavicular fossa, axillary area, or internal mammary region. Distant metastasis was defined as any relapse outside the locoregional recurrence area and was confirmed using two types of medical imaging methods (and pathology assessment, if needed). OS was calculated as a period of time from the date of diagnosis to the date of death from any cause or the date of last follow-up.
Statistical analysis
All analyses were performed using the SPSS statistical software package, version 16.0 (SPSS Inc., Chicago, IL, USA). The χ2 and Fisher’s exact probability tests were used to analyze the differences between qualitative data. Calculation of survival rates were plotted by the Kaplan–Meier method and compared using the Log-rank test. Univariate survival analysis was conducted using the Log-rank test. Stepwise Cox regression analysis was used for multivariate analysis. Factors that were significant indicators of endpoints in the univariate analysis were included in the stepwise Cox regression analysis. A value of P<0.05 was considered statistically significant.
Results
Patient characteristics and treatment protocol
A total of 221 patients met the inclusion criteria and were enrolled in the study. The median age was 32 years (range 20–35). All patients underwent mastectomy and axillary lymph node dissection (level II or III), 46 (20.8%) received radical mastectomy, and 175 (79.2%) underwent modified radical mastectomy. The median number of removed lymph nodes was 16 (range 2–47), and the median number of positive lymph nodes was three (range 1–37). According to the UICC/AJCC staging system, 116 patients (52.5%) were classified as pT1–2-N1-M0 (one to three positive nodes) and 105 (47.5%) as pT1–4-N2–3-M0 (four or more positive nodes). All patients received adjuvant chemotherapy, 24 (10.9%) were treated with a CMF (cyclophosphamide, methotrexate, and 5-fluorouracil [5-Fu]) regimen, and 197 (89.1%) received regimens with anthracycline and/or a taxane. The median number of chemotherapy cycles was 6 (range 4–8). A total of 110 patients (53.8%) with positive hormone receptor status were treated with endocrine therapy using tamoxifen following radiotherapy, and 17 were treated with luteinizing hormone-releasing hormone agonists (eg, goserelin). None of the patients with human epidermal growth factor receptor (HER)-2 positive cancers received trastuzumab. A total of 92 patients (41.6%) received adjuvant radiotherapy, with a median dose of radiation of 50 Gy (range 46–50) after adjuvant chemotherapy (Tables 1 and 2).
Table 1.
Patient characteristics and treatment protocols
| Characteristic | n | PMRT
|
P-value | |
|---|---|---|---|---|
| None (%) | Yes (%) | |||
| Age (y) | ||||
| Median (range) | 221 | 32 (23–35) | 33 (20–35) | – |
| Tumor stage | ||||
| pT1 | 54 | 36 (66.7) | 18 (33.3) | 0.024 |
| pT2 | 134 | 81 (60.4) | 53 (39.6) | |
| pT3 | 23 | 7 (30.4) | 16 (69.6) | |
| pT4 | 10 | 5 (50.0) | 5 (50.0) | |
| Nodal stage | ||||
| pN1 | 116 | 92 (79.3) | 24 (20.7) | <0.001 |
| pN2 | 54 | 19 (35.2) | 35 (64.8) | |
| pN3 | 51 | 18 (35.3) | 33 (64.7) | |
| TNM stage | ||||
| II | 116 | 92 (79.3) | 24 (20.7) | <0.001 |
| III | 105 | 37 (35.2) | 68 (64.8) | |
| ER statusa | ||||
| Negative | 95 | 48 (50.5) | 47 (49.5) | 0.060 |
| Positive | 119 | 75 (63.0) | 44 (37.0) | |
| Unknown | 7 | 6 (85.7) | 1 (14.3) | |
| PR statusa | ||||
| Negative | 71 | 38 (53.5) | 33 (36.5) | 0.234 |
| Positive | 143 | 85 (59.4) | 58 (40.6) | |
| Unknown | 7 | 6 (85.7) | 1 (14.3) | |
| HER-2 statusb | ||||
| Negative | 129 | 78 (60.4) | 51 (39.6) | 0.181 |
| Positive | 71 | 36 (50.7) | 35 (49.3) | |
| Unknown | 21 | 15 (71.4) | 6 (28.6) | |
| Adjuvant chemotherapy regimen | ||||
| CMF | 24 | 16 (66.7) | 8 (33.3) | 0.383 |
| Anthracycline and/or taxane | 197 | 113 (57.4) | 84 (42.6) | |
Notes:
Positive status refers to 10% immuno-stained cells by immunohistochemical results.
