Abstract
Many internal organs develop distinct left and right sides that are essential for their functions. In several vertebrate embryos, motile cilia generate an asymmetric fluid flow that plays an important role in establishing left-right (LR) signaling cascades. These ‘LR cilia’ are found in the ventral node and posterior notochordal plate in mammals, the gastrocoel roof plate in amphibians and Kupffer’s vesicle in teleost fish. I consider these transient ciliated structures as the ‘organ of asymmetry’ that directs LR patterning of the developing embryo. Variations in size and morphology of the organ of asymmetry in different vertebrate species have raised questions regarding the fundamental features that are required for LR determination. Here, I review current models for how LR asymmetry is established in vertebrates, discuss the cellular architecture of the ciliated organ of asymmetry and then propose key features of this organ that are critical for orienting the LR body axis.
Keywords: Kupffer’s vesicle, Left-right asymmetry, calcium ion flux, cilia, congenital heart defects, gastrocoel roof plate, posterior notochordal plate
Left-Right Asymmetries in the Vertebrate Body Plan
Left-right (LR) asymmetry is a common feature of visceral organs. In the normal arrangement, called situs solitus, the heart is positioned on the left side of the body and has distinct left and right chambers with specific vascular connections, the left lung has two lobes while the right lung has three, the stomach and spleen are positioned on the left side of the body and the liver is on the right side. Situs inversus totalis is a complete mirror-reversal of organ LR asymmetry, but this condition has a low risk of phenotypic consequences1 since all organs remain in concordant alignment. In contrast, defects during embryogenesis that perturb LR asymmetry of only a subset of organs cause a broad spectrum of congenital malformations that compromise organ function. This situs ambiguus (also known as heterotaxy) occurs ~1:10,000 live births and usually results in life-threatening complex congenital heart defects.2-4 LR asymmetries are conserved among vertebrates, which allow the biochemistry, genetics and cell biology underlying LR axis determination to be studied in model systems.5 Work over the last two decades has uncovered a number of genes and signaling pathways that are involved in establishing LR asymmetry, but precise mechanisms have not been determined making it difficult to conceptualize therapeutic or preventative approaches.
A groundbreaking advancement of our understanding of LR asymmetry came with the discovery that signaling molecules are asymmetrically expressed along the LR axis in the chicken embryo at developmental stages that precede formation of visceral organs.6 Transient left-sided expression of Sonic hedgehog (Shh) near an embryonic structure called Hensen’s node was found to activate asymmetric expression of cNR-1, a homolog of the mouse Nodal gene that encodes a secreted signaling ligand in the TGF-β superfamily. At subsequent stages, an expansion of cNR-1/Nodal expression was observed exclusively on the left side of the embryo in lateral plate mesoderm (LPM) that contributes to the heart and gut. This revealed that the left and right sides of the embryo are patterned at the molecular level prior to the development of organ asymmetries. While not all molecular asymmetries identified in the chick embryo are conserved (e.g., Shh asymmetry), a left-sided Nodal signaling cascade (Fig. 1A) has been observed in all vertebrate embryos analyzed. Nodal induces its own expression in neighboring cells as well as the expression of Lefty proteins that function as diffusible Nodal antagonists to limit the Nodal expression domain.7 Nodal also activates expression of the transcription factor Pitx2 in left LPM cells. Left-sided Pitx2 expression persists in the developing heart and gut where it regulates genes that mediate asymmetric morphogenesis of these organs (Fig. 1A). Genetic and functional analyses in medaka, zebrafish, frog and mouse8-11 indicate this asymmetric Nodal signaling plays a critical and conserved role in directing LR development of visceral organs.
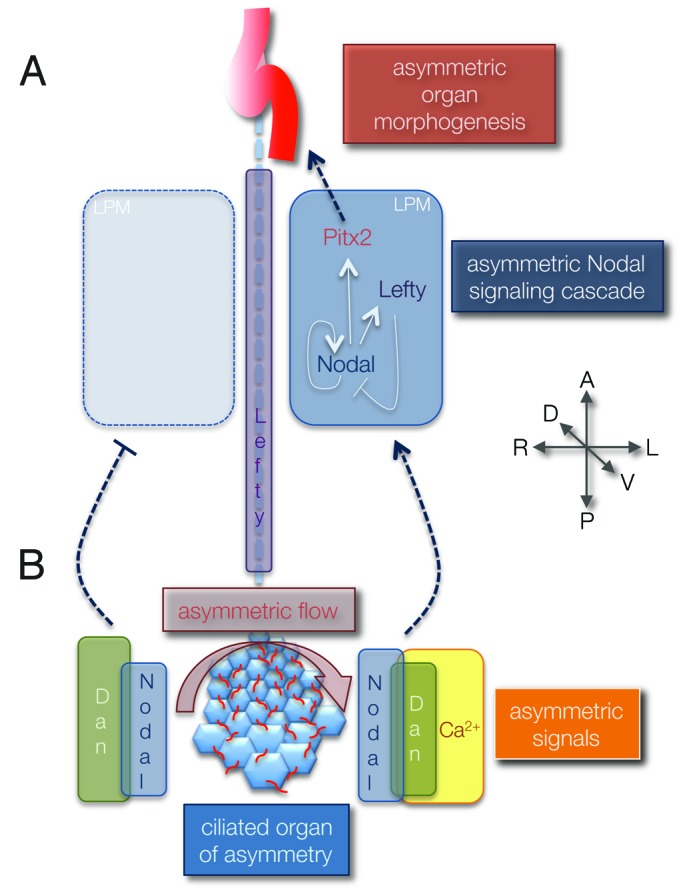
Figure 1. Working model for how LR asymmetry is established in vertebrate embryos. (A) Asymmetric Nodal signaling guides asymmetric organ development. The TGFβ signaling molecule Nodal initiates its own expression in left lateral plate mesoderm (LPM) to expand asymmetric patterning along the left side of the embryo and induces expression of Lefty and Pitx2. Lefty molecules function as Nodal antagonists in the embryonic midline (dashed line) and LPM to place boundaries on Nodal signaling. Pitx2 is a transcription factor thought to control genes involved in asymmetric morphogenesis of visceral organs. (B) Asymmetric fluid flow in the ciliated organ of asymmetry is translated into asymmetric Nodal signaling. Motile LR cilia (red) generate a leftward flow that results in increased Ca2+ signals on the left side of the organ of asymmetry and elevated expression of Cerebrus/Dan family Nodal antagonists (Dan proteins) on the right side. These flow-dependent asymmetric signals are integrated with bilateral Nodal expression to trigger Nodal signaling exclusively in the left LPM. Embryonic axes: A, anterior; P, posterior; D, dorsal; V, ventral; L, left; R, right.
A Role For Cilia
The importance of the Nodal-Lefty-Pitx2 network in establishing LR asymmetry is well recognized, but the mechanisms that lead to its activation on the left side of the embryo are not fully understood. In other words, how is left-right asymmetric Nodal signaling aligned with the previously established dorsal-ventral and anterior-posterior axes of the embryo? In several vertebrates, motile cilia are involved in directing left-sided Nodal signaling. Motile cilia are microtubule based hair-like structures that project from cells and beat in a coordinated fashion to move fluids unidirectionally in several tissues including airways, brain ventricles and fallopian tubes. A link between motile cilia and LR asymmetry was first established in the 1970s when Bjorn Afzelius discovered immotile cilia in patients with situs inversus.12 Electron microscopy revealed that cilia from these patients lacked dynein arm complexes that mediate cilia beating. This finding prompted Afzelius to predict, “asymmetry is determined through the movements of cilia of some embryonic epithelial tissues.”12
Two decades after Afzelius made his prediction, motile cilia in a transient epithelial structure in the mouse embryo analogous to Hensen’s node were implicated in establishing LR asymmetry. Motile monocilia projecting from ventral node cells13 were shown to beat with a clockwise rotational pattern and generate a leftward fluid flow across the pit-shaped node.14 In mutant embryos deficient for the microtubule motor protein Kif3B, these node cilia were missing, fluid flow was absent and LR asymmetry was randomized.14 Leftward flow was observed at developmental stages that corresponded with the initiation of asymmetric Nodal expression near the ventral node. This led to the ‘nodal flow’ hypothesis14 that cilia-driven asymmetric fluid flow orients the LR axis relative to the dorsal-ventral and anterior-posterior axes (Fig. 1B). Analogous asymmetric flows have been observed in transient ciliated epithelial organs in several other vertebrates, suggesting a conserved role for motile cilia in establishing LR asymmetry. These organs include the posterior notochordal plate in rabbit,15 gastrocoel roofplate in frog,16 and Kupffer’s vesicle in medaka17 and zebrafish.18,19 Due to differences in names and embryonic locations, I refer to these structures collectively as the ciliated ‘organ of asymmetry’ and the monocilia in these organs as ‘LR cilia.’ Interestingly, LR cilia are not found in all vertebrates. Henson’s node in the chick embryo, where asymmetric gene expression was first described, does not contain motile cilia and uses an alternative strategy to establish LR asymmetry.20,21 This review focuses on the function of the ciliated organ of asymmetry in LR patterning, but cilia-independent mechanisms will be discussed below.
