Abstract
Plasma membrane organization is under the control of cytoskeletal networks and endocytic mechanisms, and a growing literature is showing how closely these influences are interconnected. Here, we review how plasma membranes are formed around individual nuclei of the syncytial Drosophila embryo. Specifically, we outline the pathways that promote and maintain the growth of pseudocleavage and cellularization furrows, as well as specific pathways that keep furrow growth in check. This system has become important for studies of actin regulators, such as Rho1, Diaphanous, non-muscle myosin II and Arp2/3, and endocytic regulators, such as a cytohesin Arf-GEF (Steppke), clathrin, Amphiphysin and dynamin. More generally, it provides a model for understanding how cytoskeletal-endocytic cross-talk regulates the assembly of a cell.
Keywords: cytoskeleton, endocytosis, plasma membrane, actin, Rho1, cytohesin, Steppke, Amphiphysin, Drosophila, cellularization
Introduction
Like the skin of a building, the plasma membrane is essential for the architecture and function of a cell. The plasma membrane, however, is continually regulated, either for its steady-state maintenance or for it to adopt new architectures and functions. Understanding this regulation requires consideration of the greater cell cortex made up of both the plasma membrane and cytoskeletal networks. Endocytosis and exocytosis are critical for controlling the quantity and composition of the plasma membrane. Additionally, several dynamically interconnected cytoskeletal networks coat the inner leaflet of the plasma membrane. Actin networks are the best studied and can be protrusive—pushing the plasma membrane outward to form filopodia or lamellipodia—or contractile—pulling the plasma membrane inward for cell division or apical constriction. Since actin networks cover the cytosolic face of the plasma membrane, interplay with membrane trafficking machinery is also inevitable. In this review, we focus on interplay between the actin cytoskeleton and endocytosis.
Studies of mammalian and yeast cells have revealed two main ways that actin networks can affect endocytosis—they can help or hinder the inward bending of membrane needed for membrane internalization. The effect of actin networks depends on their organization relative to endocytic machinery. Specifically, local assembly of actin networks can help clathrin coats bend plasma membrane domains inward for endocytosis.1,2 Such help appears especially important when cells are under elevated osmotic pressure (an outward pressure on the plasma membrane).3 Local actin networks can help core endocytic machinery overcome such counteracting forces. On the other hand, actin networks spread widely over the plasma membrane can themselves create tension that hinders endocytosis.4,5 For example, an increase in widespread cortical actin inhibits endocytosis during mitotic cell rounding and apparently does so both by increasing membrane tension and by sequestering actin away from endocytic machinery.6 Thus, the effect of actin on endocytosis seems to depend on the combined effects of its various local and global networks organized over the inner face of the plasma membrane.
The effects of endocytosis on actin networks are less clear. It is well-established that the local assembly of actin networks associated with clathrin coats occurs in response to signals for organizing endocytic machinery.1,2 However, effects of endocytic pathways on widespread actin networks were undocumented until the discovery of such regulation by an Arf-GEF during plasma membrane furrow ingression in the syncytial Drosophila embryo.7 Steppke, a member of the cytohesin family of Arf-GEFs, plays a critical role in restraining the membrane cytoskeleton to help orchestrate proper plasma membrane growth in this system. This process involves a complex set of interconnected cytoskeletal and membrane trafficking activities, and thus provides an excellent model for understanding how conserved molecular complexes interact to regulate plasma membrane organization in a developing animal.
Plasma Membrane Furrows of the Syncytial Drosophila Embryo
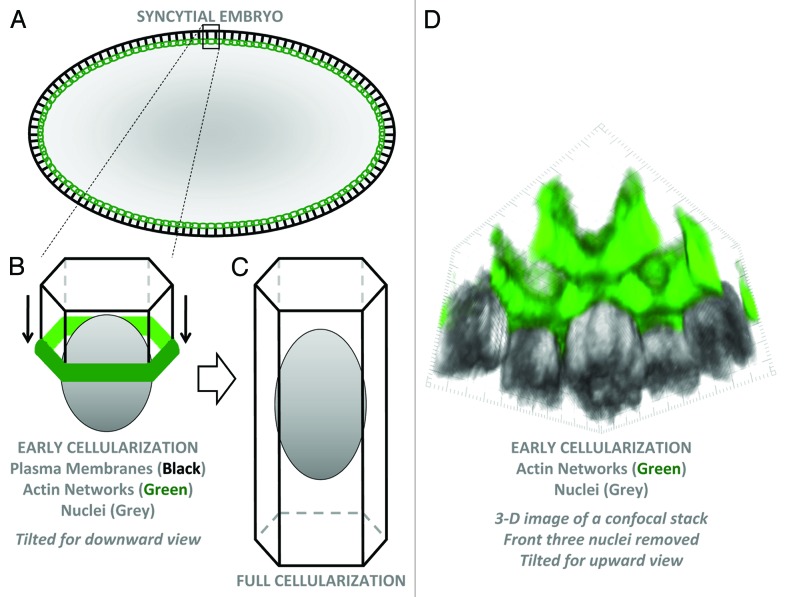
