Summary
Paradoxically, aging leads to both decreased regenerative capacity in the brain and an increased risk of tumorigenesis, particularly the most common adult-onset brain tumor, glioma. A shared factor contributing to both phenomena is thought to be agerelated alterations in neural progenitor cells (NPCs), which function normally to produce new neurons and glia, but are also considered likely cells of origin for malignant glioma. Upon oncogenic transformation, cells acquire characteristics known as the hallmarks of cancer, including unlimited replication, altered responses to growth and anti-growth factors, increased capacity for angiogenesis, potential for invasion, genetic instability, apoptotic evasion, escape from immune surveillance, and an adaptive metabolic phenotype. The precise molecular pathogenesis and temporal acquisition of these malignant characteristics is largely a mystery. Recent studies characterizing NPCs during normal aging, however, have begun to elucidate mechanisms underlying the age-associated increase in their malignant potential. Aging cells are dependent upon multiple compensatory pathways to maintain cell cycle control, normal niche interactions, genetic stability, programmed cell death, and oxidative metabolism. A few multi-functional proteins act as ‘critical nodes’ in the coordination of these various cellular activities, although both intracellular signaling and elements within the brain environment are critical to maintaining a balance between senescence and tumorigenesis. Here, we provide an overview of recent progress in our understanding of how mechanisms underlying cellular aging inform on glioma pathogenesis and malignancy.
Keywords: aging, AMP-activated protein kinase, malignant potential, mitochondria, neural stem cells, p16, p53, replicative senescence
Gliomas: how aging impacts the clinical problem
The incidence of glioma, the most common form of adult-onset primary brain tumor, increases with age. The grade III anaplastic astrocytoma and grade IV astrocytoma (or glioblastoma, GBM) are collectively referred to as the ‘malignant gliomas’; these comprise up to 90% of all adult gliomas and have exceptionally dismal prognosis with median survival of 3 and 1 years, respectively (Ohgaki & Kleihues, 2005). Of importance, the outcomes for malignant gliomas and GBM in particular, have not improved significantly since the introduction of routine radiotherapy over three decades ago, providing only modest, albeit measurable, benefit.
Increased age is strongly associated with increased cancer incidence and malignancy for all grades and types of glioma (Barker et al., 2001; Porter et al., 2010). Age is, in fact, among the most robust single predictive factors in glioma incidence, malignancy, and patient survival (Ohgaki & Kleihues, 2005; CBTRUS, 2009; Porter et al., 2010). In particular, high-grade gliomas and tumors containing multiple genomic abnormalities are more common in the elderly. Correspondingly, patients over 70 respond poorly to conventional therapy compared with younger patients. Yet, despite the strong associations between patient age and glioma outcomes, little is known about how aging increases glioma malignancy. However, recent advances in our understanding of cellular aging provide a starting point toward discovering the mechanisms by which age contributes to tumor incidence and progression.
Cells which originate and sustain the growth of brain tumors
It is currently accepted that malignant gliomas likely originate from neural progenitor cells (NPCs) or their glial progenitor progeny: accumulation of oncogenic mutations in this lineage give rise to malignant tumors (Holland et al., 2000; Alcantara Llaguno et al., 2009; Wang et al., 2009), recruited NPCs form the bulk of the tumor (Assanah et al., 2006), proliferative cells within the tumor share some immunomarkers with NPCs (Yuan et al., 2004; Stiles & Rowitch, 2008), and gliomas initiate and cluster near germinal centers of the brain (Lim et al., 2007). Although this evidence strongly implicates the tissue-specific progenitor cell in gliomagenesis, one recent study demonstrated that astrocytes and even neurons can be forced to undergo oncogenic transformation upon promoter-specific expression of short hairpin RNAs targeting tumor suppressor pathways (Friedmann-Morvinski et al., 2012).
Neural progenitor cells have an advantage over other cells in the brain for acquiring an oncogenic phenotype: they already exist in proliferative state, producing a variety of fate-restricted cell types which migrate through the brain during differentiation (Altman & Das, 1966). Yet, increasing lineage specificity and cellular maturity may not prevent oncogenic transformation but merely influence tumor behavior; indeed, variation in the cellular origin, as well as the underlying genetic lesions, of histopathologically similar tumors may result in differential growth and invasiveness, with clinical implications.
Regardless of the identity of the cell of origin of glioma, the ‘cancer stem cell’ is a helpful concept in describing the radiation-resistant population of cells which recapitulate the tumor after treatment (Cheng et al., 2010). These cells are not necessarily the cells that originate the tumor and may bear only superficial resemblance to cells within the normal brain, yet they utilize signaling pathways that have already been characterized in a normal context. The identification of molecular markers for this population, such as CD133, has allowed investigation into the mechanisms by which this slowly dividing population is able to survive and flourish despite clinical intervention in both human tumors and mouse models of glioma (Singh et al., 2004; Bao et al., 2006). For example, CD133+ cells within gliomas, which are localized to the perivascular niche, co-express integrin alpha 6; ablation of this extra-cellular matrix protein has been shown to reduce proliferation in mouse models (Lathia et al., 2010). In addition, transcriptional regulators such as NSRF/REST, a zinc finger transcription factor, have been shown to support a pluripotent phenotype and promote neuronal gene silencing in both normal neural stem cells and GBM stem cells (Zhang et al., 2008; Covey et al., 2012; Kamal et al., 2012).These studies demonstrate how discoveries in stem cell biology have crossover benefits in cancer biology, despite the controversy remaining about the direct lineage relationship between cancer cells and cell types within the normal brain.
Oncogenesis and the aging neural progenitor cell
Because age is a strong predictive factor in glioma occurrence, it is worth investigating how the process of aging might itself promote malignant potential in the putative tumor-originating cells. Aged mouse NPCs that have been oncogenically transformed by overexpression of Ras and simultaneous abrogation of tumor suppressor proteins p53 and Rb, form more malignant gliomas in vivo than their younger counterparts (Mikheev et al., 2009). The negative impact of increased age on outcome in this model is cell intrinsic; the age of transplanted cells, but not the host age, determines the degree of malignancy based on survival, invasiveness, genomic stability, and responses to genotoxic and hypoxic stress (Mikheev et al., 2012). These data suggest that normal aging predisposes NPCs to increased malignant potential.
At a population level, NPCs are less regenerative with age. Rates of new neuron production decline in both the subventricular zone (SVZ) and hippocampal dentate gyrus during normal aging (Kuhn et al., 1996; Luo et al., 2006). Decreases in neurogenesis in vivo correspond to a reduction in the number of neurospheres that can be cultured from aged rodents (Maslov et al., 2004), suggesting that the cellular deficit is due to decreased number of stem-like cells (Molofsky et al., 2006; Ahlenius et al., 2009). Neurogenesis in the aging brain is also hampered by impaired cellular survival and neuronal differentiation (Ahlenius et al., 2009). Although a smaller number of cells are observed to be dividing at a given time in the aged forebrain, the cell cycle is not slowed down in aged NPCs (Stoll et al., 2011a). Interestingly, cycling cells that remain in the aged SVZ are actually more proliferative than young NPCs, due to increased rates of cell cycle re-entry (Stoll et al., 2011a). Yet increased proliferative capacity alone is not enough to initiate tumor formation.
In order for a cell to undergo oncogenesis, multiple transforming events must occur to dysregulate both intracellular signaling pathways and the cellular response to the brain microenvironment (Hanahan & Weinberg, 2000). Upon oncogenic transformation, cells acquire characteristics known as the hallmarks of cancer, including unlimited replication, altered responses to growth and anti-growth factors, increased capacity for angiogenesis, potential for invasion, genetic instability, apoptotic evasion, escape from immune surveillance, and an adaptive metabolic phenotype (Hanahan & Weinberg, 2011). Yet an important feature of aging NPCs (and other tissue-specific stem cells) is their increased tendency to undergo senescence as opposed to proliferation and self-renewal in response to growth signals.
Senescence and the development of malignant potential
By striking a balance in favor of senescence, oncogenic risk would presumably be reduced as this process is broadly tumor suppressive. Yet although senescence is associated with declining proliferative capacity, it is an imperfect barrier to oncogenic transformation. For example, in a mouse model of pancreatic cancer, a weak senescence imposed by Akt activation has been shown to override the strong p16-mediated senescence normally imposed by KRAS activation. Therefore, a so-called ‘second-hit’ mutation which allows constitutive KRAS activation would affect a cell with activated Akt differently than a cell that had not undergone such Akt activation (Kennedy et al., 2011). The effects of such altered signaling may thus be context-dependent; mutations that affect KRAS expression may induce proliferative arrest in some cells while a senescent phenotype may be bypassed or overcome in another context.
