Abstract
OBJECTIVE
To examine the history of superovulation for ovulation induction, its contributions to reproductive medicine and its impact on multiple births.
DESIGN
A search of the relevant literature using Pubmed and other online tools.
RESULT(S)
Infertility has been a condition known and studied for thousands of years. However, it was not until this past century that effective treatments were developed. With the advancement of our knowledge of the hypothalamic-pituitary axis, therapies utilizing gonadotropins were developed to stimulate ovulation. Not only were we now able to treat anovulatory infertility, but also induce superovulation for in vitro fertilization. With these successes came consequences, including increased multiple pregnancies. Several countries recognized the high costs associated with multiple births and implemented regulations on the infertility industry. The rate of triplet and higher-order multiples has declined over the past decade. This is largely attributed to a decreased number of embryos transferred. Nonetheless, the twin rate has remained consistently high.
CONCLUSION(S)
Superovulation has become a routine medical therapy used for ovulation induction and in vitro fertilization. With the development of this technology have come effective therapies for infertility and new ethical and medical challenges. Since the advent of gonadotropin therapy we have already developed technologies to improve monitoring and decrease hyperstimulation and high order multiple pregnancies. In the future, we anticipate new tools devised to optimize one embryo for one singleton live birth.
Keywords: Gonadotropin, Ovarian Stimulation, Ovulation Induction, History, Multiple Births
Introduction: Infertility throughout the millennia
The affliction of infertility has been well known since ancient times. In the first known gynecologic medical text, the Kahoun papyrus (2200-1950 B.C.), the ancient Egyptian’s describe infertility as a disruption in the continuity between the reproductive organs and the digestive tract. Diagnosis was based on physical examination, focusing on symptoms such as flatulence and vomiting, but no treatment was described. Hippocrates adopted these teachings and in his treatises he described several causes of infertility and potential therapies. He prescribed “[when] a cervix is closed too tightly the inner orifice must be opened using a special mixture of red nitre, cumin, resin and honey…[or] it could be dilated by inserting a hollow leaden probe into the uterus enabling emollient substances to be poured in”(1). Hippocrates was prolific in his writings, publishing over 60 treatises, which were studied throughout ancient to medieval times.
The Renaissance marked a period of undeniable scientific and medical progress. Vesale published an anatomic atlas, Humani Corporis Fabrica in 1543, in which he included anatomic cross sections of female genital organs. In 1672 De Graaf wrote De Mullerium Organis in which he described the ovary and follicular function, although he mistakenly interpreted the follicle to be the egg. Martin Naboth, in 1707, published De Sterilitate, in which he claimed that ovarian sclerosis and tubal blockage caused infertility. In 1769, Morgani added follicular absence or agenesis as a cause for infertility. While a greater understanding of the etiology of infertility was gained during this time, the Renaissance physician continued to struggle to find an effective treatment to offer their patients.
The 19th and 20th century was marked with numerous advances which now define what is known as modern medicine today. Reproductive endocrinology was born with the discovery of gonadotropins and their regulation of the menstrual cycle. In 1927, Ascheim and Zondek isolated human chorionic gonadotropin (hCG) from the urine of pregnant women. Soon thereafter in 1930, Cole and Hart discovered equine chorionic gonadotropin, which became known as pregnant mare serum gonadotropin (PMSG)(2). From these discoveries, the technique of superovulation was born. Methods were developed to superovulate immature mice using PMSG followed by hCG to induce estrus and ovulation. Foreshadowing the ethical and medical complications of ovarian stimulation, Edwards and Fowler described an increase in liter size and fetal mortality during superovulation of mice(3). First described in the late 1920s to early 1930s, it took 40 years for the technique to be described as a treatment of anovulation in humans.
Isolation and purification of gonadotropins
It was during the 20th century that medical research advanced our understanding of the pituitary and the role of gonadotropins in the control of ovulation and reproduction. The species specificity of gonadotropins delayed the use of these hormones for ovulation induction in humans. Early studies looking at PMSG in women was complicated by the development of antibodies to the gonadotropins, resulting in treatment failure, and some patients developed cross-reacting antibodies with their own gonadotropins.
The development of methods for the isolation and purification of gonadotropins of human origin in quantities sufficient to treat patients was a milestone in the history of ovulation induction and the treatment of ovulatory dysfunction. Although it was recognized that follicle stimulating hormone (FSH) and luteinizing hormone (LH) could be found in the urine of menopausal women, initially the amount of urine required was too great and the extraction method too complex for urine to be a valid source of human gonadotropin. Therefore, attention was turned to deriving gonadotropins from the pituitary gland.
