The Genus Chrysosporium: Its Clinical Importance
The anamorphic (asexual) genus Chrysosporium Corda includes mostly keratinophilic species that live on the remains of hair and feathers in soil. These fungi are rarely reported as animal pathogens, apart from in reptiles, and only a few species have been involved in mycoses. In a comprehensive review of opportunistic mycoses published by Smith [1], only one case of a Chrysosporium-incited cutaneous abscess in a snake was cited with no additional details provided. A few other cases have been published in a small range of animal species including C. pannicola (formerly C. evolceanui) from affected skin of a dog [2], a case of keratomycosis in a horse [3], a probable case of mycosis caused by C. tropicum in two breeds of chickens [4], and a disseminated infection in a dog [5] by a pair of Chrysosporium isolates not identified to the species level. Some species also occasionally infect humans with reports of C. keratinophilum and C. pannicola in skin and nail infections and some deep infections by C. zonatum [6]. However, in recent years there has been a noticeable increase in mycoses caused by some Chrysosporium-related fungi in reptiles. These fungal pathogens are, however, unrelated to the chytrids (Phylum: Chytridiomycota), such as Batrachochytrium dendrobatidis, which is causing the current catastrophic die-off of amphibians, i.e., chytridiomycosis in frogs [7].
Chrysosporium-Related Fungi: Incidence, Pathogenicity, and Potential Causes of Disease in Reptiles
Organisms identified as the Chrysosporium anamorph of Nannizziopsis vriesii (unsuitably abbreviated as CANV in some reports) and similar undescribed species [8]–[18], C. guarroi [19], [20] (now a synonym of Nannizziopsis guarroi; Figure 1A [21]) and C. ophiodiicola [22] (now a synonym of Ophidiomyces ophiodiicola; Figure 1B [23]) have been isolated with some frequency from reptiles in recent years. These fungi are the cause of superficial and deep mycoses that affect pets as well as captive and wild animals. Several reports indicate that these organisms are an emerging cause of fungal disease in bearded dragons and iguanas [8], [10], [11], [19], [24]. As these species of reptiles continue to gain popularity as pets, the disease is being found worldwide with cases reported thus far in Asia, Australia, Europe, and North America. The source(s) of the etiologic agents of this contagious mycosis, however, are yet unknown. One survey of the skin of healthy squamate (covered with scales) reptiles from zoological and veterinary institutions revealed that Chrysosporium-related fungi are present in very low numbers in the cutaneous mycobiota of reptiles [25].
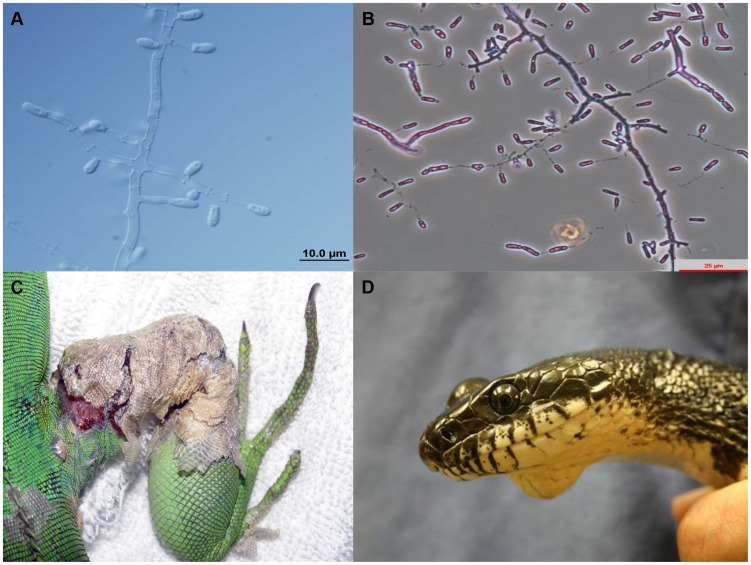
Figure 1. Microscopic morphology of Nannizziopsis guarroi (A) and Ophidiomyces ophiodiicola (B).