The idea that senescence might actually ‘precondition’ cells for oncogenic transformation is gaining traction recently: markers of senescence are evident in premalignant adenomas, but not in advanced adenocarcinomas in a mouse model of RAS-induced tumorigenesis (Collado et al., 2005). Interestingly, the malignant preconditioning associated with senescence is not entirely cell-autonomous: cells induced to undergo senescence upon genotoxic stress have been shown to secrete pro-oncogenic paracrine factors IL-6 and IL-8, particularly in the presence of constitutively active RAS (Coppe et al., 2008). It has also been suggested that senescence may be a necessary step in the progression to immortality in cell culture. One recent study has shown that many pluripotency genes are hypermethylated in mouse embryonic fibroblasts during immortalization in vitro, in patterns similar to those observed in cancer cells (Tommasi et al., 2013). Yet a direct comparison of the methylation patterns in senescent and transformed mammalian cells is still lacking. Therefore, it is not currently clear whether cells actually pass through a state of senescence or are able to bypass senescence completely during the process of oncogenic transformation. Perhaps epigenetic preconditioning during cellular senescence translates into an advantage in the acquisition of a malignant phenotype; if the process of senescence actually prepares a cell epigenetically for oncogenic transformation, chromatin modifiers may prove to be excellent targets in reducing malignant progression clinically. Alternatively, if a malignant phenotype is more easily attainable in cells resistant to senescence, promoting such a phenotype through chromatin modification may also be a sensible therapeutic strategy.
The relationship between senescence and oncogenic potential in aging NPCs in particular has yet to be resolved. Indeed, markers of senescence such as beta-galactosidase have not been investigated in this cell type during aging and malignant transformation. Despite the existing gaps in our understanding, recent observations support the hypothesis that the process of normal aging in NPCs, the presumed glioma cells of origin, contributes to the observed age-related increases in gliomagenesis, malignancy, treatment resistance, and tumor progression. The characteristics of aging NPCs, which display both a senescent phenotype and increased oncogenic potential, are summarized in Table 1. Below, we provide an overview of our current understanding of the behavior of aging NPCs and discuss molecular mechanisms that may contribute to the age-associated increase in their malignant potential.
Table 1.
Characteristics of aging NPCs compared with an expanded list of the hallmarks of cancer
| Expanded list of the hallmarks of cancer |
Actual characteristics of aged NPCs and their niche | Potential mechanisms underlying age-related changes |
|---|---|---|
| Increased cell cycle progression | Large quiescent population; increased cell cycle re-entry in the remaining cycling population (Stoll et al., 2011a) | Decreased p53 expression (Mikheev et al., 2009); increased p16 expression (Molofsky et al., 2006) |
| Self-sufficiency in growth factor signaling | Retained sensitivity to growth factors FGF and EGF; fewer growth factors in aged neurogenic niche (Jin et al., 2003) | Aged astrocytes produce fewer growth factors (Jin et al., 2003) |
| Insensitivity to antigrowth signals | Retained responses to anti-growth signal TGFβ; more anti-growth factors in aged neurogenic niche (Buckwalter et al., 2006) | Aged astrocytes produce more TGFb (Buckwalter et al., 2006) |
| Genetic instability | Increased chromosomal loss of heterozygosity (Bailey et al., 2004) | Accumulation of mutations over time; loss of telomeres (Ferron et al., 2009) |
| Escape from apoptosis | Increased apoptosis (Ahlenius et al., 2009) | Not known |
| Angiogenesis and neovascularization | Decreased vascularity and cerebral blood flow (Aanerud et al., 2012) | Loss of pro-angiogenic VEGF and other factors (Shetty et al., 2005) |
| Invasion into surrounding tissue | No change in migratory potential of actively cycling cells (Stoll et al., 2011a) | |
| Escape from immune destruction | Increased microglia and cytokine production (Kuzumaki et al., 2010) | Not known |
| Decreased aerobic respiration and increased glycolysis | Decreased aerobic respiration but no compensatory glycolytic switch (Stoll et al., 2011b) | Lower mitochondrial content (Stoll et al., 2011b) |
NPC, neural progenitor cells; VEGF, vascular endothelial growth factor. Listed is an updated list of the hallmarks of cancer, originally escribed by Hanahan and Weinberg, with the addition of several features that have recently been recognized as key events during oncogenesis. The observed cellular activities of aging NPCs are listed; these characteristics largely describe a senescent phenotype both within the aging cell and throughout the aging brain. Compensatory mechanisms must balance the forces of senescence and oncogenesis in NPCs. For example, multiple pathways may be particularly important in the case of metabolism, Aged NPCs consume less oxygen, but do not secrete more lactate than young adult NPCs, suggesting that these cells have deficits in aerobic metabolism but have not acquired a greater dependence upon glycolytic metabolism.
Acquisition of a highly proliferative phenotype
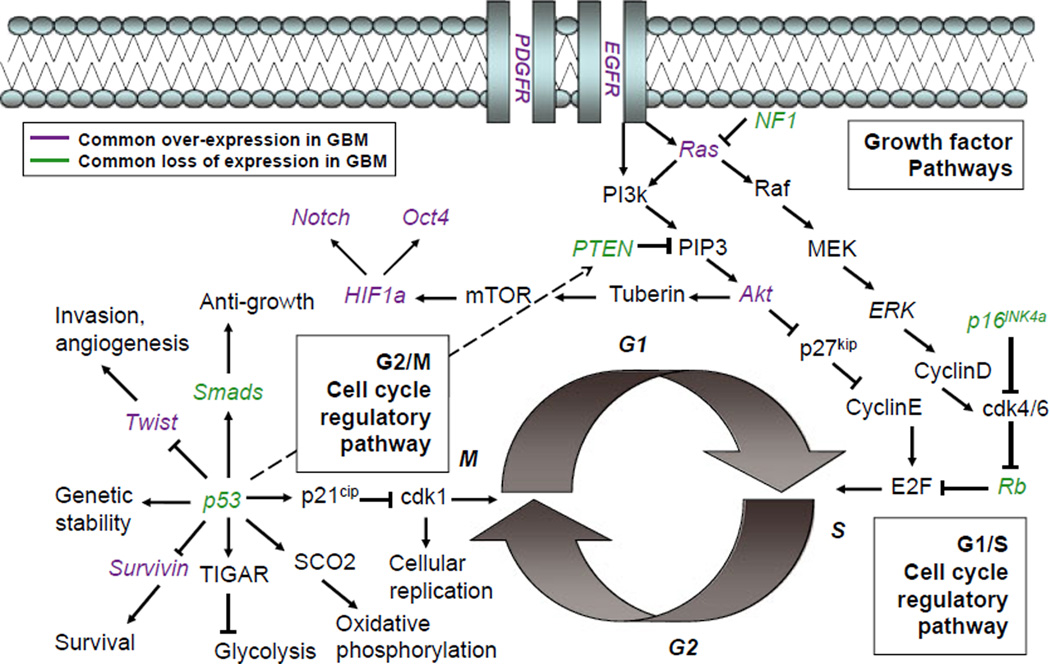
Mitotic activity is regulated by multiple, often convergent, pathways. Progression through the G1/S checkpoint, before DNA replication, is regulated by serial phosphorylation events of cyclin-dependent kinases and tumor suppressor proteins within the p16Ink4a pathway. The tumor suppressor p53 similarly prevents progression through the G2/M cell cycle checkpoint by decreasing the activity of cdk1 via p21 (Chung & Bunz, 2010). These regulatory proteins work in concert to ensure that cells do not replicate their DNA or undergo mitosis unless they are viable enough to do so. As confirmed through analysis of TCGA databases (TCGA, 2008), mutations are commonly found throughout these intracellular pathways in human GBMs (Fig. 1).
Fig. 1.
Common mutations in glioblastoma are observed throughout cell cycle regulatory pathways. The multifunctional protein p53, which acts as a tumor suppressor regulating the G2/M cell cycle checkpoint, is commonly observed to be lost in patient samples of glioma. MDM2, a regulatory protein that ubiquitinates p53, targeting it for degradation, is observed to be overexpressed in a subset of gliomas; this regulatory protein is itself transcriptionally regulated by p53. Several direct transcriptional and post-translational targets of p53 are commonly affected in glioma. Most other molecular lesions, due to either genetic mutation or expression changes, are observed in the p16/Rb pathway, which regulates the G1/S cell cycle checkpoint. Upon loss of p53, aging neural progenitor cells (NPCs) may be highly dependent on such compensatory pathways to maintain normal cellular activities. This schematic is not an exhaustive description of the complex interactions between many convergent intracellular signaling pathways, but illustrates how a few multifunctional proteins can act as nodes coordinating many cellular activities.
At least 59% of low-grade gliomas have p53 mutations and approximately 35% of primary GBMs have p53 mutations (Purow & Schiff, 2009). p53 not only halts cell cycle progression, but also plays a regulatory role in many other cellular processes, promoting apoptosis, genetic stability, and aerobic respiration, while inhibiting pro-invasive, pro-angiogenic, and glycolytic gene expression (Wang et al., 2009). Several thousand genes contain p53 response elements, but may be differentially responsive to wild-type and mutant forms of the protein (for review, see Strano et al., 2007). Interestingly, we have found that expression and inducibility of p53 is dramatically decreased in NPCs within the normal aging mouse brain (Mikheev et al., 2009). Lost or altered function of such a critical node may predispose oncogenic transformation, leading cells to become more reliant upon proteins with compensatory function to maintain cell cycle control and other cellular activities. A resulting reliance upon a more limited set of tumor suppressor pathways may render NPCs more susceptible to tumorigenesis upon subsequent loss or disruption of proteins with functionally redundant roles. Indeed, multiple semi-redundant pathways must be disrupted to allow a cell to realize its malignant potential. In particular, loss of both G1/S and G2/M cell cycle control is required for tumorigenesis (Chow et al., 2011).
