Abstract
Background
We carried out a systematic review and meta-analysis to evaluate the impact of prophylactic dexamethasone on post-operative nausea and vomiting (PONV), post-operative pain, and complications in patients undergoing thyroidectomy.
Methods
We searched Pubmed, Embase, and Cochrane Library databases for randomized controlled trials (RCTs) that evaluated the prophylactic effect of dexamethasone versus placebo with or without other antiemetics for PONV in patients undergoing thyroidectomy. Meta-analyses were performed using RevMan 5.0 software.
Results
Thirteen RCTs that considered high quality evidence including 2,180 patients were analyzed. The meta-analysis demonstrated a significant decrease in the incidence of PONV (RR 0.52, 95% CI 0.43 to 0.63, P<0.00001), the need for rescue anti-emetics (RR 0.42, 95% CI 0.30 to 0.57, P<0.00001), post-operative pain scores (WMD –1.17, 95% CI –1.91 to –0.44, P = 0.002), and the need for rescue analgesics (RR 0.65, 95% CI 0.50–0.83, P = 0.0008) in patients receiving dexamethasone compared to placebo, with or without concomitant antiemetics. Dexamethasone 8–10mg had a significantly greater effect for reducing the incidence of PONV than dexamethasone 1.25–5mg. Dexamethasone was as effective as other anti-emetics for reducing PONV (RR 1.25, 95% CI 0.86–1.81, P = 0.24). A significantly higher level of blood glucose during the immediate post-operative period in patients receiving dexamethasone compared to controls was the only adverse event.
Conclusions
Prophylactic dexamethasone 8–10mg administered intravenously before induction of anesthesia should be recommended as a safe and effective strategy for reducing the incidence of PONV, the need for rescue anti-emetics, post-operative pain, and the need for rescue analgesia in thyroidectomy patients, except those that are pregnant, have diabetes mellitus, hyperglycemia, or contraindications for dexamethasone. More high quality trials are warranted to define the benefits and risks of prophylactic dexamethasone in potential patients with a high risk for PONV.
Introduction
Post-operative nausea and vomiting (PONV) is a common and distressing complication associated with surgery. The overall incidence of PONV ranges from 20 to 30% in general surgery and up to 80% in high-risk surgical patients when no prophylactic anti-emetic is given [1], [2]. For patients undergoing thyroidectomy, PONV is a risk factor for post-operative bleeding [3], [4], and prophylactic anti-emetics may be beneficial.
Previous studies have shown that prophylactic dexamethasone has anti-emetic and analgesic effects. Glucocorticoids are anti-inflammatory and immunosuppressive agents, and dexamethasone may exert its therapeutic actions through central inhibition of prostaglandin synthesis, by decreasing serotonin turnover in the central nervous system, and by influencing the systemic inflammatory response in favor of anti-inflammatory mediators [5]–[9].
A systematic review demonstrated that prophylactic dexamethasone was safe and effective for reducing the incidence of PONV and post-operative pain in patients undergoing laparoscopic cholecystectomy compared to placebo [10]. In patients undergoing thyroidectomy, a previous meta-analysis demonstrated a significant reduction of PONV in patients treated with a single dose of dexamethasone versus placebo [11]. However, the relatively small sample size included in this review precluded the authors from drawing definitive conclusions, and the optimal dose and timing of dexamethasone administration, and efficacy of combining dexamethasone with other anti-emetics, remains unclear.
The objective of the current study was to confirm, and continue to investigate the impact of prophylactic corticosteroid administration on PONV, post-operative pain, and complications following thyroidectomy.
Methods
This systematic review and meta-analysis is reported in accordance with the recommendations of the PRISMA statement [12].
2.1 Outcome measures
2.1.1 Primary outcome measure
Incidence of PONV during the immediate 24h post-operative period, dichotomized as no nausea versus others; this was evaluated according to a 3-point ordinal scale: no nausea; nausea; retching and/or vomiting
2.1.2 Secondary outcome measures
Post-operative pain scores
Need for rescue anti-emetic or analgesic agent(s)
Incidence of steroid-related complications, including hyperglycemia, wound infection, delayed wound healing, headaches, dizziness, facial flushing, constipation, and abdominal pain
2.2 Data collection and analysis
2.2.1 Searches
We searched PubMed, Embase, and Cochrane Library databases from their inception to October 1, 2013 using Cochrane Highly Sensitive Search Strategies to identify randomized controlled trials (RCTs) for potential inclusion in our review [13]. We used the following MeSH terms and keywords: thyroid surgery OR thyroidectomy AND corticosteroid, glucocorticoid, steroid, OR dexamethasone. The search strategy is summarized in Table S1. Authors’ names were entered as search terms in the PubMed database to check for additional studies. Trials were also identified using the “related articles” function in PubMed. We hand-searched reference lists from articles identified by the electronic search and from previous meta-analyses. This process was performed iteratively until no additional articles could be identified.
2.2.2 Inclusion and Exclusion Criteria
We included RCTs that: evaluated the prophylactic effect of dexamethasone versus placebo without other anti-emetics, dexamethasone versus placebo plus concomitant administration of a different anti-emetic, dexamethasone versus a different anti-emetic, and comparisons using different doses of dexamethasone for PONV in patients undergoing thyroidectomy. The included trials reported at least one of our outcome measures, and clearly reported patient inclusion and exclusion criteria, anesthetic technique, protocols for administration of the experimental drugs, and a definition and evaluation of nausea and vomiting. Studies were excluded if they were not RCTs, included patients who were undergoing other surgical procedures concomitantly, reported insufficient data, or were duplicate studies.
2.2.3 Selection of studies
Two reviewers (ZH Zou and YM Jiang) independently examined titles and abstracts to select eligible RCTs. We removed records that were ongoing or unpublished studies, or were published as abstracts or conference proceedings. Where datasets were overlapping or duplicated, only the most recent information was included. We retrieved the full text of potentially relevant studies. Two reviewers (ZH Zou and YM Jiang) independently examined the full text records to determine which studies met the inclusion criteria. We resolved disagreements about selection of studies by discussion and consensus.
2.2.4 Data extraction and management
Two reviewers (ZH Zou and YM Jiang) independently extracted data from eligible RCTs including details describing study population, interventions, and outcomes. We resolved disagreements about data extraction by discussion and consensus.
