Abstract
Fetal cells persist in mothers for decades after delivery: in a phenomenon called fetal microchimerism. While persistent fetal cells were first implicated in autoimmune disease, parallel studies in animal and human pregnancy now suggest that microchimeric fetal cells play a role in the response to tissue injury. The aim of this study was to investigate the impact of fetal microchimeric cells in the adult wound, using caesarean section (CS) as a model of wound healing in pregnancy. XY-FISH (fluorescence in situ hybridization) and immunostaining was used in multiple tissue sections from CS skin biopsies from 70 women, to locate, quantitate and characterize microchimeric male presumed-fetal cells. Y-FISH and Nested PCR was used to confirm XY-FISH results. XY-FISH demonstrated the presence of isolated 0–9 male fetal cells per section in the epidermis of the healed CS scars from only those women who had their first male child by CS. Both Y-FISH and Y-PCR confirmed the presence of fetal cells in CS scars. Combined FISH and immunostaining showed all male fetal cells present were keratinocytes, as they expressed cytokeratin, and were almost exclusively located in epidermis. Microchimeric fetal cells also expressed Collagen I, III, and TGF-β3 in healed maternal scars. Identification of male–presumed fetal cells in healed maternal CS scars after pregnancy suggests that, possibly in response to signals produced by maternal skin injury at CS, fetal cells migrate to the site of damage to become involved in maternal tissue repair, or proliferate locally.
Keywords: fetal microchimerism, pregnancy, Caesarean section, skin, fluorescence in situ hybridization
Introduction
After skin injury in an adult, a well-coordinated sequence of events starts in order to patch the defect. Wound healing has three phases—inflammation, tissue formation, and tissue remodelling. Over six to 12 months following the acute injury, the extracellular matrix is strengthened by active remodelling from the main type 3 collagen to type 1 collagen.1 However, the healed wound never regains the properties of uninjured skin.2
Skin wounds in the early mammalian embryo heal without scar formation and with complete restitution of the normal skin architecture.3 There are many differences between fetal and adult wound healing processes, including differences in intrinsic functions of adult and fetal dermal fibroblasts,4-6 extracellular matrix component,7-9 the inflammatory response to the tissue injury,10,11 intracellular signal transduction,12 as well as cytokine and growth factor profiles.13-15 In the adult, age-related delayed wound healing has been blamed on altered angiogenesis and inflammatory responses, and declining fibroblast function.
Many events occur during pregnancy that not only influence the health of the child into adulthood, but may also have long-term effects on the health of the mother. One such event is the passage of fetal cells into the maternal circulation during pregnancy.16,17 Trafficking of fetal cells into the maternal circulation begins very early in pregnancy and the effects of this cell traffic are long lasting. These fetal cells can be located in maternal tissues during and after pregnancy, and persist as microchimeric cells for decades in bone marrow and other organs. While persistent fetal cells were first implicated in the pathogenesis of maternal autoimmune disease, subsequent reports found microchimeric cells in healthy tissues. Parallel studies in animal and human pregnancy now suggest instead that microchimeric fetal cells play a role in the response to tissue injury.18,19
Previous studies have shown that mobilization and differentiation of fetal stem cells sequestered in the mother’s bone marrow and other organs may require acute and/or chronic tissue injury.20,21 Fetal cells have been demonstrated in skin lesions of women affected with PEP (Polymorphic Eruption of Pregnancy), an inflammatory skin disease of pregnancy.22 Murine models of skin inflammation in pregnancy have confirmed that fetal cells are significantly more frequent in inflamed tissues compared with healthy skin, and suggested maternal recruitment of functional fetal endothelial stem cells which participated in angiogenesis.23
Adult bone marrow contains many cells that have potential to promote healing and reconstitute skin and it is accepted that adult bone marrow-derived stem cells play a crucial role in wound healing. Experimental work has shown that whole marrow, as well as cultured marrow stem cells, accelerates wound healing.24 It is biologically plausible that engrafted microchimeric fetal stem cells are recruited from marrow to sites of skin injury along with the endogenous stem cell population, but there is as yet no evidence to support a beneficial effect of these stem cells on wound healing in the adult. Controlling delivery systems of fetal cells to the skin also has potential to improve wound healing as fetal cells are differentiated cells with high expansion, regeneration, and low immunogenic properties,25,26 the vast number of additional growth factors normally necessary are not needed for cell culture and expansion, and these cells are not known to dedifferentiate once placed into the in vivo environment.26-29
Caesarean section (CS) is a common surgical procedure, and as it has become safer and electively scheduled, functional and cosmetic aspects have gained increased importance. Wound complications after CS delivery are also a significant economic and psychological burden. CS scars provide the ideal tissue to investigate the association between adult wound healing and the presence of fetal cells in the wound. Understanding this association may lead to strategies to manipulate adult healing to become more fetal-like.
The hypothesis of this study is that wound healing in women becomes more fetal-like after pregnancy; and that this may be due to the transplacental trafficking of microchimeric fetal cells with regenerative capacity. Our aims were to show that microchimeric fetal cells were present in sites of maternal tissue injury after pregnancy and to demonstrate that these cells contributed to maternal tissue repair. We also aimed to investigate whether microchimeric fetal cells improved scarring after pregnancy and finally planned to examine microchimeric cell phenotype in these models of tissue injury and repair.
Results
Study population
A total of 70 skin biopsies, consisting of 31 normal (unwounded) skin samples obtained at the first CS and 39 caesarean scars (CS) were harvested during routine surgical procedures. Based on different reproductive histories, we categorized the 70 women into three different groups: women without miscarriages (A1–A27) (Group 1), women with previous miscarriages (B1-–B12) (Group 2) and normal skin/ uninjured healthy skin - controls (C1–C31) (Group 3). All patients were coded from A-C to maintain anonymity. The mean maternal age was 32.9 y (range 23–42 y) for Group 1, 35.8 y (range 32–42 y) for Group 2, and 31.9 y (range 18–40 y) for group 3 (controls). These women were entirely healthy and had never had a transfusion or organ transplantation.
Identification of male cells in CS scars by XY FISH
FISH using the DXZ1/DYZ1 probe was performed on two hundred and 80 slides from the one hundred and 40 tissue blocks (2 blocks per patient and 2 slides per block). Each slide contained one or more tissue sections. Microchimeric fetal cells were identified in CS scars of the women who delivered their first male child by caesarean section (Figs. 1 and 2) (Table 1) but not in the skin biopsies of those who had their first male child by the vaginal route (Table 2). Ninety male cells were detected in total in the one hundred and 15 sections analyzed. This was a mean of 1.2 male fetal cells/section with a range of 0–7 fetal cells in the combination of two male pregnancies delivered by CS.
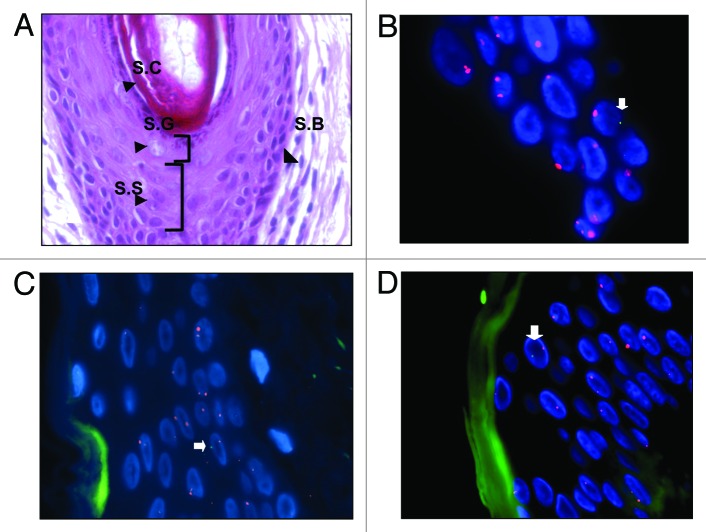
Figure 1. XY-FISH on caesarean scar showing male cells (XY) in epidermis. (A) H&E staining of skin showing four layers of epidermis - Stratum Basale (S.B), Stratum Spinosum (S.S), Stratum Granulosum (S.G), and Stratum Corneum (S.C). (B–D) XY-FISH showing male cells (XY) in epidermis of caesarean scars [arrows]. Blue represents DAPI stained nuclei. Green autofluorescence in XY-FISH in figure C and D was artifact from the Stratum Corneum layer of the skin.
