Abstract
Background
Octopus vulgaris is a highly valuable species of great commercial interest and excellent candidate for aquaculture diversification; however, the octopus’ well-being is impaired by pathogens, of which the gastrointestinal coccidian parasite Aggregata octopiana is one of the most important. The knowledge of the molecular mechanisms of the immune response in cephalopods, especially in octopus is scarce. The transcriptome of the hemocytes of O. vulgaris was de novo sequenced using the high-throughput paired-end Illumina technology to identify genes involved in immune defense and to understand the molecular basis of octopus tolerance/resistance to coccidiosis.
Results
A bi-directional mRNA library was constructed from hemocytes of two groups of octopus according to the infection by A. octopiana, sick octopus, suffering coccidiosis, and healthy octopus, and reads were de novo assembled together. The differential expression of transcripts was analysed using the general assembly as a reference for mapping the reads from each condition. After sequencing, a total of 75,571,280 high quality reads were obtained from the sick octopus group and 74,731,646 from the healthy group. The general transcriptome of the O. vulgaris hemocytes was assembled in 254,506 contigs. A total of 48,225 contigs were successfully identified, and 538 transcripts exhibited differential expression between groups of infection. The general transcriptome revealed genes involved in pathways like NF-kB, TLR and Complement. Differential expression of TLR-2, PGRP, C1q and PRDX genes due to infection was validated using RT-qPCR. In sick octopuses, only TLR-2 was up-regulated in hemocytes, but all of them were up-regulated in caecum and gills.
Conclusion
The transcriptome reported here de novo establishes the first molecular clues to understand how the octopus immune system works and interacts with a highly pathogenic coccidian. The data provided here will contribute to identification of biomarkers for octopus resistance against pathogens, which could improve octopus farming in the near future.
Introduction
Octopus vulgaris is the most important octopus species in worldwide fisheries [1], [2], and represents a major protein resource in most fish-eating countries. It is of great commercial importance in Mediterranean, South American and Asian countries as well as in the NW Atlantic coasts of Spain and Portugal [3]. However, in the last 10 years cephalopod fishery has increased due to the mollusc high price in the market, which has in turn favoured the development of octopus on-growing on an industrial scale [4]. The octopus on-growing is currently developed in tanks and in floating cages [4]–[6] with favourable results. However, high mortality has also been recorded [7]–[9] and as a result, several studies about the aetiology and prevention of diseases caused by different pathogens have been encouraged [10]–[12].
The gastrointestinal coccidian parasite Aggregata octopiana (Protozoa: Apicomplexa) has been noted as the most important epizootiological agent in wild and cultured octopus stocks from European waters [12], [13]. The infection by A. octopiana induces ulceration of the epithelium of caecum and intestine, partial destruction of the digestive tract, and decrease or malfunction of the absorption enzymes [10], [14]. Although enteric coccidiosis is not a primary cause of death, the induced malabsorption syndrome may impair octopus growth and health [10].
Hemocytes are the circulating cells of the hemolymph. They play a major role in processes like wound repair and nutrient transport, but are also important in the cellular defense against pathogens [15]. Although molluscs lack a specific immune system, the innate response mediated by circulating hemocytes and molecular effectors allows efficient and rapid responses to aggressors. The role of the hemolymph and the hemocytes in physiological functions and the immune system of bivalve molluscs have been the objective of a large amount of studies [15]–[19]. In contrast, only few studies related to cephalopod immuno-biology are available to date. Most of them report functional immune assays on the white octopus Eledone cirrhosa [20]–[22], the Pacific sepiola Euprymna scolopes (the only one detailing some molecular data) [23]–[27] and the common octopus, O. vulgaris [28]–[30].
Cephalopods are invertebrates showing innovative traits, such as no larval phase in ontogenesis, a vertebrate-like eye, a highly centralized nervous system and a close circulatory system, where the hemolymph is restricted to blood vessels and capillaries. All these characteristics indicate cephalopods as a highly evolved branch of molluscs, thus making them interesting models for neurobiological [31], learning [32], [33] and circulatory system studies [34]. However, molecular studies in cephalopods are at still at their beginning. In particular, the genome of O. vulgaris, has not yet been sequenced and no data exists about the molecular defense mechanisms underlying octopus-pathogen interactions. Nevertheless, a strategic plan aimed to promote the genome sequencing of different cephalopod species has recently been stated [35]. In the absence of genomic data, the high-throughput sequencing of total mRNA is a viable strategy for the study of the genes expressed in Octopus vulgaris [36]. Next Generation Sequencing, and Illumina short reads in particular, has successfully been used to build transcriptomic datasets in non-model species [37], [38]. The assembly of short reads data into contiguous sequences demonstrates that the assembly of long, potentially full-length transcripts assemblies is indeed possible [38].
Currently, transcriptomic studies on cephalopods have been restricted to an ecological framework in the sepiolid E. scolopes through cDNA libraries and EST collections [24], [39], [40]. The 454 pyrosequencing approach has been employed to understand the role of the circulating hemocytes of E. scolopes (colonized by the symbiotic bacteria Vibrio fischeri) in the squid/Vibrio association [41]. Only two transcriptomic studies have been performed to date related to the common octopus. The first one was an analysis of gene expression carried out through an EST collection of the O. vulgaris ocular chamber [42]. Recently, Illumina next generation sequencing technology was employed to characterize the transcriptome of the O. vulgaris central nervous system [43]. Due to its relatively low cost and good results obtained in octopus and other organisms, the Illumina RNA-Seq technology paired-end is a promising tool to study the octopus immune system as well.
In this study, we present the first hemocyte transcriptomic analysis of the cephalopod O. vulgaris by de novo sequencing and annotation of the data generated by high-throughput sequencing Illumina platform (GAII). The new data is expected to increase the publicly available sequence records of cephalopods substantially, especially considering genes involved in the cellular immune defensive activities of octopus hemocytes during coccidiosis.
Results and Discussion
Illumina sequencing and reads assembly
We used the paired-end Illumina sequencing platform to obtain the de novo transcriptome of the circulating hemocytes from adult octopus, and to analyse the octopus gene expression profile against infection by the parasite A. octopiana. Wild octopuses collected for the analysis were divided in two groups, one of five healthy octopuses harboring 0–2×103 sporocyst per gram of digestive tissue tract (spor/g) and without digestive tissue damage, and a second group of five sick octopuses infected by 6×106 to 2×107 spor/g, showing inflammation, distention and necrosis of digestive tract tissue.
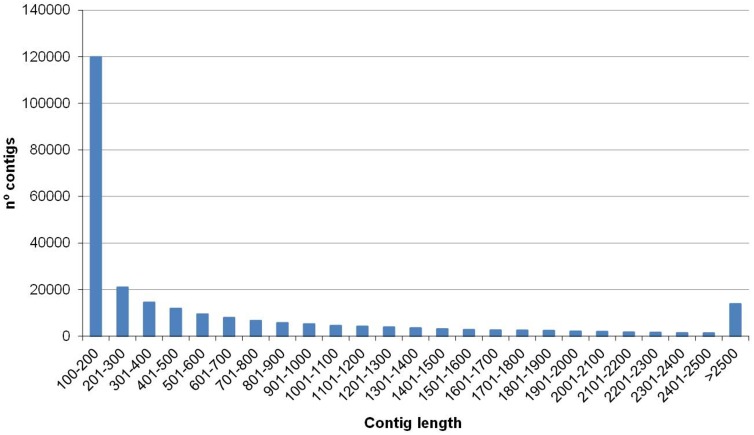
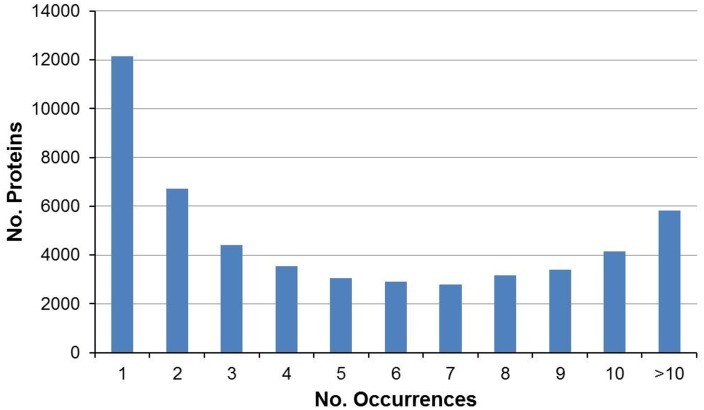
A total of 150,302,926 reads (sequences) of 105 bp were generated by the Illumina sequencer. These reads correspond to the raw data of the experiment (Table1). The Q20 percentage (sequences of high quality indicator) was 97.6% (75,571,280 reads) for the pool of hemocytes from sick individuals (highly infected by the parasite A. octopiana, showing high parasite load) and 97% (74,731,646 reads) for the pool of healthy ones (showing null or low parasite load). After filtering to remove low quality reads, a total of 127,019,711 (84.5%) clean reads were obtained from both pooled hemocyte samples. Reads from both levels of infection were sequentially assembled together with Trinity [38] and Velvet [44]. In this manner, the transcriptome reflects specific transcripts from sick and healthy octopuses plus additional transcripts putatively common to both conditions. Further alignment of sequences belonging to both sick and healthy octopuses against the entire transcriptome allowed us to detect the transcript profile of each condition. Posteriorly, a comparative analysis of gene expression was performed between sick and healthy conditions. Through assembly, 254, 506 contigs (grouped in 228,314 clusters) with a mean length of 669 bp and a maximum of 19,120 bp were generated (Table 1). Hence, the theoretical transcriptome length for O. vulgaris was 170.24 Mb. Figure 1 shows the distribution of contig lengths. The frequency of contigs showing similarity to known proteins in the NCBI database is shown in Figure 2.
Table 1. Summary statistics of sequencing and assembly for O. vulgaris hemocytes transcriptome.
| EST database summary | |
| Sequences before Filtering | |
| Number of reads | 150,302,926 |
| Total Megabases | 15,781.8 |
| Sequences after Filtering | |
| Number of reads | 127,019,711 |
| Total Megabases | 13,180.8 |
| Assembly Statistics | |
| Number of reads assembled | 42,826,899 |
| Number of contigs | 254,506 |
| Total consensus Megabases | 170.24 |
| Average contig length | 669 |
| N50 contig length | 1,632 |
| Range contig length | 100–19,120 |
| Number of contigs >500 bp | 87,408 |
| Number of clusters | 228,314 |
| Number of clusters with 1 contig | 214,607 |
| Number of clusters with >1 contig | 13,707 |
| Percentage of contigs annotated by SwissProt | 18.9% |
| Percentage of contigs functionally annotated | 13.7% |
Figure 1. Length distribution of transcripts obtained from O. vulgaris hemocytes transcriptome library.
Figure 2. Frequency of contigs showing similarity to known proteins in the NCBI database.