The early Drosophila embryo provides an attractive system to study the assembly, remodelling and maintenance of plasma membranes.8-12 Before gastrulation, the embryo develops as a syncytium. The diploid nucleus produced from fertilization undergoes 13 rounds of highly synchronous nuclear division, without cytokinesis. The first nine rounds occur at the center of the embryo and are thus detached from the plasma membrane covering the embryo surface. Most nuclei then migrate to the embryo periphery and divide four more times just below the plasma membrane. These divisions are closely linked to dramatic plasma membrane changes—as the nuclei enter mitosis plasma membrane furrows (pseudocleavage furrows) synchronously ingress, engage and separate mitotic spindles, and then regress upon interphase re-entry. Following the final round of nuclear division, plasma membrane furrows form and separate each nucleus again, but now ingress deeply into the embryo to encapsulate ~6000 nuclei into discrete columnar cells (Fig. 1). The completion of cellularization involves a unique form of cell division during which the base of each cell constricts to synchronously separate ~6000 blastoderm cells from a single large yolk cell at the center of the embryo. The assembly of pseudocleavage and cellularization furrows occurs with great speed—in ~10 min and ~60 min, respectively. The disassembly of pseudocleavage furrows is equally dramatic and implicates a carefully regulated steady-state system. The scale of the plasma growth is also incredible—with a 25-fold expansion of the cell cortex over cellularization.
Figure 1. Plasma membrane organization of the syncytial Drosophila embryo. (A) Schematic cross-section of an early cellularization embryo. Black lines are plasma membranes. Green circles show actin networks forming a supracellular 3-D oval connecting all furrow tips around the embryo. (B) A 3-D schematic of a single hexagonal cell compartment containing a peripheral nucleus at early cellularization. Plasma membranes are invaginating from the surface with actin at the basal furrow tips. (C) A fully-formed cell. (D) 3-D imaging of F-actin (phalloidin staining) and nuclei (Nucleoporin 50-staining) of an early cellularization embryo using published materials and methods.7 The image is tilted for a view from the embryo interior toward the peripheral nuclei. The three front nuclei were deleted to allow a clear view of actin-coated plasma membranes and furrow tips.
Supplying Furrows with Plasma Membrane Material
Cellularization requires membrane addition to ingressing furrows. The biosynthetic system provides this material. Each forming cell contains a nucleus and a pair of apical centrosomes close to the embryo surface. The centrosomes assemble an inverted basket of microtubules that extend basally around the nucleus and organize a secretory membrane system for each cell compartment.13 Thus, each cell compartment has its own nucleus, microtubule network and secretory system, but lateral and basal plasma membranes have not yet formed (Fig. 2A).
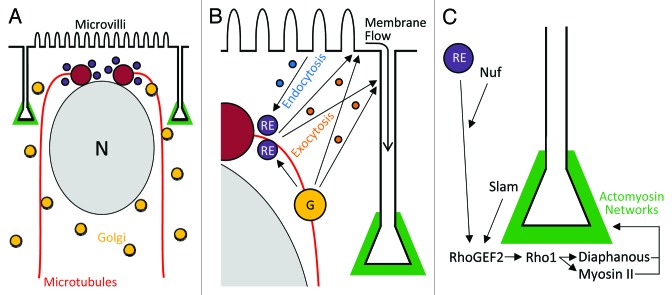
Figure 2. Mechanisms for plasma membrane furrow growth and maintenance. (A) A cross-sectional view of a single hexagonal cell compartment containing a peripheral nucleus at early cellularization showing two centrosomes above the nucleus, an inverted basket of microtubules around the nucleus, and Golgi vesicles associated with the microtubule network. (B) A close view of the apicolateral corner of the compartment showing membrane trafficking routes fuelling membrane growth: Golgi (G)-to-plasma membrane via exocytosis; Golgi-to-recycling endosome (RE)-to-plasma membrane via exocytosis; endocytosis from the apical plasma membrane and transcytosis to lateral plasma membranes via the recycling endosome; and basal flow of exocytosed and microvillar membrane. (C) A close view of the basal furrow tip showing recruitment of RhoGEF2 and downstream activation of the Rho1-Diaphanous/non-muscle myosin II pathway for forming basal actomyosin networks that maintain and coordinate furrow growth, and ultimately close off the base of the cell by driving cytokinesis.