Green iguana showing ulcerative dermatitis in the right leg caused by N. guarroi (C). Black rat snake showing a mycotic granuloma caused by O. ophiodiicola (D). Figure 1D was published previously by Rajeev et al. [22].
Bearded dragons kept in captivity suffer a sometimes fatal dermatological condition known in the pet trade as “yellow fungus disease,” and it has been suggested recently that N. guarroi and some other related species are the etiological agents [10], [21], [23]. The circumstances under which mycotic diseases in these species occur are not yet known, although inadequate diet and husbandry, environmental stresses, trauma, and existing dermatitis are all likely contributors [26]. Furthermore, some Chrysosporium-related fungi, especially N. dermatitidis, can act as primary pathogens in veiled chameleons [23], [27]. Infection by these species in bearded dragons begins as a cutaneous disease often characterized by vesicular lesions and bullae. Necrosis, sloughing, and ulceration then follow, progressing to involve muscle and bone. The infection can disseminate with a fatal outcome [10], [12], [23], [28]. In captive and pet green iguanas, cases of superficial dermatomycoses and other more severe infections that progress to involve muscle and bone have been recently reported [11], [19], [20]. Most of these cases were probably caused by N. guarroi (Figure 1C) [21], [23]. Table 1 summarizes other Chrysosporium-related fungi involved in cutaneous and/or systemic mycoses in a variety of other captive and wildlife reptile species (Figure 1D). Infections caused by Nannizziopsis, Paranannizziopsis, and Ophidiomyces species are contagious among reptiles [23].
Table 1. Proposed species causing infection in reptiles [21], [23].
| Chrysosporium-related fungi | Reptile species |
| Nannizziopsis Currah (1985) | |
| N. arthrosporioides Stchigel et al. (2013) | Water dragon (Physignathus sp.) |
| N. barbata Sigler et al. (2013) | Coastal bearded dragon (Pogona barbata) |
| N. chlamydospora Stchigel et al. (2013) | Inland bearded dragon (Pogona vitticeps) |
| N. crocodili Sigler et al. (2013) | Saltwater crocodile (Crocodylus porosus) |
| N. dermatitidis Sigler et al. (2013) | Chameleons, geckos |
| N. draconii Cabañes et al. (2013) | Inland bearded dragon |
| N. guarroi Cabañes et al. (2013) | Green iguana (Iguana iguana), inland bearded dragon, lizard (Agama agama), snake |
| N. pluriseptata Stchigel et al. (2013) | Skink lizard (Eumeces inexpectatus) |
| N. vriesii Currah (1985) | Lizard (Ameiva sp.) |
| Paranannizziopsis Sigler et al. (2013) | |
| P. australiensis Sigler et al. (2013) | Northern tuatara (Sphenodon punctatus punctatus), coastal bearded dragon, aquatic file snake (Acrochordus sp.) |
| P. californiensis Sigler et al. (2013) | Tentacled snake (Erpeton tentaculatum) |
| P. crustacea Sigler et al. (2013) | Tentacled snake |
| P. longispora Sigler et al. (2013) | Tentacled snake |
| Ophidiomyces Sigler et al. (2013) | |
| O. ophiodiicola Sigler et al. (2013) | Snakes |
A recent essay [29] hypothesized that fungal proliferation after the devastation of the Cretaceous-Tertiary (K-T) event preferentially selected for the fungal-resistant endothermic mammals and hindered the reemergence of a second reptilian age. The darkened skies and cooler temperatures that accompanied the K-T cataclysm would have shielded the sun and reduced the ability of ectothermic creatures such as reptiles to induce fevers by insolation, a necessary activity for protection against fungal diseases. Historically, mycotic infections in reptiles have likely remained underdiagnosed. Most recognize fungal infections as secondary infections resulting primarily from poor husbandry and underlying chronic comorbidities. Along with good food and proper husbandry, adequate light and heat are also essential to reptile health [30].
