Abstract
A point mutation [technical knockout25t (tko25t)] in the Drosophila gene coding for mitoribosomal protein S12 generates a phenotype of developmental delay and bang sensitivity. tko25t has been intensively studied as an animal model for human mitochondrial diseases associated with deficiency of mitochondrial protein synthesis and consequent multiple respiratory chain defects. Transgenic expression in Drosophila of the alternative oxidase (AOX) derived from Ciona intestinalis has previously been shown to mitigate the toxicity of respiratory chain inhibitors and to rescue mutant and knockdown phenotypes associated with cytochrome oxidase deficiency. We therefore tested whether AOX expression could compensate the mutant phenotype of tko25t using the GeneSwitch system to activate expression at different times in development. The developmental delay of tko25t was not mitigated by expression of AOX throughout development. AOX expression for 1 d after eclosion, or continuously throughout development, had no effect on the bang sensitivity of tko25t adults, and continued expression in adults older than 30 d also produced no amelioration of the phenotype. In contrast, transgenic expression of the yeast alternative NADH dehydrogenase Ndi1 was synthetically semi-lethal with tko25t and was lethal when combined with both AOX and tko25t. We conclude that AOX does not rescue tko25t and that the mutant phenotype is not solely due to limitations on electron flow in the respiratory chain, but rather to a more complex metabolic defect. The future therapeutic use of AOX in disorders of mitochondrial translation may thus be of limited value.
Keywords: mitochondrial disease, oxidative phosphorylation, gene therapy, seizures, developmental delay
Drosophila provides a useful animal model for human genetic diseases (Lloyd and Taylor 2010; Lu and Vogel 2009), including those associated with mitochondrial dysfunction (Sánchez-Martinez et al. 2006, Palladino 2010). Prominent among the latter are the many diseases caused by deficiency or malfunction of components of the machinery of mitochondrial protein synthesis (Pearce et al. 2013). These can be caused by point mutations of mitochondrial DNA (mtDNA), by large mtDNA deletions, or by nuclear gene lesions, and can involve interactions with environmental factors, including some antibiotics. Although their clinical phenotypes vary, a common thread is deficiency of multiple respiratory chain complexes, including ATP synthase, which include mtDNA-encoded subunits. The resulting metabolic crisis then produces a developmental and physiological disease condition, which can be widespread, severe, and often fatal.
We have previously investigated a Drosophila model of such diseases; tko25t carries a (recessive) point mutation in the gene for mitoribosomal protein S12 (Royden et al. 1987; Shah et al. 1997). tko25t flies exhibit developmental delay, sensitivity to seizures induced by mechanical stress (“bang sensitivity”), and a set of linked phenotypes that share features with human mitochondrial disease, including hearing impairment and sensitivity to antibiotics that impair mitochondrial protein synthesis (Toivonen et al. 2001). At the molecular level, tko25t shows decreased abundance of mitoribosomal small subunits, multiple respiratory chain and ATP synthase deficiency (Toivonen et al. 2001), and altered gene expression indicative of a metabolic shift toward glycolytic lactate production and anaplerotic pathways (Fernández-Ayala et al. 2010).
The phenotype of tko25t flies can be partially suppressed by segmental duplication of the mutant gene in its natural chromosomal milieu (Kemppainen et al. 2009), by cybridization to specific suppressor cytoplasmic (mtDNA) backgrounds (Chen et al. 2012), or by overexpression of spargel (Chen et al. 2012), the Drosophila homolog of PGC1-α, proposed to function as a master regulator of mitochondrial biogenesis (Scarpulla 2011). In other studies, we found that toxic inhibition of complex III (cIII) by antimycin or cIV by cyanide, or phenotypes resulting from mutations or knockdown of cIV subunits or the cIV assembly factor Surf1 in Drosophila, could be mitigated by concomitant expression of the mitochondrial alternative oxidase (AOX) from Ciona intestinalis (Fernández-Ayala et al. 2009; Kemppainen et al. 2014).
AOX is widespread in eukaryotes, being found in plants, fungi, and many animal phyla, although not in arthropods or vertebrates (McDonald et al. 2009). It provides a nonproton-translocating bypass of the cytochrome segment of the mitochondrial respiratory chain, maintaining electron flow under conditions in which it would be inhibited by high membrane potential, toxic inhibition, or insufficient capacity of cIII and/or cIV. tko25t flies exhibit multiple respiratory chain deficiency, including profoundly decreased activity of both cIII and cIV (Toivonen et al. 2001). However, whereas lactate dehydrogenase can theoretically compensate, at least in part, for the lack of cI (Fernández-Ayala et al. 2010), ubiquinone-linked dehydrogenases, such as succinate dehydrogenase (complex II, cII), require the cytochrome chain for onward electron transfer to oxygen to reoxidize ubiquinol. Thus, even though it cannot directly support ATP production, AOX expression in tko25t should facilitate intermediary metabolism, leading to an amelioration of the mutant phenotype if that phenotype is due to limitations on electron flow through cIII and cIV.
We therefore set out to test whether expression of Ciona AOX in Drosophila at different times in the life-cycle could correct the major organismal phenotypes of tko25t, namely bang sensitivity and developmental delay.
Materials and Methods
Flies, maintenance, and behavioral assays
Drosophila lines were as described previously (Toivonen et al. 2001; Fernández-Ayala et al. 2009; Sanz et al. 2010a). Flies were maintained at 25° on standard medium with supplements, as previously described (Fernández-Ayala et al. 2009), including RU486 (Mifepristone), with indicated time to eclosion and bang sensitivity at 25° measured as previously described (Toivonen et al. 2001).
RNA isolation and analysis
RNA extraction and QRTPCR were performed as previously described (Fernández-Ayala et al. 2009). RNA isolations were performed in triplicate from batches of 40 males or 30 virgin females. For QRTPCR, cDNA was synthesized using High-Capacity cDNA Reverse-Transcription kit (Life Technologies, Carlsbad, CA). Analysis used a StepOnePlus instrument (Life Technologies) with the manufacturer’s SYBR Green PCR reagents and customized AOX primers and normalization to RpL32 RNA as previously described (Fernández-Ayala et al. 2009).