BLASTx search in Swiss-Prot database
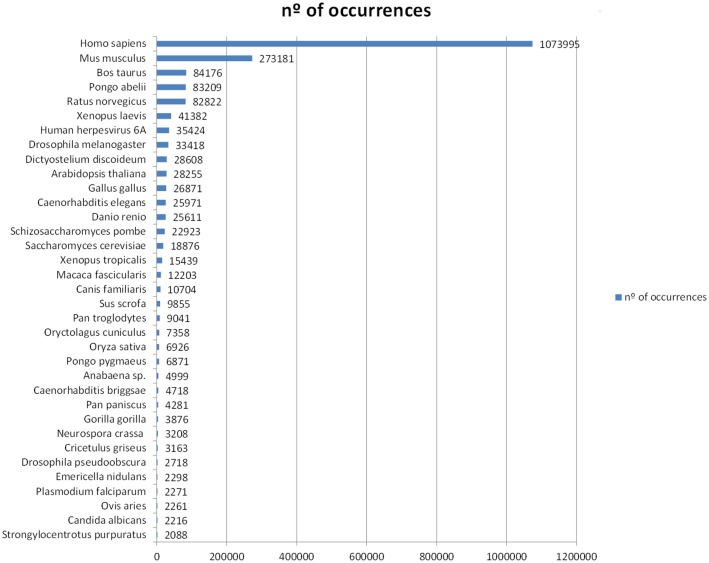
Contig gene annotation was performed through BLASTx search against the SwissProt database using a cut-off e-value of 1e−3. Using this approach, a total of 48,225 (18.95%) contigs presented a significant BLASTx hit (e-value<1e−3) and were selected for annotation. The remaining, 81.05% of the assembled sequences did not match any known proteins probably because of the lack of molecular data of cephalopod species. Therefore, a high number of potentially novel genes could be included, but more genetic studies are needed to annotate them correctly. The species that were found with the most matching sequences was Homo sapiens (1,073,995 occurrences), whereas the sea urchin Strongylocentrotus purpuratus (with 2,088 occurrences at position 35) was the single marine invertebrate homologue to sequences of the common octopus in the top 35 species represented (Figure 3). Despite other marine mollusc species matched to our library, they were all below the top 35.
Figure 3. Top 35 hit sequences matching O. vulgaris assembled sequences.
Functional annotation based on GO
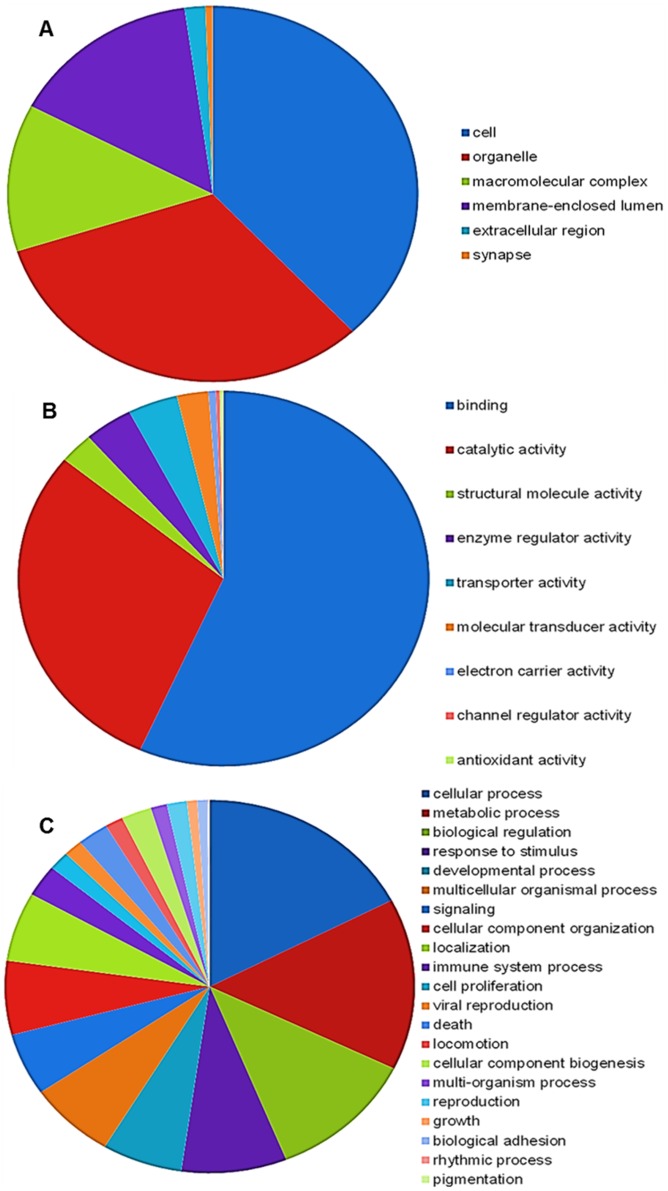
Gene Ontology (GO) assignments were carried out at level 2 to classify the proteins putatively identified after blasting the Swissprot database based on sequences homology, into three main ontology categories: cellular component, molecular function and biological process. Relative to cellular components (Figure 4A), the highest percentage of GO corresponded to cell and organelle proteins, with 38% and 32% respectively. Within the molecular function category (Figure 4B), binding and catalytic activity were the most represented groups, with 57% and 29% respectively. Related to the biological process (Figure 4C) cellular (17%) and metabolic process (15%) were the highest represented groups; in addition, biological (12%) and response to stimulus (8%) also showed a high percentage of representative sequences.
Figure 4. Distribution of second level GO annotation in three categories: (A) cellular component, (B) molecular function and (C) biological process.
Comparative analysis of the O. vulgaris transcriptome
In order to understand similarities among the O. vulgaris transcriptome and their molluscan relatives, the general transcriptome of the hemocytes of O. vulgaris was compared to the bivalve species Crassostrea gigas, Mytilus galloprovincialis and Ruditapes philippinarum, which are well represented in public databases, and the cephalopod species O. vulgaris and E. scolopes which have been the subject of other studies. The transcripts obtained in this study matched 20% (6,402 hits) with the O. vulgaris sequences available in NCBI and 0.85% (301 hits) with those available for E. scolopes. The comparison with other mollusc species showed a match of 0.06% with Crassostrea gigas (135 hits), 0.40% with Mytilus galloprovincialis (79 hits) and 3.80% with Ruditapes philippinarum (900 hits).
These results clearly indicate the limited representation of molluscs, specifically cephalopods, in public databases. The mollusc sequences used for the comparative analysis were derived from different tissues, and only few of them come from hemocytes. In fact, for O. vulgaris, only 32,304 nucleotide sequences, 35 ESTs, 257 proteins and 13 genes are deposited in the GenBank databases to date. Most of these sequences are derived from taxonomic and central nervous system studies. Consequently, the results provided in this study highlight the need to increase the number of annotated sequences from cephalopods in public databases, which will help to discover new genes that would allow further understanding of the entire molluscan cephalopod biology. An additional file containing the largest contigs of each representative locus or gene selected for annotation is provided in Table S1.
Immune transcriptome analysis
Hemocytes are the key effectors of cellular defense activities against invading agents. When challenged by pathogens, the octopus raises a strong and effective innate immune response [45], [46] and therefore, immune genes are of particular interest to understand i) how the host-cell biological processes are altered by pathogens, specifically by the natural infection of the coccidia A. octopiana, and in consequence, ii) how the host immune system faces the infection. A selection of GO immune-related terms allowed us to identify more than 3% of the predicted proteins with a possible immune function. Among the different transcripts identified, a significant number of putative immune-related genes involved in several pathways like NFκB, TLR signalling pathway, complement cascade and apoptosis were recognized (Figure 5,6,7), suggesting that similar ancient mechanisms are shared with other molluscs.
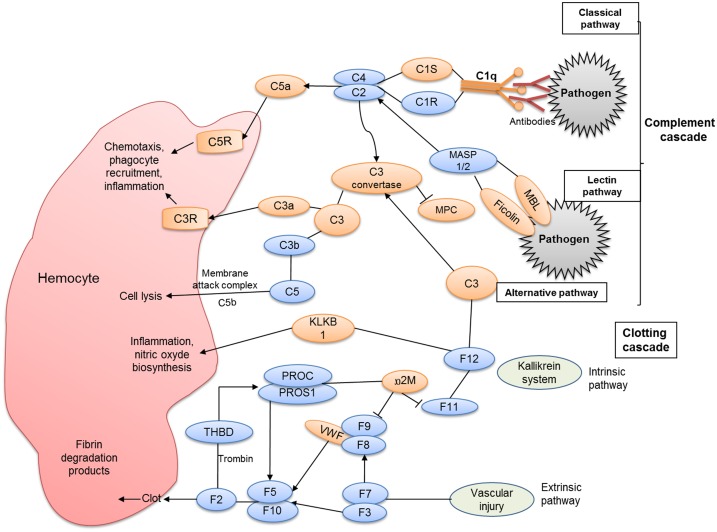
Figure 5. Complement and clotting pathway.
Brown figures indicate proteins identified in the O. vulgaris library; and blue figures indicate the absent ones. C1q: Complement C1q binding protein; C1R: C1r subcomponent; C1S: Complement C1 subcomponent; C2: Complement component 2; C4: Complement component 4; C3: Complement component 3; C3a: anaphylatoxin subcomponent 3a; C3b: Opsonin subcomponent 3b; C5: Complement component; C3R: C3 receptor; C5R: C5 receptor; MBL: Manose-binding lectin; MASP1/2: Mannan-binding lectin serine protease 1/2; F12: factor 12; F11: Factor 11; α2M: Alpha-macroglobulin; F2,3,5,7,8,9,10: Coagulation factors 2,3,5,7,8,9,10; MPC: CD46, membrane cofactor protein; VWF: Von Willebrand factor; KLKB1: kallikrein B1; PROC: protein C; PROS1: protein S (alpha); THBD: trombomodulin.
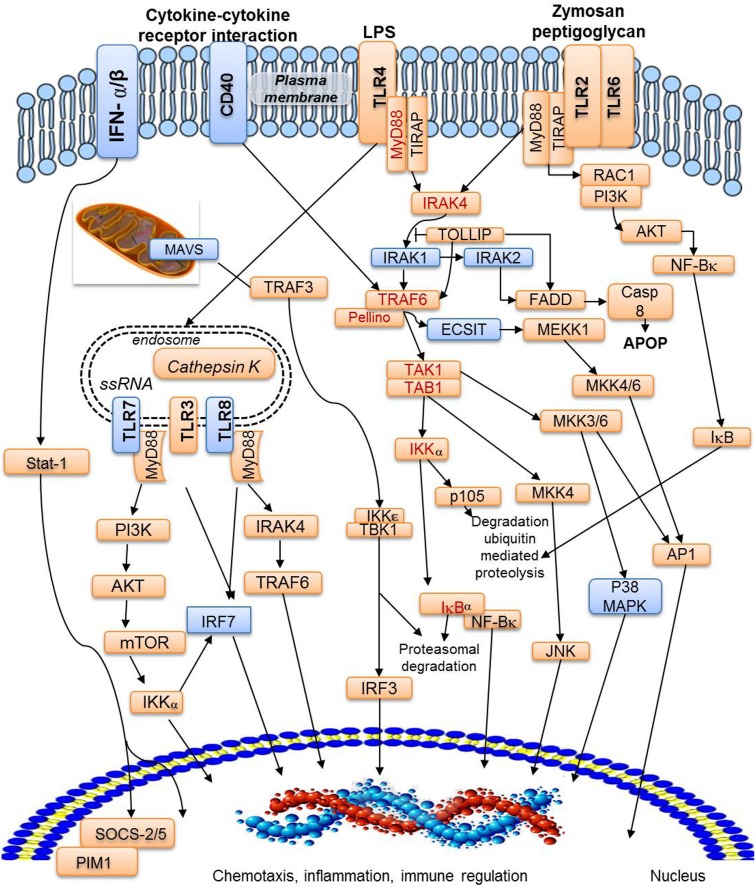
Figure 6. TLR/NF-κB signaling pathway.