2.2.5 Assessment of quality of evidence in included studies
Two reviewers (ZH Zou and YM Jiang) independently assessed RCT quality and risk of bias using tools provided by the Cochrane Collaboration [14]. The reviewers examined six domains including sequence generation, allocation concealment, double-blind evaluation (blinding), complete outcome data, selective outcome reporting, and baseline comparability of groups. The risk of bias was categorized as low, high, or unclear. RCTs with high risk of bias in at least three of six domains were not included in the meta-analysis. Baseline comparability of groups was assessed using seven matching criteria: age, sex, history of motion sickness, previous post-operative emesis, anesthetic technique, operation type (partial or total thyroidectomy), and duration of surgery. Baseline incomparability was defined as non-matching in at least three of the seven criteria. We resolved disagreements about quality of evidence by discussion and consensus.
2.2.6 Statistical analysis
Statistical analyses were performed using RevMan (ver. 5.0; The Cochrane Collaboration, Oxford, UK) software and STATA (ver. 11.2; STATA Corporation, College Station, TX, USA) software. Weighted mean differences (WMDs) with 95% confidence intervals (CIs) were calculated for continuous variables, and risk ratios (RRs) with 95% CIs were calculated for dichotomous variables. A random-effects model was used to pool studies with significant heterogeneity, as determined by the chi-squared test (P≤0.10) and the inconsistency index (I2≥50%). Potential sources of statistical heterogeneity were explored by carrying out subgroup and sensitivity analyses. Subgroup analyses were performed by stratifying patients according to dose of corticosteroid and timing of dexamethasone administration; sensitivity analyses explored the impact of excluding outlying results. The presence of publication bias was comprehensively assessed using Begg’s funnel plot and Begg’s rank correlation test of asymmetry. Publication bias was thought to be present when the continuity-corrected Pr>|z| value was ≤0.1 [15]. The GRADE system was used to summarize the overall quality of evidence [16], [17].
Results
3.1 Trial identification
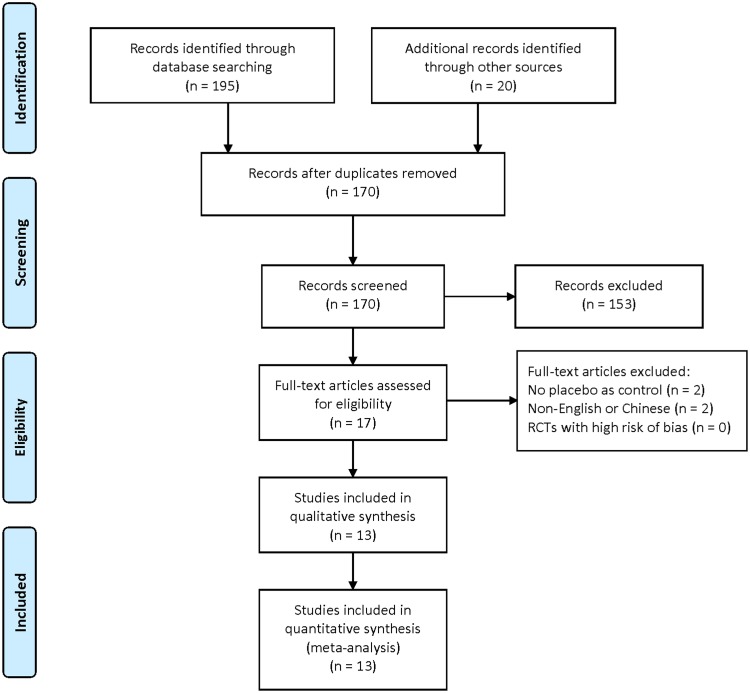
The searches identified 195 articles. We screened titles and abstracts, and 17 were identified as potentially eligible for inclusion. We retrieved the full text articles. After analyzing the full text articles, 4 studies were excluded and 13 RCTs [18]–[30] were found eligible for inclusion according to our criteria for considering studies in this review (Fig. 1).
Figure 1. Flow chart for selecting the trials.
On the basis of the search strategy, 195 articles were identified by the initial search, and 17 required further assessment. Finally, 13 articles were included in this review.
3.2 Characteristics of included studies
The characteristics of the included studies are shown in Table 1. The 13 eligible RCTs included 2,180 patients who underwent general anesthesia for thyroidectomy. The majority of RCTs included patients classified as American Society of Anesthesiologists (ASA) class I or II. Exclusion criteria were: pregnant women, patients with insulin-dependent diabetes mellitus, obesity, and patients with a high risk for PONV. Dexamethasone was administered intravenously in a single or combination dose ranging from 1.25–18mg. Timing of administration varied from 90minutes before skin incision to the end of surgery. Controls included placebo, droperidol, granisetron, ondansetron, tropisetron or a combination of these medications. Confounders such as anesthetic technique and rescue analgesics and anti-emetics were standardized within studies (Table 2). Risk of bias was low across all RCTs (Table 3).
Table 1. Characteristics of trials included in the meta-analysis.
