Abstract
Self-organization of dynamic microtubules via interactions with associated motors plays a critical role in spindle formation. The microtubule-based mechanisms underlying other aspects of cellular morphogenesis, such as the formation and development of protrusions from neuronal cells is less well understood. In a recent study, we investigated the molecular mechanism that underlies the massive reorganization of microtubules induced in non-neuronal cells by expression of the neuronal microtubule stabilizer MAP2c. In that study we directly observed cortical dynein complexes and how they affect the dynamic behavior of motile microtubules in living cells. We found that stationary dynein complexes transiently associate with motile microtubules near the cell cortex and that their rapid turnover facilitates efficient microtubule transport. Here, we discuss our findings in the larger context of cellular morphogenesis with specific focus on self-organizing principles from which cellular shape patterns such as the thin protrusions of neurons can emerge.
Keywords: self-organization, cortical dynein, cytoplasmic dynein, cellular morphogenesis, neuromorphogenesis, neurite, microtubules
Introduction
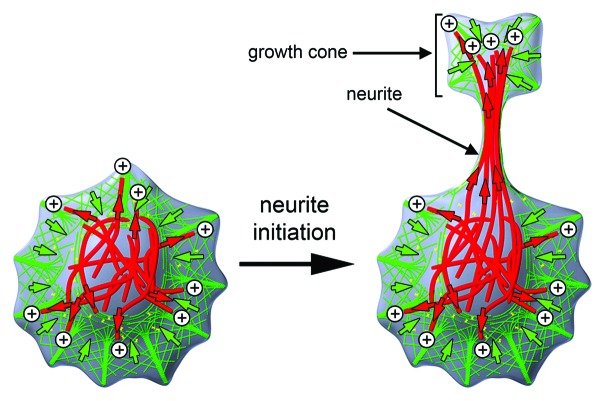
During their development, neurons form thin protrusions, called neurites that form the basis of neuronal networks in the brain. The cellular forces that drive the protrusion of neurites primarily originate from dynamic reorganization mechanisms of filamentous proteins that constitute the cytoskeleton (Fig. 1). Classically, the two major components of the cytoskeleton, filamentous actin (F-actin) and microtubules, were thought to play distinct roles in cellular function: While F-actin can form various supramolecular structures together with associated proteins to drive cell protrusion or cell contraction,1 microtubules are best known for their ability to form directional tracks for subcellular transport of cargo.2 During their initial formation, neurites contain a tight array of parallel microtubules in their shaft and an actin-rich growth cone at their tips.3 According to the classic view, the actin-rich growth cone provides the main driving force behind neurite formation while microtubules merely take a supporting role.4 However, both components also play additional roles. F-actin is also used as a directional track for cargo transport5 and microtubules can also form supramolecular structures that organize contractile or protrusive processes in cells.6-8
Figure 1. Cytoskeletal organization of neurons before and after neurite formation. Neurites are thin protrusions that originate from neuronal cell bodies. They are characterized by a microtubule-rich shaft and tipped by an F-actin-rich growth cone. Microtubules: red; F-actin: green; red and green arrows: forces based on microtubules and the F-actin cytoskeleton, respectively. Microtubule plus tips are indicated by ⊕ symbols.
The best-studied example for a microtubule-based supramolecular structure is the mitotic spindle. Overlapping microtubules at the center of the spindle can interact with multimeric microtubule motors to drive contraction of the spindle similar to contraction in overlapping actin filaments interacting with multimeric actin motors.9,10 Ultimately, the shape of the mitotic spindle emerges via a self-organizing mechanism from many local, molecular interactions including several microtubule motor species that interact with dynamic microtubules via complex feedback mechanisms.11,12
Microtubule dependent protrusive processes were first described centuries ago, but less well characterized.13-15 Letourneau and colleagues observed that neurons form thin neurite-like cell protrusions after combined treatment with drugs that stabilize microtubules and disrupt actin.15 In a more recent study that combined those pharmacological treatments with live cell imaging of protrusion dynamics, we observed neurite-like protrusions shortly after cessation of actin dynamics.16 Those observations suggest that actin dynamics are not only dispensable for the formation of neurite-like protrusions, but in fact inhibitory for this process. This raises the question: How can microtubules generate the force to drive cell protrusion?
