Abstract
Cell surface expansion is a necessary part of cell shape change. One long-standing hypothesis proposes that membrane for this expansion comes from the flattening out of cell surface projections such as microvilli and membrane folds. Correlative EM data of cells undergoing phagocytosis, cytokinesis, and morphogenesis has hinted at the existence of such an unfolding mechanism for decades; but unfolding has only recently been confirmed using live-cell imaging and biophysical approaches. Considering the wide range of cells in which plasma membrane unfolding has now been reported, it likely represents a fundamental mechanism of cell shape change.
Keywords: cell shape change, microvilli, cell surface area regulation, plasma membrane tension, actin, morphogenesis, exocytosis, cellularization
Introduction
Cell shape change often requires cell surface expansion. For example, cytokinesis of a simple spherical cell requires a 28% increase in surface area.1 So where does new plasma membrane come from for this expansion? With the advent of the molecular era, biologists started thinking of cell surface remodeling predominantly in terms of endo- and exocytosis.2 However, in some cases perturbations in vesicle trafficking only modestly influence surface expansion, suggesting there may be more to the story.3-5 Decades ago, researchers viewing cell shape change by scanning electron microscopy (SEM) came up with an alternative idea about where new membrane comes from. Those keen observers noted that events of cell surface expansion, like cytokinesis and cell spreading, are accompanied by loss of cell surface projections, including microvilli and folds. They suggested that the projections serve as membrane stores that unfold to fuel cell surface expansion.1,6-11 Building on those prescient SEM snapshots and insights, modern biologists and biophysicists have now validated that plasma membrane unfolding can be a driving mechanism in cell surface remodeling. In this review, we will discuss the evidence supporting plasma membrane unfolding, provide clues as to how unfolding is regulated and balanced with endo- and exocytosis, and discuss how unfolding might occur in the context of intact tissues.
Plasma Membrane Unfolding in Cell Shape Change
Snapshots from SEM hint at an unfolding mechanism
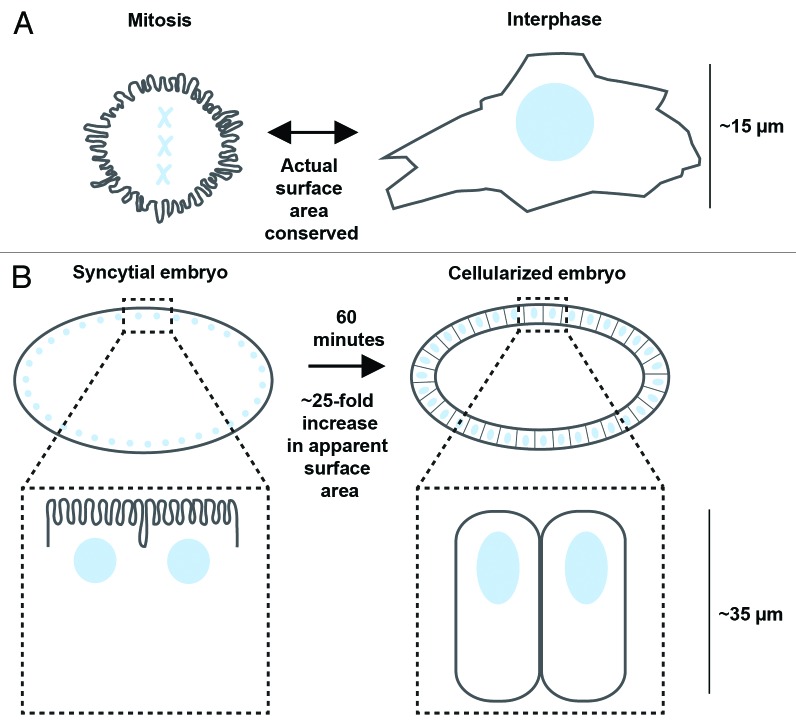
SEM micrographs documenting cell shape change were collected many decades ago, and provided the first correlative support for the idea that microvilli, finger-like membrane projections with F-actin cores, could function as membrane “storage organelles.”1 One major example of this came from cultured cells, which transition from a spherical shape during mitosis to a spread disc shape during interphase. The rounded mitotic cell requires less surface area than its spread interphase counterpart of the same volume; and numerous labs reported that the spherical cells contain a dense coating of microvilli, while spread cells have a smoother surface devoid of microvilli (Fig. 1A).1,6,7,12 Thus, it was suggested that actual surface area may remain relatively constant, but the microvilli fold or unfold to achieve a spherical or spread cell shape, respectively.1,7
Figure 1. Microvilli are lost or gained coincident with cell surface expansion or shrinkage, respectively. Many cultured cells (A) alternate between a spherical shape in mitosis (left), and a flat spread shape in interphase (right). Spherical cells are decorated with dense microvilli, whereas their spread counterparts display a smoother surface, devoid of microvilli. In cellularization (B), the first tissue-building event in the Drosophila embryo, furrows form at the syncytial embryo’s surface (left) and ingress between ~6000 nuclei to generate a single layer of epithelial cells (right). This expands the apparent membrane surface area ~25-fold in one hour. At the beginning of cellularization, the embryo surface is covered in dense microvilli; by the end of cellularization, the microvilli are gone. (A-B: mitotic chromosomes or nuclei are shown in blue)
In this case of mitotic vs. interphase cells, the density of folds and microvilli was shown to be more strongly linked to the physical geometry of the cell than to the stage of the cell cycle. That is, P815Y mastocytoma cells cultured in suspension remain round throughout the cell cycle and maintain their microvilli, with microvillar loss only accompanying plasma membrane expansion during cytokinesis.9 Conversely, adherent PtK2 cells maintain a highly spread morphology until late mitosis, rather than rounding up at early mitosis like other cultured cells. Yet, the increase in microvillar density in PtK2 cells still approximately coincides with the apparent cell surface shrinkage during cell rounding, not mitosis onset.12
SEM data similarly showed a strong correlation between loss of microvilli and expansion of the plasma membrane in other cell shape changes, including mammalian phagocytosis,10 cleavage of Drosophila and human embryos,8,13 mouse blastomere compaction,14 epiboly,15 and cell swelling in response to hypotonic solution or viral infection.11 Thus, the relationship between high microvillar density and reduced apparent cell surface area, and vice versa, was seen to play out in many contexts and cell types, suggesting that plasma membrane unfolding could be a broadly conserved mechanism of cell shape change.