Brown rectangles indicate proteins identified in the present Octopus vulgaris library and blue rectangles indicate the absent ones. Brown rectangles with red letters indicate proteins in the NF-κB pathway. AKT: RAC-alpha serine/threonine-protein kinase; API1: Transcription factor AP-1; Casp8: Caspase 8; FADD: FAS-associated via death domain; IκB: Inhibitor of NF-κB; IKKε: Inhibitor of nuclear factor kappa-B kinase subunit epsilon; IRAK4: Interleukin-1 receptor-associated kinase 4; IRF3: Interferon regulatory factor 3; IκBα: NF-kappa-B inhibitor alpha; JNK: c-Jun N-terminal kinase; MEKK1: Mitogen-activated protein kinase knase 1; MKK4/6: Mitogen-activated protein kinase kinase 4/6; MyD88: Myeloid differentiation primary response protein 88; Mtor: Mechanistic target of rapamycin; NF-Κb: Nuclear factor kappa-B; PI3K: Phosphatidylinositol 3 kinase; PIM1: Proto-oncogene serine/threonine-protein kinase pim-1; p105: Nuclear factor NF-kappa-B p105 subunit; RAC1: Ras related C3 botulinum toxin substrate; Stat-1: Signal transducer and activator of transcription 1; SOCS-2/5: Suppressor of cytokine signaling; TAB1: TAK1-binding protein1; TAK1: TGF-beta activated protein kinase kinase 1; TIRAP: Toll-interleukin 1 receptor domain-containing adaptor protein; TLR2: Toll-like receptor 2; TLR4: Toll like receptor 4; TOLLIP: Toll interacting protein (├ direct inhibition); TRAF3: TNF receptor-associated factor 3; TRAF6: TNF receptor-associated factor 6; MAVS: Mitochondrial antiviral signaling protein that activates NF-kappa B and IRF 3; INFα/β: Interferon alpha/beta receptor; IRAK1–2: Interleukin receptor associated kinase 1, 2; IRF7: Interferon regulatory factor. P38MAPK: p38 mitogen-activated protein kinases; ECSIT: Evolutionarily conserved signaling intermediate in Toll pathways.
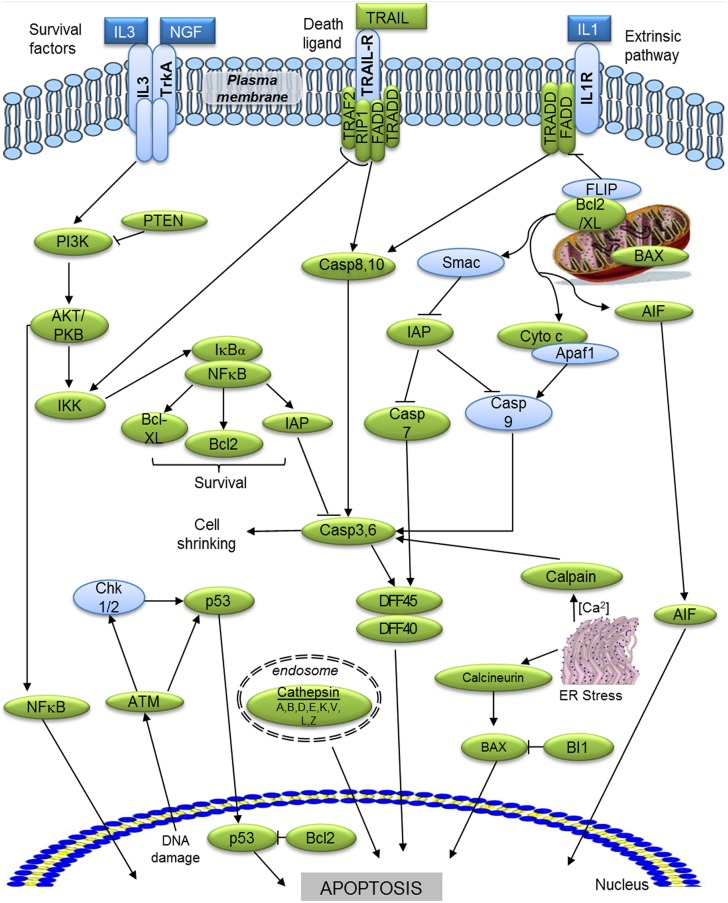
Figure 7. Apoptosis pathway.
Green ellipse indicates proteins identified in the present O. vulgaris library and blue ones indicate absence. (├ direct inhibition). AKT/PKB: RAC-alpha serine/thereonine-protein kinse/Protein kinase B; AIF: Apoptosis-inducing factor 1 mitochondiral; ATM: Ataxia telangiectasia mutated protein; BAX: Apoptosis regulator BAX. Bcl2: Apoptosis regulator Bcl-2; Bcl-XL: Bcl-2 like protein 1; BI1: BAX inhibitor-1; Casp 3, 6, 7, 8, 10: Caspase 3, 6, 7, 8, 10; Cytc: Cytochrome C; DFF40, 45: DNA fragmentation factor of 40 kD, 45 kD; FADD: FAS-associated via death domain; IAP: Inhibitor of apoptosis; IKK: Inhibitor of nuclear factor kappa-Bkinase; IκBα: MyD88: Myeloid differentiation primary response protein MyD88; NF-kappa-B inhibitor alpha. IL3R: Interleukin 3 receptor; NFκB: Nuclear factor kappa-B; PI3K: Phosphatidylinositol 3-kinase; p53: Tumor suppressor p53. RIP1: Receptor interacting serine/threonine-protein kinase1; TRADD: TNF receptor superfamily 1 alpha-associated via death domain; TRAF2: TNF-receptor-associated factor 2; TRAIL: TNF-related apoptosis-inducing ligand; Apaf1: Apoptotic protease-activating factor; FLIP: FADD-like apoptosis regulator; PTEN: Phosphatidylinositol-3, 4, 5-trisphosphate 3 phosphatase and dual specificity protein phosphatase PTEN; Smac: Second mitochondria-derived activator of caspase; Chk1/2: Checkpoint kinases 1, 2.
1. Complement pathway and related proteins
The complement system is a pathway comprised of more than 30 plasma and membrane-associated proteins that interact to the enhancement of cellular responses. Three different pathways activate complement: classical, lectin and alternative. All three have the component C3 in common, the central molecule where known activation pathways converge [47], [48]. Homologs to the complement C3 have been identified in marine organisms including the horseshoe crab Carcinoscorpius rotundicauda [49], the sea urchin S. purpuratus [50], the carpet-shell clam Ruditapes decussatus [51], the mussel Mytilus galloprovincialis [52] and the sea cucumber Apostichopus japonicus [53]. To date, the knowledge of the complement component C3 in cephalopods has been limited to identification and characterization in the sepiolid E. scolopes [39], [40], [54].
The O. vulgaris database presented herein contains putative homolog molecules of the complement signaling pathway (C3, C3R, C5R, C1S, MBL, Ficolin, C1q binding protein) (Figure 5). Additionally, highly important molecules including α2-Macroglobulin (3 transcripts) and fibronectin (2 transcripts) were also recorded in our O. vulgaris library (see Table S1).
2. Pattern recognition receptors (PRRs)
Lectins: Lectins are sugar-specific binding proteins that take part in different roles such as in cell-to-cell interaction, signal transduction and protein folding, but they also take part in self/non-self-recognition [55], having a direct role in innate immune functions as LPS-binding molecules, agglutination, recognition and phagocytosis through opsonisation and complement-activating factors. Therefore, lectins are valuable to recognize potential invaders and may be critical to the internal defense of marine molluscs [56], [57]. Few reports regarding the isolation and biochemical characterization of lectins and their ability to recognise non-self molecules were identified in cephalopods. One lectin was biochemically characterized in O. vulgaris [58] and two others in Octopus maya [59], [60]. In the O. vulgaris library reported herein, homolog sequences of mannose binding C-lectin (MBL) (2 transcripts), galectin (1 transcript) and a different carbohydrate binding lectin (malectin) (1 transcript) have been putatively found.
Peptidoglycan recognition proteins: Peptidoglycan recognition proteins (PGRPs) specifically recognize bacterial peptidoglycan from Gram-positive and Gram-negative bacteria. This group of proteins is conserved from insects to mammals and has diverse functions in antimicrobial defense [61], [62]. To date, five PGRP transcripts with different characteristics and location are known in cephalopods, all of them identified in a cDNA library from the sepiolid E. scolopes [24], [41]. The analysis of the O. vulgaris library led to the identification of three PGRPs in the circulating hemocytes of the octopus for the first time. Further characterization of O. vulgaris PGRPs as well as studies to determinate their specific localization are required.
Toll-like receptors: Toll-like receptors are responsible for initiating inflammatory responses against invading pathogens in invertebrates and vertebrates. The Toll receptors provide the trans-membrane molecular link between the extracellular and intracellular compartments [63], [64]. Toll-like receptors and additional genes involved in this pathway have recently been described in M. galloprovincialis [65], [66] while in cephalopods several genes involved in this pathway were described in the light organ of E. scolopes [24]. The results obtained from our sequences showed transcripts encoding homologues to TLR-2, TLR-3, TLR-4 and TLR-6 (1 transcript respectively). In addition, most of the central proteins belonging to the TLR signalling pathway (the adaptor MyD88, IRAK and TRAF6 proteins) have also been identified in this transcriptomic analysis (Figure 6). Furthermore, several transcripts containing leucine rich repeat (LRR) domains and some immunoglobulin superfamily members also containing LRR have been identified in our library (48 transcripts).
3. Cytokines
Cytokines are cell-signaling proteins that regulate inflammation and infection in the body [67]. They can be released through complement receptor-mediated signaling or by pathogens through a wide array of pattern recognition receptors (PRR) [68]. Two putatively identified transcripts of IL-17 were found in our O. vulgaris library. IL-17 is involved in the inflammatory process during infection and in the pathogenesis of chronic inflammation in autoimmune diseases. It is also capable of activating the NF-κB transcription factor in different cell types like macrophages or intestinal epithelial cells [69], [70]. In addition, the growth factors granulin (1 transcript), fibroblast growth factor 1 (FGF1) (1 transcript), fibroblast growth factor receptor 2 (FGRF2) (2 transcripts), transforming growth factor beta receptors (TGFβ) (2 transcripts), vascular endothelial growth factor (VEGF) (1 transcript), epidermal growth factor (EGF) (2 transcripts) and bone morphogenic protein (BMP) were found in the O. vulgaris transcriptome.