Microtubule-Based Mechanisms that Drive Cell Protrusion
A hint at possible molecular mechanisms that might underlie microtubule-based cell protrusion came from studies in non-neuronal cells that express the neuronal microtubule stabilizer MAP2c. In such cells, microtubules were shown to form thin protrusions that were increased if actin filaments were depolymerized.17,18 Direct comparison between such MAP2c-induced neurites and the corresponding protrusions from primary neurons revealed that their cytoskeletal organization and dynamics are similar, suggesting that they can serve as a model for neurite formation.16 Interestingly, the MAP2c-induced thin protrusions contained tight bundles of microtubules that had uniform orientation with distal plus ends,19 similar as in nascent neurites.20 This suggests that either a motor-based transport process or a process based on dynamic microtubule polymerization/depolymerization might be responsible for this specific MAP2c-induced organization of microtubules that ultimately leads to cell protrusion.
Actin-dependent mechanisms that drive cell protrusion are thought to be driven by a molecular ratchet based polymerization mechanism.21 Similarly, MAP2c-induced cell protrusions could also be driven by microtubule polymerization. However, mechanisms based on molecular motors that push filaments toward the leading edge are also plausible. In the case of neurite-like protrusions, cytoplasmic dynein is a prime candidate for such a molecular motor-based direct pushing mechanism. A subpopulation of this motor commonly referred to as “cortical dynein” is known to be anchored to the cell cortex.22 Cortical dynein can exert forces on interacting microtubules, and those forces are known to drive large-scale structural changes inside cells, such as positioning of the mitotic spindle23 or centering of the microtubule organizing center (MTOC).24 Indeed, we found that dynein is required both for MAP2c-induced formation of neurite-like protrusions in non-neuronal cells, as well as the spontaneous formation of neurites in primary hippocampal neurons.25
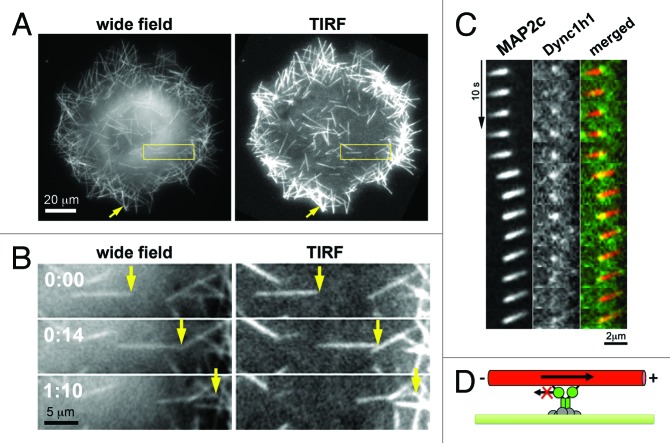
To study this process in more detail, we employed a nocodazole washout procedure in combination with live cell imaging to directly observe MAP2c-induced, dynein dependent microtubule reorganization in cells.25 Without this washout procedure, microtubules reorganize into tight bundles that are oriented toward the cell periphery within an extended time-course of several hours, which is difficult to monitor via fluorescence microscopy at high temporal resolution. However, shortly after a cycle of microtubule depolymerization and repolymerization using nocodazole and subsequent washout, short microtubules appear randomly distributed within cells with random orientation. Within minutes, those microtubules then move directionally with leading plus ends inside cells26 (Fig. 2A). Importantly, speckle microscopy revealed that the velocity of instantaneous microtubule movements was 40-fold higher than that of microtubule treadmilling, i.e., polymerization at microtubule plus-tips and depolymerization at their minus-ends.25 Thus, microtubule movements represent translocation of preassembled microtubules. Furthermore, this translocation was reversed by inhibition of cytoplasmic dynein.25
Figure 2. Direct observation of microtubule pushing by cortical dynein in living cells. (A) Images of COS7 cells transfected with the neuronal microtubule stabilizer MAP2c obtained shortly after nocodazole washout. In those conditions, short microtubules formed that were transported directionally within a distance up to ~200nm from the plasma membrane as observed by evanescent wave illumination in total internal refelction fluorescence (TIRF) microscopy. (B) Individual frames from video-microscopic observation of the boxed region in A. (C) Simultaneous observation of MAP2c-decorated short microtubules (left) and dynein heavy chain subunits (Dync1h1, middle) via TIRF microscopy. As expected for a direct, physical interaction between those components, the fluorescence signal derived from MAP2c-decorated microtubules is strongest at the localization of cortical dynein, suggesting that this microtubule region is closest to the plasma membrane and therefore excited most strongly by the evanescent wave of the TIRF microscope. (D) Schematic of the dynein-mediated microtubule pushing mechanism. This image was taken from26 (http://www.molbiolcell.org/content/25/1/95).
