Abstract
Background and aims
Acid-suppressive medications, particularly proton pump inhibitors (PPIs), may decrease the risk of oesophageal adenocarcinoma (OAC) in patients with Barrett’s oesophagus (BO). We performed a systematic review with meta-analysis of studies evaluating the association between acid-suppressive medications (PPIs and histamine receptor antagonists (H2RAs)) and risk of OAC or high-grade dysplasia (BO-HGD) in patients with BO.
Methods
We performed a systematic search of multiple electronic databases and conference proceedings up to June 2013 to identify studies reporting the association between use of acid-suppressive medications and risk of OAC and/or BO-HGD in patients with BO. Summary ORs with 95% CIs were estimated.
Results
We identified seven observational studies (2813 patients with BO, 317 cases of OAC or BO-HGD, 84.4% PPI users). On meta-analysis, PPI use was associated with a 71% reduction in risk of OAC and/or BO-HGD in patients with BO (adjusted OR 0.29; 95% CI 0.12 to 0.79). There was a trend towards a dose–response relationship with PPI use for >2–3 years protective against OAC or BO-HGD (three studies; PPI use >2–3 years vs <2–3 years: OR 0.45 (95% CI 0.19 to 1.06) vs 1.09 (0.47 to 2.56)). Considerable heterogeneity was observed. Two studies reported the association between H2RA use and risk of OAC and/or BO-HGD (1352 patients with BO, 156 cases of OAC, 25.4% on H2RAs), and both studies did not show a significant effect.
Conclusions
Based on meta-analysis of observational studies, the use of PPIs is associated with a decreased risk of OAC and/or BO-HGD in patients with BO. None of the studies showed an increased risk of OAC. PPI use should be considered in BO, and chemopreventive trials of PPIs in patients with BO are warranted.
INTRODUCTION
The incidence of oesophageal adenocarcinoma (OAC) has increased more than sixfold in the last three decades in the USA.1 Barrett’s oesophagus (BO) is precursor lesion for OAC and confers a 30–125-fold higher risk of OAC. However, only a small proportion of patients have BO that progresses to OAC. Routine endoscopic surveillance of patients with BO and endoscopic eradication therapy for a subset of patients with high-grade dysplasia (BO-HGD) is recommended.2 However, this strategy is expensive and limited by suboptimal adherence and access. Hence, there is a great interest in identifying relatively inexpensive and effective chemopreventive strategies for patients with BO.3–5
Acid-suppressive medications such as proton pump inhibitors (PPIs) and histamine receptor antagonists (H2RAs) are the most commonly used medications in the management of gastroesophageal reflux disease (GERD). Preclinical studies and early phase biomarker-based chemoprevention trials have shown that PPIs may prevent or delay progression of dysplasia in BO.6,7 However, PPI-related acid suppression induced hypergastrinemia and consequent proliferation have led to concerns about oncogenic potential of long-term PPI therapy.8 Epidemiological studies of the association between acid-suppressive therapy and OAC risk have been conflicting. A large population-based nested case–control study from the UK reported an increased risk of OAC in patients on long-term acid-suppressive therapy, but not independent of underlying GERD symptoms (which prompted acid-suppressive therapy).9 In contrast, several small observational studies have reported a protective association between PPI therapy and risk of progression to OAC and/or BO-HGD in a cohort of patients with BO.10,11 However, these studies have been limited by the small number of events, precluding a robust estimation of the true association between acid-suppressive medications and risk of OAC.
To better understand this issue, we performed a systematic review with meta-analysis of all studies that investigated the association between acid-suppressive medications, PPIs and H2RAs, and OAC and/or BO-HGD in patients with BO.
METHODS
This systematic review was conducted and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.12 The process followed a priori established protocol.
Selection criteria
We included randomised controlled trials (RCTs) or observational studies (cohort and case–control design) that met the following inclusion criteria: evaluated and clearly defined exposure to PPIs or H2RAs (exposed and unexposed group); reported OAC and/or BO-HGD risk in patients with established BO; and reported HR, relative risk (RR) or OR, or provided data for their calculation. Inclusion was not otherwise restricted by study size, language or publication type. We excluded cross-sectional studies, studies performed in the general population without knowledge of BO status, studies with insufficient information on histological progression to OAC or BO-HGD, and studies comparing medical and surgical therapy for GERD or BO. When there were multiple publications from the same population, we only included data from the most recent comprehensive report.