Positive status refers to 3+ or 2+ score by immunohistochemical results that is confirmed to be positive by FISH test.
Abbreviations: CMF, cyclophosphamide, methotrexate, and 5-Fu; ER, estrogen receptor; FISH, fluorescence in situ hybridization; HER-2, human epidermal growth factor receptor-2; PMRT, post-mastectomy radiotherapy; PR, progesterone receptor; TNM, tumor, lymph nodes, metastasis; 5-Fu, 5-fluorouracil.
Table 2.
Clinical characteristics of patients with one to three positive nodes and four or more positive nodes
| Characteristic | One to three positive nodes
|
Four or more positive nodes
|
||||||
|---|---|---|---|---|---|---|---|---|
| n | PMRT
|
P-value | n | PMRT
|
P-value | |||
| None (%) | Yes (%) | None (%) | Yes (%) | |||||
| Tumor stage | ||||||||
| pT1 | 38 | 30 (78.9) | 8 (21.1) | 0.564 | 16 | 6 (37.5) | 10 (62.5) | 0.737 |
| pT2 | 78 | 62 (79.5) | 16 (20.5) | 56 | 19 (33.9) | 37 (66.1) | ||
| pT3 | – | – | – | 23 | 7 (30.4) | 16 (69.6) | ||
| pT4 | – | – | – | 10 | 5 (50.0) | 5 (50.0) | ||
| Nodal stage | ||||||||
| pN1 | 116 | 92 (79.3) | 24 (20.7) | – | – | – | – | – |
| pN2 | – | – | – | 54 | 19 (35.2) | 35 (64.8) | 0.991 | |
| pN3 | – | – | – | 51 | 18 (35.3) | 33 (64.7) | ||
| ER status | ||||||||
| Negative | 41 | 30 (73.1) | 11 (26.9) | 0.482 | 54 | 18 (33.3) | 36 (66.7) | 0.380 |
| Positive | 69 | 57 (82.6) | 12 (17.4) | 50 | 18 (36.0) | 32 (64.0) | ||
| Unknown | 6 | 5 (83.3) | 1 (16.7) | 1 | 1 (100) | 0 (0) | ||
| PR status | ||||||||
| Negative | 34 | 25 (73.5) | 9 (26.5) | 0.610 | 37 | 13 (35.1) | 24 (64.9) | 0.394 |
| Positive | 76 | 62 (81.6) | 14 (18.4) | 67 | 23 (34.3) | 44 (65.7) | ||
| Unknown | 6 | 5 (83.3) | 1 (16.7) | 1 | 1 (100) | 0 (0) | ||
| HER-2 status | ||||||||
| Negative | 61 | 56 (91.8) | 5 (9.2) | 0.002 | 68 | 22 (32.4) | 46 (67.4) | 0.040 |
| Positive | 40 | 26 (65.0) | 14 (35.0) | 31 | 10 (32.3) | 21 (67.7) | ||
| Unknown | 15 | 10 (66.7) | 5 (33.3) | 6 | 5 (83.3) | 1 (16.7) | ||
| Adjuvant chemotherapy regimen | ||||||||
| CMF | 15 | 11 (73.3) | 4 (26.7) | 0.540 | 9 | 5 (55.6) | 4 (44.4) | 0.166 |
| Anthracycline and/or taxane | 101 | 81 (80.2) | 20 (19.8) | 96 | 32 (33.3) | 64 (66.7) | ||
Note: P-values for comparison between PMRT group and non-PMRT group with chi-square test.
Abbreviations: CMF, cyclophosphamide, methotrexate, and 5-Fu; ER, estrogen receptor; HER-2, human epidermal growth factor receptor-2; PMRT, post-mastectomy radiotherapy; PR, progesterone receptor; 5-Fu, 5-fluorouracil.