Metabolic assays
ATP levels in adult female flies were measured as previously described (Chen et al., 2012), along with ATP standards. Mitochondrial reactive oxygen species (ROS) production was measured essentially according to Ballard et al. (2007) as hydrogen peroxide produced in whole-body mitochondrial extracts from 2- to 5-d-old females using a substrate mix of 5 mM pyruvate, 5 mM proline, 20 mM sn-glycerol-3-phosphate, and 1 mM ADP.
Results
Transgenic expression of AOX in Drosophila using an inducible driver
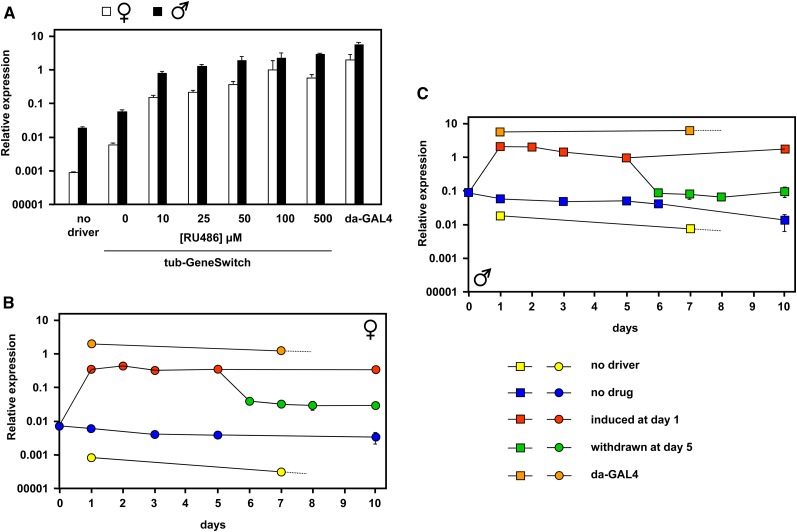
We previously documented the amount of expression of AOX at the RNA level in transgenic flies containing single and double copies of the UAS-AOX transgene activated by different ubiquitously acting drivers (Fernandez-Ayala et al. 2009). In the same study, using the drug-inducible tubulin-GeneSwitch driver (tub-GS), we determined the minimal level of the inducing drug RU486 (10 μM) that would sustain maximal AOX expression throughout development when flies were cultured in drug-containing food. To be able to induce and sustain AOX expression at different times during adult life, we first conducted further tests using the tub-GS driver (Figure 1). Expression of AOX was induced in 1-d-old adults using different concentrations of RU486 and was measured 24 hr later using UAS-AOX–bearing flies with no driver or with the highly active da-GAL4 driver as controls (Figure 1A). Even without drug, the tub-GS driver supported AOX expression at a three-fold to 10-fold higher level than in the absence of any driver. As observed previously using various drivers (Fernandez-Ayala et al. 2009), expression in males was always approximately three-fold higher than in females, which is probably a feature of the standard UAS transgenic construct and/or dosage compensation elements associated with the linked mini-white marker gene. RU486 even at low doses increased expression at least 10-fold further, and expression reached a plateau at a drug concentration of 100 μM. To be sure of fully activating expression, we thereafter routinely used 200 μM RU486 as the activating condition.
Figure 1.
AOX expression in adult flies driven by tubulin-GeneSwitch. (A) Relative AOX expression, determined by QRTPCR normalized to RpL32 control RNA, in 2-d-old UAS-AOX flies bearing the drivers indicated exposed to different concentrations of RU486 for 24 hr. Means ± SD of three biological replicates. Note the logarithmic scale. (B and C) Relative AOX expression in adult UAS-AOX flies bearing the indicated drivers and exposed to 200 μM RU486 as shown. Means ± SD of three biological replicates.
Next, we determined the kinetics of induced expression and the effects of sustained drug exposure or its withdrawal (Figure 1, B and C). AOX expression already reached a plateau level after 1 d of drug exposure in females (Figure 1B) and males (Figure 1C); thereafter, it remained constant if flies were maintained on drug-containing food. If drug was withdrawn by switching to drug-free food at day five, then expression decreased to a new plateau level by 1 d later. However, this level was two-fold to three-fold higher than that of flies never exposed to drug. Flies endowed with UAS-AOX and tub-GS were cultured continuously on RU486-containing food for many weeks and remained phenotypically indistinguishable from flies grown on drug-free food.
Adult-specific induction of AOX does not rescue bang sensitivity of tko25t
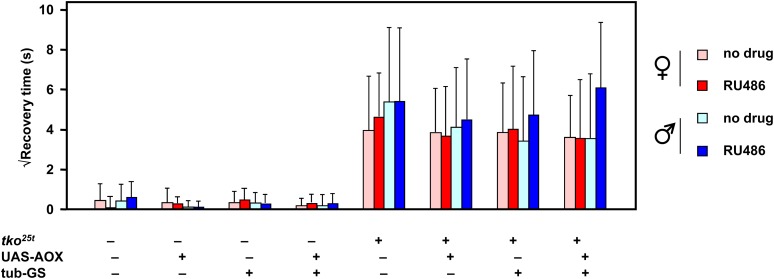
Bang sensitivity is generally considered to arise from a functional defect of nerve conduction during high-frequency stimulation in the giant fiber pathway (Pavlidis and Tanouye 1995; Lee and Wu 2002; Fergestad et al. 2006; Ueda et al. 2008). Bang-sensitive mutants with an underlying mitochondrial defect, including kdn (citrate synthase) and sesB1 (adenine nucleotide translocase) as well as tko25t display a characteristic seizure pattern (Fergestad et al. 2006). We therefore decided to test whether expression of AOX in tko25t mutant flies could compensate for the mitochondrial defect and thus alleviate bang sensitivity. We crossed tub-GS into the tko25t background using a balancer chromosome strategy to analyze progeny from a single experimental cross that generated flies carrying tko25t, tub-GS, and/or UAS-AOX in all eight possible combinations. Bang sensitivity was tested in 2-d-old males and females of each class, either with or without transfer 24 hr earlier to food containing 200 μM RU486 (Figure 2). Unambiguously, the results indicate that AOX is unable to modify the bang-sensitive phenotype of tko25t adults, and it does not induce any detectable bang sensitivity in control flies. In fact, applying Student’s t test with Bonferroni correction confirmed that there were no significant differences between any of the classes that were mutant for tko25t, irrespective of sex, transgene, driver, or RU486 induction. Similarly, there were no significant differences between any of the classes that were wild-type for the tko gene, irrespective of these other parameters. As expected, the difference between tko25t mutant flies of each class and the corresponding class without tko25t was significant (P < 0.01) in every case.