The p16 tumor suppressor pathway may ‘compensate’ for the loss of p53 activity in aging NPCs.Weand others have observed higher expression of p16 in NPCs within the normal aging mouse brain (Molofsky et al., 2006; Mikheev et al., 2009). The age-associated increase in p16 expression is due to reduction of its transcriptional regulator Hgma2, which exhibits a microRNA-mediated decline with age (Nishino et al., 2008). Transcriptome-based comparisons between human tumors and mouse models have demonstrated that p16 is a critical tumor suppressive mechanism, and its loss is a driving mutation in ependymoma (Johnson et al., 2010). Likewise, in human glioma, mutations are common in critical regulators of the p16/Rb cell cycle control pathway, which is subject to deregulation by alterations in growth factor receptor activation and kinase signaling (Fig. 1). Intriguingly, mutations of p53 or p16 themselves are highly dependent on patient age and mirror their differential expression patterns in normal NPCs during aging. For example, mutations of p53 predominate in pediatric gliomas and in lower grade astrocytomas of younger patients while p16 mutations are more commonly seen in malignant gliomas of older patients (Sung et al., 2000; Ohgaki & Kleihues, 2005). It is therefore tempting to speculate that this pattern reflects the requirement of NPCs to override the prevailing mechanism constraining proliferation in the face of genomic instability as a prerequisite to full malignant transformation.
In addition to changes in tumor suppressor activity, kinase signaling is often altered in glioma. Growth factor receptors, such as EGFR and PDGFR, are often constitutively active in primary GBM (Ohgaki & Kleihues, 2007; Hegi et al., 2011), as are membrane-bound GTPases such as Ras (Brennan et al., 2009). These oncogenic mutations, which allow for cell division in the absence of growth factors, are predicted to occur after the loss of p53 (Attolini et al., 2010). In a fraction of GBMs, Neurofibromin 1 (NF1) and Phosphatase and Tensin Homolog (PTEN), which inhibit signaling downstream of growth factor receptors, are observed to be lost or mutated, with a net effect of promoting G1/S progression (Reilly et al., 2000; Ermoian et al., 2002). NF1 inhibits RAS activity, while PTEN dephosphorylates phosphotidylinositol-3,4,5-trisphosphate; both thereby inhibit the downstream phosphorylation of Akt. Mutations which lead to constitutive activation of EGFR, or the loss of NF1 or PTEN, allow stabilization of Akt, which activates cyclin D1 through NFkappa B and disinhibits cyclin E through phosphorylation of p27kip. An alternate pathway recently implicated in GBM stem cell identity and self-renewal capacity involves the nonreceptor tyrosine kinase BMX, which activates Stat3 to promote a proliferative tumor phenotype (Guryanova et al., 2011). Expression and functionality of G1/S regulatory kinases such as PI3 kinase/Akt or alternative pathways such as BMX/Stat3 have not been assessed in normal aging NPCs. Mutations throughout these pathways are likely to be much more devastating in aged NPCs, which may have already deregulated p53 function.
The astonishing redundancy of intracellular signaling pathways not only exerts a protective effect by maintaining cell cycle control after loss of a critical regulator, but also broadly aids cellular function. Information theory-based analysis of stochastic biochemical networks support the role of redundant pathways in reducing noise within a system (Ziv et al., 2007). The loss of redundancy upon bi-directional changes in tumor suppressor protein expression during normal aging is therefore predicted to increase inefficiency in signaling. Cellular stress resulting from this process and other events during aging may cause important contextual changes in the cell, which are then carried forward during oncogenic transformation to define the phenotype of the resulting malignancy. In the absence of oncogenic mutation, a cell subjected to this stress may acquire a characteristically senescent phenotype.
Neural progenitor cells are also subject to proliferative control by growth and anti-growth signals derived from neighboring cells. Yet mitotic cells within a tumor often do not respond to extracellular factors which normally promote cell cycle exit, such as the TGFβfamily of proteins (Kjellman et al., 2000). TGFβ inhibits growth of normal cells by promoting nuclear localization and transcriptional activity of downstream factors, particularly Smads 1/5/8, which can promote differentiation (Shukla et al., 2009). TGFβreceptor-activated intracellular signaling pathways can however be pro-oncogenic in cells with missing or mutant p53 (Adorno et al., 2009). TGFβoverexpression reduces proliferation in both the adult and aged hippocampus (Buckwalter et al., 2006), suggesting that aging NPCs retain normal responses to this signal. However, increased TGFβexpression is observed in the aged neurogenic niche (Buckwalter et al., 2006). Similarly, aged NPCs appear to retain sensitivity to the secreted protein Wnt3, although support cells express much lower levels of this and other pro-proliferative factors (Jin et al., 2003; Shetty et al., 2005; Okamoto et al., 2011). Expression of Wnt inhibitory factor has not been investigated in normal aged NPCs; this anti-proliferative signal is often lost in GBM (Lambiv et al., 2011).
Overall, these studies suggest that paracrine signals within the aged brain are not conducive for growth. This may provide a selective advantage to cells that are able to bypass these signals. Computational modeling has predicted that NPCs that are able to proliferate without growth factors could be selected for in this environment, but would have no such competitive advantage in a younger brain with more growth factor availability (Anderson et al., 2009). Although dysfunction in intracellular signaling as a result of genetic mutation is considered to be the major cause of oncogenic transformation, malignant cells are also able to co-opt the surrounding tissue to create a favorable environment for the growing tumor.
Interactions between tumor-initiating cells and the surrounding niche
Vascular occlusion events within gliomas commonly cause localized hypoxia, which drives migration of tumor cells away from the throm-bosed vessel, giving rise to the histological feature of pseudopalisading necrosis (for review, see Rong et al., 2006). Hypoxic conditions also induce expression of intracellular hypoxia-inducible factors (HIFs) and are associated with increased glioma cell proliferation (Heddleston et al., 2009; Qiang et al., 2012). We have also observed increased proliferation in normal aging mouse NPCs under hypoxic conditions (Stoll et al., 2011b), although it is not known whether HIFs mediate this response.
In addition to promoting proliferation and a pluripotent phenotype, HIFs induce production and secretion of vascular endothelial growth factor (VEGF; Jensen et al., 2006). VEGF secretion causes nearby vasculature to sprout, allowing more cells within the tumor to access oxygen and available nutrients from highly permeable newly formed blood vessels. Abnormal, remodeled blood vessels, often in aggregates of proliferating microvessels called glomeruloid bodies, are more common in older patients with GBM, although overall vascular density decreases with age (Izycka-Swieszewska et al., 2003). Similarly, tortuous vessels are commonly observed in the normal aged brain, particularly in white matter (Brown & Thore, 2011). In addition, age is associated with lower vascular density, cerebral blood flow, and oxygen consumption rates (Aanerud et al., 2012). Yet, despite the interesting connections between age and pathological vasculature, it remains unclear how changes in blood vessel morphology during normal aging might influence the proliferation or invasiveness of malignantly transformed cells within the brain.
Gliomas are remarkably invasive within the central nervous system. This characteristic renders surgical resection ultimately futile, as the leading edge of the tumor, which intercalates with normal brain tissue, is never completely obliterated. In vitro studies show that actively cycling NPCs derived from the aged mouse forebrain migrate at similar rates to actively cycling NPCs from the young adult mouse forebrain, while noncycling cells migrate more slowly with age (Stoll et al., 2011a). By contrast, in a syngeneic transplant model, aging-transformed NPCs demonstrated markedly increased invasive potential in vitro and in vivo compared with young transformed NPCs (Mikheev et al., 2012). These findings suggest that age-related differences in normal NPCs that are either amplified or ‘unmasked’ upon oncogenic transformation result in age-related increases in invasive potential in mouse models. In human studies, the degree of glioma cell invasiveness and motility directly correlates with higher malignant grade (Chicoine & Silbergeld, 1995). Because higher malignant grades are more common in older patients, it is possible that aging contributes to increased tumor invasiveness in human glioma, but further study is required to clarify this relationship.
Glioma cell invasion is an extremely complex biological process with numerous mechanisms likely to contribute to a possible age-dependent invasion phenotype. Among these, age-dependent differences in hypoxic response and cellular metabolism may contribute (Mikheev et al., 2012), as these mechanisms are known to regulate invasiveness in glioma and other cancers (Jensen, 2009; Sottnik et al., 2011). The decline in p53 activity associated with aging in NPCs (Mikheev et al., 2009) may also contribute to differential invasiveness, as wild-type p53 inhibits cell migration and invasion (Mukhopadhyay et al., 2009) while gain-of-function p53 mutants associated with cancer can promote cell invasion (Muller et al., 2009). While these associations suggest intriguing possibilities by which NPC aging may influence glioma invasiveness, these putative mechanisms require further characterization in animal models of glioma and additional verification of clinical phenotypes.