Data sources and search strategy
First, we conducted a systematic literature search of Medline, Embase, Web of Science and Scopus, from inception through 15 June 2013, with the help of an expert medical librarian, to identify all relevant articles on the association between acid-suppressive medication and risk of OAC in patients with BO. Details of the search strategy are available in the online supplementary appendix. Briefly, a combination of keywords and medical subject heading terms were used, including ‘proton pump inhibitor*’, ‘PPI’, ‘acid suppress*’, ‘omeprazole’, ‘panto-prazole’, ‘esomeprazole’, ‘lansoprazole’, ‘dexlansoprazole’, ‘histamine receptor antagonists’, ‘histamine receptor blockers’, ‘ranitidine’, ‘cimetidine’ AND ‘barrett’s’ OR ‘oesophageal’ AND ‘neoplasia’, ‘oesophageal adenocarcinoma’. Subsequently, two authors independently reviewed the title and abstract of studies identified in the search to exclude studies that did not answer the research question of interest, based on prespecified inclusion and exclusion criteria. The full text of the remaining articles was again independently reviewed to determine whether it contained relevant information. Next, we manually searched the bibliographies of the selected articles and review articles on the topic for additional articles. Third, we performed a manual search of conference proceedings from major gastroenterology meetings (Digestive Diseases Week, United European Gastroenterology Week and Annual Meeting of the American College of Gastroenterology; from 2008 to 2012) for additional abstracts on the topic.
Data abstraction and quality assessment
After study identification, data on study and patient characteristics, exposure and outcome assessment, potential confounding variables and estimates of association were independently abstracted onto a standardised form by two authors. Details of data abstraction are reported in the online supplementary appendix. To estimate the duration–response relationship, using non-users as reference, we measured the association between patients exposed to acid-suppressive medication for a short period of time (<2–3 years) and non-use, and the association between long duration of medication use (>2–3 years) and non-use. Conflicts in data abstraction were resolved by consensus, referring back to the original article. The methodological quality of case–control and cohort studies was assessed by two authors independently (SS and SK) using the Newcastle–Ottawa scale.13 Any discrepancies were addressed by a joint re-evaluation of the original article.
Outcomes assessed
The primary analysis focused on assessing the risk of progression to OAC and/or BO-HGD in patients with BO, among PPI users (and H2RA users) compared with non-users. We also analysed the time to progression to any neoplasia (OAC or BO-HGD or Barrett’s oesophagus with low-grade dysplasia (BO-LGD)) in patients with non-dysplastic BO (time-to-event analysis) based on PPI use, as reported in cohort studies.
We performed pre-planned subgroup analysis based on study design (cohort vs case–control), study location (USA vs non-USA), method of exposure ascertainment (pharmacy prescription database vs self-report vs medical record review) and proportion of patients exposed to medication in entire BO cohort at time of enrolment (>90% vs <90%). To assess the presence of a reflux-independent association between acid-suppressive medication use and risk of progression to OAC and/or BO-HGD, we performed sensitivity analysis restricting analysis to studies which adjusted for the presence of erosive esophagitis or reflux symptoms; likewise, to assess the presence of an independent chemopreventive association, we restricted analysis to studies which adjusted for concomitant use of non-steroidal anti-inflammatory drugs (NSAIDs)/aspirin or statins.
Statistical analysis
We used the random-effects model described by DerSimonian and Laird to calculate summary ORs and 95% CIs.14 Since outcomes were relatively rare, ORs were considered approximations of RRs or HRs. Maximally adjusted OR, when reported in studies, was used for analysis to account for confounding variables. To estimate what proportion of total variation across studies was due to heterogeneity rather than chance, I2 statistic was calculated. In this, values of <30%, 30–60%, 60–75% and >75% were suggestive of low, moderate, substantial and considerable heterogeneity, respectively.15,16 Once heterogeneity was noted, between-study sources of heterogeneity were investigated using subgroup analyses by stratifying original estimates according to study characteristics (as described above). In this analysis also, a p value for differences between subgroups of <0.10 was considered statistically significant. Given the small number of studies identified in our analysis, statistical tests for assessing publications bias were not performed.17 All p values were two tailed. For all tests (except for heterogeneity), a probability level <0.05 was considered statistically significant. All calculations and graphs were performed using Comprehensive Meta-Analysis (CMA) V.2 (Biostat, Englewood, New Jersey, USA).