To directly study the role of molecular motors in this process, we observed this microtubule reorganization process in the presence of fluorescently labeled cytoplasmic dynein subunits.26 By using total internal reflection fluorescence (TIRF) microscopy, combined with sensitive detection methods that are compatible with low expression levels, individual cortical dynein complexes were observed near the plasma membrane. The dynein complexes contained few subunits of the dynein heavy chain and they were characterized by highly dynamic association kinetics with the plasma membrane. The colocalization of cortical dynein complexes with microtubules correlated with their dynamic behavior, including their directional movement (Fig. 2C).26 Interestingly, motile microtubules were also observed to kink or pivot around cortical dynein complexes, suggesting their direct physical interaction inside living cells.26
Role of Microtubules in Early Neuromorphogenesis
In order to study the role of all (~418) microtubule regulating genes in neurite formation, we performed a high-content screen using RNA interference in mouse stem cells.27 As expected for a critical component, at least 8 subunits of the cytoplasmic dynein motor stood out in the set of candidate microtubule regulators as being essential for neurite formation. Dynein is composed of several subunits that are encoded by several distinct gene families.28 Only a specific subset of those subunits were essential, while others were dispensable.27 Interestingly, the plus-end directed motor conventional kinesin was also found to play a role in neurite formation in Drosophila by affecting microtubule sliding against each other,29 indicating that multiple microtubule motors might play a role in neuromorphogenesis.
Apart from motor driven microtubule translocation, their dynamics also need to be regulated during neuromorphogenesis. In accordance with this idea, we found that the microtubule stability regulators EB1 and EB2 compete for limited binding sites on microtubules, and that this competition appears to play a critical role during neurite formation by regulating microtubule stability.27 Furthermore, as observed earlier in other cell systems,30 ROCK-dependent contractility of actin and the associated myosin motor was inhibitory to neurite formation.27 This suggests a potential mechanism for neurite formation, in which stabilized microtubules are pushed by cortical dynein toward the cell periphery to overcome contractile acto-myosin mediated forces.27
To be pushed efficiently by cortical dynein, microtubules have to be free to move within the cell. In non-neuronal cells, this is often not the case, as many microtubules are linked with their minus ends to the microtubule organizing center (MTOC). However, particularly in neuronal cells many MTOC independent microtubules exist, which could originate from extracentrosomal nucleation, katanin-mediated microtubule severing or microtubule breakage.31
Cellular Mechanisms of Microtubule Self-Organization
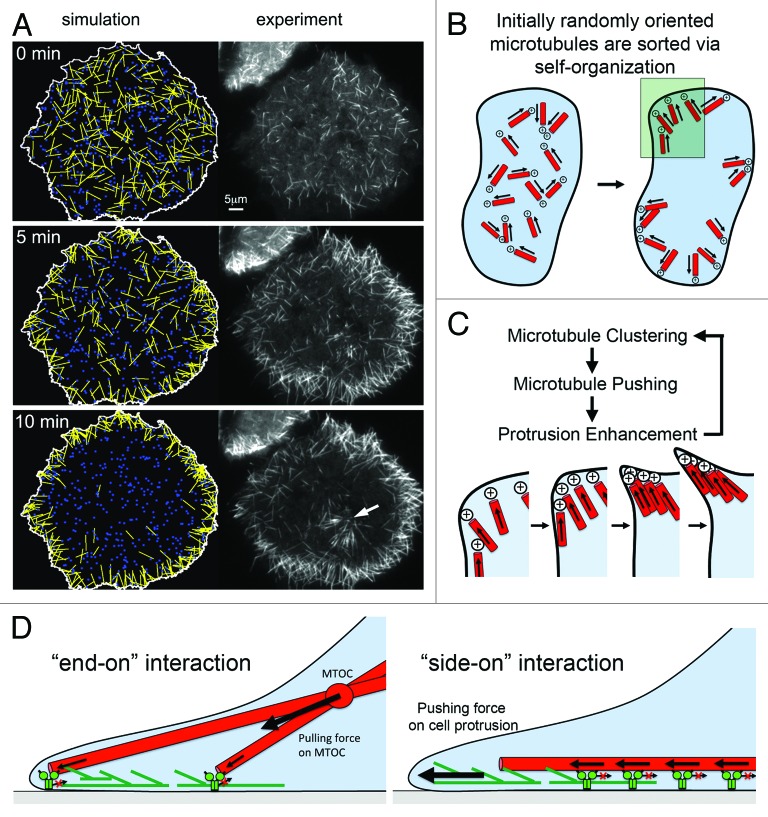
In nocodazole washout experiments, MTOC independent microtubules move directionally until they encounter an obstacle, such as the cell border, where they then accumulate to form small cell protrusions.25,26 The directional transport with leading plus ends drives the reorganization of initially randomly oriented microtubules to end up with uniform polarity orientation, with microtubule plus ends pointing toward the cell periphery19,26 – similar as in nascent neurites.20 We also observed a fairly sparse distribution of cytoplasmic dynein at the cell cortex that rapidly exchanged with a cytoplasmic pool. Mathematical modeling of the microtubule reorganization process revealed that this dynamic cortex association of dynein facilitates a rapid search to capture microtubules for directional transport in cells.26 Also, simulations on the basis of this model were able to closely mimic experimental observations, thus suggesting that they represent the essential components and rules that underlie this cellular process26 (Fig. 3A).