| Study | Samplesize | Interventions | Studies divided |
| Wang1999 [18] | 120 | D 10 mg vs. Droperidol1.25mg vs. Placebo, all IVat 1minute before induction | Wang 1999 D 10: D 10 mg vs.Placebo; Wang 1999: D 10 mg vs.Droperidol 1.25 mg |
| Wang2000 [19] | 225 | D 10 mg vs. D 5 mg vs. D2.5 mg vs. D 1.25 mg vs. Placebo,all IV immediately after induction | Wang 2000 D 10: D 10 mg vs.Placebo; Wang 2000 D 5: D 5 mg vs.Placebo; Wang 2000 D 2.5: D 2.5 mg vs.Placebo; Wang 2000 D 1.25: D 1.25 mg vs. Placebo |
| Lee2001 [20] | 135 | D 8 mg vs. D 5 mg vs. Placebo,all IV before anesthesia | Lee 2001 D 8: D 10 mg vs.Placebo; Lee 2001 D 5: D 5 mg vs. Placebo |
| Fujji2007 [21] | 75 | D 8 mg vs. D 4 mg vs. Placebo,all IV at the end of surgery | Fujji 2007 D8: D 8 mg vs.Placebo; Fujji 2007 D4: D 4 mg vs. Placebo |
| Worni2008 [22] | 70 | D 8 mg vs. Placebo, both IVat 45minutes before anesthesia | Worni 2008 D8: D 8 mg vs. Placebo |
| Feroci2010 [23] | 102 | D 8 mg vs. Placebo, both IVat 20minutes before induction | Feroci 2010 D8: D 8 mg vs. Placebo |
| Doksrod2012 [24] | 120 | D 0.3 mg/kg vs. D 0.15 mg/kg vs.Placebo, all IV within10 min after induction | Doksrod 2012 D18: D 0.3 mg/kg vs. Placebo;Doksrod 2012 D9: D 0.15 mg/kg vs. Placebo |
| Song2013 [25] | 123 | D 10 mg vs. Ramosetron0.3 mg vs. Placebo, both IVimmediately after anesthesia | Song 2013 D 10: D10: D 10 mg vs.Placebo; Song 2013: D 10 mg vs.Ramosetron 0.3 mg |
| Barros2013 [26] | 40 | D 4 mg vs. Placebo, bothIV immediately after induction | Barros 2013 D4: D 4 mg vs. Placebo |
| Schietroma2013 [27] | 328 | D 8 mg vs. Placebo, bothIV at 90minutes before skin incision | Schietroma 2013 D8: D 8 mg vs. Placebo |
| Zhou2012 [28] | 150 | D 8 mg + T 5 mg vs. D 8 mg vs.T 5 mg, all IVimmediately before induction | Zhou 2012 Tropisetron:D 8 mg + T 5 mg vs.T 5 mg;Zhou 2012: D 8 mg vs. T 5 mg |
| Bononi2010 [29] | 562 | D 4 mg +O 4 mg vs.O 4 mg, D IV at inductionand ondansetron IV at15minutes before tracheal extubation | Bononi 2010 Ondansetron:D 4 mg +O 4 mg vs. O 4 mg |
| Fujji2000 [30] | 130 | D 8 mg + G 40 ug/kg vs.G 40 ug/kg, both IVimmediately before induction | Fujji 2000 Granisetron:D 8 mg + G 40 ug/kg vs. G 40 ug/kg |
IV: intravenous; ASA, American Society of Anesthesiologists; D: dexamethasone; T: tropisetron; O: ondansetron; G: Granisetron.
Table 2. Details of anesthetic technique, and rescue analgesics and anti-emetics in the included trials.
| Study | Anesthetic technique | Rescue analgesics | Rescue antiemetics |
| Wang 1999 [18] | Propofol 2.0–2.5mg/kg,glycopyrrolate 0.2mg,fentanyl 2.0 ug/kg IVmaintained with 1.0%–2.5%isoflurane in oxygen | Diclofenac75mg IM q12h | Ondansetron 4mg IV |
| Wang 2000 [19] | Propofol 2.0–2.5mg/kg,glycopyrrolate 0.2mg,fentanyl 2.0 ug/kg IVmaintained with 1.0%–2.5%isoflurane in oxygen | Diclofenac75mg IM q12h | Ondansetron 4mg IV |
| Lee 2001 [20] | Glycopyrrolate 0.2mg,fentanyl 2 ug/kg, thiopental5mg/kg IV maintainedwith desflurane in oxygen | Ketorolac 15mg IV q6h | Droperidol 1.25mg IV |
| Fujji 2007 [21] | Propofol 2mg/kg,fentanyl 2 ug/kg, vecuronium0.1mg/kg IV maintainedwith 1–3% sevoflurane in oxygen | Indomethacin50 mg rectally | Ranitidine 150mg orally |
| Worni 2008 [22] | Propofol/thiopental,atracurium, isoflurane, orsevoflurane andfentanyl 5–10 ug/kg | Acetaminophen4g/day; second-linemetamizole1g or morphine | Ondansetron 4mg IV; second-line droperidol 0.625mg IV |
| Feroci 2010 [23] | Propofol 2mg/kg,fentanyl 2 ug/kg,vecuronium 0.1mg/kg IVmaintained withsevoflurane in oxygen | Paracetamol1000mg IVq8h;second-lineketorolac 30mgIV q12h | Metoclopramide 10mg IV; second-line ondansetron 4mg IV |
| Doksrod 2012 [24] | Propofol, fentanyl,vecuronium IV maintainedwith desflurane (4–8%) anditrous oxide (60%) in oxygen | Oxycodone 5mgorally; second-linemetamizole ormorphine 2.5mgIV | Metoclopramide 20mg IV; second-line ondansetron 4mg IV |
| Song 2013 [25] | Remifentanil 1 ug/kg,propofol 1–2mg/kg,rocuroniumin 0.9mg/kg IV,maintained with desfluranein oxygen–air mixture | Ketorolac30mgIV | Metoclopramide 10mg IV |
| Barros 2013 [26] | Propofol, fentanyl 2.0 ug/kg,cisatracurium 0.15mg/kg,maintained withsevoflurane in oxygen | Ketorolac 30mgorparecoxib 40mg IV | Ondansetron 4mg IV |
| Schietroma 2013 [27] | Sodium thiopental5mg/kg,atracuriumbesylate 0.5mg/kg,maintained with oxygenin air, sevoflurane, andremifentanil hydrochloride | Ketorolac tromethamine30mg IV q6h | Ondansetron hydrochloride 4mg IV |
| Zhou 2012 [28] | Propofol 1.5–2.5mg/kg,midazolam 0.1–0.2mg/kg,fentanyl 1.0–2.0 ug/kg,maintained with 1.0–3.0%sevoflurane in oxygen | Pethidine 25mgIV | Metoclopramide 10mg IV; second-line tropisetron 5mg IV |
| Bononi 2010 [29] | Not stateda | Not stated | Not stated |
| Fujji 2000 [30] | Thiopentone 5 mg/kg,fentanyl 2 ug/kg,vecuronium0.2 mg/kg maintainedwith isoflurane (1.0%–3.0%)and nitrous oxide(66%) in oxygen | Indomethacin50mg rectally formoderae painand buprenorphiine 0.2 mg IM forsevere pain | Domperidone retally |
IV, intravenous; IM, intramuscular; ano difference.
