Abstract
Purpose
To evaluate prophylactic salpingo-oophorectomy uptake and timing among BRCA1/2 mutation carriers in a cancer risk assessment program.
Methods
Clinical records of female BRCA1/2 mutation carriers who received cancer genetic counseling between 1996 and 2003 were reviewed to determine the completion and the timing of prophylactic salpingo-oophorectomy. Logistic regression models evaluated associations between subject characteristics and surgery. Survival analysis methods were used to estimate the distribution of time to surgery.
Results
Among 88 women, 70% underwent prophylactic salpingo-oophorectomy. Prophylactic salpingo-oophorectomy was associated with older age, white race, having children, and a family history of ovarian cancer. Many women waited more than 12 months to undergo surgery and some delayed by several years. Younger age and not having children were associated with delays to surgery.
Conclusion
Prophylactic salpingo-ooporectomy is an acceptable risk reduction measure for many BRCA1/2 mutation carriers. Some women make this decision many years after genetic testing. Continued discussion of the risks and benefits of risk reduction options may facilitate the uptake of recommended risk reduction interventions among BRCA mutation carriers.
Keywords: BRCA1, BRCA2, prophylactic salpingo-oophorectomy
Women who carry a BRCA1 or BRCA2 mutation have a 31–87% risk of developing breast cancer and a 15–40% risk of developing ovarian cancer1–4 compared with the risk in the general population of 12.5% and 1.5%, respectively.5 In light of these risks, BRCA mutation carriers are counseled regarding available risk reduction methods, including prophylactic surgery, increased surveillance, and chemoprevention. One of these options, bilateral prophylactic salpingo-oophorectomy (BSO), has been shown to decrease the risk of ovarian cancer in BRCA mutation carriers by 85–96% and the risk of breast cancer by 50%.6–8 In addition, surgical morbidity and mortality has decreased with the advent of laparoscopic surgical techniques.9 Thus, BSO is currently recommended to BRCA mutation carriers between 35 and 40 years of age or at completion of childbearing.5,10 Despite this, many women and clinicians are concerned about the effects of premature menopause after surgical prophylaxis.11,12 Although there is some evidence suggesting that short-term hormone replacement therapy (HRT) does not increase breast cancer risks in BRCA mutation carriers, long-term prospective data are not available and many physicians are reluctant to provide, and many women are reluctant to consider postsurgical HRT.13 Thus, some women elect not to undergo BSO and receive ovarian cancer surveillance with transvaginal ultrasound, serum CA-125, and clinical pelvic examination, although studies have suggested that the ability to detect early cancers with such screening is poor.14,15
Despite these recommendations and considering the controversy surrounding postsurgical HRT, the acceptability of BSO as a risk reduction method among BRCA mutation carriers has been questioned.16,17 Reported rates of BSO among BRCA mutation carriers have varied from 13% to 75%.6,16–24 Several studies have reported that the majority (50–75%) of mutation carriers undergo prophylactic salpingo-oophorectomy.6,18,19,21,23 In contrast, other studies have reported lower rates (13–27%) of uptake of BSO among BRCA mutation carriers.16,17 In these studies, surgical decision was assessed 12 months after genetic test results. Long-term decision-making regarding prophylactic salpingo-oophorectomy among mutation carriers has not been well described.
We hypothesized that some BRCA mutation carriers elect to undergo prophylactic salpingo-oophorectomy many years after test disclosure, based on age, concerns regarding premature menopause, and childbearing plans. If so, short-term assessments of BSO uptake may underestimate BSO rates and fail to accurately reflect the acceptability of this risk reduction measure among BRCA mutation carriers. In addition, delays in surgical decision-making could indicate a need for health care professionals to repeatedly discuss the risks and benefits of surgical prophylaxis over multiple clinical encounters. In this study, we evaluate salpingo-oophorectomy rates, time to BSO from test result disclosure, and factors associated with uptake of BSO among BRCA mutation carriers evaluated in a cancer risk assessment and prevention program.
METHODS
Study design and sample
We used a retrospective cohort design to evaluate the long-term uptake of BSO among BRCA mutation carriers who received clinical services at the University of Chicago Cancer Risk Clinic between January 1996 and December 2003. This study was approved by the Institutional Review Board at the University of Chicago.
