Abstract
During gestation, maternal cells traffic to the fetus leading to the natural phenomenon of microchimerism. Although their persistence in offspring has been associated with several autoimmune disorders, the precise role of maternal cells in these disorders remains unclear. We aimed to evaluate whether alloreactive maternal T cells could directly trigger a graft-vs.-host like reaction or indirectly influence the development of the offspring’s regulatory T cells (Treg) favoring autoimmunity. In a specific breeding strategy, we recently reported that maternal allogeneic T cells changed fetal Treg development and their quantities in mesenteric lymph nodes, leading to early signs of inflammation in the gut later in life. Although maternal microchimeric T cells were found in newborn tissues, we could not detect any cells in the gut from adult offspring where the inflammation occurred. Thus, strongly alloreactive maternal microchimeric T cells may indirectly drive the offspring to gut inflammation. We believe these results suggest a new mechanism for predisposition to auto-immunity.
Keywords: maternal microchimerism, pregnancy, regulatory T cells, autoimmunity, self-antigens
Maternal microchimerism (MMc) is a frequent phenomenon occurring during all pregnancies. A higher frequency of maternal microchimeric (Mc) cells in the circulation and affected tissues from children has been associated with autoimmune diseases such as diabetes, systemic sclerosis, skin inflammatory disorders or juvenile dermatomyositis.1-3 However, it is not clearly understood whether MMc is a direct or indirect cause or if it is simply a bystander associated phenomenon. To date, it has been suggested that MMc acts by direct reactivity targeting offspring’s tissues.4,5 However, some evidence favors the hypothesis that MMc may shape the fetal immunity toward self-antigen inducing inflammation indirectly in offspring’s tissues.6
Although, the possibility of modification of tolerance to self-antigens via maternal Mc cells has yet to be addressed, the influence of maternal Mc cells on the offspring’s immunity toward alloantigens has been well established. Indeed, the exposure to non-inherited maternal antigens (NIMA) during fetal life and nursing was shown to induce alloantigen-specific natural and adaptive regulatory T cells (Tregs) and this was associated with a high level of maternal Mc cells suggesting that NIMA specific Tregs develop upon exposure to MMc.7 Together, these data suggest a role for maternal Mc cells in inducing NIMA-specific Tregs and their persistence in adult life could allow the achievement of tolerance toward NIMA allografts. Indeed in allogeneic solid organ transplantation from parents to child, recent studies showed that the presence of MMc directly changed the fetal tolerance toward NIMA by affecting Treg development which leads to better transplantation outcome and reduced graft rejection.8-11
Previous indications strongly suggested the importance of maternal Mc cells in triggering an auto-immune response in a model of juvenile diabetes.6 RIP-OVA mice, which express low level of ovalbumin (OVA) in pancreatic β cells, when exposed to maternal OT2 T cells, reactive against the OVA antigen, had significantly higher rates of peri-insulitis in pancreatic islets six weeks after birth when compared with controls. Importantly, most, if not all of the infiltrating T cells in the pancreas were from the offspring and not from the mother, indicating that maternal OT2 T cells had influenced the host T cells to infiltrate the pancreas. Based on these results, it was speculated that maternal Mc T cells did not act directly on the tissue but had changed fetal immune responses toward self-antigens.
Based on these data, we hypothesized that maternal Mc T cells acquired during pregnancy may be the potential key actors that interfere with the developing fetal immune system and therefore may modify Treg development predisposing the offspring to autoimmunity.12 We aimed to investigate whether allogeneic maternal Mc T cells would directly trigger a graft-vs.-host (GVH) like reaction in the offspring and examined whether allogeneic maternal Mc T cells change Treg development between offspring from strongly alloreactive, weakly alloreactive or immunodeficient mothers.
