Ibrutinib is a potent inhibitor of Bruton’s tyrosine kinase (BTK). Studies published in the New England Journal of Medicine report that patients with chronic lymphocytic leukemia (CLL) have durable responses to ibrutinib, but they also describe the advent of bypass mutations that result in ibrutinib resistance and progressive disease.
Signaling through the B cell receptor (BCR) can promote tumor cell survival in B cell malignancies, including CLL, mantle cell lymphoma (MCL) and the activated B cell-like (ABC) subtype of diffuse large B cell lymphoma (DLBCL). The BCR consists of immunoglobulin heavy (IgH) and light (IgL) chains coupled to a CD79A-CD79B heterodimer that transduces signals by engaging downstream non-receptor kinases, including BTK (Young and Staudt, 2013). These kinases offer a wealth of therapeutic targets, and drugs targeting SYK, BTK and phosphatidylinositol 3-kinase (PI3K) are in clinical trials to evaluate their efficacy against a variety of human lymphomas.
Ibrutinib (PCI-32765, Imbruvica) is an irreversible inhibitor of BTK that works by forming a covalent bond with cysteine 481 (C481) in the BTK active site, rendering the drug potent and highly selective, thereby limiting side effects. Several clinical trials are now evaluating ibrutinib in human lymphomas, and the drug has been granted breakthrough status by the United States Food and Drug Administration for the treatment of refractory MCL and high-risk CLL. Since activating mutations in BTK have not been observed in these lymphomas, it is likely that upstream signaling from the BCR is the culprit.
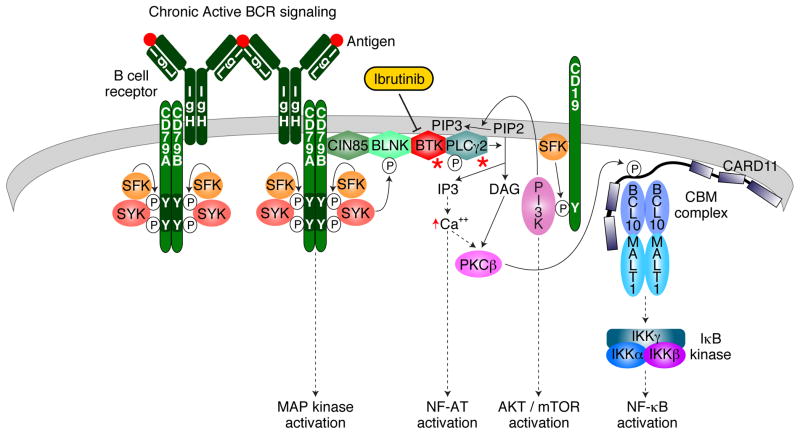
BCR expression is obligatory in normal B cells and in most malignant B cells. In CLL, analysis of the antigen recognition portion of the BCR revealed preferential usage of a small subset of immunoglobulin variable gene segments, suggesting that the BCRs may react with an antigen. In support of this notion, different CLL and MCL patients can have “stereotypic” BCRs with virtually identical antigen recognition sites (Agathangelidis et al., 2012). The first direct evidence for BCR-dependent survival signaling was obtained in ABC DLBCL (Davis et al., 2010). RNA interference screening revealed that BCR components and downstream signaling effectors (SYK, BTK, PLCγ2) are required for ABC DLBCL cell survival. Microscopy revealed BCR clusters on the surface of ABC DLBCL cells that are similar to those induced by antigen engagement of the BCR in normal B cells. Recurrent gain-of-function mutations in CD79A and CD79B augment BCR signaling in a subset of ABC DLBCL cases, providing genetic evidence that the BCR pathway is important in the pathogenesis of this lymphoma subtype. The “chronic active” form of BCR signaling in ABC DLBCL is sensitive to ibrutinib and therefore may be mechanistically similar to BCR signaling in CLL and MCL (Figure 1).
Figure 1.
