Abstract
Development of multicellular organisms depends on patterning and growth mechanisms encoded in the genome, but also on the physical properties and mechanical interactions of the constituent cells that interpret these genetic cues. This fundamental biological problem requires integrated studies at multiple levels of biological organization: from genes, to cell behaviors, to tissue morphogenesis. We have recently combined functional genetics with live imaging approaches in embryos of the insect Tribolium castaneum, in order to understand their remarkable transformation from a uniform single-layered blastoderm into a condensed multi-layered embryo covered by extensive extra-embryonic tissues. We first developed a quick and reliable methodology to fluorescently label various cell components in entire Tribolium embryos. Live imaging of labeled embryos at single cell resolution provided detailed descriptions of cell behaviors and tissue movements during normal embryogenesis. We then compared cell and tissue dynamics between wild-type and genetically perturbed embryos that exhibited altered relative proportions of constituent tissues. This systematic comparison led to a qualitative model of the molecular, cellular and tissue interactions that orchestrate the observed epithelial rearrangements. We expect this work to establish the Tribolium embryo as a powerful and attractive model system for biologists and biophysicists interested in the molecular, cellular and mechanical control of tissue morphogenesis.
Keywords: Tribolium castaneum, Drosophila melanogaster, arthropods, fluorescence live imaging, functional genetics, embryo morphogenesis, extraembryonic development, cell shape, cell intercalation, convergence and extension
Tribolium castaneum, an Emerging Model System to Study Embryonic Morphogenesis
During embryogenesis, multicellular organisms become organized into increasingly elaborate and specialized tissues and organs. Thanks to the various implementations of fluorescence microscopy, development of intact embryos can be imaged in 3D over time from a broad tissue level down to a sub-cellular resolution.1-3 Based on these recordings, gene expression dynamics, cell pedigrees and a variety of cell behaviors can be analyzed in vivo during cell fate specification and in the context of tissue and organ morphogenesis. These studies have been performed predominantly with established model organisms (nematode, fruit fly, zebrafish, mouse, Arabidopsis) that are supported by extensive imaging resources, in particular, suitable transgenic lines expressing fluorescent markers that label cellular components, such as chromatin, cell membranes or the cytoskeleton. Biological research would benefit in many ways from adapting live imaging methodologies for other emerging non-model organisms.4 These technical breakthroughs would allow the in-depth study of developmental processes that are not present, or are highly modified in classical experimental models, and also contribute to our understanding of the evolution of developmental mechanisms that have shaped animal and plant diversity.5,6
One such prominent case is the red flour beetle Tribolium castaneum, which is arguably the second best studied insect after Drosophila melanogaster.7,8 Starting with the seminal work by the geneticist Alexander Sokoloff in the 1960s,9 Tribolium has become an increasingly popular and powerful system for developmental genetic studies. Tribolium lends itself to forward and reverse genetic approaches,10-13 is amenable to transgenic manipulations,14-17 and is supported by a sequenced genome and other resources for genome-wide research.18
Early embryogenesis in Drosophila, Tribolium and most other insects is fairly stereotypical.19,20 The zygote nucleus of the fertilized egg undergoes rapid and synchronous mitotic divisions, before nuclei migrate from the yolky interior to the egg periphery, where they form a syncytial blastoderm. After several more rounds of synchronous divisions, the syncytial blastoderm cellularizes, resulting in a mono-layered blastoderm surrounding the egg yolk. Beyond these very early similarities, the Tribolium embryo exhibits many features that are common to most insects (and other arthropods), but not to Drosophila, which exhibits many derived characters.21,22 