Abstract
Ferns are the only major lineage of vascular plants not represented by a sequenced nuclear genome. This lack of genome sequence information significantly impedes our ability to understand and reconstruct genome evolution not only in ferns, but across all land plants. Azolla and Ceratopteris are ideal and complementary candidates to be the first ferns to have their nuclear genomes sequenced. They differ dramatically in genome size, life history, and habit, and thus represent the immense diversity of extant ferns. Together, this pair of genomes will facilitate myriad large-scale comparative analyses across ferns and all land plants. Here we review the unique biological characteristics of ferns and describe a number of outstanding questions in plant biology that will benefit from the addition of ferns to the set of taxa with sequenced nuclear genomes. We explain why the fern clade is pivotal for understanding genome evolution across land plants, and we provide a rationale for how knowledge of fern genomes will enable progress in research beyond the ferns themselves.
Keywords: Azolla, Ceratopteris, Comparative analyses, Ferns, Genomics, Land plants, Monilophytes
Introduction
Ferns (Monilophyta) are an ancient lineage of land plants that comprise a significant component of the Earth’s terrestrial flora. They are the second largest group of vascular plants, with more than 10,000 species [1], and play a major role in shaping community assembly and ecological processes in many biomes. For example, ferns shape ecosystem regeneration, persistence, and biomass production in eastern North American temperate forests [2-4]; play keystone roles in tropical rainforest canopies [5,6], heathlands [7], after landslides [8], and on islands [9]; and include several invasive species with significant economic impact [10-12]. Phylogenetically, ferns are sister to the seed plant clade (Spermatophyta) that includes the ecologically dominant flowering plants. Thus, the phylogenetic position of ferns makes them pivotal in the evolutionary history of land plants (Embryophyta), and essential for a comprehensive understanding of the origin and diversification of numerous traits found in seed plant crops and model species, such as rice and Arabidopsis[13,14].
Review
In a broad sense, ferns include four main clades: psilotoids (whisk ferns) + ophioglossoids, equisetoids (horsetails), marattioids, and leptosporangiates (Figure 1). The leptosporangiate ferns are the most species-rich clade by far, with over 9,000 species [15,16] that include the majority of fern species found in temperate and tropical regions. Ferns and seed plants diverged from a common ancestor around 380 million years ago (mya) (the oldest fern fossils date to ca. 350 mya [17]), and the most recent common ancestor (MRCA) of the leptosporangiate ferns arose ca. 280 mya [17,18]. Several fern lineages diverged from one another prior to the divergence of the angiosperm and gymnosperm sister clades (Figure 1).
Figure 1.
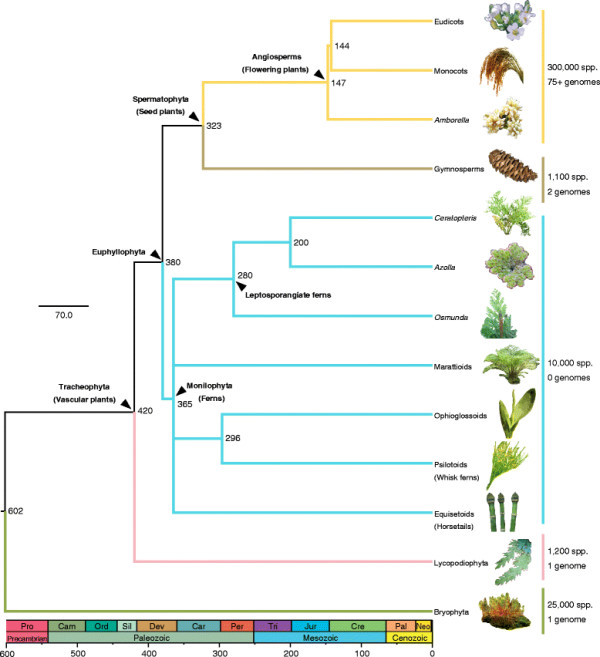
Phylogeny of major groups of land plants. Based on [13,15,19,20]. Approximate numbers of species and available genome sequences are given, and approximate times of major divergences are indicated. Ferns as a whole include lineages that diverged from one another prior to the divergence of the major seed plant clades. The most recent common ancestor of all leptosporangiates arose approximately 280 mya [17,18]. The ancestors of Ceratopteris and Azolla diverged from each other ca. 200 mya, well before the divergence of monocots and eudicots. Dates obtained from TimeTree [21,22].
Despite the ubiquity of ferns and their ecological and evolutionary importance, genomic resources for the group remain sparse. Ferns are the only major clade of vascular land plants for which a complete nuclear genome has not yet been sequenced. This gap is particularly acute in light of recent efforts to sequence the transcriptomes of all major lineages of green plants [23,24]. The assembly, analysis, and interpretation of these transcriptomes would benefit enormously from the availability of well-annotated fern genomes. Recent innovations in sequencing technologies and the resulting torrent of whole-genome sequencing projects have fueled a renaissance in comparative genetic and genomic analyses, and each genome sequenced yields new insights into plant evolution. For example, the recently-sequenced genome of Amborella trichopoda[25]—the sister taxon to all other angiosperms—has revealed much about the conservation of synteny across flowering plants and about genome organization, as well as gene content in the ancestral angiosperm. It has also facilitated inference of ancient genome doubling events in angiosperms. Ferns, with their large genomes, high chromosome numbers, independent gametophyte phase, and mix of heterosporous and homosporous taxa, offer unparalleled opportunities for groundbreaking comparative genetic and genomic analyses across land plants as a whole.