Finally, calculations based on the available SEM data also provided quantitative support for plasma membrane unfolding. In many cases, researchers made careful measurements of the amount of membrane contained in microvilli and the amount of membrane required for cell surface expansion. In spherical mitotic cells, the membrane incorporated into microvilli can account for the difference in apparent surface area between the spherical and spread shapes.1,7 Likewise, in suspended mastocytoma cells, the calculated surface area to volume ratio is conserved throughout cell division, suggesting that “the mechanism of cytokinesis [may be] a physical one, involving the unfolding of previously accumulated microvilli.”9 For phagocytosis, Petty, et al. estimated that, the disappearance of surface folds could account for around 25% of the membrane required to form the phagosome.10 For cellularization, the first tissue-building event in the Drosophila embryo, microvilli could supply >40% of the membrane required to build the ingressing furrows.16 Thus, these calculations show that microvilli contain sufficient membrane to make significant contributions to surface area expansion during a wide variety of cell shape changes.
Kinetics and membrane tracking from live-cell imaging validate the unfolding mechanism
Although SEM observations pointed to the possibility that membrane projections may serve as a membrane reservoir for cell shape change, the need to fix the cells for imaging precluded definitive demonstrations of this mechanism. More recent use of light microscopy has filled this gap by allowing researchers to observe the surface of living cells. As we will review below, live cell imaging has given us a better idea of the time scale and kinetics of the unfolding process, as well as its reversibility. Most importantly, live-cell imaging has allowed us to perform direct tracking to observe the fate of labeled microvillar membrane over time, and so confirm that the unfolding mechanism does happen.
Leading the charge, several cultured cell studies have documented and validated the plasma membrane unfolding mechanism. First, Gauthier and Sheetz labeled the plasma membrane with the lipophilic dye FM1–43 and performed simultaneous differential interference contrast (DIC) and epifluorescence microscopy to track membrane folds during lamellipodial protrusion.17 Using this technique, they observed a loss of fluorescence intensity in folded regions in sync with a corresponding gain of fluorescence intensity in the extending lamellipodia, strongly suggesting that the membrane folds are disassembled to fuel lamellipodial protrusion.17 In another study, using time-lapse confocal imaging of a plasma membrane-GFP probe during phagocytosis, Masters and Gauthier generated 3D renderings of the cell surface. Here, it was observed that membrane folds are lost coincident with cell surface expansion to form the phagocytic cup.18 Likewise, Kapustina, et al. found that periodic bulge-like protrusions formed in CHO cells are driven by compression and expansion of the plasma membrane and underlying F-actin, reminiscent of the bellows of an accordion.19 Two striking features in this latter work are (i) the rapidity of the plasma membrane folding and unfolding events, acting at time-scales incompatible with contributions from endo- and exocytosis, and (ii) the reversibility of the folding mechanism.
A limitation of these cultured cell studies was the inability to selectively label only the microvillar or folded membrane and then watch its trajectory over time. But our lab recently managed this experiment in the intact fly embryo.16 That is, given the unique architecture of Drosophila cellularization, we were able to use a plasma membrane pulse-labeling strategy to demonstrate that microvilli unfold to fuel surface area expansion during cleavage furrow ingression.16 In the Drosophila embryo, the first 13 mitoses occur without intervening cytokinesis. In interphase 14, membrane furrows form at the embryo’s surface and ingress to cleave the embryo into a layer of approximately 6000 epithelial cells, requiring an approximately 25-fold increase in apparent membrane surface area (Fig. 1B).20 Microvilli decorate the surface of the embryo prior to cellularization, but are gone afterwards; and the microvilli have long been proposed to provide the membrane for the ingressing furrows.8,21 Because the microvilli are exposed on the embryo’s surface, we selectively labeled them by applying a pulse of fluorescent wheat germ agglutinin (WGA), which binds glycosylated transmembrane proteins. During the “chase,” we used time-lapse imaging to follow the labeled microvillar membrane, and saw it slide along the cell surface into the forming furrows (Fig. 2). Remarkably, the front of the labeled membrane moved at a rate similar to furrow ingression itself, and membrane translocation from microvilli to ingressing furrows was independent of endocytosis.16 Thus, live cell imaging has now demonstratively shown what the early SEM data alluded to long ago: convolutions in the plasma membrane can unfold to make a direct contribution to cell surface expansion, even in the context of intact embryos/tissues.