Figure 2.
Bang sensitivity is unaffected by AOX induction in adult flies. Bang sensitivity (square-root of recovery time from vortexing) of 2-d-old flies of the sex and genotype indicated, with or without 24 hr of prior treatment with 200 μM RU486. Means ± SD for groups of 30 individually analyzed flies.
Continuous induction of AOX throughout development does not rescue tko25t
Considering an alternative hypothesis, that the bang-sensitive phenotype of tko25t is established during development, we conducted similar crosses but used fly food containing RU486. In our previous study (Fernandez-Ayala et al. 2009), we established that 10 μM RU486 was sufficient to induce maximal transgene expression during the larval stages, so we used this concentration of the drug along with drug-free control vials. This procedure allowed us also to analyze effects on the second canonical phenotype of tko25t, developmental delay, which was previously found to occur uniquely during the larval (growth) stages (Toivonen et al. 2001).
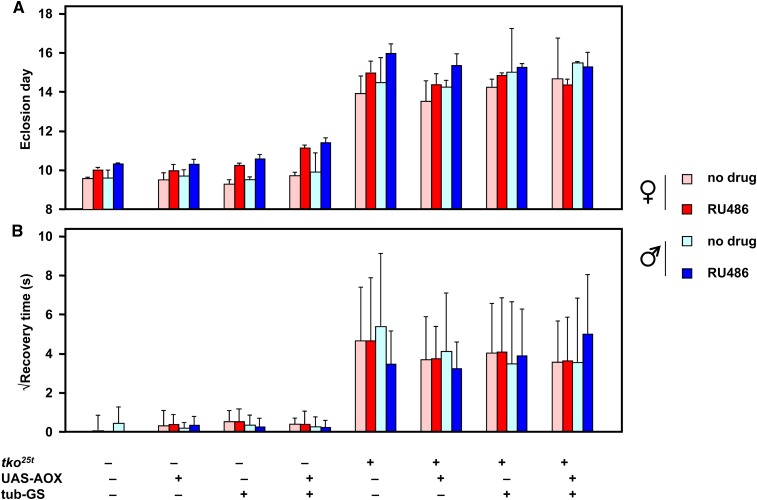
Once again, we observed no rescue of the mutant phenotype that was attributable to AOX expression (Figure 3). The developmental delay of tko25t mutant flies (Figure 3A) was slightly greater in males than in females, as observed previously (Kemppainen et al. 2009), and an additional delay of approximately 1 d was produced in flies of all genotypes and both sexes by the presence of RU486 in the food. The UAS-AOX transgene, the tub-GS driver, and the two in combination did not produce any significant change in developmental timing of tko25t mutant flies, although there was a slight delay produced by AOX expression in wild-type flies, as reported previously using the da-GAL4 driver. The bang sensitivity of the progeny flies showed no significant change according to any of the parameters tested, except for the presence of the tko25t mutation itself (Figure 3B).
Figure 3.
Phenotype of tko25t is unaffected by AOX expression throughout development. (A) Eclosion day and (B) bang sensitivity of 1-d-old flies of the sex and genotype indicated, cultured throughout development on medium with or without 10 μM RU486. Means ± SD based on eclosion data from three replicate experiments and bang sensitivity of groups of 50 individually analyzed flies.
Prolonged adult induction of AOX does not rescue bang sensitivity of tko25t
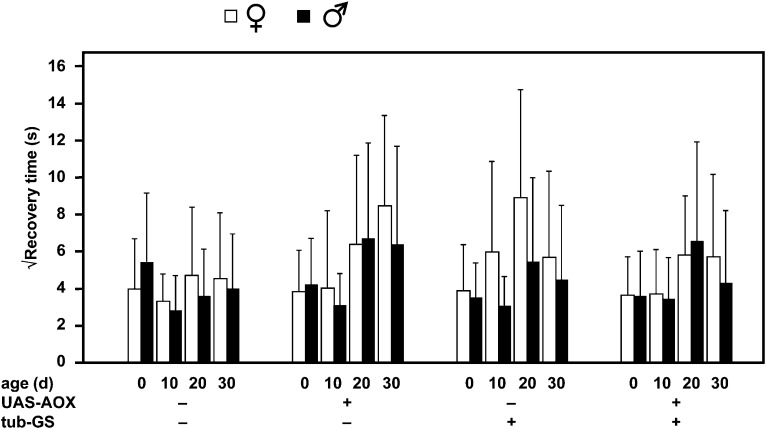
To test whether correction of the tko25t phenotype in adult flies requires long-term expression of AOX, we cultured tko25t flies generated in the previous crosses continuously for a period of 30 d on food either with or without RU486 at the inducing concentration of 200 μM, noting the previous result that sustained expression requires continuous exposure to the drug. This also enabled us to check the stability of the phenotype during adult life, which, to our knowledge, has not previously been studied systematically.
Bang sensitivity was unaffected by any of the parameters tested in this experiment (Figure 4). There was no rescue (or worsening) of the phenotype either by basal or by induced AOX expression, no effect of age, no difference between the sexes, and no effect of tub-GS.
Figure 4.