Chrysosporium-Related Fungi: Taxonomy
The genus Chrysosporium is polyphyletic, having affiliation with at least two orders of the Ascomycota; however, rDNA sequencing studies which included a representative number of reference Chrysosporium and related species indicate that it should be restricted to anamorphs (asexual states) in the order Onygenales [31]. Relevant fungal pathogens that produce important mycoses such as blastomycosis, coccidioidomycosis, dermatophytosis, histoplasmosis, and paracoccidioidomycosis are also grouped in this order. These species usually produce white to yellowish colonies and poorly differentiated fertile hyphae with terminal, lateral, and usually one-celled conidia. These conidia are broader than the diameter of the supporting hyphae and are mainly clavate or pyriform with a truncated base, sessile or borne on short protrusions or side branches of the vegetative hyphae [6]. Species of Chrysosporium are not easy to identify since their conidia are similar to those of other anamorphic genera such as Blastomyces, Emmonsia, Geomyces, Malbranchea, and Myceliophthora, and also to some species in the dermatophyte genus Trichophyton that produce only microconidia. About 65 Chrysosporium species are currently accepted and their sexual morphs (teleomorphs) are found in a variety of genera such as Aphanoascus, Arthroderma, or Nannizziopsis, among others [32].
Nannizziopsis vriesii (Apinis) Currah (Ascomycota, Onygenales, Onygenaceae) has white ascomata, asperulate peridial hyphae constricted at septa, hyaline and globose ascospores, and a Chrysosporium asexual morph. The ex type strain of this species was isolated from the skin and lungs of a lizard [33], [34]. Most fungal isolates from reptiles have been considered to belong to the Chrysoporium anamorph of N. vriesii because of morphological similarities of the anamorph with those of this ascomycete. However, no sexual structures of N. vriesii have been obtained in these case reports and some phenotypic and molecular differences among isolates from reptiles have been detected. This corroborates what was suggested several years ago from preliminary molecular phylogenetic analysis that the Chrysoporium anamorph of N. vriesii actually represented a species complex, rather than a single species, containing members that could be allied to specific hosts [26].
Recently, Stchigel et al. [21] and Sigler et al. [23] published the latest taxonomic revisions regarding Chrysosporium-related fungi, also noting relationships between specific fungal species and different reptile hosts. These new revisions also comply with the recent changes in the International Code of Nomenclature for algae, fungi, and plants (one fungus, one name) [35]. In these papers [21], [23], one new family (Nannizziopsiaceae), two new genera (Ophidiomyces and Paranannizziopsis), and 15 new species were proposed. Table 1 highlights these new Chrysosporium-related fungi and the reptile hosts they are known to infect.
Given the difficulty in identifying Chrysosporium species morphologically, and prior to our knowledge of closely related genera, several older reports of infection in reptiles by species such as C. keratinophilum and C. tropicum [17] may have actually been caused by the Chrysosporium anamorph of N. vriesii. Another example is the C. queenslandicum isolate from a case report of a mycosis in a snake [36], which upon reexamination of the fungus was indeed found to be the Chrysosporium anamorph of N. vriesii [28].
Treatment of Chrysosporium-Related Infections in Reptiles
Treatment of fungal infections in reptiles includes the administration of effective antifungal agents for a minimum of 2 to 4 weeks, together with the correction of inappropriate environmental conditions. As most cases of mycotic diseases in reptiles are diagnosed at necropsy, there are relatively few reports that discuss effective dosages and dosage intervals of antifungal agents. The systemic drugs of choice for use in reptiles diagnosed with infection caused by filamentous fungi include ketoconazole and itraconazole [26]. Voriconazole seems to be also a safe and effective antimycotic drug to eliminate these infections in bearded dragons [24]. However, treatment using antifungals has shown mixed results [37]. As mentioned earlier, adequate light and heat are also essential to reptile health and largely influence clinical recovery, given that the reptile's immune response, metabolism of drugs, and use of fluid therapy are heat dependent [30].