Specific membrane trafficking pathways coordinate plasma membrane growth (Fig. 2B). The Golgi apparatus is fragmented in this system with Golgi vesicles found throughout the cytosol. These vesicles, however, are organized by the microtubule networks and undergo dynein-dependent transport to the centrosomes above the nucleus and in proximity to the plasma membrane, which is initially a flat sheet covering the embryo surface.12,14 From here, the material is directed to the plasma membrane by the exocyst complex and SNAREs,15,16 and at least in part, this traffic occurs via peri-centrosomal recycling endosomes and Rab11 activity.17 Once at the plasma membrane, material can follow one of two main routes. One route is a simple flow of membrane from the embryo surface basally around each nucleus and microtubule basket to form lateral plasma membranes.12 In fact, there is an additional supply of membrane over the embryo surface, initially sequestered in microvilli, that flows to lateral membranes as well.18 Another route is a transcytosis pathway in which membrane is endocytosed from the apical domain through the actions of clathrin, dynamin and Rab5, trafficked through the endomembrane system, and redeployed to augment the growth of lateral plasma membranes.7,17 Presumably cargo from the biosynthetic pathway would also join lateral supply routes as furrows elongate, although such trafficking appears to be polarized apically.12
One major question is how the sites for lateral plasma membrane formation are determined. Studies of conventional cell division have implicated sites of overlap between microtubule arrays in designating cleavage furrow position.19 Since each cell compartment in the early Drosophila embryo contains its own microtubule array, regions of overlap between these arrays could similarly specify sites of pseudocleavage and cellularization furrow formation. Indeed, a recent paper has implicated this mechanism.20 Additionally, the Drosophila-specific (FlyBase) protein Slow as Molasses (Slam) localizes to initial sites of plasma membrane ingression and is critical for ingression initiation.21
Coordinating Furrow Tips with Large-Scale Actomyosin Networks
As the furrows ingress, they are stabilized at their basal tips by cytoskeletal networks that integrate dynamically to form a supracellular 3-D oval connecting all furrow tips of the embryo. Actin networks are formed downstream of both a SCAR-Arp2/3 pathway22,23 and a RhoGEF2-Rho1-Diaphanous/non-muscle myosin II pathway.24-27 Additionally, they are supported by spectrin networks,28 septin and anillin networks,29,30 as well as Src64 and Tec29 tyrosine kinase activities,31 and a number of Drosophila-specific proteins.9 Disrupting these players leads to furrow de-stabilization and loss, or to failures in the final network contractility needed to close off the base of each cell at the end of cellularization.
The Rho1 pathway is the best understood regulator of the furrow actin networks (Fig. 2C). Intriguingly, it is linked to the endomembrane system. Nuclear fallout (Nuf) is an arfophilin that functions with recycling endosomes to target RhoGEF2 to ingressing plasma membrane furrows.32 Further interactions with Slam help recruit RhoGEF2 to the furrows.33 Once recruited, RhoGEF2 would activate Rho1 and as a result local Diaphanous-induced actin polymerization and non-muscle myosin II activation would occur.24-27,32 During cellularization, the Drosophila-specific (FlyBase) protein Nullo modifies the networks and is required for forming distinctive, inverted funnel-shaped furrow canals at the basal tips of the furrows.34,35 Once the lateral membranes reach their full-length, the basal actomyosin networks constrict as contractile rings to form basal plasma membrane as they separate each cell from the yolk cell beneath.11
Keeping Furrow Tips in Check
Thus far, we have discussed mechanisms driving and maintaining furrow growth. However, this plasma membrane growth is tightly regulated. The need for regulation is evident from the rapid cycling between furrow ingression and regression during the peripheral nuclear division cycles. Additionally, mutant analyses have revealed three distinct mechanisms that prevent furrow overgrowth. The first two, an Amphiphysin-based mechanism and an Arf-GEF-induced mechanism, both involve endocytic events at the base of pseudocleavage and early cellularization furrows. The third mechanism is specific for mid-late cellularization, and involves the Drosophila-specific (FlyBase) protein Bottleneck.
Endocytic events were identified at or near the base of pseudocleavage and early cellularization furrows through the localization of endocytic machinery (clathrin adaptor and coat proteins and dynamin), the live imaging of plasma membrane internalization events, and the detection of Amphiphysin-positive tubules at the base of the plasma membrane furrows36 (Amphiphysin is a conserved BAR-domain containing protein that binds curved endocytic membranes in many systems37). The extended Amphiphysin-positive tubules are prominent features at these stages, but resemble tubular structures often associated with delayed or defective endocytosis (e.g., refs. 38 and 39). Indeed, reducing dynamin activity increases their numbers, suggesting that scission failures lead to extended endocytic tubules.36 Interestingly, increasing endocytic activity through overexpression of the Arf-GEF Steppke can induce tubule formation,7 suggesting that high demands on a limited supply of endocytic machinery can also lead to endocytic delays and the tubules. Additionally, weakening actin increases tubule numbers, possibly because of defects in individual actin-dependent endocytic events,36 or because of a general weakening of the actin cortex that leads to greater endocytic activity and thus a greater demand on limited supplies of endocytic machinery.40 The fact that Amphiphysin-positive tubules form during normal syncytial development, and that they change their numbers with the cell cycle,36 suggests the overall demand for endocytic machinery (i.e., the sum of signals recruiting the machinery) can become very high in the natural system.
