Abstract
Showerheads support the development multi-species biofilms that can be unsightly, produce malodor, and may harbor pathogens. The outer surface spray plates of many showerheads support visible biofilms that likely contain a mixture of bacteria from freshwater and potentially from human users. Coaggregation, a mechanism by which genetically distinct bacteria specifically recognize one another, may contribute to the retention and enrichment of different species within these biofilms. The aim of this work was to identify the bacterial composition of outer spray plate biofilms of three domestic shower heads and to determine the inter- and intra-biofilm coaggregation ability of each culturable isolate. The bacterial composition of the three biofilms was determined by using bacterial tag-encoded FLX amplicon pyrosequencing (bTEFAP) and by culturing on R2A medium. An average of 31 genera per biofilm were identified using bTEFAP and a total of 30 isolates were cultured. Even though the microbial diversity of each showerhead biofilm differed, every cultured isolate was able to coaggregate with at least one other isolate from the same or different showerhead biofilm. Promiscuous coaggregating isolates belonged to the genera Brevundimonas, Micrococcus, and Lysobacter. This work suggests that coaggregation may be a common feature of showerhead biofilms. Characterization of the mechanisms mediating coaggregation, and the inter-species interactions they facilitate, may allow for novel strategies to inhibit biofilm development.
Keywords: Showerhead, biofilm, coaggregation, autoaggregation, pyrosequencing, phylogeny
INTRODUCTION
Showering is a common bathing method in the developed world (Chauvet et al, 1994). Showerheads can enrich for bacteria present in potable water by facilitating bacterial adhesion to exposed surfaces and to pre-attached bacteria (Feazel et al, 2009). Over time, these adherent bacteria grow and recruit additional species on outer and inner surfaces to form complex, multi-species biofilm communities (Costerton et al, 1995).
The development of showerhead biofilms has recently received increased attention because of the potential to enrich bacterial species including those that may have the ability to cause disease (Bauer et al, 2008; Exner et al, 2005; Falkinham et al, 2008; Nishiuchi et al, 2009; Schoen & Ashbolt, 2011). Notably, work by Feazel et al. demonstrated that numerous species can be enriched within showerhead biofilms including non-tuberculous mycobacteria and members of the genera Methylobacterium, Escherichia, Blastomonas, Pseudomonas, Methylocystitis, Staphylococcus, and Sphingomonas (Feazel et al, 2009). Members of some of these genera have been reported to cause opportunistic infections, especially in immunocompromised individuals (Cooksey et al, 2008; Ferroni et al, 2008). In addition to the potential to harbor pathogens, biofilms on and within showerheads can be unsightly, cause damage to the showerheads through microbial influenced corrosion, reduce water quality and increase bacterial load in aerosols, as well as produce malodor (Bloetscher et al, 2010; Bollin et al, 1985; Marras et al, 2005). Understanding how bacteria form multi-species biofilms may allow for improved strategies to control their development on inner and outer showerhead surfaces.
Coaggregation is the highly specific recognition and adhesion of genetically distinct bacteria. Specificity is mediated by complementary protein adhesins and polysaccharide receptors on the cell surface of coaggregating cells (Kolenbrander, 2000; Rickard et al, 2003a). This phenomenon is distinct from autoaggregation, which is the recognition and adhesion of genetically identical bacteria (Khemaleelakul et al, 2006; Rickard et al, 2003b; Van Houdt & Michiels, 2005). Coaggregation was first described between human dental plaque bacteria in 1970 (Gibbons & Nygaard, 1970), and work over the last two decades has shown that it also occurs between bacteria isolated from the human gut, the human urogenital tract, in wastewater flocs, and freshwater biofilms (Ledder et al, 2008; Phuong et al, 2011; Reid et al, 1988; Rickard et al, 2000; Simoes et al, 2008). Coaggregation may promote biofilm development, changes in biofilm architecture, and alter species composition of biofilms (Hojo et al, 2009; Kolenbrander et al, 2006; Rickard et al, 2003a). Coaggregation has also been shown to occur among numerous taxonomically distinct fresh water species (Rickard et al, 2002; Rickard et al, 2003b; Rickard et al, 2004). Studies of coaggregation between the freshwater bacterium Sphingomonas (Blastomonas) natatoria and Micrococcus luteus demonstrated that the ability of a species to coaggregate influences dual-species biofilm development in both flowing and static environments (Min & Rickard, 2009; Min et al, 2010). In addition, coaggregation has been suggested to play a role in promoting or hindering the integration of pathogenic species into freshwater biofilms (Buswell et al, 1998). Increasing evidence for such a possibility can be found in studies of dental plaque biofilms where coaggregation has been indicated to promote the integration of oral pathogens such as Porphyromonas gingivalis (Kolenbrander et al, 2006; Whitmore & Lamont, 2011). In constrast, studies of interactions between bacteria in the human urogenital tract indicate that coaggregation may promote juxtaposition and killing of E. coli by Lactobacillus reuteri RC-14 (McMillan et al, 2011).
The aim of this work was to determine if coaggregation occurs between bacterial species present on the surface of the outer spray plate of three domestic showerhead biofilms. This required the sampling of real-world biofilms (as opposed to model systems) which were subject to highly fluctuating conditions with respect to the amount, flow-rate, temperature and duration of water use. In addition, the type of water source used to feed the showerheads were different (two showerheads were fed metropolitan water and one was fed water from a well). A culture-independent and a culture-dependent approach was used to describe the microbial composition of three showerhead biofilms; bacterial composition was determined by bTEFAP 454 FLX titanium based massively parallel amplicon pyrosequencing and cultured isolates were identified by partial 16S rRNA gene sequencing. Isolates were evaluated for coaggregation ability, propensity to autoaggregate, and whole-cell hydrophobicity. The findings from this work demonstrate that coaggregation occurs both between bacteria isolated from the same showerhead biofilm and between those isolated from different showerhead biofilms. This finding indicates that coaggregation may be a common phenomenon within showerhead biofilms and thus a potential target for biofilm control strategies.
MATERIALS AND METHODS
Sample Collection and Bacterial Culturing
Three showerheads that supported visible biofilms on the outer spray plate surface were chosen for study. All three were located within domestic residences that were ~2.5–10 miles apart and had not received any chemical or physical cleaning regiments for >2 months prior to sampling. One domestic residence (showerhead UH) received well water while the other two residences (showerheads AH and HM) were geographically closer to one another and received water from the same metropolitan water supply. Showerhead biofilm samples were collected from the three domestic showerheads using BBL™ CultureSwab™ swabs (BD, Franklin Lakes, NJ). These swabs were wetted in autoclaved filter sterilized tap water and approximately 5cm2 of the outer surface of the showerhead was sampled. The swabs were suspended in 1.5ml of a sterile 50% v/v glycerol solution. Tubes were agitated for 10 seconds to re-suspend the biofilm bacteria and then stored at −80°C.
For culturing, suspended biofilm samples were serially diluted in sterile distilled water to extinction. Dilutions were plated on R2A agar (Reasoner & Geldreich, 1985), a medium for the cultivation of bacteria from potable water sources, and incubated for 14 days at 23°C. Colonies differentiated based on colony color, size, and morphology were isolated and purity streaked as they appeared from 72 h of incubation onwards. Only R2A agar was used in order to reduce the likelihood of re-isolating the same species that displayed culture-medium dependent colony morphologies and because R2A broth was used to grow bacteria for coaggregation assays. Dominant species were isolated and re-plated on solid R2A media, incubated for 72 hours at 30°C, and stored at 4°C. For long-term storage, isolates were stored at 80°C in 50% v/v glycerol solution.
Bacterial Isolate Identification via partial 16S rRNA PCR Amplification and Sequencing
For 16S rRNA sequencing, single colonies were suspended in 100μl of PCR certified water (Promega, Madison, WI) and heated to 85°C for 7 minutes. The suspension was centrifuged for 10 seconds at 6,400 RPM and 5μl of each suspension was used as the template for PCR amplification of the 16S rRNA gene. PCR was performed using a mix of 25μl GoTaq® Green Master Mix (Promega, Madison, WI), 18μl PCR certified water (Promega, Madison, WI), and 1μl each of forward primer (8FPL, AGTTTGATCCTGGCTCAG) and reverse primer (806R, GGACTACCAGGGTATCTAAT). The amplification protocol was identical to that used by Rickard et. al. (Rickard et al, 2004) except that a TC-5000 Thermo Cycler (Techne, Burlington, NJ) was used. PCR products were cleaned using the QIAquick PCR Purification System (Qiagen, Valencia, CA) according to the manufacturer’s protocol. The purified DNA was checked for quantity and purity using a NanoDrop 2000c spectrophotometer (Thermo Scientific, Watham, MA). Sequencing was performed by the DNA Sequencing Core at the University of Michigan (Ann Arbor, MI) using Applied Biosystems 3730xl DNA Analyzers (Applied Biosystmes, Carlsbad, CA), BigDyev3.1 chemistry (MCLAB, San Francisco, CA), and the protocols recommended by the manufacturer. Resulting partial 16s rRNA gene sequences were analyzed using CHROMAS (Technelysium Pty. Ltd., Australia) and compared to known sequences in the National Center for Biotechnology (NCBI) database using the Basic Local Alignment Search Tool (BLAST).
Phylogenetic Analysis
CLUSTALX v. 2.1 (Larkin et al, 2007) was used to initially align the partial 16s rRNA gene sequences with closely related strains in the NCBI database. Sequences of 620 nucleotides in length were used for tree construction. Aligned sequences were analyzed using TREECON v. 1.3b (Van de Peer & De Wachter, 1993) using the Jukes and Cantor (Jukes & Cantor, 1969) substitution model. The 16S rRNA gene sequence from Thermus thermophilus (EMBL accession no. X07998) was used as an out-group.
