Abstract
Cadherin-mediated cell adhesion at Adherens Junctions (AJs) and its dynamic connections with the microtubule (MT) cytoskeleton are important regulators of cellular architecture. However, the functional relevance of these interactions and the molecular players involved in different cellular contexts and cellular compartments are still not completely understood. Here, we comment on our recent findings showing that the MT plus-end binding protein CLASP2 interacts with the AJ component p120-catenin (p120) specifically in progenitor epidermal cells. Absence of either protein leads to alterations in MT dynamics and AJ functionality. These findings represent a novel mechanism of MT targeting to AJs that may be relevant for the maintenance of proper epidermal progenitor cell homeostasis. We also discuss the potential implication of other MT binding proteins previously associated to AJs in the wider context of epithelial tissues. We hypothesize the existence of adaptation mechanisms that regulate the formation and stability of AJs in different cellular contexts to allow the dynamic behavior of these complexes during tissue homeostasis and remodeling.
Keywords: microtubules, Adherens Junctions, p120-catenin, CLASP2, cadherins, epidermis, keratinocytes
Introduction
Cadherin-based structures are fundamental for the maintenance of cell-cell adhesion, tissue architecture and for the integration of signaling cues that preserve tissue homeostasis.1 They were initially described in 1963 by Marilyn Farquhar and George Palade using electron microscopy,2 but it was not until the late 70s when the groups led by Takeichi, Kemler and Jacob identified and characterized the first component of the AJs: the transmembrane protein cadherin.3-5
Cadherins exert their functions via a group of intracellular proteins termed catenins. At adhesion sites, p120 binds directly to the juxtamembrane domain of the cadherin tail and controls its stability at the membrane.6 β-catenin, a transcriptional coactivator of the Wnt pathway, also binds the C-terminal domain of cadherins mediating the connection with α-catenin.7 In turn, α-catenin interacts with actin binding proteins connecting the cadherin complex to the actin cytoskeleton. This connection has been thoroughly studied and is fundamental for cells within tissues to function as cohesive sheets.8
The dynamic regulation of cadherin cell adhesion is critical during development and adult tissue homeostasis. At the organismal level, absence of E-Cadherin (ECad) is embryonically lethal at the blastocyst stage,9 whereas in adult tissues, alterations in cadherins have a causal role in cancer progression and metastasis.10,11
Due to the relevance of cadherins in human disease, substantial efforts have been made to understand the mechanisms that dynamically regulate the surface levels of cadherins. Phosphorylation, proteolytic cleavage, regulation of actomyosin contractility and trafficking of cadherins are all dynamic processes that impact on cadherin levels at the membrane, and thus, on the adhesive properties of cells.12 However, the role of microtubules and their associated proteins in the dynamic regulation of cadherins at the membrane is still not well understood. More importantly, one of the challenges in the field is to understand how the MT-AJ connection preserves cell-cell adhesion in the physiological context of tissues.
In recent years, several findings have started to illustrate the organization of microtubule networks in the epidermis.13 This self-renewing stratified tissue is able to regenerate due to the presence of progenitor cells located in the innermost basal layer, that undergo terminal differentiation to form the suprabasal postmitotic layers and sustain the epidermal barrier function of skin.
We have recently identified a novel interaction between the MT binding protein CLASP2 and the AJ component p120. This interaction mediates MT targeting to AJs specifically in progenitor cells of the epidermis, where it controls the dynamics of AJ components and impacts on the adhesive properties of these cells.14 In this commentary, we will highlight the major findings of our work and globally discuss the role of MTs in the maintenance of adhesion in different cellular contexts.
Microtubules and Adherens Junctions
Simple epithelial cells exhibit polarized apical and basolateral surface domains, which present a distinct composition of proteins and lipids. This organization is accompanied by non-centrosomal arrays of MTs that align parallel to the apical-basolateral polarity axis.15 In addition, a number of MTs that emanate from the apical centrosome projecting their plus-ends to the cell cortex can be identified.16 In the epidermis, this organization is established across the stratified tissue. Basal progenitor cells present a centrosomal MT network, while suprabasal postmitotic cells exhibit non-centrosomal MT arrays at the cell cortex.13
Using simple epithelial cells in culture, diverse mechanisms of MT interactions with AJ components have been proposed.17-22 Indeed, both MT plus-ends and minus-ends have been observed at AJs by electron microscopy.23 However, the specific functions that MTs play at AJs remain under debate.