Survival of patients
The median follow-up duration of the 221 patients was 61 months (range 10–154). Locoregional recurrence occurred in 34 cases: six in the chest wall, 19 in the supraclavicular fossa, one in the axillary lymph nodes, one in the internal mammary node, and seven in more than two sites. The median time to recurrence was 27.8 months (range 7–110). The 5- and 10-year LRFS for all patients was 84.1% and 76.4%, respectively. A total of 78 patients developed distant metastases. The detail of the distant metastases events are shown in Table 3. For all patients, the 5- and 10-year DMFS was 65.2% and 53.9%, respectively. The 5- and 10-year DFS was 61.4% and 49.4%, respectively. A total of 60 patients died of breast cancer during the follow-up; one patient died in a car accident. The 5- and 10-year OS rates were 77.2% and 60.0%, respectively.
Table 3.
Distribution of the first event by specific distant metastases sites
| Distant metastases site | n | % |
|---|---|---|
| Isolated lung | 10 | 12.8 |
| Isolated bone | 19 | 24.4 |
| Isolated liver | 14 | 17.9 |
| Isolated soft tissue | 10 | 12.8 |
| Isolated brain | 6 | 7.7 |
| Multiple sites | 19 | 24.4 |
Prognostic factors for locoregional recurrence-free survival
The univariate analysis results for prognostic factors are summarized in Table 4. According to the univariate analysis, patients with higher pN stage (P=0.041) and patients without PMRT (P=0.003) had lower LRFS. The pT stage; the estrogen receptor (ER), progesterone receptor (PR), and HER-2 status; and the adjuvant chemotherapy regimen had no significant effect on LRFS (Table 4). Multivariate analysis also supported that pN stage and PMRT were independent prognostic factors of LRFS (Table 5).
Table 4.
Univariate analysis of locoregional recurrence-free survival
| Characteristic | 5-year LRFS (%) | P-value |
|---|---|---|
| Tumor stage | ||
| pT1–2 | 84.4 | 0.555 |
| pT3–4 | 82.2 | |
| Nodal stage | ||
| pN1 | 86.0 | 0.041 |
| pN2 | 93.2 | |
| pN3 | 70.1 | |
| ER status | ||
| Negative | 87.9 | 0.484 |
| Positive | 80.1 | |
| PR status | ||
| Negative | 88.6 | 0.406 |
| Positive | 81.1 | |
| HER-2 status | ||
| Negative | 81.0 | 0.641 |
| Positive | 86.3 | |
| Adjuvant chemotherapy regimen | ||
| CMF | 86.8 | 0.823 |
| Anthracycline and/or taxane | 83.7 | |
| PMRT | ||
| None | 76.3 | 0.003 |
| Yes | 94.4 | |
Note: P-value was calculated using the Log-rank test.
Abbreviations: CMF, cyclophosphamide, methotrexate, and 5-Fu; ER, estrogen receptor; HER-2, human epidermal growth factor receptor-2; LRFS, locoregional recurrence-free survival; PMRT, post-mastectomy radiotherapy; PR, progesterone receptor; 5-Fu, 5-fluorouracil.
Table 5.
Multivariate analyses of locoregional recurrence-free survival
| Characteristic | HR | 95% CI | P-value |
|---|---|---|---|
| Nodal stage | |||
| pN1 | 1 | ||
| pN2 | 1.511 | 0.543–4.205 | 0.430 |
| pN3 | 4.631 | 2.093–10.247 | <0.001 |
| PMRT | |||
| None vs yes | 5.553 | 2.263–13.627 | <0.001 |
Abbreviations: CI, confidence interval; HR, hazard ratio; PMRT, post-mastectomy radiotherapy.