In 1958, Gemzell was the first to report induction of ovulation with pituitary derived gonadotropins (4). Buxton and his colleagues at Yale University confirmed Gemzell’s work in the United States in 1960 (5). In their work, induction of ovulation was accomplished using pituitary extracts, which had primarily FSH activity, and hCG as a substitute for LH. Although successful in stimulating follicular development, the technique was fraught with problems. First off, the pituitary glands from 10 individuals were needed to yield sufficient quantities of gonadotopin to stimulate one patient for one cycle. This spurred the United States Public Health Service and the Veterans Administration to recruit a large number of hospitals for the extraction and purification of the pituitary tissue. Secondly, there were problems with purification and standardization of the dose from one batch to the next. In addition, while unknown at the time, some of these women were infected with Jacob Creutzfeld disease and died from this condition several years after treatment.
In 1964 Donini reported the extraction of human gonadotropins (HMG) from human menopausal urine in sufficient quantities; and later introduced the use of HMG for ovarian stimulation as a treatment for amenorrhea (6). In these studies, he identified forms of hyperstimulation and described the untoward side effect of multiple pregnancies as a result of the use of HMG and hCG for ovulation induction. While the development of the technique to extract urinary derived gonadotropins provided a safer and more easily available source of FSH and LH, the purity of the product was still low. The high levels of co-purified contaminant proteins resulted in hypersensitivity and injection site reactions. In addition, similar to purified pituitary, there were problems with the standardization of the dose from one batch to the next.
The desire to develop a monotherapy with FSH alone was driven by the hopes to provide a more consistent and better-defined dose. This led to the development of urinary FSH with minimal LH activity by Serono, marketed under the named Metrodin. This formulation was used successfully in patients with PCOS (7) and later for superovulation in IVF (8). While reproductive endocrinologists continued to isolate, purify, and study the regulation and function of hormones, the field of molecular and genetic science was exploding. In 1953 Watson and Crick published their classic paper that first described the double helical structure of DNA(9). The central dogma of molecular biology, a gene is transcribed to mRNA and mRNA is translated to protein, was proposed by Crick in 1958(10). These two monumental theories ushered in the molecular genetic age. In 1972, the first complete gene sequence was obtained and published by Walter Fiers(11). During this time, Shome and Parlow proposed the amino acid sequence of FSH in 1974(12). This was soon followed by the cloning and DNA sequencing of the common alpha subunit (13) then the hormone specific B subunit (14). These early studies led the way to the expression of human FSH by Chinese hamster ovary cells, providing a source of bioactive FSH not only for experimental use but also for clinical (15).
The first pregnancy using recombinant human FSH was reported in 1992 during ovarian stimulation for in vitro fertilization (16,17). Quickly following, a 27 year old woman who had been infertile due to chronic clomiphene-resistant anovulation secondary to PCOS, was reported to be pregnant following controlled ovarian stimulation with recombinant FSH (18). Subsequent studies showed that low-dose administration of FSH allowed safe stimulation of patient’s with polycystic ovarian syndrome (19). The development of recombinant FSH provided great advantages to the urine derived HMG available at that time. Not only did it not require the donation of millions of gallons of urine a year for its synthesis, but it provided a highly purified product free from contaminating proteins, and it allowed for a reliable standardization of dose.
It was not until the development of immunoaffinity purification and high performance liquid chromatography that urinary derived gonadotropins could be obtained with low levels (<1%) of co-purified non-gonadotropin proteins. This finally allowed for a reliable standard dose of combined human gonadotropins. Currently there are multiple gonadotropin formulations available for ovulation induction, recombinant FSH and LH in addition to combined gonadotropins purified from urine. This allows the physician great flexibility in determining individualized stimulation protocols.
Gonadotropins and in vitro fertilization
The groundwork for in vitro fertilization (IVF) was first laid back in 1890 when Heap isolated and briefly cultured embryos from an Angora female rabbit, transferred the embryos into the reproductive tract of a Belgian hare recipient, and obtained offspring from the transfer (20). Subsequently, multiple scientists studying the process of reproduction stimulated ovulation, retrieved oocytes, and fertilized them in vitro in animal models (21,22). During this time, similar work was being conducted in humans. In 1944, Harvard physician John Rock published the first report claiming successful in vitro fertilization and cleavage of a human oocyte (23). In 1955, Shettles repeated Rocks work and reported successful culture to the morula stage in vitro of a human embryo(24). Shettles accomplishment in supporting embryo development is likely due to his use of co-culture of the fresh human oocyte with portions of human tubal mucosa.