4. NFκB pathway
The nuclear factor-κB (NF-κB) is rapidly activated by a wide group of agents and cellular stress conditions [71]. The NF-κB pathway seems to be an evolutionary conserved innate immune pathway that is also present in molluscs. Proteins of this pathway like Rel have been characterized in C. gigas [72] and Haliotis diversicolor supertexta hemocytes [73]. Likewise, IκB gene was characterized in the pearl oyster P. fucata [74] and recently, molecules like IKK, IκB and KKγ/NEMO were characterized in M. galloprovincialis [66]. In cephalopods, molecules belonging to the NF-κB pathway like IKKg, TRAF6 or IRAK4 were identified from juvenile E. scolopes light organs [24]. The transcripts found in this O. vulgaris library have a high similarity with the previous findings identified in E. scolopes. In addition, we have putatively identified molecules that have never before been reported in cephalopods including TRAF2, TRAF3, TRAF5, IKKα, IKKβ, RIP and TAK1 (Figure 6; Table S1).
5. Antimicrobial peptides (AMPs)
Antimicrobial peptides are proteins with the broad ability to kill or neutralize Gram-negative and Gram-positive bacteria, fungi, parasites or viruses, interacting with and crossing cell envelope membranes by a multihit mechanism [75]. The bactericidal permeability-increasing protein (BPI) is an AMP produced by polymorphonuclear leukocytes, but also by epithelial cells. Mucosal epithelia that co-exist with microbes and microbial products expressing BPI probably contribute to the maintenance of immunologic homeostasis at mucosal surfaces [76]. At least three light-organ proteins in the BPI/LBP (lipopolysaccharide-binding protein) family have been sequenced from E. scolopes [77]. A single transcript of the BPI protein is provided in this O. vulgaris library. Additional studies are needed to understand the role of this protein in the octopus cellular defense.
6. Stress response genes
Hemocytes are the primary line of defense against pathogens and one of the strategies to avoid infections is the release of reactive oxygen and nitrogen species to kill pathogens [17]. Related to cytotoxicity, one transcript of nitric oxide synthase (NOS) and nitric oxide synthase trafficker (NOSTRIN), respectively, were putatively identified in the O. vulgaris library. In addition, 3 transcripts homologous to superoxide dismutase (SOD), 1 transcript homologue to peroxiredoxins (Prxs), both involved in the antioxidant system [78], [79] were also recorded. Other redox factors such as peroxisome (3 transcripts) were also observed. Previous records found abundant transcripts of myeloperoxidase in the E. scolopes symbiotic light organ [80] as well as SOD, peroxirredoxins, peroxidases and glutathione peroxidase [41], [54].
Heat-shock proteins (HSPs) serve as molecular chaperones that protect cells from the toxic effects of heat and modulate the stress response [81], [82]. In addition, their activity is closely related to the innate immune response [83]. In the O. vulgaris library HSP13, HSP27, HSP70, HSP71, HSP74, HSP76, HSP83, HSP85 and HSP90 were putatively identified.
7. Apoptosis
Apoptosis is a common physiological process to remove damaged or potentially dangerous cells, but it is also a major defense mechanism against pathogens [84]. The central components of the apoptosis pathway are the proteases caspases. Initiator caspases (caspase 2, 8, 9 and 10) cleave and activate the effector caspases (3, 6 and 7) [85]. Apoptosis has been studied in marine invertebrates such as the abalone Haliotis diversicolor [85], the mussel M. galloprovincialis [86]–[88] or the shrimp Penaeus monodon [89], but it has not been studied before in cephalopods. The analysis of the here reported O. vulgaris library led to the putative identification of two initiator caspases, namely caspase 8 (3 transcripts) and 10 (1 transcript); and three effector caspases, caspases 3 (4 transcripts), 6 (1 transcript) and 7 (4 transcript) (Figure 7).
8. Other proteins
Serin protease inhibitor (SERPIN) proteins are important elements of the host defense to inactivate proteases secreted by pathogens and restrict their invasion [89], [90]. Protease inhibitors have been found in Crassostrea virginica, C. gigas [91], Chlamys farreri [92] and Ruditapes philippinarum [93], but have not been described in cephalopods. A total of 6 transcripts corresponding to SERPIN were putatively identified in the O. vulgaris library. Biochemical, functional and molecular characterization of SERPIN is needed to understand whether and how the octopus’ hemocytes use this protein to counteract coccidiosis.
Angiopoietin is a protein that regulates angiogenesis, the process of formation of new blood vessels from other pre-existent ones [94]. A protein putatively similar to angiopoietin-like 4 (2 transcripts) was identified in our O. vulgaris library, which is not surprising since cephalopods possess the most complex circulatory system of all invertebrates.
Peroxisome proliferator-activated receptors (PPARS) are, in general, anti-inflammatory and can interact with transcription factors involved in inflammation such as NF-κB, activator protein-1 (AP-1) and STAT [95]. A total of 3 transcripts corresponding to PPARS were found in the O. vulgaris library.
Cluster of differentiation (CDs) are cell surface molecules expressed on various cell types in the immune system. They have a defined structure that is recognized by a group of monoclonal antibodies and are used to associate cells with specific immune functions. Using this approach, certain CD markers have been revealed in small coelomocytes of the earthworm Eisenia foetida [96], [97], the purple sea urchin Arbacia punctulata [98], and the leech Hirudo intestinalis [99]. In cephalopods, the CD63 molecule (3 transcripts) was previously found in E. scolopes hemocytes [41]. In the present O. vulgaris library, sequences putatively similar to different CDs were recorded. However, further studies will be needed to characterize them.
LPS-induced TNF-α factor (LITAF) is a transcription factor that regulates inflammatory cytokines in response to LPS stimulation, and thus controls TNF-α expression. This gene has been identified in gastropods [100] and bivalves species [52], [93], [101]. In this study, we have found two transcripts similar to LITAF, which have not been reported in cephalopods before.
Allograft inflammatory factor-1 (AIF-1) is a cytokine-responsive macrophage molecule, inducible by such cytokines as IFN-g, IL1β or IL-18 [102]. AIF-1 has been characterized in the coelomocytes from the Antarctic sea urchin Sterechinus neumayeri [103] and the pearl oyster, Pinctada martensii [104]. In the present study, one transcript of AIF-1 is reported for the first time in cephalopods.
Results reported so far provide a general overview of the proteins putatively found encoded by the common octopus hemocytes. Cellular components and proteins involved in metabolic processes have been commonly found. However, the information provided here is mainly focused on the immune proteins expressed by the O. vulgaris hemocytes.
Differentially expressed transcripts in response to coccidian infection
Tophat and Cufflinks programs were used to analyse the reads of both infection conditions and report differentially expressed transcripts using a rigorous statistical analysis. From the two sample groups, the assembled contigs were transformed into FPKM (Fragments per Kilo bases per Million reads) to calculate abundance differences of each gene with further false discovery rate analysis. Thus, a set of 538 transcripts was differentially expressed (p<0.05) between sick and healthy octopuses. Significant transcripts included molecules related to cell structure (actin, tubulin, filamin) and metabolism (NADH). However, transcripts related to immune system and involved in pathogen recognition (C1q, TLR, PGRP), apoptosis (BAX inhibitor) and antioxidant system (Peroxiredoxin PRDX), among others, were also recorded (Table S2). Of them, a total of 312 transcripts were successfully identified in public databases. The remaining 226 assembled sequences did not match to any known proteins, probably due to the scarcity of the molecular representation of cephalopod species.
RT-qPCR of selected genes
To quantify differences in gene expression between sick and healthy octopuses, RT-qPCR was performed on genes such as PRRs (TLR, PGRP, C1q) and cell antioxidant system (PRDX) using specific primers (Table 2). Gene selection was based on gene implication in the host-immune response to pathogens and the differential expression observed in the transcriptomic library. Figure 8 demonstrated that the mRNA expression tested by RT-qPCR, followed the same trend of gene expression (in terms of up or down regulation pathway) as in the RNA-seq analysis. Consequently, RT-qPCR data supports the sequencing results and provides data about the suitability of using the Illumina sequencing approach for de novo assembly of the O. vulgaris hemocytes transcriptome without a genome reference.
Table 2. Primer sequences used for RT-qPCR.
| Primer | Primer sequence 5′–3′ | Amplicon Bp |
| TLR F | TATGGGTACCTGCAGATGGT | 137 |
| TLR R | TGAAAGCTGCTCATGTGAAA | |
| PGRP-F | GAGCTGCTCCACAACTGC | 119 |
| PGRP-R | CGACACCATTTCCACCA | |
| C1q-F | ACCAAGGTGGCACTGAGA | 130 |
| C1q-R | TCGCCCTCATGGAGAGT | |
| PRDX-F | CCAGTGCCAGTCTCTTTGAACA | 100 |
| PRDX-R | AGTGCACCTGGTACACCAAAAA |
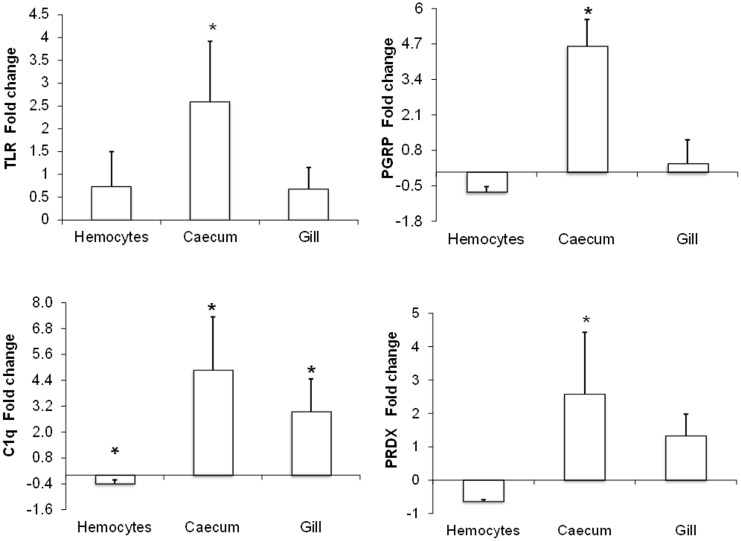
Figure 8. Fold change in gene expression analysis by RT-qPCR.
Tissue expression profiles of immune genes in O. vulgaris. Data represent the fold change in expression of the analyzed transcripts relative to β-acting transcript level of sick octopuses (highly infected by A. octopiana), referred to healthy octopuses (null or lowly infected by A. octopiana). Results are mean ± standard deviation. Asterisks denotes significant differences (P<0.05).
In order to get an insight about the molecular effect of coccidiosis on the octopus immune defense, three different tissues were selected to perform the RT-qPCR analysis: hemocytes, responsible for the cellular defensive mechanisms [17], caecum, which is the target organ of the A. octopiana infection, and the gills, which are in permanent contact with the surrounding environment and potential pathogenic agents [105].
