Abstract
We introduce a whole-mount immunohistochemistry method for analyzing intricate vascular network formation in mouse embryonic tissues. Laser scanning confocal microscopy with multiple labeling allows for robust imaging of blood and lymphatic vessel branching morphogenesis with excellent resolution.
Keywords: Confocal microscopy, Whole-mount immunohistochemistry, Mouse embryo, Blood vessel, Lymphatic vessel, Fluorescence, Antibody, Patterning
1. Introduction
The circulatory system is crucial for organ development during embryogenesis, as well as for organ maintenance and reproductive function in the adult. The specific pattern of blood vessel branching in each organ is achieved by a complex process, termed angiogenesis, in which a preexisting primitive capillary plexus is reorganized into a hierarchically branched vascular network. Emerging evidence in mouse genetics demonstrates that a variety of intercellular signaling systems are implicated in patterning the vascular network.
Whole-mount immunohistochemical analysis for imaging the entire vasculature is pivotal for understanding the cellular mechanisms of branching morphogenesis. The confocal micro-scope is a powerful tool to visualize intact blood vessels as well as their cellular components including endothelial cells, pericytes, and smooth muscle cells, using specific fluorescent markers. This chapter describes a simple and robust protocol to stain intact blood vessels with vascular-specific antibodies and fluorescent secondary antibodies, which is applicable for vascularized embryonic organs where we are able to follow the process of vascular development. We will provide examples from embryonic limb skin (1) and heart (manuscript in preparation) for whole-mount multiple imuuno-fluorescence confocal microscopy (see Note 1).
2 Materials
2.1. Tissues
E13.5~E17.5 mouse embryos for harvesting limb skin and heart specimen
2.1.1. Antibodies for Vascular Markers
see Table 1.
Table 1.
Summary of vascular-specific antibodies for whole-mount staining of mouse tissues
| Antibody | Species | Company | Working condition |
|---|---|---|---|
| Pan-endothelial cell marker | |||
| PECAM-1 | Armenian hamster (M) | Chemicon (MAB1398Z) | 1:100 dilutiona |
| PECAM-1 | Rat (M) | BD Pharmingen (553369) | 1:300 dilution |
| VEGFR2 | Rat (M) | eBioscience (14-5821-82) | 1:200 dilution |
| CD34 | Rat (M) | eBioscience (13-0341) | 1:300 dilution |
| Collagen IV | Rabbk (P) | AbD Serotec (2150-1470) | 1:300 dilutionb |
| Arterial endothelial cell marker | |||
| Neuropilin-l | Rabbit (P) | The Alex Kolodkin labc | 1:3,000 dilution |
| Unc5H2 | Goat (P) | R&D (AF1006) | 1:200 dilution |
| Venous endothelial cell marker | |||
| EphB4 | Goat (P) | R&D (AF446) | 1:100 dilution |
| Lymphatic endothelial cell marker | |||
| LYVE-ld | Rabbit (P) | Abcam (abl4917) | 1:200 dilution |
| LYVE-1d | Rat (M) | MBL (D225-3) | 1:300 dilution |
| Prox-1 | Rabbit (P) | Chemicon (AB5475) | 1:1,000 dilution |
| Prox-1 | Goat (P) | R&D (AF2727) | 1:50 dilution |
| Neuropilin-2 | Rabbit (P) | Cell signaling (3366) | 1:100 dilution |
| Podoplanin | Syrian hamster (M) | Hybridoma bank (8.1.1) | 1:200 dilution |
| Smooth muscle cell/pericyte marker | |||
| αSMA-Cy3 | Mouse (M)e | Sigma (c-6198) | 1:500 dilutionf |
| NG2 | Rabbit (P) | Chemicon (AB5320) | 1:200 dilution |
| SM22α | Rabbit (P) | Abcam (abl4106) | 1:200 dilution |
P polyclonal antibody; M monoclonal antibody
Goat anti-Armenian hamster-Cy3 (Jackson ImmunoResearch 127-165-160) antibody should be used as a secondary antibody
The collagen IV antibody can be used to detect blood vessels after in situ hybridization
The neuropilin-1 antibody was kindly provided by the Alex Kolodkin lab in the Johns Hopkins University
The LYVE-1 antibodies also detect a subset of macrophages in the embryonic skin and heart
The anti-αSMA antibody is mouse IgG2a monoclonal antibody
The Cy3-donjugated αSMA antibody is incubated for 1 h at room temperature together with secondary antibodies for other primary antibodies
2.2.2. Antibodies for Reporter Genes (GFP and lacZ)
see Table 2.