Ferns provide a stark contrast to other lineages of land plants in several key biological features. For example, angiosperms and gymnosperms are both dominated by a diploid, spore-bearing (sporophyte) stage of the life cycle. Their haploid sexual stage, the gametophyte, is extremely reduced (microscopic in angiosperms) and completely dependent on the sporophyte for nutrition. On the other hand, in bryophytes (mosses, liverworts and hornworts), it is the sporophyte that is dependent at maturity on the dominant, macroscopic and photosynthetic gametophyte. Ferns and lycophytes are the only land plants where, for most taxa, both gametophytes and sporophytes are independent, free-living organisms that can each be long-lived. Unlike seed plants, which are exclusively heterosporous, ferns include both heterosporous and homosporous species. The latter group includes the majority of extant fern diversity, in which only one spore type is produced that develops into a gametophyte that is either bisexual, or whose sex is determined by non-genetic aspects of development (e.g., pheromones from surrounding gametophytes). The evolution from homospory to heterospory—in which a megaspore develops into a female gametophyte that includes one or more egg cells, and a microspore develops into a male gametophyte that includes sperm—is among the most important transitions in the evolution of plants, with profound effects on plant reproduction and the life cycle [26]. Nevertheless, the nuclear genome of a homosporous vascular plant has yet to be sequenced.
Cytological studies throughout the twentieth century revealed that ferns, especially homosporous species (which include up to 99% of extant ferns [1]), have significantly higher chromosome numbers than other plants [27-30]. Homosporous ferns average n = 57.05 chromosomes, compared to n = 15.99 for flowering plants [31], and the highest chromosome number known for any multicellular organism (2n = 1440) is that of the homosporous fern Ophioglossum reticulatum[32]. However, heterosporous ferns possess an average of only n = 13.62 chromosomes, very close to the average of flowering plants—another heterosporous lineage. To date, no explanatory hypothesis for this cross-lineage discrepancy in chromosome numbers vs. spore type has survived rigorous testing [33]. Along with their high chromosome numbers, many homosporous ferns have extremely large genomes [34-39], and homosporous ferns are the only land plants to show a strong positive correlation between chromosome number and genome size [40].
Because of their high chromosome numbers [41,42], homosporous ferns were initially assumed to have experienced many rounds of ancient whole-genome duplication (polyploidy) [31], events that have likely influenced the structures of all land plant genomes. In addition, two decades of experiments have consistently shown that homosporous ferns possessing the putative base chromosome numbers of their genus—even if those numbers are high compared to those of angiosperms—behave genetically as diploids (e.g., [43-52]). Ferns also lack chromosome-level evidence of extensive ancient polyploidy, such as syntenic chromosomal blocks [53,54]. This combination of high chromosome numbers and lack of evidence for extensive polyploidy in homosporous ferns has been referred to as the “polyploidy paradox” [55]. Whole-genome data are essential for resolving this paradox and also for understanding basic aspects of genome organization and different pathways for genome streamlining and diploidization—acting post-polyploidization—that may operate in ferns vs. angiosperms.
Paleopolyploidy events have been inferred in the histories of all angiosperm lineages studied to date (e.g., [56]) and are implicated in the ancestral angiosperm and ancestral seed plant genomes [25,57]. Thus, even contemporary flowering plant taxa with relatively small genomes, such as the model species Arabidopsis thaliana (n = 5, 125 Mb [58]), often belong to lineages that have experienced multiple rounds of polyploidy. Arabidopsis is thought to have experienced five such events, including the ancestral seed plant and angiosperm duplications [57,59]. Various groups have evidently responded to these events in different ways, and data from ferns are the key to understanding these differences. Using these data, we can ask, for example: how do the various genomic components (e.g., repetitive elements) differ across land plant lineages, and how do their fates differ following polyploidy? What mechanisms are responsible for the universally smaller numbers of chromosomes in heterosporous vs. homosporous lineages, and how do these relate to the transitions among mating systems across land plants? What genomic changes underlie trends in gametophyte reduction and the shift from haploid-dominant to diploid-dominant life cycles across land plants? Do the free-living, haploid gametophytes of ferns experience strong purifying selection? Ferns are the crucial missing clade for understanding all of these evolutionary paradoxes. Most importantly, the addition of ferns to the set of sequenced land plant genomes will also facilitate reconstruction of the ancestral euphyllophyte (ferns plus seed plants; Euphyllophyta) and vascular plant (Tracheophyta) genomes, and will inform efforts to reconstruct the ancestral seed plant genome by providing an outgroup that is more suitable for comparative analyses than are the currently available lycophyte [60] and moss [61] genomes. Improved understanding of genomic changes during the evolution of seed plants will provide a new perspective for examining key evolutionary innovations in that clade, such as the seed itself.
To capture and characterize the genetic, genomic, and ecological diversity of ferns, we recommend two candidates for genome sequencing: Azolla (Azollaceae: Salviniales) and Ceratopteris (Pteridaceae: Polypodiales). Both have been promoted as model ferns for genome sequencing [14,40,62,63] and together, Azolla and Ceratopteris are a powerful combination. They cumulatively represent more than 400 million years of independent evolution (MRCA 200 mya [16]), and embody the key genomic and life-history characteristics of interest for fern genome sequencing.