Table 3. Quality of evidence in included studies.
| Included studies | Country | Sequencegeneration | Allocationconcealment | Doubleblinding | Completeoutcome data | No selectivereporting | Baselinecomparability | Risk ofbias |
| Wang 1999 [18] | China | Adequate | Unclear | Yes | Yes | Yes | Yes | Low |
| Wang 2000 [19] | China | Adequate | Unclear | Yes | Yes | Yes | Yes | Low |
| Lee 2001 [20] | China | Adequate | Adequate | Yes | Yes | Yes | Yes | Low |
| Fujii 2007 [21] | Japan | Adequate | Adequate | Yes | Yes | Yes | Yes | Low |
| Worni 2008 [22] | Switzerland | Adequate | Adequate | Yes | Yes | Yes | Yes | Low |
| Feroci 2011 [23] | Italy | Adequate | Adequate | Yes | Yes | Yes | Yes | Low |
| Doksrod 2012 [24] | Norway | Adequate | Adequate | Yes | Yes | Yes | Yes | Low |
| Song 2013 [25] | Korea | Adequate | Adequate | Unclear | Yes | Yes | Yes | Low |
| Barros 2013 [26] | Portugal | Adequate | Adequate | Yes | Yes | Yes | Yes | Low |
| Schietroma 2013 [27] | Italy | Adequate | Adequate | Yes | Yes | Yes | Yes | Low |
| Zhou 2012 [28] | China | Adequate | Adequate | Unclear | Yes | Yes | Yes | Low |
| Bononi 2010 [29] | Italy | Adequate | Adequate | Unclear | Yes | Yes | Yes | Low |
| Fujii 2000 [30] | Japan | Adequate | Adequate | Yes | Yes | Yes | Yes | Low |
3.3 Treatment effects
3.3.1 Primary outcome
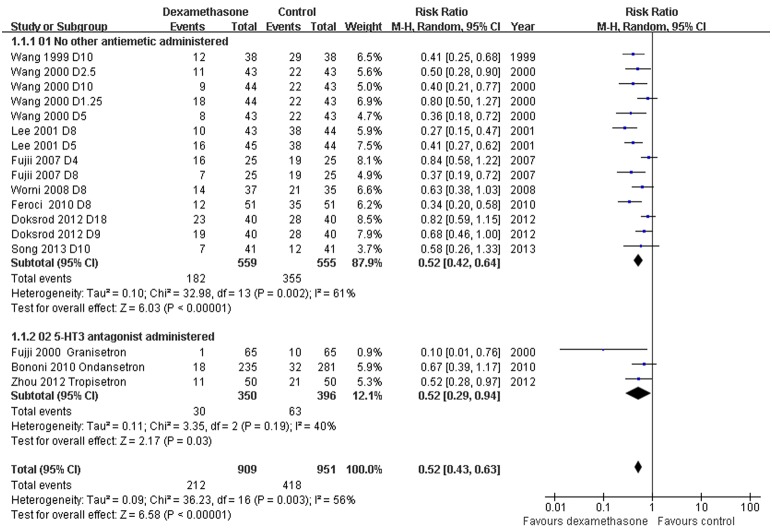
Incidence of PONV: Dexamethasone versus placebo, with or without concomitant anti-emetics - Data reporting on the incidence of PONV in thyroidectomy patients treated with dexamethasone versus placebo with or without concomitant anti-emetics are described in 11 RCTs [18]–[25], [28]–[30]. The meta-analysis demonstrated a significant decrease in the incidence of PONV in patients receiving dexamethasone compared to placebo, with or without concomitant anti-emetics (RR 0.52, 95% CI 0.43 to 0.63, P<0.00001; Fig. 2). There was evidence of significant heterogeneity between studies (P = 0.003, I2 = 56%). The dose-response gradient may have caused most of the variation between RCTs (Fig. 3; Fig.S1).
Figure 2. Incidence of PONV grouped by concomitant anti-emetics.
Eleven studies described the incidence of PONV in thyroidectomy patients treated with dexamethasone versus placebo with or without concomitant anti-emetics (RR 0.52, 95% CI 0.43 to 0.63, P<0.00001). There was evidence of significant heterogeneity between studies (P = 0.003, I2 = 56%).
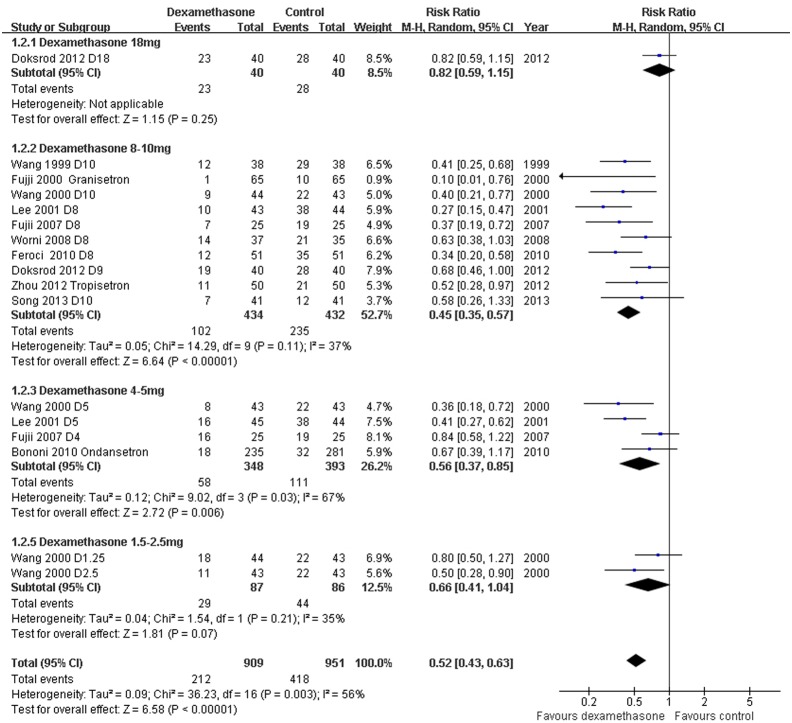
Figure 3. PONV according to dexamethasone dose.
Higher dexamethasone doses (8–10mg) were significantly more effective than lower dexamethasone doses (1.25–5mg) (P = 0.02).