Bang sensitivity is unaffected by continuous AOX expression over 30 d. Bang sensitivity of flies of the sex, genotype, and age indicated, with or without continuous growth as adults on media containing 200 μM RU486. Means ± SD for groups of 50 individually analyzed flies.
Ndi1 expression during development is lethal to tko25t
Because AOX expression at any stage of the fly life-cycle had no effect on the major phenotypic features of tko25t mutants, we considered the hypothesis that the steps in mitochondrial electron flow that AOX bypasses may not be crucial determinants of the phenotype. The tko25t mutation impacts all four of the enzymatic complexes of the oxidative phosphorylation (OXPHOS) system that contain mitochondrial translation components (Toivonen et al. 2001), but it is unclear which is limiting for respiration or ATP synthesis. Because complex I (cI) activity is severely affected by the mutation, we considered the alternative hypothesis that a decreased capacity for electron flow through cI alone underlies the tko25t mutant phenotype, and that decreased capacity of complexes III and/or IV is immaterial, thus accounting for a failure of AOX expression to modify the phenotype.
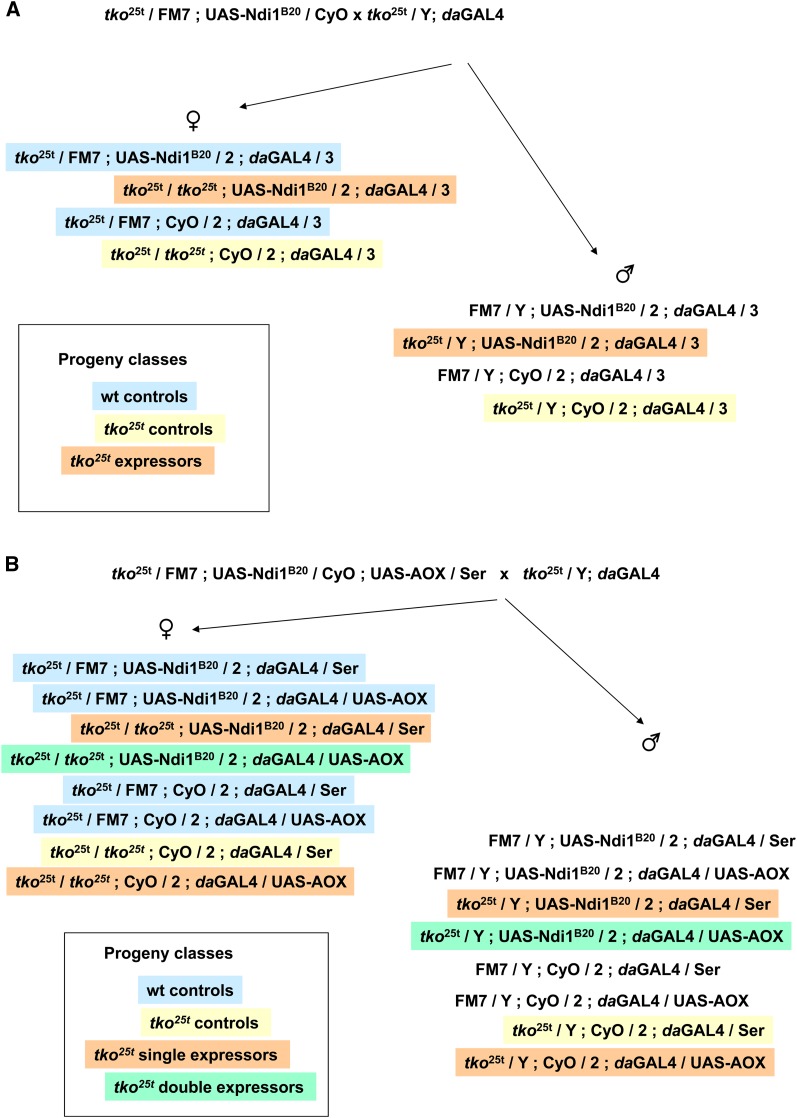
To test this idea, we set-up a genetic cross (Figure 5A) to investigate whether an analogous bypass of cI using the nonproton-pumping NADH dehydrogenase from yeast (Ndi1) could rescue the phenotype. Ndi1 expression was shown previously to be benign in Drosophila and to rescue the lethality of severe knockdown of cI subunits (Sanz et al. 2010b). We introduced the ubiquitously acting da-GAL4 driver and a UAS-Ndi1 transgene separately into the tko25t mutant strain and then crossed females heterozygous both for tko25t and UAS-Ndi1 with tko25t males carrying da-GAL4 (Figure 5A). The cross repeatedly gave a low number of tko25t progeny (Table 1). However, almost all of them carried the balancer marker in place of UAS-Ndi1, indicating that the combination of da-GAL4, tko25t and UAS-Ndi1 is semi-lethal. Expression of Ndi1 in tko25t heterozygotes had a far less dramatic effect. We conclude that, far from rescuing tko25t, expression of Ndi1 is selectively deleterious to tko25t mutant flies.
Figure 5.
Genetic crosses used to test rescue of tko25t. Crosses used to test rescue by (A) Ndi1 or (B) Ndi1 plus AOX combined. Progeny classes are color-coded as indicated to denote their meaning in the experiment. The results of the cross are shown in Table 1. Note that FM7 / Y males do not contain an unmanipulated X-chromosome, so they are not strictly a wild-type control.
Table 1. Test of ability of Ndi1 expression to rescue tko25t.
| Genotypea | Sex | Number of Progenyb |
|---|---|---|
| tko25t / FM7 ; CyO / 2 ; daGAL4 / 3 | Female | 152 |
| tko25t / FM7 ; UAS-Ndi1B20 / 2 ; daGAL4 / 3 | Female | 72 |
| tko25t / tko25t ; CyO / 2 ; daGAL4 / 3 | Female | 57 |
| tko25t / tko25t ; UAS-Ndi1B20 / 2 ; daGAL4 / 3 | Female | 1 |
| FM7 / Y ; CyO / 2 ; daGAL4 / 3 | Male | 65 |
| FM7 / Y ; UAS-Ndi1B20 / 2 ; daGAL4 / 3 | Male | 25 |
| tko25t / Y ; CyO / 2 ; daGAL4 / 3 | Male | 20 |
| tko25t / Y ; UAS-Ndi1B20 / 2 ; daGAL4 / 3 | Male | 1 |
Output from cross shown in Fig. 5A.