Among all the 141 female BRCA1 and BRCA2 mutation carriers evaluated in the University of Chicago Cancer Risk Clinic between January 1996 and December 2003, those with a history of ovarian cancer (n = 17), metastatic cancer at the time of evaluation (n = 4), or a prior history of salpingo-oophorectomy for gynecologic reasons (n = 8) were excluded. An additional 3 BRCA mutation carriers who elected not to obtain their genetic test results were excluded. Of 109 female mutation carriers (with at-risk ovarian tissue), 21 were excluded because of insufficient data or follow-up. The final cohort of 88 BRCA mutation carriers represents 81% of eligible women. There were no significant differences in ethnicity, mutation status (BRCA1 versus BRCA2), or personal history of breast cancer between the 21 excluded BRCA1/2 mutation carriers and those included in the analysis.
An extensive analysis of family and personal medical history was completed for all patients referred to the Cancer Risk Clinic to identify families suggestive of familial or hereditary cancer. Once identified, genetic counseling as well as recommendations for cancer risk reduction interventions are provided. Some participants, but not all, elect to have formal genetic testing. Risk reduction recommendations are conveyed in person during the initial genetic counseling session and after genetic testing and test disclosure. In addition, these recommendations are included in a patient letter that is sent after disclosure of genetic test results. Consistent with current guidelines,10 BSO is routinely recommended to mutation carriers older than 35 years or at the completion of childbearing. Alternative ovarian risk reduction options routinely discussed include ovarian cancer screening (pelvic exam, transvaginal ultrasound, and CA-125) and oral contraceptive use. Prophylactic surgeries are recorded in the patient chart, and pathology reports are requested for all prophylactic surgeries.
Medical records were reviewed for all eligible female BRCA1/2 mutation carriers with at-risk ovarian tissue. Age, mutation status, self-reported race, genetic test date, test disclosure date, personal history of breast cancer and/or mastectomy, number of children, number of first-degree and second-degree relatives with breast and ovarian cancer, completion of prophylactic salpingo-oophorectomy, date of BSO, and date of last follow-up were recorded.
Statistical analysis
Logistic regression models were used to evaluate the association between each participant characteristic and surgery. In these models, robust variance estimates were used to account for possible clustering effects due to some participants in the study cohort being related.25 These univariate analyses were followed by evaluation of multiple characteristics jointly as predictors of surgery, using all characteristics that showed association at P < 0.10. Results for associations are reported as odds ratios with confidence intervals.
For the analysis of surgery timing, censored data methods were used. The distribution of time to surgery or last follow-up was estimated using the Kaplan-Meier method.26 To evaluate factors associated with time to surgery, the Cox proportional hazards model was used, incorporating variance adjustment for observations clustered by family relation.26
RESULTS
Sample characteristics and uptake of prophylactic bilateral salpingo-oophorectomy
Characteristics of the study sample are described in Table 1. The median age of women in the sample was 42 years (range, 23–71 years). Fifty-eight percent of women were older than 39 years, and 75% were older than 34 years when they received their genetic test results. Among 88 BRCA mutation carriers, 62 (70%) had undergone BSO. The median age at the time of surgery was 44 (range 30–68) years. Sixteen women elected to have BSO before undergoing genetic testing, accounting for 26% of the total sample who had a BSO.
Table 1.
Characteristics of BRCA mutation carriers eligible for prophylactic salpingo-oophorectomy (n = 88)
| N (%) | |
|---|---|
| Age at testing, median (range) | 42 (23–71) |
| Under 40 years | 37 (42) |
| 40 or older | 51 (58) |
| Mutation status | |
| BRCA1 | 62 (70) |
| BRCA2 | 26 (30) |
| Ethnicity | |
| White | 78 (89) |
| Black | 8 (9) |
| Hispanic | 2 (2) |
| Personal history of breast cancer | |
| Yes | 52 (59) |
| No | 36 (41) |
| History of mastectomy | |
| No mastectomy | 42 (48) |
| Treatment mastectomya | 26 (30) |
| Prophylactic mastectomya | 31 (35) |
| No. children | |
| None | 17 (19) |
| One child | 14 (16) |
| 2–3 children | 50 (57) |
| 4 or more children | 7 (8) |
| No. FDRs and SDRs with breast cancer | |
| None | 5 (6) |
| 1–2 | 44 (50) |
| 3–4 | 26 (30) |
| 5 or more | 13 (15) |
| No. FDRs and SDRs with ovarian cancer | |
| None | 35 (40) |
| 1 | 23 (26) |
| 2 | 20 (23) |
| 3 or more | 10 (11) |
Women could have had both a treatment and prophylactic mastectomy.