How fluid flow in the organ of asymmetry is detected and translated into molecular asymmetries in neighboring cells remains unclear. It has been proposed that flow generates LR signals by 1) creating a left-to-right morphogen gradient,14 2) moving membrane-covered ‘nodal vesicle parcels’ containing signal molecules to the left22 or 3) activating cilia that are mechanosensory23 or chemosensory24 on the left side of the embryo. Each of these potential mechanisms, which have been recently reviewed in detail,25-27 has been postulated to trigger asymmetric calcium ion (Ca2+) flux on the left periphery of the organ of asymmetry (Fig. 1B).22,23,28 The function of Ca2+ in this context is not known, but this asymmetric signal is somehow integrated with conserved domains of Nodal and Lefty expression around the organ of asymmetry to establish a self-enhancement lateral-inhibition system that biases Nodal activation in the left LPM.29 Flow-dependent right-sided expression of Cerebrus/Dan family of Nodal antagonists—Cerebrus like 2 (Cerl2) in mouse,30 Coco in frog31 and charon in fish17,32,33—are thought to inhibit Nodal initiation in the right LPM (Fig. 1B). Notch signaling is involved in establishing Nodal expression at the organ of asymmetry34 as well as left-biased Wnt3 expression35 that has been shown to regulate Cerl2 asymmetry by promoting the degradation of Cerl2 mRNA in response to flow.36 Studies in mouse using Cerl2 antibodies indicate the distribution of the secreted Cerl2 protein first shows a right-sided bias matching the mRNA expression pattern, but later in development accumulates on the left side of the node where it likely functions to turn off asymmetric Nodal signaling.37 These examples of the complex regulation of Nodal that occurs at the organ of asymmetry underscore the need to understand the cellular and molecular biology of this transient structure.
Ciliated Organ of Asymmetry Architecture
The size and gross morphology of the ciliated organ of asymmetry varies considerably among vertebrate embryos. Comparative anatomy of the organ of asymmetry, as well as the location of this structure in different embryos, has been presented previously in excellent reviews,38-40 therefore I provide here only a brief overview of the cellular architectures of these organs in selected model vertebrates (summarized in Table 1) and then focus on key features. In the mouse embryo, monociliated epithelial cells in the posterior notochordal plate and ventral node form a pit-like indentation. Cells in the pit of the node have motile monocilia projecting from small apical surfaces and are surrounded by large crown cells that have a mixed population of motile and immotile cilia.41 Fluid moves leftward near the surface of the node and then recirculates back to the right well above the surface and closer to Reichardt’s membrane that covers the ventral node.15 Analysis of a second mammal, the rabbit embryo, revealed LR cilia in the posterior notochordal plate (PNC) adjacent to the node, which does not contain cells with motile cilia.15,42 It has been proposed that the PNC is also the site of functional asymmetric flow in the mouse embryo,42 so I refer to the mouse organ of asymmetry as the node/PNC. In contrast to the concave pit structure in the mouse, ciliated cells form a convex ridge along the rabbit PNC and drive fluid flow leftward across the ridge that then returns to the right at the posterior end of the PNC.15 Also different from the mouse, the rabbit PNC is not covered by a membrane, but rather the cilia contact fluid of the yolk sac cavity.42
Table 1. Features of the ciliated organ of asymmetry in selected vertebrates.
| Species | Organ of Asymmetry |
Developmental Stages Present |
Organ Structure | Number of Cilia | Pattern of Cilia-Driven Fluid Flow |
|---|---|---|---|---|---|
| Mouse | Ventral node/ Posterior notochordal plate (node/PNC) |
LB-EHF stage to 8 somite stage86 | Concave pit-like structure; ~60 μm wide; Pit cells with small apical surfaces are surrounded by large crown cells13,39 | 200–30038 | Clockwise rotation of cilia; Strong leftward flow near surface; Slow rightward flow 20 μm above surface14,15 |
| Rabbit | Posterior notochordal plate (PNC) |
Stage 5 to 8 somite stage65 |
Groove-like structure; ~100 μm wide; Ciliated cells form a convex surface15,38,42 | ~80038 | Clockwise rotation of cilia; Strong leftward flow across convex ridge; Slow rightward flow at posterior end15 |
| Frog | Gastrocoel roof plate (GRP) | Stage 13 to stage 1916 |
Elongated triangular structure; ~175 μm wide; Ciliated cells are flanked by large non-ciliated lateral endoderm crest cells16,43 | 240–27016 | Clockwise rotation of cilia; Strong leftward flow across GRP16 |
| Zebrafish | Kupffer’s vesicle (KV) |
2 somite stage to 20 somite stage116,117 | Sphere-like structure; ~65 μm diameter; Cilia concentrated on dorsal-anterior surface46,47 | 50–6045 | Clockwise rotation of cilia; Strong leftward flow at anterior end; Slow rightward flow at posterior end19,47,48 |
| Medaka | Kupffer’s vesicle (KV) |
2 somite stage (st. 19) to 14 somite stage (st. 23)17 | Sphere-like structure; ~150 μm diameter; Cilia positioned on a dorsal convex surface15,17 | ~15038 | Clockwise rotation of cilia; Strong leftward flow; Slow rightward flow at posterior end15 |
The ciliated organ of asymmetry in the frog Xenopus laevis is known as the gastrocoel roof plate (GRP).16 The GRP is a transient ciliated epithelium flanked by large non-ciliated lateral endoderm crest cells43 that line the gastrocoel cavity. Two populations of ciliated GRP cells have been described: peripheral cells that express Nodal and Coco signaling molecules and more centrally positioned cells that do not express these markers.31 Similar to the mouse node/PNC, the central cells contain predominantly motile cilia and the peripheral cells have a higher concentration of immotile cilia.44 These different cell types and their relative positions in and around the GRP are likely important for generating and detecting flow in this organ. In contrast to mammals and frogs, asymmetric flow in the teleost fish medaka and zebrafish is generated in an enclosed spherical organ called Kupffer’s vesicle (KV).15,18,19 In Medaka, epithelial cells on the dorsal roof of KV project motile cilia into the spherical fluid-filled lumen that is otherwise enclosed by a yolk membrane on the ventral and lateral sides.17 In zebrafish, the entire KV lumen is lined by ciliated epithelial cells,45 but there is a concentration of ciliated cells in the dorsal-anterior region of KV46,47 where strong leftward flow is observed.19,47,48 The significant differences in size, shape—and even the number of ciliated cells (Table 1)—indicate these features of the organ of asymmetry are not under evolutionary pressure and may not be critical for establishing LR asymmetry.
Salient Features of the Ciliated Organ of Asymmetry
A striking take home message from comparing architectures of the ciliated organ of asymmetry in different vertebrates is that although the conceptual design is the same—a transient ciliated epithelium generates an asymmetric fluid flow across the midline of the embryo to convey LR positional information—the locations, sizes and shapes of these organs are quite different. This raises the important question: what are the key architectural features that are essential for establishing LR asymmetry? I propose four features of ciliated organs of asymmetry that are critical for LR patterning (Fig. 2). First, properly assembled motile monocilia are necessary to generate fluid flow. Second, the cilia must be tilted toward the posterior to create unidirectional flow. Third, Ca2+ flux that responds to flow is necessary to translate mechanical stimulus of flow into molecular signals. Finally, the spatial organization of cells relative to one another is important for both generating and sensing flow. Each of these features is discussed below.

Figure 2. Salient features of the ciliated organ of asymmetry. (1–2) Close-up view of ciliated epithelial cells in the organ of asymmetry. (1) Motile monocilia (red) use a vortical motion to generate fluid flow. (2) Motile cilia must be tilted posteriorly to create leftward flow. Positioning of the basal body (yellow) at the posterior end of the cell is involved in cilia tilting. (3–4) Overview of the ciliated organ of asymmetry. (3) Asymmetric fluid flow (arrow) induces a transient left-sided calcium ion (Ca2+) flux that is essential for establishing asymmetric Nodal signaling. (4) The arrangement of ciliated cells is important for generating and sensing fluid flow. Shown as an example is a gradient of cell morphologies along the anteroposterior axis in the zebrafish Kupffer’s vesicle, where cells with small apical surfaces are tightly packed into the anterior pole and fewer, larger cells are placed in the posterior region. Embryonic axes: A, anterior; P, posterior; D, dorsal; V, ventral; L, left; R, right.