Pioneering experiments using MT depolymerizing drugs such as Nocodazole (noc) have undoubtedly implicated MTs in AJ homeostasis, but depending on the cellular context different outcomes have been observed. MT depolymerization compromised AJ formation in PtK2 kidney epithelial cells,19 but had no effect in AJ formation in L cells expressing ECad or MDCK kidney cells.24,25 In terms of maintenance of AJs, MT depolymerization in monolayers of primary thyroid cells or newt lung epithelial cells induced AJ discontinuities.26,27 The opposite effect was observed in human colonic epithelial cells (SK-CO-15). MT depolymerization inhibited junction disassembly, suggesting that MTs are required for cadherin endocytosis in this cellular system.28
In our work using confluent primary mouse keratinocytes (mKer) we observed that treatment with noc did not disassemble AJs. In fact, the surface levels of cadherins increased with time, suggesting that most likely cadherins were not internalized.14 In terms of AJ formation we did not observe any apparent abnormalities in their assembly (unpublished observations). This resembles the behavior observed at cell-extracellular matrix (ECM) adhesion sites termed Focal Adhesions (FAs), in which noc treatment induces FA formation via RhoGTPase, and MT regrowth after noc induces FA disassembly.29
The diverse effects observed in different cell types may reside in the use of different noc concentrations, incomplete MT depolymerization and/or cell context. However, these results globally underscore the relevance of MTs in the assembly/disassembly of cadherin complexes depending on the cellular system. One of the current challenges in the field is to find scaffolding molecules in specific tissues that link MTs to AJs and regulate their functional activity.
p120 and CLASP2: A Novel Link Between MTs and AJs in Primary Mouse Keratinocytes
p120 is best known for its role as a regulator of cadherin stability at the membrane, but in the last decade a number of connections between p120 and MTs have been documented, placing p120 at the crossroads of cell adhesion and MT regulation.
p120 has been shown to bind directly the kinesin motor KIF5 and this link may be relevant for cadherin trafficking.30,31 p120 also interacts directly with MTs throughout the entire MT lattice,32 leading to an increased MT stability in a cadherin-independent manner.33 Interestingly, p120 was found to capture MT at AJs in simple epithelial cells. In this scenario, p120 interacts with non-centrosomal MT minus-ends via the MT minus-end binding protein Nezha and its associated protein PLEKHA7.21
How are MTs targeted to AJs in basal mKer? To answer this question, we performed a yeast-two hybrid screen searching for novel p120 interactors that could mediate MT targeting to AJs in basal mKer, and we identified the MT binding protein CLASP2 (CLIP-associated protein 2). CLASP2 belongs to the +TIP family of proteins, which track only the plus-ends of MTs.34 In particular, CLASP2, together with CLASP1, are major MT stabilizing factors at the cortex, preventing MT catastrophe and promoting MT pausing in a polarized manner.35,36 Thus, CLASP2 was an ideal candidate to mediate interactions between MT plus-ends and AJs in basal epidermal cells.
According to the “search and capture” hypothesis, MT attachment to cortical sites leads to MT stabilization.37 Thus, we envisioned a working model in which MT targeting to AJs via the CLASP2-p120 interaction would lead to MT stabilization at sites of cell-cell adhesion. Indeed, CLASPs have already been proposed to mediate cortical capture of MTs at cell-ECM adhesion sites. CLASPs interact with proteins located in the vicinity of FAs (LL5α and LL5β).38 The absence of CLASPs or LL5s leads to a decrease in MT density at cell-ECM adhesion sites and an increase in MT growth rate.39 In the same way, we observed a decrease in MT density and stability and an increase in MT growth rate at AJs in the absence of either CLASP2 or p120. Therefore, our results expand the role of CLASP2 as a cortical MT anchor to AJs.