Table 2.
Summary of antibodies for reporter genes (GFP and lacZ)
| Antibody | Species | Company | Working condition |
|---|---|---|---|
| GFP reporter | |||
| GFP | Rabbit (P) | Invitrogen (A11122) | 1:300 dilution |
| GFP | Rat(M) | Nacalai tesque (04404-84) | 1:1,000 dilution |
| GFP | Chick (P) | Chemicon (P42212) | 1:300 dilution |
| LacZ reporter | |||
| β-gal | Rabbit (P) | MP biomedical (55976) | 1:5,000 dilution |
| β-gal | Goat (P) | AbD Serotec (4600-1409) | 1:500 dilution |
| β-gal | Chick (P) | Abcam (ab9361) | 1:200 dilution |
P polyclonal antibody; M monoclonal antibody
2.2. Solutions
70% Ethanol (EtOH) in Water.
Hanks’ Balanced Salt Solution (HBSS).
Phosphate Buffer Saline (PBS). 4. Triton X-100 (TX100).
Triton X-100 (TX100).
Goat Serum (heat inactivation in 56°C for 30 min).
Donkey Serum.
4% (w/v) Paraformaldehyde in PBS (PFA) (freshly prepare from 16% stock).
PBT (PBS + 0.2%TX100): add 0.2 mL of Triton X-100 to 100 mL of PBS.
75%, 50%, 25%Methanol (MeOH)/PBT.
Blocking buffer for goat secondary antibodies: 10%HIGS (Heat Inactivated Goat Serum)/PBS + 0.2%TX100 (keep at 4°C for 2 weeks).
Washing buffer for goat secondary antibodies: 2%HIGS/PBS + 0.2%TX100 (keep at 4°C for 2 weeks).
Blocking buffer for donkey secondary antibodies: 10%DS (Donkey Serum)/PBS + 0.2% TX100 (keep at 4°C for 2 weeks).
Washing buffer for donkey secondary antibodies: 2%DS/ PBS + 0.2%TX100 (keep at 4°C for 2 weeks).
Nuclear counterstaining solution: To-Pro-3 1:3,000 dilution.
2.3. Dissection Tools
Microdissecting forceps.
Microdissecting scissors.
Microdissecting fine tweezers (Inox #5).
Ring forceps.
2.4. Supplies for Dissection and Staining
Bench paper.
Paper towel.
100 × 15-mm Petri dish.
35 × 10-mm Petri dish.
24-well plate cell culture plate.
5-mL Polypropylene round-bottom tube.
2-mL Conical screw-cap microcentrifuge tube.
0.22-mm PVDF membrane syringe filters.
2.5. Supplies for Mounting
Anti-fade mounting media (Prolong Gold, Invitrogen).
Spacer for mounting (Secure-Seal™ spacer, 9 mm diameter, 0.12 mm deep, Invitrogen).
Microscopic slides with adhesive coating (Matsunami MAS-GP, EverMark Select EMS200W+).
Cover glass 25 × 25 mm.
Kimwipe.
2.6. Equipment
Dissecting stereomicroscope.
Fiber optic illumination system for reflected light.
Gentle shaker.
3. Methods
3.1. Collecting Specimen
Euthanize plugged females by approved procedure.
Lay the euthanized animal on an absorbent paper towel and soak it thoroughly in 70% EtOH/H2O from a squeeze bottle.
Dissect the uterus intact and place it in a 100 × 15-mm Petri dish containing ice-cold HBSS to wash out blood.
Separate and dissect the embryo. Remove the very thin amnion from the embryo.
(Option) Dissect a single embryo in a 35 × 10-mm Petri dish if each embryo needs to be genotyped.
Cut off the forelimbs of embryo and dissect the heart from the embryo under the microscope.
Transfer these tissues (forelimbs and hearts) by a ring forceps into 24-well plate containing 2 mL of ice-cold fresh 4%PFA in PBS.
Fix the tissues with gentle mixing on the Nutator Mixer at 4°C overnight.
On the following day, remove the PFA and wash the tissues three times for 5 min in 2 mL of PBS with gentle mixing on the Nutator Mixer at room temperature.
Stock the tissues in 100% MeOH at −20°C enzyme freezer (the freezer with critical temperature control and without automatic defrost function) (see Note 2). Primary antibodies listed in Tables work after the 100% MeOH treatment.
(Forelimb skin) Peel off skin from the forelimb using fine tweezers in 100% MeOH (see the details in Fig. 1).
Fig. 1.