While in vitro maturation of animal oocytes became a technique used during in vitro fertilization in the laboratory, difficulty was encountered when attempted in humans. In 1965, Robert Edwards obtained a fellowship to work with Georgeanna and Howard Jones at Johns Hopkins Hospital on in vitro maturation and fertilization of human oocytes. Unsuccessful, he turned his attention to the retrieval of mature oocytes in vivo. Upon his return to England he forged a collaboration with Patrick Steptoe, a pioneer of laparoscopy. Initially, they did not use gonadotropins to stimulate ovulation (natural cycle) during the IVF cycle. However, thinking that the more oocytes retrieved, the greater the chance of achieving a pregnancy, they switched to using HMG during the follicular phase to stimulate the growth of several mature follicles. Ovulation was triggered with hCG followed by laparoscopic retrieval of the mature oocytes. Embryo transfer began in the early 1970s. In 1976, they achieved their first pregnancy however it was an ectopic pregnancy (25). By this time they had performed well over 100 stimulated cycles, and were able to transfer embryos into approximately 70 women without success (26). Concerned that their failure was due to the hormonal stimulation and resulting luteal phase defect, they returned to natural cycle IVF. Two years later they announced the delivery of the first IVF baby, Louise Brown(27).
The year 1978 was also notable, as that was the year that Georgeanna and Howard Jones left Johns Hopkins and moved to Norfolk to start a clinical program of IVF. Due to the success encountered by Edwards and Steptoe, they initially decided to use natural cycle IVF. During the first year, laparoscopic retrieval of oocytes was attempted in over 40 patients but only 19 mature eggs were obtained, and no pregnancies occurred(26). In 1981 they made the decision to use hMG and after 13 attempted cycles, Elizabeth Carr was conceived and born(28). During the year of 1981, there were 55 laparoscopies, 31 transfers and 7 pregnancies which all carried to term. In an article written by Howard Jones looking back upon this time in history he noted “these initial results pointed clearly to the advantages of COH for IVF” (26). Following the Jones success and the initial reports of multiple follicular development for IVF, multiple stimulation regimens have been described, including clomiphene alone, hMG, clomiphene plus hMG, FSH, pulsatile gonadotropin releasing hormone, FSH plus hMG, just to name a few. The use of agonists and antagonists of GnRH have improved the pregnancy rate by preventing natural ovulation. The disadvantages of using these medications to induce multifollicular development for IVF and ovulation induction include multiple pregnancy and hyperstimulation syndrome.
Oral agents to induce increased endogenous gonadotropins
As Gemzell looked at pituitary derived gonadotropins and Donini worked at isolating urinary gonadotropins for ovulation induction, clomiphene arose unexpectedly into the clinical realm. Clomiphene was first synthesized in 1956 and was found to be both an estrogen receptor agonist and antagonist. Early rat studies predicted a suppressive function of clomiphene on the pituitary and an anti-fertility effect. Therefore, the initial studies looked at clomiphene as a potential oral contraceptive. To the surprise of the clinical researchers, early studies in humans demonstrated that ovulation was stimulated and not suppressed (29). Subsequently, the use of clomiphene to induce ovulation in women with ovulatory failure was reported and the first pregnancy appeared in the literature in 1963 (30). The convenience and effectiveness of this oral medication quickly made it a popular treatment option for infertile women.
Since the FDA approved clomiphene in 1967 for ovulation induction, no other oral medication has gained approval. Two alternative medications, tamoxifen and letrozole, have been studied and may be as effective as clomiphene and may also provide additional benefits (See McClamrock et al in this Views and Reviews section)”.
Tamoxifen is a selective estrogen receptor modulator used as an adjuvant therapy in the treatment of breast cancer. Similar to clomiphene, tamoxifen acts primarily by binding with estrogen receptors at the hypothalamus, resulting in a perceived drop in endogenous estrogen levels, and leading to an increase of endogenous gonadotropin secretion. Unlike clomiphene, tamoxifen acts as an estrogen agonist on the receptors in the vagina and endometrium. In 1973, Williamson and Ellis reported induction of ovulation with tamoxifen (31). Nearly 10 years later, Messinis and Nillius found that tamoxifen was just as effective as clomiphene in inducing ovulation in women with anovulation (32). Further studies suggest that tamoxifen may be an alternative to clomiphene, especially in circumstances when women fail to ovulate on clomiphene or in cases of fertility preservation for young women undergoing breast cancer therapy.