RESULTS
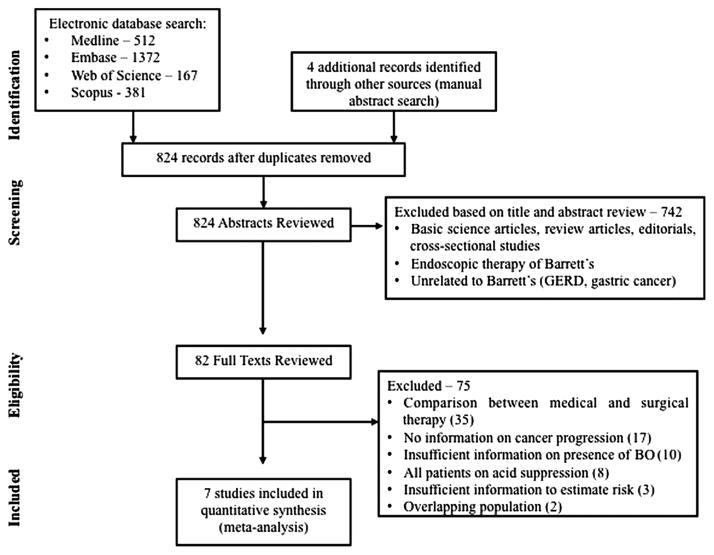
From 824 unique studies identified using our search strategy, seven studies (five cohort, two case–control studies) reporting on the association between PPIs and risk of OAC and/or BO-HGD,10,11,18–22 and two studies (one cohort and one case–control) reporting on the association between H2RAs and relevant outcomes were included.10,20 The flow diagram summarising study identification and selection is shown in figure 1. There were no RCTs comparing effect of acid-suppressive medications (with no acid-suppressive medications) addressing this question. Four studies were from two overlapping populations,10,11,23,24 and hence, only two of the more comprehensive studies were included.10,11 In three studies, there was insufficient information to calculate a measure of association.25–27
Figure 1.
Flow sheet summarising study identification and selection. BO, Barrett’s oesophagus; GERD, gastroesophageal reflux disease.
Characteristics of included studies
Seven studies reported on 2813 patients with non-dysplastic BO (or BO-LGD), of whom 317 progressed to OAC and/or BO-HGD; 84.4% of patients in these studies were PPI users.10,11,18–22 Table 1 shows the baseline characteristics of the studies. Four of these studies were conducted in the USA, two studies were conducted in Europe and one in Australia. Only one of the US studies was population based at low risk for selection bias22 and two Dutch studies were multicentred, conducted in a mix of academic and private practices10,18; three of the American studies were conducted in the military veteran population.11,20,21 A majority of these patients had non-dysplastic BO (88.5% of patients in cohort studies); one nested case–control study from the national Veteran Affairs cohort did not report baseline dysplasia status of patients with BO.20 Systematic information on surveillance of BO was provided in only two studies.10,19 Three studies used record linkage with the pharmacy prescription database for exposure assessment10,11,20; three studies relied on medical record review19,21,22 and one on patient self-report.18
Table 1.