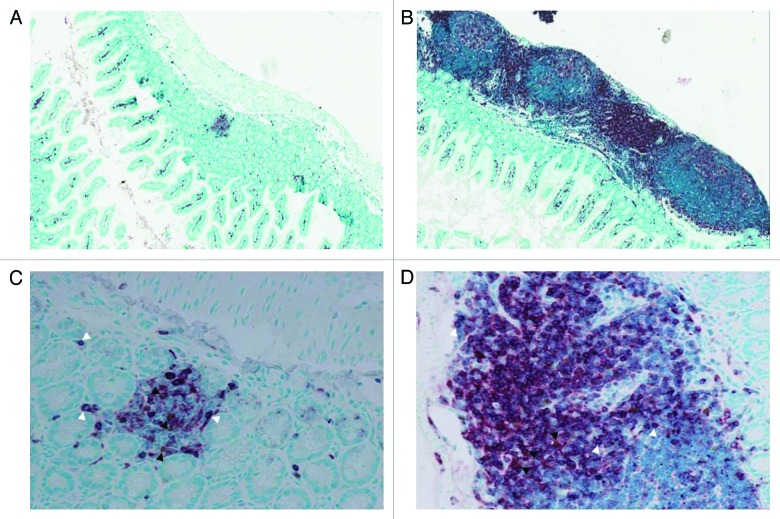
We first reported the possibility of persistence of highly alloreactive maternal Mc T cells in semi-allogeneic offspring.13 Although, we could detect large numbers of alloreactive maternal Mc T cells in newborn mice, these cells did not persist in adulthood except in the thymus and skin. Autoimmune diseases and graft-vs.-host disease (GVHD) share common features and target organs as skin, liver and gut. However, the alloreactive model used in our study targeted more specifically the gut. We compared tissue inflammation of offspring from different breeding combinations. Adult offspring from strongly alloreactive and allogeneic mothers displayed prominent lymphoid aggregates in the gut compared with the offspring from immunodeficient non-reactive mothers. Figure 1 shows a marked increase in infiltrating T cells as labeled by CD3. The liver and skin were normal. In newborn offspring, no inflammation/GVHD reaction was detected. Since the maternal alloreactive Mc T cells could not be detected in the gut of adult offspring, these data supported the hypothesis that alloreactive maternal Mc T cells could not directly be responsible for the tissue specific inflammation.
Figure 1. Enrichment in lymphoid aggregates in the small intestine of 6–8 weeks old offspring from strongly alloreactive mothers. Staining for CD3 (red, white arrowheads) and Foxp3 (brown, dark arrowheads) was performed on small intestine sections from offspring of (A-C) immunodeficient and (B-D) strongly alloreactive mothers. The absolute number of Tregs was not different between the different conditions, but the effector to regulator ratio was strongly in favor of effectors in strongly alloreactive mothers. Representative pictures of mice with presence of CD3+ lymphoid aggregates are shown at x100 magnification (A-B) and x400 magnification (C-D).
Interestingly, the frequency of Tregs was reduced in the mesenteric lymph node (mLN) but not in the spleen from adult offspring of highly alloreactive mothers. Similarly in gut sections, although Foxp3 staining displayed similar numbers of Tregs between different conditions, the effector to regulator ratio was strongly in favor of effectors in strongly alloreactive and alloreactive mothers. Altogether, these data provide some evidence that alloreactive maternal T cells possibly change the fetal immune responses at early stages by affecting the development of Tregs toward self-antigens in the offspring later in life. As the reduced number of Tregs was found in the mLN and not in the spleen of adult offspring, this data could explain the selective gut inflammation in these animals.
This mechanism could occur in a narrow window in utero in which maternal Mc T cells as well as other effector cells could create a pro-inflammatory environment. Although, the cytokine profile of the T cells in spleen of the offspring was not changed, maternal T cells could be strongly alloreactive toward non-shared fetal antigens and produce inflammatory cytokines such as IFNγ, IL-6 or TNFα that would modify the tolerogenic environment where TGF-β is critical for Treg development.14 Intra-amniotic LPS injections in sheep directly affects the number of Tregs long-term after birth.15 Thus, it could be postulated that chronic persistence of fetal autoreactive T cells would escape the appropriate suppression by Tregs, consequently leading to autoimmunity. In this report, we found that maternal cells, naturally acquired during fetal life, alter the offspring’s response to self-antigens and trigger a selective organ inflammation predisposing to an auto-immune response later in life (Figure. 2). However, some limitations appear as this study was performed on naïve mice and therefore further investigations are needed to understand this mechanism when the offspring is immunologically challenged. This paper provided new insights in the balance between tolerance and autoimmunity occurring in the fetus during pregnancy and defined a common mechanism for predisposition to auto-immune diseases that now needs to be further evaluated.
