Abstract
Background
Identifying immunologic mechanisms that contribute to premature cardiovascular disease (CVD) among HIV-positive patients will inform prevention strategies.
Methods
Coronary artery calcium (CAC) progression was studied in an HIV cohort. Immunophenotypes were measured on baseline cryopreserved peripheral blood mononuclear cells using multi-color flow cytometry. Logistic regression identified predictors of CAC progression after adjusting for traditional and HIV -related risk factors.
Results
Baseline characteristics for the analysis cohort (n=436) were: median age 42 years, median CD4 count 481cells/mm3, and 78% receiving ART. Higher frequencies of CD16+ monocytes were associated with greater likelihood of CAC progression, after adjusting for traditional and HIV risk factors (OR per doubling was 1.66 for CD14+/CD16+ [p=0.02], 1.36 for CD14dim/CD16+ [p=0.06], and 1.69 for CD14var/CD16+ [p=0.01]). Associations for CD16+ monocytes persisted when restricted to participants with viral suppression. We found no significant associations for CAC progression with other cellular phenotypes, including T-cell activation and senescence markers.
Conclusions
Circulating CD16+ monocytes, potentially reflecting a more pro-atherogenic subpopulation, independently predicted greater CAC progression among HIV-infected persons at low risk for AIDS. In contrast to T-cell abnormalities classically associated with AIDS-related disease progression, these data highlight the potential role of monocyte activation in HIV-related CVD risk.
Keywords: HIV, cardiovascular disease, coronary artery calcium, immune activation, monocyte activation, inflammation
INTRODUCTION
HIV-positive persons are at increased risk for premature atherosclerotic cardiovascular disease (CVD), which is now a leading cause of morbidity and mortality among contemporary patients with access to effective combination antiretroviral therapy (ART) [1, 2]. Pro-atherogenic factors among HIV-positive patients include a greater prevalence of traditional risk factors (e.g., smoking), consequences of HIV replication, and exposure to certain antiretroviral medications [3–5]. We have shown that both HIV replication and exposure to protease inhibitors are associated with greater progression of subclinical atherosclerotic disease among participants in the SUN Study (Study to Understand the Natural History of HIV/AIDS in the Era of Effective Therapy) [6].
Recent data suggest that chronic inflammation may partly account the excess CVD risk attributable to HIV infection [7, 8]. However, few data exist on the immunologic mechanisms underlying inflammation-CVD risk associations among HIV positive patients. Cardiac computed tomography (CT) estimates of coronary artery calcified plaque (CAC) provide a non-invasive assessment of subclinical atherosclerosis that also correlates with the extent of histologically confirmed non-calcified plaque [9, 10]. In addition, CAC progression is independently associated with future risk for atherosclerotic CVD events and all-cause mortality, and adding CAC assessments to traditional risk factor prediction significantly improves risk classification for coronary heart disease or stroke [11–14].
We conducted a longitudinal analysis of participants in the SUN Study, and hypothesized that pro-inflammatory cellular phenotypes would predict CAC progression independent of traditional and HIV infection-related clinical factors. The most widely studied measure of immune activation in HIV-positive persons is the frequency of CD4+ or CD8+ T-cells expressing activation markers (e.g., CD38/HLA-DR), which independently predicts risk for AIDS progression and has more recently been associated with subclinical CVD [15, 16]. Abnormalities in innate, or non-specific, immunity have also been described among HIV-positive patients, such as a higher prevalence of circulating monocytes that express CD16+ [17, 18]. Three distinct monocyte subpopulations have been defined, based on expression of CD14 and CD16: classical (CD14+/CD16−), intermediate (CD14+/CD16+), and non-classical (CD14dim/CD16+) phenotypes [19]. Intermediate and non-classical monocyte phenotypes may reflect a more activated immunologic state, demonstrating greater release of pro-inflammatory cytokines and an affinity for attaching to vascular surfaces, respectively [20–23]. These data, along with the well-accepted role of monocytes in CVD pathogenesis [24], motivated our decision to study monocytes subpopulations along with more traditional T-cell phenotypes associated with HIV disease risk.
METHODS
Study Design
The SUN Study is a Centers for Disease Control and Prevention (CDC)-funded prospective observational cohort study of HIV-infected participants enrolled at seven clinics in four U.S. cities (Denver, Minneapolis, Providence, and St. Louis) between March 2004 and June 2006 [25]. The protocol was approved by ethics committees at the CDC and each clinical site. Participants provided written informed consent. The SUN Study design and cohort have been previously described [25]. Participant visits occurred at baseline and every six months thereafter, with a CAC measurement obtained at the baseline and year two visit. Participants enrolled in the SUN Study were either naÔve to ART or their prior antiretroviral exposure had consisted solely of combination ART (≥ three nucleoside reverse transcriptase inhibitors [NRTI] or ≥ three antiretroviral drugs from at least two different classes), and were expected to survive at least two years. Participants were included in this analysis if they had CAC measurements at both the baseline and two-year visit.
Clinical data, including all medications and diagnoses, were abstracted and entered into a database (Clinical Practice Analyst; Cerner Corporation, Vienna, VA), with additional data provided by a study-specific physical examination, laboratory testing, and an audio computer-assisted self-interview (ACASI). The presence of hypertension was defined as a blood pressure (BP) >140/90 mmHg, prescription of antihypertensives, or a diagnosis of hypertension. We estimated glomerular filtration rate (GFR) using the Cockcroft-Gault equation and Framingham 10-year coronary heart disease (CHD) risk score (FRS) using published score sheets [26].
Laboratory Measurements
Following each visit, fasting whole blood and plasma specimens were shipped overnight to the CDC. Peripheral blood mononuclear cells (PBMCs) were isolated and cryopreserved in liquid nitrogen centrally at a CDC lab within 30 hours of blood draw. Clinical site laboratory testing included measurement of fasting serum lipids, plasma HIV RNA viral load (VL), and CD4 T-cell counts.
Immunophenotyping was performed on cryopreserved PBMCs using multi-color flow cytometry. Panels of fluorochrome-conjugated antibodies for cell surface markers not affected by cryopreservation (and viability dye to exclude non-viable cells) have been validated [27]. The fluorochrome-conjugated antibodies used to characterize cellular phenotypes were: anti-CD38 PE-Cy7(Clone:HIT2), anti-CX3CR1 PE(Clone:2A9-1), anti-CD28 PE-Cy7(Clone:CD28.2), anti-CD4 efluor605(Clone:OKT4), and anti-HLADR efluor605(Clone:LN3) from eBioscience (San Diego, CA), anti-CD57 APC(Clone:HCD57), anti-CD27 Ax700(Clone:O323), anti-CX3CR1 APC(Clone:2A9-1), anti-CD16 PE-Cy7(Clone:3G8), and anti-CCR2 PerCp-Cy5.5(Clone:TG5) from BioLegend (San Diego, CA), anti-HLADR PE(Clone:G46-6), anti-CD3 APC-Cy7(Clone:SK7), anti-CD8 PB(Clone:RPA-T8), anti-CD14 PE(Clone:M5E2), anti-CCR5 APC-Cy7(Clone:2D7), and anti-CD56 PB(Clone:B159) from BD Biosciences (San Jose, CA), anti-TF FITC(Clone:VIC7) from American Diagnostica (Stamford, CT), and anti-CD45RO ECD(Clone:UCHL1) from Beckman Coulter (Brea, CA), and Live/Dead Fixable Blue Dead Cell Stain Kit with UV excitation from Invitrogen (Grand Island, NY). Samples were acquired on an LSR-II flow cytometer (BD Diagnostic Systems; Franklin Lakes, NJ) and data were analyzed using FlowJo software version 9.5.3 (Treestar Inc., Ashland, OR).