Azolla is a heterosporous, free-floating water fern with a compact, 750 Mb (1C) genome and n = 22 chromosomes [38,64]. It has long been valued in Southeast Asia as a green fertilizer due to its symbiotic relationship with Nostoc azollae, a cyanobacterium that lives in cavities enclosed by the leaf tissue of Azolla[65] and renders it capable of nitrogen fixation [66]. Azolla also has promise as a biofuel and bioremediator in carbon sequestration efforts [63]. In addition, Azolla has been implicated as the cause of a massive shift in Earth’s climate approximately 50 mya [67], when atmospheric carbon dioxide levels were apparently halved by Azolla-driven carbon sequestration [68-70]. A genome sequence for Azolla will allow us to explore its relationship with its symbionts and may facilitate efforts to harness its nitrogen-fixing ability on a scale large enough to provide an inexpensive source of nitrogen-rich fertilizer [71].
Recently, the BGI (formerly Beijing Genomics Institute) agreed to complete the first fern genome sequencing project, for Azolla, in collaboration with principal investigator K.M. Pryer and colleagues (see [72,73]). Supplemental funds were also raised through crowdfunding [74,75], and the PIs are currently gathering material for the project. This planned sequencing of Azolla will provide initial and much-needed genomic resources for ferns, but given the deep divergence times, variation in life-history characteristics, and diversity within this clade, one fern genome is simply not enough to address the full range of outstanding genomic questions in ferns and across land plants.
Ceratopteris provides an ideal contrast to Azolla. It is homosporous, and its genome is 11.26Gb (1C; DB Marchant, unpublished), an order of magnitude larger than that of Azolla. This size is more typical of genome sizes found in leptosporangiate ferns and is closer to the size scale of conifer genomes than to Azolla. Ceratopteris is the “Arabidopsis of the fern world”: it can be readily transformed with recombinant DNA [76,77] and has a fast life cycle, features that have made it an ideal genetic model system for studying sex expression and mating systems [78-81], spore and gametophyte development [82-87], and even plant responses to gravity during space flight [88]. In addition, a rapidly developing strain of Ceratopteris has been used extensively as an educational model system in undergraduate and K-12 biology instruction worldwide [89,90].
The earliest candidates for genome sequencing in plants tended to be those with small and simple genomes that could be assembled with relative ease. As the trend towards whole-genome sequencing intensifies, an increasing number of taxa with large or complex genomes will be of interest for complete nuclear genome sequencing. It is likely that most large fern genomes will not assemble easily using current techniques, making them important test cases for improved sequencing strategies, mapping, and especially assembly approaches, such as those recently developed for sequencing of the 22Gb (1C) loblolly pine [91,92] and 20Gb (1C) Norway spruce [93] genomes [94]. Ceratopteris will provide such an opportunity, and genetic resources for this species already exist to facilitate the assembly process. These include a genetic linkage map and mapping population comprising ~500 doubled haploid lines (DHLs) [53], which will allow efficient de novo sequencing and high-quality assembly, leveraging, for example, the recombinant population genome construction approach of Hahn et al. [95]. Azolla will provide a novel opportunity to sequence a plant nuclear genome that has co-evolved for more than 70 million years along with the genomes of its obligate, vertically-inherited symbiotic microbiome. The genome of one such symbiont has been sequenced [66], but additional components of the fern microbiome are not well characterized.
Conclusions
Ferns are a phylogenetically pivotal and evolutionarily critical group of plants, yet they remain a group for which we lack extensive nuclear genomic resources. This is an astonishing reality, given the progress that has been made to date elsewhere across the tree of life. Transcriptome sequencing efforts such as the 1,000 Plants Project [23] have vastly expanded the gene sequence resources available for plants, but genes alone are insufficient to answer the most pressing questions in fern and land plant genome evolution. Ferns are crucial for understanding many aspects of plant development, physiology, metabolism, and evolution, and they hold the answers to key questions that have puzzled evolutionary and comparative biologists for more than a century. Between these two ferns—Ceratopteris and Azolla—evolution has operated for 400 million years, providing tremendous opportunity for differences to accumulate, both between these genomes and between ferns and other extant plants. Simultaneous sequencing of Azolla and Ceratopteris will close the phylogenetic gap in available plant genomes, and more importantly, will complete the critical framework necessary for rigorous comparative studies of genome structure and function across land plants.
Abbreviations
Gb: Gigabases; Mb: Megabases; MRCA: Most recent common ancestor; mya: Million years ago.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
EBS and PGW conceived and drafted the paper; all other authors edited, contributed comments to, and read and approved the final manuscript.
Contributor Information
Emily B Sessa, Email: emilysessa@ufl.edu.
Jo Ann Banks, Email: banksj@purdue.edu.
Michael S Barker, Email: msbarker@email.arizona.edu.
Joshua P Der, Email: jpd18@psu.edu.
Aaron M Duffy, Email: aduffy70@gmail.com.
Sean W Graham, Email: swgraham@mail.ubc.ca.
Mitsuyasu Hasebe, Email: mhasebe@nibb.ac.jp.