Even with a model of Amphiphysin-positive tubules forming as a by-product of elevated endocytic activity, they do appear to regulate plasma membrane growth.41 Using septins as separate markers for the tubules, it was found that the tubules are absent in null amphiphysin mutants. Quantification of furrow ingression rates in these mutants revealed a faster rate of plasma membrane growth toward the embryo center. These data led to a model in which the tubules help sequester membrane material to slow furrow ingression—perhaps acting as reservoirs to buffer membrane addition to apical and lateral plasma membrane domains41 (Fig. 3A). One can imagine two buffering mechanisms in the normal embryo: (1) effects of individual endocytic events until machinery becomes limiting, and then (2) effects of the Amphiphysin-positive tubules. In the absence of Amphiphysin, the membrane material presumably remains part of the plasma membranes, which thus grow faster. However, the developmental significance of this regulatory mechanism is unclear as amphiphysin mutants complete cellularization and become viable and fertile adults.42
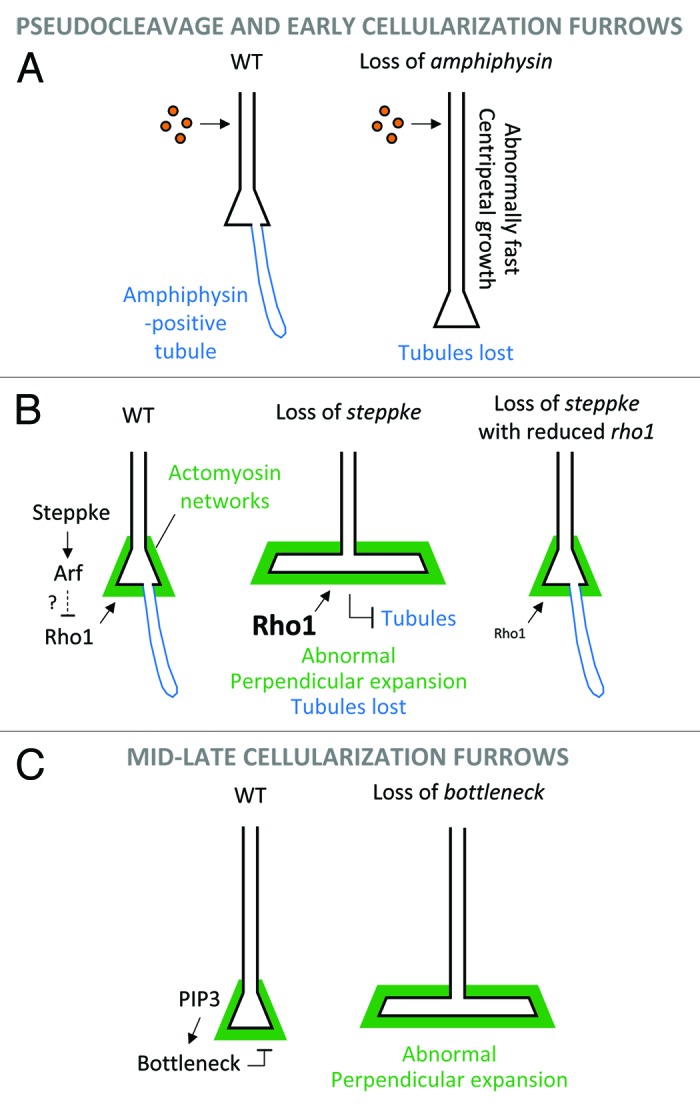
Figure 3. Regulatory mechanisms keeping furrow growth in check. (A) Reduction of centripetal plasma membrane growth by Amphiphysin-dependent endocytic tubules that seem to act as basal reservoirs to buffer apicolateral membrane addition. (B) Inhibition of the Rho1-Diaphanous/non-muscle myosin II pathway by the Arf-GEF Steppke to prevent premature perpendicular expansion of basal furrow tips during peripheral nuclear division cycles and early cellularization. (C) Restraint of actomyosin rings by a PIP3-Bottleneck pathway to prevent premature perpendicular expansion of basal furrow tips during mid-late cellularization.
A separate endocytic mechanism regulates plasma membrane growth, but in a distinct way (Fig. 3B). This mechanism is controlled by Steppke, the sole Drosophila member of the cytohesin family of Arf-GEFs. Cytohesins are conserved across animals and activate Arf G proteins and downstream endocytosis.43,44 Steppke has been shown to regulate insulin and EGF signaling during post-embryonic Drosophila stages,45,46 and its depletion from early embryos has a striking effect on pseudocleavage and early cellularization furrows.7 Specifically, the furrow tips extend perpendicularly into taut sheets of membrane that invade spaces occupied by nuclei which are displaced upwards or out of the forming cells. The expanded membrane is coated with actin, non-muscle myosin II, the septin Peanut, and Anillin. The presence of these cytoskeletal networks together with the taut structure of the expansions suggested that they might be driven by abnormal cytoskeletal activity. Indeed, suppression experiments indicated that the steppke phenotype is due to elevated activity of the Rho1-Diaphanous/non-muscle myosin II pathway. Imaging of Rho1 showed that Steppke somehow reduces Rho1 protein levels near the base of furrows, but no changes to RhoGEF2 were observed, suggesting that Steppke antagonizes a distinct Rho1 localization mechanism. A GFP-Steppke construct rescued the overall steppke furrow phenotype and was enriched toward the base of the furrows, suggesting Steppke acts locally to restrain the perpendicular growth of furrow tips. A GEF-inactive form of Steppke failed to rescue the phenotype, indicating that Steppke restrains plasma membrane growth by activating Arf G proteins and their downstream endocytic pathways.