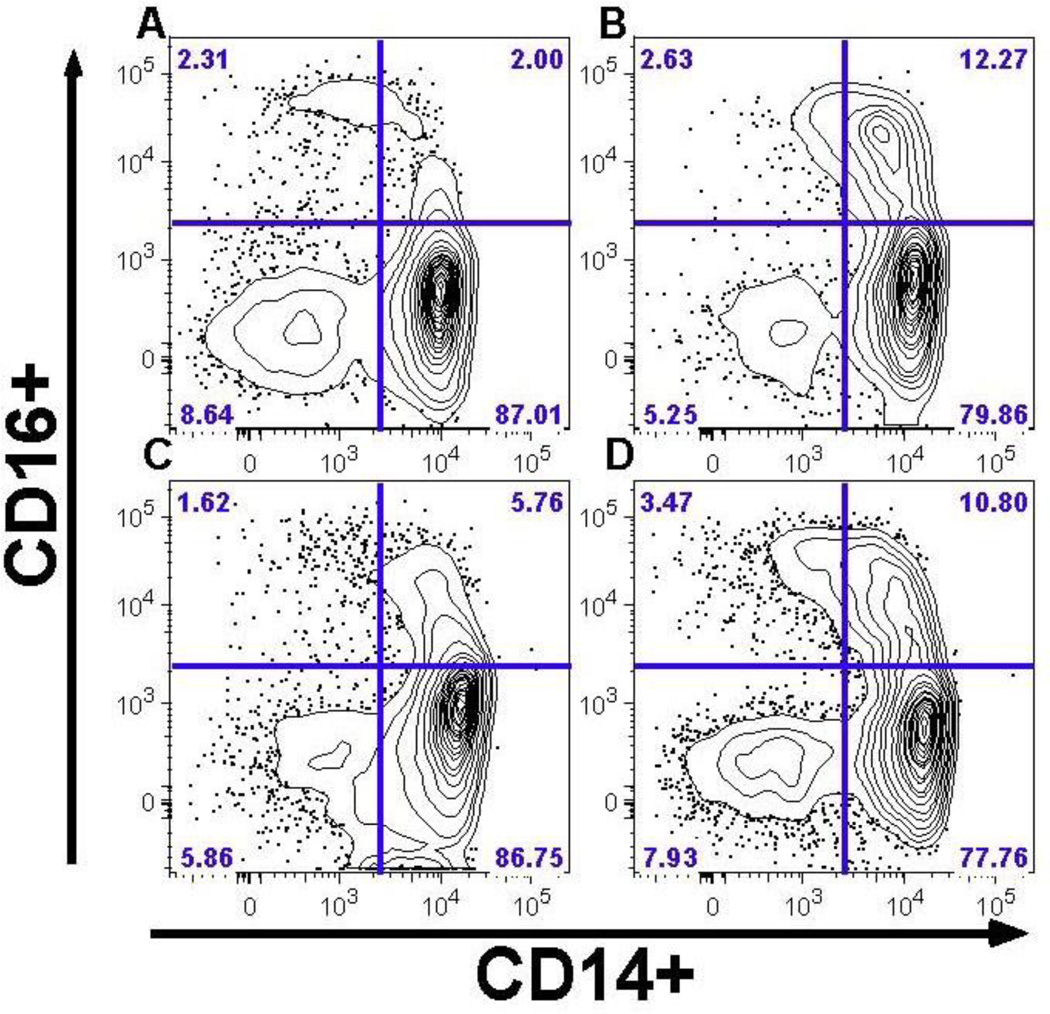
The following monocyte cell phenotypes were characterized and expressed as proportional percentages: classical phenotype (CD14+/CD16−), intermediate phenotype (CD14+/CD16+), non-classical phenotype (CD14dim/CD16+), CD14var/CD16+ (intermediate and non-classical combined, or CD16+), tissue factor expression (TF+), and expression of tissue migration markers (CCR2+, CCR5+, CX3CR1+). CD4+ and CD8+ T-cells phenotypes included: CX3CR1 expression, activated phenotype (HLA-DR+/ CD38+), and senescence phenotype (CD57+/CD38+). Representative flow cytometry plots are shown in Figure 1. Only gated live cells were included in analyses; all cryopreserved samples had >75% viability.
Figure 1. Monocyte Phenotype by CAC Outcome.
After gating on live cells that express HLA-DR and lack expression of markers of T, B, and NK cells (CD2, CD3, CD19, CD20, and CD56), the proportions of monocytes expressing CD14 (horizontal axis) and CD16 (vertical axis) are shown for representative patients with: A) No CAC: absent at baseline and at year 2 follow up, B) Incident CAC: absent at baseline but detectable at year 2 follow up, C) Stable CAC: detectable at baseline but without significant increase over 2 years of follow up, and D) Increased CAC: detectable at baseline with a significant increase over 2 years of follow up. The percent of cells for each quadrant are reported in the outer most corner. Greater frequencies of CD14+/CD16+ (upper right quadrant) and CD14var/CD16+ (both upper quadrants combined) are seen with subsequent CAC progression (‘B’ and ‘D’).
The following soluble biomarkers were measured from stored plasma at the Diabetes Research and Training Center Radioimmunoassay Core Laboratory (Washington University School of Medicine, St. Louis, MO): high-sensitivity C-reactive protein (hsCRP; Kamiya Biomedical Company, Seattle, WA) and D-dimer (Roche Diagnostics, Indianapolis, IN) using immunoturbidometric assays on a Hitachi 917 analyzer; interleukin-6 (IL-6) using electrochemiluminescence assay on Immulite system (Siemens, Erlangen, Germany), and soluble CD14 by ELISA (R&D Systems, Minneapolis, MN).
Coronary Artery Calcium Measures
CAC was measured from multi-slice CT scans performed at the baseline and year two SUN Study visits. Thirty to forty contiguous tomographic slices were obtained at 3-mm intervals beginning 1 cm below the carina and progressing caudally to include the entire coronary tree. All scans were analyzed with a commercially available software package (Neo Imagery Technologies, City of Industry, CA). An attenuation threshold of 130 Hounsfield units (HU) and a minimum of three contiguous pixels were utilized to identify a calcific lesion. Each focus was scored using the algorithm developed by Agatston, [28] and the total CAC score was determined by summing individual lesion scores from each of four anatomical sites: the left main, left anterior descending, left circumflex, and right coronary arteries [9]. An expert reader, blinded to all clinical and demographic information, read each case for total and per-vessel CAC score.
Statistical Methods
We analyzed baseline predictors of progression in CAC over two years. Significant CAC progression was defined as: a) incident CAC (score of 0 at baseline and >0 at year two), or b) increase in CAC (score >0 at baseline with a positive change at year two greater than the measurement error). Previously established methods were applied that defined a threshold for CAC increase beyond measurement error [12, 29]. For this approach, data were square-root transformed and the difference between the CAC values at year two and baseline was then calculated (“SQRT method”). A threshold of ≥ 2.5mm from the SQRT method corresponded to > 99th percentile for inter-scan variability. Given that the majority of the cohort had no detectable CAC, additional methods for studying changes in CAC on a continuous scale could not be applied.
Descriptive statistics of characteristics at baseline and year two are presented as medians with interquartile range (IQR) or frequency (number and percent). Logistic regression models were used to explore the predictors of CAC progression. Univariate models examined associations with baseline characteristics and HIV-related parameters. The frequencies of monocyte and T-cell phenotypes were then studied as predictors of CAC progression (versus no progression) after adjustment for traditional and HIV-related risk factors. Unless otherwise stated, fully adjusted models included the following covariates: age, gender, race/ethnicity, tobacco smoker, diabetes, hepatitis B or C co-infection, treatment with blood pressure or lipid lowering therapy, baseline CD4 cell count and plasma HIV RNA level (undetectable versus not). Analyses were performed using SAS 9.3 (SAS Institute, Cary NC); plots were generated with R statistical software 2.10.1 (http://www.R-project.org).
RESULTS
Study Population
Of the 691 SUN Study participants, baseline or year-two CAC data were not available for 255 participants, resulting in 436 SUN Study participants with paired baseline and two-year follow-up CAC measurements. Of those participants excluded from the analysis (n=255), the most common reasons for missing data were: missed CAC measure at either baseline or year two but continued in the study (n = 72), withdrew from study (n = 59), moved (n=36), lost to follow-up (n = 27), and death (n = 11). Compared to any SUN Study participant excluded from this analysis, the analysis cohort was slightly older (mean age 42 vs. 40 years; p=0.01), fewer baseline tobacco smokers (41% versus 48%; p = 0.10), and a lower prevalence of prior injection drug use (12% vs. 17%; p = 0.07), respectively (characteristics for excluded from analyses presented along with the analysis cohort in Appendix Table A).