Motile cilia
Since Afzelius first associated motile cilia with LR asymmetry, mutations in several genes have been identified that disrupt cilia motility in patients affected by what is now known to be a genetically heterogeneous disorder called primary ciliary dyskinesia (PCD).49 About half of the individuals with PCD have situs inversus, which suggests the LR body axis is randomly oriented in the absence of motile cilia. Interestingly, ~6% of PCD patients present with situs ambiguus/heterotaxy,50 but mechanisms underlying these phenotypes are not clear. Genetic analyses in animal models agree that correct LR patterning depends on genes involved in assembling motile cilia (previously reviewed in51,52). These genes encode structural components of cilia such as dynein axonemal heavy chains that are required for cilia beating, as well as signaling molecules, transcription factors and transport proteins that regulate cilia formation and maintenance. An example is the inversus viscerum (iv) mutant mouse in which cilia are paralyzed due to loss of the dynein axonemal heavy chain Dnah11, which is also called Left-right dynein (Lrd).53 In Lrd mutant mice,54 and other animal models with cilia defects, LR patterning defects correlated with perturbed cilia-driven flow in the organ of asymmetry. However, since loss of gene function in both patients and animal models was global it was not possible to rule out roles for cilia genes in tissues other than the organ of asymmetry. This was first addressed in zebrafish, in which antisense morpholino oligonucleotides (MO) can be targeted to KV precursor cells to knock down genes specifically in the organ of asymmetry.55 Depletion of the dynein axonemal heavy chain Dnah918 or the intraflagellar transport gene Ift8856 in KV demonstrated genes involved in the movement or formation of motile cilia are required cell autonomously in the zebrafish ciliated organ of asymmetry to establish normal LR patterning. Similarly, targeting MOs that deplete expression of Dnah9 or Dnah5 to the cells that give rise to GRP in the frog embryo disrupted cilia motility, asymmetric flow and LR asymmetry.57 Finally, restoring motility to a subset of LR cilia in Lrd mutant mice was sufficient to partially rescue LR asymmetry defects.58 These results provide compelling evidence that motile cilia located in the organ of asymmetry are required for establishing LR asymmetry.
Since perturbing genes that control cilia motility could potentially affect other cellular processes, it was important to test the requirement for cilia-driven flow using non-genetic methods. In an elegant set of experiments, mouse embryos placed in a custom-built culture system were exposed to artificial laminar fluid flow of the culture media.59 Strong rightward flow reversed LR patterning in wild-type embryos, and strong leftward flow rescued LR defects in mutants that lack motile cilia. This was the first direct evidence that fluid flow is involved in establishing LR asymmetry. To interfere with endogenous flow in the frog embryo, a viscous solution of 1% methylcellulose was injected into GRP that would impede cilia movement.16 In these experiments, disrupting flow at specific developmental stages altered LR asymmetry. Similar results were observed when mouse embryos were cultured in 1% methylcellulose.60 These embryological manipulations compliment the genetic studies described above to demonstrate that fluid flow generated by motile cilia is an essential feature of the organ of asymmetry.
Recent studies have sought to pinpoint properties of asymmetric flow that are necessary and sufficient to establish LR asymmetry. Taking advantage of the ability to target injected molecules to either the left or right side of the GRP in frog, antisense MO depletion of Dnah9 was used to paralyze cilia and abolish flow on one side or the other. This analysis showed that only flow on the left side of the GRP was required to activate normal LR gene expression,57 indicating not all LR cilia are necessary to generate an effective flow. To determine the threshold of cilia activity needed to establish LR asymmetry, live imaging of cilia was followed by assessment of LR markers in individual mutant mouse embryos (Dpcd−/− or Rfx3−/−) that exhibit cilia motility defects with incomplete penetrance.60 Embryos with one or zero motile cilia showed LR defects, whereas embryos with at least two motile cilia developed normal LR asymmetry. Thus, a surprisingly low number of cilia that generate a weak leftward flow are sufficient to establish LR asymmetry in the mouse embryo. These results are consistent with the rescue experiments described above in which re-activation of motility in only a subpopulation of LR cilia in Lrd mutant mice was sufficient to restore normal LR patterning.58 Together, these findings emphasize the necessity of motile cilia in orienting the LR axis and reveal that the number of functional cilia needed to create an effective flow in the mouse embryo is far fewer than the number present. This reinforces the idea that the number of cilia is not a critical feature of the ciliated organ of asymmetry.
Tilted cilia
The observation that LR cilia beat with a vortical motion (Fig. 2–1) raised the question of how these monocilia generate unidirectional flow. Mathematical modeling of fluid dynamics61 predicted that rotating cilia do not project straight up and down, but rather must be tilted toward the posterior of the embryo to generate a net leftward flow. High-speed imaging in live mouse embryos revealed that indeed the majority of LR cilia are tilted posteriorly.15,62 The clockwise rotation of posteriorly tilted cilia is thought to result in an ineffective left-to-right stroke near the cell surface and an effective right-to-left stroke that generates a net leftward flow. Tilting of LR cilia appears to be mediated by the positioning of the cilium base, or basal body, at the posterior pole of the cell (Fig. 2–2), which suggests an intriguing mechanism for aligning the LR axis with the existing anterior-posterior axis.63 In frog and/or mouse embryos, loss of Inversin15 or Bicaudal C64 was found to alter the posterior positioning of LR cilia and disrupt asymmetric flow. These observations provided in vivo evidence that tilted cilia are important for generating coordinated fluid flow and LR signals. Although the cellular architecture of the organ of asymmetry varies considerably among vertebrates, posterior projection of LR cilia is a common feature observed in rabbit15,65 frog,16 medaka15 and zebrafish.19,66
Planar cell polarity (PCP) signaling has been implicated in posterior basal body positioning and LR cilia tilting. A conserved PCP network of proteins regulates multiple cellular processes, including the polarization of epithelial cells within a plane.67 The PCP proteins Disheveled (Dvl), Van gogh like 1 (Vangl1) and Prickle 2 show polarized localizations in node/PNC cells in mouse embryos,68,69 but upstream signals that establish this polarity remain unknown. Basal bodies failed to polarize along the anteroposterior axis in the node/PNC of mice carrying mutations in three Dvl genes68 or two Vangl genes (Vangl1 and Vangl2),70 which resulted in aberrant flow and LR defects. Similar phenotypes were observed in mouse embryos treated with a small molecule inhibitor of Rac1, a downstream effector of PCP signals.68 Analysis of double mouse mutants lacking Vangl2 and the actin severing protein Cofilin indicated actin rearrangements are involved in trafficking PCP proteins in the node/PNC.71 A role for PCP in LR cilia polarization appears to be conserved among vertebrates, as depletion of Vangl2 in frog inhibited posterior positioning of LR cilia and disrupted LR patterning69 and cilia tilting and asymmetric flow were altered in zebrafish mutants that lack all Vangl2 (maternal and zygotic) expression.66 Zebrafish LR cilia are positioned such that the majority point toward the posterior using a Vangl2 (PCP) dependent mechanism, but whether individual cilia project from the posterior end of the cell is unclear.47 Taken together, these results from several vertebrate models indicate PCP-mediated posterior tilting of cilia is critical for generating unidirectional flow in the ciliated organ of asymmetry.
Calcium ion flux
There is strong evidence that Ca2+ flux on the left side of the organ of asymmetry (Fig. 2 part 3) is necessary for normal LR pattering. Mouse embryos cultured in the presence of cell-permeable fluorescent Ca2+ indicator dyes revealed asymmetric intracellular Ca2+ signals in endodermal cells at the left periphery of the node/PNC at stages concomitant with asymmetric flow and prior to Nodal asymmetry.23 Similar experiments using a different fluorescent Ca2+ dye that loaded more efficiently into node/PNC cells identified predominantly left-sided dynamic Ca2+ ‘flashes’ within the organ of asymmetry.72 Perturbation of Ca2+ signaling with small molecules that change intracellular Ca2+ levels, block transient receptor potential (TRP) ion channels or inhibit inositol trisphosphate (Ins(1,4,5)P3) receptors disrupted LR asymmetry at the node/PNC.41,72 Left-sided Ca2+ flux in endoderm was lost in Lrd mutant embryos with paralyzed LR cilia or mutant embryos that lacked the Ca2+ permeable TRP channel Polycystin 2 (Pkd2).23 These findings indicated fluid flow is critical for establishing Ca2+ asymmetry and suggested Pkd2 functions as a Ca2+ channel that opens on the left side of the organ of asymmetry in response to flow. Consistent with this idea, Pkd2 protein was found to localize to LR cilia in the mouse node/PNC, including both motile cilia and immotile cilia in surrounding crown cells.23 More recently, rescue experiments showed that expression of Pkd2 exclusively in the crown cells was sufficient to restore LR signaling in Pkd2 mutant mice.41 Moreover, cilia were needed only in these crown cells to sense artificial flow.41 These observations support the hypothesis that fluid flow bends a population of immotile cilia containing Pkd2 channels to allow Ca2+ entry into the cell,23,73,74 reminiscent of the role of mechanosensory cilia in kidney cells.75
A transient Ca2+ flux has been observed in zebrafish embryos on the left side of KV just before the onset of asymmetric Nodal signaling,28 suggesting a conserved role for Ca2+ signals in LR determination. Pkd2 localizes to LR cilia in the frog GRP16 and medaka KV24 and is necessary for normal LR patterning in zebrafish.56,76 Although it was initially confusing that Pkd1—a protein that forms a complex with Pkd2 in cilia—is not involved in LR signaling,77 it was later discovered that the close homolog Pkd1l1 (Pkd1-like1) interacts with Pkd2 and mediates LR asymmetry in mouse78 and fish.24 It is interesting to note that in contrast to the mouse node/PNC that has populations of both motile and immotile cilia, it appears all cilia in the medaka KV are motile and contain Pkd2 and Pkd1l1.24 This observation led to the hypothesis that LR cilia in fish are both motile and sensory and support proposals that Pkd2-Pkd1l1 complexes are chemosensory rather than mechanosensory. In zebrafish, phosphorylation of calmodulin dependent kinase II (CaMKII) on the left side of KV was identified as a target of Ca2+ flux that may participate in transferring LR signals generated at the organ of asymmetry.79 Potential mechanisms for how Ca2+ flux impacts asymmetric Nodal signaling have been reviewed recently25 and may involve Ca2+ moving through the endoderm via gap junctions from the organ of asymmetry to LPM. Mouse mutants lacking the transcription factor Sox17 develop endoderm defects and fail to effectively transmit LR asymmetries from the node/PNC to the LPM.80,81 More specifically, gap junctions that would allow Ca2+ signals to propagate through the endoderm were disrupted in these mutants. Intracellular Ca2+ communication in the left endoderm could function to facilitate efficient extracellular transfer of secreted Nodal protein to the LPM. Alternatively, differential Ca2+ levels may influence modulators of nodal signaling, such as Gdf1,82 Dvr183 or Cerl2.41 Uncovering mechanisms and pathways downstream of asymmetric Ca2+ flux is an exciting area of investigation that is likely to provide new insight into the function of the ciliated organ of asymmetry.