FDR, first degree relative; SDR, second degree relative.
Predictors of prophylactic bilateral salpingo-oophorectomy
Several factors were associated with BSO in a univariate analysis that took into account clustering among related participants (Table 2). These included older age at genetic testing, having children, a personal history of breast cancer, non-Hispanic white race, history of mastectomy (treatment or prophylactic), and a family history of ovarian cancer. Participants with more than three relatives with a history of breast cancer were less likely to undergo BSO in the univariate analysis. In the multivariate analysis, older age, non-Hispanic white race, having children, and a family history of ovarian cancer remained statistically significant. Women with three or more relatives with breast cancer again were significantly less likely to have undergone BSO.
Table 2.
Associations with bilateral prophylactic salpingo-oophorectomy (n = 88)
| Univariate test of associationa |
Multivariate test of associationb |
|||||||
|---|---|---|---|---|---|---|---|---|
| Had BSO, N (%) | No BSO, N (%) | Odds ratio | Confidence interval | P | Odds ratio | Confidence interval | P | |
| Age <40 yr | 19 (51) | 18 (49) | 1.00 | — | 1.00 | — | ||
| Age ≥40 yr | 43 (84) | 8 (16) | 5.09 | 1.82–14.24 | 0.002 | 12.77 | 2.22–73.50 | 0.004 |
| Unaffected | 20 (56) | 16 (44) | 1.00 | — | 1.00 | — | ||
| Had breast cancer | 42 (81) | 10 (19) | 3.36 | 1.33–8.51 | 0.011 | 2.13 | 0.49–9.16 | 0.311 |
| BRCA1 | 44 (71) | 18 (29) | 1.00 | — | ||||
| BRCA2 | 18 (69) | 8 (31) | 0.92 | 0.36–2.435 | 0.862 | — | ||
| Non-White | 4 (40) | 6 (60) | 1.00 | — | 1.00 | — | ||
| White | 58 (74) | 20 (26) | 4.35 | 1.15–16.40 | 0.023 | 13.80 | 3.02–63.04 | <0.001 |
| No mastectomy | 25 (60) | 17 (40) | 1.00 | — | 1.00 | — | ||
| Mastectomy | 35 (80) | 9 (20) | 2.45 | 0.91–6.61 | 0.077 | 1.48 | 0.30–7.26 | 0.632 |
| No children | 8 (47) | 9 (53) | 1.00 | — | 1.00 | — | ||
| Have children | 54 (76) | 17 (24) | 3.57 | 1.19–10.74 | 0.023 | 7.47 | 1.32–42.24 | 0.023 |
| Family history of ovarian cancer | ||||||||
| No | 18 (51) | 17 (49) | 1.00 | — | 1.00 | — | ||
| Yes | 44 (83) | 9 (17) | 4.62 | 1.63–13.12 | 0.004 | 6.46 | 1.11–37.63 | 0.038 |
| No. relatives with breast cancer | ||||||||
| 0 | 22 (81) | 5 (19) | 1.00 | — | 0.009 | 1.00 | — | 0.016 |
| 1–2 | 32 (80) | 10 (20) | 0.91 | 0.24–3.45 | 2.65 | 0.63–11.16 | ||
| 3 or more | 8 (38) | 13 (62) | 0.14 | 0.03–0.74 | 0.22 | 0.03–1.85 | ||
Chi-squared tests to assess associations between prophylactic oophorectomy and sample characteristics were computed accounting for clustering by family unit via robust variance estimates.