Figure 3. Model for MAP2c and dynein-mediated self-organization of microtubules. (A) Simulations based on mathematical modeling of cortical dynein mediated microtubule transport closely mimic experimental observations after nocodazole washout in the presence of MAP2c. Both the overall redistribution (shown here) and saltatory movements of individual microtubules are observed in those simulations.26 The white arrow points to microtubules radiating from a microtubule organizing center that was not included in simulations. (B) Shortly after washout of nocodaole, small microtubules with random initial orientation nucleate in the cytosol. Those microtubules are transported directionally with leading plus ends via cortical dynein complexes until they encounter an obstacle, which ultimately will be the plasma membrane. This will orient microtubule plus ends to point toward the cell periphery. Microtubules will then push against the outer edge of the cell, which can induce a small convex cell protrusion. (C) Enlarged region marked in B. In a process related to stigmergy, the convex cell protrusion can self-amplify by collecting and trapping more microtubules, which push further against the cell periphery. (D) Distinct modes of interaction between microtubules and cortical dynein. Right: The “end-on” dynein-microtubule interaction produces pulling forces that are integrated to pull the MTOC toward regions of higher cortical dynein density. At homogenous densities, pulling forces can lead to MTOC centering. Left: The “side-on” dynein-microtubule interaction produces pushing forces that are integrated to push microtubules toward the cell periphery where they can stimulate cell protrusions via a “clutch”-like mechanism. Panel A was taken from26 (http://www.molbiolcell.org/content/25/1/95).
Overall, the process of microtubule reorganization represents an illustrative example for cellular self-organization, as increased order in microtubule organization from initial random orientation toward a state, in which microtubules point toward the cellular periphery, is achieved in an energy consuming, motor-driven process (Fig. 3B). In addition, microtubules can accumulate within small initial protrusions in a process related to stigmergy, a building principle of termites.32 Stigmergy is a self-amplifying process, in which a positive feedback between termites and the structure that is built by them leads to pattern formation. A similar self-amplifying process can stimulate neurite formation, as more microtubules get trapped in the convex geometry of cell protrusions that they induce33,34 (Fig. 3C). Taken together, those processes can act together to constitute a pattern forming process, in which the structure of a neuron emerges from local interactions of active agents, i.e., microtubules and associated motor proteins. Additional motor activities that can drive lateral microtubule association10 might facilitate the formation of parallel microtubule arrays commonly observed in neurons or MAP2c transfected non-neuronal cells and might cooperate with those pattern forming mechanisms.