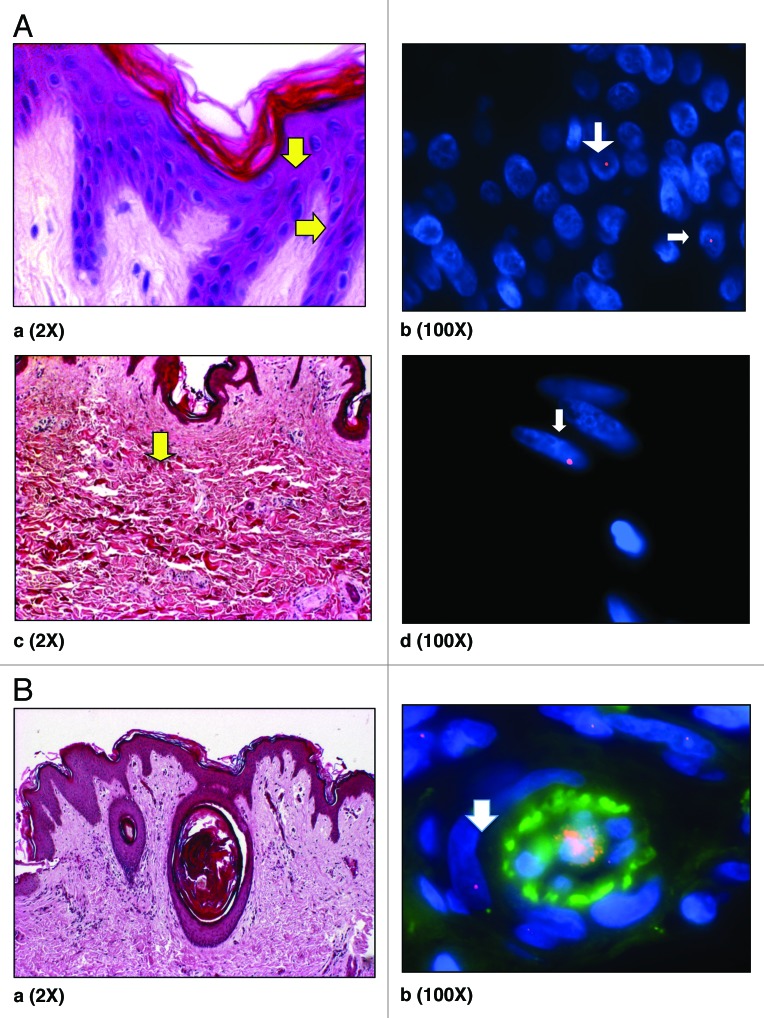
Figure 2. (A) H&E staining of skin showing epidermis (a). Y -FISH on Caesarean scar of women with previous male pregnancy showing male cells in epidermis (b) (arrows). H&E staining of skin showing dermis (c) and male cell in dermis (d) (arrows). Arrows (yellow; aandc) demonstrate putative location of these cells in H&E stained sections. Male cells identified in the female caesarean scar, bearing a Y chromosome labeled with SpectrumOrange and blue represents DAPI stained nuclei. (B) (a) H&E staining of skin showing blood vessels in dermis and (b) XY-FISH showing a male cell around a blood vessel in dermis, Male cell bearing an X chromosome labeled with SpectrumOrange and a Y chromosome labeled with SpectrumGreen. Green in the center of blood vessel is autofluorescence caused by blood. All chromosomes may not appear in the same plane of focus due to the thickness of sections (5 µm).
Table 1. Women with repeat caesarean section (Group 1; A 1- A27).
| No. Of patients | Previous pregnancy With CS |
Current pregnancy With CS |
Slides analyzed | Sections analyzed per slide | Total Male cells (n) |
|---|---|---|---|---|---|
| 4 | Female | Female | 16 | 16 | 0 |
| 5 | Female | Male | 20 | 20 | 2 |
| 11 | Male | Female | 44 | 49 | 52 |
| 7 | Male | Male | 28 | 30 | 36 |
| Σ = 27 | Σ = 108 | Σ = 115 | Σ = 90 |
Table 2. Women delivering first child by vaginal delivery (Uninjured healthy skin –subset of group 3; C1–32).
| Previous pregnancy delivered vaginally |
Current pregnancy With CS |
Slides analyzed | Sections analyzed per slide | Total Male cells (n) | |
|---|---|---|---|---|---|
| 1 | Female | Female | 4 | 4 | 0 |
| 2 | Female | Male | 8 | 10 | 0 |
| 1 | Male | Male | 4 | 8 | 0 |
| 2 | Male | Female | 8 | 10 | 0 |
| Σ = 6 | Σ = 24 | Σ = 32 | Σ = 0 |
Male fetal cells were also found in women who had only one or both female children and no sons but had miscarriage of unknown gender. More male cells were seen in caesarean scars of women who had previous miscarriages especially when surgical evacuation of retained products of conception was performed than women without previous miscarriages (Table 3). A mean of 1.33 male fetal cells/section were seen, with a range of 0 – 4 fetal cells in group 2 – a combination of previous male with CS and current male pregnancy with CS. However, this difference was not statistically significant: 1.33 male cells per section vs. 1.2 male cells per section in patients with two male pregnancies with and without previous miscarriages.
Table 3. Women with repeat caesarean section and previous miscarriages (Group 2; B1-B12).
| No. Of Patients |
Previous pregnancy With CS |
Current pregnancy With CS |
Slides Analyzed |
Sections analyzed | Total Male cells (n) |
|---|---|---|---|---|---|
| 5 | Female | Female | 20 | 22 | 26 |
| 3 | Male | Female | 12 | 12 | 11 |
| 1 | Female | Male | 4 | 4 | 4 |
| 3 | Male | Male | 12 | 12 | 16 |
| Σ = 12 | Σ = 48 | Σ = 50 | Σ = 57 |
Male fetal cells were also found in the skin biopsies of women removed during caesarean delivery of first male baby and also in skin biopsies of women who had only one female baby but had had a miscarriage of unknown fetal gender before the term pregnancy (Table 4). This skin was just like unwounded skin and was removed before the process of wound healing had started. The numbers of fetal presumed male cells were very small in uninjured skin (mean: 0.1–0.5 cells per section).
Table 4. Uninjured healthy skin – subset of group 3.
| No. of women | Parity | Slides analyzed |
Total Male cells (n) |
|
|---|---|---|---|---|
| 1 | 3 | 1st male baby | 12 | 6 |
| 2. | 3 | 1st CS male baby, Previous miscarriage |
12 | 4 |
| 3. | 7 | 1st female baby | 7 | 0 |
| 4. | 6 | 1st CS female baby, Previous miscarriage |
24 | 2 |
| Σ = 19 | Σ = 55 | Σ = 12 |
Location and phenotyping of male-presumed fetal microchimeric cells
Male cells of presumed fetal origin were seen throughout the epidermis, around blood vessels as endothelial cells and as spindle shaped cells in dermis of healed caesarean scars of women who delivered their first male child by CS (Figs. 1, 2A, and 2B). To characterize the identified male cells in skin and what they produced within different skin layers, we looked for different cell markers in the skin. Six antibodies were used for phenotypic characterization: anti-pan Cytokeratin, anti-collagen I and III, anti-fibronectin, anti-TGF-β1, and anti-TGF-β3. Fluorescent immunohistochemistry identified that anti-pan Cytokeratin binds to epithelial and trichocytic cells of skin. Collagen I is the most abundant collagen of skin, and anti-collagen I stains more in reticular dermis while anti-collagen III stains more in papillary dermis of skin. Like collagen III, fibronectin stains greatest in the papillary dermis. TGF β1 and anti-TGF β3 stains positive around keratinocytes in the epidermis.