Characteristics of individual studies included in the meta-analysis
| First author, ref. | Location; setting | Time period; follow-up | Total no. of patients with BO with baseline dysplasia status | Incident OAC and/or HGD | Primary outcome of interest | Patients on PPI
|
Patients not on PPI
|
Variables adjusted for | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Incident OAC and/or HGD | Total no. of patients on PPI | Incident OAC and/or BO-HGD | Total no. of patients not on PPI | |||||||
| Cohort studies | ||||||||||
| Kastelein10 | Rotterdam, The Netherlands; multicentre, hospital based | 2003–2009; mean 5.2 years | 540 NDBO—86% LGD—14% |
HGD—28 OAC—12 Combined—40 |
Progression to OAC and/or HGD | 28 | 462* (85.6%) | 12 | 78 (14.4%) | 1, 2, 7, 8, 9, 10, 11 |
| Jung22 | Minnesota, USA; population based | 1976–2006; median 5.9 years | 355 NDBO—83% LGD—17% |
HGD—12 OAC—7 Combined—9 |
Progression to OAC and/or HGD | Univariate HR for progression to OAC and/or HGD, for PPI users (PPI non-users as reference): 0.23 (95% CI 0.03 to 1.72) | N/A | |||
| Hillman19 | Canberra, Australia; 2 centres, hospital based | 1981–2001; median 7.4 years |
350† NDBO—85.4% LGD—14.6% |
HGD—9 OAC—7 Combined—11 |
Progression to OAC and/or HGD | 216 (61.7%) on PPI at start of surveillance; during follow-up all patients started on PPI; using time to PPI use as a time-dependent covariate, >2-year delay in starting PPI after BO diagnosis, resulted in 21-fold higher risk of OAC and/or HGD compared with use at time of BO diagnosis (HR 20.9; 95% CI 2.78 to 158) | 1, 2, 10 | |||
| Nguyen11 | Arizona, USA; multicentre, hospital based | 1982–2004; mean 7.6 years | 344 NDBO—100% LGD—0 |
HGD—20 OAC—13 Combined—33 |
Progression to OAC and/or HGD | 17 | 231 (67.2%) | 16 | 113 (32.8%) | 1, 2, 7, 11 |
| Altawil21 (abstract) | Michigan, USA; single centre, hospital based | 2004–2010; NR | 77 NDBO—100% LGD—0 |
17 (any dysplasia or OAC) | Progression to OAC and/or HGD and/or LGD | 7 | 49 (63.6%) | 10 | 28 (26.4%) | NR |
| Case–control studies | ||||||||||
| Nguyen20 (nested case-control study) | Nationwide VA, USA; multicentre, hospital based | 2000–2002; NA | 812 | OAC—116 | Development of OAC | 110 | 763 (94.0%) | 6 | 49 (6.0%) | 1, 2, 3, 9, 12, 13, 14 |
| de Jonge18 | Rotterdam, The Netherlands; multicentre, hospital based | 2003–2005; NA | 335 | OAC—91 | Development of OAC | 43 | 270 (81.6%) | 44 | 61 (18.4%) | 1, 2, 4, 5, 10 |
Variables adjusted for age, sex, race, smoking, alcohol use, obesity, BO length, baseline dysplasia status, time of BO diagnosis, esophagitis or reflux symptoms, medications (NSAIDs/aspirin/statins), healthcare encounters, comorbidities, socioeconomic status; studies arranged by study design, and within OAC study type, by size of BO cohort.
A total of 462 patients were on PPI at time of enrolment in cohort, with an additional 70 patients starting PPI during surveillance.
Only 299 patients with NDBO used for analysis.
BO, Barrett’s oesophagus; HGD, Barrett’s oesophagus with high-grade dysplasia; LGD, Barrett’s oesophagus with low-grade dysplasia; NA, not applicable; NDBO, non-dysplastic Barrett’s oesophagus; NR, not reported; NSAID, non-steroidal anti-inflammatory drug; PPI, proton pump inhibitor; OAC, oesophageal adenocarcinoma.
Four cohort studies reported the risk of progression to advanced neoplasia (OAC or BO-HGD)10,11,19,22 (with no information on risk of progression to OAC or BO-HGD separately), and one study reported the risk of progression to any grade of neoplasia (OAC or BO-HGD or BO-LGD).21 Two case–control studies included only patients with OAC as cases.18,20 The primary outcome was OAC in 39 and BO-HGD in 69 patients, from 1589 patients. One of the studies by Nguyen et al20 was a nested case–control study within a cohort of patients with BO, and risk estimate was reported as incidence density ratio.
Table 2 shows the baseline characteristics of the patients included in these studies. The mean age at BO diagnosis ranged from 58 to 63 years in cohort studies and the mean age at OAC diagnosis was 64 years. Approximately 82.1% of the patients with BO in these studies were men. More than 75% of the patients in the studies were Caucasians. The proportion of patients with long-segment BO (>3 cm) ranged from 29% to 59% when reported11,19,22; Kastelein and colleagues only included patients with BO segment length >2 cm.10 In three studies, there was no information on baseline length of BO segment.18,20,21 Only three studies reported on the presence of erosive esophagitis in the cohort (present in 9–88% of BO cohort)10,19,22; three studies reported on the presence of reflux symptoms (present in 29–77% of cohort).10,18,22 About 22–58% of patients with BO in the included studies were concomitantly on NSAIDs/aspirin and 19–46% were on statins.
Table 2.