Spatio-Temporal Control of Cortical Dynein in Cellular Morphogenesis
The rapid cortex association dynamics of cytoplasmic dynein enables tight regulation of its activity in time and space.26,35,36 Interestingly, multiple, distinct types of cortical dynein exist, that are regulated by distinct molecular mechanisms.37 In the metaphase and anaphase spindle, a detailed cellular and molecular mechanism was described, in which microtubules are attached “end-on” and pulled by cortical dynein complexes.38 This type of cortical dynein complex associates with the heterotrimeric G protein α subunit Gαi at the cell cortex via the adaptor proteins NUMA and LGN.37,39,40 In contrast, at earlier stages of mitosis, during pronuclear migration and centrosome centering, cortical dynein translocates microtubules preferably via a “side-on” gliding mode, which is independent of heterotrimeric G proteins but dependent on the dynein regulator dynactin.37 Our observation of microtubules in nocadazole washout experiments that are pushed along the cell cortex via cortical dynein resembles the “side-on” gliding mode and not the “end-on” mode and is therefore also expected to be independent of heterotrimeric G proteins but instead dependent on dynactin. Also, the “side-on” gliding mode can effectively transfer forces from single or multiple dynein motors to microtubule tips and is therefore suitable to push microtubules against the cell periphery to induce cell protrusions (Fig. 3D). The “end-on” pulling mode does not allow for such a direct protrusive force transduction mechanism. Thus, the role of dynein in morphogenic processes that are based on cell protrusion, such as neurite formation is likely mediated by the “side-on” gliding mode and similar mechanisms might operate in other processes shown to be dependent on cytoplasmic dynein, such as axon outgrowth41 or growth cone turning.42 Although microtubule tips seldom reach the very border of the cell, i.e., the plasma membrane itself, they can still affect cell protrusion by their physical interaction with cortical actin structures. Earlier, we suggested that single or bundled microtubules that are more rigid due to their interaction with neuronal stabilizers such as MAP2c, and that are physically associated with actin filaments,43 can transform non-productive actin retrograde flow in growth cones into productive protrusion.16 This mechanism is in analogy to the classical clutch mechanism proposed by Mitchison and Kirschner,44 in which the actin cytoskeleton is linked to the rigid extracellular matrix instead of the rigid intracellular microtubule array.
Due to this diversity of dynein regulatory mechanisms, the link between microtubule-based cell protrusion and dynein activity is currently unclear. In particular, during neurite formation only roles for Ndel1, LIS1, dynein and dynactin complex subunits were proposed so far,27,45-47 however, their exact role in the spatio-temporal microtubule reorganization and force transduction is unclear. Techniques that enable the analysis of local activity states of signal networks, such as fluorescent resonant energy transfer (FRET)-based live cell activity sensors48,49 or intracellular protein interaction arrays50 might aid future studies to uncover regulatory pathways that control local activity patterns of cytoplasmic dynein that steer the spatial organization of microtubule pushing forces.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Blanchoin L, Boujemaa-Paterski R, Sykes C, Plastino J. Actin dynamics, architecture, and mechanics in cell motility. Physiol Rev. 2014;94:235–63. doi: 10.1152/physrev.00018.2013. [DOI] [PubMed] [Google Scholar]