Letrozole is an aromatase inhibitor approved for use in postmenopausal women as adjuvant breast cancer therapy. Inhibition of aromatase results in lower levels of circulating estrogen and decreased negative feedback on the hypothalamus and pituitary, therefore increasing endogenous gonadotropin secretion. Similar to tamoxifen, letrozole was found to be as effective as clomiphene for ovulation induction. In addition, letrozole may be effective in inducing ovulation in women resistant to clomiphene and result in a lower multiple gestation rate(33).
Complications of superovulation: hyperstimulation and multifetal pregnancies
On January 26th, 2009 Nadya Suleman gave birth to octuplets, increasing scrutiny on the infertility field and the lack of legal regulation. The popular press was a beat with stories concerning the treatments Ms. Suleman received. News articles questioned the risks of assisted reproduction, citing the high rate of multiple pregnancies and the hazards to both mother and child they pose.
This was not the first time that the impacts of high order multiple pregnancies as a result of infertility treatment came into question. Soon after gonadotropin therapy was discovered and used to treat anovulatory infertility, reports appeared in the literature and popular press describing the premature delivery of two sets of quintuplets (34).
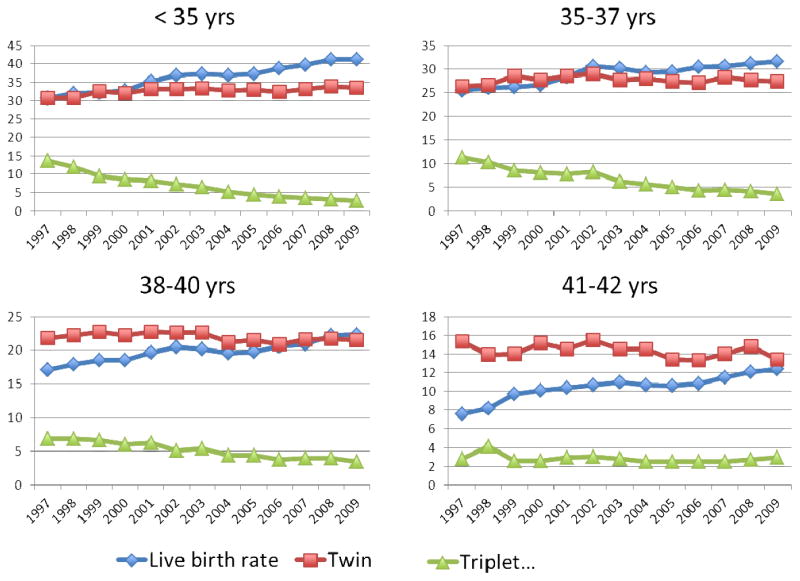
Since the first successful IVF pregnancy in the United States in 1981, there has been a marked increase in the use of assisted reproductive technologies in the treatment of infertility. Between 1997 and 2009, the use of IVF has increased 84%, with 101,090 fresh cycles performed in 2009. With the increased utilization there has also been a marked increase in success. In 1997, the overall live birth rate was 24% versus 30.4% in 2009. Simultaneously, there has been a decrease in the number of embryos transferred from 3.8 to 2.4 per cycle. The decrease in number of embryos transferred has resulted in 62% lower high order multiple pregnancies. A similar trend has been seen worldwide (Table 2) with the mean number of embryos transferred correlating with the triplet rate, but not the delivery rate (35). However, there has not been a decrease in the percent of twin pregnancies (Figure 1), and considering that the number of couples undergoing assisted reproduction has almost doubled, the overall number of twin pregnancies nationwide has increased as well (Figure 2).
Table 2.
ICMART 2003 World Report Pregnancy Outcomes of Assisted Reproductive Technologies by Region
| Region | Delivery rate per retrieval (%) | Twin Pregnancy (%) | Triplet or higher order multiple pregnancy (%) | Average number embryos transferred |
|---|---|---|---|---|
| Canada | 25.9 | 29.7 | 1.6 | 2.36 |
| United States | 32.6 | 31.1 | 3.1 | 2.8 |
| Asia | 18.4 | 21.1 | 0.8 | 2.67 |
| Australia/New Zealand | NA | 21.3 | 0.4 | 1.78 |
| Europe | 21.1 | 22.0 | 1.2 | 2.16 |
| Central and South America | 24.3 | 26.1 | 6.6 | 3.01 |
| Middle East | 26.1 | 26.0 | 3.6 | 2.97 |
| Total | 23.1 | 24.8 | 2.0 | 2.37 |
NA = not available
Figure 1.