Culture-Independent Analysis
For culture-independent analysis, via pyrosequencing, dry BBLTM CultureSwab™ (BD, Franklin Lakes, NJ) swabs were used to sample the three showerheads selected for study at the same time as samples were taken for culture-dependent analyses. Swab tips were removed and placed in labeled sterile 2.0ml vials. These biofilm samples were frozen at −80°C until needed for pyrosequencing which relied upon the use of the primers 939F (TTGACGGGGGCCCGCAC) and 1492R (TACCTTGTTACGACTT) for pyrosequencing. DNA was extracted using a modified qiagen DNA preparation kit, which included incubating the swab in lysis buffer for 1 hr, bead beating utilizing a Qiagen Tissue lyser, and column capture, purification and elution of DNA. A single step PCR were conducted to generate bar coded amplicons with linkers. To prepare for FLX sequencing, the size and concentration of DNA fragments were determined by using DNA chips within a Bio-Rad Experion Automated Electrophoresis Station (Bio-Rad Laboratories, Hercules, CA) and a TBS-380 Fluorometer (Promega Corporation, Madison, WI). A 9.6 × 106 sample of double-stranded DNA molecules/μl with an average size of 625 bp were mixed with 9.6 million DNA capture beads, and subsequently amplified by emulsion PCR. After bead recovery and enrichment, the bead-attached DNAs were denatured with NaOH, and sequencing primers were annealed. A two-region 454 sequencing run was performed on a 70 × 75 GS PicoTiterPlate using a Genome Sequencer FLX System (Roche, Nutley, NJ). Following sequencing, all failed sequence reads, low quality sequence ends (Avg Q25), short reads < 150 bp (final mean length 412bp) and tags and primers were removed. Sequence collections were then depleted of any non-bacterial sequences, sequences with ambiguous base calls, sequences with homopolymers > 5bp in length, and chimeras as has been described previously (Bailey et al, 2010; Callaway et al, 2010; Capone et al, 2011; Handl et al, 2011; Ishak et al, 2011; Pitta et al, 2010). To determine the predicted identity of microorganisms in the remaining sequences, they were de-noised, de-replicated, and OTU clustering performed using uClust (www.drive5.com) and then queried using BLASTn against a highly curated custom database of high quality 16s bacterial sequences derived and manually curated from NCBI. Using a NET analysis pipeline, the resulting BLASTn outputs were compiled and data reduction analysis as described previously (Bailey et al, 2010; Callaway et al, 2010; Capone et al, 2011; Handl et al, 2011; Ishak et al, 2011; Pitta et al, 2010). Bacteria were classified at the closest well-characterized genus (Bailey et al, 2010; Callaway et al, 2010; Capone et al, 2011; Handl et al, 2011; Ishak et al, 2011; Pitta et al, 2010). Rarefaction analysis was conducted using QIIME (Caporaso et al, 2010) sequences were de-noised, chimeras removed using UCHIME (Edgar et al, 2011), sequences < 250bp removed, sequences > 250bp trimmed to 250bp, sequences were normalized to 1200 and 10 iterations of rarefaction performed to evaluate number of species present. The average rarefaction curve was then plotted based upon observed species. A Richard’s equation was utilized to predict the maximum OTU based upon the rarefaction curves as previously described (Acosta-Martinez et al, 2010).
Whole-Cell Hydrophobicity Assays
The surface hydrophobicity of the isolates was determined by using a modified approach of measuring bacterial adhesion to hexadecane described by Rosenberg (Rosenberg, 1981; Rosenberg & Rosenberg, 1981). Isolates were cultured in R2A broth for 48 hours at 30°C in a rotary shaker-incubator shaking at 225 rpm. Cells were subsequently washed three times in sterile tap water by centrifugation (3,000 × g for 10 min), normalized to an OD600 of 1.0, and mixed in equal volumes with hexadecane (Sigma, 99%). Suspensions were vortexed for 60s, and the two phases were allowed to separate for 10 min. Whole-cell hydrophobicity was expressed as the percent reduction in optical density difference between the original OD600 of cells in tap water and the OD600 of the hexadecane treated suspension.
Visual Aggregation Assays
For visual coaggregation assays, isolates were cultured in R2A broth for 48 hours at 30°C in a rotary shaker-incubator shaking at 225 rpm. Cell suspensions were subsequently washed and mixed in pairwise combinations according to the method of Rickard et al. (Rickard et al, 2002). The extent of coaggregation was scored using the protocol described by Cisar et. al (Cisar et al, 1979) and this was performed for at-least three independent experiments with the most consistent score being assigned. Specifically, coaggregation between isolates was scored from 0 to 4 where: 0 – no visible coaggregation in cell suspension; 1 – small uniform coaggregates in suspension; 2 – large coaggregates but suspension remains turbid; 3 – large coaggregates which settle rapidly leaving some turbidity in the supernatant; 4 – large coaggregates that settle immediately, leaving a clear supernatant. The cell suspensions containing single isolates were scored by the same manner to determine autoaggregation (self-aggregation) according to the method of Rickard et al (Rickard et al, 2003b). Autoaggregation was scored by using the same criteria as those used for coaggregation: the visual score assigned to the pair was determined by observing the relative drop in mixture turbidity and relative increase in aggregate flock size.
In order to gauge the ability of an isolate to coaggregate, each isolate was assigned a composite score based on the number of partners and relative strength of the individual coaggregation interactions. The resulting score was referred to as the coaggregation index (CI). This score was calculated using the following equation:
Where:
CI = Coaggregation index of an isolate
nx = The number of coaggregations displayed by that isolate (n) with a visual coaggregation score of x. The visual coaggregation score (x) can be 4, 3, 2, or 1.
Microscopy and Imaging
Coaggregates were visualized using transmitted light and solid state lasers on a Leica Microsystems TCS SPE confocal laser scanning microscope (Leica, Exon, PA) and the LAS-AF acquisition software (Leica, Exon, PA). Isolates were grown under the same conditions as described for coaggregation assays. After growth, cultures were centrifuged for 4 minutes at 9,000xg, and washed 3 times with sterilized distilled water. Isolates were labeled with nucleic acid stains. Specifically coaggregating partners were with either 3.34 μM SYTO® 9 or 5.0 μM SYTO® 59 according to manufacturer protocols (Invitrogen, Carlsbad, CA). This allowed the cells of each isolated to be differentiated within coaggregates (excitation and emission parameters for SYTO® 9 and SYTO® 59 were 488/510–550 and 635/640–680) and their relative spatial positions evaluated. Equal volumes of stained cells were then combined in a glass culture tubes and agitated to allow for coaggregation. Following approximately 30 s of gentle agitation, 100μl of the coaggregated pair was transferred to a glass microscope slide (VWR, Radnor, PA). To reduce disintegration of coaggregates and alteration of coaggregate structure, wells were created on microscope slides by layering 4 layers of parafilm M™(Marathon, Menasha, WI) on the glass surfaces and excising a 10mm X 10mm square with a razorblade. Pair-wise combinations with high >2 coaggregation scores were studied. Coaggregates were initially examined using the microscope in transmitted light mode and then visualized using the solid state lasers (with laser lines at 488 and 635nm). Excitation and emission wavelengths were selected for SYTO® 9 and SYTO® 59 (488/510–550 and 635/640–680, respectively) in accordance with manufacturer specifications (Invitrogen, Carlsbad, CA). The resulting images were visualized and inspected in 3-dimensions using IMARIS (Bitplane, Zurich, Switzerland). Captured renderings were assembled in CORELDRAW v. X4 (Corel, Mountain View, CA).
RESULTS
Culture-Independent Analysis
The three showerhead biofilms were examined to determine their bacterial composition using bTEFAP FLX massively parallel pyrosequencing. An average of 31 genera per biofilm were identified using bTEFAP and the twenty most dominant genera are shown in Table 1. Each showerhead biofilm possessed a unique bacterial community at the genus level. The most dominant bacterial genera within the showerhead biofilms were members of the Xanthomonas, Methylobacterium, Lysobacter, Brevundimonas, and Flavobacterium. Each showerhead biofilm also possessed genera that were unique to that given showerhead (e.g. Enhydrobacter was present at 13.1% in shower head biofilm AH but absent in both other shower head biofilms). Genus-level differences extended to the level of bacterial class and upon averaging across the three showerhead biofilms, 50.3% of the sequences derived from bTEFAP were members of the gamma-proteobacteria (HM, 82.0%; AH, 28.0%; UH, 41.06%), 28.0% were members of the alpha-proteobacteria (HM, 11.4%; AH, 64.4%; UH, 8.3%), 7.53% were flavobacteria (HM, 1.3%; AH, 2.0%; UH, 19.3%), 5.1% were beta-proteobacteria (HM, 2.1%; AH, 0.2%; UH, 12.9%), 4.0% were bacilli (HM, 0.0%; AH, 0.1%; UH, 11.8%), and 2.4% were actinobacteria (HM, 1.8%; AH, 5.0%; UH, 1.2%). The remaining 2.7% comprised 10 other bacterial classes. DNA sequences from opportunistic pathogens, e.g. Staphylococcus and Pseudomonas, were detected in the showerhead biofilms (Table 1). Legionella sequences and Mycobacterium sequences were detected in the showerhead biofilm HM, but only at 0.1% and 0.2%, respectively (data not presented). Rarefaction analysis was performed to determine how much of the microbiome was evaluated. It was determined based upon maximum OTU prediction in the AH, HM, and UH samples that there were 95, 126, and 43 species of bacteria. Rarefaction curves (supplemental Fig. 1) of the sequencing data identified 87, 100 and 39 species in each of the samples. Thus, the percentage of each population evaluated was predicted to be 92%, 80%, and 90% respectively.