- 2.Caviston JP, Holzbaur EL. Microtubule motors at the intersection of trafficking and transport. Trends Cell Biol. 2006;16:530–7. doi: 10.1016/j.tcb.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 3.Dehmelt L, Halpain S. Actin and microtubules in neurite initiation: are MAPs the missing link? J Neurobiol. 2004;58:18–33. doi: 10.1002/neu.10284. [DOI] [PubMed] [Google Scholar]
- 4.da Silva JS, Dotti CG. Breaking the neuronal sphere: regulation of the actin cytoskeleton in neuritogenesis. Nat Rev Neurosci. 2002;3:694–704. doi: 10.1038/nrn918. [DOI] [PubMed] [Google Scholar]
- 5.Cheney RE, Mooseker MS. Unconventional myosins. Curr Opin Cell Biol. 1992;4:27–35. doi: 10.1016/0955-0674(92)90055-H. [DOI] [PubMed] [Google Scholar]
- 6.Hoogenraad CC, Bradke F. Control of neuronal polarity and plasticity--a renaissance for microtubules? Trends Cell Biol. 2009;19:669–76. doi: 10.1016/j.tcb.2009.08.006. [DOI] [PubMed] [Google Scholar]
- 7.Kirschner M, Mitchison T. Beyond self-assembly: from microtubules to morphogenesis. Cell. 1986;45:329–42. doi: 10.1016/0092-8674(86)90318-1. [DOI] [PubMed] [Google Scholar]
- 8.Flynn KC. The cytoskeleton and neurite initiation. Bioarchitecture. 2013;3:86–109. doi: 10.4161/bioa.26259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mcintosh JR, Hepler PK, Van Wie DG. Model for Mitosis. Nature. 1969;224:659–63. doi: 10.1038/224659a0. [DOI] [Google Scholar]
- 10.Kapitein LC, Peterman EJ, Kwok BH, Kim JH, Kapoor TM, Schmidt CF. The bipolar mitotic kinesin Eg5 moves on both microtubules that it crosslinks. Nature. 2005;435:114–8. doi: 10.1038/nature03503. [DOI] [PubMed] [Google Scholar]
- 11.Karsenti E, Vernos I. The mitotic spindle: a self-made machine. Science. 2001;294:543–7. doi: 10.1126/science.1063488. [DOI] [PubMed] [Google Scholar]
- 12.Dumont S, Mitchison TJ. Force and length in the mitotic spindle. Curr Biol. 2009;19:R749–61. doi: 10.1016/j.cub.2009.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gallo G, Letourneau PC. Different contributions of microtubule dynamics and transport to the growth of axons and collateral sprouts. J Neurosci. 1999;19:3860–73. doi: 10.1523/JNEUROSCI.19-10-03860.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spero DA, Roisen FJ. Neuro-2a neuroblastoma cells form neurites in the presence of taxol and cytochalasin D. Brain Res. 1985;355:155–9. doi: 10.1016/0165-3806(85)90016-1. [DOI] [PubMed] [Google Scholar]
- 15.Letourneau PC, Shattuck TA, Ressler AH. “Pull” and “push” in neurite elongation: observations on the effects of different concentrations of cytochalasin B and taxol. Cell Motil Cytoskeleton. 1987;8:193–209. doi: 10.1002/cm.970080302. [DOI] [PubMed] [Google Scholar]
- 16.Dehmelt L, Smart FM, Ozer RS, Halpain S. The role of microtubule-associated protein 2c in the reorganization of microtubules and lamellipodia during neurite initiation. J Neurosci. 2003;23:9479–90. doi: 10.1523/JNEUROSCI.23-29-09479.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weisshaar B, Doll T, Matus A. Reorganisation of the microtubular cytoskeleton by embryonic microtubule-associated protein 2 (MAP2c) Development. 1992;116:1151–61. doi: 10.1242/dev.116.4.1151. [DOI] [PubMed] [Google Scholar]
- 18.Edson K, Weisshaar B, Matus A. Actin depolymerisation induces process formation on MAP2-transfected non-neuronal cells. Development. 1993;117:689–700. doi: 10.1242/dev.117.2.689. [DOI] [PubMed] [Google Scholar]