In Tribolium, only the posterior and ventral part of the blastoderm condenses to give rise to the embryo proper (Fig. 1A and B).23,24 The rest of the blastoderm differentiates into two extra-embryonic epithelia; the serosa, which spreads to surround the entire egg surface, and the amnion, which folds around the embryo to cover its ventral surface (Fig. 1A and B).25,26 In contrast to Tribolium, the Drosophila embryo rudiment has undergone an expansion during evolution, such that it occupies almost the entire blastoderm surface, while the extra-embryonic tissue has been reduced and is restricted to the dorsal-most region.27 Due to this expansion, and the fact that the embryo patterns all of its body units (segments) simultaneously, Drosophila embryogenesis is classed as being long germ, whereas short germ embryos like Tribolium specify their anterior segments at the blastoderm stage and the more posterior segments later in development from a posterior growth zone.28,29
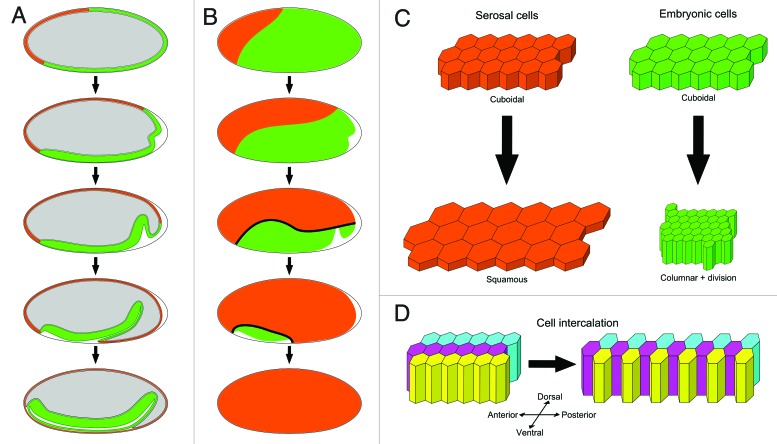
Figure 1. Schematic representation of tissue movements and cell behaviors during Tribolium embryo morphogenesis. (A) Midsagittal sections showing from top to bottom: the uniform blastoderm stage, the differentiated blastoderm stage, the posterior amniotic fold stage, the oval amniotic fold stage, and the amnion/serosa separation stage. The outer vitelline membrane is shown with a gray line, the expanding serosa rudiment in orange, the condensing embryonic rudiment in green, the deforming yolksac in gray, and the amniotic cavity in white in the last stage. (B) Embryo surface views staged and color-coded as in A. The thick black line indicates the actomyosin cable at the amnion/serosa boundary that is evident from the posterior amniotic fold stage through to completion of serosa epiboly. (C) Differential cell behaviors in the serosa and embryonic epithelia. Both cell types start out with the same cuboidal shape in the uniform blastoderm. Upon blastoderm differentiation, non-dividing serosa cells (orange) become squamous, while embryonic cells (green) divide once and become columnar. (D) Embryo convergence and extension. During embryonic condensation, mediolateral cell intercalation elongates the tissue in the anterior-posterior axis and narrows it in the dorsal-ventral axis. Note that the polygonal cell pattern is more variable, and cells are displayed with hexagonal contours for simplicity.
Overall, these fate map differences lead to much more dramatic cell movements and epithelial rearrangements during early Tribolium embryogenesis compared with Drosophila.24 During the last two decades, developmental biologists have compared early embryonic patterning and segmentation between these two insects, and have discovered many similarities, but also striking differences in the underlying molecular genetic mechanisms.29-31 However, despite the major differences in the shape and structure of the embryos, the analysis of cell and tissue dynamics during Tribolium embryogenesis has largely been neglected. As a result, it has not been easy to relate gene expression and function to these salient features of Tribolium embryo morphogenesis.
Fluorescence Live Imaging of Transiently Labeled Tribolium Embryos
In a recent article published in the journal Development, we addressed this limitation by first demonstrating transient fluorescent labeling of Tribolium embryos by microinjecting pre-blastoderm embryos with synthesized capped mRNAs encoding nuclear, membrane or cytoskeletal fluorescent markers.32 Transient methods have been widely used to fluorescently label animal embryos with different tempos and modes of development (including crustaceans, spiders, annelids, sea urchins, ascidians and diverse vertebrates), but have found limited application in labeling the superficially cleaving insect embryos. This can be attributed in part to the ease of generating and maintaining suitable stable transgenic lines in D. melanogaster, but also to the fast tempo of D. melanogaster blastoderm cellularization. Although this approach might not be very useful for D. melanogaster itself, transient labeling methodology will be valuable for other Drosophila species and, more widely, for other insect and arthropod species that have limited or no established genetic tools.