Jane Langdale, Email: jane.langdale@plants.ox.ac.uk.
Fay-Wei Li, Email: fl43@duke.edu.
D Blaine Marchant, Email: dbmarchant@gmail.com.
Kathleen M Pryer, Email: pryer@duke.edu.
Carl J Rothfels, Email: crothfels@yahoo.ca.
Stanley J Roux, Email: sroux@uts.cc.utexas.edu.
Mari L Salmi, Email: marilsalmi@utexas.edu.
Erin M Sigel, Email: erin.sigel@duke.edu.
Douglas E Soltis, Email: dsoltis@ufl.edu.
Pamela S Soltis, Email: psoltis@flmnh.ufl.edu.
Dennis W Stevenson, Email: dws@nybg.org.
Paul G Wolf, Email: paul.wolf@usu.edu.
Acknowledgements
The title alludes to the popular internet comedy series “Between Two Ferns with Zach Galifianakis”: http://www.funnyordie.com/between_two_ferns.
Note from the Editors
A related discussion by Fay-Wei Li and Kathleen Pryer on crowdfunding efforts to sequence the Azolla fern genome is published alongside this article [75].
References
- Smith AR, Pryer K, Schuettpelz E, Korall P, Schneider H, Wolf P. A classification for extant ferns. Taxon. 2006;55:705–731. doi: 10.2307/25065646. [DOI] [Google Scholar]
- George LO, Bazzaz FA. The fern understory as an ecological filter: Emergence and establishment of canopy-tree seedlings. Ecology. 1999;80:833–845. doi: 10.1890/0012-9658(1999)080[0833:TFUAAE]2.0.CO;2. [DOI] [Google Scholar]
- George LO, Bazzaz FA. The fern understory as an ecological filter: Growth and survival of canopy-tree seedlings. Ecology. 1999;80:846–856. doi: 10.1890/0012-9658(1999)080[0846:TFUAAE]2.0.CO;2. [DOI] [Google Scholar]
- Siccama TG, Bormann FH, Likens GE. The Hubbard Brook ecosystem study: Productivity, nutrients, and phytosociology of the herbaceous layer. Ecol Monogr. 1970;40:389–402. doi: 10.2307/1942337. [DOI] [Google Scholar]
- Ellwood MDF, Foster WA. Doubling the estimate of invertebrate biomass in a rainforest canopy. Nature. 2004;429:549–551. doi: 10.1038/nature02560. [DOI] [PubMed] [Google Scholar]
- Watkins JE Jr, Cardelús CL. Ferns in an angiosperm world: Cretaceous radiation into the epiphytic niche and diversification on the forest floor. Int J Plant Sci. 2012;173:695–710. doi: 10.1086/665974. [DOI] [Google Scholar]
- Marrs RH, Le Duc MG, Mitchell RJ, Goddard D, Paterson S, Pakeman RJ. The ecology of Bracken: Its role in succession and implications for control. Ann Bot. 2000;85:3–15. [Google Scholar]
- Walker LR. Effects of fern thickets on woodland development on landslides in Puerto Rico. J Veg Sci. 1994;5:525–532. doi: 10.2307/3235979. [DOI] [Google Scholar]
- Tryon RM. Development and evolution of fern floras of oceanic islands. Biotropica. 1970;2:76–84. doi: 10.2307/2989765. [DOI] [Google Scholar]
- Allison SD, Vitousek PM. Rapid nutrient cycling in leaf litter from invasive plants in Hawai'i. Oecologia. 2004;141:612–619. doi: 10.1007/s00442-004-1679-z. [DOI] [PubMed] [Google Scholar]
- Pemberton RW, Ferriter AP. Old World climbing fern (Lygodium microphyllum), a dangerous invasive weed in Florida. American Fern Journal. 1998;88:165–175. doi: 10.2307/1547769. [DOI] [Google Scholar]
- de AL l C, Kelty MJ. Establishment and control of hay-scented fern: a native invasive species. Biol Invasions. 1999;1:223–236. doi: 10.1023/A:1010098316832. [DOI] [Google Scholar]
- Pryer KM, Schneider H, Smith AR, Cranfill R, Wolf PG, Hunt JS, Sipes SD. Horsetails and ferns are a monophyletic group and the closest living relatives to seed plants. Nature. 2001;409:618–622. doi: 10.1038/35054555. [DOI] [PubMed] [Google Scholar]
- Pryer KM, Schneider H, Zimmer EA, Banks JA. Deciding among green plants for whole genome studies. Trends Plant Sci. 2002;7:550–554. doi: 10.1016/S1360-1385(02)02375-0. [DOI] [PubMed] [Google Scholar]
- Pryer KM, Schuettpelz E, Wolf PG, Schneider H, Smith AR, Cranfill R. Phylogeny and evolution of ferns (Monilophytes) with a focus on the early leptosporangiate divergences. Am J Bot. 2004;91:1582–1598. doi: 10.3732/ajb.91.10.1582. [DOI] [PubMed] [Google Scholar]
- Schuettpelz E, Pryer KM. Evidence for a Cenozoic radiation of ferns in an angiosperm-dominated canopy. Proc Natl Acad Sci. 2009;106:11200–11205. doi: 10.1073/pnas.0811136106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider H, Schuettpelz E, Pryer K, Cranfill R, Magallón SA, Lupia R. Ferns diversified in the shadow of angiosperms. Nature. 2004;428:554–557. doi: 10.1038/nature02361. [DOI] [PubMed] [Google Scholar]