A repeat experiment gave similar results.
This result raises the possibility that although neither Ndi1 nor AOX can individually rescue tko25t, the co-expression of both transgenes might do so. This would be the case, for example, if the tko25t phenotype were due to a combined limitation on electron flow at both cI and at cIII+cIV of similar magnitude. Although co-expression of Ndi1 and AOX at 25° was previously shown to be synthetically lethal even in wild-type flies (Sanz et al. 2010b), in trial experiments we were able to obtain co-expressing flies when cultured at 18°. We therefore implemented the experimental cross illustrated in Figure 5B to determine whether Ndi1 and AOX co-expression can rescue tko25t. As shown in Table 2, although control flies were now obtained, and again there were only a few Ndi1-expressing flies in the tko25t mutant background, no doubly expressing tko25t flies eclosed. We conclude that, far from rescuing tko25t, combined expression of the two transgenes is more deleterious than of either alone.
Table 2. Test of ability of Ndi1 and AOX co-expression to rescue tko25t.
| Genotypea | Sex | Number of Progenyb |
|---|---|---|
| tko25t / FM7 ; CyO / 2 ; daGAL4 / Ser | Female | 54 |
| tko25t / FM7 ; CyO / 2 ; daGAL4 / UAS-AOX | Female | 48 |
| tko25t / FM7 ; UAS-Ndi1B20 / 2 ; daGAL4 / Ser | Female | 34 |
| tko25t / FM7 ; UAS-Ndi1B20 / 2 ; daGAL4 / UAS-AOX | Female | 35 |
| tko25t / tko25t ; CyO / 2 ; daGAL4 / Ser | Female | 23 |
| tko25t / tko25t ; CyO / 2 ; daGAL4 / UAS-AOX | Female | 17 |
| tko25t / tko25t ; UAS-Ndi1B20 / 2 ; daGAL4 / Ser | Female | 5 |
| tko25t / tko25t ; UAS-Ndi1B20 / 2 ; daGAL4 / UAS-AOX | Female | 0 |
| FM7 / Y ; CyO / 2 ; daGAL4 / Ser | Male | 26 |
| FM7 / Y ; CyO / 2 ; daGAL4 / UAS-AOX | Male | 27 |
| FM7 / Y ; UAS-Ndi1B20 / 2 ; daGAL4 / Ser | Male | 8 |
| FM7 / Y ; UAS-Ndi1B20 / 2 ; daGAL4 / UAS-AOX | Male | 7 |
| tko25t / Y ; CyO / 2 ; daGAL4 / Ser | Male | 19 |
| tko25t / Y ; CyO / 2 ; daGAL4 / UAS-AOX | Male | 14 |
| tko25t / Y ; UAS-Ndi1B20 / 2 ; daGAL4 / Ser | Male | 6 |
| tko25t / Y ; UAS-Ndi1B20 / 2 ; daGAL4 / UAS-AOX | Male | 0 |
Output from cross shown in Fig. 5B.
A repeat experiment gave similar results.
Effects on ATP or ROS do not correlate with modulation of tko25t phenotype
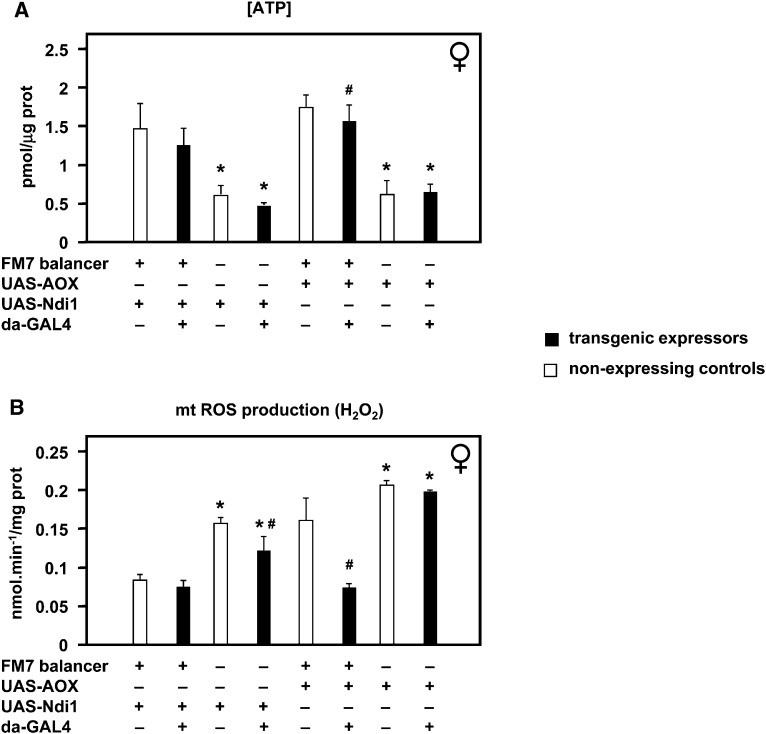
In previous studies we found decreased steady-state ATP levels in extracts from tko25t mutant flies, as well as elevated production of ROS in isolated tko25t mitochondria (Chen et al. 2012). However, the relevance of these observations to the organismal phenotype remains to be conclusively demonstrated. The effects of AOX and Ndi1 expression on the tko25t phenotype provided an opportunity to test this relationship further. To obtain a sufficient number of tko25t flies expressing Ndi1 to conduct this experiment, flies were reared at 18° instead of 25° (see previous section).