Table 1.
Estimated relative abundance of bacterial genera (%), as determined by bTEFAP, of showerhead biofilms. The relative percentage of sequences assigned to a given taxonomic classification (genera) for each individual showerhead sample are arranged from highest to lowest across three showerheads.
| Genus | Average | Showerhead Biofilm HM (%) | Showerhead Biofilm AH (%) | Showerhead Biofilm UH (%) |
|---|---|---|---|---|
| Xanthomonas | 18.67 | 37.48 | 3.92 | 14.60 |
| Methylobacterium | 12.58 | 3.98 | 33.77* | - |
| Lysobacter | 7.21 | 12.01* | 1.69 | 7.94 |
| Brevundimonas | 6.90 | 1.98* | 18.73* | -* |
| Flavobacterium | 6.87 | 1.30 | - | 19.31 |
| Pseudoxanthomonas | 6.86 | 13.55 | 2.59* | 4.44 |
| Methylophaga | 5.43 | 11.24 | 3.25 | 1.80 |
| Enhydrobacter | 4.35 | - | 13.06 | - |
| Curvibacter | 3.30 | - | - | 9.89 |
| Stella | 2.22 | - | - | 6.67 |
| Bacillus | 2.08 | -* | - | 6.24 |
| Staphylococcus | 1.83 | - | -* | 5.50 |
| Serratia | 1.50 | - | - | 4.50 |
| Sphingomonas | 1.38 | 1.02 | 3.13 | -* |
| Xylella | 1.12 | 2.72 | - | 0.63 |
| Enterobacter | 0.92 | - | - | 2.75 |
| Pseudomonas | 0.90 | 2.32 | 0.40 | - |
| Fulvimarina | 0.79 | - | 2.36 | - |
| Microbacterium | 0.75 | -* | 2.24* | - |
| Sphingobium | 0.68 | - | 2.03 | - |
| Chryseobacterium | 0.66 | -* | 1.98 | - |
| Geobacter | 0.65 | - | - | 1.96 |
| Bdellovibrio | 0.63 | - | - | 1.90 |
| Moraxella | 0.51 | - | 1.54 | - |
| Brevibacterium | 0.49 | - | 1.46 | - |
| Photobacterium | 0.46 | - | - | 1.38 |
| Caulobacter | 0.42 | - | 1.26 | - |
| Aeromonas | 0.41 | - | - | 1.22 |
| Methylophilus | 0.35 | - | - | 1.06 |
| Rhodoferax | 0.35 | - | - | 1.06 |
| Micrococcus | 0.34 | - | 1.02* | -* |
| Propionibacterium | 0.32 | - | - | 0.95 |
| Rhizobium | 0.31 | - | 0.92 | - |
| Pedobacter | 0.23 | 0.68 | - | - |
| Phenylobacterium | 0.23 | 0.68 | - | - |
| Roseomonas | 0.23 | - | 0.68 | - |
| Dokdonella | 0.21 | 0.62 | - | - |
| Blastochloris | 0.20 | - | 0.61 | - |
| Xenophilus | 0.20 | 0.59 | - | - |
| Acidobacterium | 0.19 | - | - | 0.58 |
| Afipia | 0.16 | 0.49 | - | - |
| Stenotrophomonas | 0.15 | 0.46* | - | - |
| Paracoccus | 0.13 | 0.40 | -* | - |
| Paracraurococcus | 0.13 | 0.40 | - | - |
| Methyloversatilis | 0.12 | 0.37 | - | - |
| Novosphingobium | 0.12 | 0.37 | - | - |
| Other genera | 5.44 | 7.35 | 3.37 | 5.61 |
A star (*) indicates that a member of this genus was cultured from that showerhead biofilm (Table 2). The 20 most abundant genera in each showerhead biofilm are shown and those that are at low levels are grouped as together as “other genera”.
Culture-Dependent Analysis
Culturing and plate counts on R2A agar indicated that all three showerhead outer spray plates supported adherent bacteria, albeit at extremely different culturable densities. Showerhead biofilm HM contained 1.02×106 CFU/cm2, showerhead AH contained 1.41×104 CFU/cm2, and showerhead UH contained 6.9CFU/cm2.
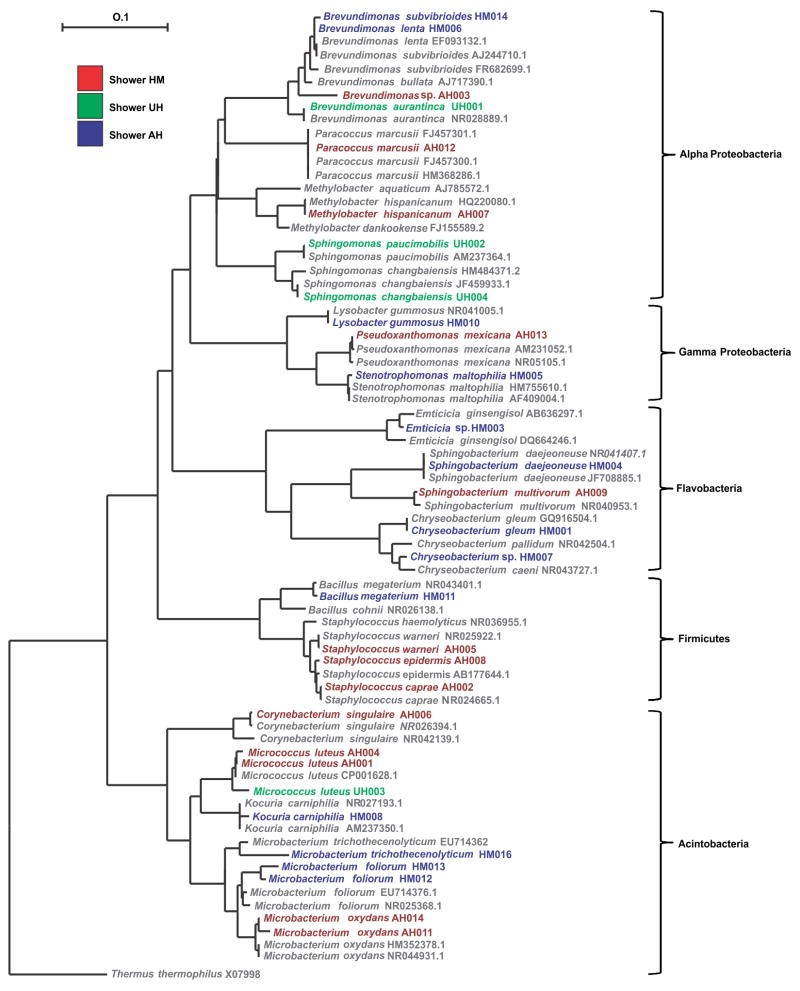
A total of 30 isolates, with differing colony morphologies, were obtained from the showerhead biofilms. Four isolates were cultured from showerhead UH, which received water from a potable well. Thirteen isolates each were isolated from showerheads AH and HM, which received water from the metropolitan water system. Isolates were identified as being from four phyla: the Actinobacteria, Firmicutes, Flavobacteria, and the Proteobacteria (alpha and gamma) (Fig. 1). Isolates belonging to the phyla Firmicutes and Flavobacteria as well as members of the gamma-Proteobacteria were exclusive to the two showerheads that were fed from the metropolitan water system (AH and HM). Only members of the genus Brevundimonas were isolated from all three showerheads (Fig. 1, Table 2). Twenty-seven of the 30 isolates were identified as having >97% identity to published bacterial 16S rRNA gene sequences (Table 2). These were consequently assigned species epithets.
Fig. 1.
Neighbor-joining phylogenetic tree of the partial 16S rRNA gene sequences from isolates cultured from the three showerhead biofilms. Colors relate to the showerhead biofilm from which the isolate was cultured. Scale bar represents one substitution for every 10 nucleotides. The outgroup sequence is from Thermus thermophilus (accession number AJ251638).
Table 2.
Identification of the showerhead biofilm isolates by alignment with 16S rRNA gene sequences of published bacterial species in the NCBI database.