- Smith SA, Beaulieu JM, Donoghue MJ. An uncorrelated relaxed-clock analysis suggests an earlier origin for flowering plants. Proc Natl Acad Sci. 2010;107:5897–5902. doi: 10.1073/pnas.1001225107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grewe F, Guo W, Gubbels EA, Hansen AK, Mower JP. Complete plastid genomes from Ophioglossum californicum, Psilotum nudum, and Equisetum hyemale reveal an ancestral land plant genome structure and resolve the position of Equisetales among monilophytes. BMC Evol Biol. 2013;13:8. doi: 10.1186/1471-2148-13-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rai HS, Graham SW. Utility of a large, multigene plastid data set in inferring higher-order relationships in ferns and relatives (monilophytes) Am J Bot. 2010;97:1444–1456. doi: 10.3732/ajb.0900305. [DOI] [PubMed] [Google Scholar]
- TimeTree. [ http://timetree.org]
- Hedges SB, Dudley J, Kumar. TimeTree: A public knowledge-base of divergence times among organisms. Bioinformatics. 2006;22:2971–2972. doi: 10.1093/bioinformatics/btl505. [DOI] [PubMed] [Google Scholar]
- The 1000 Plants Project. [ http://www.onekp.com]
- Wickett NJ, Mirarab S, Nguyen N, Warnow T, Carpenter EJ, Matasci N, Ayyampalayam S, Barker MS, Burleigh JG, Gitzendanner MA, Ruhfel B, Wafula E, Der JP, Graham SW, Mathews S, Melkonian M, Soltis DE, Soltis PS, Rothfels C, Pokorny L, Shaw J, DeGironimo L, Stevenson DW, Surek B, Villarreal JC, Roure B, Philippe H, dePamphilis CW, Chen T, Deyholos MK, A phylotranscriptomics analysis of the origin and early diversification of land plants. Proc Natl Acad Sci. 2014. (In Press) [DOI] [PMC free article] [PubMed]
- Amborella Genome Project. The Amborella genome and the evolution of flowering plants. Science. 2013;342:1241089-1–10. doi: 10.1126/science.1241089. [DOI] [PubMed] [Google Scholar]
- Bateman RM, DiMichele WA. Heterospory: the most iterative key innovation in the evolutionary history of the plant kingdom. Biol Rev. 1994;69:345–417. doi: 10.1111/j.1469-185X.1994.tb01276.x. [DOI] [Google Scholar]
- Britton DM. Chromosome studies on ferns. Am J Bot. 1953;40:575–583. doi: 10.2307/2438442. [DOI] [Google Scholar]
- Britton DM. The significance of chromosome numbers in ferns. Ann Mo Bot Gard. 1974;61:310–317. doi: 10.2307/2395059. [DOI] [Google Scholar]
- Love A, Love D, Pichi-Sermolli REG. Cytotaxonomical Atlas of the Pteridophyta. J. Cramer: Vaduz, Liechtenstein; 1977. [Google Scholar]
- Manton I. Problems of Cytology and Evolution in the Pteridophyta. Cambridge: Cambridge University Press; 1950. [Google Scholar]
- Klekowski EJ, Baker HG. Evolutionary significance of polyploidy in the Pteridophyta. Science. 1966;153:305–307. doi: 10.1126/science.153.3733.305. [DOI] [PubMed] [Google Scholar]
- Ghatak J. Biosystematic survey of the pteridophytes from Shevaroy Hills, South India. Nucleus. 1977;20:105–108. [Google Scholar]
- Barker MS, Wolf PG. Unfurling fern biology in the genomics age. Bioscience. 2010;60:177–185. doi: 10.1525/bio.2010.60.3.4. [DOI] [Google Scholar]
- Bainard JD, Henry TA, Bainard LD, Newmaster SG. DNA content variation in monilophytes and lycophytes: large genomes that are not endopolyploid. Chromosome Res. 2011;19:763–775. doi: 10.1007/s10577-011-9228-1. [DOI] [PubMed] [Google Scholar]
- Bennett MD, Leitch IJ. Nuclear DNA amounts in pteridophytes. Ann Bot. 2001;87:335–345. doi: 10.1006/anbo.2000.1339. [DOI] [Google Scholar]
- Garcia S, Leitch IJ, Anadon-Rosell A, Canela MA, Galvez F, Garnatje T, Gras A, Hidalgo O, Johnston E, Mas de Xaxars G, Pellicer J, Siljak-Yakovlev S, Vallés J, Vitales D, Bennett MD. Recent updates and developments to plant genome size databases. Nucleic Acids Res. 2013;42:D1159–D1166. doi: 10.1093/nar/gkt1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson L, Leitch IJ. DNA amounts for five pteridophyte species fill phylogenetic gaps in C-value data. Bot J Linn Soc. 2002;140:169–173. doi: 10.1046/j.1095-8339.2002.00083.x. [DOI] [Google Scholar]
- Obermayer R, Leitch IJ, Hanson L, Bennett MD. Nuclear DNA C-values in 30 species double the familial representation in pteridophytes. Ann Bot. 2002;90:209–217. doi: 10.1093/aob/mcf167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitch IJ, Soltis DE, Soltis PS, Bennett MD. Evolution of DNA amounts across land plants (Embryophyta) Ann Bot. 2005;95:207–217. doi: 10.1093/aob/mci014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazato T, Barker MS, Rieseberg LH, Gastony GJ. In: Biology and Evolution of Ferns and Lycophytes. Ranker TA, Haufler CH, editor. Cambridge, UK: Cambridge University Press; 2008. Evolution of the nuclear genome of ferns and lycophytes. [Google Scholar]