B cell Receptor Signaling in malignant B cells.
Chronic active BCR signaling is shown. Ibrutinib is shown to inhibit BTK. Red asterisks denote signaling effectors that are the target of ibrutinib resistance mutations in CLL patients.
Three reports in the New England Journal of Medicine examined ibrutinib treatment in CLL patients. The first study evaluated ibrutinib monotherapy in patients with relapsed and high-risk CLL versus ofatumumab, an anti-CD20 antibody that is the current standard therapy for these patients. Ibrutinib produced a 70% response rate compared with only 21% for ofatumumab, and ibrutinib was also superior to ofatumumab with respect to progression-free and overall survival (Byrd et al., 2014).
However, ibrutinib does not eliminate the malignant clone in CLL, and the latter two studies describe resistance mechanisms in CLL patients who had progressive disease while on ibrutinib therapy (Woyach et al., 2014) (Furman et al., 2014). Whole-exome sequencing of pre-treatment and relapse samples from six CLL patients identified a mutation that changed the cysteine at BTK position 481 to serine (C481S) in five of the six patients. BTK C481S prevents the drug from covalently binding BTK, rendering cells substantially more resistant to ibrutinib. By contrast, cells with wild type or mutant BTK were equally sensitive to dasatinib, a reversible BTK inhibitor that does not act on C481. Additionally, CLL cells from two patients had gain-of-function mutations targeting PLCγ2, a direct downstream target of BTK phosphorylation. These data imply that the efficacy of ibrutinib in CLL is due to inhibition of BTK in the malignant cells rather than other potential effects on non-malignant cells. It is unclear at present how frequently these mutations cause ibrutinib resistance in CLL and it seems likely that other resistance mechanisms remain to be discovered.
These results are reminiscent of resistance mutations that occur with other tyrosine kinase inhibitors, including the T315I mutation in BCR-ABL that disrupts a hydrogen bond with imatinib (Gorre et al., 2001) and the T790M mutation in EGFR that sterically excludes erlotinib from the active site (Pao et al., 2005). A variety of next-generation inhibitors have been developed to circumvent these mutations. Similarly, ATP-competitive BTK inhibitors could be developed that do not require C481 for activity, although it will be a challenge to identify molecules with the potency of ibrutinib. The detection of PLCG2 mutations may indicate that a CLL clone could bypass the need for BTK activity altogether. Perhaps arguing against this, one CLL case had both PLCG2 and BTK C481S mutations.
One wonders about the origins of the BTK and PLCG2 mutations. Are these mutations present because of the low ongoing rate of mutagenesis within the tumor? Substantial intraclonal diversity has been reported in CLL and other malignancies, and it is known that standard cytotoxic chemotherapies promote the outgrowth of tumor subclones (Landau et al., 2013). Alternatively, mutations in BTK and PLCG2 may confer gain-of-function phenotypes that lead to their enrichment prior to therapy. Of note, one of the PLCG2 mutations in CLL (S707Y) was previously identified as a germline-encoded mutation that causes an autoinflammatory disorder due to its ability to enhance synthesis of inositol trisphosphate (IP3) and promote calcium flux after receptor stimulation (Zhou et al., 2012). Two other PLCG2 mutants in CLL (R665W, L845F) increase calcium flux in response to IgM cross-linking (Woyach et al., 2014), raising the possibility that they augment chronic active BCR signaling and increase the abundance of the subclone prior to therapy by increasing tumor cell proliferation and/or survival.