Furthermore, MTs have been proposed to stabilize cell-cell adhesions.22,27 In this regard, our results indicate a role for CLASP2 in the formation and maintenance of proper AJ dynamics. In the absence of CLASP2, AJs do not properly form and p120 has decreased dynamics at cell-cell contacts. So far, the role of CLASP2 in terms of formation and stability of FAs has not been evaluated. Thus, to our knowledge this is the first time a role for CLASP2 in the maintenance of adhesion homeostasis is described, and future research will clarify whether CLASP2 functions are shared both at FAs and AJs.
MT Plus-Ends as Platforms for Protein Interactions at AJs
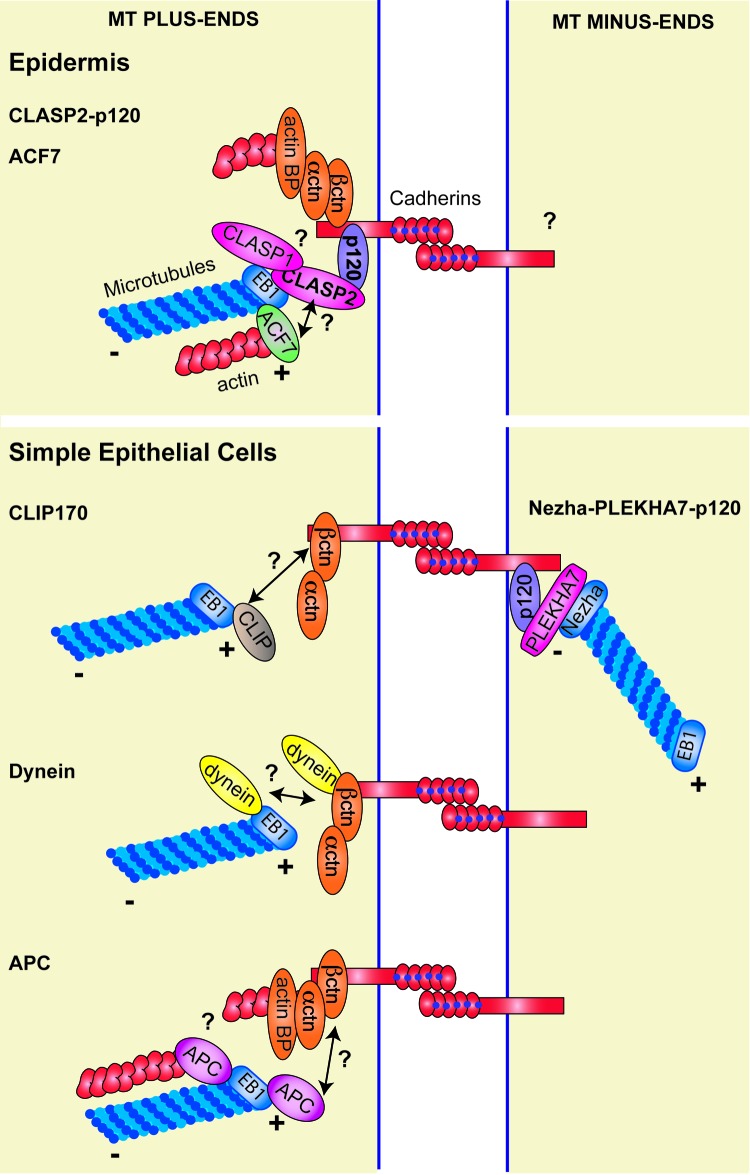
CLASPs were described as CLIP interacting proteins that bind MT plus-ends in an EB1 dependent manner.36,40 Additional studies have shown that Drosophila CLASP interacts with ACF7 (Actin crosslinking family 7)41 and in mammalian cells CLASP2 functions downstream of ACF7 during polarized cell migration.35 CLASP2 also interacts with the Rac and Cdc42 effector IQGAP1, which has fundamental roles during cell-cell contact formation.42 Which other MT binding proteins have been studied in the context of AJs? And how do they fit in our model? (Fig. 1).