Baseline characteristics for analysis cohort are presented in Table 1. Median age was 42 years, the majority were male, over half were non-Hispanic whites, and 41% of participants smoked cigarettes at baseline. Median total cholesterol (TC) and low-density lipoprotein cholesterol (LDL-C) were within desired ranges for persons without known CVD (67% with TC <200 mg/dL and 73% with LDL-C <130 mg/dL), [30] and Framingham risk scores were consistent with a low CHD risk as estimated by traditional risk factors.
Table 1.
Characteristics of SUN Participants with Paired Coronary Artery Calcium Assessments (N = 436)
| Demographics | Baseline Visit | 2-Year Visit | ||
|---|---|---|---|---|
| Median age, years (IQR) | 42 | (36–48) | 44 | (38–50) |
| Male gender, n (%) | 339 | (78) | -- | |
| Race/ethnicity, n (%) | ||||
| White, non-Hispanic | 258 | (59) | -- | |
| Black, non-Hispanic | 119 | (27) | -- | |
| Hispanic | 48 | (11) | -- | |
| Other | 11 | (3) | -- | |
| Clinical Characteristics | ||||
| Median body mass index, kg/m2 (IQR) | 26 | (23–29) | 26 | (23–30) |
| Hepatitis B or C co-infection, n (%) | 70 | (16) | 79 | (18) |
| Tobacco smoking, n (%) | 175 | (41) | 176 | (40) |
| Injection drug use (ever), n (%) | 52 | (12) | -- | |
| Diabetes (diagnosis or treatment), n (%) | 40 | (9) | 38 | (9) |
| Prescribed antihypertensive therapy, n (%) | 89 | (20) | 89 | (20) |
| Prescribed lipid-lowering therapy, n (%) | 48 | (11) | 77 | (18) |
| 10-year Framingham Risk Score, median (IQR) | 4.5 | (2.0–8.2) | 5.4 | (2.2–8.7) |
| Clinical Labs | ||||
| Total cholesterol, median mg/dL (IQR) | 180 | (155–209) | 183 | (158–210) |
| Triglycerides, median mg/dL (IQR) | 141 | (97–213) | 136 | (92–211) |
| LDL-C, median mg/dL (IQR) | 104 | (84–130) | 104 | (86–131) |
| HDL-C, median mg/dL (IQR) | 41 | (34–50) | 43 | (36–52) |
| GFR, median mL/min/1.73m2 (IQR) | 100 | (86–111) | 99 | (84–111) |
| HIV Parameters | ||||
| Median years with HIV diagnosis (IQR) | 4.7 | (2.2–7.9) | 6.7 | (4.2–10.0) |
| Prior AIDS-defining event, n (%) | 106 | (24) | 113 | (26) |
| Median baseline CD4 cell count, cells/mm3 (IQR) | 481 | (339–685) | 516 | (369–695) |
| Median nadir CD4 cell count, cells/mm3 (IQR) | 205 | (77–312) | 188 | (74–296) |
| HIV RNA viral load <400 copies/mL, n (%) | 314 | (72) | 358 | (84) |
| Median years of ART exposure (IQR) | 2.8 | (1.1–5.4) | 4.3 | (2.6–6.8) |
| Baseline ART exposure, n(%) | 339 | (78) | 366 | (84) |
| Abacavir use, n (%) | 100 | (29) | 97 | (27) |
| Tenofovir use, n (%) | 161 | (47) | 219 | (60) |
| NNRTI use, n (%) | 165 | (49) | 181 | (49) |
| PI use, n (%) | 152 | (45) | 175 | (48) |
BP = blood pressure; hypertension = clinical diagnosis, prescribed BP lowering medication, or BP >140/90 mmHg; ART = combination antiretroviral therapy; GFR = glomerular filtration rate; HDL-C = high-density lipoprotein cholesterol; hepatitis C = IgG antibody positive; LDL-C = low-density lipoprotein cholesterol; NNRTI = non-nucleoside reverse transcriptase inhibitor; PI = protease inhibitor
At baseline, 78% of participants were prescribed ART. Of these, 88% had suppressed plasma HIV RNA VL at baseline and 65% maintained a suppressed HIV RNA VL at baseline and year 2. The proportion of participants prescribed non-nucleoside reverse transcriptase inhibitor- (NNRTI-) or protease inhibitor (PI)-based ART were similar at baseline, and among those prescribed a PI atazanavir (35%) and lopinavir (46%) were the most common. Sixty percent of participants not prescribed ART at baseline started ART after baseline.
Baseline median (IQR) frequencies for monocyte and T-cell phenotypes, and levels of soluble plasma biomarkers, for all SUN Study participants are shown in Appendix Table B. When compared to participants excluded from analyses, the analysis cohort did not differ significantly with respect to the frequency of any of the cellular immunophenotypes studied.
Two-Year CAC Progression
Most participants had no detectable CAC at baseline (82%), and similarly at year two (75%). Median (IQR) detectable CAC score was 39 (8–136) Agatston units at baseline, and 39 (7–114) Agatston units at year 2. Fifty-five participants (12.6%) fulfilled criteria for CAC progression (6.4% with incident CAC and 6.2% with an increase in detectable CAC). The frequency of detectable CAC at baseline and CAC progression over 2 years are presented for subgroups in Table 2. From unadjusted models, traditional CVD risk factors associated with significant CAC progression included older age, male gender, prescription of blood pressure lowering therapy, prescription of lipid lowering therapy, and higher Framingham risk score (Table 3). The duration of HIV diagnosis and duration of ART exposure were each associated with greater frequency of CAC progression (Table 3). PI-based ART was associated with greater CAC progression, but having a detectable HIV viral load (versus <400 copies/mL) was not.
Table 2.
Presence of Coronary Artery Calcium (CAC) at Baseline and 2-Year Progression by Subgroups (n=436)
| Baseline Variable | CAC at Baseline | *CAC Progression | ||
|---|---|---|---|---|
| Yes | No | Yes | No | |
| Totals for Analysis Cohort, # (%) | 80 (18.3%) | 356 (81.7%) | 55 (12.6%) | 381 (87.4%) |
| Age | ||||
| <40 years | 6 (7.5%) | 164 (46.1%) | 14 (25.5%) | 156 (40.9%) |
| 40–49 years | 38 (47.5%) | 150 (42.1%) | 21 (38.2%) | 167 (43.8%) |
| >50 years | 36 (45.0%) | 42 (11.8%) | 20 (36.4%) | 58 (15.2%) |
| Duration of HIV Diagnosis | ||||
| 0.1–2.2 years (1st quartile) | 11 (13.8%) | 97 (27.6%) | 7 (13.0%) | 101 (26.7%) |
| 2.2–4.7 years (2nd quartile) | 21 (26.3%) | 87 (24.7%) | 8 (14.8%) | 100 (26.5%) |
| 4.7–7.9 years (3rd quartile) | 28 (35.0%) | 81 (23.0%) | 17 (31.5%) | 92 (24.3%) |
| 7.9–21.6 years (4th quartile) | 20 (25.0%) | 87 (24.7%) | 22 (40.7%) | 85 (22.5%) |
| CD4 Cell Count | ||||
| <350 cells/mm3 | 20 (25.0%) | 99 (28.0%) | 13 (23.6%) | 106 (28.0%) |
| 350–499 cells/mm3 | 20 (25.0%) | 96 (27.2%) | 12 (21.8%) | 104 (27.5%) |
| ≥500 cells/mm3 | 40 (50.0%) | 158 (44.8%) | 30 (54.5%) | 168 (44.4%) |
| HIV Viral Load | ||||
| <400 copies/mL | 65 (81.3%) | 249 (70.3%) | 41 (74.5%) | 273 (72.0%) |
| ≥400 copies/mL | 15 (18.8%) | 105 (29.7%) | 14 (25.5%) | 106 (28.0%) |
| Lipid Lowering Therapy | ||||
| Yes | 20 (25.0%) | 28 (7.9%) | 12 (21.8%) | 36 (9.4%) |
| No | 60 (75.0%) | 328 (92.1%) | 43 (78.2%) | 345 (90.6%) |
| Blood Pressure Lowering Therapy | ||||
| Yes | 26 (32.5%) | 63 (17.7%) | 17 (30.9%) | 72 (18.9%) |
| No | 54 (67.5%) | 293 (82.3%) | 38 (69.1%) | 309 (81.1%) |
CAC progression defined as either incident (change from 0 to detectable) or significant increase from a detectable measure at baseline (difference ≥ 2.5 on square root scale)
Table 3.