Cellular interactions observed in patient samples of glioma also highlight the inherent susceptibility of the aged brain microenvironment. In particular, the loss of immune surveillance, due to ‘immunosenescence’, may contribute to age-related increases in glioma incidence. One recent study showed that decreased production of CD8+ T cells is associated with increased glioma malignancy in both aged human patients and a knockout mouse model (Wheeler et al., 2003). While bone marrow-derived immune cells decrease in number during normal aging, immune activity increases within the brain. A recent hetero-chronic parabiosis experiment demonstrated that increased levels of chemokines in the systemic mileau are partially responsible for age-related neurogenic decline (Villeda et al., 2011). Greater numbers of chemokine-secreting microglia are observed in the aged brain (Kuzumaki et al., 2010), yet results have differed as to whether these cells are anti-tumoral or pro-tumoral (Chiu et al., 2011; Zhai et al., 2011). One recent study may have resolved this debate by showing that gliomas activate microglia, but inhibit their phagocytotic activity and enhance expression of pro-migratory metalloproteases (Held-Feindt et al., 2010). Interestingly, normal NPCs themselves are anti-tumorigenic; the age-related decline of this population has been shown to allow unchecked tumor growth, which can be reversed by injection of adult NPCs (Glass et al., 2005). While this effect appeared to be due to apoptotic induction of glioma cells, it is not clear whether normal NPCs inhibit tumor activity directly or indirectly, perhaps through competition for resources such as metabolic substrates.
Regulation of energy metabolism
Gliomas, like other solid tumors, are thought to adopt a highly glycolytic metabolism. Instead of converting the end product of glycolysis, pyruvate, into acetyl CoA for use in the citric acid cycle and electron transport chain, tumor cells convert pyruvate into lactate which is secreted into the extracellular space, creating a highly acidic microenvironment. This phenomenon, discovered by Otto Warburg in the 1930s, has been termed the ‘Warburg Effect’ (Warburg, 1956).
Abandoning oxidative respiration for alternative metabolic strategies has been hypothesized to confer an advantage upon cancer cells. The reliance upon glycolysis has been proposed to increase energy efficiency, perhaps by increasing the speed of ATP production without necessitating accurate transcription and translation of the large number of enzymes required for aerobic respiration. Recently, one group has proposed that ATP is not a limiting factor in cancer cells, so aerobic glycolysis is undertaken not to produce energy, but primarily to recycle carbon into the raw materials necessary for synthesis of nucleic acids, amino acids, and lipids (Vander Heiden et al., 2009). This hypothesis is supported by findings that many cancer cells readily metabolize glutamine in the absence of glucose, using both as carbon substrates for the production of NADPH and lactate (DeBerardinis et al., 2007). Mitochondria-independent respiration could be favored for either energy production or biomass production, given the hypoxic conditions within a tumor.
Multiple pathways converge to regulate a cell’s metabolic strategy. p53 promotes oxidative respiration through SCO2, which organizes assembly of cytochrome C oxidase (Matoba et al., 2006). p53 also promotes transcription of TP53-induced glycolysis and apoptosis regulator, which suppresses glycolysis by decreasing levels of fructose-2, 6-bisphosphate (Bensaad et al., 2006). Therefore, loss of p53 during normal aging may both suppress oxidative phosphorylation and disinhibit glycolytic activity. Indeed, NPCs within the aged mouse brain consume less oxygen and have a higher rotenone tolerance, although they do not compensate by boosting glycolytic activity (Stoll et al., 2011b). Decreased p53 expression may put additional pressure on the Akt pathway, which not only promotes cell cycle progression at the G1/S checkpoint but also prolongs activation of hypoxia-inducible transcription factors (HIFs). Hypoxia-inducible factors proteins have oxygen-dependent degradation domains and are stabilized in the event of hypoxia, allowing nuclear localization and transcriptional upregulation of a wide variety of genes promoting glucose metabolism as well as Notch and Oct4 which promote the cancer stem cell phenotype (Hjelmeland et al., 2011; Mathieu et al., 2011; Yoshimoto et al., 2011; Qiang et al., 2012). Loss of critical regulatory mechanisms, such as PTEN and NF1, during oncogenic transformation may not only cause uncontrolled cell proliferation, but also stimulate metabolic remodeling and pro-survival signaling, primarily through mammalian target of rapamycin (mTOR; Akhavan et al., 2010). These inhibitors of Akt signaling therefore play a critical role in regulating proliferation, apoptosis, and metabolism especially in the absence of p53.
Mammalian target of rapamycin has been implicated in the process of cellular aging (Cornu et al., 2013). This protein, which both activates Akt as part of the complex mTORC2 and is in turn disinhibited by Akt as part of the complex mTORC1, plays a role in multiple cellular functions in nervous tissues, notably promoting translational activity and glycolysis [reviewed in (Garelick & Kennedy (2011) and Rafalski & Brunet (2011)]. This kinase also promotes proliferative activity through activation of cyclin E; inhibition of mTOR with rapamycin in NPCs induces a quiescence that is reversible upon EGF treatment (Paliouras et al., 2012). Studies have shown that nontumorigenic cell lines slow proliferation upon treatment with rapamycin, while cell lines from rat liver carcinomas are insensitive to the growth-inhibitory effects of this drug (Jimenez et al., 2009). Although the NPCs in the aged brain remain sensitive to both the mTOR-mediated pro-proliferative effects of EGF and the growth-inhibitory effects of rapamycin (Paliouras et al., 2012), we have observed that aged NPCs with higher rates of cell cycle progression have increased mTOR expression (unpublished observation). Such age-related changes may confer malignant potential, as overexpression of mTOR regulatory subunits in GFAP+ cells is sufficient to induce glioma formation (Bashir et al., 2012).
AMP-activated protein kinase (AMPK) acts as another critical node in the regulation of proliferation and metabolism. Activated by low ATP levels, AMPK in turn stabilizes p57, which regulates G1/S progression (Owusu-Ansah et al., 2008), and p53, which regulates G2/M progression (Jones et al., 2005). Simultaneously, activated AMPK promotes glycolysis through activation of phosphofructokinase 2 and fatty acid oxidation through disinhibition of the carnitine transporter CPT1. ATP produced during the breakdown of carbohydrates and lipids then rebalances the AMP/ATP ratio, allowing the inactivation of AMPK and the degradation of tumor suppressors p57 and p53. Thus AMPK regulates a switch between ATP-producing catabolic activity (such as glycolysis) and ATP-consuming anabolic activity (such as cell division). However, in the case of mitochondrial dysfunction, ATP could never be replenished and AMPK might be expected to be constitutively active. Conversely, if cells are glycolytic, continuous ATP production may favor inactivation of AMPK, leading to unconstrained cell cycle progression and the accumulation of unchecked errors. Interestingly, both over-expression and ablation of AMPK cause cell cycle dysfunction, specifically formation of multinucleated cells (Banko et al., 2011). Further research is needed to understand how this metabolic sensor might coordinate the balance between cell cycle arrest and cell cycle progression in aging and oncogenic NPCs.
The connection between metabolic activity and pluripotency is further highlighted by the incidence of mutations within genes encoding IDH1 and IDH2. Common to low-grade glioma in younger patients (Kim et al., 2010), these mutations render cells incapable of producing α-ketoglutarate as part of the citric acid cycle (Yan et al., 2009). IDH1 mutations also bestow gain-of-function properties through the production of oncometabolite 2-hydroxyglutarate, which causes widespread DNA methylation (Liu et al., 2012). It would be useful to test whether the presence of this mutation predicts clinical response to therapies aimed at reversing chromatin modification in patients with glioma.
Although gliomas are thought to rely primarily on glycolysis, like other tumors, it is not clear at which point this metabolic strategy might be acquired. One view holds that glycolytic dependency evolves within a growing tumor (Gatenby & Gillies, 2004). As cells proliferate quickly without effective DNA repair, mutations accumulate and aerobic respiratory enzymes may be rendered dysfunctional. Cells are subject to environmental pressures within a tumor, particularly hypoxia, and those that can exploit the niche may be favored. These cells might survive and create more progeny, leading the tumor as a whole to acquire a glycolytic phenotype. Such genetic diversity and subsequent selection have provided the basis for mathematical models of tumor evolution, which predict the adoption of glycolysis over time (Gerlee & Anderson, 2008). Alternatively, molecular mechanisms may explain why glycolytic metabolism is observed in many types of tumor tissue. Due to extensive cross talk in pathways regulating cell division and aerobic metabolism, any disruption in cell cycle control due to a mutation in a ‘key node’ such as p53 or Akt may inevitably lead to metabolic dysfunction. In the latter case, a glycolytic switch may often be one of the earliest events in the creation of a tumor. The reliability of the observation across many tissue types suggests that acquisition of a glycolytic metabolic strategy may be an integral step in tumor initiation or progression.
Genetic stability and the survival of the fittest
Although large-scale chromosomal abnormalities and point mutations in the nuclear genome drive oncogenic transformation, it is not entirely clear how these changes arise. Genetic instability may simply arise stochastically with DNA replication errors during somatic stem cell proliferation over the course of the lifespan. In fact, the relative rarity of this tumor type may be due to a small stem cell population with a slow rate of cell division, rendering the accumulation of detrimental mutations within a single cell unlikely to be sufficient for oncogenic transformation. Yet, as NPCs continue to proliferate over the lifespan, increased genetic heterogeneity may augment the likelihood that genomically unstable cells acquire an advantage in exploiting the nutrient-rich environment of the brain.