In hemocytes, only TLR-2 was up-regulated in sick octopuses (Figure 8), suggesting that these cells could detect parasite-derived ligands or endogenous molecules such as HSP and thus trigger inflammatory response [106]. From transcripts analyzed, only peroxiredoxin has been previously recorded weakly expressed at proteomic level in O. vulgaris hemocytes of octopuses highly infected by A. octopiana [107] providing evidence of the negative effect of severe coccidiosis in the octopus cellular immune defense.
Significantly high expression of PRRs and antioxidant genes was recorded in caecum of sick octopuses. TLR-2 has a crucial role in tolerance against commensal flora, recognizing pathogens and maintaining gastrointestinal homeostasis [108]. Likewise, PGRP also regulates the microbiota inside the gut [62]. High expression of TLR-2 (2.58 fold increase) is usually related to chronic inflammatory diseases, such as inflammatory bowel disease [109]. Thus, up-regulation of TLR-2 could be induced by tissue rupture and hemocytic infiltration originated by coccidiosis [14] in a disease specific manner similar to inflammatory bowel disease. Derived from high infection, microbiota is no longer controlled by PGRP and that could be the reason for such up-regulation (4.61 fold increase). However, an attempt to maintain homeostasis inside sick octopuses seems to be present. C1q is known to be produced in response to infection as inducers of pro-inflammatory activators [110] and seems to be activated in caecum of sick octopuses (4.86 fold increase) to induce proinflammatory response. At the same time, an additional cytotoxic defensive mechanism could be run to face coccidiosis and could be responsible for up-regulation (4.61 fold increase) of antioxidant proteins like PRDX to regulate the levels of toxic radicals that can also damage the host tissue [111].
From gills, C1q (2.93 fold increase) and PRDX (1.32 fold increase) were the highest up-regulated genes observed in sick octopuses. C1q could putatively work as an opsonising protein. Similar up-regulation was observed in protease inhibitors and PRDX, suggesting that the octopus cellular defense is acting against potential pathogens present in the seawater. The target organ of A. octopiana infection is the octopus digestive tract. Gills are not a target site of A. octopiana, but they can also be found infected [112], [113]. However, gills represent the main interface between aquatic organisms and the surrounding environment. Therefore, in molluscs, gills are not only valuable for oxygenation, but are also an important defense against infections [114], and thus the expression of the immune related genes in the octopus’ gills also has to be taken into account when studying immune response.
Conclusion
The present study applied the high-throughput sequencing Illumina technology to provide the first data of the O. vulgaris immune system transcriptome. The successful results allowed the identification of a great number of new transcripts related to metabolic, functional and cellular components, but also transcripts of putatively new genes involved in the octopus immune response, which are herein provided for the first time. Sequences of molecules highly important for pathogen recognition and cellular homeostasis, belonging to pathways like complement, TLR and apoptosis were identified.
The inventory of the O. vulgaris genes involved in immunity evidenced that coccidiosis by A. octopiana induces differential expression profiles. Thus, the first insights of the effect of the A. octopiana infection at transcriptomic level, promoting the over-expression of some immune-related genes is herein provided. Finally, the information herein obtained would be useful i) to develop comparative immunology studies, ii) to better understanding of cephalopod’s immune response against pathogens, iii) and to characterize immune-relevant genes at the molecular and functional level in order to seek genetic markers of resistance that, in turn, will allow the development of future selective breeding programs to improve octopus farming.
Materials and Methods
Animal sampling, hemolymph extraction and selecting groups of infection
Specimens of O. vulgaris naturally infected by A. octopiana were collected by traps, an artisanal fishing gear used by local fishermen from the Ria of Vigo, Spain (24° 14.09′N, 8° 47.18′W). All the octopuses analyzed were sampled at the same locality, exposed to the same environmental conditions, showing similar length (DML: dorsal mantle length), and weighing around 1 kg (minimum legal weight of collection). Octopuses were maintained in tanks filled with filtered seawater at 15°C during 24 h. All experiments were carried out in accordance with the principles published in the European Animal directive (2010/63/EU) for the protection of experimental animals and approved by the Consejo Superior de Investigaciones Científicas (CSIC) ethics committee (Project number 10PXIB402116PR). Before hemolymph extraction, each octopus was anaesthetized using 7.5% magnesium chloride (MgCl2) according to Messenger [115], in strict accordance with ethical procedures and recommendations in order to minimize suffering [116], [117]. A dorsal incision was made through the skin and mantle muscle behind the head to withdraw hemolymph with a disposable syringe (1 ml) inserted directly into the cephalic aorta. One milliliter of hemolymph from each octopus was centrifuged at 12000×g, 4°C for 5 min. The pellet of hemocytes was re-suspended in 1 ml of Trizol reagent (Invitrogen) and stored at −80°C until the analysis was performed. The sacrifice was performed by immersion in frozen seawater (<1°C).
The digestive tract from each octopus was dissected and homogenized in 10 ml of filtered seawater (FSW) 1% Tween80 using an electric tissue grinder (IKA-Ultra Turrax T-25). After filtering, the number of sporocyst was counted in a Neubauer chamber. The sporocyst number is referred to as the number of parasites infecting a unit gram of octopus digestive tract (spor/g) in order to state the intensity of infection. The intensity of infection as well the histopathology produced was confirmed through the observation of caecum sections processed by standard histological methods [118]. Hence, taking into account both parameters (the intensity of infection and the histopathological damage), octopus were divided in two groups: the first one, showing a high parasite load, high intensity of infection (6×106 to 2×107 spor/g) and strong histological caecum damage; termed sick octopus group; and the second one, having a null or low parasite load, low intensity of infection (0 to 2×103 spor/g), without histological caecum damage; termed healthy octopus group. The validity of both groups of infection were confirmed using a Student’s t-test analysis (p<0.05) over the sporocyst number date, performed in Statistica 6.0 software.
RNA isolation, paired-end mRNA library preparation and sequencing
Total RNA from the hemocytes of 5 sick and 5 healthy octopuses selected from each group was extracted according to the Invitrogen protocol. After RNA extraction, samples were treated with Turbo DNase free (Ambion) to eliminate DNA. The RNA samples were purified using RNeasy Mini Kit (Qiagen), quantified using a NanoDrop ND1000 spectrophotometer and the RNA quality was assessed by Nano and Pico Chips Bioanalyzer (Agilent). A total of 1.5 µg of RNA from each of the 5 animals per group was pooled to construct the mRNA libraries according to the Illumina standard protocol. Thus, two mRNA libraries (one from the pool of sick octopus, and one from the pool of healthy octopus) were analyzed in a Genome Analyzer (GAII). In short, mRNA was purified using oligo (dT) probes and then fragmented into small pieces using divalent cations under a high temperature. The cleaved RNA fragments were used for first strand cDNA synthesis using random primers, modified and enriched for attachment to the Illumina flow cell. The two hemocyte libraries were generated using the mRNA sequencing sample preparation kit (Illumina). The libraries were validated by processing an Agilent DNA 1000 chip on a 2100 Bioanalyzer (Agilent) and quantified by qPCR using complementary primers of the library adapters with the KAPA SyBR FAST Universal qPCR kit (KAPA Biosystems). The cDNA libraries were sequenced on the Illumina sequencing platform (GAII equipped with a paired-end module) performing 105 cycles per read on two flow cell lanes.
The raw data are accessible in the NCBI Short Read Project (Accession number: SRP043705).
De novo Transcriptome generation: transcript assembly
Prior to the assembly, filters to remove low quality reads and bases were applied using ConDeTri [119]. Base trimming was done from the 3′end of each read to remove bases with a quality less than Q20 up to a minimum length of 80 bases. Reads not reaching the 80 nucleotides in length were removed before further analysis. ConDeTri allows filtering in a paired manner. The filtered Illumina paired-end and remaining orphan reads from both sequenced samples were used together for assembly. First, an initial assembly was performed using Trinity [38]. The Trinity assembly was then used as a long sequence to guide re-assembly with Velvet [44]. The use of both software allowed us to test a wide range of K-mer lengths (25 for Trinity and 31, 35, 39, 43 for Velvet) and algorithms for assembly, and to obtain a consensus transcriptome that may cover the hemocyte transcriptome spectrum. Finally, Oases was used to produce a set of putative transcripts grouped in different genes or loci [120]. CD-HIT v4.5.4 [121], [122] was used to group similar transcripts into clusters. Two transcripts were grouped if at least 95% of the positions had at least 95% identity.
Assembly validation and Functional annotation
To assess the coverage of the assembly, a homology search of the assembled transcriptome was performed against the Swissprot using BLASTx with an e-value threshold of 1e−3. BLASTx results were passed through a custom Perl script that merged the assembly Fasta sequence and summarized information to produce a table. Functional annotation was performed using Blast2GO v2.5.0 [123]–[125] with the default annotation parameters (Blast e-value threshold of 1e−3, Gene Ontology (GO) annotation threshold of 55). The GO terms associations for “Biological process”, “Molecular function” and “Cellular component” were performed using BLASTx algorithm against the Swissprot database.
Comparative analysis
The library of the O. vulgaris hemocytes here generated was compared with sequences of the cephalopods E. scolopes (35,420 ESTs) and O. vulgaris (31,929 ESTs); and the bivalves M. galloprovincialis, (19,617 ESTs), C. gigas (206,388 ESTs) and R. philippinarum (23,649 ESTs) deposited in the NCBI public database (accessed 5/6/2013). BLASTn algorithm was performed to test the sequence similarity with a threshold e-value less than 1e−5. The sequences were compared with the longest contig from each of the transcripts identified in O. vulgaris hemocytes.
Identification of immune-related genes
To identify the putative genes involved in the immune response, the sequences obtained in this study were screened using the GO terms at level 2 assigned to each sequence after annotation and confirmation of its relationship with the immune response. They were also revised based on an immune system process and response to the stimulus keyword list elaborated in our lab. BLASTx was used to identify the putative immune related transcripts looking for these specific keywords in the hit descriptions of proteins of the NCBI database, which had shown to be involved in immune response. An important number of immune-related genes identified from our high-throughput sequencing results were grouped in 4 different pathways following the KEGG reference pathways [126], and related to: Complement system, Toll-like receptor, NF-κB and apoptosis.
Transcripts differentially expressed against the infection
The differential expression of transcripts from sick and healthy animals was evaluated with TopHat [127] and Cufflinks [128] using the generated assembly as reference for mapping the reads from each condition and determining the relative transcript abundance by measuring FPKM (expected fragments per kilobase of transcript per million fragments). All p-values were adjusted with a false-discovery rate (FDR) correction for multiple testing according to the Benjamini-Hochberg method [129]. The transcripts were considered significant at p<0.05.