Several arguments support a model in which Steppke promotes a specific subset of endocytic events occurring in the cell compartments.7 First, the ability of Steppke overexpression to induce Amphiphysin-positive tubules argues that Steppke-Arf signaling is one of multiple competitors for endocytic machinery. Second, Amphiphysin-positive tubules can still occur with Steppke loss, as can individual internalizations of labeled plasma membrane. Third, Steppke can recruit the clathrin adaptor AP-2α, but is not required for the protein to localize to furrows. Fourth, the plasma membrane mis-regulation that occurs with Steppke loss is different from that which occurs with Amphiphysin loss (faster centripetal membrane growth), or with loss of clathrin adaptors or dynamin (impaired transcytosis and reduced membrane growth). Thus, it appears that Steppke-Arf G protein signaling is one inducer of clathrin recruitment, but that others exist as well. How various endocytic pathways are coordinated remains a major outstanding question in this system and others.
A third, apparently distinct mechanism for restraining plasma membrane furrows is based upon the protein Bottleneck (Fig. 3C). Similar to Steppke, Bottleneck is important for preventing the premature perpendicular expansion of the basal tips of plasma membrane furrows.47 In contrast to the Steppke-Arf pathway, the Bottleneck pathway has no effect on pseudocleavage furrows or early cellularization furrows, but specifically prevents the premature contraction of basal actomyosin rings during mid-late cellularization. Since Bottleneck has no recognizable domains, its exact mechanism of action is unclear, but recent work has documented its ability to bind and modify the structure of actin bundles.48
With these three regulatory mechanisms identified, it is important to investigate their upstream control. One can consider mechanisms for individual protein activation and localization. For Amphiphysin, the protein might be recruited to nascent endocytic tubules through the affinity of its BAR domain for curved membranes,37 and this domain is indeed required for forming the extended tubules at furrow tips.41 For Steppke-related cytohesins, plasma membrane recruitment through PH domain binding to PIP3 or Arf-GTP is evident in mammalian cells.44 Additionally, counteraction of Steppke Arf-GEF activity by Arf-GAP activity should be considered. For Bottleneck, PIP3 has recently been shown to stabilize its furrow association.48 Interestingly, manipulations of PIP3 only affected cellularization furrow structure, consistent with alterations to Bottleneck activity, but had no effect on pseudocleavage furrow structure,48 arguing that PIP3 is not essential for the Steppke-Arf pathway to act in this system.
A Complexity of Feedback Mechanisms at Furrow Tips
In the Introduction, we described the positive role of local actin networks on endocytosis and the negative impact of global actin networks on endocytosis. Both effects are also apparent at plasma membrane furrows of the early Drosophila embryo. Thus, this system offers an appealing model to study conserved molecular mechanisms controlling bi-directional cross-talk between endocytosis and the cytoskeleton. However, dissecting such cross-talk is challenging.
As discussed above, loss of Steppke leads to a loss of Amphiphysin-positive tubule numbers, but this effect appears to be indirect. The loss of Steppke elevates Rho1-Diaphanous/non-muscle myosin II pathway activity which then inhibits overall endocytosis at furrow tips. With this cytoskeletal-endocytic cross-talk in place, it is difficult to identify the specific endocytic events that require Steppke. Nonetheless, genetic approaches can be used to remove Steppke plus the resulting downstream cytoskeletal over-activity to allow analyses of Steppke’s more direct effects.7
Reduced Arp2/3 activity also lowers Amphiphysin-positive tubule numbers during cellularization, consistent with local Arp2/3 networks promoting endocytosis in other systems. Thus, Arp2/3-dependent actin networks may directly contribute to membrane bending and invagination.40 Alternatively, cytoskeletal cross-talk should also be considered. For example, competition among actin nucleators for a limited supply of network components has recently been implicated as one mechanism of cross-talk between actin networks.49 Thus, loss of Arp2/3 might increase the pool of available network components and thereby increase the bulk of competing actomyosin networks that inhibit endocytosis.
In contrast to Arp2/3, Diaphanous seems to assemble actin networks that antagonize the Amphiphysin-positive tubules. Specifically, loss of Diaphanous alone increased Amphiphysin-positive tubule numbers.40 Additionally, the absence of Amphiphysin-positive tubules with the loss of Steppke is due to abnormally increased activity of the Rho1-Diaphanous/non-muscle myosin II pathway.7 These results are consistent with long-range actomyosin networks antagonizing the local bending of the plasma membrane needed for endocytosis, but other molecular cross-talk is also possible.