From a model including all variables shown.
Timing of prophylactic salpingo-oophorectomy
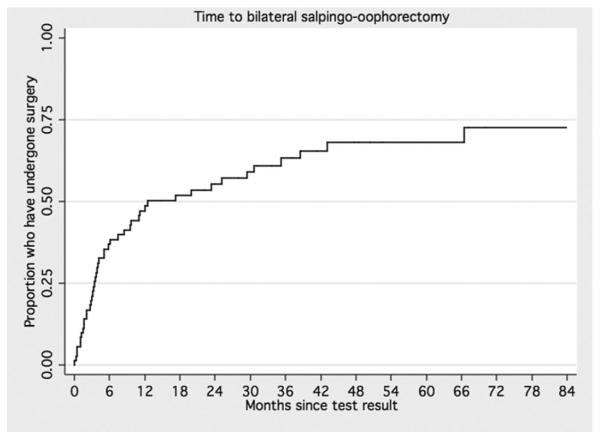
Among all the 46 mutation carriers who underwent BSO after receiving their genetic test results, the majority had their surgery within 15 months of their test result. Yet, many women waited more than 12 months to undergo surgery (from 17 to 112 months), and four women underwent BSO more than 3 years after receiving their genetic test results. The distribution of time to BSO was estimated, and factors related to timing of surgery were investigated (excluding those who underwent surgery before genetic testing [16 women]). Among these 72 women, the median follow-up from testing was 48 months (range <1–112 months). Median time to surgery was approximately 12.5 months (Fig. 1). Factors from Table 1 were examined for association with surgery timing. Among these, younger age at testing and not having children were most strongly associated with deferral of surgery (Fig. 2, A and B).
Fig. 1.
Time to bilateral salpingo-oophorectomy.
Fig. 2.
Time to bilateral salpingo-oophorectomy (A) by age at testing and (B) by child bearing history.
Ovarian cancers
Among the 62 BRCA mutation carriers who underwent BSO, two epithelial ovarian cancers and one tumor of low malignant potential were detected on pathologic review of the surgical specimens. A papillary serous tumor of low malignant potential was identified in a 48-year-old woman. The other two ovarian cancers were high-grade papillary serous tumors in two women who were 35 and 61 years old at the time of surgery.
DISCUSSION
In this study we found that the majority of BRCA mutation carriers elect to undergo BSO, although many make this decision over time and in some cases many years after receiving genetic test results. The high uptake of BSO among our population (70%) is consistent with several previous studies6,18,19,23,24,27 and is higher than those reporting low rates of BSO uptake.16,17,28 Differences in published rates of BSO use among mutation carriers may be related to several factors. The extended follow-up of many participants in our study, with women electing to undergo BSO several years after their genetic testing, could contribute to our higher rate of BSO use and underscores the need for long-term follow-up among mutation carriers. Studies evaluating short-term surgical decision-making may underestimate BSO use if women delay surgery, especially among cohorts with younger nulliparous women. In addition, differences among the populations studied could contribute to the variability in BSO rates. Women enrolled in research programs offering free genetic testing may differ significantly from those who present for clinical genetic testing, where testing is covered either by medical insurance or out-of-pocket payment. In addition, BSO rates may be higher in studies conducted after 2002, when the first prospective study suggested that prophylactic salpingo-oophorectomy also reduces breast cancer risk among BRCA mutation carriers,6–8 potentially increasing the acceptability of BSO among BRCA mutation carriers or their health care providers. Thus, studies with short-term follow-up, assessments before 2002, and select populations may have underestimated the acceptability of BSO among BRCA mutation carriers. Our data suggest that BSO is an acceptable risk reduction measure for the majority of BRCA mutation carriers seeking evaluation and care in a clinical setting.