Expression analysis of selected genes by quantitative real time PCR (RT-qPCR)
The differential expression of four genes selected from the transcriptome library and related to the innate immune response were analysed by RT-qPCR from three different tissues. Total RNA was extracted from the hemocytes, caecum and gills of 5 individual octopuses from each group (sick and healthy) using TRIZOL reagent (Invitrogen) and following the manufacture’s instruction. The RNA concentration was quantified using a NanoDrop ND2000 spectrophotometer (Thermo Scientific). First strand cDNA was synthesized using Maxima First Strand cDNA Synthesis Kit for RT-PCR (Thermo Scientific) using 1 µg of total RNA, treated with DNAse (QIAGEN) to remove the remaining genomic DNA. For each of the selected genes, forward and reverse primers were designed using primer 3 software (http://biotools.umassmed.edu/bioapps/primer3_www.cgi). PCR efficacy (E) was calculated for each primer pair by determining the slopes of standard curves according to Pfaffl [130]. The β-actin gene was determined as the best reference gene (HKG) through the NormFinder [131], geNorm [132] and Bestkeeeper [133] algorithms. RT-qPCR reactions were performed in triplicate with a total volume of 25 µl using a 7500 FAST Thermocycler (Applied Biosystems) sequence detector in 96-microwell plates. Each well contained 1 µl of cDNA (dilution 1/10), 12.5 µl of SYBR green PCR master mix (Thermo Scientific) and 0.5 µl of each diluted primer (10 µM). The standard cycling conditions were a two-step method: 95°C for 10 min and then 40 cycles of 95°C 15 s, and 60°C for 1 min. The expression of the selected genes was normalized using the β-actin gene and analysed following the Pfaffl method [130]. Results were expressed as the mean ± standard deviation. Fold units were calculated dividing the normalized expression values of tissues samples in sick individuals by the normalized expression values of healthy ones. Data were analyzed using a Student’s t-test and differences were considered statistically significant at p<0.05.
Supporting Information
List of transcripts including the largest contig of each representative locus (e-values<1e−3) of Octopus vulgaris selected for annotation.
(XLSX)
List of transcripts differentially expressed (P<0.05) between sick (S) and healthy (H) octopuses. FC: Rate change. Expression rates of sick octopuses respect to the healthy. NA: Transcripts not identified in public databases. (#) denotes transcripts tested by RT-qPCR showed the same trend of gene expression as in the RNA-seq analysis, but without statistical significance (P>0.05).
(XLS)
Acknowledgments
The authors thank Codi Gharagouzloo, Department BioEngineering, Northeastern University, Boston, for polishing the English of this manuscript.
Funding Statement
This work has been funded by Xunta de Galicia (10PXIB402116PR). SCM wishes to acknowledge additional funding from CONACyT (Mexico). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Boyle P, Rodhouse P (2005) Cephalopods: Ecology and fisheries. Iowa: Blackwell Science. 452 p. [Google Scholar]
- 2.ICES (2012) Report of the working group on cephalopod fisheries and life history (WGCEPH). Cadiz: ICES. 241 p. [Google Scholar]
- 3. Otero J, Rocha F, González AF, García J, Guerra A (2005) Modelling artisanal coastal fisheries of Galicia (NW Spain) based on data obtained from fishers: the case of Octopus vulgaris . Sci Mar 69: 577–585. [Google Scholar]
- 4. Iglesias J, Sánchez FJ, Bersanob JGF, Carrasco JF, Dhont J, et al. (2007) Rearing of Octopus vulgaris paralarvae: Present status, bottlenecks and trends. Aquaculture 266: 1–15. [Google Scholar]
- 5.Sendao JC, Carvalho V, Borges TC (1998) Rearing octopus (Octopus vulgaris, Cuvier) with three different diets. Livro de resumos, VI Congreso Nacional de Aquacultura. Viana de Castelo, Portugal, October 15–16.
- 6. Chapela A, González AF, Dawe EG, Rocha FJ, Guerra A (2006) Growth of common octopus (Octopus vulgaris) in cages suspended from rafts. Sci Mar 70: 121–129. [Google Scholar]
- 7. García-García B, Cerezo-Valverde J (2006) Optimal proportions of crabs and fish in diet for common octopus (Octopus vulgaris) on-growing. Aquaculture 211: 171–182. [Google Scholar]
- 8. Prato E, Portacci G, Biandolino F (2010) Effect of diet on growth performance, feed efficiency and nutritional composition of Octopus vulgaris . Aquaculture 309: 203–211. [Google Scholar]
- 9. Estefanell J, Socorro J, Tuya F, Izquierdo M, Roo J (2011) Growth, protein retention and biochemical composition in Octopus vulgaris fed on different diets based on crustaceans and aquaculture by-products. Aquaculture 322–323: 91–98. [Google Scholar]
- 10. Gestal C, de la Cadena MP, Pascual S (2002) Malabsorption syndrome observed in the common octopus Octopus vulgaris infected with Aggregata octopiana (Protista: Apicomplexa). Dis Aquat Org 51: 61–65. [DOI] [PubMed] [Google Scholar]
- 11. Gestal C, Guerra A, Pascual S (2007) Aggregata octopiana (Protista: Apicomplexa): a dangerous pathogen during commercial Octopus vulgaris on-growing. ICES J Mar Sci 64: 1743–1748. [Google Scholar]
- 12. Pascual S, Gestal C, Estévez J, Rodríguez H, Soto M, et al. (1996) Parasites in commercially-exploited cephalopods (Mollusca: Cephalopodoa) in Spain: an update perspective. Aquaculture 142: 1–10. [Google Scholar]
- 13.Gestal C (2000) Epidemiología y patología de las coccidiosis en cefalópodos. Ph.D. Thesis, University of Vigo. 157 p. [Google Scholar]
- 14. Gestal C, Abollo E, Pascual S (2002) Observations on associated histopathology with Aggregata octopiana infection (Protista: Apicomplexa) in Octopus vulgaris . Dis Aquat Org 50: 45–49. [DOI] [PubMed] [Google Scholar]
- 15. Cheng TC (1975) Functional morphology and biochemistry of molluscan phagocytes. Ann NY Acad Sci 266: 343–379. [DOI] [PubMed] [Google Scholar]
- 16. Pipe RK (1992) Generation of reactive oxygen metabolites by the haemocytes of the mussel Mytilus edulis . Dev Comp Immunol 16: 111–122. [DOI] [PubMed] [Google Scholar]
- 17.Chu FL (2000) Defense mechanism of marine bivalves. In: Fingerman M, Nagabhushanam R, editors. Recent advances in marine biotechnology. Immunology and pathology: Science Publishers Inc. pp. 1–42.
- 18. Canesi L, Gallo G, Gavioli M, Pruzzo C (2002) Bacteria-hemocyte interactions and phagocytosis in marine bivalves. Microsc Res Tech 57: 469–476. [DOI] [PubMed] [Google Scholar]
- 19. Comesaña P, Casas SM, Cao A, Abollo E, Arzul I, et al. (2012) Comparison of haemocytic parameters among flat oyster Ostrea edulis stocks with different susceptibility to bonamiosis and the Pacific oyster Crassostrea gigas . J Inv Path 109: 274–286. [DOI] [PubMed] [Google Scholar]
- 20. Malham SK, Runham NW, Secombes CJ (1997) Phagocytosis by haemocytes from the lesser octopus Eledone cirrhosa . Iberus 15: 1–11. [DOI] [PubMed] [Google Scholar]
- 21. Malham SK, Runham NW, Secombes CJ (1998) Lysozyme and antiprotease activity in the lesser octopus Eledone cirrhosa (Lam.) (Cephalopoda). Dev Comp Immunol 22: 27–37. [DOI] [PubMed] [Google Scholar]
- 22. Malham SK, Lacoste A, Gélébart F, Cueff A, Poulet SA (2002) A first insight into stress-induced neuroendocrine and immune changes in the octopus Eledone cirrhosa . Aquat Living Resour 15: 187–192. [Google Scholar]
- 23. Davidson SK, Koropatnick TA, Kossmehl R, Sycuro L, McFall-Ngai MJ (2004) NO means ‘yes’ in the squid-Vibrio symbiosis: nitric oxide (NO) during the initial stages of a beneficial association. Cell Microbiol 6: 1139–1151. [DOI] [PubMed] [Google Scholar]
- 24. Goodson MS, Kojadinovic M, Troll JV, Scheetz TE, Casavant TL, et al. (2005) Identifying components of the NF- κB pathway in the beneficial Euprymna scolopes-Vibrio fischeri light organ symbiosis. Appl Environ Microbiol 71: 6934–6946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Koropatnick TA, Kimbell JR, McFall-Ngai MJ (2007) Responses of host hemocytes during the initiation of the squid-Vibrio symbiosis. Biol Bull 212: 29–39. [DOI] [PubMed] [Google Scholar]
- 26. Nyholm SV (2009) Peptidoglycan monomer release and Vibrio fischeri . J Bacteriol 191: 1997–1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Altura MA, Stabb E, Goldman W, Apicella M, McFall-Ngai MJ (2011) Attenuation of host NO production by MAMPs potentiates development of the host in the squid-Vibrio simbiosis. Cell Microbiol 13: 527–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rodríguez-Domínguez H, Soto-Búa M, Iglesias-Blanco R, Crespo-González C, Arias-Fernández C, et al. (2006) Preliminary study on the phagocytic ability of Octopus vulgaris Cuvier, 1797 (Mollusca: Cephalopoda) haemocytes in vitro . Aquaculture 254: 563–570. [Google Scholar]
- 29. Novoa B, Tafalla C, Guerra A, Figueras A (2002) Cellular immunological parameters of the octopus, Octopus vulgaris . J Shellfish Res 21: 243–248. [Google Scholar]
- 30. Castellanos-Martínez S, Prado-Alvarez M, Lobo-da-Cunha A, Azevedo C, Gestal C (2014) Morphologic, cytometric and functional characterization of the common octopus (Octopus vulgaris) hemocytes. Dev Comp Immunol 44: 50–58. [DOI] [PubMed] [Google Scholar]
- 31. Grant P, Zheng Y, Pant H (2006) Squid (Loligo pealei) giant fiber system: a model for studying neurodegeneration and dementia? Biol Bull 210: 318–333. [DOI] [PubMed] [Google Scholar]
- 32. Robertson DJ, Bonaventura J, Kohnm AP (1994) Nitric oxide is required for tactile learning in Octopus vulgaris . Proc R Soc Lond B 256: 269–273. [DOI] [PubMed] [Google Scholar]
- 33. Robertson DJ, Bonaventura J, Kohnm AP, Hiscat M (2006) Nitric oxide is necessary for visual learning in Octopus vulgaris . Proc R Soc Lond B 263: 1739–1743. [DOI] [PubMed] [Google Scholar]
- 34. Wells MJ, Smith PJS (1987) The performance of the octopus circulatory system: a triumph of engineering over design. Experientia 43: 487–499. [Google Scholar]
- 35. Albertin CB, Bonnaud L, Brown TC, Crookes-Goodson WJ, da Fonseca RR, et al. (2012) Cephalopod genomics: a plan of strategies and organization. Stand Genomic Sci 7: 175–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Feldmeyer B, Wheat CW, Krezdom N, Rotter B, Pfenninger M (2011) Short read Illumina data for the de novo assembly of a non-model snail species transcriptome (Radix balthica, Basommatophora, Pulmonata), and a comparison of assembler performance. BMC Genomics 12: 317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Riesgo A, Andrade SCS, Sharma PP, Novo M, Pérez-Porro AR, et al. (2012) Comparative description of ten transcriptomes of newly sequenced invertebrates and efficiency estimation of genomic sampling in non-model taxa. Front Zool 9: 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Grabherr MG, Haas BJ, Yassour M, Levin JZ, Thomson DA, et al. (2011) Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat Biotechnol 29: 644–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chun CK, Scheetz TE, Bonaldo MF, Brown B, Clemens A, et al. (2006) An annotated cDNA library of juvenile Euprymna scolopes with and without colonization by the symbiont Vibrio fischeri . BMC Genomics 7: 154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Castillo MG, Goodson MS, McFall-Ngai MJ (2009) Identification and molecular characterization of a complement C3 molecule on a lophotrochozoan, the Hawaiian bobtail squid Euprymna scolopes . Dev Comp Immunol 33: 69–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Collins AJ, Schleicher TR, Rader BA, Nyholm SV (2012) Understanding the role of host hemocytes in a squid/Vibrio symbiosis using transcriptomics and proteomics. Front Immunol 3: 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ogura A, Ikeo K, Gojobori T (2004) Comparative analysis of gene expression for convergent evolution of camera eye between octopus and human. Genome Res 14: 1555–1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zhang X, Mao Y, Huang Z, Qu M, Chen J, et al. (2012) Transcriptome analysis of the Octopus vulgaris central nervous system. PloS One 7: e40320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zerbino DR, Birney E (2008) Velvet: algorithms for de novo short read assembly using the Bruijn graphs. Genome Res 18: 821–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ford LA (1992) Host defense mechanisms of cephalopods. Ann Rev Fish Dis 2: 25–41. [Google Scholar]