Final Comments
We have reviewed the amazing interplay between membrane trafficking pathways and cytoskeletal networks needed for plasma membrane growth in the early Drosophila embryo. In particular, we have focused on endocytic and cytoskeletal activities at the basal tips of growing furrows. Studies of this system have highlighted a number of possible complexities: multiple endocytic mechanisms competing for supplies, multiple actin networks competing for supplies, impacts of specific actin networks on endocytosis, impacts of specific endocytic pathways on the membrane cytoskeleton, etc. Dissection of these complexities will be aided by the identification of specific regulators. For example, Steppke-induced endocytic events appear to antagonize the membrane cytoskeleton,7 and the F-BAR domain-containing protein CIP4 can regulate choices between Arp2/3- and Diaphanous-dependent actin networks in proximity to endocytic sites.40 Defining such pathways will provide a systematic understanding of this model of endocytic-cytoskeletal coordination.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank Francisco Rodrigues for comments on the manuscript. D.M.L. was supported by an Ontario Graduate Scholarship and T.J.C.H. holds a Tier 2 Canada Research Chair. Our work in this area is supported by a Canadian Institutes of Health Research operating grant.
References
- 1.Mooren OL, Galletta BJ, Cooper JA. Roles for actin assembly in endocytosis. Annu Rev Biochem. 2012;81:661–86. doi: 10.1146/annurev-biochem-060910-094416. [DOI] [PubMed] [Google Scholar]
- 2.Anitei M, Hoflack B. Bridging membrane and cytoskeleton dynamics in the secretory and endocytic pathways. Nat Cell Biol. 2012;14:11–9. doi: 10.1038/ncb2409. [DOI] [PubMed] [Google Scholar]
- 3.Aghamohammadzadeh S, Ayscough KR. Differential requirements for actin during yeast and mammalian endocytosis. Nat Cell Biol. 2009;11:1039–42. doi: 10.1038/ncb1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gauthier NC, Masters TA, Sheetz MP. Mechanical feedback between membrane tension and dynamics. Trends Cell Biol. 2012;22:527–35. doi: 10.1016/j.tcb.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 5.Boulant S, Kural C, Zeeh JC, Ubelmann F, Kirchhausen T. Actin dynamics counteract membrane tension during clathrin-mediated endocytosis. Nat Cell Biol. 2011;13:1124–31. doi: 10.1038/ncb2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaur S, Fielding AB, Gassner G, Carter NJ, Royle SJ. An unmet actin requirement explains the mitotic inhibition of clathrin-mediated endocytosis. Elife. 2014;3:e00829. doi: 10.7554/eLife.00829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee DM, Harris TJ. An Arf-GEF regulates antagonism between endocytosis and the cytoskeleton for Drosophila blastoderm development. Curr Biol. 2013;23:2110–20. doi: 10.1016/j.cub.2013.08.058. [DOI] [PubMed] [Google Scholar]
- 8.Lecuit T. Junctions and vesicular trafficking during Drosophila cellularization. J Cell Sci. 2004;117:3427–33. doi: 10.1242/jcs.01312. [DOI] [PubMed] [Google Scholar]
- 9.Mazumdar A, Mazumdar M. How one becomes many: blastoderm cellularization in Drosophila melanogaster. Bioessays. 2002;24:1012–22. doi: 10.1002/bies.10184. [DOI] [PubMed] [Google Scholar]
- 10.Harris TJ, Sawyer JK, Peifer M. How the cytoskeleton helps build the embryonic body plan: models of morphogenesis from Drosophila. Curr Top Dev Biol. 2009;89:55–85. doi: 10.1016/S0070-2153(09)89003-0. [DOI] [PubMed] [Google Scholar]
- 11.Foe VE, Alberts BM. Studies of nuclear and cytoplasmic behaviour during the five mitotic cycles that precede gastrulation in Drosophila embryogenesis. J Cell Sci. 1983;61:31–70. doi: 10.1242/jcs.61.1.31. [DOI] [PubMed] [Google Scholar]
- 12.Lecuit T, Wieschaus E. Polarized insertion of new membrane from a cytoplasmic reservoir during cleavage of the Drosophila embryo. J Cell Biol. 2000;150:849–60. doi: 10.1083/jcb.150.4.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frescas D, Mavrakis M, Lorenz H, Delotto R, Lippincott-Schwartz J. The secretory membrane system in the Drosophila syncytial blastoderm embryo exists as functionally compartmentalized units around individual nuclei. J Cell Biol. 2006;173:219–30. doi: 10.1083/jcb.200601156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Papoulas O, Hays TS, Sisson JC. The golgin Lava lamp mediates dynein-based Golgi movements during Drosophila cellularization. Nat Cell Biol. 2005;7:612–8. doi: 10.1038/ncb1264. [DOI] [PubMed] [Google Scholar]