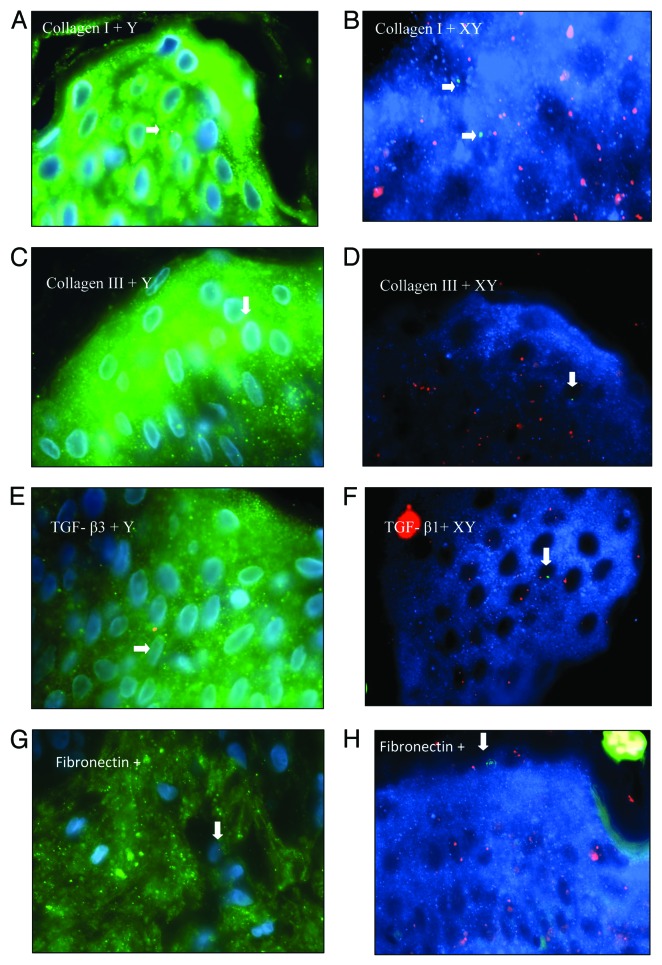
By combining Y-FISH with pan-Cytokeratin, we were able to identify male cells in the epidermis of healed caesarean scars. Male cells present in epidermis of scars were all positive for pan-Cytokeratin, implicating them as Keratinocytes of the epidermis (Fig. 3A). By combining XY-FISH with pan Cytokeratin-AMCA staining, we were once again able to identify male cells in the epidermis of caesarean scars (Fig. 3B) and were also able to show that these male presumed-microchimeric cells were Cytokeratin positive just like the adjacent female cells in epidermis of skin. Combined FISH and immunostaining showed that these male cells also expressed Collagen I, Collagen III, Fibronectin, TGF β1, and TGF β3 in healed caesarean scars of parous women (Fig. 4).
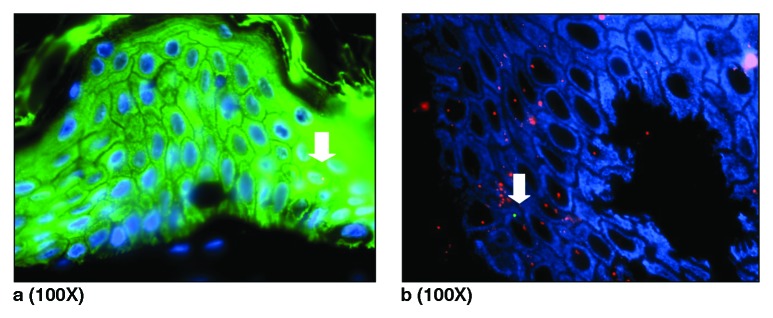
Figure 3. (A) Combined Y-FISH and pan Cytokeratin staining identified male cell in epidermis of caesarean scar. Blue represents DAPI stained nuclei, green is cytokeratin staining of epidermal layer of skin caused by fluorescein Streptavidin. The white arrows indicate Y+ positive Cytokeratin positive cell. (B) Combined XY-FISH and pan Cytokeratin (AMCA). Arrow shows pan Cytokeratin positive male cell in epidermis of caesarean scar from a pregnant women.
Figure 4. Combined FISH and immunostaining of Caesarean scars from pregnant women. All 100x. (AandB) Collagen I positive male cells in epidermis. (CandD) Collagen III positive male cells in epidermis. (E) TGF-β3+ male cell bearing a Y chromosome (red). (F) TGF-β3+ male cell with X chromosome labeled with SpectrumOrange and Y chromosome labeled with SpectrumGreen. (G) Fibronectin+ male cell bearing a Y chromosome (red) in dermis. (H) Fibronectin+ male cell (XY) in epidermis of skin. In A, C, E, andG, blue represents the DAPI staining of nuclei and green is staining of antibody conjugated with fluorescein Streptavidin, whereas in B, D, F, andH, blue represents staining of antibody conjugated with fluorescein AMCA
Identification of male-presumed fetal cells by Y-FISH and SRY-PCR
DXZ1/DYZ1 probe was used as a standard probe for XY-FISH, but to confirm the results of XY-FISH, an alternative probe was used. A single DYZ1 probe labeled with Spectrum Orange (red signal) allowed the identification of only male cells in the tissue, which made screening easier. FISH using the single DYZ1 probe was performed only on those skin sections from blocks where male cells were already seen by XY-FISH. Therefore a smaller number of sections were analyzed by Y-FISH as compared with XY-FISH. This is because XY-FISH was the main approach to identify and quantify male cells in skin sections and single DYZ1 probe was mainly used to confirm the presence of already seen male cells. This technique was later combined with immunohistochemistry to identify the phenotype of male cells. Similarly to the results obtained by XY-FISH, male cells were identified throughout the epidermis and in the dermis of healed CS scars of women, who had their first male delivery by CS (Fig. 2).
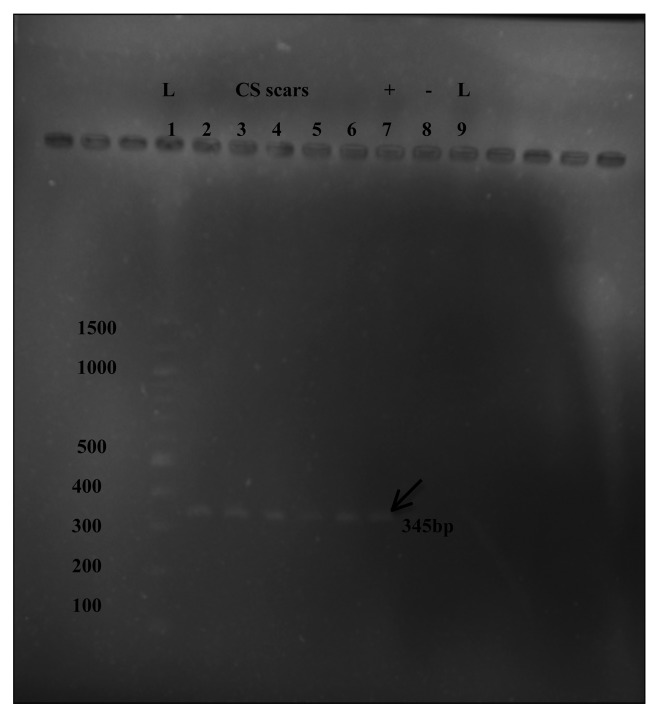
As well as using the alternative DYZ1 probe to confirm the male cells seen by XY-FISH, we next performed RT-PCR to amplify the SRY gene of the male cells in maternal CS scars. These experiments were performed on CS scar samples, where we had previously identified male cells both by XY-FISH and Y-FISH. By using a nested PCR technique, we were able to detect male genetic material in all CS scar samples tested (Fig. 5).
Figure 5. Nested PCR products of 345bp assessed by electrophoresis in a 1.2% Agarose gel. L = 100bp DNA ladder (Promega, UK), (2–6) CS-scars, (7) Positive control using placental tissue and (8) Negative control using nulliparous skin.
Discussion
In this study, we demonstrated the presence of male cells of presumed fetal origin in caesarean scars of parous women, implicating their role in the healing process after CS. XY-FISH analysis allowed the identification of male cells in CS scars from most of the women with their first male pregnancy delivered by CS (n = 27) but not in the controls, women who delivered their first male child vaginally (n = 6). Male cells were also identified in the CS scars of women with no sons, but who had previous miscarriages of unknown gender. Y-FISH analysis using the single DYZ1 probe and nested SRY-PCR confirmed the results of XY-FISH.
XY-FISH showed that the frequency of male cells in the caesarean scars of women ranged between 0–7 (mean 1.2), higher than the 0–2 male cells (mean 0.5) found in healthy uninjured skin. From our results, we suggest that injury of skin at CS recruits more fetal cells to contribute in the wound healing process. Male fetal cells seen in uninjured skin are most likely due to the random distribution of fetal cells to various maternal organs, as has been shown in other studies. Animal models have also previously demonstrated that fetal cells are recruited to the skin as a result of injury and can also be seen in healthy skin.23,24,30
Five women with no proven previous male pregnancy had male presumed-fetal cells (mean 1.18, range 0–6) in CS scars (Table 3). This could be explained by the fact that these women had previous miscarriages of unknown gender. Most of these women had surgical management of their miscarriage and it has been demonstrated that significant fetomaternal hemorrhage occurs at similar surgical management of termination of pregnancy.31
Combined FISH and immunostaining showed male cells present both in epidermis as well as in the dermis of CS scars. Male cells present in the epidermis were positive for pan-Cytokeratin indicating that these male cells were keratinocytes of the epidermis. Moreover when the scar tissue was stained for collagen I, collagen III, fibronectin, TGF-β1, and TGF-β3, male cells of presumed fetal origin in both epidermis and dermis were found to be positive for all these markers. This confirms that microchimeric fetal cells after entering maternal skin during the process of wound healing not only differentiate into local skin cells but may also take an active part in wound healing, as they produce markers of wound healing.