We confirmed the previous observation of decreased ATP levels in tko25t homozygotes compared with heterozygous controls (Figure 6A) but found no significant alteration thereof when either AOX or Ndi1 was expressed. Mitochondrial ROS production in tko25t homozygotes was also elevated in every case compared with heterozygous controls (Figure 6B). This was unaffected by expression of AOX but modestly alleviated by Ndi1 expression, despite the fact that the effect of Ndi1 on the overall organismal phenotype was deleterious. This, plus the wide variation in ROS production according to genetic background (reflecting different balancer chromosomes), implies that the tko25t organismal phenotype is also not directly determined by ROS.
Figure 6.
Altered ATP and ROS levels do not account for phenotypic effects of AOX or Ndi1. Effects of Ndi1 and AOX expression on (A) ATP levels and (B) mitochondrial ROS production of female tko25t flies of the indicated genotypes, reared at 18°. Flies were homozygous for tko25t, except those carrying the FM7 balancer, which are phenotypically wild-type. Means ± SD for three or more biological replicates of each genotype.*Significant differences between tko25t homozygotes and heterozygotes of otherwise identical genotypes, P < 0.01, Student’s t test, two-tailed. #Significant differences between Ndi1 or AOX expressors and nonexpressors of otherwise identical genotypes, P < 0.05, Student’s t test, two-tailed.
Discussion
In this work we set out to determine whether AOX from Ciona intestinalis can ameliorate the mutant phenotype of tko25t, which carries a mutation in mitoribosomal protein S12, resulting in globally decreased OXPHOS capacity. We found that induced AOX expression, whether during development, in freshly eclosed adults, or maintained in adults over a period of 30 d, has no effect on tko25t, nor does it produce a phenocopy of tko25t in wild-type flies. In contrast, ubiquitous expression of Ndi1, the alternative NADH dehydrogenase from yeast, was highly deleterious to tko25t during development and was lethal when combined with both tko25t and AOX.
Failure of AOX rescue suggests that a complex metabolic defect underlies the tko25t phenotype
tko25t exhibits a functional deficiency of all four OXPHOS complexes containing mitochondrial translation products (Toivonen et al. 2001), but it is unclear which of these is limiting for electron transfer. Because AOX provides a functional bypass of complexes III and IV, its failure to rescue the organismal phenotype can be interpreted in one of several ways. The first would be that the residual activity of cIII/cIV is not limiting for mitochondrial electron transport in tko25t, and that the phenotype is entirely due to cI dysfunction. The second postulates that AOX is unable to rescue tko25t because, as a nonproton-motive enzyme, it does not support the synthesis of ATP, and ATP deficiency is what underlies the mutant phenotype. A third possibility is that the phenotype is a consequence of one or more processes on which AOX does not impinge, such as elevated ROS production, or proteotoxicity due to the protein synthesis defect. Although none of these can be entirely eliminated, the fact that Ndi1 expression worsens the phenotype, either alone or in combination with AOX, and that changes in ATP level or mitochondrial ROS production do not correlate with it, suggest that the mutant phenotype is determined either by a complex interplay of factors or by other metabolic effects that are as yet unknown. Disrupted redox homeostasis resulting from a cI defect should be rescuable by Ndi1. A combined limitation on electron flow at cI and cIII and/or cIV should be alleviated by combined expression of Ndi1 and AOX. Manifestly, these predictions are inconsistent with our findings.
Ndi1 is constitutively active (Sanz et al. 2010b), consistent with the fact that in its natural setting (in budding yeast) cI is absent. By diverting electrons away from cI, it may act to decrease net ATP production still further, but this seems unlikely to be the explanation for its effect on tko25t because the apparent additional decrease in ATP level (Figure 6A) was modest and not statistically significant. However, the low number of successfully eclosing flies may represent the tail of a distribution, with those individuals suffering further ATP depletion simply unable to complete development. Effects on mitochondrial ROS production also did not correlate with the organismal phenotype. Although we confirmed elevated ROS production in tko25t flies (Figure 6B), it was more affected by genetic background than by the expression of the alternative respiratory chain enzymes, and the effect of Ndi1 was again paradoxical. Note, however, that all metabolic assays were conducted on materials from flies reared at 18°, whereas for most of the phenotypic experiments reported here flies were cultured at 25°. This may have some bearing on the findings.
Proteotoxicity due to imbalance between cytosolic and mitochondrial protein synthesis has been implicated as a longevity mechanism, acting hormetically via the induction of the mitochondrial unfolded protein response (Houtkooper et al. 2013; Arnsburg and Kirstein-Miles 2014). However, decreased levels of NAD+ are associated with a failure of this mechanism (Mouchiroud et al. 2013). The deleterious effect produced by Ndi1 expression is again not consistent with this being the primary mechanism underlying the tko25t phenotype.
The failure of AOX to rescue bang sensitivity and developmental delay in tko25t reflects a similar finding for a second mutant affecting mitochondrial ATP production, sesB1 (Vartiainen et al. 2014). sesB1 carries a mutation in the gene encoding the major adult isoform of the adenine nucleotide translocase (Zhang et al. 1999) and, like tko25t, sesB1 mutant flies show decreased steady-state ATP levels as well as bang sensitivity and developmental delay (Vartiainen et al. 2014). For these reasons, as well as the arguments stated above, we feel the “ATP hypothesis” cannot be entirely discounted, although other metabolic effects need to be further investigated as well.