In our experience, a number of important parameters need to be optimized to apply this method in a new species of interest. First, a method for intracellular administration of the mRNA into the eggs or cells of interest is required (e.g., microinjection, electroporation). Second, a sufficiently high volume and concentration of mRNA has to be delivered and allowed to diffuse intracellularly for optimal labeling of cells. Third, great care should be taken that the labeling treatments (i.e., microinjection and overexpression of the fluorescent markers) together with the imaging procedures (i.e., mounting conditions and illumination of the specimen) interfere minimally with normal embryo development.
In the case of Tribolium, transient expression of mRNA resulted in strong, homogeneous and persistent fluorescent labeling of developing embryos.32 We were able to image these embryos at single cell resolution with confocal laser scanning microscopy for over 15 hours of development, spanning the uniform blastoderm stage through to embryo elongation stages. This approach allowed us to formulate, for the first time, a qualitative model for the cell behaviors and tissue interactions that orchestrate the observed epithelial rearrangements during early Tribolium embryogenesis. Our scenario (described below) was based on careful consideration of cell and tissue dynamics in wild-type embryos, and systematic comparisons to genetically perturbed embryos with altered relative proportions of constituent tissues.32
A Qualitative Model for Cell and Tissue Dynamics Driving Epithelial Reorganization in Tribolium
After 12 rounds of synchronous nuclear divisions, the Tribolium blastoderm completes cellularization during the interphase of the 13th cycle. All formed cells in the blastoderm initially have a uniform cuboidal shape, and it is not possible to discriminate between the prospective embryonic and extra-embryonic domains based on cell morphology until the onset of 13th divisions (Fig. 1C).24,32 During the next stage of blastoderm differentiation, the asynchronous cell divisions of the 13th cycle take place in the presumptive embryo and amnion territory (called the embryonic or germ rudiment), but not in the presumptive serosa (Fig. 1C).24,32,33 In addition to cell divisions, prominent changes in cell shape differentiate cells further; dividing embryonic cells start to contract and become columnar (i.e., lengthen in the apical-basal direction), while non-dividing serosa cells start to flatten and become squamous (i.e., shorten in the apical-basal direction) (Fig. 1C).24,32,33 The consequences of these changes at the tissue level are that the embryonic domain progressively thickens and condenses ventrally, while the serosal domain thins and expands during its epibolic movement around the egg surface (Fig. 1A and B). Genetic evidence suggests that contraction of the embryonic rudiment is an autonomous process that is not dependent on forces originating from the expanding serosa. RNAi-mediated knock-down of the Tribolium zerknüllt-1 (Tc-zen1) gene, which specifies serosal cell fate, results in embryos that completely lack the serosa but still condense ventrally and even survive to hatching!25
Recent experiments suggest that the extra-embryonic serosa is an evolutionary novelty of insect eggs to protect them against desiccation.34 But does it play an active role during early embryonic morphogenesis? While it is still an open question whether initiation of serosa expansion is an active process or merely a consequence of tensile forces exerted by the contracting embryonic rudiment, our Tribolium imaging studies suggested that the serosa is needed during the later stages of epithelial rearrangement. In wild-type embryos, the periphery of the condensing embryonic rudiment that abuts the expanding serosa gives rise to the other extra-embryonic tissue, the amnion.24,25 During blastoderm remodeling, this tissue folds ventrally, first at the posterior end (posterior amniotic fold), then along the lateral margins of the germband (horseshoe amniotic fold) and finally over the head lobes anteriorly (oval amniotic fold) (Fig. 1A).32 The rim of the oval amniotic fold undergoes pulsatile contractions until it completely closes the amniotic cavity at the ventral surface of the germband (Fig. 1A).24,32 In addition, this closure disrupts the continuity between the amnion and serosa epithelia, separating the outer serosal envelope from the inner amnion (Fig. 1A). Labeling of developing Tribolium embryos with membrane or actin fluorescent markers suggested the presence of an intercellular actomyosin cable at the boundary between the amnion and the serosa (Fig. 1B).32 Actomyosin cables are known to produce contractile forces in several developmental contexts, including insect embryo repositioning and dorsal closure.35-37 In our live recordings, the progressive constriction of this actomyosin cable coincided precisely with the expansion of the amniotic fold and the closure of the amniotic cavity, strongly suggesting a causal association (Fig. 1A and B). Based on these observations, we proposed that cable contraction pulls the amnion over the lateral edges of the germband (forming the horseshoe amniotic fold) and over the anterior margin of the head (forming the oval amniotic fold), and finally drives closure of the amniotic cavity.32 Congruent with our model, in serosa-less Tc-zen1 knocked-down embryos, the amnion initiates folding at the posterior end but fails to expand anteriorly and cover the remaining germband.25