The mutational load in genomic DNA does appear to increase over the lifespan. Loss of heterozygosity (LOH) occurs in NPCs during normal aging (Bailey et al., 2004). Genomic instability may be exacerbated by the loss or mutation of p53 (Honma et al., 2000; Morris, 2002); aged NPCs with decreased p53 activity display increased numbers of micronuclei and H2AX foci (Mikheev et al., 2012). In addition, age-related shortening of telomeres may contribute to loss of genetic stability (Ferron et al., 2009). Genomic instability, particularly LOH on chromosome 10, has been well documented within glioma (Ohgaki & Kleihues, 2007). Although an increased mutation load is associated in turn with better cellular survival, aged NPCs are highly prone to apoptosis (Ahlenius et al., 2009).
Gliomas, like many tumors, contain a highly necrotic core, but the cells capable of generating the tumor are resistant to cell death. Neither the local hypoxic environment nor insults due to radiation and chemotherapy readily affect the viability of this hardy population. Tumor suppressor proteins, in addition to their roles in cell cycle control, promote apoptosis by repressing transcription of pro-survival genes (Raj et al., 2008; Yamaguchi et al., 2009). Dysregulation of these pathways during oncogenic transformation may alter the propensity of NPCs to initiate programmed cell death, a key defense against tumor growth and progression.
Although normal aging is associated with a host of proto-oncogenic changes in NPCs, including increased genetic instability, altered cell cycle control, and a loss of aerobic metabolism (Bailey et al., 2004; Ahlenius et al., 2009), other observations, such as increased apoptosis, are more characteristic of a senescent cell. Apoptotic escape may be a key progression toward tumorigenesis. However, the underlying mechanisms by which cancer cells avoid apoptosis are not yet understood. Mitochondrial quantity declines during normal aging (Stoll et al., 2011b); perhaps a threshold of mitochondrial insufficiency is reached under pathological circumstances, rendering cells both entirely dependent upon glycolysis and incapable of mitochondrial-initiated apoptosis.
Conclusions and future directions
It is not clear how many mutations have to occur to initiate tumorigenesis, although it has been postulated that approximately six to seven key mutations are needed to achieve the hallmarks of cancer (Hanahan & Weinberg, 2000). Yet the loss of a multifunctional protein such as p53 in a stem-like cell during normal aging would make fewer additional mutational events necessary to complete oncogenic transformation. Further investigation is warranted to identify age-related changes in other proteins, such as Akt, mTOR, and AMPK, that act as critical nodes in regulating multiple intracellular pathways. The promiscuity of such key factors may render the balance between healthy regeneration, senescence, and tumorigenesis especially precarious in aging cells. Additionally, interactions of NPCs with other cells within the neurogenic niche can boost or hamper proliferative activity. An appreciation of the ecological nature of the brain environment, allowing the selection of advantageous cellular phenotypes, has recently been embraced in the context of tumor progression. Less understood is the way somatic gene mutations and large-scale chromatin remodeling might play a role in cellular adaptation to the changing environment of the aging brain.
In this review, we have outlined key findings regarding age-related changes within NPCs themselves and in their microenvironment that might contribute to malignant potential within the aged brain. These results implicate a few ‘critical nodes’ in the balance between senescence and oncogenesis: multi-functional proteins that play compensatory roles and are vital to the proper function of numerous cellular activities. This context makes apparent the connections between the various hallmarks of cancer, such as changes in energy metabolism that are commonly observed with increased proliferative capacity. The emergence of such a conceptual framework may help to resolve the paradox between ostensibly senescent phenotypes such as decreased regenerative capacity and the increased oncogenic potential observed in the aging brain. This understanding could be usefully employed in directing future research and in developing preclinical models that may better predict therapeutic responses in human patients by accounting for age-related mechanisms affecting malignancy. In addition, the identification of biomarkers, or signatures of aging, might uniquely inform on patient prognosis and selection of patient-specific therapies.
References
- Aanerud J, Borghammer P, Chakravarty MM, Vang K, Rodell AB, Jonsdottir KY, Moller A, Ashkanian M, Vafaee MS, Iversen P, Johannsen P, Gjedde A. Brain energy metabolism and blood flow differences in healthy aging. J. Cereb. Blood Flow Metab. 2012;32:1177–1187. doi: 10.1038/jcbfm.2012.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adorno M, Cordenonsi M, Montagner M, Dupont S, Wong C, Hann B, Solari A, Bobisse S, Rondina MB, Guzzardo V, Parenti AR, Rosato A, Bicciato S, Balmain A, Piccolo S. A mutant-p53/Smad complex opposes p63 to empower TGFbeta-induced metastasis. Cell. 2009;137:87–98. doi: 10.1016/j.cell.2009.01.039. [DOI] [PubMed] [Google Scholar]
- Ahlenius H, Visan V, Kokaia M, Lindvall O, Kokaia Z. Neural stem and progenitor cells retain their potential for proliferation and differentiation into functional neurons despite lower number in aged brain. J. Neurosci. 2009;29:4408–4419. doi: 10.1523/JNEUROSCI.6003-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhavan D, Cloughesy TF, Mischel PS. mTOR signaling in glioblastoma: lessons learned from bench to bedside. Neuro Oncol. 2010;12:882–889. doi: 10.1093/neuonc/noq052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcantara Llaguno S, Chen J, Kwon CH, Jackson EL, Li Y, Burns DK, Alvarez-Buylla A, Parada LF. Malignant astrocytomas originate from neural stem/progenitor cells in a somatic tumor suppressor mouse model. Cancer Cell. 2009;15:45–56. doi: 10.1016/j.ccr.2008.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman J, Das GD. Autoradiographic and histological studies of postnatal neurogenesis. J. Comp. Neurol. 1966;126:337–389. doi: 10.1002/cne.901260302. [DOI] [PubMed] [Google Scholar]
- Anderson AR, Hassanein M, Branch KM, Lu J, Lobdell NA, Maier J, Basanta D, Weidow B, Narasanna A, Arteaga CL, Reynolds AB, Quaranta V, Estrada L, Weaver AM. Microenvironmental independence associated with tumor progression. Cancer Res. 2009;69:8797–8806. doi: 10.1158/0008-5472.CAN-09-0437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assanah M, Lochhead R, Ogden A, Bruce J, Goldman J, Canoll P. Glial progenitors in adult white matter are driven to form malignant gliomas by platelet-derived growth factor-expressing retroviruses. J. Neurosci. 2006;26:6781–6790. doi: 10.1523/JNEUROSCI.0514-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attolini CS, Cheng YK, Beroukhim R, Getz G, Abdel-Wahab O, Levine RL, Mellinghoff IK, Michor F. A mathematical framework to determine the temporal sequence of somatic genetic events in cancer. Proc. Natl Acad. Sci. USA. 2010;107:17604–17609. doi: 10.1073/pnas.1009117107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey KJ, Maslov AY, Pruitt SC. Accumulation of mutations and somatic selection in aging neural stem/progenitor cells. Aging Cell. 2004;3:391–397. doi: 10.1111/j.1474-9728.2004.00128.x. [DOI] [PubMed] [Google Scholar]
- Banko MR, Allen JJ, Schaffer BE, Wilker EW, Tsou P, White JL, Villen J, Wang B, Kim SR, Sakamoto K, Gygi SP, Cantley LC, Yaffe MB, Shokat KM, Brunet A. Chemical genetic screen for AMPKalpha2 substrates uncovers a network of proteins involved in mitosis. Mol. Cell. 2011;44:878–892. doi: 10.1016/j.molcel.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, Dewhirst MW, Bigner DD, Rich JN. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756–760. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- Barker FG, 2nd, Chang SM, Larson DA, Sneed PK, Wara WM, Wilson CB, Prados MD. Age and radiation response in glioblastoma multiforme. Neurosurgery. 2001;49:1288–1297. doi: 10.1097/00006123-200112000-00002. discussion 1297-8. [DOI] [PubMed] [Google Scholar]