| Isolate | Sequence Length (b.p.) | Closest Published Database Accession | Proposed Identity | % Identity | Accession Numb |
|---|---|---|---|---|---|
| Showerhead Biofilm HM | |||||
| HM001 | 727 | AM159535.1 | Chryseobacteriumgleum | 100 | HE800565 |
| HM003 | 714 | AB636297.1 | Emticicia sp. | 96 | HE800566 |
| HM004 | 744 | NR041407.1 | Sphingobacteriumdaejeonense | 99 | HE800567 |
| HM005 | 742 | HM755619.1 | Stenotrophomonasmaltophilia | 99 | HE800568 |
| HM006 | 681 | EF093132.1 | Brevundimonaslenta | 99 | HE800569 |
| HM007 | 739 | NR043727.1 | Chryseobacterium sp. | 96 | HE800570 |
| HM008 | 727 | NR027193.1 | Kocuriacarniphila* | 98 | HE800571 |
| HM010 | 754 | NR041005.1 | Lysobactergummosus | 99 | HE800572 |
| HM011 | 749 | FJ380122.1 | Bacillus megaterium | 99 | HE800573 |
| HM012 | 722 | HM233981.1 | Microbacterium foliorum | 99 | HE800574 |
| HM013 | 732 | EU714376.1 | Microbacterium foliorum | 99 | HE800575 |
| HM014 | 658 | EF093132.1 | Brevundimonassubvibrioides | 99 | HE800576 |
| HM016 | 628 | EU714362 | Microbacterium trichothecenolyticum | 99 | HE800577 |
| Showerhead Biofilm AH | |||||
| AH001 | 702 | HM584233.1 | Micrococcus luteus | 99 | HE800578 |
| AH002 | 693 | JN644490.1 | Staphylococcus caprae | 99 | HE800579 |
| AH003 | 668 | AJ717390.1 | Brevundimonas sp. | 95 | HE800580 |
| AH004 | 691 | HM584251.1 | Micrococcus luteus | 99 | HE800581 |
| AH005 | 749 | JN644590.1 | Staphylococcus warneri | 99 | HE800582 |
| AH006 | 628 | NR026394.1 | Corynebacterium singulare* | 99 | HE800583 |
| AH007 | 689 | HQ220080.1 | Methylobacterium hispanicum | 99 | HE800584 |
| AH008 | 740 | GU797281.1 | Staphylococcus epidermidis | 100 | HE800585 |
| AH009 | 754 | FJ548749.1 | Sphingobacteriummultivorum* | 99 | HE800586 |
| AH011 | 692 | HM352378.1 | Microbacteriumoxydans | 98 | HE800587 |
| AH012 | 687 | FJ457300.1 | Paracoccusmarcusii | 99 | HE800588 |
| AH013 | 738 | FM213381.2 | Pseudoxanthomonas Mexicana | 99 | HE800589 |
| AH014 | 672 | JN613481.1 | Microbacteriumoxydans | 100 | HE800590 |
| Showerhead Biofilm UH | |||||
| UH001 | 689 | GU204962.1 | Brevundimonasaurantiaca | 100 | HE800591 |
| UH002 | 679 | AY749436.1 | Sphingomonaspaucimobilis | 99 | HE800592 |
| UH003 | 726 | CP001628.1 | Micrococcus luteus | 99 | HE800593 |
| UH004 | 686 | JF459933.1 | Sphingomonas changbaiensis | 99 | HE800594 |
A star (*) indicates that this genus was not identified through culture-independent techniques (Table 1).
Autoaggregation and Whole-Cell Hydrophobicity
The propensity for a bacterium to autoaggregate may be related to whole-cell hydrophobicity and/or coaggregation ability. Autoaggregation ability was isolate-dependent and 13/30 of the isolates gave a positive visual autoaggregation score ≥ 1 (Table 3). The strongest autoaggregating isolates were Brevundimonas sp. AH003 (4), Corynebacterium singulare AH006 (4), Microbacterium hispanicum AH007 (4), Microbacterium trichothecenolyticum HM016 (4), and Sphingomonas changbaiensis UH004 (4). No relationship between autoaggregation score and % whole-cell hydrophobicity was evident (Table 3). For example, Staphylococcus warneri AH005 generated a hydrophobicity of 35.7% but did not autoaggregate while Microbacterium trichothecenolyticum HM016 generated a similar hydrophobicity of 39.9% and autoaggregated (visual score= 4). Similarly, C. singulare AH006 generated a hydrophobicity of 5.6% and autoaggregated (visual score= 4) while another isolate with a similar low percentage hydrophobicity, Emticicia sp. HM003 (26.1%), did not autoaggregate. While no link was observed to coaggregation ability, autoaggregation had the potential to mask coaggregation, and this was taken into account when performing coaggregation assays. An example of this phenomenon was evident between M. hispanicum AH007 (autoaggregation score of 4) and M. trichothecenolyticum HM016 (autoaggregation score of 4) which was assigned a coaggregation score of 4 in recognition of the visually strong coaggregation interactions between the autoaggregated flocs (Fig. 2).
Table 3.
Autoaggregation, whole-cell hydrophobicity, and coaggregation partnerships between isolates from within the same shower biofilm (intra-biofilm coaggregation) and with isolates from different shower biofilms (inter-biofilm coaggregation). The isolates from each showerhead biofilm are arranged based upon high to low values for their coaggregation index (CI). Data obtained from at-least three independent tests.
| Showerhead biofilm/Isolate | Numbers of Intra-Biofilm Coaggregations | Numbers of Inter-Biofilm Coaggregations | Autoaggregation Score | % Whole-Cell Hydrophobicity | Visual Coaggregation Scores | CI | |||
|---|---|---|---|---|---|---|---|---|---|
| 4 | 3 | 2 | 1 | ||||||
| Showerhead Biofilm HM | |||||||||
| B. lenta HM006 | 6 | 11 | 0 | 17.1 | 1 | 1 | 6 | 9 | 28 |
| L. gummosus HM010 | 6 | 8 | 0 | 4.9 | 1 | 1 | 1 | 11 | 20 |
| M. foliorum HM013 | 7 | 6 | 3 | 38.9 | 0 | 1 | 3 | 9 | 18 |
| C. gleum HM001 | 8 | 5 | 0 | 7.0 | 0 | 0 | 3 | 10 | 16 |
| K. carniphila HM008 | 4 | 7 | 0 | 0.6 | 0 | 0 | 2 | 9 | 13 |
| B. megaterium HM011 | 5 | 6 | 2 | 3.0 | 0 | 0 | 1 | 10 | 12 |
| M. foliorum HM012 | 4 | 6 | 3 | 29.4 | 0 | 0 | 2 | 8 | 12 |
| B. subvibrioides HM014 | 3 | 7 | 2 | 26.1 | 0 | 0 | 1 | 9 | 11 |
| S. maltophilia HM005 | 2 | 7 | 0 | 3.9 | 0 | 0 | 1 | 8 | 10 |
| S. daejeonense HM004 | 4 | 5 | 0 | 4.8 | 0 | 0 | 0 | 9 | 9 |
| Emticicia sp. HM003 | 2 | 6 | 0 | 26.1 | 0 | 0 | 0 | 8 | 8 |
| Chryseobacterium sp. HM007 | 5 | 1 | 0 | 4.9 | 0 | 1 | 0 | 5 | 8 |
| M. trichothecenolyticum HM016 | 0 | 2 | 4 | 39.9 | 1 | 0 | 1! | 0 | 6 |
| Showerhead Biofilm AH | |||||||||
| M. luteus AH004 | 5 | 12 | 1 | 14.0 | 0 | 2 | 4 | 11 | 25 |
| M. luteus AH001 | 6 | 9 | 1 | 2.9 | 0 | 0 | 1 | 14 | 16 |
| Brevundimonas sp. AH003 | 5 | 3 | 4 | 9.7 | 1 | 0 | 2 | 5 | 16 |
| M. hispanicum AH007 | 1 | 7 | 4 | 7.8 | 2 | 0 | 0 | 6 | 14 |
| P. mexicana AH013 | 3 | 8 | 0 | 3.0 | 0 | 0 | 1 | 10 | 12 |
| S. warneri AH005 | 5 | 5 | 0 | 35.7 | 0 | 0 | 1 | 9 | 11 |
| S. caprae AH002 | 2 | 6 | 3 | 10.4 | 0 | 0 | 2 | 6 | 10 |
| S. epidermidis AH008 | 2 | 6 | 0 | 11.0 | 0 | 0 | 0 | 8 | 8 |
| S. multivorum AH009 | 3 | 5 | 0 | 1.0 | 0 | 0 | 0 | 8 | 8 |
| C. singulare AH006 | 2 | 6 | 4 | 5.6 | 0 | 0 | 0 | 7 | 7 |
| M. oxydans AH014 | 3 | 4 | 0 | 13.6 | 0 | 0 | 0 | 7 | 7 |
| M. oxydans AH011 | 2 | 2 | 0 | 6.4 | 0 | 0 | 0 | 4 | 4 |
| P. marcusii AH012 | 1 | 2 | 1 | 9.8 | 0 | 0 | 0 | 3 | 3 |
| Showerhead Biofilm UH | |||||||||
| S. paucimobilis UH002 | 3 | 10 | 0 | 14.5 | 0 | 0 | 1 | 12 | 14 |
| B. aurantiaca UH001 | 2 | 7 | 0 | 0.8 | 0 | 0 | 1 | 8 | 10 |
| M. luteus UH003 | 3 | 6 | 0 | 11.5 | 0 | 0 | 1 | 8 | 10 |
| S. changbaiensis UH004 | 2 | 6 | 4 | 4.8 | 0 | 0 | 1 | 7 | 9 |
Coaggregation Index
Fig. 2.
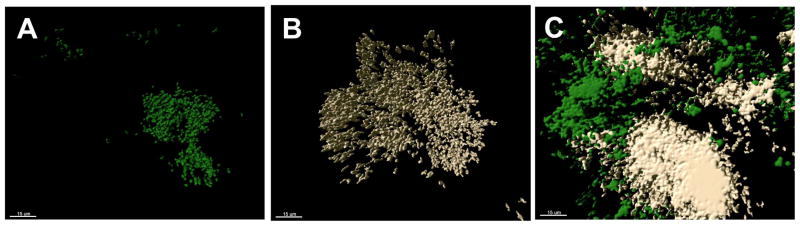
Representative confocal laser scanning microscope images showing the ability of two autoaggregating species to coaggregate. Cells have been falsely colored and surface rendered using Imaris® Surpass to aid differentiation of cells in coaggregates. Cell suspensions of (A) M. hispanicum AH007 and, (B) M. trichothecenolyticum HM016, and (C) coaggregating autoaggregates of M. hispanicum AH007 and M. trichothecenolyticum HM016 are shown. Bar represents 15 μm.