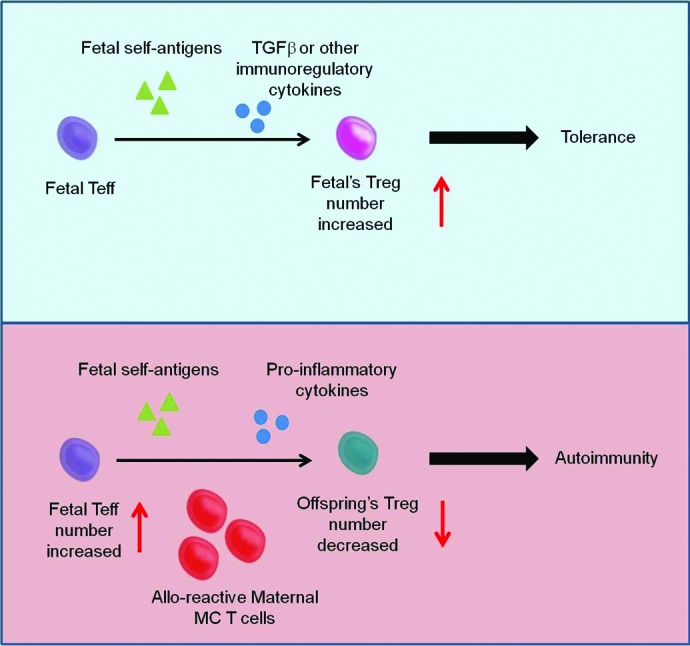
Figure 2. The roles of maternal MC cells on fetal immunity. The presence of maternal MC cells in the fetus can skew fetal immunity toward auto-reactivity. In normal human pregnancy, Mold et al.14 showed that fetuses developed Tregs specific for allo-antigens presented in utero in the presence of TGF-β leading to tolerance to the same allo-antigens later in life. Maternal MC T cells, strongly alloreactive toward any fetal antigen may produce inflammatory cytokines that would modify the tolerogenic environment via TGF-β which is critical for Treg development. Thus, it could be postulated that chronic persistence of fetal autoreactive T cells would escape the appropriate suppression by Tregs, consequently leading to autoimmunity.
References
- 1.Nelson JL, Gillespie KM, Lambert NC, Stevens AM, Loubiere LS, Rutledge JC, Leisenring WM, Erickson TD, Yan Z, Mullarkey ME, et al. Maternal microchimerism in peripheral blood in type 1 diabetes and pancreatic islet beta cell microchimerism. Proc Natl Acad Sci U S A. 2007;104:1637–42. doi: 10.1073/pnas.0606169104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reed AM, Picornell YJ, Harwood A, Kredich DW. Chimerism in children with juvenile dermatomyositis. Lancet. 2000;356:2156–7. doi: 10.1016/S0140-6736(00)03500-5. [DOI] [PubMed] [Google Scholar]
- 3.Lambert NC, Erickson TD, Yan Z, Pang JM, Guthrie KA, Furst DE, Nelson JL. Quantification of maternal microchimerism by HLA-specific real-time polymerase chain reaction: studies of healthy women and women with scleroderma. Arthritis Rheum. 2004;50:906–14. doi: 10.1002/art.20200. [DOI] [PubMed] [Google Scholar]
- 4.Reed AM, McNallan K, Wettstein P, Vehe R, Ober C. Does HLA-dependent chimerism underlie the pathogenesis of juvenile dermatomyositis? J Immunol. 2004;172:5041–6. doi: 10.4049/jimmunol.172.8.5041. [DOI] [PubMed] [Google Scholar]
- 5.Lambert N, Nelson JL. Microchimerism in autoimmune disease: more questions than answers? Autoimmun Rev. 2003;2:133–9. doi: 10.1016/S1568-9972(02)00149-0. [DOI] [PubMed] [Google Scholar]
- 6.Roy E, Leduc M, Guegan S, Rachdi L, Kluger N, Scharfmann R, Aractingi S, Khosrotehrani K. Specific maternal microchimeric T cells targeting fetal antigens in β cells predispose to auto-immune diabetes in the child. J Autoimmun. 2011;36:253–62. doi: 10.1016/j.jaut.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 7.Molitor-Dart ML, Andrassy J, Kwun J, Kayaoglu HA, Roenneburg DA, Haynes LD, Torrealba JR, Bobadilla JL, Sollinger HW, Knechtle SJ, et al. Developmental exposure to noninherited maternal antigens induces CD4+ T regulatory cells: relevance to mechanism of heart allograft tolerance. J Immunol. 2007;179:6749–61. doi: 10.4049/jimmunol.179.10.6749. [DOI] [PubMed] [Google Scholar]