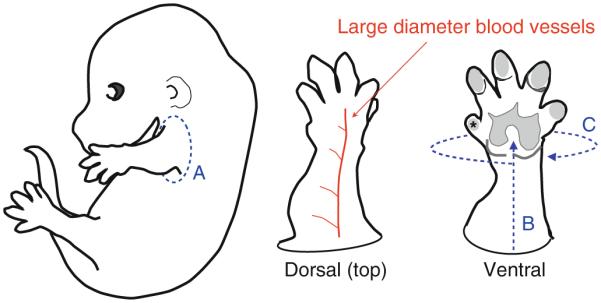
Limbs can be dissected postmethanol dehydration in a small dish containing methanol, respectively. Carefully pull apart skin at the base of the limb with tweezers, following the blue dashed line (a). Snip through muscle and bone completely. Large diameter vessels may be visible on the dorsal surface of the limb as depicted. The dissection of limb skin must be performed on MeOH-dehydrated limbs in a dish containing 100% methanol. Limb skin separates easily from the limb skin when dehydrated in this manner. To avoid dissecting through the branched vessel plexus on the dorsal surface of the limb, invert the limb so that the ventral side faces up. Using fine tweezers, dissect through skin near the base of the limb toward the paw in a straight line (b). Next dissect around the entire limb, peeling the limb skin off gently as you turn the limb (c). Note: dissect in opposite directions for the right and left limbs, and toward the digit labeled with an asterisk in the diagram.
3.2. Staining of Whole-Mount Tissues
Rehydrate the tissues in 5-mL polypropylene round-bottom tube with graded series of MeOH/PBT (75, 50, and 25%) for 5 min each and then wash twice for 5 min in PBT with gentle mixing on the Nutator Mixer at room temperature.
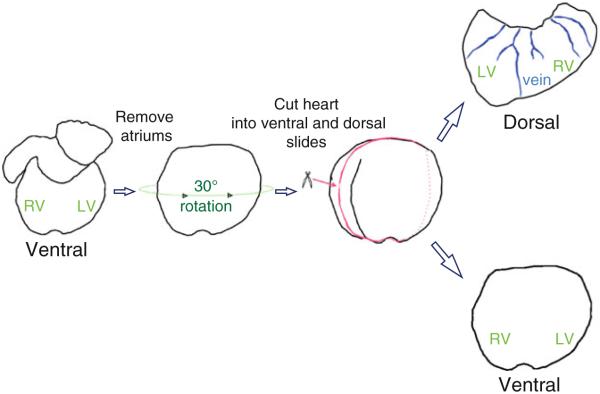
(Heart) Cut the heart to divide dorsal and ventral potions (see the details in Fig. 2).
Block the tissues with either 10%HIGS/PBS + 0.2%TX100 for goat secondary antibodies or 10%DS/PBS + 0.2%TX100 for donkey secondary antibodies for 2 h with gentle mixing on the Nutator Mixer at room temperature.
Place the tissues on a 35 × 10-mm Petri dish and transfer by a ring forceps into 2-mL microcentrifuge tube with 800 mL of primary antibodies (appropriate dilution as listed in Tables) in the blocking buffer (either 10%HIGS/PBS + 0.2%TX100 or 10%DS/PBS + 0.2%TX100) (see Note 3).
Incubate the tissues with gentle mixing on the Nutator Mixer at 4°C overnight.
Place the tissues on a 35 × 10-mm Petri dish and transfer by a ring forceps into 5-mL polypropylene round-bottom tube with 4 mL of the washing buffer (either 2%HIGS/PBS + 0.2%TX100 or 2%DS/PBS + 0.2%TX100). Wash five times for 15 min with gentle mixing on the Nutator Mixer at room temperature.
Place the tissues on a 35 × 10-mm Petri dish and transfer by a ring forceps into 2-mL microcentrifuge tube with 800 μL of secondary antibodies in the blocking buffer (either 10%HIGS/ PBS + 0.2%TX100 or 10%DS/PBS + 0.2%TX100). Filter the secondary antibody solution using 0.22-μm PVDF membrane syringe filters to remove aggregated particles of the secondary antibodies (see Note 3).
Incubate the tissues in the dark or wrapped with aluminum foil for 1 h with gentle mixing on the Nutator Mixer at room temperature.
Place the tissues on a 35 × 10-mm Petri dish and transfer by a ring forceps into 5-mL polypropylene round-bottom tube with 4 mL of the washing buffer (either 2%HIGS/PBS + 0.2%TX100 or 2%DS/PBS + 0.2%TX100). Wash five times for 15 min in the dark or wrapped with aluminum foil with gentle mixing on the Nutator Mixer at room temperature.