Univariate Associations For Baseline Traditional and HIV Infection-related Risk Factors at with Coronary Artery Calcium (CAC) Progression
| Clinical Characteristics and Labs | OR* (95% CI) | p-value |
|---|---|---|
| Age (per 10 years older) | 1.79 (1.28, 2.50) | 0.001 |
| Male (vs. female) | 2.56 (1.06, 6.18) | 0.04 |
| Race/ethnicity (white vs. other) | 1.80 (0.97, 3.34) | 0.06 |
| Smoking (current vs. not) | 1.33 (0.75, 2.38) | 0.33 |
| Injection drug use (ever vs. never) | 0.72 (0.27, 1.91) | 0.52 |
| Hepatitis B or C co-infection (vs. not) | 1.33 (0.65, 2.73) | 0.43 |
| BMI (per kg/m2 higher) | 0.62 (0.21, 1.79) | 0.37 |
| Diabetes (vs. not) | 1.86 (0.81, 4.27) | 0.15 |
| Blood pressure-lowering therapy (vs. not) | 1.92 (1.03, 3.59) | 0.04 |
| Lipid-lowering therapy (vs. not) | 2.68 (1.29, 5.53) | 0.01 |
| Framingham Risk Score (per unit higher) | 1.29 (1.05, 1.59) | 0.02 |
| LDL-C (per mg/dL higher) | 0.62 (0.35, 1.09) | 0.10 |
| Total-to-HDL-C ratio (per unit higher) | 0.99 (0.53, 1.84) | 0.97 |
| HIV Infection-related Parameters at Baseline | ||
| Duration of HIV diagnosis (per year) | 1.10 (1.04, 1.16) | <0.001 |
| Prior AIDS-defining event (vs. not) | 1.33 (0.71, 2.49) | 0.38 |
| Baseline CD4+ count (per 100 cells/mm3 higher) | 1.40 (0.95, 2.07) | 0.09 |
| Nadir CD4+ count (per 100 cells/mm3 higher) | 1.01 (0.86, 1.19) | 0.90 |
| HIV RNA viral load <400 copies/mL (vs. not) | 1.14 (0.60, 2.17) | 0.70 |
| Duration of ART exposure (per year) | 1.19 (1.07, 1.33) | 0.002 |
| Baseline ART use (vs. not) | 1.79 (0.82, 3.93) | 0.15 |
| Abacavir use (vs. not) | 0.90 (0.45, 1.79) | 0.77 |
| PI use (vs. not) | 0.47 (0.24, 0.92) | 0.03 |
OR represents likelihood of a significant 2-year change in CAC, defined as incident or significant progression in CAC; BMI = Body Mass Index; NNRTI = non-nucleoside reverse transcriptase inhibitor; PI = protease inhibitor; LDL = low-density lipoprotein; HDL = high-density lipoprotein; ART = combination antiretroviral therapy
In the fully adjusted model, a higher CD4 cell count at baseline (OR 1.12 per 100 cells/mm3 increase; 95%CI 1.00–1.24; p = 0.04) was, unexpectedly, associated with greater CAC progression. As a result, we further explored the relationship between baseline CD4 cell count and duration of HIV diagnosis. Mean (SD) duration of HIV diagnosis at baseline was 5.03 (4.93) years for participants with a CD4 count < 500 cells/mm3 (n = 235), and 6.75 (4.38) years for those with a CD4 count ≥ 500 cells/mm3 (n = 195; p = 0.002 for difference). When duration of HIV diagnosis was then added to the fully adjusted model, baseline CD4 count was no longer predictive of CAC progression (OR 1.08 per 100 cells/mm3 higher; 95%CI 0.96–1.20; p = 0.21) but duration of HIV diagnosis remained significant (OR 1.08 per year; 95%CI 1.02–1.15; p = 0.008).
Immunologic Predictors of CAC Progression
Figure 1 presents flow cytometry plots used to quantify monocyte subsets for representative participants based on presence or absence of CAC progression. After adjusting for traditional and HIV-related risk factors, greater frequencies of CD14+/CD16+ and of CD14var/CD16+ monocytes at baseline were independently associated with greater likelihood of CAC progression; the association with CD14dim/CD16+ approached significance (Table 4 column A and Appendix Figure A). Associations with monocyte phenotypes appeared to be driven largely by the subset of participants with a significant increase in CAC (Table 4 column C). When restricted to participants with an undetectable HIV VL at baseline (Table 4 column B), ORs for CAC progression became more extreme for all the CD16+ monocyte populations. Finally, adding duration of HIV diagnosis or use of PI-based ART to multivariate models did not attenuate associations between monocyte phenotypes and CAC progression (data not shown).
Table 4.
Multivariate Models for CAC Progression by Immunologic Predictors
| (A) Multivariate Model (n=436) |
(B) Restrict Cohort to Suppressed VL (n=314) |
(C) Restrict Outcome to CAC increase (n=436) |
(D) Restrict Outcome to CAC Incidence (n=436) |
|||||
|---|---|---|---|---|---|---|---|---|
| Monocytes (%) | OR* | (95% CI) | OR* | (95% CI) | OR* | (95% CI) | OR* | (95% CI) |
| CD14+/CD16− | 0.64 | (0.32, 1.25) | 0.65 | (0.32, 1.31) | 0.04 | (0.00, 0.79) | 1.53 | (0.31, 7.42) |
| CD14+/CD16+ | 1.66 | (1.09, 2.55) | 2.02 | (1.21, 3.38) | 2.87 | (1.21, 6.77) | 1.13 | (0.67, 1.89) |
| CD14dim/CD16+ | 1.36 | (0.98, 1.88) | 1.48 | (1.01, 2.17) | 1.81 | (1.01, 3.25) | 1.10 | (0.73, 1.67) |
| CD14var/CD16+ | 1.69 | (1.13, 2.55) | 1.96 | (1.21, 3.18) | 3.13 | (1.35, 7.28) | 1.16 | (0.71, 1.89) |
| TF+ | 1.13 | (0.86, 1.48) | 1.08 | (0.79, 1.48) | 1.28 | (0.80, 2.05) | 0.96 | (0.65, 1.42) |
| CCR2+ | 1.04 | (0.10, 11.34) | 0.35 | (0.03, 4.53) | 0.47 | (0.01, 16.48) | 3.78 | (0.13, 112.2) |
| CCR5+ | 1.01 | (0.99, 1.03) | 1.01 | (1.00, 1.03) | 1.02 | (0.99, 1.06) | 0.99 | (0.95, 1.02) |
| CX3CR1+ | 0.95 | (0.72, 1.26) | 1.10 | (0.78, 1.55) | 0.84 | (0.53, 1.32) | 1.08 | (0.74, 1.57) |
| T-cells (%) | ||||||||
| CD4+/HLADR+/CD38+ | 1.17 | (0.87, 1.58) | 1.10 | (0.78, 1.55) | 1.16 | (0.58, 2.31) | 1.10 | (0.74, 1.63) |
| CD4+/CD57+/CD38+ | 1.09 | (0.83, 1.41) | 1.26 | (0.93, 1.70) | 1.60 | (0.98, 2.60) | 0.83 | (0.58, 1.19) |
| CD4+/CD57+ | 1.03 | (0.80, 1.31) | 1.02 | (0.76, 1.35) | 1.43 | (0.93, 2.21) | 0.84 | (0.61, 1.14) |
| CD4+/CX3CR1+ | 1.01 | (0.80, 1.29) | 1.08 | (0.82, 1.43) | 1.49 | (0.96, 2.32) | 0.88 | (0.65, 1.20) |
| CD8+/HLADR+/CD38+ | 0.94 | (0.69, 1.28) | 0.85 | (0.60, 1.22) | 0.74 | (0.39, 1.42) | 1.05 | (0.70, 1.58) |
| CD8+/CD57+/CD38+ | 0.95 | (0.68, 1.33) | 0.93 | (0.65, 1.32) | 0.98 | (0.47, 2.05) | 0.96 | (0.63, 1.47) |
| CD8+/CD57+ | 0.76 | (0.47, 1.23) | 0.63 | (0.36, 1.09) | 1.01 | (0.42, 2.41) | 0.84 | (0.45, 1.56) |
| CD8+/CX3CR1+ | 0.94 | (0.72, 1.22) | 1.00 | (0.73, 1.35) | 1.30 | (0.78, 2.16) | 0.99 | (0.70, 1.38) |
| Plasma Biomarkers | ||||||||
| hsCRP, mg/dL | 0.83 | (0.61, 1.12) | 0.82 | (0.58, 1.15) | 0.74 | (0.39, 1.40) | 0.97 | (0.67, 1.41) |
| IL-6, pg/mL | 0.95 | (0.56, 1.62) | 0.96 | (0.53, 1.73) | 1.09 | (0.38, 3.14) | 0.96 | (0.48, 1.92) |
| sCD14, µg/mL | 0.76 | (0.33, 1.76) | 1.01 | (0.38, 2.68) | 0.58 | (0.11, 3.07) | 1.10 | (0.37, 3.28) |
| sCD163, ng/mL | 0.92 | (0.64, 1.33) | 0.91 | (0.61, 1.38) | 0.89 | (0.43, 1.82) | 0.92 | (0.56, 1.52) |
| D-dimer, µg/mL | 1.24 | (0.41, 3.77) | 0.81 | (0.19, 3.48) | 3.11 | (0.35, 27.80) | 0.93 | (0.17, 5.07) |
OR are per log-2 unit higher, which corresponds to a doubling in frequency of given monocyte phenotype, adjusted for age, gender, race/ethnicity, tobacco smoking, diabetes, hepatitis B or C co-infection, treatment for hypertension, treatment for hyperlipidemia, baseline CD4 count, and undetectable plasma HIV RNA level—except for model restricted to participants with suppressed VL (<400 copies/mL).