- Chiarugi A. Tavole chromosomiche delle Pteridophyta. Caryologia. 1960;13:27–150. [Google Scholar]
- Fabbri F. Primo supplemento alle tavole chromosomiche delle Pteridophyta di Alberto Chiarugi. Caryologia. 1963;16:237–335. [Google Scholar]
- Gastony GJ, Gottlieb LD. Genetic variation in the homosporous fern Pellaea andromedifolia. Am J Bot. 1985;72:257–267. doi: 10.2307/2443553. [DOI] [Google Scholar]
- Haufler CH, Soltis DE. Genetic evidence suggests that homosporous ferns with high chromosome numbers are diploid. Proc Natl Acad Sci. 1986;83:4389–4393. doi: 10.1073/pnas.83.12.4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haufler CH. Electrophoresis is modifying our concepts of evolution in homosporous pteridophytes. Am J Bot. 1987;74:953–966. doi: 10.2307/2443877. [DOI] [Google Scholar]
- Wolf PG, Haufler CH, Sheffield E. Electrophoretic evidence for genetic diploidy in the bracken fern (Pteridium aquilinum) Science. 1987;236:947–949. doi: 10.1126/science.236.4804.947. [DOI] [PubMed] [Google Scholar]
- Soltis DE. Genetic evidence for diploidy in Equisetum. Am J Bot. 1986;73:908–913. doi: 10.2307/2444303. [DOI] [Google Scholar]
- Soltis PS, Soltis DE. Estimated rates of intragametophytic selfing in lycopods. Am J Bot. 1988;75:248–256. doi: 10.2307/2443891. [DOI] [Google Scholar]
- McGrath JM, Hickok LG, Pichersky E. Assessment of gene copy number in the homosporous ferns Ceratopteris thalictroides and C. richardii (Parkeriaceae) by restriction fragment length polymorphims. Plant Syst Evol. 1994;189:203–210. doi: 10.1007/BF00939726. [DOI] [Google Scholar]
- DeYoung B, Weber T, Hass B, Banks JA. Generating autotetraploid sporophytes and their use in analyzing mutations affecting gametophyte development in the fern Ceratopteris. Genetics. 1997;147:809–814. doi: 10.1093/genetics/147.2.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks JA. The TRANSFORMER genes of the fern Ceratopteris simultaneously promote meristem and archegonia development and repress antheridia development in the developing gametophyte. Genetics. 1997;147:1885–1897. doi: 10.1093/genetics/147.4.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strain E, Hass B, Banks JA. Characterization of mutations that feminize gametophytes of the fern Ceratopteris. Genetics. 2001;159:1271–1281. doi: 10.1093/genetics/159.3.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakazato T, Jung MK, Housworth EA, Rieseberg LH, Gastony GJ. Genetic map-based analysis of genome structure in the homosporous fern Ceratopteris richardii. Genetics. 2006;173:1585–1597. doi: 10.1534/genetics.106.055624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker MS. Evolutionary genomic analyses of ferns reveal that high chromosome numbers are a product of high retention and fewer rounds of polyploidy relative to angiosperms. American Fern Journal. 2009;99:136–141. [Google Scholar]
- Soltis PS, Soltis DE. The role of genetic and genomic attributes in the success of polyploids. Proc Natl Acad Sci. 2000;97:7051–7057. doi: 10.1073/pnas.97.13.7051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltis DE, Albert VA, Leebens-Mack J, Bell CD, Paterson AH, Zheng C, Sankoff D, dePamphilis CW, Wall PK, Soltis PS. Polyploidy and angiosperm diversification. Am J Bot. 2009;96:336–348. doi: 10.3732/ajb.0800079. [DOI] [PubMed] [Google Scholar]
- Jiao Y, Wickett NJ, Ayyampalayam S, Chanderbali AS, Landherr L, Ralph PE, Tomsho LP, Hu Y, Liang H, Soltis PS, Soltis DE, Clifton SW, Schlarbaum SE, Schuster SC, Ma H, Leebens-Mack J, dePamphilis CW. Ancestral polyploidy in seed plants and angiosperms. Nature. 2011;473:97–100. doi: 10.1038/nature09916. [DOI] [PubMed] [Google Scholar]
- Initiative TAG. Analysis of the genome sequence of the flowering plant Arabidopsis thaliana. Nature. 2000;408:796–815. doi: 10.1038/35048692. [DOI] [PubMed] [Google Scholar]