Cutaneous wound healing involves the recruitment of various cell types in the acute phase such as neutrophils, macrophages, and fibroblasts, in addition to re-dermalisation and re-epithelialisation. Since previous studies have shown that adult bone marrow derived cells (BMDCs) may be involved in skin repair and regeneration,32 we suggest that injury at CS caused the recruitment of adult BMDCs including engrafted microchimeric fetal cells in maternal bone marrow to the site of injury. In this study, we found microchimeric fetal cells interspersed throughout the interfollicular epidermis both in the basal layer of the epidermis as well as in the suprabasal maturing progeny (epidermal proliferative unit) of the healed scar. The process of maturation of a basal cell through to desquamation takes approximately 27 d in humans and the outer keratinized layer is shed continuously, being replaced by the progressive movement and maturation of cells from the germinal layers. Therefore, for persistent male fetal cells to be present years after CS, as we found, a fetal stem cell presence in epidermal stem cell niches is essential.
In our study, male cells of presumed fetal origin were seen as isolated single cells throughout the epidermis and were never seen in clusters. Fetal cells in maternal epidermis were identified morphologically as keratinocytes by their rounded vesicular nuclei, abundant non-dendritic cytoplasm, and epithelial cell-like morphology. Moreover, they stained positive for Cytokeratin, which means that they differentiated into the local skin cells. Comparing our results with the previous study of Borue et al. in which they observed few cytokeratin positive Y- chromosome cells in skin wounds two months after skin injury,33 we observed a higher frequency of engrafted fetal cells as keratinocytes even 8 years after caesarean section. Therefore, some of these engrafted fetal cells might be self- renewing stem cells.
For engrafted fetal cells to proliferate and differentiate in maternal organs, they must have multi-lineage capacity and should have stem cell like properties.34 According to our work, it seems that these fetal stem cells are of epithelial in origin, as we observed male keratinocytes of presumed fetal origin in maternal skin. Therefore fetal stem cells of epithelial origin could have the ability to engraft within maternal skin during pregnancy and differentiate into keratinocytes upon injury to maternal skin. Alternatively, fetal stem cells could act as a common precursor that according to the area of tissue where they engrafted would differentiate into different types of cells.
Rarely in this study were fetal cells seen in the dermis. Occasional spindle shaped cells, morphologically resembling fibroblasts were seen in the healed dermis. As in this study, we examined scars many months after the initial injury; it is not surprising that none of the fetal inflammatory cells was present at that stage. Microchimeric male fetal cells were twice seen around blood vessels; this was an unusual finding as endothelial progenitor cells contribution in wound healing is transient and their number decrease as the wound matures and vascular regression occurs. In agreement with our results, Fathke et al. also observed bone marrow derived endothelial progenitor cells in a microvessel after the acute stage of wound healing was over.35
Brittan et al. found GFP –positive cells within hair follicles after skin injury.36 We never found male fetal cells in the outer root sheath of the hair follicle, which is the repository for the epidermal stem cells. As keratinocytes are actively recycling cells, the presence of male fetal cells in the scar suggests areas of fetal stem cell population in follicular skin other than the hair follicle. In keeping with this, studies have shown before that the epidermis is self-renewing and it does not depend on cells generated from multipotent stem cells of the hair follicle.37
In this study, we used stringent measures to minimize contamination to avoid false-positive results and controls without male pregnancy were consistently negative. Analysis of tissues was blinded and independently verified. We detected the presence of male cells in female skin as indicators of fetal microchimerism, using the fluorescent in situ hybridization (FISH) technique. Two variants of FISH were used. XY- FISH was the main method for the detection of male cells of presumed fetal origin and Y- FISH was used to confirm the results from XY-FISH and also for the later combined method with immunohistochemistry. Using an RT-PCR technique, we were able to confirm male genetic material in the same CS scars. Male cells could originate in female tissues from other sources; however, we confirmed that none of women had confounding sources of microchimerism such as blood transfusion or transplantation.
Finally, this work on archived human tissues cannot demonstrate that fetal cells actively participate in the wound healing process. Human studies of wound healing are limited by the difficulty in obtaining samples from various stages of wound healing, and moreover invasive surgery like ovariectomy to exclude the role of estrogen in wound healing is neither appropriate not ethical. Therefore, murine models are a better tool to understand the individual role of fetal microchimerism and estrogen in wound healing process. Using GFP mice, Khosrotehrani et al. was able to show the engraftment of fetal cells to the site of maternal skin wounds and their involvement in angiogenesis.38 Similarly in another study, estrogen deprivation in ovariectomized female mice resulted in impaired wound healing.39
Self-renewal capacity and the long-term potential of persisting fetal stem cells to form undifferentiated cell lineages has been suggested to confer a female advantage in terms of health and longevity.16,18 Previously, various studies had shown the potential involvement of fetal microchimeric stem cells in tissue repair in myocardium, thyroid, lung, and appendix tissue during pregnancy and post-pregnancy.19,21,40,41 Our study now suggests that fetal microchimeric cells might also be involved in wound healing immediately as well as many years after the delivery. Therefore, it seems reasonable that acquired fetal cells not only have potential implications in later life but also immediately after pregnancy.
In conclusion, using CS scars as a model of injury and repair in pregnant women, we were able to demonstrate male cells of presumed fetal origin in caesarean scars eight years after their last caesarean section, from women with a previous male pregnancy. These cells were present in the epidermis and differentiated into keratinocytes. We speculate that they may be fetal stem cells.
Materials and Methods
Ethical approval
With the approval of the Clinical Research Ethics Committee of Cork Teaching Hospitals (ref: CKZ47), written informed consent was obtained from each of the participants before enrolment in the study. Women undergoing elective surgeries were recruited in the antenatal wards on the day of their operation.
Study population
Skin biopsies were taken during routine operations from women undergoing their 1st CS, where a piece of the incised skin was obtained and the 2nd elective CS, where the (full-thickness) previous caesarean scar was removed. A detailed obstetric history was obtained in women with singleton pregnancies undergoing either primary or recurrent Caesarean section (CS) with particular reference to gender, mode of delivery and outcome of previous pregnancies. Maternal age, parity, use of medications, smoking status, and past medical history was also recorded. Women who had a previous blood transfusion, stem cell, or organ transplantation were excluded. From this population, the many different possible combinations of delivery and infant gender are set out in Table 5A, 5B, and 5C.
Table 5A. Group 1: Study population and reproductive histories.