Tissue factor (TF) expression on monocytes was not associated with CAC progression, nor was expression of the monocyte migration markers CCR2, CCR5, or CX3CR1 in fully adjusted models. Similarly, none of the CD8+ T-cell phenotypes studied in Table 4 demonstrated independent associations. Finally, none of the inflammatory and coagulation plasma biomarkers were associated with CAC progression.
DISCUSSION
In this cohort of HIV-infected patients with relatively restored immune function and at very low risk for AIDS complications, we describe a novel finding that higher frequencies CD16+ monocytes (intermediate and non-classical phenotypes) predicted greater CAC progression, independent of traditional and HIV-related related risk factors. We found no significant associations for other cellular phenotypes, including those reflecting T-cell activation, with CAC progression. These data suggest a potentially important role for monocyte-related cellular activation in coronary atherosclerotic progression among HIV-positive patients, and suggest that the immunologic abnormalities that contribute to risk for non-AIDS defining comorbidities like CVD among contemporary patients may not predominantly involve T-cell abnormalities that are classically associated with risk for AIDS-defining complications.
The hallmark immunologic abnormalities associated with HIV infection include immune depletion (i.e., low CD4+ T-cell counts) as well as chronic immune activation, which is most pronounced in untreated disease but remains present in persons effectively treated with ART [31, 32]. At present, epidemiologic data have been inconsistent with respect to whether CVD risk is associated with immune depletion when measured by absolute CD4 count [6, 33–36]. The classic indicator of HIV-related T-cell immune activation—the frequency of CD4+ and CD8+ T-cells with a CD38+/HLA-DR+ phenotype—independently predicts greater risk for AIDS disease progression [15]. In cross-sectional studies, T-cell activation was associated with an increased prevalence of carotid artery atherosclerotic lesions [16, 37] and greater carotid artery stiffness [38]. In contrast, we did not find that CD4+ or CD8+ T-cell phenotypes were potential predictors for greater CAC progression, and the potential association with absolute CD4 cell count was accounted for by the duration of HIV diagnosis in our analyses.
HIV infection, including treated disease, is also characterized by elevations in inflammatory markers and by abnormalities in innate, or non-specific, immunity, such as a higher prevalence of circulating monocytes that express CD16+ [17, 18, 31, 39, 40]. Although the functional characteristics of intermediate and non-classical monocyte phenotypes (combined as CD14var/CD16+, or CD16+, in these analyses) remain controversial, these subsets may be permissive to infection by HIV [41] and exhibit properties that promote atherogenesis [20–23]. Specifically, the non-classical phenotype may act as a ‘patrolling’ subset that has greater affinity for vascular surfaces and preferentially migrate into atherosclerotic lesions [20, 21]. The intermediate monocyte phenotype appears to be functionally more pro-inflammatory, with greater cytokine release after stimulation [20, 22, 23]. Consistent with CD16+ monocytes being precursors for tissue macrophages, this population has been shown to localize to tissues sites of inflammation and fibrosis, with transendothelial migration facilitated by CX3CL1 [42]. Epidemiologic data from HIV-uninfected participants at risk for CVD (n=951) also demonstrates that the intermediate monocyte phenotype independently predicts higher risk for subsequent CVD events (i.e., myocardial infarction, stroke or CVD-death) [43]. Our findings are consistent with these data and suggest that non-classical and intermediate monocyte phenotypes, reflecting greater activation and potential for transendothelial migration, may contribute to excess coronary atherosclerosis in the context of HIV infection. The relative importance of these associations in the context of other known risk factors requires further clarification. While a doubling in the frequency of CD16+ monocytes conferred risk for CAC progression similar to being 10 years older, this degree of difference in CD16+ monocytes is substantial (e.g., corresponds to a change from the 25th to 75th percentile).
Recent data support the hypothesis that monocyte-related inflammation has clinical consequences for HIV-positive patients [18, 44–47]. Soluble CD14 and CD163 levels, both reflecting monocyte activation, have been associated with greater subclinical atherosclerosis among HIV-positive patients and all cause mortality [45–47]. Data from the AIDS Clinical Trials Group report further demonstrate the degree of CIMT progression was also positively associated with plasma levels of lipopolysaccharide (LPS), potentially reflecting translocation of microbial products across damaged mucosal surfaces—a mechanism hypothesized to be an important driver of persistent immune activation among HIV-positive patients [45, 48]. Furthermore, Funderberg and colleagues have shown that LPS levels correlated with the frequency of the intermediate monocyte phenotype (CD14+/CD16+) among virally suppressed HIV-positive patients [18].
An important observation is that the rate of significant CAC progression (12.4% over 2 years) was low among this HIV positive cohort, who were at low risk for CVD (by traditional risk factors) and had high utilization of antiretrovirals that have not been associated with CVD event risk (i.e., tenofovir, atazanavir, NNRTIs) [49, 50]. Specifically, the CAC incident rate was within the expected range for HIV uninfected participants <50 years old reported in the general population cohort MESA (Multi-Ethnic Study of Atherosclerosis) [51]. However, the vast majority of SUN Study participants with a detectable CAC score at baseline would be still be considered to be at high or very high age-adjusted risk for CHD event (i.e., CAC >75th percentile for age), and would prompt some guidelines to recommend lipid lowering prevention therapy with LDL-C goal of <100 mg/dL or <70 mg/dL, respectively [52]. Only 25% of the SUN Study participants with detectable CAC at baseline were prescribed lipid-lowering therapy, of which 45% had an LDL-C <100 mg/dL. Furthermore, our subgroup analyses demonstrated that the association between CAC progression and the frequency of CD16+ monocytes was driven by participants who had a significant increase in detectable CAC (versus those with de novo incident CAC). These data emphasize the need for aggressive risk factor modification among HIV positive patients with evidence of coronary heart disease by non-invasive imaging, and suggest this target population in particular may benefit from adjunct anti-inflammatory treatment strategies.
Ultimately, the low rate of CAC progression limited our ability to detect modest associations with potentially important predictors (e.g., TF expression on monocytes and D-dimer levels). However, the lack of association between T-cell phenotypes and CAC progression specifically did not appear to be due to inadequate statistical power, as the OR estimates were very close to one. Additional limitations of our analyses included the lack of an HIV-uninfected comparison population, the inability to adjust for CMV serostatus or viremia, the uncertain clinical implications for the degree of CAC change observed, and differences in methodology precluded direct comparison of monocyte subset frequencies to other HIV uninfected populations. As with any cohort study, causation cannot be established and channeling bias and other unmeasured confounding may be present. The degree to which circulating monocytes reflect pathologic changes in tissue macrophages (e.g., those within atherosclerotic plaques) is also unclear, and our methods did not assess for earlier atherosclerotic lesions such as non-calcified plaque.