Live birth rate, twin pregnancy, and high order multiple pregnancy in women undergoing IVF 1997–2009, CDC National Summary and Fertility Clinic Report.
Figure 2.
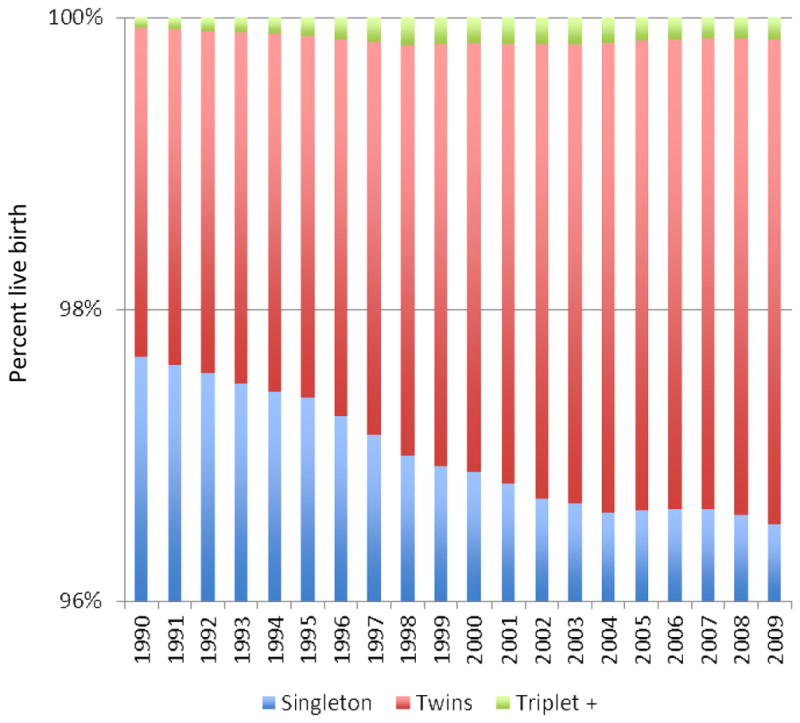
United States of America Centers for Disease Control National Birth Data 1990–2009
Why the concern? Twin and high order multiple pregnancies and births are at substantially higher risk than singletons for many adverse obstetric complications, perinatal morbidity and mortality. Multiple studies have been published showing an increased risk of gestational diabetes, pre-eclampsia, cesarean delivery, and preterm labor and delivery. With preterm delivery, infants are at risk for low birth weight, very low birth weight, respiratory distress, intracranial hemorrhage, retinopathy of prematurity, necrotizing enterocolitis, and death. Thus, not only are twin and high order multiple pregnancies dangerous for the infant, but also more dangerous for the mother.
The use of medications for ovarian stimulation may result in the syndrome of ovarian hyperstimulation. The first reports of hyperstimulation as a result of gonadotropin administration appeared in the literature in the early 1940s. Ovarian hyperstimulation syndrome (OHSS) is a rare complication characterized by rapid massive ovarian enlargement, fluid shifts with ascites and hemoconcentration. Severe cases may be associated with thromboembolic events, respiratory distress, and acute renal failure. The first fatality was reported in 1961(36). Although OHSS is rare, with the expansion of assisted reproductive technologies and the use of gonadotropins, the overall incidence of OHSS has increased worldwide.
Within the infertility field, the complications of hyperstimulation and multiple pregnancies led to a desire to be able to closely monitor gonadotropin stimulation of ovulation. Ultrasound was not available at the time when gonadotropins were first introduced into the infertility field for the treatment of anovulation. Patients were monitored by physical exam alone. It was not until 1979 that Ylostalo reported on the use of ultrasound to monitor follicular development during ovulation induction (37). During their study they also evaluated serum estradiol levels as a predictor of ovarian hyperstimulation. In 1980, Smith reported their use of following serum estradiol in conjunction with ultrasound to track follicular development for IVF(38). These studies supported the use of ultrasound and estradiol levels to provide valuable information about the number and maturity of the follicles, allowing stimulation cycles to be followed closely and to predict those patients at risk for complications.
Outside of the medical field, politicians and lawyers looked to regulate the infertility industry. The first country to enact regulatory statutes regarding IVF technology was the United Kingdom. The Human Fertilization and Embryology Act was passed in 1990 in response to the report of the Committee of Inquiry into Human Fertilization and Embryology (the Warnock report). This act resulted in the establishment of the Human Fertilization and Embryology Authority (HFEA) and its licensing and inspection procedures. Other European countries followed suit in the establishment of regulations to control how assisted reproduction was performed in their country. Some of the most restrictive laws were enacted in Italy. In 2004 Italy passed the Medically Assisted Reproduction Law, which prohibits the destruction of embryos created during IVF.