Bang sensitivity of tko25t is a developmental rather than a degenerative phenotype
Bang sensitivity is a commonly observed mutant phenotype in Drosophila and is due to lesions affecting a variety of cellular or physiological pathways, including, in addition to mitochondrial protein synthesis, adenine nucleotide transport and the TCA cycle (Fergestad et al. 2006), phospholipid metabolism (Pavlidis et al. 1994), ion pumps and channels (Schubiger et al. 1994; Kane et al. 2000; Iovchev et al. 2002; Parker et al. 2011), and proteolysis (Zhang et al. 2002). Although they manifest some similarities in their electrophysiological defects (Engel and Wu 1994), they fall into two classes depending on whether motor neurons are directly affected (Fergestad et al. 2006). Some of them show a clear degenerative phenotype with drastically shortened lifespan, whereas others, including tko25t, show only a modestly decreased lifespan and associated neuropathology (Fergestad et al. 2008). In the current study, we found no significant change in the bang sensitivity of tko25t over 30 d of adult life, in contrast to the synergistic and progressive effects on bang sensitivity seen when tko25t is combined with other bang-sensitive mutants (Fergestad et al. 2008). We conclude that the bang sensitivity of tko25t is a developmentally determined phenotype, at least in an otherwise wild-type genetic background
Therapeutic implications for AOX in mitochondrial disease
AOX has been proposed as a therapeutic tool relevant to a wide variety of mitochondrial disorders (El-Khoury et al. 2014). The present work indicates important limitations of this concept, whatever the precise link between mitochondrial translational dysfunction and the organismal phenotype in tko25t. Despite profound effects on flies exposed to toxins or mutations directly or indirectly affecting cytochrome oxidase (Fernandez-Ayala et al. 2009; Kemppainen et al. 2014), or even the pleiotropic phenotypes caused by partial knockdown of DNA polymerase γ (Humphrey et al. 2012), AOX expression produced no detectable modification to the tko25t phenotype.
tko25t has been considered as a model for mitochondrial diseases, exhibiting not only seizures and developmental delay but also hearing impairment (Toivonen et al. 2001). It is of particular relevance to those disorders where the primary defect is in the mitochondrial translation system, which applies to many of the commonest pathological mtDNA mutations such as the 3243G > A MELAS mutation, as well as an increasingly recognized subset of nuclear gene mitochondrial disorders exhibiting multiple OXPHOS deficiencies (Pearce et al. 2013). The implementation of respiratory chain bypasses such as AOX or Ndi1 should, in theory, alleviate pathological phenotypes associated with restrictions on electron transport, depending on which segments of the respiratory chain are affected. In cases where multiple OXPHOS complexes are affected, both bypasses in combination might be needed to restore electron flow. tko25t constitutes a model for such diseases, yet neither AOX nor Ndi1 ameliorated the phenotype, and Ndi1 was even deleterious. As already indicated, Ndi1 and AOX do not restore proton pumping at the respiratory chain segments that they bypass, nor can they alleviate, a priori, all other aspects of mitochondrial dysfunction. Their uses in eventual therapy for disorders of mitochondrial translation therefore may be limited and clearly requires a fuller understanding of the pathophysiological mechanism case by case.
Acknowledgments
We thank Tea Tuomela, Eveliina Kaulio, and Outi Kurronen for technical assistance, and Pierre Rustin, Eric Dufour, Alberto Sanz, and Rhoda Stefanatos for many useful discussions. Funding was provided by Academy of Finland (FinMIT Centre of Excellence, Academy Professorship to H.T.J.), Sigrid Juselius Foundation, and Tampere University Hospital Medical Research Fund. The authors declare no competing interests. H.T.J., K.K.K., and E.K. together conceived and designed the experiments, which K.K.K. and E.K. executed. All authors contributed to data analysis. H.T.J. compiled the figures and drafted the manuscript.
Footnotes
Communicating editor: H. D. Lipshitz
Literature Cited
- Arnsburg K., Kirstein-Miles J., 2014. Interrelation between protein synthesis, proteostasis and life span. Curr. Genomics 15: 66–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard J. W., Melvin R. G., Muller J. T., Katewa S. D., 2007. Sex differences in survival and mitochondrial bioenergetics during aging in Drosophila. Aging Cell 6: 699–708 [DOI] [PubMed] [Google Scholar]
- Chen S., Oliveira M. T., Sanz A., Kemppainen E., Fukuoh A., et al. , 2012. A cytoplasmic suppressor of a nuclear mutation affecting mitochondrial functions in Drosophila. Genetics 192: 483–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Khoury R., Kemppainen K. K., Dufour E., Szibor M., Jacobs H. T., et al. , 2014. Engineering the alternative oxidase gene to better understand and counteract mitochondrial defects: State of the art and perspectives. Br. J. Pharmacol. 171: 2243–2249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel J. E., Wu C. F., 1994. Altered mechanoreceptor response in Drosophila bang-sensitive mutants. J. Comp. Physiol. A. 175: 267–78 [DOI] [PubMed] [Google Scholar]
- Fergestad T., Bostwick B., Ganetzky B., 2006. Metabolic disruption in Drosophila bang-sensitive seizure mutants. Genetics 173: 1357–1364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fergestad T., Olson L., Patel K. P., Miller R., Palladino M. J., et al. , 2008. Neuropathology in Drosophila mutants with increased seizure susceptibility. Genetics 178: 947–956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Ayala D. J., Sanz A., Vartiainen S., Kemppainen K. K., Babusiak M., et al. , 2009. Expression of the Ciona intestinalis alternative oxidase (AOX) in Drosophila complements defects in mitochondrial oxidative phosphorylation. Cell Metab. 9: 449–460 [DOI] [PubMed] [Google Scholar]