Coaggregation Interactions
Every isolate from the three showerhead biofilms coaggregated with at least one other isolate, although the majority of pairwise combinations demonstrated only weak visual coaggregation scores. Of all the 435 possible pairwise combinations used to determine coaggregation, 32.8% (143/435) of pairs coaggregated to give a visual score of 1, 2, 3, or 4. Of the coaggregating partnerships, 2.1% (3/143) coaggregated at a score of 4, 2.1% (3/143) coaggregated at a score of 3, 12.6% (18/143) coaggregated at a score of 2, and 83.2% of pairs (119/143) coaggregated at a score of 1 (Table 3). Coaggregation between isolates that displayed a visual score of 2 or greater are shown in Fig. 4. With the exception of one isolate, M. trichothecenolyticum HM016, all isolates coaggregated with other isolates from the same showerhead biofilm from which it was isolated (intra-biofilm coaggregation, table 3). Every isolate was able to coaggregate with at least one other isolate from a different showerhead biofilm (inter-biofilm coaggregation, Table 3). Coaggregation also occurred at the intra-generic level, for example between Brevundimonas lenta HM006 and Brevundimonas aurantinca UH001 to give a visual score of 1, and at the inter-generic level, for example between B. lenta HM006 and M. luteus AH004 to give a visual score of 3. Isolate promiscuity can be determined by three measures: (i) number of coaggregation partners, (ii) average visual coaggregation score per partner, (iii) as a function of both the number of partners and the visual coaggregation score of each partnership. Some isolates had a high visual coaggregation score, but few partners (M. trichothecenolyticum HM016), and other isolates had a large number of partners, but the resulting visual scores were relatively low (Pseudoxanthomonas mexicana AH013). In order to categorize isolate promiscuity as a function of both the number of partners and the resulting visual score of those partnerships the coaggregation index (CI) was determined for each isolate. Based upon this index, the most promiscuous coaggregating isolates were B. lenta HM006 (17 partners, CI=28), M. luteus AH004 (17 partners, CI=25), and Lysobacter gummosus HM010 (14 partners, CI=20) (Table 3).
Fig. 4.
A diagrammatic representation of the inter- and intra-biofilm specificity of coaggregation between the showerhead biofilm isolates after growth in batch culture for 48h. Visual coaggregation scores <2 are not shown. Colors highlight the showerhead from which the isolates were actually harvested. Cells are not to scale and visual scores are depicted as connecting lines of different thickness. Thickest line (⚊) represents a visual coaggregation score of 4, line of intermediate thickness represents score of 3 (―), and thinnest dotted line (----) represents a score of 2.
Microscopic analysis of coaggregates
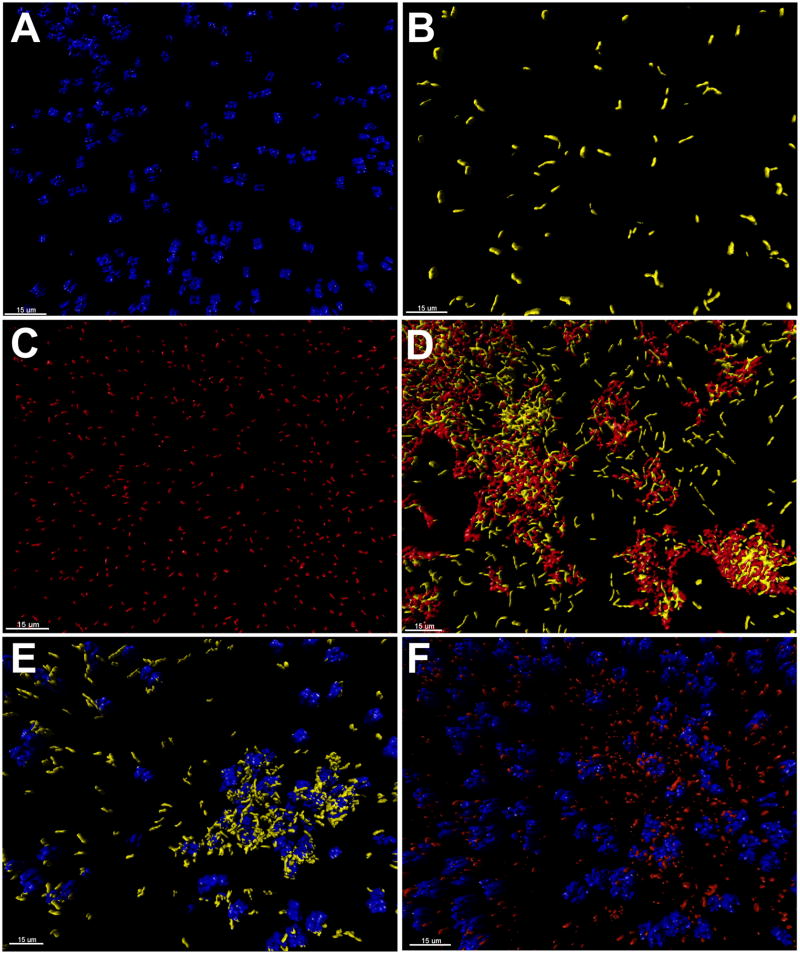
In order to discern structure, coaggregates that gave visual scores >2 were visualized using transmitted light microscopy and confocal scanning laser microscopy. Using confocal scanning laser microscopy in conjunction with different Syto® stains, single-isolate suspensions (Fig. 3A–C) and mixtures (coaggregated suspensions, Fig. 3D–F) were easily discerned. Microscopy of all coaggregates with a visual coaggregation of ≥2 showed that none of the coaggregate mixtures possessed any defined architecture with respect to shape, size (from 2–3 cells to large coaggregated flocs >100μM in diameter) and spatial position of the isolates within the coaggregate. The visual strength of coaggregation clearly influenced the size of the coaggregate that was visualized by confocal scanning laser microscopy. The lower the visual score the smaller the coaggregate that were observed by microscopy and the more susceptible were the coaggregates to disassociation. For example, coaggregation between B. lenta HM006 and L. gummosus HM010 (visual score of 4) and coaggregation between Micrococcus luteus AH004 and B lenta HM006 (visual score of 4) both yielded large densely-packed inter-digitated coaggregated flocs with isolates wrapping around each other (Fig. 3D and E). This was in stark contrast to coaggregation between M. luteus AH004 and L. gummosus HM010 (visual score of 2) that, when visualized by confocal scanning laser microscopy, were consistently mixtures of single-cells and small weakly adherent inter-digitated coaggregates (Fig. 3F).
Fig. 3.
Representative confocal laser scanning microscope images from multiple experiments demonstrating the interdigitated nature of coaggregation between three showerhead biofilm species that coaggregated with one another. Cells have been falsely colored and surface rendered using Imaris® Surpass to aid differentiation of cells in coaggregates. Cell suspensions of (A) M. luteus AH004, (B) B. lenta HM006, (C) L. gummosus HM010, and coaggregates of (D) B. lenta HM006 and L. gummosus HM010 (visual score of 4), (E) M. luteus AH004 and B. lenta HM006 (visual score of 4), and (F) M. luteus AH004 and L. gummosus HM010 (visual score of 2). Bar represents 15 μm.
DISCUSSION
The work presented here demonstrates that coaggregation occurs between bacteria isolated from biofilms present on the outer spray plate surface of showerheads. In particular, coaggregation occurred between isolates from the same showerhead biofilm and between those from different showerhead biofilms. We describe these phenomena as intra- and inter-biofilm coaggregation. Autoaggregation was also displayed by many isolates. No obvious correlation between whole-cell hydrophobicity and autoaggregation or coaggregation was observed, suggesting that species-and/or strain-specific surface-appendages were involved in these interactions. Highly specific inter-bacterial aggregative interactions may therefore contribute to multi-species bacterial biofilm development on showerheads.
Coaggregation and autoaggregation interactions are receiving increased attention across different microbiology research fields (Chen et al, 2010; Kolenbrander et al, 2006; Luthje & Brauner, 2010; Min & Rickard, 2009). Collective evidence from these studies suggests that coaggregation and autoaggregation promote the highly specific integration and expansion of bacterial populations within biofilm communities (Kolenbrander, 2000; Merritt et al, 2009; Min & Rickard, 2009; Rickard et al, 2002; Walter et al, 2008). Here we show that all 30 showerhead biofilm isolates were able to coaggregate with atleast one other isolate (Table 3), and that 13 of these were able to autoaggregate. Strong visual coaggregation scores (3–4) were less common than has been reported in other studies of coaggregation in freshwater biofilms. Work by Rickard et al. (Rickard et al, 2002) demonstrated that after mixing all pair-wise combinations of 19 strains isolated from a freshwater biofilm, 82/171 pairs (48%) coaggregated with a visual score of 1–4. Forty of these coaggregation partnerships (23%) expressed a score of ≥2. These proportions were derived from testing pairs at three different time-points during batch-culture growth, as coaggregation between freshwater bacteria can be growth-phase dependent (Rickard et al, 2000). From our showerhead data, 143/435 (32.9%) pair-wise combinations coaggregated with a visual score of 1–4 after culturing for 48h. This is a lower percentage than the previously studied freshwater biofilm system. Only 24 (5.5%) of the showerhead coaggregating pairs expressed a score ≥2 (Fig. 4). These lower values are likely a consequence of growth-phase-dependent coaggregation between the showerhead biofilm isolates. Testing for coaggregation at other periods of growth in batch-cultures may increase both the number of detected coaggregation pairs and the strength of these coaggregations. Regardless, coaggregation interactions were detected and all of the cultured showerhead biofilm isolates identified in this study coaggregated with isolates from other showerhead biofilms (Table 3). Such inter-biofilm coaggregation interactions suggest that coaggregation is a common phenomenon within showerhead biofilms, regardless of species composition. Fig. 4 shows the intra- and inter- biofilm coaggregation was observed among those that gave stronger coaggregation scores of ≥2.