In summary, we report the novel finding that higher frequencies of circulating CD16+ monocytes, reflecting an activated, pro-inflammatory and pro-atherogenic state, predicted greater short-term progression of subclinical coronary atherosclerosis as estimated by CAC. These findings, combined with the lack of a potential association between CAC progression and activated T-cell phenotypes or immune depletion per se, suggest greater focus on reducing innate immune activation is warranted as a CVD prevention strategy among HIV-positive patients.
Supplementary Material
Acknowledgements
The authors would like to sincerely thank all of the SUN Study participants, as well as all the clinical site investigators and staff.
FINANCIAL SUPPORT:
Centers for Disease Control and Prevention contract numbers 200-2002-00610, 200-2002-00611, 200-2002-00612, 200-2002-00613, 200-2007-23633, 200-2007-23634, 200-2007-23635, and 200-2007-23636. Additional support was provided by the NIH 1KL2RR033182-01. The work of EMPW, AS and IS was supported by the intramural program of NIAID.
Appendix Table
Baseline Characteristics of SUN Participants Included and Excluded in Analysis Cohort
| Demographics | Analysis Cohort (n=436) |
Excluded* (n=255) |
p-value | ||
|---|---|---|---|---|---|
| Median age, years (IQR) | 42 | (36–48) | 40 | (34, 46) | 0.01 |
| Male gender, n (%) | 339 | (78) | 188 | (74) | 0.23 |
| Race/ethnicity, n (%) | 0.12 | ||||
| White, non-Hispanic | 258 | (59) | 144 | (56) | |
| Black, non-Hispanic | 119 | (27) | 88 | (35) | |
| Hispanic | 48 | (11) | 19 | (7) | |
| Other | 11 | (3) | 4 | (2) | |
| Clinical Characteristics | |||||
| Median body mass index, kg/m2 (IQR) | 26 | (23–29) | 26 | (23, 28) | 0.40 |
| Hepatitis B or C co-infection, n (%) | 70 | (16) | 47 | (19) | 0.43 |
| Tobacco smoking, current n (%) | 175 | (41) | 116 | (48) | 0.10 |
| Injection drug use (ever), n (%) | 52 | (12) | 42 | (17) | 0.07 |
| Diabetes (diagnosis or treatment), n (%) | 40 | (9) | 19 | (7) | 0.43 |
| Prescribed antihypertensive therapy, n (%) | 89 | (20) | 44 | (17) | 0.31 |
| Prescribed lipid-lowering therapy, n (%) | 48 | (11) | 10 | (4) | 0.00 |
| 10-year Framingham Risk Score, median (IQR) | 4.5 | (2.0–8.2) | 3.4 | (1.3, 8.0) | 0.05 |
| Clinical Labs | |||||
| Total cholesterol, median mg/dL (IQR) | 180 | (155–209) | 181 | (150, 205) | 0.41 |
| Triglycerides, median mg/dL (IQR) | 141 | (97–213) | 133 | (93, 193) | 0.29 |
| LDL-C, median mg/dL (IQR) | 104 | (84–130) | 101 | (79, 126) | 0.29 |
| HDL-C, median mg/dL (IQR) | 41 | (34–50) | 41 | (34, 51) | 0.73 |
| GFR, median mL/min/1.73m2 (IQR) | 100 | (86–111) | 102 | (87, 113) | 0.46 |
| HIV Parameters | |||||
| Median years with HIV diagnosis (IQR) | 4.7 | (2.2–7.9) | 5.4 | (2.4, 8.4) | 0.21 |
| Prior AIDS-defining event, n (%) | 106 | (24) | 62 | (24) | 1.00 |
| Median baseline CD4 count, cells/mm3 (IQR) | 481 | (339–685) | 463 | (327, 674) | 0.40 |
| Median nadir CD4 count, cells/mm3 (IQR) | 205 | (77–312) | 214 | (100, 330) | 0.28 |
| HIV RNA viral load <400 copies/mL, n (%) | 314 | (72) | 188 | (74) | 0.63 |
| Median years of ART exposure (IQR) | 2.8 | (1.1–5.4) | 2.5 | (1.0, 5.5) | 0.67 |
| Baseline ART exposure, n(%) | 339 | (78) | 197 | (77) | 0.88 |
| Abacavir use, n (%) | 100 | (29) | 43 | (22) | 0.05 |
| Tenofovir use, n (%) | 161 | (47) | 99 | (50) | 0.54 |
| NNRTI use, n (%) | 165 | (49) | 95 | (48) | 0.92 |
| PI use, n (%) | 152 | (45) | 96 | (49) | 0.38 |
SUN Study participants excluded from analysis cohort included those missing CAC measures at baseline or year 2.
P-value for difference in the distribution given parameter between groups by Wilcoxon rank sum test.
BP = blood pressure; hypertension = clinical diagnosis, prescribed BP lowering medication, or BP >140/90 mmHg; ART = combination antiretroviral therapy; GFR = glomerular filtration rate; HDL-C = high-density lipoprotein cholesterol; hepatitis C = IgG antibody positive; LDL-C = low-density lipoprotein cholesterol; NNRTI = non-nucleoside reverse transcriptase inhibitor; PI = protease inhibitor
Appendix Table B
Baseline Levels of Immune Phenotypes and Plasma Biomarkers (N=434)
| Measurement | Analysis Cohort (n=436) |
Excluded* (n=255) |
p-value | ||
|---|---|---|---|---|---|
| Monocyte Phenotypes | Median (IQR) | Median (IQR) | |||
| CD14+/CD16−, % | 78.8 | (71.4, 83.8) | 80.0 | (73.4, 83.9) | 0.14 |
| CD14+/CD16+, % | 5.2 | (3.3, 7.6) | 5.4 | (3.5, 8.4) | 0.19 |
| CD14dim/CD16+, % | 6.6 | (3.8, 10.6) | 5.6 | (3.6, 9.6) | 0.12 |
| CD14var/CD16+, % | 12.7 | (8.4, 18.5) | 11.9 | (8.3, 17.2) | 0.53 |
| TF+, % | 1.6 | (0.9, 3.2) | 1.4 | (0.8, 3.0) | 0.37 |
| CCR2+, % | 88.9 | (82.9, 93.4) | 89.6 | (84.7, 93.5) | 0.19 |
| CCR5+, % | 4.3 | (2.1, 9.6) | 4.8 | (2.2, 8.2) | 0.91 |
| CX3CR1+, % | 7.5 | (3.6, 12.4) | 6.9 | 3.6, 10.9) | 0.52 |
| T-cell Phenotypes | |||||
| CD4+/HLADR+/CD38+, % | 7.6 | (4.4, 13.8) | 8.3 | (4.8, 14.9) | 0.09 |
| CD4+/CD57+/CD38+, % | 3.8 | (1.7, 8.2) | 3.0 | (1.5, 8.0) | 0.55 |
| CD4+/CD57+, % | 7.5 | (3.4, 14.7) | 7.3 | (3.2, 15.9) | 0.89 |
| CD4+/CX3CR1+, % | 2.6 | (0.9, 6.3) | 2.7 | (0.7, 5.3) | 0.56 |
| CD8+/HLADR+/CD38+, % | 19.8 | (11.3, 33.9) | 22.9 | (12.1, 35.6) | 0.13 |
| CD8+/CD57+/CD38+, % | 22.1 | (13.2, 31.3) | 22.6 | (14.3, 29.9) | 0.91 |
| CD8+/CD57+, % | 34.6 | (26.2, 44.4) | 33.4 | (26.7, 43.3) | 0.37 |
| CD8+/CX3CR1+, % | 18.9 | (10.3, 31.4) | 17.0 | (9.4, 28.3) | 0.15 |
|
Soluble Plasma Biomarkers |
|||||
| hsCRP, mg/dL | 1.75 | (0.79, 4.25) | 1.91 | (0.82, 4.94) | 0.43 |
| IL-6, pg/mL | 1.05 | (0.68, 1.66) | 1.11 | (0.72, 1.70) | 0.29 |
| sCD14, µg/mL | 1146 | (962, 1390) | 1137 | (917, 1348) | 0.43 |
| sCD163, ng/mL | 539 | (372, 788) | 523 | (368, 802) | 0.69 |
| D-dimer, µg/mL | 0.12 | (0.05, 0.24) | 0.14 | (0.06, 0.24) | 0.43 |
SUN Study participants excluded from analysis cohort included those missing CAC measures at baseline or year 2.