- Fernández-Ayala D. J., Chen S., Kemppainen E., O’Dell K. M. C., Jacobs H. T., 2010. Gene expression in a Drosophila model of mitochondrial disease. PLoS ONE 5: e8549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houtkooper R. H., Mouchiroud L., Ryu D., Moullan N., Katsyuba E., et al. , 2013. Mitonuclear protein imbalance as a conserved longevity mechanism. Nature 497: 451–457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphrey D. M., Parsons R. B., Ludlow Z. N., Riemensperger T., Esposito G., et al. , 2012. Alternative oxidase rescues mitochondria-mediated dopaminergic cell loss in Drosophila. Hum. Mol. Genet. 21: 2698–2712 [DOI] [PubMed] [Google Scholar]
- Iovchev M., Kodrov P., Wolstenholme A. J., Pak W. L., Semenov E. P., 2002. Altered drug resistance and recovery from paralysis in Drosophila melanogaster with a deficient histamine-gated chloride channel. J. Neurogenet. 16: 249–261 [DOI] [PubMed] [Google Scholar]
- Kane N. S., Hirschberg B., Qian S., Hunt D., Thomas B., et al. , 2000. Drug-resistant Drosophila indicate glutamate-gated chloride channels are targets for the antiparasitics nodulisporic acid and ivermectin. Proc. Natl. Acad. Sci. USA 97: 13949–13954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemppainen E, Fernández-Ayala D. J., Galbraith L. C., O’Dell K. M. C., and H. T. Jacobs, 2009. Phenotypic suppression of the Drosophila mitochondrial disease-like mutant tko25t by duplication of the mutant gene in its natural chromosomal context. Mitochondrion 9: 353–363 [DOI] [PubMed] [Google Scholar]
- Kemppainen K. K., Rinne J., Sriram A., Lakanmaa M., Zeb A., et al. , 2014. Expression of alternative oxidase in Drosophila ameliorates diverse phenotypes due to cytochrome oxidase deficiency. Hum. Mol. Genet. 23: 2078–2093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J., Wu C. F., 2002. Electroconvulsive seizure behavior in Drosophila: analysis of the physiological repertoire underlying a stereotyped action pattern in bang-sensitive mutants. J. Neurosci. 22: 11065–11079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd T. E., Taylor J. P., 2010. Flightless flies: Drosophila models of neuromuscular disease. Ann. N. Y. Acad. Sci. 1184: e1–e20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu B., Vogel H., 2009. Drosophila models of neurodegenerative diseases. Annu. Rev. Pathol. 4: 315–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald A. E., Vanlerberghe G. C., Staples J. F., 2009. Alternative oxidase in animals: unique characteristics and taxonomic distribution. J. Exp. Biol. 212: 2627–2634 [DOI] [PubMed] [Google Scholar]
- Mouchiroud L., Houtkooper R. H., Moullan N., Katsyuba E., Ryu D., et al. , 2013. The NAD(+)/sirtuin pathway modulates longevity through activation of mitochondrial UPR and FOXO signaling. Cell 154: 430–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palladino M. J., 2010. Modeling mitochondrial encephalomyopathy in Drosophila. Neurobiol. Dis. 40: 40–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker L., Padilla M., Du Y., Dong K., Tanouye M. A., 2011. Drosophila as a model for epilepsy: bss is a gain-of-function mutation in the para sodium channel gene that leads to seizures. Genetics 187: 523–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlidis P., Tanouye M. A., 1995. Seizures and failures in the giant fiber pathway of Drosophila bang-sensitive paralytic mutants. J. Neurosci. 15: 5810–5819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlidis P., Ramaswami M., Tanouye M. A., 1994. The Drosophila easily shocked gene: a mutation in a phospholipid synthetic pathway causes seizure, neuronal failure, and paralysis. Cell 79: 23–33 [DOI] [PubMed] [Google Scholar]
- Pearce S., Nezich C. L., Spinazzola A., 2013. Mitochondrial diseases: translation matters. Mol. Cell. Neurosci. 55: 1–12 [DOI] [PubMed] [Google Scholar]
- Royden C. S., Pirrotta V., Jan L. Y., 1987. The tko locus, site of a behavioral mutation in D. melanogaster, codes for a protein homologous to prokaryotic ribosomal protein S12. Cell 51: 165–173 [DOI] [PubMed] [Google Scholar]
- Sánchez-Martínez A., Luo N., Clemente P., Adán C., Hernández-Sierra R., et al. , 2006. Modeling human mitochondrial diseases in flies. Biochim. Biophys. Acta 1757: 1190–1198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz A., Fernández-Ayala D. J., Stefanatos R. K., Jacobs H. T., 2010. a Mitochondrial ROS production correlates with, but does not directly regulate lifespan in Drosophila. Aging 2: 200–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz A., Soikkeli M., Portero-Otín M., Wilson A., Kemppainen E., et al. , 2010b. Expression of the yeast NADH dehydrogenase Ndi1 in Drosophila confers increased lifespan independently of dietary restriction. Proc. Natl. Acad. Sci. USA 107: 9105–9110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarpulla R. C., 2011. Metabolic control of mitochondrial biogenesis through the PGC-1 family regulatory network. Biochim. Biophys. Acta 1813: 1269–1278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schubiger M., Feng Y., Fambrough D. M., Palka J., 1994. A mutation of the Drosophila sodium pump alpha subunit gene results in bang-sensitive paralysis. Neuron 12: 373–381 [DOI] [PubMed] [Google Scholar]
- Shah Z. H., O’Dell K. M. C., Miller S. C., An X., Jacobs H. T., 1997. Metazoan nuclear genes for mitoribosomal protein S12. Gene 204: 55–62 [DOI] [PubMed] [Google Scholar]
- Toivonen J. M., O’Dell K. M., Petit N., Irvine S. C., Knight G. K., et al. , 2001. technical knockout, a Drosophila model of mitochondrial deafness. Genetics 159: 241–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda A., Grabbe C., Lee J., Lee J., Palmer R. H., et al. , 2008. Mutation of Drosophila focal adhesion kinase induces bang-sensitive behavior and disrupts glial function, axonal conduction and synaptic transmission. Eur. J. Neurosci. 27: 2860–2870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vartiainen S., Chen S., George J., Tuomela T., Luoto K. R., et al. , 2014. Phenotypic rescue of a Drosophila model of mitochondrial ANT1 disease. Dis. Model. Mech. 7: 635–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Tan J., Reynolds E., Kuebler D., Faulhaber S., et al. , 2002. The Drosophila slamdance gene: a mutation in an aminopeptidase can cause seizure, paralysis and neuronal failure. Genetics 162: 1283–1299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y. Q., Roote J., Brogna S., Davis A. W., Barbash D. A., et al. , 1999. stress sensitive B encodes an Adenine Nucleotide Translocase in Drosophila melanogaster. Genetics 153: 891–903 [DOI] [PMC free article] [PubMed] [Google Scholar]