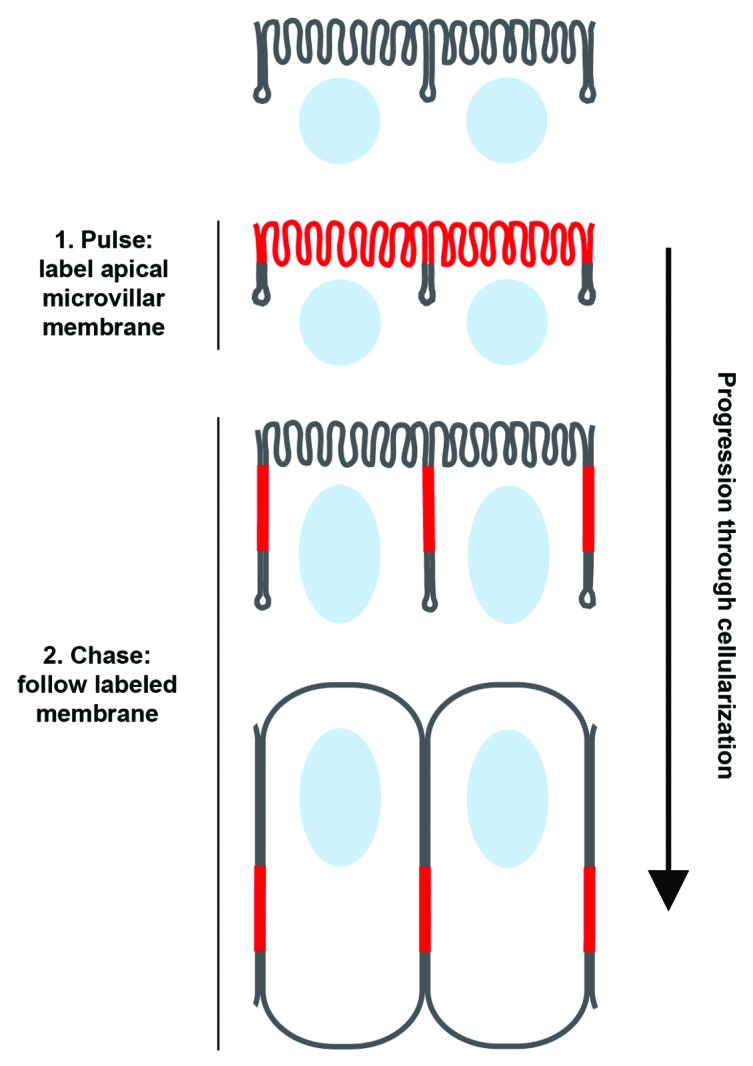
Figure 2. Microvillar membrane unfolds and slides along the embryo surface during Drosophila cellularization. Schematic depicting how red fluorescent wheat germ agglutinin (WGA) was used to pulse-label apical microvilli in cellularizing Drosophila embryos. After the microvillar membrane label was applied, time-lapse imaging during furrow ingression allowed us to follow the membrane as it slid along the plane of the cell surface and into the furrows. (Nuclei are shown in blue; WGA is shown in red).
Tension as a Potential Coordinator and Regulator of Membrane Unfolding
Mechanical properties of the plasma membrane can regulate cell shape and behavior. Specifically, plasma membrane tension has been characterized as a “global” regulator of events at the cell surface.22-26 Plasma membrane tension is a measure of the force required to deform the surface membrane of intact cells, and consists of two major components: the in-plane membrane tension, and membrane-cytoskeletal adhesion energy.22,27 As such, this tension measurement can report on stiffness or slack in the plasma membrane. Thus, plasma membrane tension can provide a read-out of sorts for the amount of membrane stored in reservoirs at the cell surface. Furthermore, because the microvilli and membrane folds that house the surface membrane reservoir are essentially membrane deformations, membrane tension is a likely candidate to regulate the plasma membrane unfolding mechanism.
Plasma membrane tension as a reporter for surface membrane reservoirs
First, we will consider the ability of membrane tension measurements to report on cell surface reservoirs. Precise measurements of plasma membrane tension can be made using optical tweezers.22,26,27 In this technique, a bead is bound to the plasma membrane of a cell and the bead is pulled by the tweezers to form a narrow, membrane-only tether between the cell and bead.26 Plasma membrane tension is proportional to the force needed to hold the bead in the laser trap.22,26 In adherent fibroblasts, membrane tethers can be pulled out to lengths of many microns without the bead being pulled out of the trap.28 That is, a constant force can be applied to pull out a long tether (~5 μm), suggesting that “excess” membrane is liberated from some cell surface reservoir.28 In mouse lung endothelial cells and human myotubes, a similar reservoir was attributed to cell surface pits, called caveolae.29 Membrane tension measurements with optical tweezers demonstrated that tension remains low in caveolae-containing-cells upon hypo-osmotic shock, but rises sharply in cells lacking caveolae.29 Thus, caveolae can serve as a membrane reservoir that unfolds to buffer tension.