Therefore, all embryos created (to a legal maximum of three) must be transferred. In the United States, there has yet to be passed a federal law to regulate the practice of assisted reproduction. The American Society of Reproductive Medicine (ASRM) has put forth a recommendation for the number of embryos transferred (Table 1). While these practice guidelines put forth a set standard of care, the physician is still allowed the ability to make individualized treatment decisions. The only federal law that regulates the industry, the Fertility Clinic Success Rate and Certification Act, requires infertility clinics to report the number of cycles performed and their outcome. While this was enacted to ensure a high level of quality provided to infertile couples, it also encourages a higher number of embryos transferred. This is due to the competitive consumer driven environment with clinics vying for patients who desire the highest chance of success.
Table 1.
American Society of Reproductive Medicine Practice Committee Guidelines for Embryo Transfer 2009
| Age (years)
| ||||
|---|---|---|---|---|
| Prognosis | <35 | 35–37 | 38–40 | 41–42 |
|
| ||||
| Day 3 | ||||
| Good | 1–2 | 2 | 3 | 5 |
| Poor | 2 | 3 | 4 | 5 |
|
| ||||
| Day 5 | ||||
| Good | 1 | 2 | 2 | 3 |
| Poor | 2 | 2 | 3 | 3 |
Good prognosis = first cycle, good quality embryos, embryos available for cryopreservation, and prior successful IVF cycle.
Summary
The last century has seen tremendous advances in the treatment of infertility. The progressive development of our understanding provided the foundation upon which new medications, assays, and techniques were developed for the management of the infertile couple. With the increased knowledge of the hormonal control of reproduction have come new ethical and medical challenges. Among these challenges are the significant issues surrounding multiple pregnancies and the great emotional, physical, and monetary cost to both the family and society.
Ovarian stimulation for intrauterine insemination or IVF is probably the most significant contributor to iatrogenic multiple pregnancies. As we move forward, we are faced with the question of how to reduce the number of multiple births as a result of assisted reproduction. Although fetal reduction is an option in the United States, this procedure is associated with its own risks and unique ethical challenges. Some believe that strict regulations that limit the number of embryos transferred during IVF would be a simple solution. Numerous observational studies looking at single embryo transfer versus double embryo transfer have been published. In general, these studies show lower multiple pregnancies and little to no decrease in the pregnancy rate. However, the patients evaluated in these studies tend to be the young patient with a good prognosis. Therefore, legislation limiting the physician’s ability to individualize treatment may have a great impact on the quality of care received by the older and most clinically challenging patient.
With freedom comes great responsibility. First and foremost in our care of patients is the dictum “primum non nocere” or first do no harm. It is well known that high order multifetal pregnancies represent the largest single cause of poor obstetric and perinatal outcome. However, infertility as a diagnosis has the potential to cause the long-term harm of childlessness. Therefore, we are left to weigh beneficence versus autonomy. In the future, these ethical challenges should dissipate as we are able to keep multiple pregnancies associated with ART to a minimum through improved monitoring, advanced culture techniques, and enhanced implantation rates. In the future the ability to predict an embryos genetic and developmental competence should be accomplished allowing us the assurance of transferring one embryo for one successful singleton pregnancy.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hippocrates. Of women’s diseases [Google Scholar]