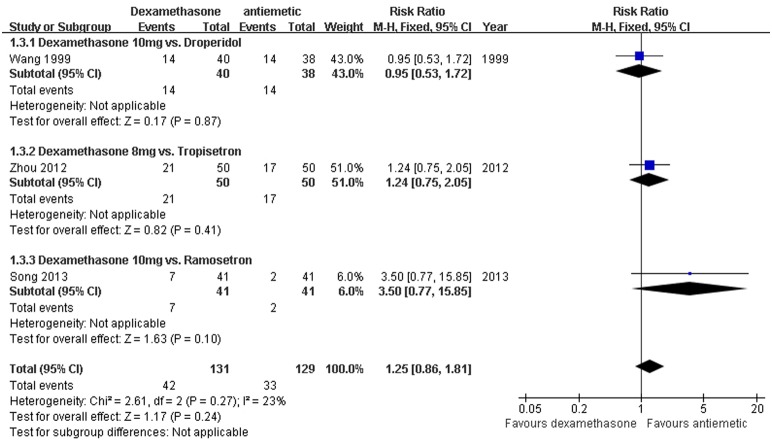
Incidence of PONV: Dexamethasone versus a different antiemetic - Data reporting on the incidence of PONV in thyroidectomy patients treated with dexamethasone versus a different anti-emetic, including droperidol, granisetron, or tropisetron, are described in three RCTs [18], [25], [28]. The meta-analysis demonstrated no significant difference in the incidence of PONV in patients receiving dexamethasone compared to these different anti-emetics (RR 1.25, 95% CI 0.86–1.81, P = 0.24; Fig. 4). There was no evidence of significant heterogeneity between RCTs (P = 0.27, I2 = 23%).
Figure 4. Comparison of dexamethasone with other anti-emetics.
Three studies described the incidence of PONV in thyroidectomy patients treated with dexamethasone versus other anti-emetics (RR 1.25, 95% CI 0.86–1.81, P = 0.24). There was no evidence of significant heterogeneity between RCTs (P = 0.27, I2 = 23%).
3.3.2 Secondary outcomes
Postoperative pain scores and need for rescue analgesia: Dexamethasone versus placebo with or without concomitant anti-emetics - Data reporting on post-operative pain scores in thyroidectomy patients treated with dexamethasone versus placebo with or without concomitant anti-emetics are described in six RCTs [18], [20], [22], [23], [25], [26]. Pain scores were evaluated based on visual analogue scales (VAS) completed by patients 24h post-operatively. Four RCTs [22], [23], [25], [26] reported data as means ± standard deviations (SDs); two RCTs [18], [20] reported data as medians (range) converted to estimated means and SDs [31]. The meta-analysis demonstrated a significantly lower post-operative VAS score in patients receiving dexamethasone compared to placebo, with or without concomitant anti-emetics (WMD –1.17, 95% CI –1.91 to –0.44, P = 0.002; Fig. 5). There was evidence of significant heterogeneity between RCTs (P<0.00001, I2 = 94%). The doses of dexamethasone may have caused most of the variation between RCTs. The need for rescue analgesia was significantly less frequent in the patients that received dexamethasone (RR 0.65, 95% CI 0.50–0.83, P = 0.0008; Fig. 6); there was no evidence of significant heterogeneity between RCTs (P = 0.25, I2 = 25%).
Figure 5. VAS post-operative pain score grouped by dexamethasone dose.
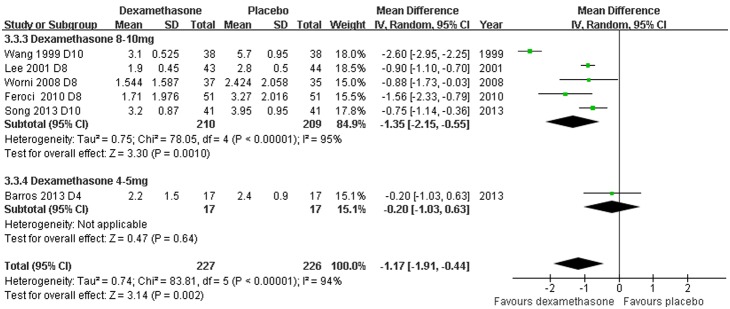
Six studies described post-operative pain scores in thyroidectomy patients treated with dexamethasone versus placebo with or without concomitant anti-emetics (WMD –1.17, 95% CI –1.91 to –0.44, P = 0.002). There was evidence of significant heterogeneity between RCTs (P<0.00001, I2 = 94%).
Figure 6. Need for rescue analgesics grouped by concomitant anti-emetics.
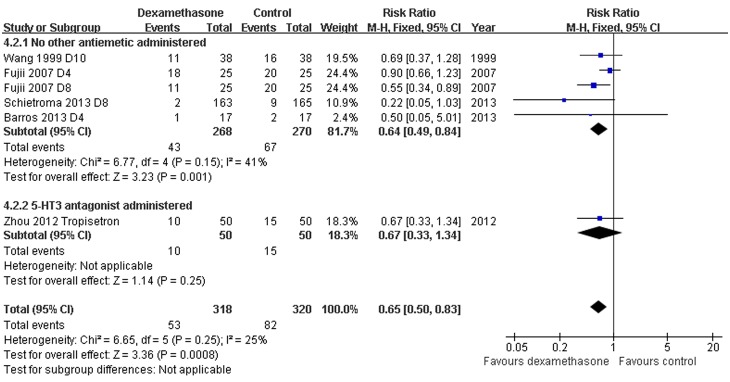
Six studies described the need for rescue analgesics in thyroidectomy patients treated with dexamethasone versus placebo with or without concomitant anti-emetics (RR 0.65, 95% CI 0.50–0.83, P = 0.0008). There was no evidence of significant heterogeneity between RCTs (P = 0.25, I 2 = 25%).
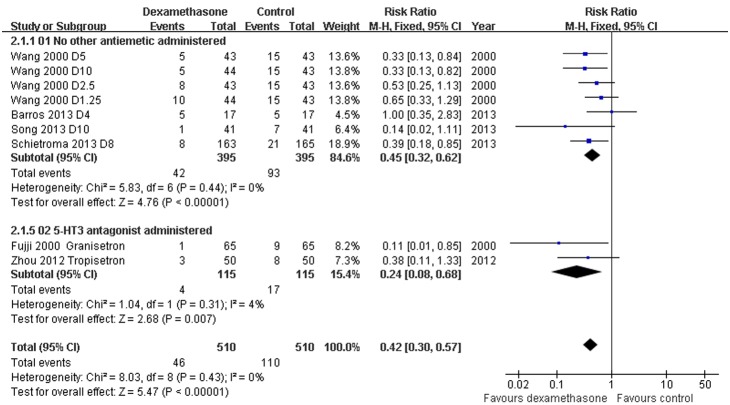
Need for rescue anti-emetic: Dexamethasone versus placebo with or without concomitant antiemetics - Data reporting on the need for rescue anti-emetics in thyroidectomy patients treated with dexamethasone versus placebo with or without concomitant anti-emetics are described in six studies [19], [25]–[28], [30]. The meta-analysis demonstrated a significant decrease in the need for rescue anti-emetics in patients receiving dexamethasone compared to placebo, with or without concomitant anti-emetics (RR 0.42, 95% CI 0.30 to 0.57, P<0.00001; Fig. 7). There was no evidence of significant heterogeneity between RCTs (P = 0.43, I2 = 0%).