However, in the above examples, the caveolae or other unnamed surface reservoir was found to amount to less than 1% of the cell’s surface area.23 While this small reservoir of membrane may be appropriate for buffering minor mechanical or osmotic fluctuations,29 it would contribute very little in the case of regulated cell shape change. But again, biophysical measurements of plasma membrane tension combined with light microscopy, suggest that much larger contributions (>10%) are made by unfolding actin-based cell surface structures like microvilli and folds. For example, consider the plasma membrane expansion that accompanies lamellipodial spreading in fibroblasts17 and particle phagocytosis by macrophage.18 In both of these systems, expansion occurs in two sequential phases. Assaying tension with membrane tethers revealed that tension remains relatively constant during the first phase of plasma membrane expansion; and this expansion is driven by flattening out membrane folds. In both fibroblasts and macrophage, this unfolding of membrane projections expands the surface area by > 20%.17,18 At the end of the first expansion phase, a spike in membrane tension indicates exhaustion of the cell surface reservoir,17,18 which triggers exocytosis and the second phase of expansion (see below).17,18 Therefore, tracking plasma membrane tension has proven an invaluable tool for detecting the presence of both small and large membrane reservoirs at the cell surface.
Plasma membrane tension as a coordinator and regulator of plasma membrane unfolding
Now we will turn to how tension can regulate the formation and retraction of actin-based membrane protrusions and projections, like filopodia, lamellipodia, pseudopodia, microvilli, membrane folds, et cetera. Recall that plasma membrane tension provides a measure of the deformability of the cell surface. Therefore, the formation and maintenance of directed protrusions (e.g., lamellipodia and pseudopodia), as well as membrane-retaining surface projections (e.g., microvilli and folds) requires a force to oppose the membrane tension. In the case of protrusions and projections, a force at odds with membrane tension is F-actin polymerization.24,25,27,30 When F-actin polymerizes, monomers add to the plus ends of filaments. In protrusions and projections, these plus ends are oriented toward and in close proximity to the plasma membrane. According to the Brownian Ratchet Model of F-actin polymerization, this proximity is only large enough to allow a monomer to add when the filament tip and membrane fluctuate away from each other, perhaps by simple Brownian motion (opening a gap of only 1 to a few nm).31,32 With the addition of monomer to the filament end, the membrane must be displaced outward.31,32 However, because high plasma membrane tension resists deformation, high tension opposes filament growth. Essentially, the plasma membrane constrains new addition of actin monomer to the filament, and due to treadmilling, the filament and structure it supports ultimately falls apart.
While many molecular details remain unclear, this well-established mechanical feedback between membrane tension and F-actin polymerization is thought to generate an “inverse relationship” between plasma membrane tension and actin-based protrusions or projections.30 For example, increasing plasma membrane tension by osmotic swelling stops or slows the rate of lamellipodial extension,17,30 while decreasing tension using small doses of detergents and lipid-intercalating dyes increases the rate and likelihood of lamellipodial extension.30 In migrating neutrophils, increasing membrane tension in the trailing edge of the cell by pipette aspiration causes retraction of the pseudopod at the leading edge.24 Conversely, when a laser is used to sever and release the cell body from its extended pseudopod, the cell body generates a new pseudopod. That is, lowering membrane tension in the cell body by releasing the extended tension-generating pseudopod, allows the cell body to make a new protrusion.24
For motile keratocytes, a simple model incorporating the interaction between lamellipodial actin network treadmilling at the leading edge and constant plasma membrane tension around the whole cell periphery recapitulates experimental observations of cell shape and motility behaviors.33 Importantly, this tension-based feedback works over length-scales encompassing the entire cell surface (~50 µm), reinforcing membrane tension’s role as an integrator of events all along the cell surface.22-26,30,33,34 Thus, plasma membrane tension not only controls F-actin polymerization and protrusion, but also integrates spatially distant events along the whole cell surface.
It follows then that plasma membrane tension is also a plausible regulator of unfolding. Like lamellipodia and pseudopodia, the folds and microvilli that comprise large reservoirs of surface membrane are supported by dynamic F-actin.35-38 Presumably this F-actin must be disassembled for unfolding. A tension-based mechanism, similar to that that stalls directed protrusions in motile cells17,18,23,24,30 may then trigger the disassembly of microvilli and surface folds in order to liberate their membrane. In a situation of high membrane tension, monomer addition at the F-actin plus end will be constrained, depolymerization will continue at the minus-end due to treadmilling, and ultimately the filament will shrink and microvillus or fold disassemble.39 Based on tension measurements made in neutrophils during phagocytosis, Herant and Dembo proposed such a tension-based model for unfolding and releasing membrane from cell surface projections.40 They proposed that during phagocytosis tension equilibrates over the whole cell surface so that all surface features experience the same tension. At a given level of tension, the smallest or weakest projections, such as microvilli or folds with sparse or poorly organized F-actin cores, would disassemble first. Meanwhile the pseudopod with its highly organized F-actin, and reinforcing cytoplasmic signals, would continue to extend.40 Only once phagocytosis is completed or the cell surface reservoir of membrane is exhausted, causing plasma membrane tension to spike, would pseudopod progression slow down.18
Of course, there are other possible ways by which unfolding could be regulated. For example, crosslinkers between F-actin and the plasma membrane in the microvilli and folds could be severed, promoting disassembly and unfolding. Or tension may somehow initiate biochemical signaling to alter the actin cytoskeleton (e.g., tension-gated ion channels).27 Many further studies of membrane tension and F-actin dynamics in a range of cell shape changes will be needed to identify the mechanism(s) by which unfolding is regulated.
Coordination Between Membrane Unfolding and Exocytosis
Of course, many studies support a role for vesicle exocytosis in surface area expansion during cell shape change.2,3,18,41 Can both membrane sources, the surface reservoir plus exocytosis from internal stores, contribute to one cell shape change? And if so, how is consumption of these membrane stores coordinated?