- Bashir T, Cloninger C, Artinian N, Anderson L, Bernath A, Holmes B, Benavides-Serrato A, Sabha N, Nishimura RN, Guha A, Gera J. Conditional astroglial Rictor overexpression induces malignant glioma in mice. PLoS ONE. 2012;7:e47741. doi: 10.1371/journal.pone.0047741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bensaad K, Tsuruta A, Selak MA, Vidal MN, Nakano K, Bartrons R, Gottlieb E, Vousden KH. TIGAR, a p53-inducible regulator of glycolysis and apoptosis. Cell. 2006;126:107–120. doi: 10.1016/j.cell.2006.05.036. [DOI] [PubMed] [Google Scholar]
- Brennan C, Momota H, Hambardzumyan D, Ozawa T, Tandon A, Pedraza A, Holland E. Glioblastoma subclasses can be defined by activity among signal transduction pathways and associated genomic alterations. PLoS ONE. 2009;4:e7752. doi: 10.1371/journal.pone.0007752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown WR, Thore CR. Review: cerebral microvascular pathology in ageing and neurodegeneration. Neuropathol. Appl. Neurobiol. 2011;37:56–74. doi: 10.1111/j.1365-2990.2010.01139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckwalter MS, Yamane M, Coleman BS, Ormerod BK, Chin JT, Palmer T, Wyss-Coray T. Chronically increased transforming growth factor-beta1 strongly inhibits hippocampal neurogenesis in aged mice. Am. J. Pathol. 2006;169:154–164. doi: 10.2353/ajpath.2006.051272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CBTRUS. Statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2004–2005. 2009 [Google Scholar]
- Cheng L, Bao S, Rich JN. Potential therapeutic implications of cancer stem cells in glioblastoma. Biochem. Pharmacol. 2010;80:654–665. doi: 10.1016/j.bcp.2010.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chicoine MR, Silbergeld DL. The in vitro motility of human gliomas increases with increasing grade of malignancy. Cancer. 1995;75:2904–2909. doi: 10.1002/1097-0142(19950615)75:12<2904::aid-cncr2820751218>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Chiu T-L, Peng C-W, Wang M-J. Enhanced anti-glioblastoma activity of microglia by AAV2-mediated IL-12 through TRAIL and phagocytosis in vitro. Oncol. Rep. 2011;25:1373–1380. doi: 10.3892/or.2011.1213. [DOI] [PubMed] [Google Scholar]
- Chow LM, Endersby R, Zhu X, Rankin S, Qu C, Zhang J, Broniscer A, Ellison DW, Baker SJ. Cooperativity within and among Pten, p53, and Rb pathways induces high-grade astrocytoma in adult brain. Cancer Cell. 2011;19:305–316. doi: 10.1016/j.ccr.2011.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung JH, Bunz F. Cdk2 is required for p53-independent G2/M checkpoint control. PLoS Genet. 2010;6:e1000863. doi: 10.1371/journal.pgen.1000863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collado M, Gil J, Efeyan A, Guerra C, Schuhmacher AJ, Barradas M, Benguria A, Zaballos A, Flores JM, Barbacid M, Beach D, Serrano M. Tumour biology: senescence in premalignant tumours. Nature. 2005;436:642. doi: 10.1038/436642a. [DOI] [PubMed] [Google Scholar]
- Coppe J-P, Patil CK, Rodier F, Sun Y, Munoz DP, Goldstein J, Nelson PS, Desprez P-Y, Campisi J. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 2008;6:2853–2868. doi: 10.1371/journal.pbio.0060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornu M, Albert V, Hall MN. mTOR in aging, metabolism, and cancer. Curr. Opin. Genet. Dev. 2013;23:53–62. doi: 10.1016/j.gde.2012.12.005. [DOI] [PubMed] [Google Scholar]
- Covey MV, Streb JW, Spektor R, Ballas N. REST regulates the pool size of the different neural lineages by restricting the generation of neurons and oligodendrocytes from neural stem/progenitor cells. Development. 2012;139:2878–2890. doi: 10.1242/dev.074765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBerardinis RJ, Mancuso A, Daikhin E, Nissim I, Yudkoff M, Wehrli S, Thompson CB. Beyond aerobic glycolysis: transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc. Natl Acad. Sci. USA. 2007;104:19345–19350. doi: 10.1073/pnas.0709747104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ermoian RP, Furniss CS, Lamborn KR, Basila D, Berger MS, Gottschalk AR, Nicholas MK, Stokoe D, Haas-Kogan DA. Dysregulation of PTEN and protein kinase B is associated with glioma histology and patient survival. Clin. Cancer Res. 2002;8:1100–1106. [PubMed] [Google Scholar]
- Ferron SR, Marques-Torrejon MA, Mira H, Flores I, Taylor K, Blasco MA, Farinas I. Telomere shortening in neural stem cells disrupts neuronal differentiation and neuritogenesis. J. Neurosci. 2009;29:14394–14407. doi: 10.1523/JNEUROSCI.3836-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedmann-Morvinski D, Bushong EA, Ke E, Soda Y, Marumoto T, Singer O, Ellisman MH, Verma IM. Dedifferentiation of neurons and astrocytes by oncogenes can induce gliomas in mice. Science. 2012;338:1080–1084. doi: 10.1126/science.1226929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garelick MG, Kennedy BK. TOR on the brain. Exp. Gerontol. 2011;46:155–163. doi: 10.1016/j.exger.2010.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatenby RA, Gillies RJ. Why do cancers have high aerobic glycolysis? Nat. Rev. Cancer. 2004;4:891–899. doi: 10.1038/nrc1478. [DOI] [PubMed] [Google Scholar]
- Gerlee P, Anderson ARA. A hybrid cellular automaton model of clonal evolution in cancer: the emergence of the glycolytic phenotype. J. Theor. Biol. 2008;250:705–722. doi: 10.1016/j.jtbi.2007.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass R, Synowitz M, Kronenberg G, Walzlein J-H, Markovic DS, Wang L-P, Gast D, Kiwit J, Kempermann G, Kettenmann H. Glioblastoma-induced attraction of endogenous neural precursor cells is associated with improved survival. J. Neurosci. 2005;25:2637–2646. doi: 10.1523/JNEUROSCI.5118-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guryanova OA, Wu Q, Cheng L, Lathia JD, Huang Z, Yang J, MacSwords J, Eyler CE, McLendon RE, Heddleston JM, Shou W, Hambardzumyan D, Lee J, Hjelmeland AB, Sloan AE, Bredel M, Stark GR, Rich JN, Bao S. Nonreceptor tyrosine kinase BMX maintains self-renewal and tumorigenic potential of glioblastoma stem cells by activating STAT3. Cancer Cell. 2011;19:498–511. doi: 10.1016/j.ccr.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Heddleston JM, Li Z, McLendon RE, Hjelmeland AB, Rich JN. The hypoxic microenvironment maintains glioblastoma stem cells and promotes reprogramming towards a cancer stem cell phenotype. Cell Cycle. 2009;8:3274–3284. doi: 10.4161/cc.8.20.9701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegi ME, Diserens A-C, Bady P, Kamoshima Y, Kouwenhoven MCM, Delorenzi M, Lambiv WL, Hamou M-F, Matter MS, Koch A, Heppner FL, Yonekawa Y, Merlo A, Frei K, Mariani L, Hofer S. Pathway analysis of glioblastoma tissue after preoperative treatment with the EGFR tyrosine kinase inhibitor gefitinib–a phase II trial. Mol. Cancer Ther. 2011;10:1102–1112. doi: 10.1158/1535-7163.MCT-11-0048. [DOI] [PubMed] [Google Scholar]
- Held-Feindt J, Hattermann K, Muerkoster SS, Wedderkopp H, Knerlich-Lukoschus F, Ungefroren H, Mehdorn HM, Mentlein R. CX3CR1 promotes recruitment of human glioma-infiltrating microglia/macrophages (GIMs) Exp. Cell Res. 2010;316:1553–1566. doi: 10.1016/j.yexcr.2010.02.018. [DOI] [PubMed] [Google Scholar]
- Hjelmeland AB, Wu Q, Heddleston JM, Choudhary GS, MacSwords J, Lathia JD, McLendon R, Lindner D, Sloan A, Rich JN. Acidic stress promotes a glioma stem cell phenotype. Cell Death Differ. 2011;18:829–840. doi: 10.1038/cdd.2010.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland EC, Celestino J, Dai C, Schaefer L, Sawaya RE, Fuller GN. Combined activation of Ras and Akt in neural progenitors induces glioblastoma formation in mice. Nat. Genet. 2000;25:55–57. doi: 10.1038/75596. [DOI] [PubMed] [Google Scholar]
- Honma M, Momose M, Tanabe H, Sakamoto H, Yu Y, Little JB, Sofuni T, Hayashi M. Requirement of wild-type p53 protein for maintenance of chromosomal integrity. Mol. Carcinog. 2000;28:203–214. doi: 10.1002/1098-2744(200008)28:4<203::aid-mc3>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Izycka-Swieszewska E, Rzepko R, Borowska-Lehman J, Stempniewicz M, Sidorowicz M. Angiogenesis in glioblastoma–analysis of intensity and relations to chosen clinical data. Folia Neuropathol. 2003;41:15–21. [PubMed] [Google Scholar]
- Jensen RL. Brain tumor hypoxia: tumorigenesis, angiogenesis, imaging, pseudoprogression, and as a therapeutic target. J. Neurooncol. 2009;92:317–335. doi: 10.1007/s11060-009-9827-2. [DOI] [PubMed] [Google Scholar]