- 2.Cole HH, Hart GH. The potency of blood serum of mares in progressive stages of pregnancy in effecting ht esexual maturity of the immature rat. Am J Physiol. 1930;83:57–68. [Google Scholar]
- 3.Edwards RG, Fowler RE. Fetal mortality in adult mice after superovulation with gonadotropins. J Exp Zool. 1959;141:299–322. doi: 10.1002/jez.1401410206. [DOI] [PubMed] [Google Scholar]
- 4.Gemzell CA, Diczfalusy E, Tillinger G. Clinical effect of human pituitary follicle-stimulating hormone (FSH) J Clin Endocrinol Metab. 1958 Dec;18(12):1333–48. doi: 10.1210/jcem-18-12-1333. [DOI] [PubMed] [Google Scholar]
- 5.Buxton CL, Herrmann W. Induction of ovulation in the human with human gonadotropins. Yale J Biol Med. 1960 Oct;33:145–7. [PMC free article] [PubMed] [Google Scholar]
- 6.Donini P, Puzzuoli D, Alessio DI. Purification of gonadotrophin from menopausal urine by gel filtration on sephadex. Acta Endocrinol (Copenh) 1964 Mar;45:329–34. doi: 10.1530/acta.0.0450329. [DOI] [PubMed] [Google Scholar]
- 7.Venturoli S, Fabbri R, Paradisi R, Magrini O, Porcu E, De Tomasi F, et al. Induction of ovulation and pregnancy with HU-FSH (Metrodin) in women with polycystic ovarian disease: summary of clinical experience. Acta Eur Fertil. 1983 Jan-Feb;14(1):29–34. [PubMed] [Google Scholar]
- 8.Shaw RW, Ndukwe G, Imoedemhe DA, Bernard AG, Burford G. Twin pregnancy after pituitary desensitisation with LHRH agonist and pure FSH. Lancet. 1985 Aug 31;2(8453):506–7. doi: 10.1016/s0140-6736(85)90444-1. [DOI] [PubMed] [Google Scholar]
- 9.Watson JD, Crick FH. Molecular structure of nucleic acids; a structure for deoxyribose nucleic acid. Nature. 1953 Apr 25;171(4356):737–8. doi: 10.1038/171737a0. [DOI] [PubMed] [Google Scholar]
- 10.Crick FH. On protein synthesis. Symp Soc Exp Biol. 1958;12:138–63. [PubMed] [Google Scholar]
- 11.Min Jou W, Haegeman G, Ysebaert M, Fiers W. Nucleotide sequence of the gene coding for the bacteriophage MS2 coat protein. Nature. 1972 May 12;237(5350):82–8. doi: 10.1038/237082a0. [DOI] [PubMed] [Google Scholar]
- 12.Shome B, Parlow AF. Human follicle stimulating hormone:first proposal for the amino acid sequence of the hormone-specific, beta subunit (hFSHb) J clin Endocrinol Metab. 1974 Jul;39(1):203–5. doi: 10.1210/jcem-39-1-203. [DOI] [PubMed] [Google Scholar]
- 13.Fiddes JC, Goodman HM. The gene encoding the common alpha subunit of the four human glycoprotein hormones. J Mol Appl Genet. 1981;1(1):3–18. [PubMed] [Google Scholar]
- 14.Esch FS, Mason AJ, Cooksey K, Mercado M, Shimasaki S. Cloning and DNA sequence analysis of the cDNA for the precursor of the beta chain of bovine follicle stimulating hormone. Proc Natl Acad Sci USA. 1986 Sep;83(10):6618–21. doi: 10.1073/pnas.83.17.6618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keene JL, Matzuk MM, Otani T, Fauser BC, Galway AB, Hsueh AJ, et al. Expression of biologically active human follitropin in Chinese hamster ovary cells. J Biol Chem. 1989 Mar 25;264(9):4769–75. [PubMed] [Google Scholar]
- 16.Devroey P, Van Steirteghem A, Mannaerts B, Coelingh Bennink H. First singleton term birth after ovarian superovulation with rhFSH. Lancet. 1992 Oct 31;340(8827):1108–9. doi: 10.1016/0140-6736(92)93141-9. [DOI] [PubMed] [Google Scholar]
- 17.Germond M, Dessole S, Senn A, Loumaye E, Howles C, Beltrami V. Successful in-vitro fertilization and embryo transfer after treatment with recombinant human FSH. Lancet. 1992 May 9;339(8802):1170. [PubMed] [Google Scholar]
- 18.Donderwinkel PF, Schoot DC, Coelingh Bennink HJ, Fauser BC. Pregnancy after induction of ovulation with recombinant human FSH in polycystic ovary syndrome. Lancet. 1992 Oct 17;340(8825):983. doi: 10.1016/0140-6736(92)92879-k. [DOI] [PubMed] [Google Scholar]