Our high rate of BSO, and specifically delayed BSO, may be related to the clinical services and structure of our cancer risk assessment program. Many women in our multidisciplinary clinic receive continued cancer screening and risk assessment, where health care providers readdress cancer risk assessment and risk reduction options at each semiannual or annual visit. For women who are candidates for BSO, this includes repeated discussions regarding the pros and cons of risk reducing prophylactic ooporectomy. In a study evaluating medical informational processing needs of BRCA mutation carriers considering prophylactic salpingo-oophorectomy, Babb et al.29 found that many women expressed a need to consider the medical implications of BSO in the context of their individual experiences, perceptions, and psychosocial needs. Miller et al.30 compared enhanced genetic counseling with standard genetic counseling and found that women in the intervention group were more likely to have sought out additional information about preventive options and to have undergone preventive surgery. Thus, the follow-up and counseling in our clinic may have contributed to the high rate of BSO use. Continued discussion of the medical and psychosocial impact of BSO may facilitate uptake of the procedure, and BSO uptake may be lower in settings where genetic services are limited to 1–2 visits with long-term follow-up relegated to the patient's oncologist or primary care physician.
In contrast to previous reports, we found that many BRCA mutation carriers delay prophylactic salpingo-oophorectomy, some for as many as several years after learning that they carry a BRCA alteration. Several studies have suggested that the majority of BRCA mutation carriers make the decision to undergo BSO shortly after receiving their genetic test results.18,19,27 In one study, 89% of BRCA mutation carriers underwent BSO within 9 months of learning their genetic test results and only 2 of 79 (<3%) waited more than 2 years.18,19 Similarly, another study reported a median time to surgery of 4.6 months.27 In contrast, many women in our cohort elected to have surgery more than 12 months after learning of their BRCA mutation, with a median time to surgery of 12.5 months, and some women waited several years to undergo surgery. Younger age and not having children were associated with delays to surgery. These data suggest that personal and childbearing factors may be important for BRCA mutation carriers considering prophylactic BSO and are consistent with a report by Ray et al.31 suggesting that concerns about the timing of surgery were a primary factor for indecision about BSO among women at high risk for breast and ovarian cancer. Our data suggest that longitudinal studies may be necessary to adequately assess the true acceptability and optimal timing of prophylactic salpingo-oophorectomy in this high-risk population.
Despite an overall high rate of BSO in our study, there are clearly BRCA mutation carriers that elect not to have this recommended risk-reducing surgery. Women who were white, older, had children, and had a family history of ovarian cancer were more likely to have undergone BSO. As age and having children were also associated with a delay to surgery, these may not be significant barriers but indicators of the importance of life stage, timing, and concerns regarding premature menopause. On the other hand, nonwhite racial background and perceived cancer risk may be barriers to the uptake of BSO among BRCA mutation carriers. Consistent with previous studies,16,27 BRCA mutation carriers with a family history of ovarian cancer were more likely to have undergone BSO than those with no experience of ovarian cancer in their family. These findings may reflect the impact of perception of risk on the decision to undergo BSO. Greater perception of ovarian cancer risk has been associated with utilization of BSO among BRCA mutation carriers.23,24 Women without a history of ovarian cancer in their family may not think that they are at sufficiently high risk to undergo prophylactic surgery. Similarly, BRCA mutation carriers with a strong family experience of breast cancer may be most worried about their risk for breast cancer and perceive their risk of ovarian cancer as low. Further evaluation of how perceived cancer risk impacts risk reduction decision-making and how interventions to facilitate accurate understanding of cancer risk impact decision-making are needed.
Very few studies have evaluated risk-reducing health behaviors in BRCA mutation carriers among African American or other minority populations. Consistent with previous studies suggesting low uptake of genetic testing among minority populations,32 we had a relatively small percentage of minority patients (11%). Regardless, this representation of minority BRCA mutation carriers is higher than most other studies evaluating BSO uptake in this population. In addition, although the number of nonwhite participants was small (n = 10) and ethnic groups were combined, there was a significant difference in uptake of BSO among white versus nonwhite participants. Although these findings need to be confirmed in larger samples with evaluations of nonwhite ethnic groups independently, there is some literature supporting racial differences in health behaviors among women at high risk for breast cancer. In a study by Kinney et al.,33 individuals from a single BRCA1 African American kindred reported a strong preference for surveillance rather than prophylactic surgery for cancer risk reduction. Similarly, Salant et al.34 reported that African American women at high-risk for breast cancer are often skeptical of primary prevention options, including prophylactic surgery. Other studies have reported racial differences in mammography use and awareness and discussion of cancer risk and cancer prevention options among minority racial groups.35,36 Thus, our findings are consistent with the body of literature suggesting the presence of racial differences in the acceptability of primary and secondary prevention of cancer, and specifically, prophylactic surgery. Although, these findings must be confirmed in prospective studies including larger numbers of minority BRCA mutation carriers, they underscore the need for further study of barriers and preferences for cancer prevention in minority populations at high risk for cancer.