- 8.Burlingham WJ, Grailer AP, Heisey DM, Claas FH, Norman D, Mohanakumar T, Brennan DC, de Fijter H, van Gelder T, Pirsch JD, et al. The effect of tolerance to noninherited maternal HLA antigens on the survival of renal transplants from sibling donors. N Engl J Med. 1998;339:1657–64. doi: 10.1056/NEJM199812033392302. [DOI] [PubMed] [Google Scholar]
- 9.Matsuoka K, Ichinohe T, Hashimoto D, Asakura S, Tanimoto M, Teshima T. Fetal tolerance to maternal antigens improves the outcome of allogeneic bone marrow transplantation by a CD4+ CD25+ T-cell-dependent mechanism. Blood. 2006;107:404–9. doi: 10.1182/blood-2005-07-3045. [DOI] [PubMed] [Google Scholar]
- 10.van Rood JJ, Loberiza FR, Jr., Zhang MJ, Oudshoorn M, Claas F, Cairo MS, Champlin RE, Gale RP, Ringdén O, Hows JM, et al. Effect of tolerance to noninherited maternal antigens on the occurrence of graft-versus-host disease after bone marrow transplantation from a parent or an HLA-haploidentical sibling. Blood. 2002;99:1572–7. doi: 10.1182/blood.V99.5.1572. [DOI] [PubMed] [Google Scholar]
- 11.Dutta P, Burlingham WJ. Tolerance to noninherited maternal antigens in mice and humans. Curr Opin Organ Transplant. 2009;14:439–47. doi: 10.1097/MOT.0b013e32832d6683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leveque L, Khosrotehrani K. Can maternal microchimeric cells influence the fetal response toward self antigens? Chimerism. 2011;2:71–7. doi: 10.4161/chim.17589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leveque L, Hodgson S, Peyton S, Koyama M, MacDonald KP, Khosrotehrani K. Selective organ specific inflammation in offspring harbouring microchimerism from strongly alloreactive mothers. J Autoimmun. 2014;50:51–8. doi: 10.1016/j.jaut.2013.10.005. [DOI] [PubMed] [Google Scholar]
- 14.Mold JE, Michaëlsson J, Burt TD, Muench MO, Beckerman KP, Busch MP, Lee TH, Nixon DF, McCune JM. Maternal alloantigens promote the development of tolerogenic fetal regulatory T cells in utero. Science. 2008;322:1562–5. doi: 10.1126/science.1164511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kunzmann S, Glogger K, Been JV, Kallapur SG, Nitsos I, Moss TJ, Speer CP, Newnham JP, Jobe AH, Kramer BW. Thymic changes after chorioamnionitis induced by intraamniotic lipopolysaccharide in fetal sheep. Am J Obstet Gynecol. 2010;202:e1–9. doi: 10.1016/j.ajog.2010.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]