P-value for difference in the distribution given parameter between groups by Wilcoxon rank sum test.
TF = tissue factor; hsCRP = high sensitivity C-reactive protein; IL-6 = interleukin-6.
Footnotes
ETHICAL CONSIDERATIONS: The investigation followed the guidelines of the U.S. Department of Health and Human Services regarding protection of human subjects. The study protocol was approved and renewed annually by each participating institutions’ ethical review board. All study participants provided written, informed consent.
CONFLICTS OF INTEREST: No author conflicts related to collection and presentation of these data.
PRIOR PRESENTATION:
Conference on Retroviruses and Opportunistic Infections, Atlanta, GA, March 3–6, 2013, Abstract #66LB
DISCLAIMER:
The findings and conclusions from this review are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
REFERENCES
- 1.Mocroft A, Reiss P, Gasiorowski J, Ledergerber B, Kowalska J, Chiesi A, et al. Serious fatal and nonfatal non-AIDS-defining illnesses in Europe. J Acquir Immune Defic Syndr. 2010;55:262–270. doi: 10.1097/QAI.0b013e3181e9be6b. [DOI] [PubMed] [Google Scholar]
- 2.Freiberg MS, Chang CC, Kuller LH, Skanderson M, Lowy E, Kraemer KL, et al. HIV infection and the risk of acute myocardial infarction. JAMA Intern Med. 2013;173:614–622. doi: 10.1001/jamainternmed.2013.3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.El-Sadr WM, Lundgren JD, Neaton JD, Gordin F, Abrams D, Arduino RC, et al. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med. 2006;355:2283–2296. doi: 10.1056/NEJMoa062360. [DOI] [PubMed] [Google Scholar]
- 4.Friis-Moller N, Reiss P, Sabin CA, Weber R, Monforte A, El-Sadr W, et al. Class of antiretroviral drugs and the risk of myocardial infarction. N Engl J Med. 2007;356:1723–1735. doi: 10.1056/NEJMoa062744. [DOI] [PubMed] [Google Scholar]
- 5.Saves M, Chene G, Ducimetiere P, Leport C, Le Moal G, Amouyel P, et al. Risk Factors for coronary heart disease in patients treated for human immunodeficiency virus infection compared with the general population. CID. 2003;37:292–298. doi: 10.1086/375844. [DOI] [PubMed] [Google Scholar]
- 6.Baker JV, Henry WK, Patel P, Bush TJ, Conley LJ, Mack WJ, et al. Progression of carotid intima-media thickness in a contemporary human immunodeficiency virus cohort. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2011;53:826–835. doi: 10.1093/cid/cir497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duprez DA, Neuhaus J, Kuller LH, Tracy R, Belloso W, De Wit S, et al. Inflammation, coagulation and cardiovascular disease in HIV-infected individuals. PLoS One. 2012;7:e44454. doi: 10.1371/journal.pone.0044454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Triant VA, Meigs JB, Grinspoon SK. Association of C-reactive protein and HIV infection with acute myocardial infarction. J Acquir Immune Defic Syndr. 2009;51:268–273. doi: 10.1097/QAI.0b013e3181a9992c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Budoff MJ, Achenbach S, Blumenthal RS, Carr JJ, Goldin JG, Greenland P, et al. Assessment of coronary artery disease by cardiac computed tomography: a scientific statement from the American Heart Association Committee on Cardiovascular Imaging and Intervention, Council on Cardiovascular Radiology and Intervention, and Committee on Cardiac Imaging, Council on Clinical Cardiology. Circulation. 2006;114:1761–1791. doi: 10.1161/CIRCULATIONAHA.106.178458. [DOI] [PubMed] [Google Scholar]
- 10.Rumberger JA, Simons DB, Fitzpatrick LA, Sheedy PF, Schwartz RS. Coronary artery calcium area by electron-beam computed tomography and coronary atherosclerotic plaque area. A histopathologic correlative study. Circulation. 1995;92:2157–2162. doi: 10.1161/01.cir.92.8.2157. [DOI] [PubMed] [Google Scholar]
- 11.Raggi P, Cooil B, Shaw LJ, Aboulhson J, Takasu J, Budoff M, et al. Progression of coronary calcium on serial electron beam tomographic scanning is greater in patients with future myocardial infarction. The American journal of cardiology. 2003;92:827–829. doi: 10.1016/s0002-9149(03)00892-0. [DOI] [PubMed] [Google Scholar]
- 12.Budoff MJ, Hokanson JE, Nasir K, Shaw LJ, Kinney GL, Chow D, et al. Progression of coronary artery calcium predicts all-cause mortality. JACC. Cardiovascular imaging. 2010;3:1229–1236. doi: 10.1016/j.jcmg.2010.08.018. [DOI] [PubMed] [Google Scholar]
- 13.Polonsky TS, McClelland RL, Jorgensen NW, Bild DE, Burke GL, Guerci AD, et al. Coronary artery calcium score and risk classification for coronary heart disease prediction. JAMA : the journal of the American Medical Association. 2010;303:1610–1616. doi: 10.1001/jama.2010.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hermann DM, Gronewold J, Lehmann N, Moebus S, Jockel KH, Bauer M, et al. Coronary Artery Calcification Is an Independent Stroke Predictor in the General Population. Stroke; a journal of cerebral circulation. 2013 doi: 10.1161/STROKEAHA.111.678078. [DOI] [PubMed] [Google Scholar]
- 15.Liu Z, Cumberland WG, Hultin LE, Prince HE, Detels R, Giorgi JV. Elevated CD38 antigen expression on CD8+ T cells is a stronger marker for the risk of chronic HIV disease progression to AIDS and death in the Multicenter AIDS Cohort Study than CD4+ cell count, soluble immune activation markers, or combinations of HLA-DR and CD38 expression. J Acquir Immune Defic Syndr Hum Retrovirol. 1997;16:83–92. doi: 10.1097/00042560-199710010-00003. [DOI] [PubMed] [Google Scholar]
- 16.Kaplan RC, Sinclair E, Landay AL, Lurain N, Sharrett AR, Gange SJ, et al. T Cell Activation and Senescence Predict Subclinical Carotid Artery Disease in HIV-Infected Women. J Infect Dis. 2011;203:452–463. doi: 10.1093/infdis/jiq071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hearps AC, Maisa A, Cheng WJ, Angelovich TA, Lichtfuss GF, Palmer CS, et al. HIV infection induces age-related changes to monocytes and innate immune activation in young men that persist despite combination antiretroviral therapy. AIDS. 2012;26:843–853. doi: 10.1097/QAD.0b013e328351f756. [DOI] [PubMed] [Google Scholar]
- 18.Funderburg NT, Zidar DA, Shive C, Lioi A, Mudd J, Musselwhite LW, et al. Shared monocyte subset phenotypes in HIV-1 infection and in uninfected subjects with acute coronary syndrome. Blood. 2012;120:4599–4608. doi: 10.1182/blood-2012-05-433946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ziegler-Heitbrock L, Ancuta P, Crowe S, Dalod M, Grau V, Hart DN, et al. Nomenclature of monocytes and dendritic cells in blood. Blood. 2010;116:e74–e80. doi: 10.1182/blood-2010-02-258558. [DOI] [PubMed] [Google Scholar]
- 20.Ziegler-Heitbrock L. The CD14+ CD16+ blood monocytes: their role in infection and inflammation. Journal of leukocyte biology. 2007;81:584–592. doi: 10.1189/jlb.0806510. [DOI] [PubMed] [Google Scholar]
- 21.Ancuta P, Rao R, Moses A, Mehle A, Shaw SK, Luscinskas FW, et al. Fractalkine preferentially mediates arrest and migration of CD16+ monocytes. The Journal of experimental medicine. 2003;197:1701–1707. doi: 10.1084/jem.20022156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Belge KU, Dayyani F, Horelt A, Siedlar M, Frankenberger M, Frankenberger B, et al. The proinflammatory CD14+CD16+DR++ monocytes are a major source of TNF. J Immunol. 2002;168:3536–3542. doi: 10.4049/jimmunol.168.7.3536. [DOI] [PubMed] [Google Scholar]
- 23.van de Veerdonk FL, Netea MG. Diversity: a hallmark of monocyte society. Immunity. 2010;33:289–291. doi: 10.1016/j.immuni.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 24.Gerszten RE, Tager AM. The monocyte in atherosclerosis--should I stay or should I go now? N Engl J Med. 2012;366:1734–1736. doi: 10.1056/NEJMcibr1200164. [DOI] [PubMed] [Google Scholar]