Human Infections Caused by Chrysosporium-Related Fungi Affecting Reptiles
As mentioned above, it has been demonstrated that various Chrysosporium-related fungi appear to be host specific. This is evidenced by the fact that three species of Nannizziopsis which have occasionally infected humans or have been found in clinical samples in the United States have never been implicated in reptile disease [23]. These species include N. hominis, recovered from groin lesions, inguinal nodes, and leg abscesses of an HIV-positive patient and from an inguinal node of an immunocompetent patient with disseminated adenopathy, N. infrequens, isolated from a bronchial wash specimen of an HIV-positive patient, and N. obscura in a case of osteomyelitis [21], [23]. This somewhat mitigates zoonotic concerns associated with handling popular pet reptiles such as green iguanas or bearded dragons, in which N. guarroi is the common etiologic agent [23]. However, the identity of a Chrysosporium-related isolate that produced a lung infiltration and a brain abscess in a Nigerian HIV-positive patient in Germany [38] is still unclear. It was originally identified as Chrysosporium anamorph of N. vriesii, but phenotypic and molecular characteristics of the isolate were not provided by the authors [38]. This isolate has been recently identified as an atypical strain of N. vriesii by Stchigel et al. [21] and as a strain close to N. obscura by Sigler et al. [23]. Most of these few human cases occurred as opportunistic infections in immunocompromised patients. In regards to infection, handling pets is no more risky for an immunosuppressed person than is contact with other people or the environment [39]. However, in these cases, a special precaution should be taken because of the fact that exotic or wild animals may harbor unusual pathogens.
Acknowledgments
We thank S. Rajeev (The University of Georgia, Tifton, US), M. van Meter (Animal Medical Center of Rome, Georgia, US), and J. Martorell (Universitat Autònoma de Barcelona, Bellaterra, Spain) for kindly providing us Figures 1C and 1D included in this paper.
Funding Statement
Financial support came from the Veterinary Bacteriology and Mycology of the Universitat Autònoma de Barcelona. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Smith JMB (1989) Opportunistic mycoses of man and other animals. Wallingford, United Kingdom: CAB International Mycological Institute. [Google Scholar]
- 2. Hajsig M, Vries GA de, Sertic V, Naglic T (1974) Chrysosporium evolceanui from pathologically changed dog skin. Veterinarski Arhiv 44: 209–211. [Google Scholar]
- 3. Grahn B, Wolfer J, Keller C, Wilcock B (1993) Equine keratomycosis: clinical and laboratory findings in 23 cases. Prog Vet Comp Ophthalmol 3: 2–7. [Google Scholar]
- 4. Saidi SA, Bhatt S, Richard JL, Sikdar A, Ghosh GR (1994) Chrysosporium tropicum as a probable cause of mycosis of poultry in India. Mycopathologia 125: 143–147. [DOI] [PubMed] [Google Scholar]
- 5. Watt PR, Robins GM, Galloway AM, O'Boyle DA (1995) Disseminated opportunistic fungal disease in dogs: 10 cases (1982–1990). J Am Vet Med Assoc 207: 67–70. [PubMed] [Google Scholar]
- 6.Hoog GS de, Guarro J, Gené J, Figueras MJ (2011) Atlas of clinical fungi, electronic version 3.1. Utrecht, The Netherlands: Centraalbureau voor Schimmelcultures/Universitat Rovira i Virgili. [Google Scholar]
- 7. Rosenblum EB, Voyles J, Poorten J, Stajich JE (2010) The deadly chytrid fungus: a story of an emerging pathogen. PLoS Pathog 6: e1000550 doi:10.1371/journal.ppat. 1000550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Abarca ML, Martorell J, Castella G, Ramis A, Cabanes FJ (2009) Dermatomycosis in a pet inland bearded dragon (Pogona vitticeps) caused by a Chrysosporium species related to Nannizziopsis vriesii . Vet Dermatol 20: 295–299. [DOI] [PubMed] [Google Scholar]