- Jensen RL, Ragel BT, Whang K, Gillespie D. Inhibition of hypoxia inducible factor-1alpha (HIF-1alpha) decreases vascular endothelial growth factor (VEGF) secretion and tumor growth in malignant gliomas. J. Neurooncol. 2006;78:233–247. doi: 10.1007/s11060-005-9103-z. [DOI] [PubMed] [Google Scholar]
- Jimenez RH, Boylan JM, Lee J-S, Francesconi M, Castellani G, Sanders JA, Gruppuso PA. Rapamycin response in tumorigenic and non-tumorigenic hepatic cell lines. PLoS ONE. 2009;4:e7373. doi: 10.1371/journal.pone.0007373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin K, Sun Y, Xie L, Batteur S, Mao XO, Smelick C, Logvinova A, Greenberg DA. Neurogenesis and aging: FGF-2 and HB-EGF restore neurogenesis in hippocampus and subventricular zone of aged mice. Aging Cell. 2003;2:175–183. doi: 10.1046/j.1474-9728.2003.00046.x. [DOI] [PubMed] [Google Scholar]
- Johnson RA, Wright KD, Poppleton H, Mohankumar KM, Finkelstein D, Pounds SB, Rand V, Leary SES, White E, Eden C, Hogg T, Northcott P, Mack S, Neale G, Wang Y-D, Coyle B, Atkinson J, DeWire M, Kranenburg TA, Gillespie Y, Allen JC, Merchant T, Boop FA, Sanford RA, Gajjar A, Ellison DW, Taylor MD, Grundy RG, Gilbertson RJ. Cross-species genomics matches driver mutations and cell compartments to model ependymoma. Nature. 2010;466:632–636. doi: 10.1038/nature09173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones RG, Plas DR, Kubek S, Buzzai M, Mu J, Xu Y, Birnbaum MJ, Thompson CB. AMP-activated protein kinase induces a p53-dependent metabolic checkpoint. Mol. Cell. 2005;18:283–293. doi: 10.1016/j.molcel.2005.03.027. [DOI] [PubMed] [Google Scholar]
- Kamal MM, Sathyan P, Singh SK, Zinn PO, Marisetty AL, Liang S, Gumin J, El-Mesallamy HO, Suki D, Colman H, Fuller GN, Lang FF, Majumder S. REST regulates oncogenic properties of glioblastoma stem cells. Stem Cells. 2012;30:405–414. doi: 10.1002/stem.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy AL, Morton JP, Manoharan I, Nelson DM, Jamieson NB, Pawlikowski JS, McBryan T, Doyle B, McKay C, Oien KA, Enders GH, Zhang R, Sansom OJ, Adams PD. Activation of the PIK3CA/AKT pathway suppresses senescence induced by an activated RAS oncogene to promote tumorigenesis. Mol. Cell. 2011;42:36–49. doi: 10.1016/j.molcel.2011.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y-H, Nobusawa S, Mittelbronn M, Paulus W, Brokinkel B, Keyvani K, Sure U, Wrede K, Nakazato Y, Tanaka Y, Vital A, Mariani L, Stawski R, Watanabe T, De Girolami U, Kleihues P, Ohgaki H. Molecular classification of low-grade diffuse gliomas. Am. J. Pathol. 2010;177:2708–2714. doi: 10.2353/ajpath.2010.100680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjellman C, Olofsson SP, Hansson O, Von Schantz T, Lindvall M, Nilsson I, Salford LG, Sjogren HO, Widegren B. Expression of TGF-beta isoforms, TGF-beta receptors, and SMAD molecules at different stages of human glioma. Int. J. Cancer. 2000;89:251–258. doi: 10.1002/1097-0215(20000520)89:3<251::aid-ijc7>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Kuhn HG, Dickinson-Anson H, Gage FH. Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J. Neurosci. 1996;15:2027–2033. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzumaki N, Ikegami D, Imai S, Narita M, Tamura R, Yajima M, Suzuki A, Miyashita K, Niikura K, Takeshima H, Ando T, Ushijima T, Suzuki T, Narita M. Enhanced IL-1beta production in response to the activation of hippocampal glial cells impairs neurogenesis in aged mice. Synapse. 2010;64:721–728. doi: 10.1002/syn.20800. [DOI] [PubMed] [Google Scholar]
- Lambiv WL, Vassallo I, Delorenzi M, Shay T, Diserens A-C, Misra A, Feuerstein B, Murat A, Migliavacca E, Hamou M-F, Sciuscio D, Burger R, Domany E, Stupp R, Hegi ME. The Wnt inhibitory factor 1 (WIF1) is targeted in glioblastoma and has a tumor suppressing function potentially by induction of senescence. Neuro Oncol. 2011;13:736–747. doi: 10.1093/neuonc/nor036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lathia JD, Gallagher J, Heddleston JM, Wang J, Eyler CE, Macswords J, Wu Q, Vasanji A, McLendon RE, Hjelmeland AB, Rich JN. Integrin alpha 6 regulates glioblastoma stem cells. Cell Stem Cell. 2010;6:421–432. doi: 10.1016/j.stem.2010.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim DA, Cha S, Mayo MC, Chen M-H, Keles E, VandenBerg S, Berger MS. Relationship of glioblastoma multiforme to neural stem cell regions predicts invasive and multifocal tumor phenotype. Neuro Oncol. 2007;9:424–429. doi: 10.1215/15228517-2007-023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Jiang W, Liu J, Zhao S, Xiong J, Mao Y, Wang Y. IDH1 mutations inhibit multiple alpha-ketoglutarate-dependent dioxygenase activities in astroglioma. J. Neurooncol. 2012;109:253–260. doi: 10.1007/s11060-012-0914-4. [DOI] [PubMed] [Google Scholar]
- Luo J, Daniels SB, Lennington JB, Notti RQ, Conover JC. The aging neurogenic subventricular zone. Aging Cell. 2006;5:139–152. doi: 10.1111/j.1474-9726.2006.00197.x. [DOI] [PubMed] [Google Scholar]
- Maslov AY, Barone TA, Plunkett RJ, Pruitt SC. Neural stem cell detection, characterization, and age-related changes in the subventricular zone of mice. J. Neurosci. 2004;24:1726–1733. doi: 10.1523/JNEUROSCI.4608-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathieu J, Zhang Z, Zhou W, Wang AJ, Heddleston JM, Pinna CMA, Hubaud A, Stadler B, Choi M, Bar M, Tewari M, Liu A, Vessella R, Rostomily R, Born D, Horwitz M, Ware C, Blau CA, Cleary MA, Rich JN, Ruohola-Baker H. HIF induces human embryonic stem cell markers in cancer cells. Cancer Res. 2011;71:4640–4652. doi: 10.1158/0008-5472.CAN-10-3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matoba S, Kang JG, Patino WD, Wragg A, Boehm M, Gavrilova O, Hurley PJ, Bunz F, Hwang PM. p53 Regulates mitochondrial respiration. Science. 2006;312:1650–1653. doi: 10.1126/science.1126863. [DOI] [PubMed] [Google Scholar]
- Mikheev AM, Stoll EA, Mikheeva SA, Maxwell JP, Jankowski PP, Ray S, Uo T, Morrison RS, Horner PJ, Rostomily RC. A syngeneic glioma model to assessthe impact of neural progenitor target cell age on tumor malignancy. Aging Cell. 2009;8:499–501. doi: 10.1111/j.1474-9726.2009.00494.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikheev AM, Ramakrishna R, Stoll EA, Mikheeva SA, Beyer RP, Plotnik DA, Schwartz JL, Rockhill JK, Silber JR, Born DE, Kosai Y, Horner PJ, Rostomily RC. Increased age of transformed mouse neural progenitor/stem cells recapitulates age-dependent clinical features of human glioma malignancy. Aging Cell. 2012;11:1027–1035. doi: 10.1111/acel.12004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molofsky AV, Slutsky SG, Joseph NM, He S, Pardal R, Krishnamurthy J, Sharpless NE, Morrison SJ. Increasing p16INK4a expression decreases forebrain progenitors and neurogenesis during ageing. Nature. 2006;443:448–452. doi: 10.1038/nature05091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris SM. A role for p53 in the frequency and mechanism of mutation. Mutat. Res. 2002;511:45–62. doi: 10.1016/s1383-5742(01)00075-8. [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay UK, Eves R, Jia L, Mooney P, Mak AS. p53 Suppresses Src-induced podosome and rosette formation and cellular invasiveness through the upregulation of caldesmon. Mol. Cell. Biol. 2009;29:3088–3098. doi: 10.1128/MCB.01816-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller PAJ, Caswell PT, Doyle B, Iwanicki MP, Tan EH, Karim S, Lukashchuk N, Gillespie DA, Ludwig RL, Gosselin P, Cromer A, Brugge JS, Sansom OJ, Norman JC, Vousden KH. Mutant p53 drives invasion by promoting integrin recycling. Cell. 2009;139:1327–1341. doi: 10.1016/j.cell.2009.11.026. [DOI] [PubMed] [Google Scholar]
- Nishino J, Kim I, Chada K, Morrison SJ. Hmga2 promotes neural stem cell self-renewal in young but not old mice by reducing p16Ink4a and p19Arf Expression. Cell. 2008;135:227–239. doi: 10.1016/j.cell.2008.09.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohgaki H, Kleihues P. Population-based studies on incidence, survival rates, and genetic alterations in astrocytic and oligodendroglial gliomas. J. Neuropathol. Exp. Neurol. 2005;64:479–489. doi: 10.1093/jnen/64.6.479. [DOI] [PubMed] [Google Scholar]