- 46. Castellanos-Martínez S, Gestal C (20013) Pathogens and immune response of cephalopods. J Exp Mar Biol Ecol 447: 14–22. [Google Scholar]
- 47. Carroll MC (2004) The complement system in regulation of adaptive immunity. Nat Immunol 5: 981–986. [DOI] [PubMed] [Google Scholar]
- 48. Dunkelberger JR, Song WC (2010) Complement and its role in innate and adaptive immune responses. Cell Res 20: 34–50. [DOI] [PubMed] [Google Scholar]
- 49. Zhu Y, Thangamani S, Ho B, Ding JL (2005) The ancient origin of the complement system. EMBO J 24: 382–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Al-Sharif WZ, Synyer JO, Lambris JD, Courtney S (1998) Sea urchin coelomocytes specifically express a homologue of the complement component C3. J Immunol 160: 2983–2997. [PubMed] [Google Scholar]
- 51. Prado-Alvarez M, Rotllant J, Gestal C, Novoa B, Figueras A (2009) Characterization of a C3 and factor B-like in the carpet-shell clam, Ruditapes decussatus . Fish Shellfish Immunol 26: 305–315. [DOI] [PubMed] [Google Scholar]
- 52. Philipp EER, Kraemer L, Melzner F, Poustka AJ, Thieme S, et al. (2012) Massively parallel RNA sequencing identifies a complex immune gene repertoire in the lophotrochozoan Mytilus edulis . PLoS ONE 7(3): e33091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zhou Z, Sun D, Yang A, Dong Y, Chen Z, et al. (2011) Molecular characterization and expression analysis of a complement component 3 in the sea cucumber (Apostichopus japonicus). Fish Shellfish Immunol 31: 540–547. [DOI] [PubMed] [Google Scholar]
- 54. Schleicher TR, Nyholm S (2011) Characterizing the host and symbiont proteomes in the association between the bobtail squid, Euprymna scolopes, and the bacterium, Vibrio fisheri . PloS One 6: e25649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Malagoli D, Sacchi S, Ottaviani E (2010) Lectins and cytokines in celomatic invertebrates: two tales with the same end. Inv Surv J 7: 1–10. [Google Scholar]
- 56. Vasta GR, Quesenberry M, Ahmed H, O’Leary N (1999) C-type lectins and galectins mediate innate and adaptive immune functions: their roles in the complement activation pathway. Dev Comp Immunol 23: 401–420. [DOI] [PubMed] [Google Scholar]
- 57. Dodd RB, Drickamer K (2001) Lectin-like proteins in model organisms: implications for evolution of carbohydrate-binding activity. Glycobiology 1185: 71R–79R. [DOI] [PubMed] [Google Scholar]
- 58. Rögener W, Renwrantz L, Uhlenbruck G (1985) Isolation and characterization of a lectin from the hemolymph of the cephalopod Octopus vulgaris (Lam) inhibited by Alpha-D-lactose and N-acetyl-lactosamine. Dev Comp Immunol 9: 605–616. [DOI] [PubMed] [Google Scholar]
- 59. Fisher WS, Dinuzzo AR (1991) Agglutination of bacteria and erythrocytes by serum from six species of marine mollusks. J Invert Pathol 57: 380–394. [DOI] [PubMed] [Google Scholar]
- 60. Alpuche J, Pereyra A, Mendoza-Hernández G, Agundis C, Rosas C, et al. (2010) Purification and partial characterization of an agglutinin from Octopus maya serum. Comp Biochem Physiol B Biochem Mol Biol 156: 1–5. [DOI] [PubMed] [Google Scholar]
- 61. Steiner H (2004) Peptidoglycan recognition proteins: on and off switches for innate immunity. Immunol Rev 198: 83–96. [DOI] [PubMed] [Google Scholar]
- 62. Royet J, Gupta D, Dziarski R (2011) Peptidoglycan recognition proteins: modulators of the microbiome and inflammation. Nat Rev Immunol 11: 837–851. [DOI] [PubMed] [Google Scholar]
- 63. Vasselon T, Detmers PA (2002) Toll receptors: a central element in innate immune responses. Infect Immun 70: 1033–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Engelmann P, Cooper EL, Németh P (2005) Anticipating innate immunity without a Toll Mol Immunol. 42: 931–942. [DOI] [PubMed] [Google Scholar]
- 65. Toubiana M, Gerdol M, Rosani U, Pallavicini A, Venier P, et al. (2013) Toll-like receptors and MyD88 adaptors in Mytilus: complete cds and gene expression levels. Dev Comp Immunol 40: 158–166. [DOI] [PubMed] [Google Scholar]
- 66. Toubiana M, Rosani U, Giambelluca S, Cammarata M, Gerdol M, et al. (2014) Toll signal transduction pathway in bivalves: complete cds of intermediate elements and related gene transcription levels in hemocytes of immune stimulated Mytilus galloprovincialis . Dev Comp Immunol 45: 300–312. [DOI] [PubMed] [Google Scholar]
- 67. Miyajima A, Hara T, Kitamura T (1992) Common subunits of cytokine receptors and the functional redundancy of cytokines. Trends Biochem Sci 17: 378–382. [DOI] [PubMed] [Google Scholar]
- 68. Lacy P, Stow JL (2011) Cytokine release from innate immune cells: association with diverse membrane trafficking pathways. Blood 118: 9–18. [DOI] [PubMed] [Google Scholar]
- 69. Witowski J, Ksiazek K, Jörres A (2004) Interleukin-17: a mediator of inflammatory responses. Cell Mol Life Sci 61: 567–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Roberts S, Gueguen Y, De Lorgeril J, Goetz F (2008) Rapid accumulation of an interleukin 17 homolog transcript on Crassostrea gigas hemocytes following bacterial exposure. Dev Comp Immunol 32: 1099–1104. [DOI] [PubMed] [Google Scholar]
- 71. Hatada EN, Krappman D, Sxheidereit C (2000) Nf-kB and the innate immune response. Curr Opin Immunol 12: 52–58. [DOI] [PubMed] [Google Scholar]
- 72. Montagnani C, Kappler C, Reichhart JM, Escoubas JM (2004) Cg-Rel, the first Rel/NF-kB homolog characterization in a mollusk, the Pacific oyster Crassostrea gigas . FEBS Lett 561: 5–82. [DOI] [PubMed] [Google Scholar]
- 73. Jiang Y, Wu X (2007) Characterization of a Rel/NF-kB homologue in a gastropod abalone, Haliotis diversicolor supertexta . Dev Comp Immunol 31: 121–131. [DOI] [PubMed] [Google Scholar]
- 74. Zhang D, Jiang S, Qiu L, Su T, Wu K, et al. (2009) Molecular characterization and expression analysis of the IκB gene from pearl oyster Pinctada fucata . Fish Shellfish Immunol 26: 84–90. [DOI] [PubMed] [Google Scholar]
- 75. Hancock REW, Scott GM (2000) The role of antimicrobial peptides in animal defenses. Proc Natl Acad Sci U S A 97: 8856–8861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Canny G, Levy O (2008) Bactericidal/permeability-increasing protein (BPI) and BPI homologs at mucosal sites. Trends Immunol 29: 469–547. [DOI] [PubMed] [Google Scholar]
- 77. Krasity BC, Troll JV, McFall-Ngai MJ (2011) Responses of host hemocytes during the initiation of the squid-Vibrio symbiosis. Biol Bull 212: 29–39. [DOI] [PubMed] [Google Scholar]
- 78. Abele D, Puntarulo S (2004) Formulation of reactive species and induction of antioxidant defense system in polar and temperature marine invertebrates and fish. Comp Biochem Physiol A 138: 405–415. [DOI] [PubMed] [Google Scholar]
- 79. Lesser MP (2006) Oxidative stress in marine environments: biochemistry and physiological ecology. Annu Rev Physiol 68: 253–278. [DOI] [PubMed] [Google Scholar]
- 80. Tomarev SI, Zinovieva RD, Weis VM, Chepelinsky AB, Piatigorsky J, et al. (1993) Abundant mRNAs in the squid light organ encode proteins with a high similarity to mammalian peroxidases. Gene 132: 219–226. [DOI] [PubMed] [Google Scholar]
- 81. Lindquist S, Craig EA (1988) The heat-shock proteins. Annu Rev Genet 22: 631–677. [DOI] [PubMed] [Google Scholar]
- 82. Parcellier A, Schmitt E, Gubuzani S, Seigneurin-Berny D, Pance A, et al. (2003) HSP27 is a ubiquitin-binding protein involved in I-kB alpha proteasomal degradation. Mol Cell Biol 23: 5790–5802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Tsan MF, Gao B (2009) Heat shock proteins and immune system. J Leukoc Biol 85: 805–910. [DOI] [PubMed] [Google Scholar]
- 84. Sahtout AH, Hassan MD, Shariff M (2001) DNA fragmentations, an indicator of apoptosis, in cultured black tiger shrimp Penaeus monodon infected with white spot syndrome virus (WSSV). Dis Aquat Org 44: 155–159. [DOI] [PubMed] [Google Scholar]
- 85. Sokolova IM (2009) Apoptosis in molluscan immune defense. Inv Surv J 6: 49–58. [Google Scholar]
- 86. Huang WB, Ren HL, Gopalakrishnan S, Xu DD, Qiao K, et al. (2010) First molecular cloning of a molluscan caspase from variously colored abalone (Haliotis diversicolor) and gene expression analysis with bacterial challenge. Fish Shellfish Immunol 28: 587–595. [DOI] [PubMed] [Google Scholar]
- 87. Romero A, Estévez-Calvar N, Dios S, Figueras A, Novoa B (2011) New insights into the apoptotic process in mollusks: characterization of caspase genes in Mytilus galloprovincialis . PLoS One 6: e17003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Estévez-Calvar N, Romero A, Figueras A, Novoa B (2013) Genes of the mitochondrial apoptotic pathway in Mytilus galloprovincialis . PLoS One 8: e61502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Xian JA, Miao YT, Li B, Guo H, Wang AL (2013) Apoptosis of tiger shrimp (Penaeus monodon) haemocytes induced by Escherichia coli lipopolysaccharide. Comp Biochem Physiol A Mol Integr Physiol 164: 301–306. [DOI] [PubMed] [Google Scholar]
- 90. Armstron PB (2006) Proteases and protease inhibitors: a balance of activities in host-pathogen interaction. Immunobiology 211: 263–281. [DOI] [PubMed] [Google Scholar]
- 91. Faisal M, MacIntyre EA, Adham KG, Tall BD, Kothary MH, et al. (1998) Evidence for the presence of protease inhibitors in eastern (Crassostrea virginica) and Pacific (Crassostrea gigas) oysters. Comp Biochem Physiol B 121: 161–168. [Google Scholar]
- 92. Wang L, Song L, Zhao J, Qiu L, Zhang H, et al. (2009) Expressed sequence tags from the zhikong scallop (Chlamys farreri): Discovery and annotation of host-defense genes. Fish Shellfish Immunol 26: 744–750. [DOI] [PubMed] [Google Scholar]
- 93. Moreira R, Balseiro P, Planas JV, Fuste B, Beltran S, et al. (2012) Transcriptomics of in vitro immune-stimulated hemocytes from the manila clam Ruditapes philippinarum using high-throughput sequencing. PloS One 7: e35009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Muñoz-Chápuli R (2011) Evolution of angiogenesis. Int J Dev Biol 55: 345–351. [DOI] [PubMed] [Google Scholar]
- 95. Anthony BJ, Allen JT, Li YS, McManus DP (2012) A role for peroxisome proliferator-activated receptors in the immunopathology of schistosomiasis?. PPAR Res 2012: 128068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Engelmann P, Pál J, Berki T, Cooper EL, Németh P (2002) Earthworm leukocytes react with different mammalian antigen-specific monoclonal antibodies. Zoology (Jena) 105: 257–265. [DOI] [PubMed] [Google Scholar]
- 97.Engelmann P, Cooper EL, Opper B, Németh P (2011) Earthworm innate immune system. In: Karaca A, editor. Biology of earthworms. Berlin: Springer-Verlag. pp. 229–245.