- 9. Bertelsen MF, Crawshaw GJ, Sigler L, Smith DA (2005) Fatal cutaneous mycosis in tentacled snakes (Erpeton tentaculatum) caused by the Chrysosporium anamorph of Nannizziopsis vriesii . J Zoo Wild Med 36: 82–87. [DOI] [PubMed] [Google Scholar]
- 10. Bowman MR, Paré JA, Sigler L, Naeser JP, Sladky KK, et al. (2007) Deep fungal dermatitis in three inland bearded dragons (Pogona vitticeps) caused by Chrysosporium anamorph of Nannizziopsis vriesii . Med Mycol 45: 371–376. [DOI] [PubMed] [Google Scholar]
- 11. Han JI, Lee SJ, Na KJ (2010) Necrotizing dermatomycosis caused by Chrysosporium spp. in three captive green iguanas (Iguana iguana) in South Korea. J Exot Pet Med 19: 240–244. [Google Scholar]
- 12. Hedley J, Eatwell K, Hume L (2010) Necrotising fungal dermatitis in a group of bearded dragons (Pogona vitticeps). Vet Rec 166: 464–465. [DOI] [PubMed] [Google Scholar]
- 13. Hellebuyck T, Baert K, Pasmans F, Waeyenberghe L van, Beernaert L, et al. (2010) Cutaneous hyalohyphomycosis in a girdled lizard (Cordylus giganteus) caused by the Chrysosporium anamorph of Nannizziopsis vriesii and successful treatment with voriconazole. Vet Dermatol 21: 429–433. [DOI] [PubMed] [Google Scholar]
- 14. Johnson RSP, Sangster CR, Sigler L, Hambletond S, Paré JA (2011) Deep fungal dermatitis caused by the Chrysosporium anamorph of Nannizziopsis vriesii in captive coastal bearded dragons (Pogona barbata). Aust Vet J 89: 515–519. [DOI] [PubMed] [Google Scholar]
- 15. Martel A, Fonteyne PA, Chiers K, Decostere A, Pasmans F (2006) Nasal Nannizziopsis vriesii granuloma in an ameiva lizard (Ameiva chaitzami). Vlaams Diergen Tijds 75: 306–307. [Google Scholar]
- 16. Nichols DK, Weyant RS, Lamirande EW, Sigler L, Mason RT (1999) Fatal mycotic dermatitis in captive brown tree snakes (Boiga irregularis). J Zoo Wildlife Med 30: 111–118. [PubMed] [Google Scholar]
- 17. Paré JA, Sigler L, Hunter DB, Summerbell RC, Smith DA, et al. (1997) Cutaneous mycoses in chameleons caused by the Chrysosporium anamorph of Nannizziopsis vriesii (Apinis) Currah. J Zoo Wildlife Med 28: 443–453. [PubMed] [Google Scholar]
- 18. Thomas AD, Sigler L, Peucker S, Norton JH, Nielan A (2002) Chrysosporium anamorph of Nannizziopsis vriesii associated with fatal cutaneous mycoses in the salt-water crocodile (Crocodylus porosus). Med Mycol 40: 143–151. [DOI] [PubMed] [Google Scholar]
- 19. Abarca ML, Martorell J, Castella G, Ramis A, Cabanes FJ (2008) Cutaneous hyalohyphomycosis caused by a Chrysosporium species related to Nannizziopsis vriesii in two green iguanas (Iguana iguana). Med Mycol 46: 349–354. [DOI] [PubMed] [Google Scholar]
- 20. Abarca ML, Martorell J, Castella G, Cabanes FJ (2010) Chrysosporium guarroi sp. nov. a new emerging pathogen of pet green iguanas (Iguana iguana). Med Mycol 48: 365–72. [DOI] [PubMed] [Google Scholar]
- 21. Stchigel AM, Sutton DA, Cano-Lira JF, Cabañes FJ, Abarca ML, et al. (2013) Phylogeny of chrysosporia infecting reptiles: proposal of the new family Nannizziopsiaceae and five new species. Persoonia 31: 86–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rajeev S, Sutton DA, Wickes BL, Miller DL, Giri D, et al. (2009) Isolation and characterization of a new fungal species, Chrysosporium ophiodiicola from a mycotic granuloma of a black rat snake (Elaphe obsolete obsolete). J Clin Microbiol 47: 1264–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sigler L, Hambleton S, Pare