| Code | Reproductive history |
Age (years) |
Ethnicity | BMI | Smoking Status |
Slides analyzed |
Section analyzed | Male cells (n) |
|---|---|---|---|---|---|---|---|---|
| A1 | 2nd CS male baby, previous female | 42 | Caucasian | 19 | N | 4 | 4 | 0 |
| A2 | 2nd CS male baby, previous female | 23 | Caucasian | 23.5 | N | 4 | 4 | 0 |
| A3 | 2nd CS male baby, previous female | 31 | Caucasian | 22 | N | 4 | 4 | 0 |
| A4 | 2nd CS male baby, previous female | 35 | Caucasian | 31.2 | N | 4 | 4 | 0 |
| A5 | 2nd CS male baby, previous female | 37 | Brazil | 21.8 | N | 4 | 4 | 2 |
| A6 | 2nd CS male baby, previous male | 33 | Caucasian | 22.6 | N | 4 | 4 | 13 |
| A7 | 2nd CS male baby, previous male | 33 | Thailand | 24 | N | 4 | 4 | 2 |
| A8 | 2nd CS male baby, previous male | 42 | Caucasian | 26 | N | 4 | 6 | 5 |
| A9 | 2nd CS male baby, previous male | 23 | Caucasian | 23.7 | Y | 4 | 4 | 6 |
| A10 | 2nd CS male baby, previous male | 29 | Caucasian | 30 | N | 4 | 4 | 2 |
| A11 | 2nd CS male baby, previous male | 34 | Caucasian | 23.3 | N | 4 | 4 | 2 |
| A12 | 2nd CS male baby, previous male | 26 | Caucasian | 41.2 | N | 4 | 4 | 6 |
| A13 | 2nd CS female baby, previous female | 35 | Caucasian | 22 | N | 4 | 4 | 0 |
| A14 | 2nd CS female baby, previous female | 34 | Caucasian | 30 | N | 4 | 4 | 0 |
| A15 | 2nd CS female baby, previous female | 29 | Caucasian | 21.3 | N | 4 | 4 | 0 |
| A16 | 2nd CS female baby, previous female | 37 | Caucasian | 22 | N | 4 | 4 | 0 |
| A17 | 2nd CS female baby, previous male | 32 | Caucasian | 38 | N | 4 | 4 | 3 |
| A18 | 2nd CS female baby, previous male | 37 | Caucasian | 36 | N | 4 | 4 | 8 |
| A19 | 2nd CS female baby, previous male | 34 | Caucasian | 28 | Y | 4 | 7 | 16 |
| A20 | 2nd CS female baby, previous male | 30 | Caucasian | 31.7 | N | 4 | 4 | 2 |
| A21 | 2nd CS female baby, previous male | 31 | Caucasian | 23.4 | N | 4 | 4 | 6 |
| A22 | 2nd CS female baby, previous male | 33 | Caucasian | 24 | N | 4 | 4 | 0 |
| A23 | 2nd CS female baby, previous male | 40 | Caucasian | 22.6 | N | 4 | 6 | 5 |
| A24 | 2nd CS female baby, previous male | 31 | Caucasian | 19.4 | N | 4 | 4 | 5 |
| A25 | 2nd CS female baby, previous male | 34 | Caucasian | 23.2 | N | 4 | 4 | 0 |
| A26 | 2nd CS female baby, previous male | 37 | Caucasian | 21.5 | N | 4 | 4 | 4 |
| A27 | 2nd CS female baby, previous male | 28 | Caucasian | 33.3 | N | 4 | 4 | 3 |
Table 5B. Group 2 (CS scar and previous miscarriages): demographics and reproductive histories.
| Code | Reproductive history | Age (years) |
Ethnicity | BMI | Smoking Status |
Slides analyzed | Section analyzed | Male cells (n) |
|---|---|---|---|---|---|---|---|---|
| B1 | 2nd CS male baby, previous female, previous miscarriage | 37 | Caucasian | 23.6 | N | 4 | 4 | 4 |
| B2 | 2nd CS male baby, previous male, previous miscarriage | 35 | Caucasian | 30 | N | 4 | 4 | 2 |
| B3 | 2nd CS male baby, previous male, previous miscarriage | 32 | Caucasian | 23 | N | 4 | 4 | 5 |
| B4 | 2nd CS male baby, previous male, previous miscarriage | 42 | Caucasian | 23 | Y | 4 | 4 | 9 |
| B5 | 2nd CS female baby, previous female, previous miscarriage | 37 | Caucasian | 24 | N | 4 | 4 | 0 |
| B6 | 2nd CS female baby, previous female, previous miscarriage | 38 | Caucasian | 44 | N | 4 | 4 | 9 |
| B7 | 2nd CS female baby, previous female, previous miscarriage | 32 | Caucasian | 20 | N | 4 | 6 | 10 |
| B8 | 2nd CS female baby, previous female, previous miscarriage | 35 | Caucasian | 30 | N | 4 | 4 | 7 |
| B9 | 2nd CS female baby, previous female, previous miscarriage | 35 | Caucasian | 22 | N | 4 | 4 | 0 |
| B10 | 2nd CS female baby, previous male, previous miscarriage. | 33 | Caucasian | 22 | N | 4 | 4 | 6 |
| B11 | 2nd CS female baby, previous male, previous miscarriage. | 39 | Caucasian | 23.3 | N | 4 | 4 | 4 |
| B12 | 2nd CS female baby, previous male, previous miscarriage. | 34 | Caucasian | 31.4 | Y | 4 | 4 | 4 |
Table 5C. Group 3 (Unwounded skin): Demographics and reproductive histories.
| Code | Reproductive history | Age | Ethnicity | BMI | Smoking status | Slides analyzed | Section analyzed | Male cells (n) |
|---|---|---|---|---|---|---|---|---|
| C1 | 1st CS male baby | 36 | Caucasian | 30 | Y | 4 | 4 | 2 |
| C2 | 1st CS male baby | 34 | Caucasian | 26 | N | 4 | 4 | 2 |
| C3 | 1st CS male baby | 35 | Caucasian | 22 | N | 4 | 4 | 2 |
| C4 | 1st CS male baby, previous miscarriage | 36 | Caucasian | 26.6 | N | 4 | 4 | 0 |
| C5 | 1st CS male baby, previous miscarriage | 48 | Caucasian | 23 | N | 4 | 4 | 2 |
| C6 | 1st CS male baby, previous miscarriage | 35 | Caucasian | 22.8 | N | 4 | 4 | 0 |
| C7 | 1st CS female baby | 28 | Caucasian | 22 | N | 1 | 1 | 0 |
| C8 | 1st CS female baby | 32 | Caucasian | 32 | N | 1 | 1 | 0 |
| C9 | 1st CS female baby | 22 | Caucasian | 23 | Y | 1 | 1 | 0 |
| C10 | 1st CS female baby | 32 | Caucasian | 26.9 | N | 1 | 1 | 0 |
| C11 | 1st CS female baby | 39 | Caucasian | 22.6 | N | 1 | 1 | 0 |
| C12 | 1st CS female baby | 18 | Caucasian | 19.7 | N | 1 | 1 | 0 |
| C13 | 1st CS female baby | 21 | Caucasian | 20 | Y | 1 | 1 | 0 |
| C14 | 1st CS female baby, previous miscarriage | 30 | Caucasian | 21 | Y | 4 | 5 | 0 |
| C15 | 1st CS female baby, previous miscarriage | 30 | Caucasian | 33 | N | 4 | 4 | 0 |
| C16 | 1st CS female baby, previous miscarriage | 30 | Caucasian | 21.7 | Y | 4 | 4 | 1 |
| C17 | 1st CS female baby, previous miscarriage | 34 | Caucasian | 21 | N | 4 | 4 | 0 |
| C18 | 1st CS female baby, previous miscarriage | 27 | Caucasian | 20.5 | N | 4 | 4 | 1 |
| C19 | 1st CS female baby, previous miscarriage | 48 | Caucasian | 20.6 | N | 4 | 4 | 0 |
| C20 | 1st CS male baby, previous female vaginal delivery (VD) | 31 | Caucasian | 26.4 | Y | 4 | 4 | 0 |
| C21 | 1st CS male baby, previous female vaginal delivery (VD) | 39 | Caucasian | 24.6 | N | 4 | 4 | 0 |
| C22 | 1st CS male baby, previous female VD, previous miscarriage | 34 | Caucasian | 26.6 | Y | 4 | 4 | 0 |
| C23 | 1st CS male baby, previous male VD | 30 | Caucasian | 28 | Y | 4 | 8 | 0 |
| C24 | 1st CS male baby, previous male VD, previous miscarriage | 30 | Caucasian | 30 | N | 4 | 4 | 1 |
| C25 | 1st CS female baby, previous female VD | 23 | Caucasian | 27.6 | Y | 4 | 4 | 0 |
| C26 | 1st CS female baby, previous female VD, previous miscarriage | 40 | Caucasian | 21 | Y | 4 | 4 | 0 |
| C27 | 1st CS female baby, previous male VD | 35 | Caucasian | 28.7 | N | 4 | 4 | 0 |
| C28 | 1st CS female baby, previous male VD | 36 | Caucasian | 23 | N | 4 | 4 | 0 |
| C29 | 1st CS female baby, previous male VD, previous miscarriage | 31 | Caucasian | 33.6 | N | 4 | 4 | 0 |
| C30 | 1st CS female baby, previous male VD, previous miscarriage | 35 | African | 31.5 | N | 4 | 4 | 0 |
| C31 | 1st CS female baby, previous male VD, previous miscarriage | 36 | Caucasian | 36 | N | 4 | 4 | 0 |
Preparation of tissue sections
Tissues were preserved in 10% buffered Formalin (KB Scientific Limited) for less than 24 h. Representative blocks were made at 2cm intervals and then embedded in paraffin wax after overnight tissue processing. Serial 5µm sections were cut, mounted on polysine-coated slides and super frost plus slides and dried at room temperature for few hours. Precautions were taken in handling the sections, which were all prepared by the female investigator and a new sterile knife was used for each specimen. The slides were cut perpendicular to the skin surface.