Figure 1. Mechanisms of MT interactions with AJs. In basal progenitor cells of the epidermis the CLASP2-p120 interaction leads to MT targeting to AJs. ACF7 localizes to cell-cell contacts in primary mKer. It remains to be addressed whether it is also associated to CLASP2 at these sites. Regarding MT minus-ends, their interaction with AJs in epidermal cells has not been experimentally validated. In simple epithelial cells different mechanisms of MT interactions with AJs have been proposed depending on the cell context. In particular, CLIP170, dynein and APC have been implicated in mediating the interaction of MT plus-ends with cadherin-based adhesions. The more mature AJs termed zonula adherens interact with MT minus-ends via the p120-PLEKHA7-Nezha complex.
The most accepted mechanism for MT plus-end capture at AJs is the interaction between dynein and β-catenin described in PtK2 cells. It has been proposed that dynein tethers MTs to AJs in a β-catenin dependent manner.19,20 The distribution of this macromolecular complex has not been explored in the epidermis, but the +TIP protein and dynein regulator LIS1localizes to desmosomes in suprabasal mKer.43 Absence of LIS1 in the epidermis leads to altered MT organization in suprabasal mKer and desmosomal defects.
The CLIP-170-IQGAP1 interaction has been described as a mechanism for MT capture at the leading edge of migrating fibroblasts.44 IQGAP1 also localizes to sites of cell-cell adhesion,42 but whether the CLIP-170-IQGAP1 interaction mediates MT targeting to AJs has not been formally tested. IQGAP1 is known to bind directly β-catenin, inhibiting the β-catenin-α-catenin interaction, and leading to cell-cell dissociation. Thus, it is unlikely that the CLIP-170-IQGAP1 interaction represents a mechanism of MT targeting to AJs. Furthermore, CLIP-170 is preferentially expressed in suprabasal epidermal cells,45 where it may potentially associate with LIS1.46 It should be noted that in the neuromuscular junction, CLIPs seem to function together with CLASP2 in targeting MTs to these sites,47 suggesting differential functions in these tissue contexts.
Another candidate is APC (Adenomatous Polyposis Coli), which is found at AJs in both Drosophila and mammalian cells.25,48 At these sites it seems to interact with cortical actin, but whether it mediates MT capture together with EB1 remains to be experimentally validated.16 ACF7 has also been observed at AJs in primary mKer but its function at this location is not yet understood.17
Whether all these MT associated proteins interact with CLASP2 at AJs, or form part of independent MT subsets associated to cell-cell contacts remains to be explored. This type of compartmentalization has been observed at the leading edge of migrating PtK1 cells in which CLASP2 and APC decorate different subsets of microtubules.49 Given the fact that MT depolymerization does not perturb CLASP2 localization to AJs, we hypothesize that CLASP2 could further interact with cortical proteins such as ACF7 or actin. The cortical localization of numerous +TIP proteins is not affected after depolymerization of the MT network with noc. This is the case for ACF7 at AJs,17 CLASPs at the leading edge of migrating cells,35,36,38 and APC both at cell-cell contacts and at the leading edge of migrating cells.25,50
Thus, we propose a model in which there are two pools of CLASP2 at AJs: a MT plus-end-associated pool, and a MT-independent pool. The latter could be forming additional complexes with actin, ACF7, APC or IQGAP1 and this may be relevant for the proper organization and dynamics of AJ components. Such a model of actin-dependent and MT-dependent pools has already been described for APC and could represent a general feature of +TIP proteins that bind both actin and MT cytoskeletons.25 From our own results and what has already been published, CLASPs, APC and ACF7 share a cortical MT-targeting function both at cell-ECM and cell-cell adhesion sites.
In addition, we have observed that although CLASP1 moderately localizes to AJs, its deficiency leads to a clear delay in the formation of AJs, a phenotype that worsens when both CLASP2 and CLASP1 are missing. Future work will shed light onto the mechanisms and functions of CLASP1 at AJs.
CLASP2 in Progenitor Versus Differentiated Epidermal Cells
MTs in the basal layer of the epidermis present a centrosomal organization, with their plus-ends projecting towards areas of cell-cell contact.13 This correlates with one of the most interesting aspects of our work, the enrichment of CLASP2 in the basal progenitor cells of the epidermis. Upon differentiation, the MT network reorganizes and concentrates at sites of cell-cell adhesions in a desmosome-dependent manner.13 We not only observed that CLASP2 levels decreased upon differentiation, but also that Nezha localized to cell-cell contacts in suprabasal cells. Interestingly, both CLASP2 and Nezha bind to the N-terminal domain of p120, which is expressed across the epidermal layers. This finding led us to hypothesize that MT plus-ends preferentially associate to AJs in basal progenitor cells via the CLASP2-p120 interaction, whereas in suprabasal differentiated cells, MT minus-ends associate to cell-cell contacts together with Nezha and p120. Whether Nezha is recruited to desmosomes or remains associated to AJs in suprabasal epidermal cells remains to be addressed.