Figure 7. Need for rescue antiemetics grouped by concomitant antiemetics.
Six studies described the need for rescue antiemetics in thyroidectomy patients treated with dexamethasone versus placebo with or without concomitant antiemetics (RR 0.42, 95% CI 0.30 to 0.57, P<0.00001). There was no evidence of significant heterogeneity between RCTs (P = 0.43, I2 = 0%).
Incidence of adverse events: Dexamethasone versus placebo with or without concomitant anti-emetics - Data reporting on blood glucose levels in thyroidectomy patients treated with dexamethasone are described in two RCTs. A significantly higher level of blood glucose was observed in patients receiving dexamethasone compared to controls during the first 8 hours post-operatively [23], [24]. No statistical differences in symptomatic transient hypocalcemia and asymptomatic transient hypocalcemia were present [23]. One RCT [27] reported that dexamethasone administration prevented recurrent laryngeal nerve palsy; however, this effect was not described elsewhere [23]. There were no significant differences in the incidences of extrapyramidal signs including headache, dizziness, constipation, and muscle pain, and other adverse events such as wound infection and delayed wound healing, in patients receiving dexamethasone compared to controls.
3.4 Subgroup analyses
Incidence of PONV: Dose of dexamethasone
Subgroup analyses stratified by dose of dexamethasone (range, 1.25 mg to 18mg) demonstrated that dexamethasone 4–5mg and 8–10mg significantly reduced the incidence of PONV compared to controls (1.25–5 mg: RR 0.59, 95% CI 0.44 to 0.79; 8–10mg: RR 0.45, 95% CI 0.35–0.57), while dexamethasone 18mg did not (RR 0.82, 95% CI 0.59–1.15) (Fig. 3). Dexamethasone 8–10mg had a significantly greater effect for reducing the incidence of PONV than dexamethasone 1.25–5mg (1.25–5mg RR 0.40, 95% CI 0.28 to 0.55; 8–10mg: RR 0.23, 95% CI 0.18–0.31; P = 0.02; Fig. S1).
Incidence of PONV: Timing of dexamethasone administration
The RCTs included in this review varied with regard to timing of dexamethasone administration. Some patients received dexamethasone 90minutes before skin incision, while others received dexamethasone postoperatively. Wang et al [32] demonstrated that dexamethasone administered before anesthesia was more effective in decreasing early PONV compared to dexamethasone administered after anesthesia. These observations are in accordance with data showing that the onset time of dexamethasone on anti-emesis is approximately 2 hours. In the current study, subgroup analysis stratified by the timing of dexamethasone administration showed that dexamethasone was most effective in preventing PONV when administered before rather than after induction of anesthesia (P = 0.0002; Fig. S2).
3.5 Sensitivity analysis
To explore the effects of individual RCTs on the pooled OR estimates, we performed a sensitivity analysis omitting one study at a time. No single RCT significantly affected the overall results of the meta-analysis.
3.6 Publication bias
Visual inspection of a Funnel plot, Egger's test, and Begg's rank correlation test revealed no significant publication bias (Begg’s rank correlation test, continuity-corrected Pr>|z| >0.1), except for RCTs reporting on the need for rescue anti-emetic in patients receiving dexamethasone versus placebo, with or without concomitant antiemetics (Pr>|z| = 0.06), (Table S2).
3.7 Quality of evidence
Quality of available evidence from RCTs, which was downgraded by inconsistency (heterogeneity between studies), indirectness (variations in study setting), or publication bias, and upgraded by dose-response gradient, varied from moderate to high (Table 4).
Table 4. GRADE evidence.
| Outcomes | Illustrative comparativerisks* (95% CI) | Relativeeffect(95% CI) | No ofParticipants(studies) | Quality of theevidence(GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Placebo | Dexamethasone | ||||
| Dexamethasone versus placebo (in addition to other antiemetics): PONV | 440 per 1000 | 229 per 1000(189 to 277) | RR 0.52(0.43 to0.63) | 1860(17 studies) | ⊕⊕⊕⊕ high 1 |
| Dexamethasone versus placebo (in addition to other antiemetics): rescue antiemetics | 216 per 1000 | 91 per 1000(65 to 123) | RR 0.42(0.3 to0.57) | 1020(9 studies) | ⊕⊕⊕ high |
| Dexamethasone comparison of doses: PONV | 440 per 1000 | 229 per 1000 (189 to 277) | RR 0.52(0.43 to0.63) | 1860(17 studies) | ⊕⊕⊕⊕ high 2 |
| Dexamethasone versus placebo: VAS pain score | The meandexamethasoneversus placebo:vas pain scorein the interventiongroups was1.17 lower(1.91 to 0.44 lower) | 453(6 studies) | ⊕⊕⊕⊖ moderate 3 | ||
| Dexamethasone versus placebo (in addtion to other antiemetics): resuce analgesic | 256 per 1000 | 167 per 1000(128 to 213) | RR 0.65(0.5 to0.83) | 638(6 studies) | ⊕⊕⊕⊖ moderate 4 |
| Dexamethasone versus a different antiemetic: PONV | 256 per 1000 | 320 per 1000(220 to 463) | RR 1.25(0.86 to1.81) | 260(3 studies) | ⊕⊕⊕⊕ high |
*The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio.
GRADE Working Group grades of evidence. High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate.
Although the PONV results demonstrated significant heterogeneity (P = 0.003, I 2 = 56%), it was partly explained by the dose of dexamethasone. 2Downgraded by not comparing higher dose with lower dose directly, but upgraded by the dose-response gradient. 3Although there was significant heterogeneity (P<0.00001, I 2 = 94%), it was partly explained by the dose of dexamethasone. 4Publication bias as Pr>|z| = 0.06.