Sequential consumption of surface vs. internal membrane stores
Several studies of cell shape change in cultured cells indicate that cells unfold their surface reservoir first, and once that is depleted, membrane tension rises, signaling exocytosis to contribute additional membrane (Fig. 3A).3,17,18,23 This interpretation is founded in a well-supported model in which plasma membrane tension is thought to act as a regulator of cell surface area, promoting exocytosis when membrane tension is high and endocytosis when membrane tension is low.22,23,34,42
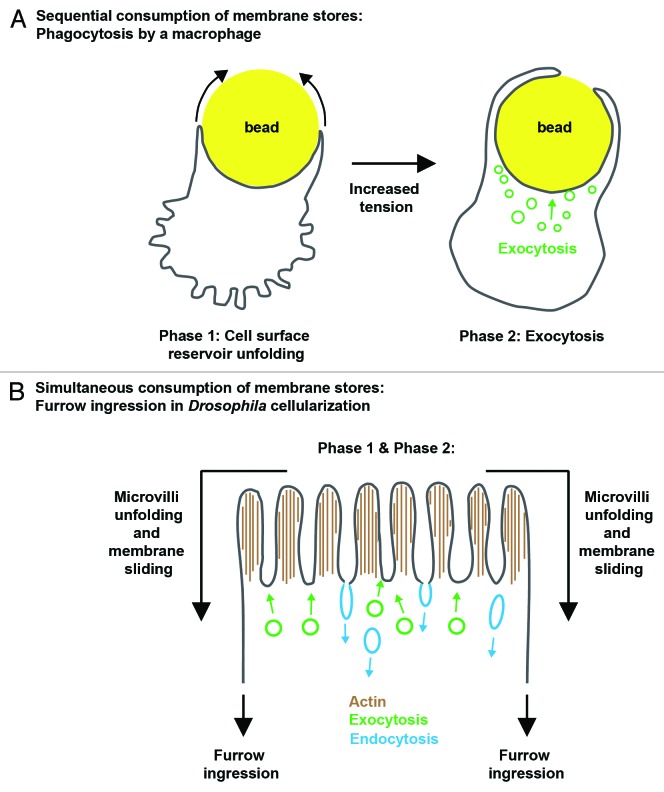
Figure 3. Membrane stores are differentially utilized in different cell types. Macrophage phagocytosis (A) occurs with two phases of membrane expansion. The first phase (left) is fueled by unfolding of membrane projections on the cell surface. After unfolding is complete, plasma membrane tension spikes, signaling the transition to a second phase, which is fueled by exocytosis. Furrow ingression in Drosophila cellularization (B) also occurs in two phases of membrane expansion. Unlike the macrophage, membrane unfolding and exocytosis fuel both phases simultaneously. Any excess membrane that is not utilized by unfolding may be pruned away by endocytosis.
As described above, fibroblast spreading proceeds in a biphasic fashion, and the two phases are fueled by two different membrane sources. In the first phase, lamellipodial extension is constant and membrane comes from unfolding a surface reservoir. Membrane for the later and slower second phase comes from exocytosis.17 The transition to the exocytic phase is triggered by a sudden increase in plasma membrane tension when the surface membrane reservoir runs out.17 Similarly, in macrophage phagocytosis, an early rapid phase of pseudopod extension is driven by unfolding a surface reservoir, whereas the second slower phase is driven by exocytosis.18 Again, the transition between phases is mediated by elevated plasma membrane tension.18 Together, these findings show that for some cell shape changes, surface area expansion is fueled by two separate membrane reservoirs in sequence: first by unfolding the surface reservoir, and then, after a rise in membrane tension, by exocytosis.
Simultaneous consumption of surface vs. internal membrane stores
But is the sequential use of membrane stores the only possibility? During furrow ingression in Drosophila cellularization, both a microvillar reservoir plus exocytosis contribute membrane to fuel the process.4,5,8,16,20,21,43-45 Also, like surface area expansion in other cell shape changes, furrow ingression during cellularization occurs with biphasic kinetics: furrows ingress slowly in the first phase, and rapidly in the second.21 Extrapolating then from cultured cells, it follows that membrane unfolding may fuel the first phase and exocytosis the second phase.46 However, several findings contradict this model. The microvilli are not exhausted during the first phase.8,16,21,47 In fact, we find that the microvilli are depleted throughout all of cellularization.16 Conversely, inferring when exocytosis occurs by displacement of a labeled plasma membrane probe suggests that vesicle addition occurs in both slow and fast phases.16,20 Therefore, it seems that both surface and internal membrane stores are used simultaneously during furrow growth in cellularization, rather than sequentially as in fibroblasts or macrophage. Thus, the coordination and consumption of different membrane stores is likely to be tailored to the specific cell type, context, or cell shape change.