The mechanism that drives initiation of the posterior amniotic fold has been the most challenging problem to address. In other developmental systems, like Drosophila mesoderm internalization or vertebrate neural tube formation, epithelial infolding is effected by the apical constriction of the invaginating cells.38 In the case of Tribolium, such wedge-shaped cells have not been described at the posterior pole, where the amnion starts folding. Therefore, we looked for alternative mechanisms that could account for posterior amniotic fold formation. The yolk of insect eggs, together with its accompanying reticular cytoplasm, yolk nuclei and membrane (collectively referred to as the yolk system or yolksac) is a source of nutrients for the developing embryo, but it has also been suggested to participate in various morphogenesis processes.19,39,40 In Tribolium, it has been known for a long time that posterior amnion folding coincides with a conspicuous change in the geometry of the yolksac.24,41 A wedge of yolk extends around the posterior pole, bends ventrally and advances anteriorly, exhibiting very similar dynamics to the posterior amniotic fold (Fig. 1A). We were able to relate tissue movements to yolk-fold dynamics by combining confocal and differential interference contrast microscopy.32 This analysis revealed that the ventral extension of the yolk-fold (during amniotic fold formation and progression), and later retraction of the yolk-fold (during posterior elongation of the germband) is tightly coupled to the movement of the posterior end of the embryo. Based on this finding, we proposed the establishment of a tight physical connection between the posterior pole of the yolksac and the posterior pole of the blastoderm.32 We envisage that during embryonic condensation, this anchor on the underlying yolksac forces the amnion to fold over the germband posteriorly. The same pulling force would drive amnion involution into the posterior amniotic fold. In this model, yolksac deformation is a passive response to external forces generated within the condensing blastoderm epithelium; the posterior yolksac fold bends ventrally and advances anteriorly dragged by the involuting amnion that is tightly attached to its surface. At this point, we cannot exclude an alternative/complementary scenario that the yolk system plays an active role in posterior amnion folding, and further analysis is required to resolve this issue.
Another powerful methodological advance in our study was the co-injection of mRNA for transient fluorescence labeling with double-stranded RNA for targeted gene knock-down by RNA interference.32 This integrated approach enabled live imaging and cell tracking in genetically perturbed embryos to make the link between gene function and morphogenetic cell/tissue behaviors during Tribolium embryogenesis. For this analysis, we focused on the Tribolium caudal gene (Tc-cad), which is known to play key roles in embryonic patterning and axial elongation.42-44 Tc-cad knock-down changed the blastoderm fate map in the opposite direction to Tc-zen1 knock-down (the serosa rudiment was expanded at the expense of the embryonic rudiment) and resulted in truncated embryos that failed to add new segments posteriorly.32 Importantly, these experiments also revealed a crucial role for the anterior-posterior patterning system in normal Tribolium embryo morphogenesis. Although embryonic cell division and contraction appeared normal, the condensed germband in Tc-cad RNAi embryos was much shorter (in the anterior-posterior axis) and wider (in the dorsal-ventral axis) compared with wild-type.32 Careful examination of the relative position of cells during embryonic condensation revealed that mediolateral cell intercalation drives embryo convergence and extension during normal condensation (Fig. 1D). Tc-cad RNAi embryos were deficient in cell intercalation, resulting in the abnormal shape of their germband.32 Thus, the universal morphogenetic mechanism of polarized cell intercalation that changes tissue proportions during development in distantly related metazoans is also at work during Tribolium germband formation.