(Option for counterstaining against nucleus) Incubate the tissues with 4 mL of the washing buffer (either 2%HIGS/ PBS + 0.2%TX100 or 2%DS/PBS + 0.2%TX100) with To-Pro-3 in the dark or wrapped with aluminum foil for 10 min with gentle mixing on the Nutator Mixer at room temperature (see Note 4). Then, wash three times for 5 min with 4 mL of the washing buffer (either 2%HIGS/PBS + 0.2%TX100 or 2%DS/PBS + 0.2%TX100) in the dark or wrapped by aluminum foil with gentle mixing on the Nutator Mixer at room temperature.
Fig. 2.
The ventral side of heart is facing the upward and dorsal side facing the downward. The heart is oriented this way to minimize the damage on the dorsal surface of heart under the stereomicroscope with low illumination to avoid extensive bleaching. The atriums are removed carefully from the heart. Rotate the heart about 30° and use microdissecting scissors to divide the heart into the ventral and dorsal side.
3.3. Mounting Limb Skin on Slide
Place the limb skins on a 35 × 10-mm Petri dish. Remove dusts, crystals, and fibers from the inner layer of the skins using fine tweezers under the stereomicroscope with low illumination to avoid extensive photo bleaching.
Transfer the limb skins to adhesive microscopic slide by a ring forceps. Place the skins with the inner layer lying upward on the slide (i.e., towards coverslip). Flatten the skins carefully using fine tweezers and remove carry-over washing buffer by Kimwipe.
Mount in anti-fade mounting media without air bubbles.
Cure on a flat surface in the dark (e.g., the samples mounted using ProLong Gold reagent are placed overnight in the dark at room temperature before viewing). For long-term storage, seal the coverslip to the slide and store at 4°C.
3.4. Mounting Heart on Slide
Place the hearts on a 35 × 10-mm Petri dish. Clean up the hearts using fine tweezers under the stereomicroscope with low illumination to avoid extensive bleaching.
Place 1 ~ 2 layers of Secure-Seal™ spacer (9 mm diameter, 0.12 mm deep) on adhesive microscopic slide.
Transfer the hearts to the slide by a ring forceps. Place the hearts with the outer surface lying upward on the slide (i.e., towards coverslip) to image coronary vasculature. Remove carry-over washing buffer by Kimwipe.
Mount in anti-fade mounting media without dirt and air bubbles.
Cure on a flat surface in the dark (e.g., The samples mounted using ProLong Gold reagent are placed for overnight in the dark at room temperature before viewing). For long-term storage, seal the coverslip to the slide and store at 4°C.
3.5. Confocal Microscopy
Set up appropriate lasers for fluorophores. We use Leica TCS SP5 confocal microscope with three laser sources including Argon 488 nm (for Alexa Fluor 488 and GFP), DPSS 561 nm (for Alexa Fluor 568 and Cy3), and HeNe 633 nm (for Alexa Fluor 633, Cy5 and To-Pro-3).
Use sequential scan tool to avoid or reduce cross talk in which all dyes in double or triple-stained samples will be excited at the same time. In the sequential scan mode, images will be recorded in a sequential order.
More general information about fluorescent dyes and lasers for excitation may be founded in “Confocal Microscopy for Biologists” by Hibbs (2004) (2).
Fig. 3.
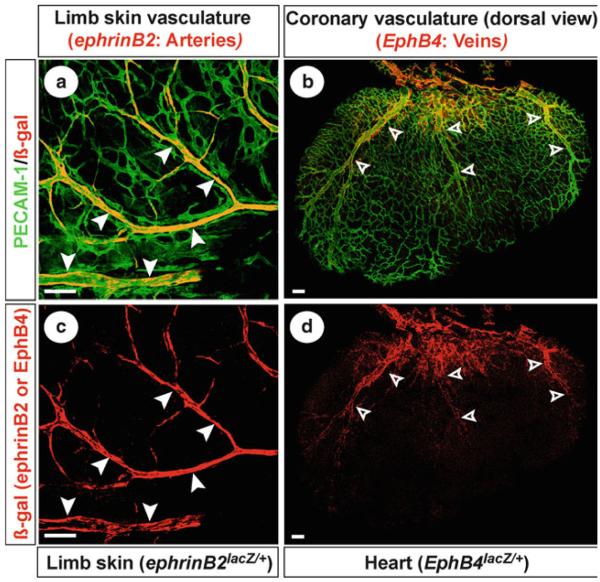
(a–b) Double-labeled confocal microscopy with anti-β-gal (red ) and PECAM-1 (green) antibodies revealed that ephrinB2+ arterial branching (white arrowheads) is seen in the limb skin of ephrinB2 lacZ /+ embryos at E15.5. The images were taken by 20× objective lens with confocal Z-series. Scale bar, 100 μm. (c–d) Double-labeling showed larger diameter EphB4+ coronary veins (open arrowheads) on the dorsal cardiac surface of EphB4 lacZ /+ embryos at E15.5. The images were taken by 20× objective lens with confocal Z-series and tiling. Scale bar, 100 μm.