A model for coordinating exocytosis, endocytosis, and unfolding in Drosophila cellularization
But could the situation of simultaneous consumption of membrane reservoirs, as in cellularization, also utilize plasma membrane tension as an integrator and regulator of cell surface expansion? We believe that it could. Building from the ideas that we have introduced in this review, we propose a model that attempts to reconcile the many threads of data concerning membrane trafficking and the use of membrane stores during Drosophila cellularization (Fig. 3B).16 Several studies indicate that vesicle exocytosis primarily takes place at the apical surface of the embryo, where the microvillar reservoir is located.4,5,20,44,45 At the same time, depletion of these microvilli is regulated by furrow ingression.16 Presuming that the F-actin cores in the microvilli are poorly organized, the elevated membrane tension generated by the ingressing furrows may integrate along the cell surface, favoring disassembly of the weakest microvilli and pulling of their membrane directly into the furrows. Thus, the rate of furrow ingression should control the rate of microvillar depletion, which we observe in our kinetic analyses of cellularization.16 Because vesicle exocytosis lowers membrane tension, apical exocytosis would permit membrane to be continuously deformed into microvilli by F-actin polymerization, even as the microvillar membrane store is depleted by furrow ingression. Consistent with this idea, microvilli are highly dynamic43,47 and secretory traffic is required to maintain the apical microvilli.5 Because reduced membrane tension also promotes endocytosis, endocytosis could also prune away excess exocytosed membrane that is not immediately drawn up into microvilli. In fact, apical endocytosis is robust,43,47 and blocking endocytosis with shibirets, a temperature-sensitive allele of Dynamin, causes elongation of the microvilli.47 (Note that blocking endocytosis does not prevent transfer of labeled microvillar membrane to the furrows.)16 Thus, plasma membrane tension may couple seemingly disparate and remote events during cellularization, including microvillar remodeling, furrow ingression, and membrane trafficking. If so, this would speak to an outstanding question of whether plasma membrane tension can integrate cell surface events during morphogenesis.48
Conclusions
The idea that the plasma membrane unfolds to expand cell surface area during cell shape change is hardly new. But the use of modern cell biology techniques has now definitively demonstrated this mechanism and has given us clues into what regulates it. Altogether, the data supporting unfolding has accrued over 40 years and includes numerous types of cell shape changes in many organisms. As such, membrane unfolding likely represents a fundamental mechanism cells use to expand their surface area during cell shape change, and thus has direct implications for development and disease.
Our observation of unfolding during Drosophila cellularization suggests that unfolding occurs even in intact tissues, not just isolated cell types. This raises a whole new set of questions: Could unfolding contribute to examples of morphogenesis beyond cellularization? Certainly, microvilli are remodeled during mouse embryo compaction and epiboly in a way suggesting that unfolding does accompany these morphogenetic events.14,15 Of course, cells within an intact tissue are most certainly subject to different mechanical and molecular signals than isolated cells. For example, intact tissues contain adhesions. Presumably the membrane unfolding mechanism would somehow have to navigate the adhesions. During cellularization, cell-cell adhesions are positioned between the microvilli and the ingressing furrow. How can unfolding and transfer of membrane play out with intervening cell-cell adhesions? Also, what forces within a tissue would generate the plasma membrane tension to trigger unfolding? In the case of the Drosophila embryo, it seems that the force from furrow ingression pulls out the microvillar membrane. In other systems might the force be generated via actomyosin contraction, as in apical constriction or convergent extension? Is plasma membrane tension adequate to regulate unfolding in morphogenetic contexts, or do other developmental or spatial cues facilitate F-actin remodeling and unfolding? It looks like we have only started the journey in appreciating the importance of plasma membrane unfolding; and we will need significant advancements in our understanding of cell and tissue mechanics to learn how unfolding is regulated and integrated with cytoskeletal remodeling and membrane trafficking.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We apologize to authors whose work was omitted due to space constraints. We thank Ido Golding (Baylor College of Medicine) and Herbert Levine (Rice University) for discussion of our model. A.M.S. and L.F. are supported by a Computational and Integrative Biomedical Research Center Seed Award and Curtis Hankamer Basic Research Fund Award (Baylor College of Medicine).
Glossary
Abbreviations:
- SEM
scanning electron microscopy
- DIC
differential interference contrast
- GFP
green fluorescent protein
- WGA
wheat germ agglutinin
- nm
nanometers
- µm
micrometers
- 3D
3-dimensional
- F-actin
filamentous actin
References
- 1.Follett EA, Goldman RD. The occurrence of microvilli during spreading and growth of BHK21-C13 fibroblasts. Exp Cell Res. 1970;59:124–36. doi: 10.1016/0014-4827(70)90631-2. [DOI] [PubMed] [Google Scholar]