In conclusion, this particular compartimentalization may be relevant for different subsets of epidermal cells (proliferating vs. differentiated) to meet their specific functional requirements. We hypothesize that basal progenitor cells undergo frequent AJ dynamic rearrangements to fuel proper epidermal regeneration, compared with those in suprabasal-differentiated layers. Interestingly, this also parallels what has been observed in simple epithelial cells, where p120 binds to the complex formed by PLEKHA7 and Nezha in a specialized and mature AJ compartment called zonula adherens but not at the more dynamic lateral AJs.21 Overall, these findings suggest the existence of a compartmentalization of MTs at AJ sites in simple and stratified epithelia, which probably regulates their dynamic state.
Conclusions
In closing, we have uncovered that the novel interaction between CLASP2 and p120 leads to MT targeting to AJs in basal progenitor mKer. Absence of either protein leads to AJ and MT alterations, suggesting that their cooperative interaction may be responsible for the dynamic regulation of cell-cell adhesion in epidermal progenitor cells.
Our findings illustrate the need to further explore how different mechanisms of MT-AJ interaction function in specific cell types within the physiological context of diverse tissues. In addition, the future development of genetically modified mouse models will help us to understand if these processes hold epidermal progenitor cells into stemness and contribute to tissue architecture and function.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Carolina Epifano and the rest of the members of the Perez-Moreno lab for helpful comments on this commentary. We also thank our many colleagues in the field for their wonderful contributions to this fascinating area of research. M.S was a recipient of a CNIO-La Caixa International Ph.D. fellowship. M.PM group is supported by a grant from the Spanish Ministry of Economy and Competitiveness (BFU2012–33910).
Glossary
Abbreviations:
- ACF7
Actin Crosslinking Family 7
- AJs
Adherens Junctions
- CLASP2
CLIP-associated protein 2
- APC
Adenomatous polyposis coli
- ECad
E-Cadherin
- ECM
Extracellular Matrix
- FAs
Focal Adhesions
- GEF
Guanine nucleotide Exchange Factor
- mKer
Mouse Keratinocytes
- MTs
Microtubules
- noc
Nocodazole
- p120
p120-catenin
References
- 1.Meng W, Takeichi M. Adherens junction: molecular architecture and regulation. Cold Spring Harb Perspect Biol. 2009;1:a002899. doi: 10.1101/cshperspect.a002899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Farquhar MG, Palade GE. Junctional complexes in various epithelia. J Cell Biol. 1963;17:375–412. doi: 10.1083/jcb.17.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hyafil F, Babinet C, Jacob F. Cell-cell interactions in early embryogenesis: a molecular approach to the role of calcium. Cell. 1981;26:447–54. doi: 10.1016/0092-8674(81)90214-2. [DOI] [PubMed] [Google Scholar]
- 4.Takeichi M. Functional correlation between cell adhesive properties and some cell surface proteins. J Cell Biol. 1977;75:464–74. doi: 10.1083/jcb.75.2.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vestweber D, Kemler R. Rabbit antiserum against a purified surface glycoprotein decompacts mouse preimplantation embryos and reacts with specific adult tissues. Exp Cell Res. 1984;152:169–78. doi: 10.1016/0014-4827(84)90241-6. [DOI] [PubMed] [Google Scholar]