Baseline characteristics of patients in the included studies
| First author, ref. | Age at BO diagnosis | Sex (% men) | Race (% Caucasian) | Obesity (% with BMI >30 kg/m2) | Smoking (% smokers) | Length of BO (% with LSBO) | Reflux symptoms or; endoscopic esophagitis | Other medication use
|
|
|---|---|---|---|---|---|---|---|---|---|
| NSAIDs/aspirin | Statins | ||||||||
| Cohort studies | |||||||||
| Kastelein10 | 61 (53–68) | 71 | NR | 19 | 19 (current) | 100* | 29%; 9% | 110 (20.4%) | 102 (18.9%) |
| Jung22† | 63 (14) | 69 | NR | 29‡ | 13 (current) | 59 | 77%; 31% | NR | NR |
| Hillman19 | 58 (12) | 71 | NR | NR | NR | 45.0 | NR; 88% | 78 (22.0%) | NR |
| Nguyen11 | 61 (12) | 94 | 90 | NR | NR | 29.1 | NR | 169 (49.1%) | 87 (25.3%) |
| Altawil21 | 60 | 96 | 75 | 28.9§ | NR | NR | NR | 20 (26.0%) | 27 (35.1%) |
| Case–control studies | |||||||||
| Nguyen20 | 65 (10)¶ | 97 | 74 | NR | NR | NR | NR | 468 (57.6%) | 377 (46.4%) |
| de Jonge18 | 62 (11)¶ | 74 | 100 | 27 | 20 | NR | 72.5%; NR | 134 (40.0%) | NR |
All patients with BO segment ≥2 cm.
For entire cohort of patients with BO.
Mean BMI.
Mean BMI among PPI users.
Age at OAC diagnosis or age of corresponding controls.
BMI, body mass index; BO, Barrett’s oesophagus; LSBO, long-segment Barrett’s oesophagus; NR, not reported; NSAID, non-steroidal anti-inflammatory drug. OAC, oesophageal adenocarcinoma; PPI, proton pump inhibitors
Quality assessment
Overall, the methodological quality of included studies was moderate to high. Online supplementary table S1 describes the quality of included studies. Only three studies accounted for the presence of reflux symptoms or erosive esophagitis,10,18,19 and only two studies adjusted for concomitant use of other putative chemopreventive medications.10,11 Systematic differences in PPI users and non-users were explored in only two studies10,21—there were no differences by patient age, sex, presence of obesity, smoking and alcohol use, characteristics of BO (segment length, baseline dysplasia) or use of NSAIDs/aspirin/statins; however, in one study, PPI users were less likely to have reflux symptoms or esophagitis, and were more likely to have prevalent BO (ie, longer duration of BO).10
PPI use and risk of advanced neoplasia in patients with BO
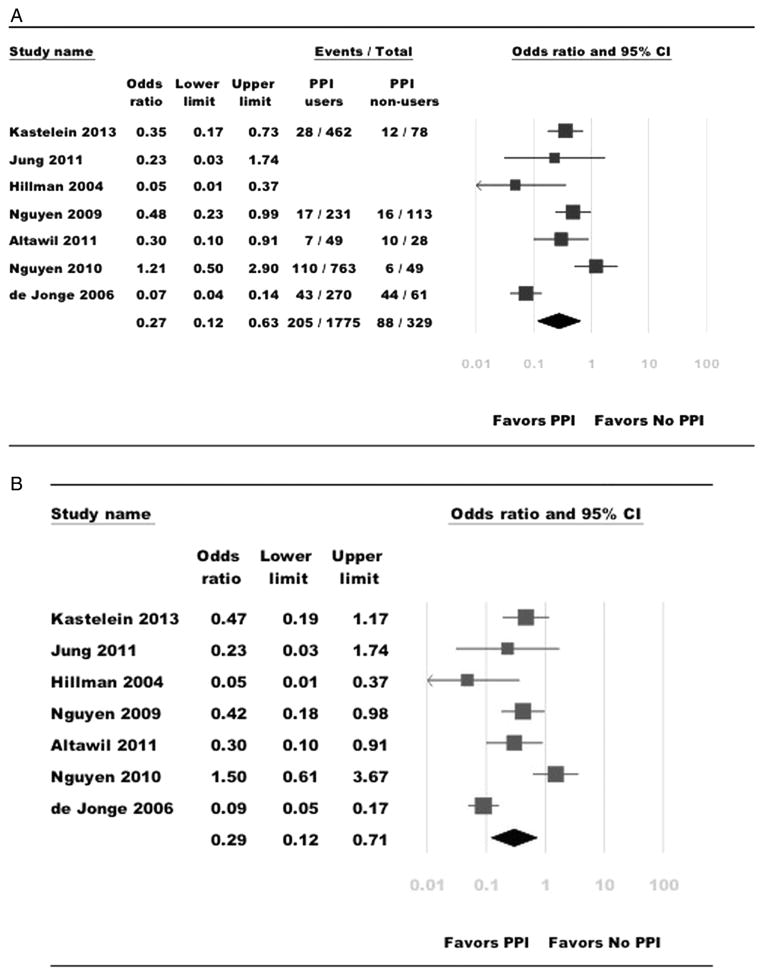
On meta-analysis of six studies that reported the endpoint of OAC and/or BO-HGD, use of PPI at time of BO diagnosis was associated with decreased risk of OAC and/or BO-HGD in patients with BO (unadjusted OR 0.26; 95% CI 0.10 to 0.71). Inclusion of one additional study, which assessed the risk of progression to any grade of dysplasia or OAC in patients with non-dysplastic BO, did not significantly change the results (unadjusted OR 0.27; 95% CI 0.12 to 0.63) (figure 2A). This protective association persisted