Cell arrangement
Genetic mutations or embryological manipulations that disrupt the morphology of the organ of asymmetry result in LR patterning defects, indicating the cellular architecture plays an important role in generating asymmetry. However, little is known about how this architecture is established. Scanning electron micrographs and immunofluorescence images of the mouse node/PNC,39 frog GRP43 and zebrafish KV48 indicate that the ciliated cells in these organs are not uniform in size or shape and are not randomly distributed within the epithelium, but rather are arranged in a particular pattern. This suggests the placement of cells in the organ of asymmetry is important for generating and/or sensing flow. In the frog GRP and mouse node/PNC, ciliated cells with small apical surfaces are flanked by much larger cells.43 In mouse, the larger crown cells have cilia that are capable of sensing flow.41 In zebrafish, ciliated KV cells are asymmetrically distributed along the anterior-posterior axis of the embryo (Fig. 2 part 4) such that more cilia are positioned in the anterior region than in the posterior region.46,47 This arrangement drives strong leftward flow at the anterior pole with a slow rightward flow at the posterior end.48 It is important to note that this feature—the arrangement of cells in the organ of asymmetry—is the least studied, and as such, the mechanisms involved remain poorly understood.
While investigating the placement of ciliated cells in zebrafish KV, we found the Rho kinase (Rock) protein Rock2b is involved in establishing the anteroposterior asymmetric arrangement of LR cilia.48 Loss of Rock2b function disrupted the distribution of ciliated cells, eliminated flow and altered LR patterning. Cilia formed normally and were motile in Rock2b depleted embryos, indicating the presence of motile cilia is not sufficient to orient the LR axis, but rather the appropriate arrangement of the cells is critical for generating coordinated flow and LR signals in zebrafish. Analysis of cell shapes during KV development revealed that anterior KV cells adopt elongated columnar morphologies with small apical surfaces to facilitate tight packing of ciliated cells, whereas posterior cells become more cuboidal with large apical surfaces.84 These shape changes, which we refer to as ‘KV remodeling,’ were disrupted by depletion of Rock2b or inhibition of non-muscle Myosin II, a known target of Rock proteins. Mathematical simulations predict that shape changes that give rise to the asymmetric arrangement of cells in KV depend on regulation of interfacial tensions that likely involves Myosin II-mediated cell contractility and/or cell-cell adhesion.84 These studies identified KV remodeling as a developmental process that positions cells in a specific arrangement that is necessary to generate asymmetric flow.
Are cell shape changes or movements involved in establishing specific cellular architectures that are critical for the function of the ciliated organ of asymmetry in other vertebrates? As observed in zebrafish, Rock2 is expressed in the Xenopus organ of asymmetry and is necessary for normal LR patterning.85 In addition, duplication of the Rock2 gene was identified in a copy number variant screen of human patients with LR defects,85 suggesting tightly regulated Rho kinase activity plays a conserved role that is critical for establishing LR asymmetry. In the mouse embryo, morphogenesis of the node/PNC depends on cell migration and highly coordinated cell rearrangements. Ciliated cells with small apical surfaces first cluster beneath a layer of larger endodermal cells and then displace the endoderm to reach the ventral surface of the embryo.39,86 These rearrangements are disrupted in embryos with mutations in the Rho family GTPase Rac187 or the FERM domain protein Lulu/Epb4.1l588 that regulate the actin cytoskeleton. Furthermore, loss of the transcription factors Noto89 or Zic390 resulted in a disorganized mixture of small ciliated cells and large endoderm cells in the node/PNC and altered LR patterning. A null mutation in the extracellular matrix (ECM) protein fibronectin, which is required for normal LR asymmetry, disrupted the layering and orientation of cells in the node/PNC,91uncovering a role for ECM in node/PNC cellular arrangement. In wild-type embryos the posterior side of the pit shaped node/PNC is transiently much steeper than the anterior side,86 suggesting an architectural anteroposterior asymmetry that is reminiscent of the zebrafish KV. Interestingly, a subset of node/PNC cells labeled by Rfx2-Cre transgene expression in the posterior region of the node/PNC migrates into the center of the pit during morphogenesis.58 More experiments are needed to determine whether programmed cell arrangements establish functional architectural features in the mouse organ of asymmetry.
Unanswered Questions
Several significant and challenging questions about the ciliated organ of asymmetry remain unanswered. Perhaps foremost are questions about the role of fluid flow. Recent results in mouse that demonstrate weak leftward flow is sufficient to generate LR signals60 reveal that our understanding of the mechanisms involved in creating and sensing functional flow remains fragmented. Is weak flow capable of creating a morphogen gradient? Or moving nodal vesicle parcels? Or bending mechanosensory cilia? It is possible that these proposed mechanisms are not mutually exclusive, but rather work in concert to amplify even the weakest mechanical stimulus. Alternatively, new hypotheses may need to be proposed and tested based on these findings. Mathematical models will likely continue to play an integral role in understanding the physical properties and consequences of asymmetric flow. Of course, it will be crucial to determine whether weak flow is sufficient to drive asymmetry in other vertebrates or this phenomenon is specific to the architecture of the mouse node/PNC. In addition, several gaps remain in our understanding of the immediate downstream effectors of asymmetric flow. Is there a connection between rapid Ca2+ flashes in the mouse node/PNC72 and stable Ca2+ signals in the endoderm?23 What are the molecular mechanisms by which the asymmetric distributions of Ca2+, Nodal and Nodal antagonists interact to ensure accurate transfer of LR information to the lateral plate mesoderm? It is clear that additional work is needed to define exactly how asymmetric flow is generated, interpreted and then translated into LR differences across the embryo.
Although motile cilia are important for LR patterning of fish, frog, mouse and human embryos, the role for cilia is not universally conserved in vertebrates. In the chick embryo, several cilia genes including left right dynein, kinesin 3B and polycystin 292,93 are expressed near Hensen’s node and monocilia have been found near the node in fixed embryos.92,94 However, motile cilia and asymmetric flow have not been observed. Similarly, the posterior notochordal plate in the pig embryo lacks cilia and does not contain a fluid-filled pit or cavity to accommodate flow.20 A mutation in the chick Talpid3 gene that eliminates primary95 and motile96 cilia does not alter LR patterning,97 indicating LR asymmetry is established without cilia. Time-lapse imaging of live chick embryos revealed a leftward migration of cells around Hensen’s node20,21 that gives rise to an asymmetric node morphology98 and creates the asymmetric domain of Shh expressing cells that activate left-sided Nodal expression. During the same stages that cell movements are establishing asymmetries in node morphology and gene expression, elevated levels of both extracellular99 and intracellular100 Ca2+ have been observed on the left side of the node. The relationship between cell rearrangements, asymmetric gene expression and Ca2+ flux in the chick embryo remains unclear, but extracellular Ca2+ signals have been proposed to activate left-sided Notch signaling upstream of Nodal.99 In addition to further characterizing this cilia-independent strategy to establish LR asymmetry in the chick embryo, it will be important to survey more vertebrate embryos to determine the prevalence of establishing asymmetry without cilia.