Future Perspectives
Accumulating evidence from Drosophila studies have started shedding light on the interplay between early patterning mechanisms and embryo morphogenesis through the polarized distribution of effector molecules (such as contractile actomyosin networks and adhesion proteins) that are required for junction remodeling and cell rearrangement in the plane of the epithelium.45-47 Our recent work has provided the foundation to address these questions in Tribolium. From an evolutionary perspective, comparative studies between Drosophila and Tribolium now have the potential to go beyond patterns of conservation and divergence at the gene regulatory level, by also taking into account the similarities and differences in morphogenetic cell behaviors guided by these genetic cues.
Our imaging study has provided qualitative descriptions of the processes and physical mechanisms postulated to drive morphogenesis of the early Tribolium embryo.32 Important aspects of our model include the hypotheses that condensation of the embryonic rudiment is autonomous and not dependent on external forces; that a tight physical association between the blastoderm and the yolksac is required for amnion folding; and that contractile forces exerted by an actomyosin cable at the amnion/serosa boundary drive extra-embryonic development. In this model, condensation of the embryonic rudiment plays a central role in remodeling the single-layered blastoderm into the multi-layered enveloped germband. Embryonic condensation in Tribolium is accompanied by cell division, cell shape change, cell rearrangement, amniotic tissue folding, and mesoderm internalization. It is plausible that the contractile forces generated within the embryonic primordium are not uniform, but regionalized and heterogeneous. To dissect in vivo the contribution of all the different cell types and processes to shaping the Tribolium embryo, more sophisticated imaging techniques will be required, allowing deeper light penetration, multi-view imaging and faster acquisition rates for higher temporal resolution.48,49 We anticipate that the Tribolium embryo will provide great scope for interdisciplinary research combining advanced bioimaging, developmental genetics, cell biology, biophysics and mathematical modeling approaches, and will contribute to our understanding of the fundamental principles of tissue morphogenesis across multiple levels of biological organization.
Disclosure of Potential Conflicts of Interest
No potential conflict of interest was disclosed.
Acknowledgments
We are especially grateful to Michael Akam, who co-authored the original research paper with us, for providing supervision and support over the years. We would also like to thank many other colleagues, who provided useful feedback in the course of these studies, in particular Michalis Averof, Pavel Tomancak, and Stephan Grill. Pavlopoulos A was supported by a Marie Curie Intra-European fellowship and by the Howard Hughes Medical Institute.