- 98. Lin W, Zhang H, Beck G (2001) Phylogeny of natural cytotoxicity: cytotoxic activity of coelomocytes of the purple sea urchin, Arbacia punctulata . J Exp Zool 290: 741–750. [DOI] [PubMed] [Google Scholar]
- 99. Macagno ER, Gaasterland T, Edsall L, Bafna V, Soares MB, et al. (2010) Construction of a medicinal leech transcriptome database an its application to the identification of leech homologs of neural and innate immune genes. BMC Genomics 11: 407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. De Zoysa M, Jung S, Lee J (2009) First molluscan TNF-α homologue of the TNF superfamily in disk abalone: molecular characterization and expression analysis. Fish Shellfish Immunol 26: 625–631. [DOI] [PubMed] [Google Scholar]
- 101. Zhu B, Wu X (2012) Identification and function of LPS induced tumor necrosis factor-alpha (LITAF) gene from Crassostrea ariakensis stimulated by Rickettsia-like organism. Afr J Microbiol Res 6: 4169–4174. [Google Scholar]
- 102. McDaniel DO, Piazza LK, Barker A, Robertson H, Moore CK, et al. (2012) AIF-1 gene expression and polymorphism in association with cardiac allograft rejection. J Clin Exp Cardiolog S 11: 002. [Google Scholar]
- 103. Ovando F, Gimpel C, Cardenas C, Machado JR, De Lorgeril J, et al. (2012) Cloning and expression analysis of allograft inflammatory factor type 1 in coelomocytes of Antarctic sea urchin (Sterechinus neumayeri). J Shellfish Res 1: 875–883. [Google Scholar]
- 104. Li J, Chen J, Zhang Y, Yu Z (2013) Expression of allograft inflammatory factor-1 (AIF-1) in response to bacterial challenge and tissue injury in the pearl oyster, Pinctada martensii . Fish Shellfish Immunol 34: 365–371. [DOI] [PubMed] [Google Scholar]
- 105.Hochberg FG (1990) Diseases of Mollusca: Cephalopoda. Diseases caused by protistans and metazoans. In: Kinne O, editor. Diseases of marine animals. Introduction, Cephalopoda, Annelida, Crustacea, Chaetognatha, Echinodermata, Urochordata, III. Hamburg: Biologische Anstalt Helgoland. pp. 47–202.
- 106. Ospelt C, Gay S (2010) TLRs and chronic inflammation. Int J Biochem Cell Biol 42: 495–505. [DOI] [PubMed] [Google Scholar]
- 107. Castellanos-Martínez S, Diz AP, Álvarez-Chaver P, Gestal C (2014) Proteomic characterization of the hemolymph of Octopus vulgaris infected by the protozoan parasite Aggregata octopiana . J Proteomics 105: 151–163. [DOI] [PubMed] [Google Scholar]
- 108. Vabulas RM, Ahmad-Nejad P, da Costa C, Miethke T, Kirschning CJ, et al. (2001) Endocytosed HSP60s use Toll-like Receptor 2 (TLR2) and TLR4 to active the Toll/Interleukin-1 receptor signalling pathway in innate immune cells. J Biol Chem 276: 1332–31339. [DOI] [PubMed] [Google Scholar]
- 109. Candia E, Díaz-Jiménez D, Langjahr P, Núñez LE, de la Fuente M, et al. (2012) Increased production of soluble TLR2 by lamina propria mononuclear cells from ulcerative colitis patients. Immunobiol 217: 634–642. [DOI] [PubMed] [Google Scholar]
- 110. Kishore U, Gaboriaud C, Waters P, Shrive AK, Greenhough TJ, et al. (2004) C1q and tumor necrosis factor superfamily: modularity and versatility. Trends Immunol 25: 551–561. [DOI] [PubMed] [Google Scholar]
- 111. Bandyopadhyay U, Das D, Banerjee RK (1999) Reactive oxygen species: oxidative damage and pathogenesis. Curr Sci 77: 658–666. [Google Scholar]
- 112. Pascual S, González A, Guerra A (2006) Unusual sites of Aggregata octopiana infecting in octopus cultured in floating cages. Aquaculture 254: 21–23. [Google Scholar]
- 113. Mladineo I, Bocina I (2007) Extraintestinal gamogony of Aggregata octopiana in the reared common octopus (Octopus vulgaris) (Cephalopoda: Octopodidae). J Invert Pathol 96: 261–264. [DOI] [PubMed] [Google Scholar]
- 114. Park EM, Kim YO, Nam BH, Kong HJ, Kim WJ, et al. (2008) Cloning, characterization and expression analysis of the gene for a putative lipopolysaccharide-induced TNF-α factor of the Pacific oyster, Crassostrea gigas . Fish Shellfish Immunol 24: 11–17. [DOI] [PubMed] [Google Scholar]
- 115. Messenger JB, Nixon M, Ryan KP (1985) Magnesium chloride as an anaesthetic for cephalopods. Comp Biochem Physiol C 82: 203–205. [DOI] [PubMed] [Google Scholar]
- 116. Moltschaniwskyj NA, Hall K, Lipinski MR, Marian J, Nishiguchi M, et al. (2007) Ethical and welfare considerations when using cephalopods as experimental animals. Rev Fish Biol Fish 17: 455–476. [Google Scholar]
- 117.EU (2010) Directive 2010/63/EU of The European parliament and of the council of 22 September 2010 on the protection of animals used for scientific purposes. Official Journal of European Union. Available: http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2010: 276: 0033: 0079:en:PDF. Accessed 7 January 2013.
- 118.Humason LG (1979) Animal tissue techniques. San Francisco: W.H. Freeman and Co. 661 p. [Google Scholar]
- 119. Smeds L, Künstner A (2011) ConDeTri – A content dependent read trimmer for Illumina data. PLoS One 6: e26314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Schulz MH, Zerbino DR, Vingron M, Birney E (2012) Oases: robust de novo RNA-seq assembly across the dynamic range of expression levels. Bioinformatics 28: 1086–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Li W, Godzik A (2006) Cd–hit: a fast program for clustering and comparing large sets of protein or nucleotide sequences. Bioinformatics 22: 1658–1659. [DOI] [PubMed] [Google Scholar]
- 122. Fu L, Niu B, Zhu Z, Wu S, Li W (2012) CD-HIT: accelerated for clustering the next generation sequencing data. Bioinformatics 28: 3150–3152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Conesa A, Götz S, García-Gómez JM, Terol J, Talón M, et al. (2005) Blast2GO: A universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics 21: 3674–3676. [DOI] [PubMed] [Google Scholar]
- 124. Conesa A, Götz S (2008) Blast2GO: A comprehensive suite for functional analysis in plant genomics. Int J Plant Genomics 2008: 619832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Götz S, García-Gómez JM, Terol J, Williams TD, Nagaraj SH, et al. (2008) High-throughput functional annotation and data mining with the Blast2GO suite. Nucleic Acids Res 36: 3420–3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Kanehisa M, Goto S (2000) KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res 28: 27–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Trapnell C, Pachter L, Salzberg SL (2009) TopHat: discovering splice junctions with RNA-Seq. Bioinformatics 25: 1105–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Trapnell C, Williams BA, Pertea G, Mortazavi A, Kwan G, et al. (2010) Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoforms switching during cell differentiation. Nat Biotechnol 28: 511–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc B 57: 289–300. [Google Scholar]
- 130. Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res 29: e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Andersen CL, Jensen JL, Orntoft TF (2004) Normalization of real-time quantitative reverse transcription-PCR data: a model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Res 64: 5245–5250. [DOI] [PubMed] [Google Scholar]
- 132. Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, et al. (2002) Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 3: RESEARCH0034.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Pfaffl MW, Tichopad A, Prgomet C, Neuvians TP (2004) Determination of stable housekeeping genes, differentially regulated target genes and sample integrity: BestKeeper – Excel-based tool using pair-wise correlations. Biotechnol Lett 26: 509–515. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
List of transcripts including the largest contig of each representative locus (e-values<1e−3) of Octopus vulgaris selected for annotation.
(XLSX)
List of transcripts differentially expressed (P<0.05) between sick (S) and healthy (H) octopuses. FC: Rate change. Expression rates of sick octopuses respect to the healthy. NA: Transcripts not identified in public databases. (#) denotes transcripts tested by RT-qPCR showed the same trend of gene expression as in the RNA-seq analysis, but without statistical significance (P>0.05).
(XLS)