PONV: post-operative nausea and vomiting; VAS: visual analogue scales.
Patient or population: patients undergoing thyroidectomy. Settings: evidence from China, Japan, Korea, Italy, Switzerland, Norway, Portugal. Intervention: dexamethasone. Comparison: placebo.
Discussion
PONV is a common and distressing complication for patients undergoing thyroidectomy; therefore, prophylactic anti-emetics may be beneficial. An optimal anti-emetic regimen should be capable of decreasing the incidence of PONV without increasing the risk for adverse events. However, most of the currently used anti-emetics, including anti-histamines, butyrophenones, and dopamine receptor antagonists cause occasional undesirable adverse events, such as excessive sedation, hypotension, dry mouth, dysphoria, hallucinations, and extrapyramidal signs [33]. 5-HT3 antagonists are effective for preventing and treating PONV in patients undergoing various types of surgery [34]. However, the use of prophylactic anti-emetic therapy with 5-HT3 antagonists has been criticized for being too expensive [35].
Our meta-analysis of 13 RCTs demonstrated that prophylactic dexamethasone is effective in reducing the incidence of PONV, post-operative pain scores, and the need for rescue analgesia and anti-emetics compared to placebo administered with or without contaminant anti-emetics in patients undergoing thyroidectomy. In addition, our findings showed that dexamethasone is as effective as other anti-emetics for reducing PONV in this patient population. However, the benefits of administering dexamethasone as a more cost-effective anti-emetic and efficacious analgesic drug35 should be weighed against the potential side effects. Our study indicated that dexamethasone administration is associated with an increase in blood glucose during the immediate post-operative period, but with no other serious adverse events.
For optimal dose and timing of dexamethasone administration, subgroup analyses showed that higher doses of dexamethasone (8–10mg) are more effective than lower doses (1.5–5mg), and dexamethasone is most effective in preventing PONV when administered before rather than after induction of anesthesia.
In terms of populations eligible for treatment, the RCTs in the current study mostly included healthy patients, and excluded pregnant women, patients with insulin-dependent diabetes mellitus, those who were obese, and patients with a high risk for PONV. As such, the impact of prophylactic dexamethasone on outcomes in high-risk patients is not known. A larger sample size and well-performed RCTs including high risk patients are required for further investigations.
This review has several limitations. First, data reporting on the effects of prophylactic dexamethasone on post-operative pain scores and need for rescue analgesics in thyroidectomy patients were limited by substantial heterogeneity and publication bias, respectively. Second, the studies in this systematic review included patients across various age groups receiving dexamethasone according to very different protocols.
Conclusion
The present meta-analysis shows that prophylactic dexamethasone is safe and effective for reducing the incidence of PONV, post-operative pain, and the need for rescue analgesia and anti-emetics in thyroidectomy patients. Prophylactic dexamethasone 8–10mg administered before induction of anesthesia should be recommended for patients undergoing thyroidectomy except for those that are pregnant, have diabetes mellitus, hyperglycaemia or contraindications for corticosteroids. More high quality trials are warranted to define the benefits and risks of prophylactic dexamethasone in potential patients with high risk for PONV.
Supporting Information
Incidence of PONV stratified according to dexamethasone dose: 8–10 mg and 1.25–5 mg.
(TIF)
Incidence of PONV stratified by timing of dexamethasone administration.
(TIF)
Search strategy from its inception to October 1, 2013.
(DOC)
Begg’s rank correlation test for publication bias.
(DOC)
PRISMA 2009 Checklist.
(DOC)
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors have no support or funding to report.
References
- 1. Sonner JM, Hynson JM, Clark O, Katz JA (1997) Nausea and vomiting following thyroid and parathyroid surgery. J Clin Anesth 9(5): 398–402. [DOI] [PubMed] [Google Scholar]
- 2. Kranke P, Eberhart LH (2011) Possibilities and limitations in the pharmacological management of postoperative nausea and vomiting. Eur J Anaesthesiol 28: 758–765. [DOI] [PubMed] [Google Scholar]
- 3. Matory YL, Spiro RH (1993) Wound bleeding after head and neck surgery. J Surg Oncol 53(1): 17–19. [DOI] [PubMed] [Google Scholar]
- 4. Schwartz AE, Clark OH, Ituarte P, Lo Gerfo P (1998) Therapeutic controversy: thyroid surgery–the choice. J Clin Endocrinol Metab 83(4): 1097–1105. [DOI] [PubMed] [Google Scholar]
- 5. Fredrikson M, Hursti T, Fürst CJ, Steineck G, Börjeson S, et al. (1992) Nausea in cancer chemotherapy is inversely related to urinary cortisol excretion. Br J Cancer 65: 779–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Aapro MS, Plezia PM, Alberts DS, Graham V, Jones SE, et al. (1984) Double-blind crossover study of the antiemetic efficacy of high-dose dexamethasone versus high-dose metoclopramide. J Clin Oncol 2: 466–471. [DOI] [PubMed] [Google Scholar]
- 7. Sapolsky RM, Romero LM, Munck AU (2000) How do glucocorticoids influence stress responses? Integrating permissive, suppressive, stimulatory, and preparative actions. Endocr Rev 21: 55–89. [DOI] [PubMed] [Google Scholar]
- 8. Hargreaves KM, Costello A (1990) Glucocorticoids suppress levels of immunoreactive bradykinin in inflamed tissue as evaluated by microdialysis probes. Clin Pharmacol Ther 48: 168–178. [DOI] [PubMed] [Google Scholar]
- 9. Hong D, Byers MR, Oswald RJ (1993) Dexamethasone treatment reduces sensory neuropeptides and nerve sprouting reactions in injured teeth. Pain 55: 171–181. [DOI] [PubMed] [Google Scholar]
- 10. Karanicolas PJ1, Smith SE, Kanbur B, Davies E, Guyatt GH (2008) The Impact of Prophylactic Dexamethasone on Nausea and Vomiting After Laparoscopic Cholecystectomy A Systematic Review and Meta-Analysis. Ann Surg 248(5): 751–762. [DOI] [PubMed] [Google Scholar]
- 11. Chen CC, Siddiqui FJ, Chen TL, Chan ES, Tam KW (2012) Dexamethasone for prevention of postoperative nausea and vomiting in patients undergoing thyroidectomy: meta-analysis of randomized controlled trials. World J Surg 36: 61–68. [DOI] [PubMed] [Google Scholar]
- 12. Moher D, Liberati A, Tetzlaff J, Altman DG (2010) PRISMA Group (2010) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg 8: 336–341. [DOI] [PubMed] [Google Scholar]
- 13.Lefebvre C, Manheimer E, Glanville J (2011) Chapter 6: searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available: www.cochrane-handbook.org.