References
- 1.Megason SG, Fraser SE. Imaging in systems biology. Cell. 2007;130:784–95. doi: 10.1016/j.cell.2007.08.031. [DOI] [PubMed] [Google Scholar]
- 2.Mavrakis M, Pourquié O, Lecuit T. Lighting up developmental mechanisms: how fluorescence imaging heralded a new era. Development. 2010;137:373–87. doi: 10.1242/dev.031690. [DOI] [PubMed] [Google Scholar]
- 3.Keller PJ. Imaging morphogenesis: technological advances and biological insights. Science. 2013;340:1234168. doi: 10.1126/science.1234168. [DOI] [PubMed] [Google Scholar]
- 4.Abzhanov A, Extavour CG, Groover A, Hodges SA, Hoekstra HE, Kramer EM, Monteiro A. Are we there yet? Tracking the development of new model systems. Trends Genet. 2008;24:353–60. doi: 10.1016/j.tig.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 5.Mallarino R, Abzhanov A. Paths less traveled: evo-devo approaches to investigating animal morphological evolution. Annu Rev Cell Dev Biol. 2012;28:743–63. doi: 10.1146/annurev-cellbio-101011-155732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Patel NH. Evolutionary crossroads in developmental biology. Development. 2012;139:2637–8. doi: 10.1242/dev.085464. [DOI] [PubMed] [Google Scholar]
- 7.Brown SJ, Shippy TD, Miller S, Bolognesi R, Beeman RW, Lorenzen MD, Bucher G, Wimmer EA, Klingler M. The red flour beetle, Tribolium castaneum (Coleoptera): a model for studies of development and pest biology. Cold Spring Harb Protoc. 2009;2009:emo126. doi: 10.1101/pdb.emo126. [DOI] [PubMed] [Google Scholar]
- 8.Schröder R, Beermann A, Wittkopp N, Lutz R. From development to biodiversity--Tribolium castaneum, an insect model organism for short germband development. Dev Genes Evol. 2008;218:119–26. doi: 10.1007/s00427-008-0214-3. [DOI] [PubMed] [Google Scholar]
- 9.Sokoloff A. The genetics of Tribolium and related species. New York: Academic Press, 1966. [Google Scholar]
- 10.Beeman RW, Stuart JJ, Haas MS, Denell RE. Genetic analysis of the homeotic gene complex (HOM-C) in the beetle Tribolium castaneum. Dev Biol. 1989;133:196–209. doi: 10.1016/0012-1606(89)90311-4. [DOI] [PubMed] [Google Scholar]
- 11.Sulston IA, Anderson KV. Embryonic patterning mutants of Tribolium castaneum. Development. 1996;122:805–14. doi: 10.1242/dev.122.3.805. [DOI] [PubMed] [Google Scholar]
- 12.Posnien N, Schinko J, Grossmann D, Shippy TD, Konopova B, Bucher G. RNAi in the red flour beetle (Tribolium) Cold Spring Harb Protoc. 2009;2009:t5256. doi: 10.1101/pdb.prot5256. [DOI] [PubMed] [Google Scholar]
- 13.Trauner J, Schinko J, Lorenzen MD, Shippy TD, Wimmer EA, Beeman RW, Klingler M, Bucher G, Brown SJ. Large-scale insertional mutagenesis of a coleopteran stored grain pest, the red flour beetle Tribolium castaneum, identifies embryonic lethal mutations and enhancer traps. BMC Biol. 2009;7:73. doi: 10.1186/1741-7007-7-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berghammer AJ, Klingler M, Wimmer EA. A universal marker for transgenic insects. Nature. 1999;402:370–1. doi: 10.1038/46463. [DOI] [PubMed] [Google Scholar]
- 15.Pavlopoulos A, Berghammer AJ, Averof M, Klingler M. Efficient transformation of the beetle Tribolium castaneum using the Minos transposable element: quantitative and qualitative analysis of genomic integration events. Genetics. 2004;167:737–46. doi: 10.1534/genetics.103.023085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schinko JB, Weber M, Viktorinova I, Kiupakis A, Averof M, Klingler M, Wimmer EA, Bucher G. Functionality of the GAL4/UAS system in Tribolium requires the use of endogenous core promoters. BMC Dev Biol. 2010;10:53. doi: 10.1186/1471-213X-10-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schinko JB, Hillebrand K, Bucher G. Heat shock-mediated misexpression of genes in the beetle Tribolium castaneum. Dev Genes Evol. 2012;222:287–98. doi: 10.1007/s00427-012-0412-x. [DOI] [PubMed] [Google Scholar]