Acknowledgments
Work supported by Intramural Research Program of National Institutes of Health. We thank Dr. Wenling Li for critical comments on this manuscript. We are also grateful to Heesuk Zang, John Hatch, and Izumi Onitsuka for their contributions to this whole-mount immunohistochemistry method.
Footnotes
1. General comment.
Whole-mount confocal microscopy with multiple labeling by vascular markers permitted us to image blood and lymphatic endothelial cells and their neighbors including smooth muscle cells and pericytes in the tissues. In addition to the vascular marker antibodies, antibodies for reporter gene products (β-galactosidase, β-gal, and green fluorescent protein, GFP, Table 2) that recapitulate the expression pattern of endogenous genes of your interest can be used. Figure 3 shows forelimb skin and heart from embryos carrying lacZ reporter targeted to the ephrinB2 (3) or EphB4 locus (4), which provides a histochemical indicator of ephrinB2 or EphB4. EphrinB2, a transmembrane ligand, is expressed by arteries but not veins, whereas its receptor, the tyrosine kinase EphB4, is preferentially expressed by veins (3). Whole-mount double-label confocal immnofluorescence microscopy with antibodies to β-galactosidase (Fig. 3 red) and the pan-endothelial marker PECAM-1 (Fig. 3 green) revealed a characteristic branching pattern of ephrinB2+ arteries in the limb skin vasculature (Fig. 3a, b) and a stereotypic pattern of EphB4+ veins in the coronary vasculature (Fig. 3c, d).
The study of different stages of these vascular systems reveals the cellular dynamics of angiogenesis including vascular branching, arterial/venous differentiation, lymphatic vessel development, and smooth muscle/pericyte coverage in the developing limb skin (1) and heart (manuscript in preparation).
2. Tissue storage in 100% methanol.
Limb skin separates easily from the limb when dehydrated. After overnight storage at −20°C, limb skin can be dissected out. According to our staining experiences, heart without MeOH-dehydration gives you a better immunostaining.
3. Primary and secondary antibodies combination.
Multiple primary antibodies derived from different species (e.g., rat monoclonal antibody + rabbit polyclonal antibody) can be used simultaneously. Different fluorescent-conjugated secondary antibodies derived from different species can be also used simultaneously.
4. To-pro-3 for nuclear counterstaining.
For a nuclear counterstaining, DAPI (4¢, 6-Diamidino-2-phenylindole) is widely used because of its high specificity for nuclear DNA. DAPI shows a blue fluorescence under ultra-violet (UV 364 nm); however, it is not suitable for a regular confocal microscope equipped with Argon-laser (488 nm excitation), DPSS-laser (561 nm excitation), and HeNe-laser (633 nm excitation) illumination system. The optimal counterstaining with DAPI needs a UV-laser. To-pro-3 is an alternative to DAPI, which provides strong and specific staining for nuclei in a specific emission (HeNe 633 nm excitation).
References
- 1.Mukouyama YS, Shin D, Britsch S, Taniguchi M, Anderson DJ. Sensory nerves determine the pattern of arterial differentiation and blood vessel branching in the skin. Cell. 2002;109:693–705. doi: 10.1016/s0092-8674(02)00757-2. [DOI] [PubMed] [Google Scholar]
- 2.Hibbs AR. Confocal Microscopy for Biologist. Springer; 2004. [Google Scholar]
- 3.Wang HU, Chen ZF, Anderson DJ. Molecular distinction and angiogenic interaction between embryonic arteries and veins revealed by ephrin-B2 and its receptor Eph-B4. Cell. 1998;93:741–53. doi: 10.1016/s0092-8674(00)81436-1. [DOI] [PubMed] [Google Scholar]
- 4.Gerety SS, Wang HU, Chen ZF, Anderson DJ. Symmetrical mutant phenotypes of the receptor EphB4 and its specific transmembrane ligand ephrin-B2 in cardiovascular development. Mol Cell. 1999;4:403–14. doi: 10.1016/s1097-2765(00)80342-1. [DOI] [PubMed] [Google Scholar]