The primary limitation of our study is the retrospective design and select population. In addition, some women may have undergone surgery, which was not recorded in their clinical record, although this would result in higher rates of BSO use. Although these findings were conducted in a clinical setting, the population still represents a highly motivated group presenting for specialized care and may be not be reflective of women presenting for genetic testing in the community. Although the racial differences noted are compelling, the number of nonwhite participants was small and future studies recruiting a higher number of minority BRCA mutation carriers are needed to confirm our findings.
In conclusion, these findings suggest that BSO is an acceptable risk reduction measure for the majority of BRCA mutation carriers. In some cases, women make this decision many years after obtaining their genetic test results. Continued discussion of the risks and benefits of risk reduction measures by health care providers may facilitate uptake of recommended risk reduction interventions among BRCA mutation carriers. In settings where genetic services are limited to 1–2 visits, continued discussion of the risks and benefits of prophylactic surgery may need to be addressed during future clinical encounters with other health care professionals. In addition, there is a need for continued study of cultural and psychosocial barriers to risk-reducing prophylactic surgery in women at high risk for breast and ovarian cancer.
ACKNOWLEDGMENTS
This work was supported by T32 CA09566 from the NCI (to A.R.B.), Doris Duke Charitable Foundation, Ralph and Marion Falk Medical Research Trust, Entertainment Industry Fund National Women Cancer Research Alliance.
Footnotes
Disclosure: The authors declare no conflict of interest.
References
- 1.Whittemore AS. Risk of breast cancer in carriers of BRCA gene mutations. N Engl J Med. 1997;337:788–789. [PubMed] [Google Scholar]
- 2.Easton DF, Ford D, Bishop DT. Breast and ovarian cancer incidence in BRCA1-mutation carriers. Breast Cancer Linkage Consortium. Am J Hum Genet. 1995;56:265–271. [PMC free article] [PubMed] [Google Scholar]
- 3.Ford D, Easton DF, Stratton M. Genetic heterogeneity and penetrance analysis of the BRCA1 and BRCA2 genes in breast cancer families. The Breast Cancer Linkage Consortium. Am J Hum Genet. 1998;62:676–689. doi: 10.1086/301749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Antoniou A, Pharoah PD, Narod S. Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case Series unselected for family history: a combined analysis of 22 studies. Am J Hum Genet. 2003;72:1117–1130. doi: 10.1086/375033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.American Cancer Society . Cancer facts and figures 2007. American Cancer Society; Oakland, CA: 2006. [Google Scholar]
- 6.Kauff ND, Satagopan JM, Robson ME. Risk-reducing salpingo-oophorectomy in women with a BRCA1 or BRCA2 mutation. N Engl J Med. 2002;346:1609–1615. doi: 10.1056/NEJMoa020119. [DOI] [PubMed] [Google Scholar]
- 7.Rebbeck TR, Levin AM, Eisen A. Breast cancer risk after bilateral prophylactic oophorectomy in BRCA1 mutation carriers. J Natl Cancer Inst. 1999;91:1475–1479. doi: 10.1093/jnci/91.17.1475. [DOI] [PubMed] [Google Scholar]
- 8.Rebbeck TR, Lynch HT, Neuhausen SL. Prophylactic oophorectomy in carriers of BRCA1 or BRCA2 mutations. N Engl J Med. 2002;346:1616–1622. doi: 10.1056/NEJMoa012158. [DOI] [PubMed] [Google Scholar]