Impact of post-mastectomy radiotherapy on survival
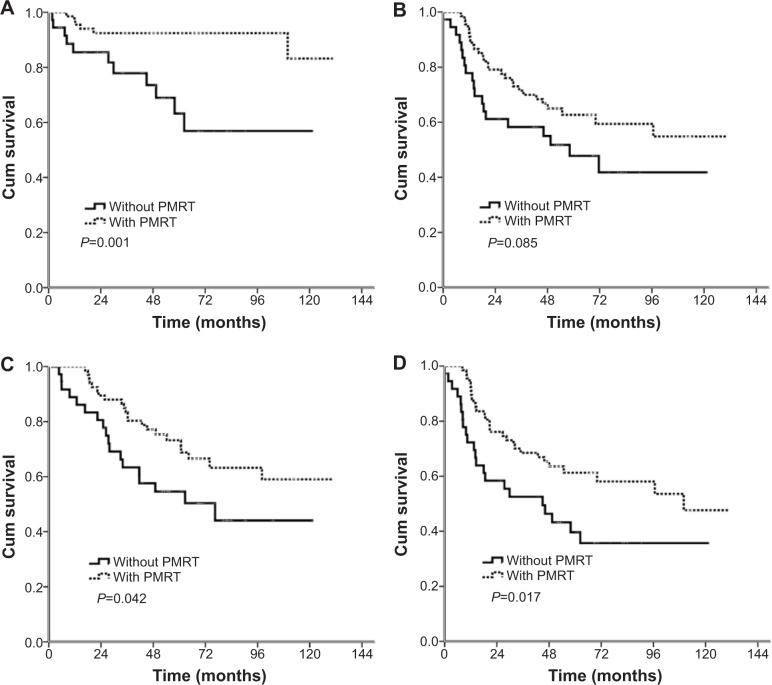
In the entire group with survival analysis, LRFS (P=0.003) was significantly better for patients who received PMRT than for those who did not, but there was no significant difference between the patients receiving and not receiving PMRT in terms of DMFS (P=0.429), DFS (P=0.146), and OS (P=0.750) (Table 6). In the subgroup analysis of patients with four or more positive nodes, the addition of PMRT improved LRFS (P=0.001), DFS (P=0.017), and OS (P=0.042). PMRT showed a trend to improve DMFS (P=0.085) (Table 6 and Figure 1). In patients with one to three positive nodes, PMRT showed a trend to improve LRFS (P=0.068) and DFS (P=0.064), but there was no significant difference between the patients receiving and not receiving PMRT in terms of DMFS (P=0.132) and OS (P=0.240) (Table 6).
Table 6.
Impact of post-mastectomy radiotherapy on survival in different subgroups
| Survival endpoint (%) | Entire
|
One to three positive nodes
|
Four or more positive nodes
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| Non-PMRT | PMRT | P-value | Non-PMRT | PMRT | P-value | Non-PMRT | PMRT | P-value | |
| 5-year LRFS | 76.3 | 94.4 | 0.003 | 80.9 | 90.9 | 0.068 | 63.2 | 92.4 | 0.001 |
| 5-year DMFS | 61.2 | 70.3 | 0.429 | 66.8 | 91.5 | 0.132 | 47.8 | 62.7 | 0.085 |
| 5-year DFS | 55.3 | 69.3 | 0.146 | 61.8 | 91.5 | 0.064 | 39.7 | 61.3 | 0.017 |
| 5-year OS | 75.6 | 79.3 | 0.750 | 84.1 | 95.7 | 0.240 | 54.6 | 73.2 | 0.042 |
Note: P-value was calculated using the Log-rank test.
Abbreviations: DFS, disease-free survival; DMFS, distant metastasis-free survival; LRFS, locoregional recurrence-free survival; OS, overall survival; PMRT, post-mastectomy radiotherapy.
Figure 1.
Comparison of Kaplan–Meier curves for patients with and without post-mastectomy radiotherapy in patients with four or more positive nodes.
Notes: (A) Locoregional recurrence-free survival; (B) distant metastasis-free survival; (C) disease-free survival; (D) overall survival.
Abbreviations: Cum, cumulative; PMRT, post-mastectomy radiotherapy.
Discussion
We retrospectively analyzed the value of PMRT in Chinese breast cancer patients aged 35 years or younger with positive axillary lymph nodes after mastectomy. We found that PMRT significantly improve LRFS but not DMFS, DFS, and OS in this patient population. Furthermore, subgroup analysis suggested that PMRT can improve the prognosis of breast cancer patients with four or more positive nodes, but has no beneficial effect on patients with one to three positive nodes.