- 19.Takemura R, Okabe S, Umeyama T, Hirokawa N. Polarity orientation and assembly process of microtubule bundles in nocodazole-treated, MAP2c-transfected COS cells. Mol Biol Cell. 1995;6:981–96. doi: 10.1091/mbc.6.8.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baas PW, Black MM, Banker GA. Changes in microtubule polarity orientation during the development of hippocampal neurons in culture. J Cell Biol. 1989;109:3085–94. doi: 10.1083/jcb.109.6.3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mogilner A, Oster G. Cell motility driven by actin polymerization. Biophys J. 1996;71:3030–45. doi: 10.1016/S0006-3495(96)79496-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moore JK, Cooper JA. Coordinating mitosis with cell polarity: Molecular motors at the cell cortex. Semin Cell Dev Biol. 2010;21:283–9. doi: 10.1016/j.semcdb.2010.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kotak S, Gönczy P. Mechanisms of spindle positioning: cortical force generators in the limelight. Curr Opin Cell Biol. 2013;25:741–8. doi: 10.1016/j.ceb.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 24.Burakov A, Nadezhdina E, Slepchenko B, Rodionov V. Centrosome positioning in interphase cells. J Cell Biol. 2003;162:963–9. doi: 10.1083/jcb.200305082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dehmelt L, Nalbant P, Steffen W, Halpain S. A microtubule-based, dynein-dependent force induces local cell protrusions: Implications for neurite initiation. Brain Cell Biol. 2006;35:39–56. doi: 10.1007/s11068-006-9001-0. [DOI] [PubMed] [Google Scholar]
- 26.Mazel T, Biesemann A, Krejczy M, Nowald J, Muller O, Dehmelt L. Direct observation of microtubule pushing by cortical dynein in living cells. Mol Biol Cell. 2014;25:95–106. doi: 10.1091/mbc.E13-07-0376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arens J, Duong TT, Dehmelt L. A morphometric screen identifies specific roles for microtubule-regulating genes in neuronal development of P19 stem cells. PLoS One. 2013;8:e79796. doi: 10.1371/journal.pone.0079796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Höök P, Vallee RB. The dynein family at a glance. J Cell Sci. 2006;119:4369–71. doi: 10.1242/jcs.03176. [DOI] [PubMed] [Google Scholar]
- 29.Lu W, Fox P, Lakonishok M, Davidson MW, Gelfand VI. Initial neurite outgrowth in Drosophila neurons is driven by kinesin-powered microtubule sliding. Curr Biol. 2013;23:1018–23. doi: 10.1016/j.cub.2013.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bito H, Furuyashiki T, Ishihara H, Shibasaki Y, Ohashi K, Mizuno K, Maekawa M, Ishizaki T, Narumiya S. A critical role for a Rho-associated kinase, p160ROCK, in determining axon outgrowth in mammalian CNS neurons. Neuron. 2000;26:431–41. doi: 10.1016/S0896-6273(00)81175-7. [DOI] [PubMed] [Google Scholar]
- 31.Kuijpers M, Hoogenraad CC. Centrosomes, microtubules and neuronal development. Mol Cell Neurosci. 2011;48:349–58. doi: 10.1016/j.mcn.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 32.Grasse PP. La reconstruction du nid et les coordinations inter-individuelles chez Bellicositermes natalensis et Cubitermes sp. La theorie de la stigmergie: Essai d'interpretation du comportement des termites constructeurs. Insectes Soc. 1959;6:41–81. doi: 10.1007/BF02223791. [DOI] [Google Scholar]
- 33.Dehmelt L, Bastiaens PI. Spatial organization of intracellular communication: insights from imaging. Nat Rev Mol Cell Biol. 2010;11:440–52. doi: 10.1038/nrm2903. [DOI] [PubMed] [Google Scholar]
- 34.Dehmelt L, Bastiaens P. Self-Organization in Cells. In: Meyer-Ortmanns H, Thurner S, eds. Principles of Evolution. Heidelberg: Springer, 2011:219-38. [Google Scholar]