- 6.Davis MA, Ireton RC, Reynolds AB. A core function for p120-catenin in cadherin turnover. J Cell Biol. 2003;163:525–34. doi: 10.1083/jcb.200307111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perez-Moreno M, Jamora C, Fuchs E. Sticky business: orchestrating cellular signals at adherens junctions. Cell. 2003;112:535–48. doi: 10.1016/S0092-8674(03)00108-9. [DOI] [PubMed] [Google Scholar]
- 8.Perez-Moreno M, Fuchs E. Catenins: keeping cells from getting their signals crossed. Dev Cell. 2006;11:601–12. doi: 10.1016/j.devcel.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Larue L, Ohsugi M, Hirchenhain J, Kemler R. E-cadherin null mutant embryos fail to form a trophectoderm epithelium. Proc Natl Acad Sci U S A. 1994;91:8263–7. doi: 10.1073/pnas.91.17.8263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 11.Perl AK, Wilgenbus P, Dahl U, Semb H, Christofori G. A causal role for E-cadherin in the transition from adenoma to carcinoma. Nature. 1998;392:190–3. doi: 10.1038/32433. [DOI] [PubMed] [Google Scholar]
- 12.Niessen CM. Tight junctions/adherens junctions: basic structure and function. J Invest Dermatol. 2007;127:2525–32. doi: 10.1038/sj.jid.5700865. [DOI] [PubMed] [Google Scholar]
- 13.Lechler T, Fuchs E. Desmoplakin: an unexpected regulator of microtubule organization in the epidermis. J Cell Biol. 2007;176:147–54. doi: 10.1083/jcb.200609109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shahbazi MN, Megias D, Epifano C, Akhmanova A, Gundersen GG, Fuchs E, Perez-Moreno M. CLASP2 interacts with p120-catenin and governs microtubule dynamics at adherens junctions. J Cell Biol. 2013;203:1043–61. doi: 10.1083/jcb.201306019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bartolini F, Gundersen GG. Generation of noncentrosomal microtubule arrays. J Cell Sci. 2006;119:4155–63. doi: 10.1242/jcs.03227. [DOI] [PubMed] [Google Scholar]
- 16.Stehbens SJ, Akhmanova A, Yap AS. Microtubules and cadherins: a neglected partnership. Front Biosci (Landmark Ed) 2009;14:3159–67. doi: 10.2741/3442. [DOI] [PubMed] [Google Scholar]
- 17.Karakesisoglou I, Yang Y, Fuchs E. An epidermal plakin that integrates actin and microtubule networks at cellular junctions. J Cell Biol. 2000;149:195–208. doi: 10.1083/jcb.149.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stehbens SJ, Paterson AD, Crampton MS, Shewan AM, Ferguson C, Akhmanova A, Parton RG, Yap AS. Dynamic microtubules regulate the local concentration of E-cadherin at cell-cell contacts. J Cell Sci. 2006;119:1801–11. doi: 10.1242/jcs.02903. [DOI] [PubMed] [Google Scholar]
- 19.Ligon LA, Holzbaur EL. Microtubules tethered at epithelial cell junctions by dynein facilitate efficient junction assembly. Traffic. 2007;8:808–19. doi: 10.1111/j.1600-0854.2007.00574.x. [DOI] [PubMed] [Google Scholar]
- 20.Ligon LA, Karki S, Tokito M, Holzbaur EL. Dynein binds to beta-catenin and may tether microtubules at adherens junctions. Nat Cell Biol. 2001;3:913–7. doi: 10.1038/ncb1001-913. [DOI] [PubMed] [Google Scholar]
- 21.Meng W, Mushika Y, Ichii T, Takeichi M. Anchorage of microtubule minus ends to adherens junctions regulates epithelial cell-cell contacts. Cell. 2008;135:948–59. doi: 10.1016/j.cell.2008.09.040. [DOI] [PubMed] [Google Scholar]
- 22.Komarova YA, Huang F, Geyer M, Daneshjou N, Garcia A, Idalino L, Kreutz B, Mehta D, Malik AB. VE-cadherin signaling induces EB3 phosphorylation to suppress microtubule growth and assemble adherens junctions. Mol Cell. 2012;48:914–25. doi: 10.1016/j.molcel.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bellett G, Carter JM, Keynton J, Goldspink D, James C, Moss DK, Mogensen MM. Microtubule plus-end and minus-end capture at adherens junctions is involved in the assembly of apico-basal arrays in polarised epithelial cells. Cell Motil Cytoskeleton. 2009;66:893–908. doi: 10.1002/cm.20393. [DOI] [PubMed] [Google Scholar]