- Barker MS, Vogel H, Schranz ME. Paleopolyploidy in the Brassicales: Analyses of the Cleome transcriptome elucidate the history of genome duplications in Arabidopsis and other Brassicales. Genome Biology and Evolution. 2009;1:391–399. doi: 10.1093/gbe/evp040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks JA, Nishiyama T, Hasebe M, Bowman JL, Gribskov M, dePamphilis CW, Albert VA, Aono N, Aoyama T, Ambrose BA, Ashton NW, Axtell MJ, Barker E, Barker MS, Bennetzen JL, Bonawitz ND, Chapple C, Cheng C, Correa LGG, Dacre M, DeBarry J, Dreyer I, Elias M, Engstrom EM, Estelle M, Feng L, Finet C, Floyd SK, Frommer WB, Fujita T. et al. The Selaginella genome identifies genetic changes associated with the evolution of vascular plants. Science. 2011;332:960–963. doi: 10.1126/science.1203810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rensing SA, Lang D, Zimmer AD, Terry A, Salamov A, HH S, Nishiyama T, Perroud P-F, Lindquist EA, Kamisugi Y, Tanahashi T, Sakakibara K, Fujita T, Oishi K, Shin-I T, Kuroki Y, Toyoda A, Suzuki Y, Hashimoto SI, Yamaguchi K, Sugano S, Kohara Y, Fujiyama A, Anterola A, Aoki S, Ashton N, Barbazuk WB, Barker E, Bennetzen JL, Blankenship R. et al. The Physcomitrella genome reveals evolutionary insights into the conquest of land by plants. Science. 2008;319:64–69. doi: 10.1126/science.1150646. [DOI] [PubMed] [Google Scholar]
- Qiu Y-L, Yu J. Azolla - a model organism for plant genomic studies. Genomics Proteomics Bioinformatics. 2003;1:15–25. doi: 10.1016/S1672-0229(03)01004-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brouwer P, Bräutigam A, Külahoglu C, Tazelaar AOE, Kurz S, Nierop KGJ, van der Werf A, Weber APM, Schluepmann H. Azolla domestication towards a biobased economy? New Phytol. 2014;202:1069–1082. doi: 10.1111/nph.12708. [DOI] [PubMed] [Google Scholar]
- Stergianou KK, Fowler K. Chromosome numbers and taxonomic implications in the fern genus Azolla (Azollaceae) Plant Syst Evol. 1990;173:223–239. doi: 10.1007/BF00940865. [DOI] [Google Scholar]
- Perkins SK, Peters GA. The Azolla—Anabaena symbiosis: endophyte continuity in the Azolla life- cycle is facilitated by epidermal trichomes. New Phytol. 1993;123:53–64. [Google Scholar]
- Ran L, Larsson J, Vigil-Stenman T, Nylander JAA, Ininbergs K, Zheng W-W, Lapidus A, Lowry S, Haselkorn R, Bergman B. Genome erosion in a nitrogen-fixing vertically transmitted endosymbiotic multicellular cyanobacterium. PLoS One. 2010;5:e11486. doi: 10.1371/journal.pone.0011486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkhuis H, Schouten S, Collinson ME, Sluijs A, Damsté JSS, Dickens GR, Huber M, Cronin TM, Onodera J, Takahashi K, Bujak JP, Stein R, van der Burgh J, Eldrett JS, Harding IC, Lotter AF, Sangiorgi F, Cittert HVK-V, de Leeuw JW, Matthiessen J, Backman J, Moran K. the Expedition 302 Scientists. Episodic fresh surface waters in the Eocene Arctic Ocean. Nature. 2006;441:606–609. doi: 10.1038/nature04692. [DOI] [PubMed] [Google Scholar]
- Bujak JP. The Azolla Story: climate change and Arctic hydrocarbons. GEO ExPro. 2007;4:66–72. [Google Scholar]
- Speelman EN, Van Kempen MML, Barke J, Brinkhuis H, Reichart GJ, Smolders AJP, Roelofs JGM, Sangiorgi F, De Leeuw JW, Lotter AF, Sinninghe Damsté JS. The Eocene Arctic Azolla bloom: environmental conditions, productivity and carbon drawdown. Geobiology. 2009;7:155–170. doi: 10.1111/j.1472-4669.2009.00195.x. [DOI] [PubMed] [Google Scholar]
- Bujak JP, Bujak AA. How a unique plant called Azolla changed our climate. Geoscientist magazine. 2014;24:10–15. [Google Scholar]
- This lowly fern has massive green potential. [ http://www.theglobeandmail.com/globe-debate/this-lowly-fern-has-massive-green-potential/article18907003/]
- The Azolla Genome Project. [ http://www.azollagenome.net/]
- Scientific American: Can the Fern That Cooled the Planet Do It Again? [ http://www.scientificamerican.com/article/can-the-fern-that-cooled-the-planet-do-it-again/]
- Experiment.com: Azolla, a Little Fern with Massive Green Potential. [ https://experiment.com/projects/azolla-a-little-fern-with-massive-green-potential]
- Li F-W, Pryer KM. Crowdfunding the Azolla fern genome project: a grassroots approach. GigaScience. 2014;3:16. doi: 10.1186/2047-217X-3-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plackett ARG, Huang L, Sanders HL, Langdale JA. High-efficiency stable transformation of the model fern species Ceratopteris richardii via microparticle bombardment. Plant Physiol. 2014;165:3–14. doi: 10.1104/pp.113.231357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthukumar B, Joyce BL, Elless MP, Stewart CN. Stable transformation of ferns using spores as targets: Pteris vittata (Chinese brake fern) and Ceratopteris thalictroides (C-fern 'Express') Plant Physiol. 2013;163:648–658. doi: 10.1104/pp.113.224675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberle J, Nemacheck J, Wen C-K, Hasebe M, Banks JA. Ceratopteris: A model system for studying sex-determining mechanisms in plants. Int J Plant Sci. 1995;156:359–366. doi: 10.1086/297257. [DOI] [Google Scholar]