- 9.Eltabbakh GH, Piver MS, Hempling RE. Laparoscopic management of women with a family history of ovarian cancer. J Surg Oncol. 1999;72:9–13. doi: 10.1002/(sici)1096-9098(199909)72:1<9::aid-jso3>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 10.The NCCN . Genetic/familial high risk assessment: breast and ovarian. version 1 2007. Clinical practice guidelines in oncology. [Google Scholar]
- 11.Armstrong K, Schwartz JS, Randall T. Hormone replacement therapy and life expectancy after prophylactic oophorectomy in women with BRCA1/2 mutations: a decision analysis. J Clin Oncol. 2004;22:1045–1054. doi: 10.1200/JCO.2004.06.090. [DOI] [PubMed] [Google Scholar]
- 12.Rebbeck TR, Friebel T, Wagner T. Effect of short-term hormone replacement therapy on breast cancer risk reduction after bilateral prophylactic oophorectomy in BRCA1 and BRCA2 mutation carriers: the PROSE Study Group. J Clin Oncol. 2005;23:7804–7810. doi: 10.1200/JCO.2004.00.8151. [DOI] [PubMed] [Google Scholar]
- 13.Rouleau I, Chiquette J, Plante M. Changes in health-related behaviours following BRCA 1/2 genetic testing: the case of hormone replacement therapy. J Obstet Gynaecol Can. 2004;26:1059–1066. doi: 10.1016/s1701-2163(16)30432-7. [DOI] [PubMed] [Google Scholar]
- 14.Burke W, Daly M, Garber J. Recommendations for follow-up care of individuals with an inherited predisposition to cancer. II. BRCA1 and BRCA2 Cancer Genetics Studies Consortium. JAMA. 1997;277:997–1003. [PubMed] [Google Scholar]
- 15.Liede A, Karlan BY, Baldwin RL. Cancer incidence in a population of Jewish women at risk of ovarian cancer. J Clin Oncol. 2002;20:1570–1577. doi: 10.1200/JCO.2002.20.6.1570. [DOI] [PubMed] [Google Scholar]
- 16.Schwartz MD, Kaufman E, Peshkin BN. Bilateral prophylactic oophorectomy and ovarian cancer screening following BRCA1/BRCA2 mutation testing. J Clin Oncol. 2003;21:4034–4041. doi: 10.1200/JCO.2003.01.088. [DOI] [PubMed] [Google Scholar]
- 17.Lerman C, Hughes C, Croyle RT. Prophylactic surgery decisions and surveillance practices one year following BRCA1/2 testing. Prev Med. 2000;31:75–80. doi: 10.1006/pmed.2000.0684. [DOI] [PubMed] [Google Scholar]
- 18.Meijers-Heijboer H, Brekelmans CT, Menke-Pluymers M. Use of genetic testing and prophylactic mastectomy and oophorectomy in women with breast or ovarian cancer from families with a BRCA1 or BRCA2 mutation. J Clin Oncol. 2003;21:1675–1681. doi: 10.1200/JCO.2003.09.052. [DOI] [PubMed] [Google Scholar]
- 19.Meijers-Heijboer EJ, Verhoog LC, Brekelmans CT. Presymptomatic DNA testing and prophylactic surgery in families with a BRCA1 or BRCA2 mutation. Lancet. 2000;355:2015–2020. doi: 10.1016/s0140-6736(00)02347-3. [DOI] [PubMed] [Google Scholar]
- 20.Lodder LN, Frets PG, Trijsburg RW. One year follow-up of women opting for presymptomatic testing for BRCA1 and BRCA2: emotional impact of the test outcome and decisions on risk management (surveillance or prophylactic surgery) Breast Cancer Res Treat. 2002;73:97–112. doi: 10.1023/a:1015269620265. [DOI] [PubMed] [Google Scholar]
- 21.Scheuer L, Kauff N, Robson M. Outcome of preventive surgery and screening for breast and ovarian cancer in BRCA mutation carriers. J Clin Oncol. 2002;20:1260–1268. doi: 10.1200/JCO.2002.20.5.1260. [DOI] [PubMed] [Google Scholar]