Fluorescence in situ hybridization (FISH)
The method adopted for FISH was initially described by Johnson et al.42,43 As reported by O’Donoghue et al.,44 skin sections (5µm) were processed in the usual way, then heat and enzyme pre-treated and fixed before hybridization. This technique was tested on normal skin, scarred and hypertrophic tissue, where the duration of heat enzyme pre-treatment was adjusted. Tissue sections were dewaxed by immersion two times in Histoclear II (Fisher Scientific) for 5 minute (min) each rehydrated in Ethanol series (100%, 95%, and 70%) for 2 min. and then finally rinsed with tap water for 2 min. For antigen retrieval, tissue sections were heat treated with 2 × SSC (sodium salted citrate) for 15 min. at 80 °C and the washed in D.W for 2 min. Sections were treated with 200µl of 40µg/ml pre-warmed proteinase K (40 units/mg, Sigma-Aldrich) at 37 °C for 6–10 min depending upon the skin samples (6 min. for normal skin, 8 min. for previous scar, and 10 min for hypertrophic scar). Sections were then washed with D.W and then with 2 × SSC twice for 3 min. Secondary fixation done with ice-cold 2:1 v/v methanol: acetone for 2 min before hybridization. Slides were then hybridized with 3 µl-undiluted probe, at 71 °C for 7 min followed by a 4-h hybridization at 37 °C. The probes used were chromosome-specific centromeric repeat probes CEP X spectrum orange/Y spectrum green or CEP Y spectrum orange (Abbott Laboratories). Following hybridization, cells were washed with 2 × SSC for 2 min, incubated with 0.4 × SSC in a water bath at 72 ± 1 °C for 2 min. and the washed with 2 × SSC/0.1% NP-40 (Nonylphenoxy-polyethoxy ethanol-40) for 2 min. During these steps after the use of probe, slides were shielded from direct light. Slides were final passed through Ethanol series (70%, 95%, and 100%), mounted in DAPI (diamidino-2 phenyl-indole; Vector laboratories, UK) and covered with 22 × 22 mm coverslips and sealed with nail varnish.
DNA probes for XY-FISH
For XY-FISH, CEP X/Y probe was used which is a mixture of a SpectrumOrange labeled CEPX probe and a SpectrumGreen labeled CEP Y probe specific for the α satellite centromeric region of chromosome X and satellite III (Yq12) region of chromosome Y. In a normal male cell, the expected pattern for nucleus hybridized with the CEP X/Y DNA probe was one red and one green signal pattern. In a normal female cell the two single red signals were seen. For Y-FISH, single CEP Y SpectrumOrange probe was used. Male cells were identified by the presence of a single red signal.
Fluorescent immunohistochemistry
Tissue sections were dewaxed by immersion two times in Histoclear II (Fisher Scientific) for five min each, rehydrated in an ethanol series (100%, 95%, and 75%), and then rinsed in distilled water. For antigen retrieval, slides were heated in 2 × SSC for 15 min. at 80 °C; washed in DW for two min and then treated with 200µl of 40µg/ml of pre-warmed proteinase K in oven for varying length, depending upon the skin type. After SSC washes, sections were incubated with 200 µl of block for one hour and then after PBS washes, sections were treated with specific blocking agent (Vector Labs). Sections were then incubated with 200µl of appropriate anti-human murine IgG – class monoclonal antibody diluted in antibody diluent (DAKO). Sections were washed twice in PBS for three minutes each and then incubated with 200µl of biotinylated goat anti-mouse secondary antibody (Vector Laboratories), diluted 1:50 in block for 30 min. Slides were again washed in PBS twice and then incubated with 200µl of fluorescent Streptavidin (Vector Laboratories), diluted 1:50 in block for 30 min. Slides were rinsed in tap water and dehydrated in 100% ethanol for 30 s. After being air-dried, the slides were mounted in DAPI (diamidino-2-phenyl-indole, Vector Laboratories), covered with 22 × 22mm coverslips, and sealed with nail varnish.16,19,42-44
Fluorescent Immunohistochemistry combined with FISH
In order to identify the phenotype of microchimeric cells found in the tissue sections, Immunohistochemistry for markers of fetal wound healing was performed with Cytokeratin, Collagen I, III, Fibronectin, Transforming growth factor β1 (TGFβ1), and Transforming growth factor β3 (TGFβ3), was done on the same sections following FISH. (Table 6). Tissue was dewaxed as described above and then heat-treated with 2x SSC for 15 min. at 80 °C, treated with 200 ml of 40µg/ml pre-warmed Proteinase-K (40 units/mg, Sigma-Aldrich) at 37 °C for 5–10 min depending upon the skin sample (5 min. for normal skin, 8 min. for previous scar and 10 min. for hypertrophic scar), and washed with 2x SSC after each step. After fixation with ice-cold 2:1 v/v methanol: acetone for 2 min., slides were hybridized with 3µl of either an XY undiluted probe or a single Y probe (1 in 1 dilution) for a minimum of 4 h. Following post-hybridization washes and an ethanol dehydration series, slides were air-dried and incubated with Block (5% BSA in PBS) for 1 h. To increase antibody staining, slides were incubated with Avidin followed by Biotin for 15 min each. The slides were then incubated with the appropriate anti-human murine IgG-class monoclonal primary antibodies (Table 6) diluted with Dako antibody diluent (1: 50) to reduce background autofluorescence, for 1 h and washed twice in PBS, 2 min each. Subsequent incubations were with biotinylated goat antimouse secondary antibody (Vector Laboratories), detected with Streptavidin conjugated with fluorescein/ Streptavidin AMCA (Vector Laboratories) both diluted 1:50 and incubated for 30 min each. Slides were finally dehydrated in 100% ethanol for 1 min and mounted in DAPI/ H1000 (Vector Laboratories) for analysis by epifluorescence microscopy.16,19,42-44
Table 6. List of antibodies and dilutions used.
| Primary Antibodies | Company | Dilution |
|---|---|---|
| Collagen I (Monoclonal) |
Sigma, Aldrich, UK | 1:50 |
| Collagen III (Monoclonal) | Sigma, Aldrich, UK | 1:50 |
| Fibronectin (Monoclonal) | Sigma, Aldrich, UK | 1:50 |
| TGFβ1 | Abcam, UK | 1:50 |
| TGβ3 | Abcam, UK | 1:50 |
| Pan cytokeratin | Abcam, UK | 1:50 |
Imaging and analysis
Following FISH, Immunohistochemistry with fluorescent Streptavidin method and Combined ImmunoFISH, slides were analyzed by epifluorescence microscopy (Zeiss Axioskope), using single band pass filters for Green, Red, and DAPI and the triple band filter set. Images were then captured using a cooled CCD camera and reviewed in Carl Zeiss Axiovision Rel. 4.8.1 software. Nuclei with two red signals were considered as female (XX) and those with one red and one green were considered as male (XY). Each slide was checked for hybridization efficiency and only analyzed if more than 75% of the nuclei showed two signals. Only those fluorescence signals were considered positive when the intensity and diameter of red and green signals were equal and inside an intact nucleus.16,42,44 To distinguish true signals from tissue autofluorescence, image intensities were captured and compared under all filter channels using high magnification (X100). Each slide was examined twice; an initial scan “trawl” of the tissue to see hybridization efficiency and tissue architecture, and a later more detailed survey of all signals.
Scoring and statistical analysis
Sections from CS wounds and controls were all analyzed for the presence of male cells without prior knowledge of histological parameters. Male cells were recognized as having 1-Y (green) chromosome within an intact blue stained nucleus. Because normal distribution of the data could not be assumed, statistical significance was determined by the Mann–Whitney Wilcoxon nonparametric or the Fisher exact test. P values < 0.05 were considered to be statistically significant.
Genomic DNA extraction
DNA was extracted from the freshly cut FFPE tissue sections, using QIAamp DNA FFPE Kit (Qiagen Ltd.). The QIAamp DNA FFPE Tissue procedure consists of 6 steps. In the first step paraffin was dissolved in xylene and removed. Sample was lysed under denaturing conditions with a short proteinase K digestion. Incubation at 90 °C reversed formalin cross-linking. DNA then binds to the membrane and contaminants flow through. Residual contaminants were washed away and pure, concentrated DNA was eluted from the membrane. The DNA was eluted from the column using 50 µl of Buffer ATE.