An age of 35 years or younger is considered to be one of the high risk factors for locoregional recurrence after breast cancer surgery,13,14 emphasizing the importance of administering locoregional therapy in young patients. Postoperative adjuvant radiotherapy is one of the essential adjuvant therapies aiming to improve locoregional control. Randomized trials have confirmed that postoperative adjuvant radiotherapy may benefit survival by improving LRFS.10–12 Our study showed that PMRT has a beneficial effect on LRFS but not on DMFS, DFS, and OS in patients aged 35 years or younger with positive lymph nodes. Our findings suggest that PMRT may enhance locoregional control but fail to prevent the development of distant metastases. This may be due to more aggressive nature of the breast cancer and a higher chance of treatment failure caused by micrometastases in patients aged 35 years or younger. It might be reasonable to hypothesize that earlier systemic therapy may improve patient prognosis. Indeed, a study conducted by Garg et al15 in stage II–III patients aged less than 35 years who received PMRT after neoadjuvant therapy showed that PMRT was able to improve LRFS and OS, suggesting that early systemic therapy may reduce distant metastases and further improve survival.
Lymph node status is an important factor that affects the selection of type of adjuvant therapy treatment for breast cancer patients. It is also one of the essential indicators for PMRT. However, whether PMRT is beneficial for patients remains controversial. The St Gallen Breast Cancer Conference16 recommends the use of PMRT in patients younger than 35 with metastases involving one to three axillary lymph nodes and patients with four or more positive nodes. In 2008, the National Comprehensive Cancer Network (NCCN)17 strongly recommended the routine use of PMRT in patients of all ages with one to three positive axillary lymph nodes as well as those with four or more positive nodes. However, the European Society of Breast Cancer Specialists18 suggested that PMRT should only be used for patients aged less than 40 years with one to three positive axillary nodes. Therefore, major controversy surrounds whether PMRT is necessary for patients with one to three lymph node metastases. The Early Breast Cancer Trialists’ Collaborative Group19 have addressed the uncertainty of PMRT in patients with one to three positive nodes and concluded it reduced both recurrence and breast cancer mortality. However, there are currently no randomized controlled trials showing the beneficial effect of postoperative adjuvant radiotherapy in patients aged 35 years or younger with metastases in one to three axillary lymph nodes. Our stratified analysis is the first to demonstrate that PMRT has no beneficial effect on patients with one to three positive lymph nodes. A randomized controlled clinical trial is currently investigating this.20 Results from the trial are expected to provide more data in patients with one to three positive lymph nodes.
Breast reconstruction is an important tool to improve cosmetic outcome after mastectomy in patients aged 35 years or younger. With the advancements in reconstruction techniques, the effect of postoperative adjuvant radiotherapy on prostheses and radiotherapy-induced skin fibrosis has gained much attention for both immediate and delayed reconstruction.21 Based on the present study, radiotherapy may be unnecessary in patients aged 35 years or younger with one to three positive lymph nodes. However, with the progress that has been made in systemic therapies that has further reduced DMFS, randomized trial results are needed to evaluate the value of PMRT.
There is little controversy surrounding the use of PMRT in patients with four or more axillary lymph node metastases. Early in 2001, the American Society of Clinical Oncology recommended the routine use of PMRT in patients with four or more positive lymph nodes.22 In 2007, the Danish Breast Cancer Cooperative Group 82b and 82c trials showed that PMRT had a beneficial effect on patients with four or more positive lymph nodes.23 In a study by Liukkonen et al24 of pT3–4 patients aged 35 years or younger with axillary lymph node metastases after mastectomy, PMRT was found to improve locoregional control. Although our study did not show a beneficial effect of PMRT on DMFS in patients with four or more positive nodes, PMRT can improve locoregional control and thereby improve DFS and OS. This result is consistent with the majority of guidelines regarding indicators for the use of PMRT in patients with four or more positive nodes.25–27
Certain limitations to this study should be considered. First, the conclusion is based on a retrospective study involving a small number of subjects and a relatively short duration of follow-up; randomized controlled trials are lacking. Second, HER-2 status differed significantly in clinical characteristics of patients with one to three positive nodes, and none of the HER-2-positive patients were treated with trastuzumab. Therefore, it remains unclear whether trastuzumab has beneficial effects in such patients.
Conclusion
Our study suggests that PMRT may reduce locoregional recurrence and improve survival in Chinese breast cancer patients aged 35 years or younger with four or more positive nodes. However, this is still uncertain and needs to be confirmed by further prospective studies to confirm the value of PMRT in patients aged 35 years or younger with one to three positive nodes.