- 14.Higgins JPT, Altman DG, Sterne JAC (2011) Chapter 8: assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available: www.cochrane-handbook.org.
- 15. Begg CB, Mazumdar M (1994) Operating characteristics of a rank correlation test for publication bias. Biometrics 50(4): 1088–1101. [PubMed] [Google Scholar]
- 16. Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, et al. (2004) GRADE Working Group (2004) Grading quality of evidence and strength of recommendations. BMJ 328: 1490–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Guyatt G, Gutterman D, Baumann MH, Addrizzo-Harris D, Hylek EM, et al. (2006) Grading strength of recommendations and quality of evidence in clinical guidelines. Chest 129: 174–181. [DOI] [PubMed] [Google Scholar]
- 18. Wang JJ, Ho ST, Lee SC, Liu YC, Liu YH, et al. (1999) The prophylactic effect of dexamethasone on postoperative nausea and vomiting in women undergoing thyroidectomy: a comparison of droperidol with saline. Anesth Analg 89: 200–203. [DOI] [PubMed] [Google Scholar]
- 19. Wang JJ, Ho ST, Lee SC, Liu YC, Ho CM (2000) The use of dexamethasone for preventing postoperative nausea and vomiting in females undergoing thyroidectomy: a dose-ranging study. Anesth Analg 91: 1404–1407. [DOI] [PubMed] [Google Scholar]
- 20. Lee Y, Lin PC, Lai HY, Huang SJ, Lin YS, et al. (2001) Prevention of PONV with dexamethasone in female patients undergoing desflurane anesthesia for thyroidectomy. Acta Anaesthesiol Sin 39: 151–156. [PubMed] [Google Scholar]
- 21. Fujii Y, Nakayama M (2007) Efficacy of dexamethasone for reducing postoperative nausea and vomiting and analgesic requirements after thyroidectomy. Otolaryngol Head Neck Surg 136: 274–277. [DOI] [PubMed] [Google Scholar]
- 22. Worni M, Schudel HH, Seifert E, Inglin R, Hagemann M, et al. (2008) Randomized controlled trial on single dose steroid before thyroidectomy for benign disease to improve postoperative nausea, pain, and vocal function. Ann Surg 248: 1060–1066. [DOI] [PubMed] [Google Scholar]
- 23. Feroci F, Rettori M, Borrelli A, Lenzi E, Ottaviano A, et al. (2011) Dexamethasone prophylaxis before thyroidectomy to reduce postoperative nausea, pain, and vocal dysfunction: a randomized clinical controlled trial. Head Neck 2011 33: 840–846. [DOI] [PubMed] [Google Scholar]
- 24. Doksrød S, Sagen Ø, Nøstdahl T, Ræder J (2012) Dexamethasone does not reduce pain or analgesic consumption after thyroid surgery; a prospective, randomized trial. Acta Anaesthesiol Scand 56: 513–519. [DOI] [PubMed] [Google Scholar]
- 25. Song YK, Lee C (2013) Effects of ramosetron and dexamethasone on postoperative nausea, vomiting, pain, and shivering in female patients undergoing thyroid surgery. J Anesth 27: 29–34. [DOI] [PubMed] [Google Scholar]
- 26. Barros A, Vale CP, Oliveira FC, Ventura C, Assunção J, et al. (2013) Dexamethasone effect on postoperative pain and tramadol requirement after thyroidectomy. Pharmacology 91: 153–157. [DOI] [PubMed] [Google Scholar]
- 27. Schietroma M, Cecilia EM, Carlei F, Sista F, De Santis G, et al. (2013) Dexamethasone for the prevention of recurrent laryngeal nerve palsy and other complications after thyroid surgery: a randomized double-blind placebo-controlled trial. JAMA Otolaryngol Head Neck Surg 139: 471–478. [DOI] [PubMed] [Google Scholar]
- 28. Zhou H, Xu H, Zhang J, Wang W, Wang Y, et al. (2012) Combination of dexamethasone and tropisetron before thyroidectomy to alleviate postoperative nausea, vomiting, and pain: randomized controlled trial. World J Surg 36: 1217–1224. [DOI] [PubMed] [Google Scholar]
- 29. Bononi M, Amore Bonapasta S, Vari A, Scarpini M, De Cesare A, et al. (2010) Incidence and circumstances of cervical hematoma complicating thyroidectomy and its relationship to postoperative vomiting. Head Neck 32: 1173–1177. [DOI] [PubMed] [Google Scholar]
- 30. Fujii Y, Tanaka H, Kobayashi N (2000) Granisetron/dexamethasone combination for the prevention of postoperative nausea and vomiting after thyroidectomy. Anaesth Intensive Care 28: 266–269. [PubMed] [Google Scholar]
- 31. Hozo SP, Djulbegovic B, Hozo I (2005) Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 5: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wang JJ, Ho ST, Tzeng JI, Tang CS (2000) The effect of timing of dexamethasone administration on its efficacy as a prophylactic antiemetic for postoperative nausea and vomiting. Anesth Analg 91(1): 136–9. [DOI] [PubMed] [Google Scholar]
- 33. Watcha MF, White PF (1992) Postoperative nausea and vomiting: its etiology, treatment, and prevention. Anesthesiology 77: 162–84. [DOI] [PubMed] [Google Scholar]
- 34. Kovac AL (2000) Prevention and treatment of postoperative nausea and vomiting. Drugs 59: 213–43. [DOI] [PubMed] [Google Scholar]
- 35. White PF, Watcha MF (1993) Are new drugs cost-effective for patients undergoing ambulatory surgery? Anesthesiology 78(1): 2–5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Incidence of PONV stratified according to dexamethasone dose: 8–10 mg and 1.25–5 mg.
(TIF)
Incidence of PONV stratified by timing of dexamethasone administration.
(TIF)
Search strategy from its inception to October 1, 2013.
(DOC)
Begg’s rank correlation test for publication bias.
(DOC)
PRISMA 2009 Checklist.
(DOC)
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.