- 19.Strowitzki T, Seehaus D, Korell M, Hepp H. Low-dose follicle stimulating hormone for ovulation induction in polycystic ovary syndrome. J Reprod Med. 1994 Jul;39(7):499–503. [PubMed] [Google Scholar]
- 20.Heap W. Preliminary note on the transplantation and growth of mammalian ova within a uterin foster mother. Proc R Soc. 1890;48:457–8. [Google Scholar]
- 21.Chang 1951 [Google Scholar]
- 22.Noyes RW. Fertilization of follicular ova. Fertil Steril. 1952 Jan-Feb;3(1):1–12. doi: 10.1016/s0015-0282(16)30780-4. [DOI] [PubMed] [Google Scholar]
- 23.Rock J, Menkin MF. In vitro fertilization and cleavage of human ovarian eggs. Science. 1944 Aug 4;100(2588):105–7. doi: 10.1126/science.100.2588.105. [DOI] [PubMed] [Google Scholar]
- 24.Shettles LB. A morula stage of human ovum developed in vitro. Fertil Steril. 1955 Jul-Aug;6(4):287–9. doi: 10.1016/s0015-0282(16)32040-4. [DOI] [PubMed] [Google Scholar]
- 25.Steptoe PC, Edwards RG. Reimplantation of a human embryo with subsequent tubal pregnancy. Lancet. 1976 Apr 24;1(7965):880–2. doi: 10.1016/s0140-6736(76)92096-1. [DOI] [PubMed] [Google Scholar]
- 26.Jones HW., Jr The use of controlled ovarian hyperstimulation (COH) in clinical in vitro fertilization: the role of Georgeanna Seegar Jones. Fertil Steril. 2008 Nov;90(5):e1–3. doi: 10.1016/j.fertnstert.2007.07.1333. [DOI] [PubMed] [Google Scholar]
- 27.Steptoe PC, Edwards RG. Birth after the reimplantation of a human embryo. Lancet. 1978 Aug 12;2(8085):366. doi: 10.1016/s0140-6736(78)92957-4. [DOI] [PubMed] [Google Scholar]
- 28.Jones HW, Acosta AA, Andrews MC, Garcia JE, Jones GS, Mayer JJS, et al. Three years of in vitro fertilization at Norfolk. Fertil Steril. 1984 Dec;42(6):826–34. doi: 10.1016/s0015-0282(16)48251-8. [DOI] [PubMed] [Google Scholar]
- 29.Greenblatt RB, Barfield WE, Jungck EC, Ray AW. Induction of ovulation with MRL/41. JAMA. 1961;178(2):101–4. doi: 10.1001/jama.1961.03040410001001. [DOI] [PubMed] [Google Scholar]
- 30.Roy S, Greenblatt RB, Mahash VB, Jungck EC. Clomiphene citrate: further observations on its use in induction of ovulation in the human and on its mode of action. Fertil Steril. 1963 Nov-Dec;14:575–95. doi: 10.1016/s0015-0282(16)35041-5. [DOI] [PubMed] [Google Scholar]
- 31.Williamson JG, Ellis JD. The induction of ovulation by tamoxifen. J Obstet Gynaecol Br Commonw. 1973 Sep;80(9):844–7. doi: 10.1111/j.1471-0528.1973.tb11230.x. [DOI] [PubMed] [Google Scholar]
- 32.Messinis IE, Nillius SJ. Comparison between tamoxifen and clomiphene for induction of ovulation. Acta Obstet Gynecol Scand. 1982;61(4):377–9. [PubMed] [Google Scholar]
- 33.Mitwally MF, Biljan MM, Casper RF. Pregnancy outcome after the use of an aromatase inhibitor for ovarian stimulation. Am J Obstet Gynecol. 2005 Feb;192(2):381–6. doi: 10.1016/j.ajog.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 34.McGavi DD. Pituitary gonadotrophins and multiple pregnancy. Lancet. 1965 Aug 7;2(7406):277. [PubMed] [Google Scholar]
- 35.Nygren KG, Sullivan E, Zegers-Hochschild F, Mansour R, Ishihara O, Adamson GD, et al. International Committee for Monitoring Assisted Reproductive Technology (ICMART) world report: assisted reproductive technology 2003. Fertil Steril. 2011 Jun;95(7):2209–22. doi: 10.1016/j.fertnstert.2011.03.058. [DOI] [PubMed] [Google Scholar]
- 36.Esteban-Altirriba J. Le syndrome d'hyperstimulation massive des ovaires. Rev Franc Gynec Obstet. 1961;56:555–64. [PubMed] [Google Scholar]
- 37.Ylostalo P, Ronnberg L, Jouppila P. Measurement of the ovarian follicle by ultrasound in ovulation induction. Fertil Steril. 1979 Jun;31(6):651–5. doi: 10.1016/s0015-0282(16)44055-0. [DOI] [PubMed] [Google Scholar]
- 38.Smith DH, Picker RH, Sinosich M, Saunders DM. Assessment of ovulation by ultrasound and estradiol levels during spontaneous and induced cycles. Fertil Steril. 1980 Apr;33(4):387–90. [PubMed] [Google Scholar]