Acknowledgments
This study was supported by a grant from the Sci-Tech Office of Guangdong Province (2008B060600019), the Youth Foundation of the First Affiliated Hospital of Xiamen University (XYY2012005) and the Education Scientific Research Foundation of Young Teachers of Fujian Province (JB13131) and Medical Scientific Research Foundation of Guangdong Province (A2010192).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Agarwal G, Pradeep PV, Aggarwal V, Yip CH, Cheung PS. Spectrum of breast cancer in Asian women. World J Surg. 2007;31(5):1031–1040. doi: 10.1007/s00268-005-0585-9. [DOI] [PubMed] [Google Scholar]
- 2.Hankey BF, Ries LA, Edwards BK. The surveillance, epidemiology, and end results program: a national resource. Cancer Epidemiol Biomarkers Prev. 1999;8(12):1117–1121. [PubMed] [Google Scholar]
- 3.Holli K, Isola J. Effect of age on the survival of breast cancer patients. Eur J Cancer. 1997;33(3):425–428. doi: 10.1016/s0959-8049(97)89017-x. [DOI] [PubMed] [Google Scholar]
- 4.Winchester DP, Osteen RT, Menck HR. The National Cancer Data Base report on breast carcinoma characteristics and outcome in relation to age. Cancer. 1996;78(8):1838–1843. doi: 10.1002/(sici)1097-0142(19961015)78:8<1838::aid-cncr27>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 5.Walker RA, Lees E, Webb MB, Dearing SJ. Breast carcinomas occurring in young women (<35 years) are different. Br J Cancer. 1996;74(11):1796–1800. doi: 10.1038/bjc.1996.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Colleoni M, Rotmensz N, Robertson C, et al. Very young women (<35 years) with operable breast cancer: features of disease at presentation. Ann Oncol. 2002;13(2):273–279. doi: 10.1093/annonc/mdf039. [DOI] [PubMed] [Google Scholar]
- 7.Rapiti E, Fioretta G, Verkooijen HM, et al. Survival of young and older breast cancer patients in Geneva from 1990 to 2001. Eur J Cancer. 2005;41(10):1446–1452. doi: 10.1016/j.ejca.2005.02.029. [DOI] [PubMed] [Google Scholar]
- 8.Peng R, Wang S, Shi Y, et al. Patients 35 years old or younger with operable breast cancer are more at risk for relapse and survival: a retrospective matched case-control study. Breast. 2011;20(6):568–573. doi: 10.1016/j.breast.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 9.Han JG, Jiang YD, Zhang CH, et al. Clinicopathologic characteristics and prognosis of young patients with breast cancer. Breast. 2011;20(4):370–372. doi: 10.1016/j.breast.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 10.Overgaard M, Hansen PS, Overgaard J, et al. Postoperative radiotherapy in high-risk premenopausal women with breast cancer who receive adjuvant chemotherapy. Danish Breast Cancer Cooperative Group 82b Trial. N Engl J Med. 1997;337(14):949–955. doi: 10.1056/NEJM199710023371401. [DOI] [PubMed] [Google Scholar]
- 11.Overgaard M, Jensen MB, Overgaard J, et al. Postoperative radiotherapy in high-risk postmenopausal breast-cancer patients given adjuvant tamoxifen: Danish Breast Cancer Cooperative Group DBCG 82c randomized trial. Lancet. 1999;353(9165):1641–1648. doi: 10.1016/S0140-6736(98)09201-0. [DOI] [PubMed] [Google Scholar]
- 12.Ragaz J, Olivotto IA, Spinelli JJ, et al. Locoregional radiation therapy in patients with high-risk breast cancer receiving adjuvant chemotherapy: 20-year results of the British Columbia randomized trial. J Natl Cancer Inst. 2005;97(2):116–126. doi: 10.1093/jnci/djh297. [DOI] [PubMed] [Google Scholar]
- 13.Elkhuizen PH, van de Vijver MJ, Hermans J, Zonderland HM, van de Velde CJ, Leer JW. Local recurrence after breast-conserving therapy for invasive breast cancer: high incidence in young patients and association with poor survival. Int J Radiat Oncol Biol Phys. 1998;40(4):859–867. doi: 10.1016/s0360-3016(97)00917-6. [DOI] [PubMed] [Google Scholar]