As highlighted in the coaggregation matrix diagram (Fig. 4) and Table 3, the three showerhead biofilm isolates with the highest CI were B. lenta HM006 (17 partners; CI=28.1), M. luteus AH004 (17 coaggregation partners; CI=24.9), and L. gummosus HM010 (14 partners; CI=20). The ability of M. luteus to coaggregate has been demonstrated in other studies (Rickard et al, 2003a) but the propensity for B. lenta and L. gummosus to coaggregate has not previously been described. Species belonging to the genus Lysobacter was detected using bTEFAP in all three showerhead biofilms although species belonging to Brevundimonas and Micrococcus were detected in two and one of the showerhead biofilms respectively. Sequences from potential pathogens that were detected by bTEFAP at notable amounts include those from the genera Staphylococcus and Pseudomonas. Staphylococcus species were cultured and shown to coaggregate with other showerhead species (Table 1, Table 3).
Many of the isolates displayed the ability to both auto- and coaggregate. Thirteen of the 30 isolates (43%) both autoaggregated to some extent and coaggregated. No correlation to whole cell-hydrophobicity was discerned suggesting that autoaggregation and coaggregation may be mediated by specific complementary cell-surface molecules that have limited effect on gross hydrophibic properties. Autoaggregation has the potential to mask coaggregation interactions (Elliott et al, 2006; Reid et al, 1988). When coaggregation was suspected between two isolates that autoaggregated, cells from both isolates were stained with different Styo® dyes prior to mixing, so they could be differentiated by fluorescence, and the autoaggregative and coaggregative abilities were assessed using confocal microscopy. Coaggregation was deemed to be occurring between the two strongly autoaggregating isolates M. hispanicum AH007 (autoaggregation score of 4, Table 3, Fig, 2) and M. trichothecenolyticum HM016 (autoaggregation score of 4, Table 3, Fig. 2). When mixed together these autoaggregating isolates adhered to one-another to form larger inter-generic flocs and, based upon microscopic and visual observations, gave a visual score of 4. Our protocol for determining the degree of coaggregation between two isolates with the ability to autoaggregate differed from previous work categorizing aggregative abilities in that a subtractive scoring would have resulting in a visual coaggregation score of a zero for the pair M. hispanicum AH007 and M. trichothecenolyticum HM016 (Elliott et al, 2006; Rickard et al, 2003b). This scoring would have been a mischaracterization of the actual interactions between the two species and the ability of these isolates to coaggregate would not have been discovered. It is possible that either one type of surface-bound multi-function polymer mediates both coaggregation and autoaggregation, or that functionally distinct surface polymers are co-expressed to perform each role.
Compared to the findings of Feazel and co-workers (Feazel et al, 2009), we did not detect high numbers of Mycobacterium species in the three showerhead biofilms using culture-dependent and culture-independent approaches. Mycobacteria were only detected using bTEFAP in showerhead biofilm HM (0.2%, data not presented). A number of reasons could be for this finding. Firstly from a culturing perspective, we only used R2A to culture the isolates and these plates were incubated for only two weeks. It is very possible that more selective agar would have allowed for the growth of mycobacteria, even if they were at low numbers as determined as determined by 454 pyrosequencing. In addition, unlike the approach of Feazel and co-workers (Feazel et al, 2009) who studied biofilms on the interior surface of the showerhead, we sampled the outer-exposed surface spray plate of the showerheads from which the water was expelled. Showerhead biofilms on outer surfaces may contain species that are distinct from those found inside and be subject to splash-back from individuals who use showers. Such a possibility could also account for the presence of the often considered human-associated genera Staphylococci, Micrococci, and Kocuria and the presence of often freshwater and soil-associated Lysobacter which can be difficult to culture and are known to lyse Gram positive bacteria (Christensen & Cook, 1978; Hayward et al, 2010). Fresh water biofilm species composition is also strongly dependent upon fluid flow/shear (Rickard et al, 2004; Rochex et al, 2008), and the extent to which diverse populations develop and display differences in coaggregation and autoaggregation have been linked to fluid shear (Rickard et al, 2004). Of course, other factors such as the microbiological content and physico-chemical properties of the source water are also relevant and may contribute to the species composition of the biofilms (Keinanen et al, 2002; Lyautey et al, 2005; Schwartz et al, 1998). This latter point might explain why the species composition of the two showerheads that received metropolitan water (AH and HM) were seemingly more similar to that which received well water (UH). While chemical analyses of the water from each showerhead was not performed, a future study that focuses on relationship of the chemical composition of water and biofilm species composition would be intriguing.
Xanthomonas, Flavobacterium, Methylophaga, and a total of 77 other genera were identified by bTEFAP as being present in the showerhead biofilms but these were not isolated by culturing (full data not presented). This disparity likely stems from the limitations of culturing microorganisms from freshwater samples. Culturing selectivity/bias is well-documented and is likely related to unsupported nutritional and environmental requirements provided by any single culture medium (Camper et al, 1998; Schloss & Handelsman, 2005; Spiegelman et al, 2005). R2A agar (Reasoner & Geldreich, 1985) was used to isolate as many taxonomically (and metabolically) diverse bacteria as possible. R2A supports the growth of a broad range of Gram-negative and Gram-positive species indigenous to freshwater communities (Cheng & Foght, 2007; Massa et al, 1998; Percival et al, 1988). As is evident in both this and other combined culture-dependent and culture-independent studies (Dowd et al, 2008; Guss et al, 2010; Na et al, 2011), a greater diversity of microbiota were identified using bTEFAP. However, bacteria were also isolated using culture-dependent methods that belong to genera which were not identified by bTEFAP (Table 1 versus Table 2). This disparity likely relates to the fact that only 3,000 bTEFAP reads were performed or to a primer induced bias. However, it should be noted that rarefaction analysis of the sequencing data indicated that we captured 92%, 80%, and 90% of the species from showerhead biofilms AH, HM, and UH, respectively. Ultimately, as suggested by Phuong and coworkers (Phuong et al, 2012) and supported here, no single approach to describing the diversity of a bacterial community may be absolutely reliable and a multifaceted (culture-dependent and culture-independent) approach can yield greater information.
From an architectural standpoint, imaging and 3-dimensional rendering of coaggregates demonstrated that all coaggregrate pairs, regardless of their ability to autoaggregate, formed complex and interdigitated structures. The lack of defined structure may be important for facilitating interactions between the component isolates. For example, when considering coaggregation between human oral bacteria, coaggregates of Streptococcus gordonii DL1 and Actinomyces naeslundii MG1 do not possess any obvious structure and are instead highly interdigitated, similar to the structures observed between the showerhead biofilm bacteria (Jakubovics et al, 2008a). Evidence has been presented that demonstrate that interdigitated coaggregation with A. oris MG1 protected S. gordonii DL1 from oxidative damage and stabilized S. gordonii DL1 expression of arginine biosynthesis (Jakubovics et al, 2008a; Jakubovics et al, 2008b). It is therefore possible that coaggregation between showerhead biofilm isolates may not only promote attachment to one another, but also allow these isolates, by virtue of their interdigitated and intimate juxtaposition, to chemically interact and communicate with one another (Kolenbrander et al, 2010; McCormick et al, 2011).
In conclusion, this study demonstrates that coaggregation may commonly occur between bacteria within biofilm communities on the outer spray plate of showerhead biofilms. In support of this, bacterial species isolated from the three different showerhead surfaces coaggregated with members of the same biofilm, from which they were isolated, and between those isolated from different showerhead biofilms. When considering that such biofilms are at a human-freshwater interface, it would be interesting to test the ability of the these bacteria to coaggregate with bacteria isolated from the human skin and from those bacteria isolated from water feeds and biofilms from the inside of such devices. Of course a future study of the microbial composition and coaggregation ability of bacteria within shower biofilms would also be interesting, especially if compared to the outer surfaces. Ultimately, a greater understanding of coaggregation between showerhead bacteria may allow for novel strategies to control biofilm development.
Supplementary Material
Acknowledgments
The authors are grateful for the support provided by the Microbial Ecology: Relationships to Human and Environmental Health group at the University of Michigan, USA and the Society for Applied Microbiology, UK for providing support via the Student into Work award program. The authors thank Adam Underwood, Dhana Rao, and Lindsay Weir (University of Michigan, Ann Arbor, MI) for technical assistance.