Studies primarily in frog embryos have implicated several molecules and pathways in establishing LR asymmetry at developmental stages that precede the appearance of LR cilia and asymmetric flow. The transmembrane heparan sulfate proteoglycan Syndecan 2 has been shown to mediate signaling between ectoderm and migrating mesoderm that is critical for LR asymmetry during early Xenopus gastrulation stages prior to GRP function.101,102 At even earlier stages of development—between fertilization and the midblastula transition—maternally supplied H+/K+ ATPase103 and vacuolar-type H+ ATPase104 ion pumps have been proposed to create an electrochemical gradient that moves Serotonin105 and potentially other LR determinants through gap junctions106 to influence asymmetric gene expression. Interestingly, several molecules implicated in these early events are critical for the development of the ciliated organ of asymmetry. In zebrafish, Syndecan 2,107 gap junctions108 and vacuolar-type H+ ATPase activity104,109 are needed for KV morphogenesis and/or ciliogenesis. In Xenopus, Serotonin110 and H+/K+ ATPase111 are necessary for Wnt signaling that mediates development of the GRP. It remains to be seen whether early factors function solely to impact development of the organ of asymmetry or have additional cilia-independent activities that act in concert with cilia to ensure correct LR axis specification.112,113
Another intriguing question is how polarity of individual cells impacts LR polarity of the embryo. A growing body of evidence suggests polarized cellular behaviors play multiple roles in establishing LR asymmetry. As described above, planar polarization of cells in the organ of asymmetry is involved in basal body positioning and LR cilia tilting that is necessary to generate asymmetric flow. In addition, the PCP proteins Wnt11 and Prickle1a regulate earlier steps of zebrafish KV development, including cellular organization and cilia formation.114 Also in zebrafish, PCP signals may be upstream regulators of Rho kinase and Myosin II activities that mediate cell shape changes during KV remodeling.84 In the chick embryo, the PCP component Vangl2 is involved in establishing LR asymmetry115 and the polarized cell movements around Hensen’s node depend on Rho kinase and Myosin II.20 These observations raise the possibility of an ancient pathway that regulates LR determination in all vertebrates and has evolved to mediate cilia-dependent and cilia-independent mechanisms.
Conclusion
Discoveries in recent years have advanced our knowledge of how the LR body axis is oriented during embryonic development, yet many of cellular and molecular aspects of this process remain enigmatic. As described above, several important animal models have been developed that will facilitate the clarification of mechanisms involved in generating and propagating LR asymmetries at the ciliated organ of asymmetry. New insights into the form and function of the organ of asymmetry will provide new opportunities to develop strategies to predict, diagnose and prevent LR defects.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
I thank members of the Pruyne, Krendel and Sirotkin laboratories for helpful discussions and members of my laboratory for critical reading of this manuscript. Research in the Amack laboratory is supported in part by a grant from the National Heart, Lung and Blood Institute (R01HL095690).
References
- 1.Peeters H, Devriendt K. Human laterality disorders. Eur J Med Genet. 2006;49:349–62. doi: 10.1016/j.ejmg.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 2.Ramsdell AF. Left-right asymmetry and congenital cardiac defects: getting to the heart of the matter in vertebrate left-right axis determination. Dev Biol. 2005;288:1–20. doi: 10.1016/j.ydbio.2005.07.038. [DOI] [PubMed] [Google Scholar]
- 3.Sutherland MJ, Ware SM. Disorders of left-right asymmetry: heterotaxy and situs inversus. Am J Med Genet C Semin Med Genet. 2009;151C:307–17. doi: 10.1002/ajmg.c.30228. [DOI] [PubMed] [Google Scholar]
- 4.Brueckner M. Heterotaxia, congenital heart disease, and primary ciliary dyskinesia. Circulation. 2007;115:2793–5. doi: 10.1161/CIRCULATIONAHA.107.699256. [DOI] [PubMed] [Google Scholar]
- 5.Bisgrove BW, Morelli SH, Yost HJ. Genetics of human laterality disorders: insights from vertebrate model systems. Annu Rev Genomics Hum Genet. 2003;4:1–32. doi: 10.1146/annurev.genom.4.070802.110428. [DOI] [PubMed] [Google Scholar]
- 6.Levin M, Johnson RL, Stern CD, Kuehn M, Tabin C. A molecular pathway determining left-right asymmetry in chick embryogenesis. Cell. 1995;82:803–14. doi: 10.1016/0092-8674(95)90477-8. [DOI] [PubMed] [Google Scholar]
- 7.Juan H, Hamada H. Roles of nodal-lefty regulatory loops in embryonic patterning of vertebrates. Genes to cells: devoted to molecular & cellular mechanisms 2001; 6:923-30. [DOI] [PubMed]
- 8.Soroldoni D, Bajoghli B, Aghaallaei N, Czerny T. Dynamic expression pattern of Nodal-related genes during left-right development in medaka. Gene Expr Patterns. 2007;7:93–101. doi: 10.1016/j.modgep.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 9.Long S, Ahmad N, Rebagliati M. The zebrafish nodal-related gene southpaw is required for visceral and diencephalic left-right asymmetry. Development. 2003;130:2303–16. doi: 10.1242/dev.00436. [DOI] [PubMed] [Google Scholar]
- 10.Lowe LA, Supp DM, Sampath K, Yokoyama T, Wright CV, Potter SS, Overbeek P, Kuehn MR. Conserved left-right asymmetry of nodal expression and alterations in murine situs inversus. Nature. 1996;381:158–61. doi: 10.1038/381158a0. [DOI] [PubMed] [Google Scholar]
- 11.Lohr JL, Danos MC, Yost HJ. Left-right asymmetry of a nodal-related gene is regulated by dorsoanterior midline structures during Xenopus development. Development. 1997;124:1465–72. doi: 10.1242/dev.124.8.1465. [DOI] [PubMed] [Google Scholar]
- 12.Afzelius BA. A human syndrome caused by immotile cilia. Science. 1976;193:317–9. doi: 10.1126/science.1084576. [DOI] [PubMed] [Google Scholar]
- 13.Sulik K, Dehart DB, Iangaki T, Carson JL, Vrablic T, Gesteland K, Schoenwolf GC. Morphogenesis of the murine node and notochordal plate. Dev Dyn. 1994;201:260–78. doi: 10.1002/aja.1002010309. [DOI] [PubMed] [Google Scholar]
- 14.Nonaka S, Tanaka Y, Okada Y, Takeda S, Harada A, Kanai Y, Kido M, Hirokawa N. Randomization of left-right asymmetry due to loss of nodal cilia generating leftward flow of extraembryonic fluid in mice lacking KIF3B motor protein. Cell. 1998;95:829–37. doi: 10.1016/S0092-8674(00)81705-5. [DOI] [PubMed] [Google Scholar]
- 15.Okada Y, Takeda S, Tanaka Y, Izpisúa Belmonte JC, Hirokawa N. Mechanism of nodal flow: a conserved symmetry breaking event in left-right axis determination. Cell. 2005;121:633–44. doi: 10.1016/j.cell.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 16.Schweickert A, Weber T, Beyer T, Vick P, Bogusch S, Feistel K, Blum M. Cilia-driven leftward flow determines laterality in Xenopus. Curr Biol. 2007;17:60–6. doi: 10.1016/j.cub.2006.10.067. [DOI] [PubMed] [Google Scholar]
- 17.Hojo M, Takashima S, Kobayashi D, Sumeragi A, Shimada A, Tsukahara T, Yokoi H, Narita T, Jindo T, Kage T, et al. Right-elevated expression of charon is regulated by fluid flow in medaka Kupffer’s vesicle. Dev Growth Differ. 2007;49:395–405. doi: 10.1111/j.1440-169X.2007.00937.x. [DOI] [PubMed] [Google Scholar]
- 18.Essner JJ, Amack JD, Nyholm MK, Harris EB, Yost HJ. Kupffer’s vesicle is a ciliated organ of asymmetry in the zebrafish embryo that initiates left-right development of the brain, heart and gut. Development. 2005;132:1247–60. doi: 10.1242/dev.01663. [DOI] [PubMed] [Google Scholar]
- 19.Kramer-Zucker AG, Olale F, Haycraft CJ, Yoder BK, Schier AF, Drummond IA. Cilia-driven fluid flow in the zebrafish pronephros, brain and Kupffer’s vesicle is required for normal organogenesis. Development. 2005;132:1907–21. doi: 10.1242/dev.01772. [DOI] [PubMed] [Google Scholar]