- 18.Richards S, Gibbs RA, Weinstock GM, Brown SJ, Denell R, Beeman RW, Gibbs R, Beeman RW, Brown SJ, Bucher G, et al. Tribolium Genome Sequencing Consortium The genome of the model beetle and pest Tribolium castaneum. Nature. 2008;452:949–55. doi: 10.1038/nature06784. [DOI] [PubMed] [Google Scholar]
- 19.Counce SJ. The Analysis of Insect Embryogenesis. Annu Rev Entomol. 1961;6:295–312. doi: 10.1146/annurev.en.06.010161.001455. [DOI] [Google Scholar]
- 20.Anderson DT. The Development of Holometabolous Insects. In: Counce SJ, Waddington CH, eds. Developmental systems Insects, Vol 1. New York: Academic Press, 1972:165-242. [Google Scholar]
- 21.Sander K. Specification of the basic body pattern in insect embryogenesis. Adv Insect Physiol. 1976;12:125–238. doi: 10.1016/S0065-2806(08)60255-6. [DOI] [Google Scholar]
- 22.Roth S. Gastrulation in Other Insects. In: Stern CD, ed. Gastrulation: From Cells to Embryo. New York: Cold Spring Harbor Laboratory Press, 2004:105-21. [Google Scholar]
- 23.Sommer RJ, Tautz D. Involvement of an orthologue of the Drosophila pair-rule gene hairy in segment formation of the short germ-band embryo of Tribolium (Coleoptera) Nature. 1993;361:448–50. doi: 10.1038/361448a0. [DOI] [PubMed] [Google Scholar]
- 24.Handel K, Grünfelder CG, Roth S, Sander K. Tribolium embryogenesis: a SEM study of cell shapes and movements from blastoderm to serosal closure. Dev Genes Evol. 2000;210:167–79. doi: 10.1007/s004270050301. [DOI] [PubMed] [Google Scholar]
- 25.van der Zee M, Berns N, Roth S. Distinct functions of the Tribolium zerknüllt genes in serosa specification and dorsal closure. Curr Biol. 2005;15:624–36. doi: 10.1016/j.cub.2005.02.057. [DOI] [PubMed] [Google Scholar]
- 26.Panfilio KA. Extraembryonic development in insects and the acrobatics of blastokinesis. Dev Biol. 2008;313:471–91. doi: 10.1016/j.ydbio.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 27.Campos-Ortega JA, Hartenstein V. The embryonic development of Drosophila melanogaster. Berlin Heidelberg New York: Springer, 1997. [Google Scholar]
- 28.Davis GK, Patel NH. Short, long, and beyond: molecular and embryological approaches to insect segmentation. Annu Rev Entomol. 2002;47:669–99. doi: 10.1146/annurev.ento.47.091201.145251. [DOI] [PubMed] [Google Scholar]
- 29.Sarrazin AF, Peel AD, Averof M. A segmentation clock with two-segment periodicity in insects. Science. 2012;336:338–41. doi: 10.1126/science.1218256. [DOI] [PubMed] [Google Scholar]
- 30.Lynch JA, Roth S. The evolution of dorsal-ventral patterning mechanisms in insects. Genes Dev. 2011;25:107–18. doi: 10.1101/gad.2010711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lynch JA, El-Sherif E, Brown SJ. Comparisons of the embryonic development of Drosophila, Nasonia, and Tribolium. Wiley Interdiscip Rev Dev Biol. 2012;1:16–39. doi: 10.1002/wdev.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Benton MA, Akam M, Pavlopoulos A. Cell and tissue dynamics during Tribolium embryogenesis revealed by versatile fluorescence labeling approaches. Development. 2013;140:3210–20. doi: 10.1242/dev.096271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Handel K, Basal A, Fan X, Roth S. Tribolium castaneum twist: gastrulation and mesoderm formation in a short-germ beetle. Dev Genes Evol. 2005;215:13–31. doi: 10.1007/s00427-004-0446-9. [DOI] [PubMed] [Google Scholar]