- 15.Murthy M, Teodoro RO, Miller TP, Schwarz TL. Sec5, a member of the exocyst complex, mediates Drosophila embryo cellularization. Development. 2010;137:2773–83. doi: 10.1242/dev.048330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burgess RW, Deitcher DL, Schwarz TL. The synaptic protein syntaxin1 is required for cellularization of Drosophila embryos. J Cell Biol. 1997;138:861–75. doi: 10.1083/jcb.138.4.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pelissier A, Chauvin JP, Lecuit T. Trafficking through Rab11 endosomes is required for cellularization during Drosophila embryogenesis. Curr Biol. 2003;13:1848–57. doi: 10.1016/j.cub.2003.10.023. [DOI] [PubMed] [Google Scholar]
- 18.Figard L, Xu H, Garcia HG, Golding I, Sokac AM. The plasma membrane flattens out to fuel cell-surface growth during Drosophila cellularization. Dev Cell. 2013;27:648–55. doi: 10.1016/j.devcel.2013.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.von Dassow G. Concurrent cues for cytokinetic furrow induction in animal cells. Trends Cell Biol. 2009;19:165–73. doi: 10.1016/j.tcb.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 20.Crest J, Concha-Moore K, Sullivan W. RhoGEF and positioning of rappaport-like furrows in the early Drosophila embryo. Curr Biol. 2012;22:2037–41. doi: 10.1016/j.cub.2012.08.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Acharya S, Laupsien P, Wenzl C, Yan S, Großhans J. Function and dynamics of slam in furrow formation in early Drosophila embryo. Dev Biol. 2014;386:371–84. doi: 10.1016/j.ydbio.2013.12.022. [DOI] [PubMed] [Google Scholar]
- 22.Zallen JA, Cohen Y, Hudson AM, Cooley L, Wieschaus E, Schejter ED. SCAR is a primary regulator of Arp2/3-dependent morphological events in Drosophila. J Cell Biol. 2002;156:689–701. doi: 10.1083/jcb.200109057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stevenson V, Hudson A, Cooley L, Theurkauf WE. Arp2/3-dependent pseudocleavage [correction of psuedocleavage] furrow assembly in syncytial Drosophila embryos. Curr Biol. 2002;12:705–11. doi: 10.1016/S0960-9822(02)00807-2. [DOI] [PubMed] [Google Scholar]
- 24.Padash Barmchi M, Rogers S, Häcker U. DRhoGEF2 regulates actin organization and contractility in the Drosophila blastoderm embryo. J Cell Biol. 2005;168:575–85. doi: 10.1083/jcb.200407124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grosshans J, Wenzl C, Herz HM, Bartoszewski S, Schnorrer F, Vogt N, Schwarz H, Müller HA. RhoGEF2 and the formin Dia control the formation of the furrow canal by directed actin assembly during Drosophila cellularisation. Development. 2005;132:1009–20. doi: 10.1242/dev.01669. [DOI] [PubMed] [Google Scholar]
- 26.Afshar K, Stuart B, Wasserman SA. Functional analysis of the Drosophila diaphanous FH protein in early embryonic development. Development. 2000;127:1887–97. doi: 10.1242/dev.127.9.1887. [DOI] [PubMed] [Google Scholar]
- 27.Royou A, Sullivan W, Karess R. Cortical recruitment of nonmuscle myosin II in early syncytial Drosophila embryos: its role in nuclear axial expansion and its regulation by Cdc2 activity. J Cell Biol. 2002;158:127–37. doi: 10.1083/jcb.200203148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zaccai M, Lipshitz HD. Role of Adducin-like (hu-li tai shao) mRNA and protein localization in regulating cytoskeletal structure and function during Drosophila Oogenesis and early embryogenesis. Dev Genet. 1996;19:249–57. doi: 10.1002/(SICI)1520-6408(1996)19:3<249::AID-DVG8>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 29.Field CM, Coughlin M, Doberstein S, Marty T, Sullivan W. Characterization of anillin mutants reveals essential roles in septin localization and plasma membrane integrity. Development. 2005;132:2849–60. doi: 10.1242/dev.01843. [DOI] [PubMed] [Google Scholar]
- 30.Adam JC, Pringle JR, Peifer M. Evidence for functional differentiation among Drosophila septins in cytokinesis and cellularization. Mol Biol Cell. 2000;11:3123–35. doi: 10.1091/mbc.11.9.3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thomas JH, Wieschaus E. src64 and tec29 are required for microfilament contraction during Drosophila cellularization. Development. 2004;131:863–71. doi: 10.1242/dev.00989. [DOI] [PubMed] [Google Scholar]