- 20.Gros J, Feistel K, Viebahn C, Blum M, Tabin CJ. Cell movements at Hensen’s node establish left/right asymmetric gene expression in the chick. Science. 2009;324:941–4. doi: 10.1126/science.1172478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cui C, Little CD, Rongish BJ. Rotation of organizer tissue contributes to left-right asymmetry. Anat Rec (Hoboken) 2009;292:557–61. doi: 10.1002/ar.20872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tanaka Y, Okada Y, Hirokawa N. FGF-induced vesicular release of Sonic hedgehog and retinoic acid in leftward nodal flow is critical for left-right determination. Nature. 2005;435:172–7. doi: 10.1038/nature03494. [DOI] [PubMed] [Google Scholar]
- 23.McGrath J, Somlo S, Makova S, Tian X, Brueckner M. Two populations of node monocilia initiate left-right asymmetry in the mouse. Cell. 2003;114:61–73. doi: 10.1016/S0092-8674(03)00511-7. [DOI] [PubMed] [Google Scholar]
- 24.Kamura K, Kobayashi D, Uehara Y, Koshida S, Iijima N, Kudo A, Yokoyama T, Takeda H. Pkd1l1 complexes with Pkd2 on motile cilia and functions to establish the left-right axis. Development. 2011;138:1121–9. doi: 10.1242/dev.058271. [DOI] [PubMed] [Google Scholar]
- 25.Norris DP. Cilia, calcium and the basis of left-right asymmetry. BMC Biol. 2012;10:102. doi: 10.1186/1741-7007-10-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hirokawa N, Tanaka Y, Okada Y. Cilia, KIF3 molecular motor and nodal flow. Curr Opin Cell Biol. 2012;24:31–9. doi: 10.1016/j.ceb.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 27.Babu D, Roy S. Left-right asymmetry: cilia stir up new surprises in the node. Open Biol. 2013;3:130052. doi: 10.1098/rsob.130052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sarmah B, Latimer AJ, Appel B, Wente SR. Inositol polyphosphates regulate zebrafish left-right asymmetry. Dev Cell. 2005;9:133–45. doi: 10.1016/j.devcel.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 29.Nakamura T, Mine N, Nakaguchi E, Mochizuki A, Yamamoto M, Yashiro K, Meno C, Hamada H. Generation of robust left-right asymmetry in the mouse embryo requires a self-enhancement and lateral-inhibition system. Dev Cell. 2006;11:495–504. doi: 10.1016/j.devcel.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 30.Marques S, Borges AC, Silva AC, Freitas S, Cordenonsi M, Belo JA. The activity of the Nodal antagonist Cerl-2 in the mouse node is required for correct L/R body axis. Genes Dev. 2004;18:2342–7. doi: 10.1101/gad.306504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schweickert A, Vick P, Getwan M, Weber T, Schneider I, Eberhardt M, Beyer T, Pachur A, Blum M. The nodal inhibitor Coco is a critical target of leftward flow in Xenopus. Curr Biol. 2010;20:738–43. doi: 10.1016/j.cub.2010.02.061. [DOI] [PubMed] [Google Scholar]
- 32.Hashimoto H, Rebagliati M, Ahmad N, Muraoka O, Kurokawa T, Hibi M, Suzuki T. The Cerberus/Dan-family protein Charon is a negative regulator of Nodal signaling during left-right patterning in zebrafish. Development. 2004;131:1741–53. doi: 10.1242/dev.01070. [DOI] [PubMed] [Google Scholar]
- 33.Schneider I, Schneider PN, Derry SW, Lin S, Barton LJ, Westfall T, Slusarski DC. Zebrafish Nkd1 promotes Dvl degradation and is required for left-right patterning. Dev Biol. 2010;348:22–33. doi: 10.1016/j.ydbio.2010.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Krebs LT, Iwai N, Nonaka S, Welsh IC, Lan Y, Jiang R, Saijoh Y, O’Brien TP, Hamada H, Gridley T. Notch signaling regulates left-right asymmetry determination by inducing Nodal expression. Genes Dev. 2003;17:1207–12. doi: 10.1101/gad.1084703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kitajima K, Oki S, Ohkawa Y, Sumi T, Meno C. Wnt signaling regulates left-right axis formation in the node of mouse embryos. Dev Biol. 2013;380:222–32. doi: 10.1016/j.ydbio.2013.05.011. [DOI] [PubMed] [Google Scholar]
- 36.Nakamura T, Saito D, Kawasumi A, Shinohara K, Asai Y, Takaoka K, Dong F, Takamatsu A, Belo JA, Mochizuki A, et al. Fluid flow and interlinked feedback loops establish left-right asymmetric decay of Cerl2 mRNA. Nat Commun. 2012;3:1322. doi: 10.1038/ncomms2319. [DOI] [PubMed] [Google Scholar]
- 37.Inácio JM, Marques S, Nakamura T, Shinohara K, Meno C, Hamada H, Belo JA. The dynamic right-to-left translocation of Cerl2 is involved in the regulation and termination of Nodal activity in the mouse node. PLoS One. 2013;8:e60406. doi: 10.1371/journal.pone.0060406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blum M, Weber T, Beyer T, Vick P. Evolution of leftward flow. Semin Cell Dev Biol. 2009;20:464–71. doi: 10.1016/j.semcdb.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 39.Lee JD, Anderson KV. Morphogenesis of the node and notochord: the cellular basis for the establishment and maintenance of left-right asymmetry in the mouse. Dev Dyn. 2008;237:3464–76. doi: 10.1002/dvdy.21598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nakamura T, Hamada H. Left-right patterning: conserved and divergent mechanisms. Development. 2012;139:3257–62. doi: 10.1242/dev.061606. [DOI] [PubMed] [Google Scholar]
- 41.Yoshiba S, Shiratori H, Kuo IY, Kawasumi A, Shinohara K, Nonaka S, Asai Y, Sasaki G, Belo JA, Sasaki H, et al. Cilia at the node of mouse embryos sense fluid flow for left-right determination via Pkd2. Science. 2012;338:226–31. doi: 10.1126/science.1222538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Blum M, Andre P, Muders K, Schweickert A, Fischer A, Bitzer E, Bogusch S, Beyer T, van Straaten HW, Viebahn C. Ciliation and gene expression distinguish between node and posterior notochord in the mammalian embryo. Differentiation. 2007;75:133–46. doi: 10.1111/j.1432-0436.2006.00124.x. [DOI] [PubMed] [Google Scholar]
- 43.Shook DR, Majer C, Keller R. Pattern and morphogenesis of presumptive superficial mesoderm in two closely related species, Xenopus laevis and Xenopus tropicalis. Dev Biol. 2004;270:163–85. doi: 10.1016/j.ydbio.2004.02.021. [DOI] [PubMed] [Google Scholar]
- 44.Boskovski MT, Yuan S, Pedersen NB, Goth CK, Makova S, Clausen H, Brueckner M, Khokha MK. The heterotaxy gene GALNT11 glycosylates Notch to orchestrate cilia type and laterality. Nature. 2013;504:456–9. doi: 10.1038/nature12723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Amack JD, Wang X, Yost HJ. Two T-box genes play independent and cooperative roles to regulate morphogenesis of ciliated Kupffer’s vesicle in zebrafish. Dev Biol. 2007;310:196–210. doi: 10.1016/j.ydbio.2007.05.039. [DOI] [PubMed] [Google Scholar]
- 46.Kreiling JA, Williams G, Creton R, Prabhat Analysis of Kupffer’s vesicle in zebrafish embryos using a cave automated virtual environment. Dev Dyn. 2007;236:1963–9. doi: 10.1002/dvdy.21191. [DOI] [PubMed] [Google Scholar]
- 47.Okabe N, Xu B, Burdine RD. Fluid dynamics in zebrafish Kupffer’s vesicle. Dev Dyn. 2008;237:3602–12. doi: 10.1002/dvdy.21730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang G, Cadwallader AB, Jang DS, Tsang M, Yost HJ, Amack JD. The Rho kinase Rock2b establishes anteroposterior asymmetry of the ciliated Kupffer’s vesicle in zebrafish. Development. 2011;138:45–54. doi: 10.1242/dev.052985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Leigh MW, Pittman JE, Carson JL, Ferkol TW, Dell SD, Davis SD, Knowles MR, Zariwala MA. Clinical and genetic aspects of primary ciliary dyskinesia/Kartagener syndrome. Genet Med. 2009;11:473–87. doi: 10.1097/GIM.0b013e3181a53562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kennedy MP, Omran H, Leigh MW, Dell S, Morgan L, Molina PL, Robinson BV, Minnix SL, Olbrich H, Severin T, et al. Congenital heart disease and other heterotaxic defects in a large cohort of patients with primary ciliary dyskinesia. Circulation. 2007;115:2814–21. doi: 10.1161/CIRCULATIONAHA.106.649038. [DOI] [PubMed] [Google Scholar]
- 51.McGrath J, Brueckner M. Cilia are at the heart of vertebrate left-right asymmetry. Curr Opin Genet Dev. 2003;13:385–92. doi: 10.1016/S0959-437X(03)00091-1. [DOI] [PubMed] [Google Scholar]
- 52.Bisgrove BW, Yost HJ. The roles of cilia in developmental disorders and disease. Development. 2006;133:4131–43. doi: 10.1242/dev.02595. [DOI] [PubMed] [Google Scholar]