- 2.Deschamps C, Echard A, Niedergang F. Phagocytosis and cytokinesis: do cells use common tools to cut and to eat? Highlights on common themes and differences. Traffic. 2013;14:355–64. doi: 10.1111/tra.12045. [DOI] [PubMed] [Google Scholar]
- 3.Groulx N, Boudreault F, Orlov SN, Grygorczyk R. Membrane reserves and hypotonic cell swelling. J Membr Biol. 2006;214:43–56. doi: 10.1007/s00232-006-0080-8. [DOI] [PubMed] [Google Scholar]
- 4.Sisson JC, Field C, Ventura R, Royou A, Sullivan W. Lava lamp, a novel peripheral golgi protein, is required for Drosophila melanogaster cellularization. J Cell Biol. 2000;151:905–18. doi: 10.1083/jcb.151.4.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Papoulas O, Hays TS, Sisson JC. The golgin Lava lamp mediates dynein-based Golgi movements during Drosophila cellularization. Nat Cell Biol. 2005;7:612–8. doi: 10.1038/ncb1264. [DOI] [PubMed] [Google Scholar]
- 6.Porter K, Prescott D, Frye J. Changes in surface morphology of Chinese hamster ovary cells during the cell cycle. J Cell Biol. 1973;57:815–36. doi: 10.1083/jcb.57.3.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Erickson CA, Trinkaus JP. Microvilli and blebs as sources of reserve surface membrane during cell spreading. Exp Cell Res. 1976;99:375–84. doi: 10.1016/0014-4827(76)90595-4. [DOI] [PubMed] [Google Scholar]
- 8.Turner FR, Mahowald AP. Scanning electron microscopy of Drosophila embryogenesis. 1. The structure of the egg envelopes and the formation of the cellular blastoderm. Dev Biol. 1976;50:95–108. doi: 10.1016/0012-1606(76)90070-1. [DOI] [PubMed] [Google Scholar]
- 9.Knutton S, Sumner MC, Pasternak CA. Role of microvilli in surface changes of synchronized P815Y mastocytoma cells. J Cell Biol. 1975;66:568–76. doi: 10.1083/jcb.66.3.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Petty HR, Hafeman DG, McConnell HM. Disappearance of macrophage surface folds after antibody-dependent phagocytosis. J Cell Biol. 1981;89:223–9. doi: 10.1083/jcb.89.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Knutton S, Jackson D, Graham JM, Micklem KJ, Pasternak CA. Microvilli and cell swelling. Nature. 1976;262:52–4. doi: 10.1038/262052a0. [DOI] [PubMed] [Google Scholar]
- 12.Sanger JM, Reingold AM, Sanger JW. Cell surface changes during mitosis and cytokinesis of epithelial cells. Cell Tissue Res. 1984;237:409–17. doi: 10.1007/BF00228425. [DOI] [PubMed] [Google Scholar]
- 13.Nikas G, Ao A, Winston RM, Handyside AH. Compaction and surface polarity in the human embryo in vitro. Biol Reprod. 1996;55:32–7. doi: 10.1095/biolreprod55.1.32. [DOI] [PubMed] [Google Scholar]
- 14.Ducibella T, Ukena T, Karnovsky M, Anderson E. Changes in cell surface and cortical cytoplasmic organization during early embryogenesis in the preimplantation mouse embryo. J Cell Biol. 1977;74:153–67. doi: 10.1083/jcb.74.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Betchaku T, Trinkaus JP. Contact relations, surface activity, and cortical microfilaments of marginal cells of the enveloping layer and of the yolk syncytial and yolk cytoplasmic layers of fundulus before and during epiboly. J Exp Zool. 1978;206:381–426. doi: 10.1002/jez.1402060310. [DOI] [PubMed] [Google Scholar]
- 16.Figard L, Xu H, Garcia HG, Golding I, Sokac AM. The plasma membrane flattens out to fuel cell-surface growth during Drosophila cellularization. Dev Cell. 2013;27:648–55. doi: 10.1016/j.devcel.2013.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gauthier NC, Fardin MA, Roca-Cusachs P, Sheetz MP. Temporary increase in plasma membrane tension coordinates the activation of exocytosis and contraction during cell spreading. Proc Natl Acad Sci U S A. 2011;108:14467–72. doi: 10.1073/pnas.1105845108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Masters TA, Pontes B, Viasnoff V, Li Y, Gauthier NC. Plasma membrane tension orchestrates membrane trafficking, cytoskeletal remodeling, and biochemical signaling during phagocytosis. Proc Natl Acad Sci U S A. 2013;110:11875–80. doi: 10.1073/pnas.1301766110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kapustina M, Elston TC, Jacobson K. Compression and dilation of the membrane-cortex layer generates rapid changes in cell shape. J Cell Biol. 2013;200:95–108. doi: 10.1083/jcb.201204157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lecuit T, Wieschaus E. Polarized insertion of new membrane from a cytoplasmic reservoir during cleavage of the Drosophila embryo. J Cell Biol. 2000;150:849–60. doi: 10.1083/jcb.150.4.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fullilove SL, Jacobson AG. Nuclear elongation and cytokinesis in Drosophila montana. Dev Biol. 1971;26:560–77. doi: 10.1016/0012-1606(71)90141-2. [DOI] [PubMed] [Google Scholar]
- 22.Sheetz MP, Dai J. Modulation of membrane dynamics and cell motility by membrane tension. Trends Cell Biol. 1996;6:85–9. doi: 10.1016/0962-8924(96)80993-7. [DOI] [PubMed] [Google Scholar]
- 23.Gauthier NC, Masters TA, Sheetz MP. Mechanical feedback between membrane tension and dynamics. Trends Cell Biol. 2012;22:527–35. doi: 10.1016/j.tcb.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 24.Houk AR, Jilkine A, Mejean CO, Boltyanskiy R, Dufresne ER, Angenent SB, Altschuler SJ, Wu LF, Weiner OD. Membrane tension maintains cell polarity by confining signals to the leading edge during neutrophil migration. Cell. 2012;148:175–88. doi: 10.1016/j.cell.2011.10.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lieber AD, Yehudai-Resheff S, Barnhart EL, Theriot JA, Keren K. Membrane tension in rapidly moving cells is determined by cytoskeletal forces. Curr Biol. 2013;23:1409–17. doi: 10.1016/j.cub.2013.05.063. [DOI] [PubMed] [Google Scholar]