- 35.Collins ES, Balchand SK, Faraci JL, Wadsworth P, Lee WL. Cell cycle-regulated cortical dynein/dynactin promotes symmetric cell division by differential pole motion in anaphase. Mol Biol Cell. 2012;23:3380–90. doi: 10.1091/mbc.E12-02-0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kiyomitsu T, Cheeseman IM. Chromosome- and spindle-pole-derived signals generate an intrinsic code for spindle position and orientation. Nat Cell Biol. 2012;14:311–7. doi: 10.1038/ncb2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gusnowski EM, Srayko M. Visualization of dynein-dependent microtubule gliding at the cell cortex: implications for spindle positioning. J Cell Biol. 2011;194:377–86. doi: 10.1083/jcb.201103128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Laan L, Pavin N, Husson J, Romet-Lemonne G, van Duijn M, López MP, Vale RD, Jülicher F, Reck-Peterson SL, Dogterom M. Cortical dynein controls microtubule dynamics to generate pulling forces that position microtubule asters. Cell. 2012;148:502–14. doi: 10.1016/j.cell.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Merdes A, Ramyar K, Vechio JD, Cleveland DW. A complex of NuMA and cytoplasmic dynein is essential for mitotic spindle assembly. Cell. 1996;87:447–58. doi: 10.1016/S0092-8674(00)81365-3. [DOI] [PubMed] [Google Scholar]
- 40.Du Q, Macara IG. Mammalian Pins is a conformational switch that links NuMA to heterotrimeric G proteins. Cell. 2004;119:503–16. doi: 10.1016/j.cell.2004.10.028. [DOI] [PubMed] [Google Scholar]
- 41.Ahmad FJ, Hughey J, Wittmann T, Hyman A, Greaser M, Baas PW. Motor proteins regulate force interactions between microtubules and microfilaments in the axon. Nat Cell Biol. 2000;2:276–80. doi: 10.1038/35010544. [DOI] [PubMed] [Google Scholar]
- 42.Myers KA, Tint I, Nadar CV, He Y, Black MM, Baas PW. Antagonistic forces generated by cytoplasmic dynein and myosin-II during growth cone turning and axonal retraction. Traffic. 2006;7:1333–51. doi: 10.1111/j.1600-0854.2006.00476.x. [DOI] [PubMed] [Google Scholar]
- 43.Schaefer AW, Kabir N, Forscher P. Filopodia and actin arcs guide the assembly and transport of two populations of microtubules with unique dynamic parameters in neuronal growth cones. J Cell Biol. 2002;158:139–52. doi: 10.1083/jcb.200203038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mitchison T, Kirschner M. Cytoskeletal dynamics and nerve growth. Neuron. 1988;1:761–72. doi: 10.1016/0896-6273(88)90124-9. [DOI] [PubMed] [Google Scholar]
- 45.Ahmad FJ, He Y, Myers KA, Hasaka TP, Francis F, Black MM, Baas PW. Effects of dynactin disruption and dynein depletion on axonal microtubules. Traffic. 2006;7:524–37. doi: 10.1111/j.1600-0854.2006.00403.x. [DOI] [PubMed] [Google Scholar]
- 46.Grabham PW, Seale GE, Bennecib M, Goldberg DJ, Vallee RB. Cytoplasmic dynein and LIS1 are required for microtubule advance during growth cone remodeling and fast axonal outgrowth. J Neurosci. 2007;27:5823–34. doi: 10.1523/JNEUROSCI.1135-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shim SY, Samuels BA, Wang J, Neumayer G, Belzil C, Ayala R, Shi Y, Shi Y, Tsai LH, Nguyen MD. Ndel1 controls the dynein-mediated transport of vimentin during neurite outgrowth. J Biol Chem. 2008;283:12232–40. doi: 10.1074/jbc.M710200200. [DOI] [PubMed] [Google Scholar]
- 48.Förster T. Zwischenmolekulare Energiewanderung und Fluoreszenz. Ann Phys. 1948;437:55–75. doi: 10.1002/andp.19484370105. [DOI] [Google Scholar]
- 49.Wouters FS, Verveer PJ, Bastiaens PI. Imaging biochemistry inside cells. Trends Cell Biol. 2001;11:203–11. doi: 10.1016/S0962-8924(01)01982-1. [DOI] [PubMed] [Google Scholar]
- 50.Gandor S, Reisewitz S, Venkatachalapathy M, Arrabito G, Reibner M, Schröder H, Ruf K, Niemeyer CM, Bastiaens PI, Dehmelt L. A protein-interaction array inside a living cell. Angew Chem Int Ed Engl. 2013;52:4790–4. doi: 10.1002/anie.201209127. [DOI] [PMC free article] [PubMed] [Google Scholar]