- 32.Cao J, Albertson R, Riggs B, Field CM, Sullivan W. Nuf, a Rab11 effector, maintains cytokinetic furrow integrity by promoting local actin polymerization. J Cell Biol. 2008;182:301–13. doi: 10.1083/jcb.200712036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wenzl C, Yan S, Laupsien P, Grosshans J. Localization of RhoGEF2 during Drosophila cellularization is developmentally controlled by Slam. Mech Dev. 2010;127:371–84. doi: 10.1016/j.mod.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 34.Simpson L, Wieschaus E. Zygotic activity of the nullo locus is required to stabilize the actin-myosin network during cellularization in Drosophila. Development. 1990;110:851–63. doi: 10.1242/dev.110.3.851. [DOI] [PubMed] [Google Scholar]
- 35.Sokac AM, Wieschaus E. Zygotically controlled F-actin establishes cortical compartments to stabilize furrows during Drosophila cellularization. J Cell Sci. 2008;121:1815–24. doi: 10.1242/jcs.025171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sokac AM, Wieschaus E. Local actin-dependent endocytosis is zygotically controlled to initiate Drosophila cellularization. Dev Cell. 2008;14:775–86. doi: 10.1016/j.devcel.2008.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Daumke O, Roux A, Haucke V. BAR domain scaffolds in dynamin-mediated membrane fission. Cell. 2014;156:882–92. doi: 10.1016/j.cell.2014.02.017. [DOI] [PubMed] [Google Scholar]
- 38.Georgiou M, Marinari E, Burden J, Baum B. Cdc42, Par6, and aPKC regulate Arp2/3-mediated endocytosis to control local adherens junction stability. Curr Biol. 2008;18:1631–8. doi: 10.1016/j.cub.2008.09.029. [DOI] [PubMed] [Google Scholar]
- 39.Leibfried A, Fricke R, Morgan MJ, Bogdan S, Bellaiche Y. Drosophila Cip4 and WASp define a branch of the Cdc42-Par6-aPKC pathway regulating E-cadherin endocytosis. Curr Biol. 2008;18:1639–48. doi: 10.1016/j.cub.2008.09.063. [DOI] [PubMed] [Google Scholar]
- 40.Yan S, Lv Z, Winterhoff M, Wenzl C, Zobel T, Faix J, Bogdan S, Grosshans J. The F-BAR protein Cip4/Toca-1 antagonizes the formin Diaphanous in membrane stabilization and compartmentalization. J Cell Sci. 2013;126:1796–805. doi: 10.1242/jcs.118422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Su J, Chow B, Boulianne GL, Wilde A. The BAR domain of amphiphysin is required for cleavage furrow tip-tubule formation during cellularization in Drosophila embryos. Mol Biol Cell. 2013;24:1444–53. doi: 10.1091/mbc.E12-12-0878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Leventis PA, Chow BM, Stewart BA, Iyengar B, Campos AR, Boulianne GL. Drosophila Amphiphysin is a post-synaptic protein required for normal locomotion but not endocytosis. Traffic. 2001;2:839–50. doi: 10.1034/j.1600-0854.2001.21113.x. [DOI] [PubMed] [Google Scholar]
- 43.Gillingham AK, Munro S. The small G proteins of the Arf family and their regulators. Annu Rev Cell Dev Biol. 2007;23:579–611. doi: 10.1146/annurev.cellbio.23.090506.123209. [DOI] [PubMed] [Google Scholar]
- 44.Donaldson JG, Jackson CL. ARF family G proteins and their regulators: roles in membrane transport, development and disease. Nat Rev Mol Cell Biol. 2011;12:362–75. doi: 10.1038/nrm3117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fuss B, Becker T, Zinke I, Hoch M. The cytohesin Steppke is essential for insulin signalling in Drosophila. Nature. 2006;444:945–8. doi: 10.1038/nature05412. [DOI] [PubMed] [Google Scholar]
- 46.Hahn I, Fuss B, Peters A, Werner T, Sieberg A, Gosejacob D, Hoch M. The Drosophila Arf GEF Steppke controls MAPK activation in EGFR signaling. J Cell Sci. 2013;126:2470–9. doi: 10.1242/jcs.120964. [DOI] [PubMed] [Google Scholar]
- 47.Schejter ED, Wieschaus E. bottleneck acts as a regulator of the microfilament network governing cellularization of the Drosophila embryo. Cell. 1993;75:373–85. doi: 10.1016/0092-8674(93)80078-S. [DOI] [PubMed] [Google Scholar]
- 48.Reversi A, Loeser E, Subramanian D, Schultz C, De Renzis S. Plasma membrane phosphoinositide balance regulates cell shape during Drosophila embryo morphogenesis. J Cell Biol. 2014 doi: 10.1083/jcb.201309079. Forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Burke TA, Christensen JR, Barone E, Suarez C, Sirotkin V, Kovar DR. Homeostatic actin cytoskeleton networks are regulated by assembly factor competition for monomers. Curr Biol. 2014;24:579–85. doi: 10.1016/j.cub.2014.01.072. [DOI] [PMC free article] [PubMed] [Google Scholar]