JA (2013) Molecular characterization of reptile pathogens currently known as members of the Chrysosporium anamorph of Nannizziopsis vriesii complex and relationship with some human-associated isolates. J Clin Microbiol 51: 3338–3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Van Waeyenberghe L, Baert K, Pasmans F, van Rooij P, Hellebuyck T, et al. (2010) Voriconazole, a safe alternative for treating infections caused by the Chrysosporium anamorph of Nannizziopsis vriesii in bearded dragons (Pogona vitticeps). Med Mycol 48: 880–885. [DOI] [PubMed] [Google Scholar]
- 25. Paré JA, Sigler L, Rypien KL, Gibas CFC (2003) Cutaneous mycobiota of captive squamate reptiles with notes on the scarcity of Chrysosporium anamorph of Nannizziopsis vriesii . J Herpetol Med Surg 13: 10–15. [Google Scholar]
- 26.Paré JA, Sigler L, Rosenthal KL, Mader DR (2006) Microbiology: fungal and bacterial diseases of reptiles. In: Mader DR, editor. Reptile medicine and surgery. St.Louis, USA: Saunders Elsevier. pp. 217–238. [Google Scholar]
- 27. Paré JA, Coyle KA, Sigler L, Maas AK, Mitchell RL (2006) Pathogenicity of the Chrysosporium anamorph of Nannizziopsis vriesii for veiled chameleons (Chamaeleo calyptratus). Med Mycol 44: 25–31. [DOI] [PubMed] [Google Scholar]
- 28.Paré JA, Jacobson E (2007) Mycotic diseases of reptiles. In: Jacobson E, editor. Infectious diseases and pathology of reptiles: color atlas and text. Boca Raton, USA: CRC Press. pp. 527–570. [Google Scholar]
- 29. Casadevall A (2012) Fungi and the rise of mammals. PLoS Pathog 8: e1002808 doi:10.1371/journal.ppat.1002808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Latney LV, Wellehan J (2013) Selected emerging infectious diseases of squamata. Vet Clin Exot Anim 16: 319–338. [DOI] [PubMed] [Google Scholar]
- 31.Vidal P, Vinuesa MA, Sánchez-Puelles JM, Guarro J (2000) Phylogeny of the anamorphic genus Chrysosporium and related taxa based on rDNA internal transcribed spacer sequences. In: Kushwaha RKS, Guarro J, editors. Biology of dermatophytes and other keratinophilic fungi. Bilbao, Spain: Revista Iberoamericana de Micología. pp. 22–29. [Google Scholar]
- 32.Seifert K, Morgan-Jones G, Gams W, Kendrick B (2011) The genera of Hyphomycetes. Utrecht, The Netherlands: Centraalbureau voor Schimmelcultures. [Google Scholar]
- 33. Currah RS (1985) Taxonomy of the Onygenales: Arthrodermataceae, Gymnoascaceae, Myxotrichaceae and Onygenaceae. Mycotaxon 24: 1–216. [Google Scholar]
- 34. Guarro J, Cano J, de Vroey C (1991) Nanniziopsis (Ascomycotina) and related genera. Mycotaxon 42: 193–200. [Google Scholar]
- 35. Norvell LL (2011) Fungal nomenclature. 1. Melbourne approves a new code. Mycotaxon 116: 481–490. [Google Scholar]
- 36. Vissiennon T, Schuppel KF, Ullrich E, Kuijpers AFA (1999) Case report. A disseminated infection due to Chrysosporium queenslandicum in a garter snake (Thamnopsis). Mycoses 42: 107–110. [DOI] [PubMed] [Google Scholar]
- 37. Mitchell MA, Walden MR (2013) Chrysosporium anamorph Nannizziopsis vriesii: an emerging fungal pathogen of captive and wild reptiles. Vet Clin North Am Exot Anim Pract 16: 659–668. [DOI] [PubMed] [Google Scholar]
- 38. Steininger C, Lunzen J van, Tintelnot K, Sobottka I, Rohde H, et al. (2005) Mycotic brain abscess caused by opportunistic reptile pathogen. Emerg Infect Dis 11: 349–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Greene CE, Levy JK (2006) Immunocompromised people and shared human animal infection: zoonoses, sapronoses, and anthroponoses. In: Greene CE, editor. Infectious diseases of the dog and cat. St. Louis, USA: Saunders Elsevier. pp.1051–1068. [Google Scholar]