- 26.Dai J, Sheetz MP. Regulation of endocytosis, exocytosis, and shape by membrane tension. Cold Spring Harb Symp Quant Biol. 1995;60:567–71. doi: 10.1101/SQB.1995.060.01.060. [DOI] [PubMed] [Google Scholar]
- 27.Diz-Muñoz A, Fletcher DA, Weiner OD. Use the force: membrane tension as an organizer of cell shape and motility. Trends Cell Biol. 2013;23:47–53. doi: 10.1016/j.tcb.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Raucher D, Sheetz MP. Characteristics of a membrane reservoir buffering membrane tension. Biophys J. 1999;77:1992–2002. doi: 10.1016/S0006-3495(99)77040-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sinha B, Köster D, Ruez R, Gonnord P, Bastiani M, Abankwa D, Stan RV, Butler-Browne G, Vedie B, Johannes L, et al. Cells respond to mechanical stress by rapid disassembly of caveolae. Cell. 2011;144:402–13. doi: 10.1016/j.cell.2010.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Raucher D, Sheetz MP. Cell spreading and lamellipodial extension rate is regulated by membrane tension. J Cell Biol. 2000;148:127–36. doi: 10.1083/jcb.148.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mogilner A, Oster G. Cell motility driven by actin polymerization. Biophys J. 1996;71:3030–45. doi: 10.1016/S0006-3495(96)79496-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mogilner A, Rubinstein B. The physics of filopodial protrusion. Biophys J. 2005;89:782–95. doi: 10.1529/biophysj.104.056515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Keren K, Pincus Z, Allen GM, Barnhart EL, Marriott G, Mogilner A, Theriot JA. Mechanism of shape determination in motile cells. Nature. 2008;453:475–80. doi: 10.1038/nature06952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dai J, Sheetz MP, Wan X, Morris CE. Membrane tension in swelling and shrinking molluscan neurons. J Neurosci. 1998;18:6681–92. doi: 10.1523/JNEUROSCI.18-17-06681.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mooseker MS, Pollard TD, Wharton KA. Nucleated polymerization of actin from the membrane-associated ends of microvillar filaments in the intestinal brush border. J Cell Biol. 1982;95:223–33. doi: 10.1083/jcb.95.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stidwill RP, Wysolmerski T, Burgess DR. The brush border cytoskeleton is not static: in vivo turnover of proteins. J Cell Biol. 1984;98:641–5. doi: 10.1083/jcb.98.2.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Loomis PA, Zheng L, Sekerková G, Changyaleket B, Mugnaini E, Bartles JR. Espin cross-links cause the elongation of microvillus-type parallel actin bundles in vivo. J Cell Biol. 2003;163:1045–55. doi: 10.1083/jcb.200309093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tyska MJ, Mooseker MS. MYO1A (brush border myosin I) dynamics in the brush border of LLC-PK1-CL4 cells. Biophys J. 2002;82:1869–83. doi: 10.1016/S0006-3495(02)75537-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pollard TD, Berro J. Mathematical models and simulations of cellular processes based on actin filaments. J Biol Chem. 2009;284:5433–7. doi: 10.1074/jbc.R800043200. [DOI] [PubMed] [Google Scholar]
- 40.Herant M, Heinrich V, Dembo M. Mechanics of neutrophil phagocytosis: behavior of the cortical tension. J Cell Sci. 2005;118:1789–97. doi: 10.1242/jcs.02275. [DOI] [PubMed] [Google Scholar]
- 41.Gauthier NC, Rossier OM, Mathur A, Hone JC, Sheetz MP. Plasma membrane area increases with spread area by exocytosis of a GPI-anchored protein compartment. Mol Biol Cell. 2009;20:3261–72. doi: 10.1091/mbc.E09-01-0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Raucher D, Sheetz MP. Membrane expansion increases endocytosis rate during mitosis. J Cell Biol. 1999;144:497–506. doi: 10.1083/jcb.144.3.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pelissier A, Chauvin JP, Lecuit T. Trafficking through Rab11 endosomes is required for cellularization during Drosophila embryogenesis. Curr Biol. 2003;13:1848–57. doi: 10.1016/j.cub.2003.10.023. [DOI] [PubMed] [Google Scholar]
- 44.Murthy M, Teodoro RO, Miller TP, Schwarz TL. Sec5, a member of the exocyst complex, mediates Drosophila embryo cellularization. Development. 2010;137:2773–83. doi: 10.1242/dev.048330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Burgess RW, Deitcher DL, Schwarz TL. The synaptic protein syntaxin1 is required for cellularization of Drosophila embryos. J Cell Biol. 1997;138:861–75. doi: 10.1083/jcb.138.4.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sommi P, Ananthakrishnan R, Cheerambathur DK, Kwon M, Morales-Mulia S, Brust-Mascher I, Mogilner A. A mitotic kinesin-6, Pav-KLP, mediates interdependent cortical reorganization and spindle dynamics in Drosophila embryos. J Cell Sci. 2010;123:1862–72. doi: 10.1242/jcs.064048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fabrowski P, Necakov AS, Mumbauer S, Loeser E, Reversi A, Streichan S, Briggs JAG, De Renzis S. Tubular endocytosis drives remodelling of the apical surface during epithelial morphogenesis in Drosophila. Nat Commun. 2013;4:2244. doi: 10.1038/ncomms3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Paluch E, Heisenberg CP. Biology and physics of cell shape changes in development. Curr Biol. 2009;19:R790–9. doi: 10.1016/j.cub.2009.07.029. [DOI] [PubMed] [Google Scholar]