- 53.Supp DM, Witte DP, Potter SS, Brueckner M. Mutation of an axonemal dynein affects left-right asymmetry in inversus viscerum mice. Nature. 1997;389:963–6. doi: 10.1038/40140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Supp DM, Brueckner M, Kuehn MR, Witte DP, Lowe LA, McGrath J, Corrales J, Potter SS. Targeted deletion of the ATP binding domain of left-right dynein confirms its role in specifying development of left-right asymmetries. Development. 1999;126:5495–504. doi: 10.1242/dev.126.23.5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Amack JD, Yost HJ. The T box transcription factor no tail in ciliated cells controls zebrafish left-right asymmetry. Curr Biol. 2004;14:685–90. doi: 10.1016/j.cub.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 56.Bisgrove BW, Snarr BS, Emrazian A, Yost HJ. Polaris and Polycystin-2 in dorsal forerunner cells and Kupffer’s vesicle are required for specification of the zebrafish left-right axis. Dev Biol. 2005;287:274–88. doi: 10.1016/j.ydbio.2005.08.047. [DOI] [PubMed] [Google Scholar]
- 57.Vick P, Schweickert A, Weber T, Eberhardt M, Mencl S, Shcherbakov D, Beyer T, Blum M. Flow on the right side of the gastrocoel roof plate is dispensable for symmetry breakage in the frog Xenopus laevis. Dev Biol. 2009;331:281–91. doi: 10.1016/j.ydbio.2009.05.547. [DOI] [PubMed] [Google Scholar]
- 58.Bisgrove BW, Makova S, Yost HJ, Brueckner M. RFX2 is essential in the ciliated organ of asymmetry and an RFX2 transgene identifies a population of ciliated cells sufficient for fluid flow. Dev Biol. 2012;363:166–78. doi: 10.1016/j.ydbio.2011.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nonaka S, Shiratori H, Saijoh Y, Hamada H. Determination of left-right patterning of the mouse embryo by artificial nodal flow. Nature. 2002;418:96–9. doi: 10.1038/nature00849. [DOI] [PubMed] [Google Scholar]
- 60.Shinohara K, Kawasumi A, Takamatsu A, Yoshiba S, Botilde Y, Motoyama N, Reith W, Durand B, Shiratori H, Hamada H. Two rotating cilia in the node cavity are sufficient to break left-right symmetry in the mouse embryo. Nat Commun. 2012;3:622. doi: 10.1038/ncomms1624. [DOI] [PubMed] [Google Scholar]
- 61.Cartwright JH, Piro O, Tuval I. Fluid-dynamical basis of the embryonic development of left-right asymmetry in vertebrates. Proc Natl Acad Sci U S A. 2004;101:7234–9. doi: 10.1073/pnas.0402001101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nonaka S, Yoshiba S, Watanabe D, Ikeuchi S, Goto T, Marshall WF, Hamada H. De novo formation of left-right asymmetry by posterior tilt of nodal cilia. PLoS Biol. 2005;3:e268. doi: 10.1371/journal.pbio.0030268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hashimoto M, Hamada H. Translation of anterior-posterior polarity into left-right polarity in the mouse embryo. Curr Opin Genet Dev. 2010;20:433–7. doi: 10.1016/j.gde.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 64.Maisonneuve C, Guilleret I, Vick P, Weber T, Andre P, Beyer T, Blum M, Constam DB. Bicaudal C, a novel regulator of Dvl signaling abutting RNA-processing bodies, controls cilia orientation and leftward flow. Development. 2009;136:3019–30. doi: 10.1242/dev.038174. [DOI] [PubMed] [Google Scholar]
- 65.Feistel K, Blum M. Three types of cilia including a novel 9+4 axoneme on the notochordal plate of the rabbit embryo. Dev Dyn. 2006;235:3348–58. doi: 10.1002/dvdy.20986. [DOI] [PubMed] [Google Scholar]
- 66.Borovina A, Superina S, Voskas D, Ciruna B. Vangl2 directs the posterior tilting and asymmetric localization of motile primary cilia. Nat Cell Biol. 2010;12:407–12. doi: 10.1038/ncb2042. [DOI] [PubMed] [Google Scholar]
- 67.Wallingford JB. Planar cell polarity and the developmental control of cell behavior in vertebrate embryos. Annu Rev Cell Dev Biol. 2012;28:627–53. doi: 10.1146/annurev-cellbio-092910-154208. [DOI] [PubMed] [Google Scholar]
- 68.Hashimoto M, Shinohara K, Wang J, Ikeuchi S, Yoshiba S, Meno C, Nonaka S, Takada S, Hatta K, Wynshaw-Boris A, et al. Planar polarization of node cells determines the rotational axis of node cilia. Nat Cell Biol. 2010;12:170–6. doi: 10.1038/ncb2020. [DOI] [PubMed] [Google Scholar]
- 69.Antic D, Stubbs JL, Suyama K, Kintner C, Scott MP, Axelrod JD. Planar cell polarity enables posterior localization of nodal cilia and left-right axis determination during mouse and Xenopus embryogenesis. PLoS One. 2010;5:e8999. doi: 10.1371/journal.pone.0008999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Song H, Hu J, Chen W, Elliott G, Andre P, Gao B, Yang Y. Planar cell polarity breaks bilateral symmetry by controlling ciliary positioning. Nature. 2010;466:378–82. doi: 10.1038/nature09129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mahaffey JP, Grego-Bessa J, Liem KF, Jr., Anderson KV. Cofilin and Vangl2 cooperate in the initiation of planar cell polarity in the mouse embryo. Development. 2013;140:1262–71. doi: 10.1242/dev.085316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Takao D, Nemoto T, Abe T, Kiyonari H, Kajiura-Kobayashi H, Shiratori H, Nonaka S. Asymmetric distribution of dynamic calcium signals in the node of mouse embryo during left-right axis formation. Dev Biol. 2013;376:23–30. doi: 10.1016/j.ydbio.2013.01.018. [DOI] [PubMed] [Google Scholar]
- 73.Tabin CJ, Vogan KJ. A two-cilia model for vertebrate left-right axis specification. Genes Dev. 2003;17:1–6. doi: 10.1101/gad.1053803. [DOI] [PubMed] [Google Scholar]
- 74.Yost HJ. Left-right asymmetry: nodal cilia make and catch a wave. Curr Biol. 2003;13:R808–9. doi: 10.1016/j.cub.2003.09.051. [DOI] [PubMed] [Google Scholar]
- 75.Nauli SM, Alenghat FJ, Luo Y, Williams E, Vassilev P, Li X, Elia AE, Lu W, Brown EM, Quinn SJ, et al. Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat Genet. 2003;33:129–37. doi: 10.1038/ng1076. [DOI] [PubMed] [Google Scholar]
- 76.Schottenfeld J, Sullivan-Brown J, Burdine RD. Zebrafish curly up encodes a Pkd2 ortholog that restricts left-side-specific expression of southpaw. Development. 2007;134:1605–15. doi: 10.1242/dev.02827. [DOI] [PubMed] [Google Scholar]
- 77.Karcher C, Fischer A, Schweickert A, Bitzer E, Horie S, Witzgall R, Blum M. Lack of a laterality phenotype in Pkd1 knock-out embryos correlates with absence of polycystin-1 in nodal cilia. Differentiation. 2005;73:425–32. doi: 10.1111/j.1432-0436.2005.00048.x. [DOI] [PubMed] [Google Scholar]
- 78.Field S, Riley KL, Grimes DT, Hilton H, Simon M, Powles-Glover N, Siggers P, Bogani D, Greenfield A, Norris DP. Pkd1l1 establishes left-right asymmetry and physically interacts with Pkd2. Development. 2011;138:1131–42. doi: 10.1242/dev.058149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Francescatto L, Rothschild SC, Myers AL, Tombes RM. The activation of membrane targeted CaMK-II in the zebrafish Kupffer’s vesicle is required for left-right asymmetry. Development. 2010;137:2753–62. doi: 10.1242/dev.049627. [DOI] [PubMed] [Google Scholar]
- 80.Viotti M, Niu L, Shi SH, Hadjantonakis AK. Role of the gut endoderm in relaying left-right patterning in mice. PLoS Biol. 2012;10:e1001276. doi: 10.1371/journal.pbio.1001276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Saund RS, Kanai-Azuma M, Kanai Y, Kim I, Lucero MT, Saijoh Y. Gut endoderm is involved in the transfer of left-right asymmetry from the node to the lateral plate mesoderm in the mouse embryo. Development. 2012;139:2426–35. doi: 10.1242/dev.079921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Tanaka C, Sakuma R, Nakamura T, Hamada H, Saijoh Y. Long-range action of Nodal requires interaction with GDF1. Genes Dev. 2007;21:3272–82. doi: 10.1101/gad.1623907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Peterson AG, Wang X, Yost HJ. Dvr1 transfers left-right asymmetric signals from Kupffer’s vesicle to lateral plate mesoderm in zebrafish. Dev Biol. 2013;382:198–208. doi: 10.1016/j.ydbio.2013.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang G, Manning ML, Amack JD. Regional cell shape changes control form and function of Kupffer’s vesicle in the zebrafish embryo. Dev Biol. 2012;370:52–62. doi: 10.1016/j.ydbio.2012.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fakhro KA, Choi M, Ware SM, Belmont JW, Towbin JA, Lifton RP, Khokha MK, Brueckner M. Rare copy number variations in congenital heart disease patients identify unique genes in left-right patterning. Proc Natl Acad Sci U S A. 2011;108:2915–20. doi: 10.1073/pnas.1019645108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yamanaka Y, Tamplin OJ, Beckers A, Gossler A, Rossant J. Live imaging and genetic analysis of mouse notochord formation reveals regional morphogenetic mechanisms. Dev Cell. 2007;13:884–96. doi: 10.1016/j.devcel.