Polymerase chain reaction (PCR)
To confirm the presence of Y chromosome in the female tissue sections, the SRY (sex determining region Y) gene was amplified by nested PCR using two pairs of primers designed to cover 609bp and 345bp around the HMG box of the gene. PCR was performed using the Hot Star Taq DNA polymerase kit (Qiagen). Each PCR reaction had a total volume of 70 µl consisting of 7µl 10× buffer, 2 µl of 40mM dNTPs, 3 µl 25 mM MgCl2, 14 µl Q solution, 1 µl DNA polymerase, 1.5 µl of the 1 mM forward hSRY-F6 primer (GACAATGCAA TCATATGCTT CTGC), 1.5 µl of the 1mM reverse hSRYB6 primer (CTGTAGCGGT CCCGTTGCTG CGGTG), and 40 µl of purified DNA. Cycling parameters were performed in a thermo cycler as follows: denaturation at 95 °C for 15 min; 35 cycles of denaturing at 95 °C for 1 min, annealing at 62 °C for 1 min and extension at 72 °C for 1 min; and final extension at 72 °C for 10 min. Genomic DNA extracted from placental tissue from a male baby was used as a positive control and DNA extracted from nulliparous skin and DEPC-treated water were used as negative controls to exclude any possibility of contamination or nonspecific amplification. The PCR product generated after the first reaction was then column purified using the Min Elute PCR purification kit (Qiagen) and DNA was eluted using 40 µl of DEPC-treated water.
Nested PCR was performed by adding the 40µl purified first round PCR product on the top of 30 µl PCR master mix prepared as described above, but this time using the hSRY-F1 (CAGTGTGAAA CGGGAGAAAA CAGT) forward primer and the hSRY-B1 (GCACTTCGCT GCAGAGTACC GAAG) reverse primer. Nested PCR amplification was performed for 35 cycles at the same conditions as described above. This strategy permits the amplification of specific sequences of DNA from a large complex mixture of DNA. Even if the wrong locus were amplified by mistake, it is very unlikely to be also amplified a second time by the second pair of primers. 15 µl nested PCR products were analyzed by electrophoresis using a 1.2% Agarose gel containing safe view dye, and visualized under UV light on a transilluminator.19,44
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Ethical approval
Clinical Research Ethics Committee of Cork Teaching Hospitals (ref: CKZ47).
Glossary
Abbreviations:
- CS
Caesarean section
- FISH
fluorescence in situ hybridization
- PCR
polymerase chain reaction
References
- 1.Lovvorn HN, 3rd, Cheung DT, Nimni ME, Perelman N, Estes JM, Adzick NS. Relative distribution and crosslinking of collagen distinguish fetal from adult sheep wound repair. J Pediatr Surg. 1999;34:218–23. doi: 10.1016/S0022-3468(99)90261-0. [DOI] [PubMed] [Google Scholar]
- 2.Levenson SM, Geever EF, Crowley LV, Oates JF, 3rd, Berard CW, Rosen H. The Healing of Rat Skin Wounds. Ann Surg. 1965;161:293–308. doi: 10.1097/00000658-196502000-00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Whitby DJ, Ferguson MW. The extracellular matrix of lip wounds in fetal, neonatal and adult mice. Development. 1991;112:651–68. doi: 10.1242/dev.112.2.651. [DOI] [PubMed] [Google Scholar]
- 4.Chen WY, Grant ME, Schor AM, Schor SL. Differences between adult and foetal fibroblasts in the regulation of hyaluronate synthesis: correlation with migratory activity. J Cell Sci. 1989;94:577–84. doi: 10.1242/jcs.94.3.577. [DOI] [PubMed] [Google Scholar]
- 5.Alaish SM, Yager D, Diegelmann RF, Cohen IK. Biology of fetal wound healing: hyaluronate receptor expression in fetal fibroblasts. J Pediatr Surg. 1994;29:1040–3. doi: 10.1016/0022-3468(94)90275-5. [DOI] [PubMed] [Google Scholar]
- 6.Lorenz HP, Lin RY, Longaker MT, Whitby DJ, Adzick NS. The fetal fibroblast: the effector cell of scarless fetal skin repair. Plast Reconstr Surg. 1995;96:1251–9, discussion 1260-1. doi: 10.1097/00006534-199511000-00001. [DOI] [PubMed] [Google Scholar]
- 7.Longaker MT, Chiu ES, Harrison MR, Crombleholme TM, Langer JC, Duncan BW, Adzick NS, Verrier ED, Stern R. Studies in fetal wound healing. IV. Hyaluronic acid-stimulating activity distinguishes fetal wound fluid from adult wound fluid. Ann Surg. 1989;210:667–72. doi: 10.1097/00000658-198911000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Longaker MT, Whitby DJ, Ferguson MW, Harrison MR, Crombleholme TM, Langer JC, Cochrum KC, Verrier ED, Stern R. Studies in fetal wound healing: III. Early deposition of fibronectin distinguishes fetal from adult wound healing. J Pediatr Surg. 1989;24:799–805. doi: 10.1016/S0022-3468(89)80540-8. [DOI] [PubMed] [Google Scholar]
- 9.Mast BA, Flood LC, Haynes JH, DePalma RL, Cohen IK, Diegelmann RF, Krummel TM. Hyaluronic acid is a major component of the matrix of fetal rabbit skin and wounds: implications for healing by regeneration. Matrix. 1991;11:63–8. doi: 10.1016/S0934-8832(11)80228-3. [DOI] [PubMed] [Google Scholar]
- 10.Robinson BW, Goss AN. Intra-uterine healing of fetal rat cheek wounds. Cleft Palate J. 1981;18:251–5. [PubMed] [Google Scholar]
- 11.Cowin AJ, Brosnan MP, Holmes TM, Ferguson MW. Endogenous inflammatory response to dermal wound healing in the fetal and adult mouse. Dev Dyn. 1998;212:385–93. doi: 10.1002/(SICI)1097-0177(199807)212:3<385::AID-AJA6>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 12.Chin GS, Kim WJ, Lee TY, Liu W, Saadeh PB, Lee S, Levinson H, Gittes GK, Longaker MT. Differential expression of receptor tyrosine kinases and Shc in fetal and adult rat fibroblasts: toward defining scarless versus scarring fibroblast phenotypes. Plast Reconstr Surg. 2000;105:972–9. doi: 10.1097/00006534-200003000-00021. [DOI] [PubMed] [Google Scholar]
- 13.Liechty KW, Crombleholme TM, Cass DL, Martin B, Adzick NS. Diminished interleukin-8 (IL-8) production in the fetal wound healing response. J Surg Res. 1998;77:80–4. doi: 10.1006/jsre.1998.5345. [DOI] [PubMed] [Google Scholar]
- 14.Liechty KW, Adzick NS, Crombleholme TM. Diminished interleukin 6 (IL-6) production during scarless human fetal wound repair. Cytokine. 2000;12:671–6. doi: 10.1006/cyto.1999.0598. [DOI] [PubMed] [Google Scholar]
- 15.Hsu M, Peled ZM, Chin GS, Liu W, Longaker MT. Ontogeny of expression of transforming growth factor-beta 1 (TGF-beta 1), TGF-beta 3, and TGF-beta receptors I and II in fetal rat fibroblasts and skin. Plast Reconstr Surg. 2001;107:1787–94, discussion 1795-6. doi: 10.1097/00006534-200106000-00023. [DOI] [PubMed] [Google Scholar]
- 16.O’Donoghue K, Chan J, de la Fuente J, Kennea N, Sandison A, Anderson JR, Roberts IA, Fisk NM. Microchimerism in female bone marrow and bone decades after fetal mesenchymal stem-cell trafficking in pregnancy. Lancet. 2004;364:179–82. doi: 10.1016/S0140-6736(04)16631-2. [DOI] [PubMed] [Google Scholar]
- 17.Bianchi DW. Robert E. Gross Lecture. Fetomaternal cell trafficking: a story that begins with prenatal diagnosis and may end with stem cell therapy. J Pediatr Surg. 2007;42:12–8. doi: 10.1016/j.jpedsurg.2006.09.047. [DOI] [PubMed] [Google Scholar]