- Banks JA, Hickok LG, Webb MA. The programming of sexual phenotype in the homosporous fern Ceratopteris richardii. Int J Plant Sci. 1993;154:522–534. doi: 10.1086/297135. [DOI] [Google Scholar]
- Nakazato T, Jung MK, Housworth EA, Rieseberg LH, Gastony GJ. A genomewide study of reproductive barriers between allopatric populations of a homosporous fern, Ceratopteris richardii. Genetics. 2007;177:1141–1150. doi: 10.1534/genetics.107.076851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janoušek B, Hobza R, Vyskot B. In: Plant Genome Diversity. Volume 2. Leitch IJ, editor. Vienna: Springer Vienna; 2012. Chromosomes and Sex Differentiation; pp. 167–186. [Google Scholar]
- Banks JA. Gametophyte development in ferns. Annu Rev Plant Physiol Plant Mol Biol. 1999;50:163–186. doi: 10.1146/annurev.arplant.50.1.163. [DOI] [PubMed] [Google Scholar]
- Salmi ML, Bushart TJ, Stout SC, Roux SJ. Profile and analysis of gene expression changes during early development in germinating spores of Ceratopteris richardii. Plant Physiol. 2005;138:1734–1745. doi: 10.1104/pp.105.062851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickok LG, Warne TR, Slocum MK. Ceratopteris richardii: Applications for experimental plant biology. Am J Bot. 1987;74:1304–1316. doi: 10.2307/2444165. [DOI] [Google Scholar]
- Hickok LG, Warne TR, Fribourg RS. The biology of the fern Ceratopteris and its use as a model system. Int J Plant Sci. 1995;156:332–345. doi: 10.1086/297255. [DOI] [Google Scholar]
- Cooke TJ, Hickey LJ, Sugai M. The fern Ceratopteris richardii as a lower plant model system for studying the genetic regulation of plant photomorphogenesis. Int J Plant Sci. 1995;156:367–373. doi: 10.1086/297258. [DOI] [Google Scholar]
- Chasan R. Ceratopteris: A model plant for the 90s. Plant Science News. 1992;4:113–115. doi: 10.1105/tpc.4.2.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmi ML, Roux SJ. Gene expression changes induced by space flight in single-cells of the fern Ceratopteris richardii. Planta. 2008;229:151–159. doi: 10.1007/s00425-008-0817-y. [DOI] [PubMed] [Google Scholar]
- Warne TR, Renzaglia KS, Hickok LG. Ceratopteris richardii - a simple model system for teaching and research. Plant Physiol. 1993;102:87. [Google Scholar]
- C-fern.org. [ http://www.c-fern.org]
- Zimin A, Stevens KA, Crepeau MW, Holtz-Morris A, Koriabine M, Marcais G, Puiu D, Roberts M, Wegrzyn JL, de Jong PJ, Neale DB, Salzberg SL, Yorke JA, Langley CH. Sequencing and assembly of the 22-Gb loblolly pine genome. Genetics. 2014;196:875–890. doi: 10.1534/genetics.113.159715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neale DB, Wegrzyn JL, Stevens KA, Zimin AV, Puiu D, Crepeau MW, Cardeno C, Koriabine M, Holtz-Morris AE, Liechty JD, Martínez-García PJ, Vasquez-Gross HA, Lin BY, Zieve JJ, Dougherty WM, Fuentes-Soriano S, Wu L-S, Gilbert D, Marçais G, Roberts M, Holt C, Yandell M, Davis JM, Smith KE, Dean JF, Lorenz WW, Whetten RW, Sederoff R, Wheeler N, McGuire PE. et al. Decoding the massive genome of loblolly pine using haploid DNA and novel assembly strategies. Genome Biol. 2014;15:R59. doi: 10.1186/gb-2014-15-3-r59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nystedt B, Street NR, Wetterbom A, Zuccolo A, Lin Y-C, Scofield DG, Vezzi F, Delhomme N, Giacomello S, Alexeyenko A, Vicedomini R, Sahlin K, Sherwood E, Elfstrand M, Gramzow L, Holmberg K, Hallman J, Keech O, Klasson L, Koriabine M, Kucukoglu M, Kaller M, Luthman J, Lysholm F, Niittyla T, Olson A, Rilakovic N, Ritland C, Rossello JA, Sena J. et al. The Norway spruce genome sequence and conifer genome evolution. Nature. 2013;497:579–584. doi: 10.1038/nature12211. [DOI] [PubMed] [Google Scholar]
- Soltis PS, Soltis DE. A conifer genome spruces up plant phylogenomics. Genome Biol. 2013;14:122. doi: 10.1186/gb-2013-14-6-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn MW, Zhang SV, Moyle LC. Sequencing, assembling, and correcting draft genomes using recombinant populations. G3 Genes, Genomes, Genetics. 2014;4:669–679. doi: 10.1534/g3.114.010264. [DOI] [PMC free article] [PubMed] [Google Scholar]