- Ohgaki H, Kleihues P. Genetic pathways to primary and secondary glioblastoma. Am. J. Pathol. 2007;170:1445–1453. doi: 10.2353/ajpath.2007.070011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto M, Inoue K, Iwamura H, Terashima K, Soya H, Asashima M, Kuwabara T. Reduction in paracrine Wnt3 factors during aging causes impaired adult neurogenesis. FASEB J. 2011;25:3570–3582. doi: 10.1096/fj.11-184697. [DOI] [PubMed] [Google Scholar]
- Owusu-Ansah E, Yavari A, Mandal S, Banerjee U. Distinct mitochondrial retrograde signals control the G1-S cell cycle checkpoint. Nat. Genet. 2008;40:356–361. doi: 10.1038/ng.2007.50. [DOI] [PubMed] [Google Scholar]
- Paliouras GN, Hamilton LK, Aumont A, Joppe SE, Barnabe-Heider F, Fernandes KJL. Mammalian target of rapamycin signaling is a key regulator of the transit-amplifying progenitor pool in the adult and aging forebrain. J. Neurosci. 2012;32:15012–15026. doi: 10.1523/JNEUROSCI.2248-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter KR, McCarthy BJ, Freels S, Kim Y, Davis FG. Prevalence estimates for primary brain tumors in the United States by age gender, behavior, and histology. Neuro Oncol. 2010;12:520–527. doi: 10.1093/neuonc/nop066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purow B, Schiff D. Advances in the genetics of glioblastoma: are we reaching critical mass? Nat. Rev. Neurol. 2009;5:419–426. doi: 10.1038/nrneurol.2009.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiang L, Wu T, Zhang HW, Lu N, Hu R, Wang YJ, Zhao L, Chen FH, Wang XT, You QD, Guo QL. HIF-1alpha is critical for hypoxia-mediated maintenance of glioblastoma stem cells by activating Notch signaling pathway. Cell Death Differ. 2012;19:284–294. doi: 10.1038/cdd.2011.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rafalski VA, Brunet A. Energy metabolism in adult neural stem cell fate. Prog. Neurobiol. 2011;93:182–203. doi: 10.1016/j.pneurobio.2010.10.007. [DOI] [PubMed] [Google Scholar]
- Raj D, Liu T, Samadashwily G, Li F, Grossman D. Survivin repression by p53, Rb and E2F2 in normal human melanocytes. Carcinogenesis. 2008;29:194–201. doi: 10.1093/carcin/bgm219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly KM, Loisel DA, Bronson RT, McLaughlin ME, Jacks T. Nf1;Trp53 mutant mice develop glioblastoma with evidence of strain-specific effects. Nat. Genet. 2000;26:109–113. doi: 10.1038/79075. [DOI] [PubMed] [Google Scholar]
- Rong Y, Durden DL, Van Meir EG, Brat DJ. ‘Pseudopalisading’ necrosis in glioblastoma: a familiar morphologic feature that links vascular pathology, hypoxia, and angiogenesis. J. Neuropathol. Exp. Neurol. 2006;65:529–539. doi: 10.1097/00005072-200606000-00001. [DOI] [PubMed] [Google Scholar]
- Shetty AK, Hattiangady B, Shetty GA. Stem/progenitor cell proliferation factors FGF-2, IGF-1, and VEGF exhibit early decline during the course of aging in the hippocampus: role of astrocytes. Glia. 2005;51:173–186. doi: 10.1002/glia.20187. [DOI] [PubMed] [Google Scholar]
- Shukla A, Malik M, Cataisson C, Ho Y, Friesen T, Suh KS, Yuspa SH. TGF-beta signalling is regulated by Schnurri-2-dependent nuclear translocation of CLIC4 and consequent stabilization of phospho-Smad2 and 3. Nat. Cell Biol. 2009;11:777–784. doi: 10.1038/ncb1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, Henkelman RM, Cusimano MD, Dirks PB. Identification of human brain tumour initiating cells. Nature. 2004;432:396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- Sottnik JL, Lori JC, Rose BJ, Thamm DH. Glycolysis inhibition by 2-deoxy-d-glucose reverts the metastatic phenotype in vitro and in vivo. Clin. Exp. Metastasis. 2011;28:865–875. doi: 10.1007/s10585-011-9417-5. [DOI] [PubMed] [Google Scholar]
- Stiles CD, Rowitch DH. Glioma stem cells: a midterm exam. Neuron. 2008;58:832–846. doi: 10.1016/j.neuron.2008.05.031. [DOI] [PubMed] [Google Scholar]
- Stoll EA, Cheung W, Mikheev AM, Sweet IR, Bielas JH, Zhang J, Rostomily RC, Horner PJ. Increased re-entry into cell cycle mitigates age-related neurogenic decline in the murine subventricular zone. Stem Cells. 2011a;29:2005–2017. doi: 10.1002/stem.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoll EA, Habibi BA, Mikheev AM, Lasiene J, Massey SC, Swanson KR, Rostomily RC, Horner PJ. Aging neural progenitor cells have decreased mitochondrial content and lower oxidative metabolism. J. Biol. Chem. 2011b;286:38592–38601. doi: 10.1074/jbc.M111.252171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strano S, Dell’Orso S, DiAgostino S, Fontemaggi G, Sacchi A, Blandino G. Mutant p53: an oncogenic transcription factor. Oncogene. 2007;26:2212–2219. doi: 10.1038/sj.onc.1210296. [DOI] [PubMed] [Google Scholar]
- Sung T, Miller DC, Hayes RL, Alonso M, Yee H, Newcomb EW. Preferential inactivation of the p53 tumor suppressor pathway and lack of EGFR amplification distinguish de novo high grade pediatric astrocytomas from de novo adult astrocytomas. Brain Pathol. 2000;10:249–259. doi: 10.1111/j.1750-3639.2000.tb00258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TCGA. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455:1061–1068. doi: 10.1038/nature07385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tommasi S, Zheng A, Weninger A, Bates SE, Li XA, Wu X, Hollstein M, Besaratinia A. Mammalian cells acquire epigenetic hallmarks of human cancer during immortalization. Nucleic Acids Res. 2013;41:182–195. doi: 10.1093/nar/gks1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villeda SA, Luo J, Mosher KI, Zou B, Britschgi M, Bieri G, Stan TM, Fainberg N, Ding Z, Eggel A, Lucin KM, Czirr E, Park J-S, Couillard-Despres S, Aigner L, Li G, Peskind ER, Kaye JA, Quinn JF, Galasko DR, Xie XS, Rando TA, Wyss-Coray T. The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature. 2011;477:90–94. doi: 10.1038/nature10357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Yang J, Zheng H, Tomasek GJ, Zhang P, McKeever PE, Lee EY, Zhu Y. Expression of mutant p53 proteins implicates a lineage relationship between neural stem cells and malignant astrocytic glioma in a murine model. Cancer Cell. 2009;15:514–526. doi: 10.1016/j.ccr.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warburg O. On respiratory impairment in cancer cells. Science. 1956;124:269–270. [PubMed] [Google Scholar]
- Wheeler CJ, Black KL, Liu G, Ying H, Yu JS, Zhang W, Lee PK. Thymic CD8+ T cell production strongly influences tumor antigen recognition and age-dependent glioma mortality. J. Immunol. 2003;171:4927–4933. doi: 10.4049/jimmunol.171.9.4927. [DOI] [PubMed] [Google Scholar]
- Yamaguchi H, Woods NT, Piluso LG, Lee HH, Chen J, Bhalla KN, Monteiro A, Liu X, Hung MC, Wang HG. p53 Acetylation is crucial for its transcription-independent proapoptotic functions. J. Biol. Chem. 2009;284:11171–11183. doi: 10.1074/jbc.M809268200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan H, Parsons DW, Jin G, McLendon R, Rasheed BA, Yuan W, Kos I, Batinic-Haberle I, Jones S, Riggins GJ, Friedman H, Friedman A, Reardon D, Herndon J, Kinzler KW, Velculescu VE, Vogelstein B, Bigner DD. IDH1 and IDH2 mutations in gliomas. N. Engl. J. Med. 2009;360:765–773. doi: 10.1056/NEJMoa0808710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimoto K, Ma X, Guan Y, Mizoguchi M, Nakamizo A, Amano T, Hata N, Kuga D, Sasaki T. Expression of stem cell marker and receptor kinase genes in glioblastoma tissue quantified by real-time RT-PCR. Brain Tumor Pathol. 2011;28:291–296. doi: 10.1007/s10014-011-0046-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan X, Curtin J, Xiong Y, Liu G, Waschsmann-Hogiu S, Farkas DL, Black KL, Yu JS. Isolation of cancer stem cells from adult glioblastoma multiforme. Oncogene. 2004;23:9392–9400. doi: 10.1038/sj.onc.1208311. [DOI] [PubMed] [Google Scholar]
- Zhai H, Heppner FL, Tsirka SE. Microglia/macrophages promote glioma progression. Glia. 2011;59:472–485. doi: 10.1002/glia.21117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P, Pazin MJ, Schwartz CM, Becker KG, Wersto RP, Dilley CM, Mattson MP. Nontelomeric TRF2-REST interaction modulates neuronal gene silencing and fate of tumor and stem cells. Curr. Biol. 2008;18:1489–1494. doi: 10.1016/j.cub.2008.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziv E, Nemenman I, Wiggins CH. Optimal signal processing in small stochastic biochemical networks. PLoS ONE. 2007;2:e1077. doi: 10.1371/journal.pone.0001077. [DOI] [PMC free article] [PubMed] [Google Scholar]