- 14.Yildirim E, Berberoglu U. Local recurrence in breast carcinoma patients with T(1–2) and 1–3 positive nodes: indications for radiotherapy. Eur J Surg Oncol. 2007;33(1):28–32. doi: 10.1016/j.ejso.2006.10.022. [DOI] [PubMed] [Google Scholar]
- 15.Garg AK, Oh JL, Oswald MJ, et al. Effect of postmastectomy radiotherapy in patients <35 years old with stage II-III breast cancer treated with doxorubicin-based neoadjuvant chemotherapy and mastectomy. Int J Radiat Oncol Biol Phys. 2007;69(5):1478–1483. doi: 10.1016/j.ijrobp.2007.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goldhirsch A, Wood WC, Coates AS, Gelber RD, Thürlimann B, Senn JH, Panel members Strategies for subtypes – dealing with the diversity of breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol. 2011;22(8):1736–1747. doi: 10.1093/annonc/mdr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu JM, Wang YS. Breast cancer radiotherapy: controversies and prospectives. Chin Med J (Engl) 2008;121(20):1957–1959. [PubMed] [Google Scholar]
- 18.Cardoso F, Loibl S, Pagani O, et al. European Society of Breast Cancer Specialists The European Society of Breast Cancer Specialists recommendations for the management of young women with breast cancer. Eur J Cancer. 2012;48(18):3355–3377. doi: 10.1016/j.ejca.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 19.EBCTCG (Early Breast Cancer Trialists’ Collaborative Group) McGale P, Taylor C, Correa C, et al. Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet. 2014;383(9935):2127–2135. doi: 10.1016/S0140-6736(14)60488-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kunkler IH, Canney P, van Tienhoven G, Russell NS, MRC/EORTC (BIG 2-04) SUPREMO Trial Management Group Elucidating the role of chest wall irradiation in ‘intermediate-risk’ breast cancer: the MRC/EORTC SUPREMO trial. Clin Oncol (R Coll Radiol) 2008;20(1):31–34. doi: 10.1016/j.clon.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 21.Shah C, Kundu N, Arthur D, Vicini F. Radiation therapy following postmastectomy reconstruction: a systematic review. Ann Surg Oncol. 2013;20(4):1313–1322. doi: 10.1245/s10434-012-2689-4. [DOI] [PubMed] [Google Scholar]
- 22.Recht A, Edge SB, Solin LJ, et al. American Society of Clinical Oncology Postmastectomy radiotherapy: clinical practice guidelines of the American Society of Clinical Oncology. J Clin Oncol. 2001;19(5):1539–1569. doi: 10.1200/JCO.2001.19.5.1539. [DOI] [PubMed] [Google Scholar]
- 23.Overgaard M, Nielsen HM, Overgaard J. Is the benefit of postmastectomy irradiation limited to patients with four or more positive nodes, as recommended in international consensus reports? A subgroup analysis of the DBCG 82 b&c randomized trials. Radiother Oncol. 2007;82(3):247–253. doi: 10.1016/j.radonc.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 24.Liukkonen S, Leidenius M, Saarto T, Sjöström-Mattson J. Breast cancer in very young women. Eur J Surg Oncol. 2011;37(12):1030–1037. doi: 10.1016/j.ejso.2011.08.133. [DOI] [PubMed] [Google Scholar]
- 25.Aebi S, Davidson T, Gruber G, Cardoso F, ESMO Guidelines Working Group Primary breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2011;22(Suppl 6):vi12–vi24. doi: 10.1093/annonc/mdr371. [DOI] [PubMed] [Google Scholar]
- 26.Taylor ME, Haffty BG, Rabinovitch R, et al. ACR appropriateness criteria on postmastectomy radiotherapy expert panel on radiation oncology-breast. Int J Radiat Oncol Biol Phys. 2009;73(4):997–1002. doi: 10.1016/j.ijrobp.2008.10.080. [DOI] [PubMed] [Google Scholar]
- 27.Sautter-Bihl ML, Souchon R, Budach W, et al. DEGRO practical guidelines for radiotherapy of breast cancer II. Post-mastectomy radiotherapy, irradiation of regional lymphatics, and treatment of locally advanced disease. Strahlenther Onkol. 2008;184(7):347–353. doi: 10.1007/s00066-008-1901-8. [DOI] [PubMed] [Google Scholar]