References
- Acosta-Martinez V, Dowd SE, Bell CW, Lasconao R, Booker JD, Zobeck TM, DRU Microbial Community Composition as Affected by Dryland Cropping Systems and Tillage in a Semiarid Sandy Soil. Diversity. 2010;2:910–931. [Google Scholar]
- Bailey MT, Dowd SE, Parry NM, Galley JD, Schauer DB, Lyte M. Stressor exposure disrupts commensal microbial populations in the intestines and leads to increased colonization by Citrobacter rodentium. Infect Immun. 2010;78:1509–1519. doi: 10.1128/IAI.00862-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer M, Mathieu L, Deloge-Abarkan M, Remen T, Tossa P, Hartemann P, Zmirou-Navier D. Legionella bacteria in shower aerosols increase the risk of Pontiac fever among older people in retirement homes. J Epidemiol Community Health. 2008;62:913–920. doi: 10.1136/jech.2007.063784. [DOI] [PubMed] [Google Scholar]
- Bloetscher F, Meeroff DE, Pisani JV, Long SC. Resolving problematic biofilms in buildings and compounds. Environmental Engineering Science. 2010;27:767–776. [Google Scholar]
- Bollin GE, Plouffe JF, Para MF, Hackman B. Aerosols containing Legionella pneumophila generated by shower heads and hot-water faucets. Appl Environ Microbiol. 1985;50:1128–1131. doi: 10.1128/aem.50.5.1128-1131.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buswell CM, Herlihy YM, Lawrence LM, McGuiggan JT, Marsh PD, Keevil CW, Leach SA. Extended survival and persistence of Campylobacter spp. in water and aquatic biofilms and their detection by immunofluorescent-antibody and -rRNA staining. Appl Environ Microbiol. 1998;64:733–741. doi: 10.1128/aem.64.2.733-741.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaway TR, Dowd SE, Edrington TS, Anderson RC, Krueger N, Bauer N, Kononoff PJ, Nisbet DJ. Evaluation of the bacterial diversity in the rumen and feces of cattle fed diets containing levels of dried distiller’s grains plus solubles using bacterial tag-encoded FLX amplicon pyrosequencing (bTEFAP) J Anim Sci. 2010 doi: 10.2527/jas.2010-2900. [DOI] [PubMed] [Google Scholar]
- Camper A, Burr M, Ellis B, Butterfield P, Abernathy C. Development and structure of drinking water biofilms and techniques for their study. J Appl Microbiol. 1998;85(Suppl 1):1S–12S. doi: 10.1111/j.1365-2672.1998.tb05277.x. [DOI] [PubMed] [Google Scholar]
- Capone KA, Dowd SE, Stamatas GN, Nikolovski J. Diversity of the Human Skin Microbiome Early in Life. J Invest Dermatol. 2011 doi: 10.1038/jid.2011.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Pena AG, Goodrich JK, Gordon JI, Huttley GA, Kelley ST, Knights D, Koenig JE, Ley RE, Lozupone CA, McDonald D, Muegge BD, Pirrung M, Reeder J, Sevinsky JR, Turnbaugh PJ, Walters WA, Widmann J, Yatsunenko T, Zaneveld J, Knight R. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010;7:335–336. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauvet LP, Probert SD, Nevrala DJ. Thermal-energy stores for supplying domestic hot-water and space-heating. Applied Energy. 1994;48:163–190. [Google Scholar]
- Chen X, Tian F, Liu X, Zhao J, Zhang HP, Zhang H, Chen W. In vitro screening of lactobacilli with antagonistic activity against Helicobacter pylori from traditionally fermented foods. J Dairy Sci. 2010;93:5627–5634. doi: 10.3168/jds.2010-3449. [DOI] [PubMed] [Google Scholar]
- Cheng SM, Foght JM. Cultivation-independent and -dependent characterization of bacteria resident beneath John Evans Glacier. FEMS Microbiol Ecol. 2007;59:318–330. doi: 10.1111/j.1574-6941.2006.00267.x. [DOI] [PubMed] [Google Scholar]
- Christensen P, Cook FD. Lysobacter, a new genus of nonfruiting, gliding bacteria with a high base ratio. Int J Syst Bacteriol. 1978;28:367–393. [Google Scholar]
- Cisar JO, Kolenbrander PE, McIntire FC. Specificity of coaggregation reactions between human oral streptococci and strains of Actinomyces viscosus or Actinomyces naeslundii. Infect Immun. 1979;24:742–752. doi: 10.1128/iai.24.3.742-752.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooksey RC, Jhung MA, Yakrus MA, Butler WR, Adekambi T, Morlock GP, Williams M, Shams AM, Jensen BJ, Morey RE, Charles N, Toney SR, Jost KC, Jr, Dunbar DF, Bennett V, Kuan M, Srinivasan A. Multiphasic approach reveals genetic diversity of environmental and patient isolates of Mycobacterium mucogenicum and Mycobacterium phocaicum associated with an outbreak of bacteremias at a Texas hospital. Appl Environ Microbiol. 2008;74:2480–2487. doi: 10.1128/AEM.02476-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costerton JW, Lewandowski Z, Caldwell DE, Korber DR, Lappin-Scott HM. Microbial biofilms. Annu Rev Microbiol. 1995;49:711–745. doi: 10.1146/annurev.mi.49.100195.003431. [DOI] [PubMed] [Google Scholar]
- Dowd SE, Wolcott RD, Sun Y, McKeehan T, Smith E, Rhoads D. Polymicrobial nature of chronic diabetic foot ulcer biofilm infections determined using bacterial tag encoded FLX amplicon pyrosequencing (bTEFAP) PLoS One. 2008;3:e3326. doi: 10.1371/journal.pone.0003326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar RC, Haas BJ, Clemente JC, Quince C, Knight R. UCHIME improves sensitivity and speed of chimera detection. Bioinformatics. 2011;27:2194–2200. doi: 10.1093/bioinformatics/btr381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott DR, Wilson M, Buckley CM, Spratt DA. Aggregative behavior of bacteria isolated from canine dental plaque. Appl Environ Microbiol. 2006;72:5211–5217. doi: 10.1128/AEM.01060-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Exner M, Kramer A, Lajoie L, Gebel J, Engelhart S, Hartemann P. Prevention and control of health care-associated waterborne infections in health care facilities. Am J Infect Control. 2005;33:S26–40. doi: 10.1016/j.ajic.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Falkinham JO, 3rd, Iseman MD, de Haas P, van Soolingen D. Mycobacterium avium in a shower linked to pulmonary disease. J Water Health. 2008;6:209–213. doi: 10.2166/wh.2008.032. [DOI] [PubMed] [Google Scholar]
- Feazel LM, Baumgartner LK, Peterson KL, Frank DN, Harris JK, Pace NR. Opportunistic pathogens enriched in showerhead biofilms. Proc Natl Acad Sci U S A. 2009;106:16393–16399. doi: 10.1073/pnas.0908446106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferroni A, Werkhauser-Bertrand A, Le Bourgeois M, Beauvais R, Vrielynck S, Durand C, Lenoir G, Berche P, Sermet-Gaudelus I. Bacterial contamination in the environment of hospitalised children with cystic fibrosis. J Cyst Fibros. 2008;7:477–482. doi: 10.1016/j.jcf.2008.05.001. [DOI] [PubMed] [Google Scholar]
- Gibbons RJ, Nygaard M. Interbacterial aggregation of plaque bacteria. Arch Oral Biol. 1970;15:1397–1400. doi: 10.1016/0003-9969(70)90031-2. [DOI] [PubMed] [Google Scholar]
- Guss AM, Roeselers G, Newton IL, Young CR, Klepac-Ceraj V, Lory S, Cavanaugh CM. Phylogenetic and metabolic diversity of bacteria associated with cystic fibrosis. Isme J. 2010;5:20–29. doi: 10.1038/ismej.2010.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handl S, Dowd SE, Garcia-Mazcorro JF, Steiner JM, Suchodolski JS. Massive parallel 16S rRNA gene pyrosequencing reveals highly diverse fecal bacterial and fungal communities in healthy dogs and cats. FEMS Microbiol Ecol. 2011 doi: 10.1111/j.1574-6941.2011.01058.x. [DOI] [PubMed] [Google Scholar]
- Hayward AC, Fegan N, Fegan M, Stirling GR. Stenotrophomonas and Lysobacter: ubiquitous plant-associated gamma-proteobacteria of developing significance in applied microbiology. Journal of applied microbiology. 2010;108:756–770. doi: 10.1111/j.1365-2672.2009.04471.x. [DOI] [PubMed] [Google Scholar]
- Hojo K, Nagaoka S, Ohshima T, Maeda N. Bacterial interactions in dental biofilm development. J Dent Res. 2009;88:982–990. doi: 10.1177/0022034509346811. [DOI] [PubMed] [Google Scholar]
- Ishak HD, Plowes R, Sen R, Kellner K, Meyer E, Estrada DA, Dowd SE, Mueller UG. Bacterial Diversity in Solenopsis invicta and Solenopsis geminata Ant Colonies Characterized by 16S amplicon 454 Pyrosequencing. Microb Ecol. 2011;61:821–831. doi: 10.1007/s00248-010-9793-4. [DOI] [PubMed] [Google Scholar]
- Jakubovics NS, Gill SR, Iobst SE, Vickerman MM, Kolenbrander PE. Regulation of gene expression in a mixed-genus community: stabilized arginine biosynthesis in Streptococcus gordonii by coaggregation with Actinomyces naeslundii. J Bacteriol. 2008a;190:3646–3657. doi: 10.1128/JB.00088-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakubovics NS, Gill SR, Vickerman MM, Kolenbrander PE. Role of hydrogen peroxide in competition and cooperation between Streptococcus gordonii and Actinomyces naeslundii. FEMS Microbiol Ecol. 2008b;66:637–644. doi: 10.1111/j.1574-6941.2008.00585.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jukes TH, Cantor CR. Evolution of protein molecules. In: Munro HN, editor. Mammalian protein metabolism. New York: Academic Press Inc; 1969. pp. 21–132. [Google Scholar]
- Keinanen MM, Korhonen LK, Lehtola MJ, Miettinen IT, Martikainen PJ, Vartiainen T, Suutari MH. The microbial community structure of drinking water biofilms can be affected by phosphorus availability. Appl Environ Microbiol. 2002;68:434–439. doi: 10.1128/AEM.68.1.434-439.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khemaleelakul S, Baumgartner JC, Pruksakom S. Autoaggregation and coaggregation of bacteria associated with acute endodontic infections. J Endod. 2006;32:312–318. doi: 10.1016/j.joen.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Kolenbrander PE. Oral microbial communities: biofilms, interactions, and genetic systems. Annu Rev Microbiol. 2000;54:413–437. doi: 10.1146/annurev.micro.54.1.413. [DOI] [PubMed] [Google Scholar]