- 18.O'Donoghue K. Fetal microchimerism and maternal health during and after pregnancy. Obstetric medicine. 2008;1:56–64. doi: 10.1258/om.2008.080008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Santos MA, O’Donoghue K, Wyatt-Ashmead J, Fisk NM. Fetal cells in the maternal appendix: a marker of inflammation or fetal tissue repair? Hum Reprod. 2008;23:2319–25. doi: 10.1093/humrep/den261. [DOI] [PubMed] [Google Scholar]
- 20.Johnson KL, Nelson JL, Furst DE, McSweeney PA, Roberts DJ, Zhen DK, Bianchi DW. Fetal cell microchimerism in tissue from multiple sites in women with systemic sclerosis. Arthritis Rheum. 2001;44:1848–54. doi: 10.1002/1529-0131(200108)44:8<1848::AID-ART323>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 21.Srivatsa B, Srivatsa S, Johnson KL, Samura O, Lee SL, Bianchi DW. Microchimerism of presumed fetal origin in thyroid specimens from women: a case-control study. Lancet. 2001;358:2034–8. doi: 10.1016/S0140-6736(01)07099-4. [DOI] [PubMed] [Google Scholar]
- 22.Aractingi S, Berkane N, Bertheau P, Le Goué C, Dausset J, Uzan S, Carosella ED. Fetal DNA in skin of polymorphic eruptions of pregnancy. Lancet. 1998;352:1898–901. doi: 10.1016/S0140-6736(98)05121-6. [DOI] [PubMed] [Google Scholar]
- 23.Nassar D, Droitcourt C, Mathieu-d’Argent E, Kim MJ, Khosrotehrani K, Aractingi S. Fetal progenitor cells naturally transferred through pregnancy participate in inflammation and angiogenesis during wound healing. FASEB J. 2012;26:149–57. doi: 10.1096/fj.11-180695. [DOI] [PubMed] [Google Scholar]
- 24.Sasaki M, Abe R, Fujita Y, Ando S, Inokuma D, Shimizu H. Mesenchymal stem cells are recruited into wounded skin and contribute to wound repair by transdifferentiation into multiple skin cell type. J Immunol. 2008;180:2581–7. doi: 10.4049/jimmunol.180.4.2581. [DOI] [PubMed] [Google Scholar]
- 25.Quintin A, Hirt-Burri N, Scaletta C, Schizas C, Pioletti DP, Applegate LA. Consistency and safety of cell banks for research and clinical use: preliminary analysis of fetal skin banks. Cell Transplant. 2007;16:675–84. doi: 10.3727/000000007783465127. [DOI] [PubMed] [Google Scholar]
- 26.Applegate LA, Scaletta C, Hirt-Burri N, Raffoul W, Pioletti D. Whole-cell bioprocessing of human fetal cells for tissue engineering of skin. Skin Pharmacol Physiol. 2009;22:63–73. doi: 10.1159/000178865. [DOI] [PubMed] [Google Scholar]
- 27.Hohlfeld J, de Buys Roessingh A, Hirt-Burri N, Chaubert P, Gerber S, Scaletta C, Hohlfeld P, Applegate LA. Tissue engineered fetal skin constructs for paediatric burns. Lancet. 2005;366:840–2. doi: 10.1016/S0140-6736(05)67107-3. [DOI] [PubMed] [Google Scholar]
- 28.De Buys Roessingh AS, Hohlfeld J, Scaletta C, Hirt-Burri N, Gerber S, Hohlfeld P, Gebbers JO, Applegate LA. Development, characterization, and use of a fetal skin cell bank for tissue engineering in wound healing. Cell Transplant. 2006;15:823–34. doi: 10.3727/000000006783981459. [DOI] [PubMed] [Google Scholar]
- 29.Ramelet AA, Hirt-Burri N, Raffoul W, Scaletta C, Pioletti DP, Offord E, Mansourian R, Applegate LA. Chronic wound healing by fetal cell therapy may be explained by differential gene profiling observed in fetal versus old skin cells. Exp Gerontol. 2009;44:208–18. doi: 10.1016/j.exger.2008.11.004. [DOI] [PubMed] [Google Scholar]
- 30.Koopmans M, Kremer Hovinga IC, Baelde HJ, Harvey MS, de Heer E, Bruijn JA, Bajema IM. Chimerism occurs in thyroid, lung, skin and lymph nodes of women with sons. J Reprod Immunol. 2008;78:68–75. doi: 10.1016/j.jri.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 31.Bianchi DW, Farina A, Weber W, Delli-Bovi LC, Deriso M, Williams JM, Klinger KW. Significant fetal-maternal hemorrhage after termination of pregnancy: implications for development of fetal cell microchimerism. Am J Obstet Gynecol. 2001;184:703–6. doi: 10.1067/mob.2001.111072. [DOI] [PubMed] [Google Scholar]
- 32.Wu Y, Wang J, Scott PG, Tredget EE. Bone marrow-derived stem cells in wound healing: a review. Wound Repair Regen. 2007;15(Suppl 1):S18–26. doi: 10.1111/j.1524-475X.2007.00221.x. [DOI] [PubMed] [Google Scholar]
- 33.Borue X, Lee S, Grove J, Herzog EL, Harris R, Diflo T, Glusac E, Hyman K, Theise ND, Krause DS. Bone marrow-derived cells contribute to epithelial engraftment during wound healing. Am J Pathol. 2004;165:1767–72. doi: 10.1016/S0002-9440(10)63431-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Khosrotehrani K, Bianchi DW. Multi-lineage potential of fetal cells in maternal tissue: a legacy in reverse. J Cell Sci. 2005;118:1559–63. doi: 10.1242/jcs.02332. [DOI] [PubMed] [Google Scholar]
- 35.Fathke C, Wilson L, Hutter J, Kapoor V, Smith A, Hocking A, Isik F. Contribution of bone marrow-derived cells to skin: collagen deposition and wound repair. Stem Cells. 2004;22:812–22. doi: 10.1634/stemcells.22-5-812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brittan M, Braun KM, Reynolds LE, Conti FJ, Reynolds AR, Poulsom R, Alison MR, Wright NA, Hodivala-Dilke KM. Bone marrow cells engraft within the epidermis and proliferate in vivo with no evidence of cell fusion. J Pathol. 2005;205:1–13. doi: 10.1002/path.1682. [DOI] [PubMed] [Google Scholar]
- 37.Levy V, Lindon C, Harfe BD, Morgan BA. Distinct stem cell populations regenerate the follicle and interfollicular epidermis. Dev Cell. 2005;9:855–61. doi: 10.1016/j.devcel.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 38.Nguyen Huu S, Oster M, Uzan S, Chareyre F, Aractingi S, Khosrotehrani K. Maternal neoangiogenesis during pregnancy partly derives from fetal endothelial progenitor cells. Proc Natl Acad Sci U S A. 2007;104:1871–6. doi: 10.1073/pnas.0606490104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gilliver SC, Ashworth JJ, Ashcroft GS. The hormonal regulation of cutaneous wound healing. Clin Dermatol. 2007;25:56–62. doi: 10.1016/j.clindermatol.2006.09.012. [DOI] [PubMed] [Google Scholar]
- 40.Choolani M, O’Donoghue K, Talbert D, Kumar S, Roberts I, Letsky E, Bennett PR, Fisk NM. Characterization of first trimester fetal erythroblasts for non-invasive prenatal diagnosis. Mol Hum Reprod. 2003;9:227–35. doi: 10.1093/molehr/gag027. [DOI] [PubMed] [Google Scholar]
- 41.Bayes-Genis A, Bellosillo B, de la Calle O, Salido M, Roura S, Ristol FS, Soler C, Martinez M, Espinet B, Serrano S, et al. Identification of male cardiomyocytes of extracardiac origin in the hearts of women with male progeny: male fetal cell microchimerism of the heart. J Heart Lung Transplant. 2005;24:2179–83. doi: 10.1016/j.healun.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 42.Johnson KL, Zhen DK, Bianchi DW. The use of fluorescence in situ hybridization (FISH) on paraffin-embedded tissue sections for the study of microchimerism. Biotechniques. 2000;29:1220–4. doi: 10.2144/00296st01. [DOI] [PubMed] [Google Scholar]
- 43.Khosrotehrani K, Stroh H, Bianchi DW, Johnson KL. Combined FISH and immunolabeling on paraffin-embedded tissue sections for the study of microchimerism. Biotechniques. 2003;34:242–4. doi: 10.2144/03342bm01. [DOI] [PubMed] [Google Scholar]
- 44.O’Donoghue K, Sultan HA, Al-Allaf FA, Anderson JR, Wyatt-Ashmead J, Fisk NM. Microchimeric fetal cells cluster at sites of tissue injury in lung decades after pregnancy. Reprod Biomed Online. 2008;16:382–90. doi: 10.1016/S1472-6483(10)60600-1. [DOI] [PubMed] [Google Scholar]