- 24.Angres B, Barth A, Nelson WJ. Mechanism for transition from initial to stable cell-cell adhesion: kinetic analysis of E-cadherin-mediated adhesion using a quantitative adhesion assay. J Cell Biol. 1996;134:549–57. doi: 10.1083/jcb.134.2.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rosin-Arbesfeld R, Ihrke G, Bienz M. Actin-dependent membrane association of the APC tumour suppressor in polarized mammalian epithelial cells. EMBO J. 2001;20:5929–39. doi: 10.1093/emboj/20.21.5929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yap AS, Stevenson BR, Abel KC, Cragoe EJ, Jr., Manley SW. Microtubule integrity is necessary for the epithelial barrier function of cultured thyroid cell monolayers. Exp Cell Res. 1995;218:540–50. doi: 10.1006/excr.1995.1189. [DOI] [PubMed] [Google Scholar]
- 27.Waterman-Storer CM, Salmon WC, Salmon ED. Feedback interactions between cell-cell adherens junctions and cytoskeletal dynamics in newt lung epithelial cells. Mol Biol Cell. 2000;11:2471–83. doi: 10.1091/mbc.11.7.2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ivanov AI, McCall IC, Babbin B, Samarin SN, Nusrat A, Parkos CA. Microtubules regulate disassembly of epithelial apical junctions. BMC Cell Biol. 2006;7:12. doi: 10.1186/1471-2121-7-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ezratty EJ, Partridge MA, Gundersen GG. Microtubule-induced focal adhesion disassembly is mediated by dynamin and focal adhesion kinase. Nat Cell Biol. 2005;7:581–90. doi: 10.1038/ncb1262. [DOI] [PubMed] [Google Scholar]
- 30.Chen X, Kojima S, Borisy GG, Green KJ. p120 catenin associates with kinesin and facilitates the transport of cadherin-catenin complexes to intercellular junctions. J Cell Biol. 2003;163:547–57. doi: 10.1083/jcb.200305137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yanagisawa M, Kaverina IN, Wang A, Fujita Y, Reynolds AB, Anastasiadis PZ. A novel interaction between kinesin and p120 modulates p120 localization and function. J Biol Chem. 2004;279:9512–21. doi: 10.1074/jbc.M310895200. [DOI] [PubMed] [Google Scholar]
- 32.Franz CM, Ridley AJ. p120 catenin associates with microtubules: inverse relationship between microtubule binding and Rho GTPase regulation. J Biol Chem. 2004;279:6588–94. doi: 10.1074/jbc.M312812200. [DOI] [PubMed] [Google Scholar]
- 33.Ichii T, Takeichi M. p120-catenin regulates microtubule dynamics and cell migration in a cadherin-independent manner. Genes Cells. 2007;12:827–39. doi: 10.1111/j.1365-2443.2007.01095.x. [DOI] [PubMed] [Google Scholar]
- 34.Akhmanova A, Steinmetz MO. Microtubule +TIPs at a glance. J Cell Sci. 2010;123:3415–9. doi: 10.1242/jcs.062414. [DOI] [PubMed] [Google Scholar]
- 35.Drabek K, van Ham M, Stepanova T, Draegestein K, van Horssen R, Sayas CL, Akhmanova A, Ten Hagen T, Smits R, Fodde R, et al. Role of CLASP2 in microtubule stabilization and the regulation of persistent motility. Curr Biol. 2006;16:2259–64. doi: 10.1016/j.cub.2006.09.065. [DOI] [PubMed] [Google Scholar]
- 36.Mimori-Kiyosue Y, Grigoriev I, Lansbergen G, Sasaki H, Matsui C, Severin F, Galjart N, Grosveld F, Vorobjev I, Tsukita S, et al. CLASP1 and CLASP2 bind to EB1 and regulate microtubule plus-end dynamics at the cell cortex. J Cell Biol. 2005;168:141–53. doi: 10.1083/jcb.200405094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kirschner M, Mitchison T. Beyond self-assembly: from microtubules to morphogenesis. Cell. 1986;45:329–42. doi: 10.1016/0092-8674(86)90318-1. [DOI] [PubMed] [Google Scholar]