- 22.Botkin JR, Smith KR, Croyle RT. Genetic testing for a BRCA1 mutation: prophylactic surgery and screening behavior in women 2 years post testing. Am J Med Genet A. 2003;118:201–209. doi: 10.1002/ajmg.a.10102. [DOI] [PubMed] [Google Scholar]
- 23.Claes E, Evers-Kiebooms G, Decruyenaere M. Surveillance behavior and prophylactic surgery after predictive testing for hereditary breast/ovarian cancer. Behav Med. 2005;31:93–105. doi: 10.3200/BMED.31.3.93-106. [DOI] [PubMed] [Google Scholar]
- 24.Madalinska JB, van Beurden M, Bleiker EM. Predictors of prophylactic bilateral salpingo-oophorectomy compared with gynecologic screening use in BRCA1/2 mutation carriers. J Clin Oncol. 2007;25:301–307. doi: 10.1200/JCO.2006.07.4922. [DOI] [PubMed] [Google Scholar]
- 25.Hosmer DW, Lameshow S. Applied logistic regression. 2nd ed Wiley-Interscience; New York: 2000. [Google Scholar]
- 26.Klein J, Moeschberger M. Survival analysis: techniques for censored and truncated data. 2nd ed Springer; New York: 2003. [Google Scholar]
- 27.Schmeler KM, Sun CC, Bodurka DC. Prophylactic bilateral salpingo-oophorectomy compared with surveillance in women with BRCA mutations. Obstet Gynecol. 2006;108:515–520. doi: 10.1097/01.AOG.0000228959.30577.13. [DOI] [PubMed] [Google Scholar]
- 28.Uyei A, Peterson SK, Erlichman J. Association between clinical characteristics and risk-reduction interventions in women who underwent BRCA1 and BRCA2 testing: a single-institution study. Cancer. 2006;107:2745–2751. doi: 10.1002/cncr.22352. [DOI] [PubMed] [Google Scholar]
- 29.Babb SA, Swisher EM, Heller HN. Qualitative evaluation of medical information processing needs of 60 women choosing ovarian cancer surveillance or prophylactic oophorectomy. J Genet Couns. 2002;11:81–96. doi: 10.1023/A:1014571420844. [DOI] [PubMed] [Google Scholar]
- 30.Miller SM, Roussi P, Daly MB. Enhanced counseling for women undergoing BRCA1/2 testing: impact on subsequent decision making about risk reduction behaviors. Health Educ Behav. 2005;32:654–667. doi: 10.1177/1090198105278758. [DOI] [PubMed] [Google Scholar]
- 31.Ray JA, Loescher LJ, Brewer M. Risk-reduction surgery decisions in high-risk women seen for genetic counseling. J Genet Couns. 2005;14:473–484. doi: 10.1007/s10897-005-5833-5. [DOI] [PubMed] [Google Scholar]
- 32.Armstrong K, Micco E, Carney A. Racial differences in the use of BRCA1/2 testing among women with a family history of breast or ovarian cancer. JAMA. 2005;293:1729–1736. doi: 10.1001/jama.293.14.1729. [DOI] [PubMed] [Google Scholar]
- 33.Kinney AY, Simonsen SE, Baty BJ. Risk reduction behaviors and provider communication following genetic counseling and BRCA1 mutation testing in an African American kindred. J Genet Couns. 2006;15:293–305. doi: 10.1007/s10897-006-9026-7. [DOI] [PubMed] [Google Scholar]
- 34.Salant T, Ganschow PS, Olopade OI. “Why take it if you don't have anything?” breast cancer risk perceptions and prevention choices at a public hospital. J Gen Intern Med. 2006;21:779–785. doi: 10.1111/j.1525-1497.2006.00461.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kaplan CP, Haas JS, Perez-Stable EJ. Breast cancer risk reduction options: awareness, discussion, and use among women from four ethnic groups. Cancer Epidemiol Biomarkers Prev. 2006;15:162–166. doi: 10.1158/1055-9965.EPI-04-0758. [DOI] [PubMed] [Google Scholar]
- 36.Pearlman DN, Rakowski W, Ehrich B. Breast cancer screening practices among black, Hispanic, and white women: reassessing differences. Am J Prev Med. 1996;12:327–337. [PubMed] [Google Scholar]