- 34.Jacobs CG, Rezende GL, Lamers GE, van der Zee M. The extraembryonic serosa protects the insect egg against desiccation. Proc Biol Sci. 2013;280:20131082. doi: 10.1098/rspb.2013.1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kiehart DP, Galbraith CG, Edwards KA, Rickoll WL, Montague RA. Multiple forces contribute to cell sheet morphogenesis for dorsal closure in Drosophila. J Cell Biol. 2000;149:471–90. doi: 10.1083/jcb.149.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Panfilio KA, Oberhofer G, Roth S. High plasticity in epithelial morphogenesis during insect dorsal closure. Biol Open. 2013;2:1108–18. doi: 10.1242/bio.20136072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Panfilio KA, Roth S. Epithelial reorganization events during late extraembryonic development in a hemimetabolous insect. Dev Biol. 2010;340:100–15. doi: 10.1016/j.ydbio.2009.12.034. [DOI] [PubMed] [Google Scholar]
- 38.Sawyer JM, Harrell JR, Shemer G, Sullivan-Brown J, Roh-Johnson M, Goldstein B. Apical constriction: a cell shape change that can drive morphogenesis. Dev Biol. 2010;341:5–19. doi: 10.1016/j.ydbio.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Anderson DT. The Development of Hemimetabolous Insects. In: Counce SJ, Waddington CH, eds. Developmental systems Insects, Vol 1. New York: Academic Press, 1972:95-163. [Google Scholar]
- 40.Reed BH, Wilk R, Schöck F, Lipshitz HD. Integrin-dependent apposition of Drosophila extraembryonic membranes promotes morphogenesis and prevents anoikis. Curr Biol. 2004;14:372–80. doi: 10.1016/j.cub.2004.02.029. [DOI] [PubMed] [Google Scholar]
- 41.Brown SJ, Patel NH, Denell RE. Embryonic expression of the single Tribolium engrailed homolog. Dev Genet. 1994;15:7–18. doi: 10.1002/dvg.1020150103. [DOI] [PubMed] [Google Scholar]
- 42.Schulz C, Schröder R, Hausdorf B, Wolff C, Tautz D. A caudal homologue in the short germ band beetle Tribolium shows similarities to both, the Drosophila and the vertebrate caudal expression patterns. Dev Genes Evol. 1998;208:283–9. doi: 10.1007/s004270050183. [DOI] [PubMed] [Google Scholar]
- 43.Copf T, Schröder R, Averof M. Ancestral role of caudal genes in axis elongation and segmentation. Proc Natl Acad Sci U S A. 2004;101:17711–5. doi: 10.1073/pnas.0407327102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schoppmeier M, Fischer S, Schmitt-Engel C, Löhr U, Klingler M. An ancient anterior patterning system promotes caudal repression and head formation in ecdysozoa. Curr Biol. 2009;19:1811–5. doi: 10.1016/j.cub.2009.09.026. [DOI] [PubMed] [Google Scholar]
- 45.Bertet C, Sulak L, Lecuit T. Myosin-dependent junction remodelling controls planar cell intercalation and axis elongation. Nature. 2004;429:667–71. doi: 10.1038/nature02590. [DOI] [PubMed] [Google Scholar]
- 46.Zallen JA, Wieschaus E. Patterned gene expression directs bipolar planar polarity in Drosophila. Dev Cell. 2004;6:343–55. doi: 10.1016/S1534-5807(04)00060-7. [DOI] [PubMed] [Google Scholar]
- 47.Rauzi M, Lenne PF, Lecuit T. Planar polarized actomyosin contractile flows control epithelial junction remodelling. Nature. 2010;468:1110–4. doi: 10.1038/nature09566. [DOI] [PubMed] [Google Scholar]
- 48.Khairy K, Keller PJ. Reconstructing embryonic development. Genesis. 2011;49:488–513. doi: 10.1002/dvg.20698. [DOI] [PubMed] [Google Scholar]
- 49.Weber M, Huisken J. Omnidirectional microscopy. Nat Methods. 2012;9:656–7. doi: 10.1038/nmeth.2022. [DOI] [PubMed] [Google Scholar]