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 87.Migeotte I, Grego-Bessa J, Anderson KV. Rac1 mediates morphogenetic responses to intercellular signals in the gastrulating mouse embryo. Development. 2011;138:3011–20. doi: 10.1242/dev.059766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lee JD, Migeotte I, Anderson KV. Left-right patterning in the mouse requires Epb4.1l5-dependent morphogenesis of the node and midline. Dev Biol. 2010;346:237–46. doi: 10.1016/j.ydbio.2010.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Beckers A, Alten L, Viebahn C, Andre P, Gossler A. The mouse homeobox gene Noto regulates node morphogenesis, notochordal ciliogenesis, and left right patterning. Proc Natl Acad Sci U S A. 2007;104:15765–70. doi: 10.1073/pnas.0704344104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sutherland MJ, Wang S, Quinn ME, Haaning A, Ware SM. Zic3 is required in the migrating primitive streak for node morphogenesis and left-right patterning. Hum Mol Genet. 2013;22:1913–23. doi: 10.1093/hmg/ddt001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pulina MV, Hou SY, Mittal A, Julich D, Whittaker CA, Holley SA, Hynes RO, Astrof S. Essential roles of fibronectin in the development of the left-right embryonic body plan. Dev Biol. 2011;354:208–20. doi: 10.1016/j.ydbio.2011.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Essner JJ, Vogan KJ, Wagner MK, Tabin CJ, Yost HJ, Brueckner M. Conserved function for embryonic nodal cilia. Nature. 2002;418:37–8. doi: 10.1038/418037a. [DOI] [PubMed] [Google Scholar]
- 93.Qiu D, Cheng SM, Wozniak L, McSweeney M, Perrone E, Levin M. Localization and loss-of-function implicates ciliary proteins in early, cytoplasmic roles in left-right asymmetry. Dev Dyn. 2005;234:176–89. doi: 10.1002/dvdy.20509. [DOI] [PubMed] [Google Scholar]
- 94.Männer J. Does an equivalent of the “ventral node” exist in chick embryos? A scanning electron microscopic study. Anat Embryol (Berl) 2001;203:481–90. doi: 10.1007/s004290100183. [DOI] [PubMed] [Google Scholar]
- 95.Yin Y, Bangs F, Paton IR, Prescott A, James J, Davey MG, Whitley P, Genikhovich G, Technau U, Burt DW, et al. The Talpid3 gene (KIAA0586) encodes a centrosomal protein that is essential for primary cilia formation. Development. 2009;136:655–64. doi: 10.1242/dev.028464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Stephen LA, Davis GM, McTeir KE, James J, McTeir L, Kierans M, Bain A, Davey MG. Failure of centrosome migration causes a loss of motile cilia in talpid(3) mutants. Dev Dyn. 2013;242:923–31. doi: 10.1002/dvdy.23980. [DOI] [PubMed] [Google Scholar]
- 97.Bangs F, Antonio N, Thongnuek P, Welten M, Davey MG, Briscoe J, Tickle C. Generation of mice with functional inactivation of talpid3, a gene first identified in chicken. Development. 2011;138:3261–72. doi: 10.1242/dev.063602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Dathe V, Gamel A, Männer J, Brand-Saberi B, Christ B. Morphological left-right asymmetry of Hensen’s node precedes the asymmetric expression of Shh and Fgf8 in the chick embryo. Anat Embryol (Berl) 2002;205:343–54. doi: 10.1007/s00429-002-0269-2. [DOI] [PubMed] [Google Scholar]
- 99.Raya A, Kawakami Y, Rodríguez-Esteban C, Ibañes M, Rasskin-Gutman D, Rodríguez-León J, Büscher D, Feijó JA, Izpisúa Belmonte JC. Notch activity acts as a sensor for extracellular calcium during vertebrate left-right determination. Nature. 2004;427:121–8. doi: 10.1038/nature02190. [DOI] [PubMed] [Google Scholar]
- 100.Garic-Stankovic A, Hernandez M, Flentke GR, Zile MH, Smith SM. A ryanodine receptor-dependent Ca(i)(2+) asymmetry at Hensen’s node mediates avian lateral identity. Development. 2008;135:3271–80. doi: 10.1242/dev.018861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kramer KL, Yost HJ. Ectodermal syndecan-2 mediates left-right axis formation in migrating mesoderm as a cell-nonautonomous Vg1 cofactor. Dev Cell. 2002;2:115–24. doi: 10.1016/S1534-5807(01)00107-1. [DOI] [PubMed] [Google Scholar]
- 102.Kramer KL, Barnette JE, Yost HJ. PKCgamma regulates syndecan-2 inside-out signaling during xenopus left-right development. Cell. 2002;111:981–90. doi: 10.1016/S0092-8674(02)01200-X. [DOI] [PubMed] [Google Scholar]
- 103.Levin M, Thorlin T, Robinson KR, Nogi T, Mercola M. Asymmetries in H+/K+-ATPase and cell membrane potentials comprise a very early step in left-right patterning. Cell. 2002;111:77–89. doi: 10.1016/S0092-8674(02)00939-X. [DOI] [PubMed] [Google Scholar]
- 104.Adams DS, Robinson KR, Fukumoto T, Yuan S, Albertson RC, Yelick P, Kuo L, McSweeney M, Levin M. Early, H+-V-ATPase-dependent proton flux is necessary for consistent left-right patterning of non-mammalian vertebrates. Development. 2006;133:1657–71. doi: 10.1242/dev.02341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Fukumoto T, Kema IP, Levin M. Serotonin signaling is a very early step in patterning of the left-right axis in chick and frog embryos. Curr Biol. 2005;15:794–803. doi: 10.1016/j.cub.2005.03.044. [DOI] [PubMed] [Google Scholar]
- 106.Levin M, Mercola M. Gap junctions are involved in the early generation of left-right asymmetry. Dev Biol. 1998;203:90–105. doi: 10.1006/dbio.1998.9024. [DOI] [PubMed] [Google Scholar]
- 107.Arrington CB, Peterson AG, Yost HJ. Sdc2 and Tbx16 regulate Fgf2-dependent epithelial cell morphogenesis in the ciliated organ of asymmetry. Development. 2013;140:4102–9. doi: 10.1242/dev.096933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hatler JM, Essner JJ, Johnson RG. A gap junction connexin is required in the vertebrate left-right organizer. Dev Biol. 2009;336:183–91. doi: 10.1016/j.ydbio.2009.09.035. [DOI] [PubMed] [Google Scholar]
- 109.Chen Y, Wu B, Xu L, Li H, Xia J, Yin W, Li Z, Shi D, Li S, Lin S, et al. A SNX10/V-ATPase pathway regulates ciliogenesis in vitro and in vivo. Cell Res. 2012;22:333–45. doi: 10.1038/cr.2011.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Beyer T, Danilchik M, Thumberger T, Vick P, Tisler M, Schneider I, Bogusch S, Andre P, Ulmer B, Walentek P, et al. Serotonin signaling is required for Wnt-dependent GRP specification and leftward flow in Xenopus. Curr Biol. 2012;22:33–9. doi: 10.1016/j.cub.2011.11.027. [DOI] [PubMed] [Google Scholar]
- 111.Walentek P, Beyer T, Thumberger T, Schweickert A, Blum M. ATP4a is required for Wnt-dependent Foxj1 expression and leftward flow in Xenopus left-right development. Cell Rep. 2012;1:516–27. doi: 10.1016/j.celrep.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 112.Schweickert A, Walentek P, Thumberger T, Danilchik M. Linking early determinants and cilia-driven leftward flow in left-right axis specification of Xenopus laevis: a theoretical approach. Differentiation. 2012;83:S67–77. doi: 10.1016/j.diff.2011.11.005. [DOI] [PubMed] [Google Scholar]
- 113.Vandenberg LN, Levin M. A unified model for left-right asymmetry? Comparison and synthesis of molecular models of embryonic laterality. Dev Biol. 2013;379:1–15. doi: 10.1016/j.ydbio.2013.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Oteiza P, Köppen M, Krieg M, Pulgar E, Farias C, Melo C, Preibisch S, Müller D, Tada M, Hartel S, et al. Planar cell polarity signalling regulates cell adhesion properties in progenitors of the zebrafish laterality organ. Development. 2010;137:3459–68. doi: 10.1242/dev.049981. [DOI] [PubMed] [Google Scholar]
- 115.Zhang Y, Levin M. Left-right asymmetry in the chick embryo requires core planar cell polarity protein Vangl2. Genesis. 2009;47:719–28. doi: 10.1002/dvg.20551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Oteíza P, Köppen M, Concha ML, Heisenberg CP. Origin and shaping of the laterality organ in zebrafish. Development. 2008;135:2807–13. doi: 10.1242/dev.022228. [DOI] [PubMed] [Google Scholar]
- 117.Cooper MS, D’Amico LA. A cluster of noninvoluting endocytic cells at the margin of the zebrafish blastoderm marks the site of embryonic shield formation. Dev Biol. 1996;180:184–98. doi: 10.1006/dbio.1996.0294. [DOI] [PubMed] [Google Scholar]