- 25.Vellozzi C, Brooks JT, Bush TJ, Conley LJ, Henry K, Carpenter CC, et al. The study to understand the natural history of HIV and AIDS in the era of effective therapy (SUN Study) Am J Epidemiol. 2009;169:642–652. doi: 10.1093/aje/kwn361. [DOI] [PubMed] [Google Scholar]
- 26.Anderson KM, Wilson PW, Odell PM, Kannel WB. An updated coronary risk profile. A statement for health professionals. Circulation. 1991;83:356–362. doi: 10.1161/01.cir.83.1.356. [DOI] [PubMed] [Google Scholar]
- 27.Ford ES, Greenwald JH, Richterman AG, Rupert A, Dutcher L, Badralmaa Y, et al. Traditional risk factors and D-dimer predict incident cardiovascular disease events in chronic HIV infection. AIDS. 2010;24:1509–1517. doi: 10.1097/QAD.0b013e32833ad914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–832. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 29.Hokanson JE, MacKenzie T, Kinney G, Snell-Bergeon JK, Dabelea D, Ehrlich J, et al. Evaluating changes in coronary artery calcium: an analytic method that accounts for interscan variability. AJR Am J Roentgenol. 2004;182:1327–1332. doi: 10.2214/ajr.182.5.1821327. [DOI] [PubMed] [Google Scholar]
- 30.Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 31.Neuhaus J, Jacobs DR, Jr, Baker JV, Calmy A, Duprez D, La Rosa A, et al. Markers of inflammation, coagulation, and renal function are elevated in adults with HIV infection. J Infect Dis. 2010;201:1788–1795. doi: 10.1086/652749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hunt PW, Brenchley J, Sinclair E, McCune JM, Roland M, Page-Shafer K, et al. Relationship between T cell activation and CD4+ T cell count in HIV-seropositive individuals with undetectable plasma HIV RNA levels in the absence of therapy. J Infect Dis. 2008;197:126–133. doi: 10.1086/524143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Triant VA, Regan S, Lee H, Sax PE, Meigs JB, Grinspoon SK. Association of immunologic and virologic factors with myocardial infarction rates in a US healthcare system. Journal of acquired immune deficiency syndromes. 2010;55:615–619. doi: 10.1097/QAI.0b013e3181f4b752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sabin C the D:A:D Study Group. Associations between markers of immuneosuppression and the risk of CVD; 19th Conference on Retroviruses and Opportunistic Infections; Seattle, WA. 2012. [Google Scholar]
- 35.Kaplan RC, Kingsley LA, Gange SJ, Benning L, Jacobson LP, Lazar J, et al. Low CD4+ T-cell count as a major atherosclerosis risk factor in HIV-infected women and men. AIDS. 2008;22:1615–1624. doi: 10.1097/QAD.0b013e328300581d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lichtenstein KA, Armon C, Buchacz K, Chmiel JS, Buckner K, Tedaldi EM, et al. Low CD4+ T cell count is a risk factor for cardiovascular disease events in the HIV outpatient study. Clin Infect Dis. 2010;51:435–447. doi: 10.1086/655144. [DOI] [PubMed] [Google Scholar]
- 37.Longenecker CT, Funderburg NT, Jiang Y, Debanne S, Storer N, Labbato DE, et al. Markers of inflammation and CD8 T-cell activation, but not monocyte activation, are associated with subclinical carotid artery disease in HIV-infected individuals. HIV Med. 2013;14:385–390. doi: 10.1111/hiv.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kaplan RC, Sinclair E, Landay AL, Lurain N, Sharrett AR, Gange SJ, et al. T cell activation predicts carotid artery stiffness among HIV-infected women. Atherosclerosis. 2011;217:207–213. doi: 10.1016/j.atherosclerosis.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Armah KA, McGinnis K, Baker J, Gibert C, Butt AA, Bryant KJ, et al. HIV Status, Burden of Comorbid Disease and Biomarkers of Inflammation, Altered Coagulation and Monocyte Activation. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2012;55:126–136. doi: 10.1093/cid/cis406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martin GE, Gouillou M, Hearps AC, Angelovich TA, Cheng AC, Lynch F, et al. Age-associated changes in monocyte and innate immune activation markers occur more rapidly in HIV infected women. PLoS One. 2013;8:e55279. doi: 10.1371/journal.pone.0055279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ellery PJ, Tippett E, Chiu YL, Paukovics G, Cameron PU, Solomon A, et al. The CD16+ monocyte subset is more permissive to infection and preferentially harbors HIV-1 in vivo. J Immunol. 2007;178:6581–6589. doi: 10.4049/jimmunol.178.10.6581. [DOI] [PubMed] [Google Scholar]
- 42.Aspinall AI, Curbishley SM, Lalor PF, Weston CJ, Miroslava B, Liaskou E, et al. CX(3)CR1 and vascular adhesion protein-1-dependent recruitment of CD16(+) monocytes across human liver sinusoidal endothelium. Hepatology. 2010;51:2030–2039. doi: 10.1002/hep.23591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rogacev KS, Cremers B, Zawada AM, Seiler S, Binder N, Ege P, et al. CD14++CD16+ monocytes independently predict cardiovascular events: a cohort study of 951 patients referred for elective coronary angiography. Journal of the American College of Cardiology. 2012;60:1512–1520. doi: 10.1016/j.jacc.2012.07.019. [DOI] [PubMed] [Google Scholar]
- 44.Crowe SM, Westhorpe CL, Mukhamedova N, Jaworowski A, Sviridov D, Bukrinsky M. The macrophage: the intersection between HIV infection and atherosclerosis. J Leukoc Biol. 2010;87:589–598. doi: 10.1189/jlb.0809580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kelesidis T, Kendall MA, Yang OO, Hodis HN, Currier JS. Biomarkers of microbial translocation and macrophage activation: association with progression of subclinical atherosclerosis in HIV-1 infection. The Journal of infectious diseases. 2012;206:1558–1567. doi: 10.1093/infdis/jis545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Burdo TH, Lo J, Abbara S, Wei J, DeLelys ME, Preffer F, et al. Soluble CD163, a novel marker of activated macrophages, is elevated and associated with noncalcified coronary plaque in HIV-infected patients. The Journal of infectious diseases. 2011;204:1227–1236. doi: 10.1093/infdis/jir520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sandler NG, Wand H, Roque A, Law M, Nason MC, Nixon DE, et al. Plasma levels of soluble CD14 independently predict mortality in HIV infection. J Infect Dis. 2011;203:780–790. doi: 10.1093/infdis/jiq118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brenchley JM, Price DA, Schacker TW, Asher TE, Silvestri G, Rao S, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12:1365–1371. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 49.Worm SW, Sabin C, Weber R, Reiss P, El-Sadr W, Dabis F, et al. Risk of myocardial infarction in patients with HIV infection exposed to specific individual antiretroviral drugs from the 3 major drug classes: the data collection on adverse events of anti-HIV drugs (D:A:D) study. J Infect Dis. 2010;201:318–330. doi: 10.1086/649897. [DOI] [PubMed] [Google Scholar]
- 50.Monforte AD, Reiss P, Ryom L, El-Sadr W, Dabis F, De Wit S, et al. Atazanavir is not associated with an increased risk of cardio or cerebrovascular disease events. AIDS. 2013;27:407–415. doi: 10.1097/QAD.0b013e32835b2ef1. [DOI] [PubMed] [Google Scholar]
- 51.Kronmal RA, McClelland RL, Detrano R, Shea S, Lima JA, Cushman M, et al. Risk factors for the progression of coronary artery calcification in asymptomatic subjects: results from the Multi-Ethnic Study of Atherosclerosis (MESA) Circulation. 2007;115:2722–2730. doi: 10.1161/CIRCULATIONAHA.106.674143. [DOI] [PubMed] [Google Scholar]
- 52.Naghavi M, Falk E, Hecht HS, Jamieson MJ, Kaul S, Berman D, et al. From vulnerable plaque to vulnerable patient--Part III: Executive summary of the Screening for Heart Attack Prevention and Education (SHAPE) Task Force report. Am J Cardiol. 2006;98:2H–15H. doi: 10.1016/j.amjcard.2006.03.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.