- 38.Lansbergen G, Grigoriev I, Mimori-Kiyosue Y, Ohtsuka T, Higa S, Kitajima I, Demmers J, Galjart N, Houtsmuller AB, Grosveld F, et al. CLASPs attach microtubule plus ends to the cell cortex through a complex with LL5beta. Dev Cell. 2006;11:21–32. doi: 10.1016/j.devcel.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 39.Hotta A, Kawakatsu T, Nakatani T, Sato T, Matsui C, Sukezane T, Akagi T, Hamaji T, Grigoriev I, Akhmanova A, et al. Laminin-based cell adhesion anchors microtubule plus ends to the epithelial cell basal cortex through LL5alpha/beta. J Cell Biol. 2010;189:901–17. doi: 10.1083/jcb.200910095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Akhmanova A, Hoogenraad CC, Drabek K, Stepanova T, Dortland B, Verkerk T, Vermeulen W, Burgering BM, De Zeeuw CI, Grosveld F, et al. Clasps are CLIP-115 and -170 associating proteins involved in the regional regulation of microtubule dynamics in motile fibroblasts. Cell. 2001;104:923–35. doi: 10.1016/S0092-8674(01)00288-4. [DOI] [PubMed] [Google Scholar]
- 41.Long JB, Bagonis M, Lowery LA, Lee H, Danuser G, Van Vactor D. Multiparametric analysis of CLASP-interacting protein functions during interphase microtubule dynamics. Mol Cell Biol. 2013;33:1528–45. doi: 10.1128/MCB.01442-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Noritake J, Watanabe T, Sato K, Wang S, Kaibuchi K. IQGAP1: a key regulator of adhesion and migration. J Cell Sci. 2005;118:2085–92. doi: 10.1242/jcs.02379. [DOI] [PubMed] [Google Scholar]
- 43.Sumigray KD, Chen H, Lechler T. Lis1 is essential for cortical microtubule organization and desmosome stability in the epidermis. J Cell Biol. 2011;194:631–42. doi: 10.1083/jcb.201104009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fukata M, Watanabe T, Noritake J, Nakagawa M, Yamaga M, Kuroda S, Matsuura Y, Iwamatsu A, Perez F, Kaibuchi K. Rac1 and Cdc42 capture microtubules through IQGAP1 and CLIP-170. Cell. 2002;109:873–85. doi: 10.1016/S0092-8674(02)00800-0. [DOI] [PubMed] [Google Scholar]
- 45.Wacker IU, Rickard JE, De Mey JR, Kreis TE. Accumulation of a microtubule-binding protein, pp170, at desmosomal plaques. J Cell Biol. 1992;117:813–24. doi: 10.1083/jcb.117.4.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gouveia SM, Akhmanova A. Cell and molecular biology of microtubule plus end tracking proteins: end binding proteins and their partners. Int Rev Cell Mol Biol. 2010;285:1–74. doi: 10.1016/B978-0-12-381047-2.00001-3. [DOI] [PubMed] [Google Scholar]
- 47.Schmidt N, Basu S, Sladecek S, Gatti S, van Haren J, Treves S, Pielage J, Galjart N, Brenner HR. Agrin regulates CLASP2-mediated capture of microtubules at the neuromuscular junction synaptic membrane. J Cell Biol. 2012;198:421–37. doi: 10.1083/jcb.201111130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Townsley FM, Bienz M. Actin-dependent membrane association of a Drosophila epithelial APC protein and its effect on junctional Armadillo. Curr Biol. 2000;10:1339–48. doi: 10.1016/S0960-9822(00)00770-3. [DOI] [PubMed] [Google Scholar]
- 49.Wittmann T, Waterman-Storer CM. Spatial regulation of CLASP affinity for microtubules by Rac1 and GSK3beta in migrating epithelial cells. J Cell Biol. 2005;169:929–39. doi: 10.1083/jcb.200412114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zaoui K, Benseddik K, Daou P, Salaün D, Badache A. ErbB2 receptor controls microtubule capture by recruiting ACF7 to the plasma membrane of migrating cells. Proc Natl Acad Sci U S A. 2010;107:18517–22. doi: 10.1073/pnas.1000975107. [DOI] [PMC free article] [PubMed] [Google Scholar]