Roughly 20% of patients with CLL have persistent lymphocytosis during ibrutinib therapy and most patients are likely to have residual tumor cells, providing ample opportunity for the emergence of resistant subclones. This necessitates the development of drug combinations that limit the avenues available to the malignant cells for proliferation and survival. A recent chemical genomics screen in ABC DLBCL revealed that ibrutinib synergizes with inhibitors of SYK, BCL2, and multiple components of the PI3K pathway (Mathews Griner et al., 2014). The PI3K delta inhibitor idelalisib and the BCL2 inhibitor navitoclax have already shown single agent activity in CLL, supporting their evaluation in combination with ibrutinib. Thus, although CLL may have won the battle with ibrutinib in some patients, our ever-increasing armamentarium of drugs targeting the BCR pathway should allow us to win the war.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agathangelidis A, Darzentas N, Hadzidimitriou A, Brochet X, Murray F, Yan XJ, Davis Z, van Gastel-Mol EJ, Tresoldi C, Chu CC, et al. Stereotyped B-cell receptors in one-third of chronic lymphocytic leukemia: a molecular classification with implications for targeted therapies. Blood. 2012;119:4467–4475. doi: 10.1182/blood-2011-11-393694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrd JC, Brown JR, O’Brien S, Barrientos JC, Kay NE, Reddy NM, Coutre S, Tam CS, Mulligan SP, Jaeger U, et al. Ibrutinib versus Ofatumumab in Previously Treated Chronic Lymphoid Leukemia. The New England journal of medicine. 2014 doi: 10.1056/NEJMoa1400376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis RE, Ngo VN, Lenz G, Tolar P, Young RM, Romesser PB, Kohlhammer H, Lamy L, Zhao H, Yang Y, et al. Chronic active B-cell-receptor signalling in diffuse large B-cell lymphoma. Nature. 2010;463:88–92. doi: 10.1038/nature08638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furman RR, Cheng S, Lu P, Setty M, Perez AR, Guo A, Racchumi J, Xu G, Wu H, Ma J, et al. Ibrutinib resistance in chronic lymphocytic leukemia. The New England journal of medicine. 2014;370:2352–2354. doi: 10.1056/NEJMc1402716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorre ME, Mohammed M, Ellwood K, Hsu N, Paquette R, Rao PN, Sawyers CL. Clinical resistance to STI-571 cancer therapy caused by BCR-ABL gene mutation or amplification. Science. 2001;293:876–880. doi: 10.1126/science.1062538. [DOI] [PubMed] [Google Scholar]
- Landau DA, Carter SL, Stojanov P, McKenna A, Stevenson K, Lawrence MS, Sougnez C, Stewart C, Sivachenko A, Wang L, et al. Evolution and impact of subclonal mutations in chronic lymphocytic leukemia. Cell. 2013;152:714–726. doi: 10.1016/j.cell.2013.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews Griner LA, Guha R, Shinn P, Young RM, Keller JM, Liu D, Goldlust IS, Yasgar A, McKnight C, Boxer MB, et al. High-throughput combinatorial screening identifies drugs that cooperate with ibrutinib to kill activated B-cell-like diffuse large B-cell lymphoma cells. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:2349–2354. doi: 10.1073/pnas.1311846111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pao W, Miller VA, Politi KA, Riely GJ, Somwar R, Zakowski MF, Kris MG, Varmus H. Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. PLoS medicine. 2005;2:e73. doi: 10.1371/journal.pmed.0020073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woyach JA, Furman RR, Liu TM, Ozer HG, Zapatka M, Ruppert AS, Xue L, Li DH, Steggerda SM, Versele M, et al. Resistance mechanisms for the Bruton’s tyrosine kinase inhibitor ibrutinib. The New England journal of medicine. 2014;370:2286–2294. doi: 10.1056/NEJMoa1400029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young RM, Staudt LM. Targeting pathological B cell receptor signalling in lymphoid malignancies. Nature reviews Drug discovery. 2013;12:229–243. doi: 10.1038/nrd3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Lee GS, Brady J, Datta S, Katan M, Sheikh A, Martins MS, Bunney TD, Santich BH, Moir S, et al. A hypermorphic missense mutation in PLCG2, encoding phospholipase Cgamma2, causes a dominantly inherited autoinflammatory disease with immunodeficiency. American journal of human genetics. 2012;91:713–720. doi: 10.1016/j.ajhg.2012.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]