- Kolenbrander PE, Palmer RJ, Jr, Periasamy S, Jakubovics NS. Oral multispecies biofilm development and the key role of cell-cell distance. Nat Rev Microbiol. 2010;8:471–480. doi: 10.1038/nrmicro2381. [DOI] [PubMed] [Google Scholar]
- Kolenbrander PE, Palmer RJ, Jr, Rickard AH, Jakubovics NS, Chalmers NI, Diaz PI. Bacterial interactions and successions during plaque development. Periodontol 2000. 2006;42:47–79. doi: 10.1111/j.1600-0757.2006.00187.x. [DOI] [PubMed] [Google Scholar]
- Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, McWilliam H, Valentin F, Wallace IM, Wilm A, Lopez R, Thompson JD, Gibson TJ, Higgins DG. Clustal W and Clustal X version 2.0. Bioinformatics. 2007;23:2947–2948. doi: 10.1093/bioinformatics/btm404. [DOI] [PubMed] [Google Scholar]
- Ledder RG, Timperley AS, Friswell MK, Macfarlane S, McBain AJ. Coaggregation between and among human intestinal and oral bacteria. FEMS Microbiol Ecol. 2008;66:630–636. doi: 10.1111/j.1574-6941.2008.00525.x. [DOI] [PubMed] [Google Scholar]
- Luthje P, Brauner A. Ag43 promotes persistence of uropathogenic Escherichia coli isolates in the urinary tract. J Clin Microbiol. 2010;48:2316–2317. doi: 10.1128/JCM.00611-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyautey E, Jackson CR, Cayrou J, Rols JL, Garabetian F. Bacterial community succession in natural river biofilm assemblages. Microb Ecol. 2005;50:589–601. doi: 10.1007/s00248-005-5032-9. [DOI] [PubMed] [Google Scholar]
- Marras TK, Wallace RJ, Jr, Koth LL, Stulbarg MS, Cowl CT, Daley CL. Hypersensitivity pneumonitis reaction to Mycobacterium avium in household water. Chest. 2005;127:664–671. doi: 10.1378/chest.127.2.664. [DOI] [PubMed] [Google Scholar]
- Massa S, Caruso M, Trovatelli F, Tosques M. Comparison of plate count agar and R2A medium for enumeration of heterotrophic bacteria in natural mineral water. World Journal of Microbiology and Biotechnology. 1998;14:727–730. [Google Scholar]
- McCormick DW, Stevens MRE, Boles BR, Rickard AH. Does it take Two to Tango? The Importance of Coaggregation in Multi-Species Biofilms. Culture. 2011;32:1–5. [Google Scholar]
- McMillan A, Dell M, Zellar MP, Cribby S, Martz S, Hong E, Fu J, Abbas A, Dang T, Miller W, Reid G. Disruption of urogenital biofilms by lactobacilli. Colloids Surf B Biointerfaces. 2011;86:58–64. doi: 10.1016/j.colsurfb.2011.03.016. [DOI] [PubMed] [Google Scholar]
- Merritt J, Niu G, Okinaga T, Qi F. Autoaggregation response of Fusobacterium nucleatum. Appl Environ Microbiol. 2009;75:7725–7733. doi: 10.1128/AEM.00916-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min KR, Rickard AH. Coaggregation by the freshwater bacterium Sphingomonas natatoria alters dual-species biofilm formation. Appl Environ Microbiol. 2009;75:3987–3997. doi: 10.1128/AEM.02843-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min KR, Zimmer MN, Rickard AH. Physicochemical parameters influencing coaggregation between the freshwater bacteria Sphingomonas natatoria 2.1 and Micrococcus luteus 2.13. Biofouling. 2010;26:931–940. doi: 10.1080/08927014.2010.531128. [DOI] [PubMed] [Google Scholar]
- Na H, Kim OS, Yoon SH, Kim Y, Chun J. Comparative approach to capture bacterial diversity of coastal waters. J Microbiol. 2011;49:729–740. doi: 10.1007/s12275-011-1205-z. [DOI] [PubMed] [Google Scholar]
- Nishiuchi Y, Tamura A, Kitada S, Taguri T, Matsumoto S, Tateishi Y, Yoshimura M, Ozeki Y, Matsumura N, Ogura H, Maekura R. Mycobacterium avium complex organisms predominantly colonize in the bathtub inlets of patients’ bathrooms. Jpn J Infect Dis. 2009;62:182–186. [PubMed] [Google Scholar]
- Percival SL, Knapp JS, Edyveab R, Wales DS. Biofilm development on stainless steel in mains water. Water Research. 1988;32:243–253. [Google Scholar]
- Phuong K, Hanazaki S, Kakii K, Nikata T. Involvement of Acinetobacter sp. in the floc-formation in activated sludge process. J Biotechnol. 2011 doi: 10.1016/j.jbiotec.2011.09.024. [DOI] [PubMed] [Google Scholar]
- Pitta DW, Pinchak E, Dowd SE, Osterstock J, Gontcharova V, Youn E, Dorton K, Yoon I, Min BR, Fulford JD, Wickersham TA, Malinowski DP. Rumen bacterial diversity dynamics associated with changing from bermudagrass hay to grazed winter wheat diets. Microb Ecol. 2010;59:511–522. doi: 10.1007/s00248-009-9609-6. [DOI] [PubMed] [Google Scholar]
- Reasoner DJ, Geldreich EE. A new medium for the enumeration and subculture of bacteria from potable water. Appl Environ Microbiol. 1985;49:1–7. doi: 10.1128/aem.49.1.1-7.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid G, McGroarty JA, Angotti R, Cook RL. Lactobacillus inhibitor production against Escherichia coli and coaggregation ability with uropathogens. Can J Microbiol. 1988;34:344–351. doi: 10.1139/m88-063. [DOI] [PubMed] [Google Scholar]
- Rickard AH, Gilbert P, High NJ, Kolenbrander PE, Handley PS. Bacterial coaggregation: an integral process in the development of multi-species biofilms. Trends Microbiol. 2003a;11:94–100. doi: 10.1016/s0966-842x(02)00034-3. [DOI] [PubMed] [Google Scholar]
- Rickard AH, Leach SA, Buswell CM, High NJ, Handley PS. Coaggregation between aquatic bacteria is mediated by specific-growth-phase-dependent lectin-saccharide interactions. Appl Environ Microbiol. 2000;66:431–434. doi: 10.1128/aem.66.1.431-434.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickard AH, Leach SA, Hall LS, Buswell CM, High NJ, Handley PS. Phylogenetic relationships and coaggregation ability of freshwater biofilm bacteria. Appl Environ Microbiol. 2002;68:3644–3650. doi: 10.1128/AEM.68.7.3644-3650.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rickard AH, McBain AJ, Ledder RG, Handley PS, Gilbert P. Coaggregation between freshwater bacteria within biofilm and planktonic communities. FEMS Microbiol Lett. 2003b;220:133–140. doi: 10.1016/S0378-1097(03)00094-6. [DOI] [PubMed] [Google Scholar]
- Rickard AH, McBain AJ, Stead AT, Gilbert P. Shear rate moderates community diversity in freshwater biofilms. Appl Environ Microbiol. 2004;70:7426–7435. doi: 10.1128/AEM.70.12.7426-7435.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochex A, Godon JJ, Bernet N, Escudie R. Role of shear stress on composition, diversity and dynamics of biofilm bacterial communities. Water Res. 2008;42:4915–4922. doi: 10.1016/j.watres.2008.09.015. [DOI] [PubMed] [Google Scholar]
- Rosenberg M. Bacterial adherence to polystyrene: a replica method of screening for bacterial hydrophobicity. Appl Environ Microbiol. 1981;42:375–377. doi: 10.1128/aem.42.2.375-377.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg M, Rosenberg E. Role of adherence in growth of Acinetobacter calcoaceticus RAG-1 on hexadecane. J Bacteriol. 1981;148:51–57. doi: 10.1128/jb.148.1.51-57.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schloss PD, Handelsman J. Metagenomics for studying unculturable microorganisms: cutting the Gordian knot. Genome Biol. 2005;6:229. doi: 10.1186/gb-2005-6-8-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoen ME, Ashbolt NJ. An in-premise model for Legionella exposure during showering events. Water Res. 2011;45:5826–5836. doi: 10.1016/j.watres.2011.08.031. [DOI] [PubMed] [Google Scholar]
- Schwartz T, Hoffmann S, Obst U. Formation and bacterial composition of young, natural biofilms obtained from public bank-filtered drinking water systems. Water Research. 1998;32:2787–2797. [Google Scholar]
- Simoes LC, Simoes M, Vieira MJ. Intergeneric coaggregation among drinking water bacteria: evidence of a role for Acinetobacter calcoaceticus as a bridging bacterium. Appl Environ Microbiol. 2008;74:1259–1263. doi: 10.1128/AEM.01747-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegelman D, Whissell G, Greer CW. A survey of the methods for the characterization of microbial consortia and communities. Can J Microbiol. 2005;51:355–386. doi: 10.1139/w05-003. [DOI] [PubMed] [Google Scholar]
- Van de Peer Y, De Wachter R. TREECON: a software package for the construction and drawing of evolutionary trees. Comput Appl Biosci. 1993;9:177–182. doi: 10.1093/bioinformatics/9.2.177. [DOI] [PubMed] [Google Scholar]
- Van Houdt R, Michiels CW. Role of bacterial cell surface structures in Escherichia coli biofilm formation. Res Microbiol. 2005;156:626–633. doi: 10.1016/j.resmic.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Walter J, Schwab C, Loach DM, Ganzle MG, Tannock GW. Glucosyltransferase A (GtfA) and inulosucrase (Inu) of Lactobacillus reuteri TMW1.106 contribute to cell aggregation, in vitro biofilm formation, and colonization of the mouse gastrointestinal tract. Microbiology. 2008;154:72–80. doi: 10.1099/mic.0.2007/010637-0. [DOI] [PubMed] [Google Scholar]
- Whitmore SE, Lamont RJ. The pathogenic persona of community-associated oral streptococci. Mol Microbiol. 2011;81:305–314. doi: 10.1111/j.1365-2958.2011.07707.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.