on using the maximally adjusted risk estimates reported in individual studies, with a 71% lower risk of OAC and/or BO-HGD in PPI users (adjusted OR 0.29; 95% CI 0.12 to 0.79; figure 2B). In four studies, which reported the time to progression to OAC or BO-HGD in a cohort of patients with BO, PPI users were also significantly less likely to progress to OAC or BO-HGD (adjusted HR 0.32; 95% CI 0.15 to 0.67).10,11,19,22 In three studies, which reported the risk of progression to any degree of dysplasia in a cohort of patients with non-dysplastic BO, PPI use was protective (adjusted OR 0.37; 95% CI 0.15 to 0.96).11,19,21 There was insufficient information in these studies to allow estimation of PPIs’ effect on risk of progression to OAC alone and BO-HGD alone.
Figure 2.
(A) Summary of unadjusted ORs assessing the risk of oesophageal adenocarcinoma (OAC) and/or high-grade dysplasia (HGD) in patients with Barrett’s oesophagus (BO) with proton-pump inhibitor (PPI) exposure in all included studies. The size of the box corresponds to the weight of the given study. (B) Summary of adjusted ORs assessing the risk of OAC and/or HGD in patients with BO with PPI exposure in all included studies. The size of the box corresponds to the weight of the given study.
There was considerable heterogeneity in the overall analysis (I2=81%), although this was observed primarily due to two case–control studies with divergent results18,20; on meta-analysis of five cohort studies, the use of PPIs was consistently and strongly associated with a lower risk of any dysplasia in patients with BO (adjusted OR 0.33; 95% CI 0.19 to 0.58; I2=10%).
Subgroup and sensitivity analysis
The association between PPIs and risk of OAC or BO-HGD was stable across study design and study location (table 3). The heterogeneity observed in the overall analysis could be partly explained based on the method of exposure ascertainment as well as what proportion of patients in the entire BO cohort were on PPIs; statistically significant risk estimates were not noted when >90% of patients in the entire BO cohort were on PPIs.
Table 3.
Subgroup analyses and dose–response relationship on the association of PPI use and risk of OAC and/or BO-HGD in patients with BO
| Groups | Categories | No. of studies | Adjusted OR | 95% CI | Heterogeneity within groups (I2) | Pinteraction |
|---|---|---|---|---|---|---|
| Study design | Case–control | 2 | 0.36 | 0.02 to 5.67 | 96% | 0.96 |
| Cohort | 5 | 0.33 | 0.19 to 0.58 | 9% | ||
| Study location | USA | 4 | 0.52 | 0.22 to 1.53 | 57% | 0.11 |
| Non-USA | 3 | 0.15 | 0.04 to 0.55 | 80% | ||
| Proportion of patients on PPI | >90% | 1 | 1.50 | 0.61 to 3.67 | – | <0.01 |
| <90% | 6 | 0.22 | 0.09 to 0.50 | 74% | ||
| Method of exposure assessment | Pharmacy prescription records | 3 | 0.66 | 0.30 to 1.47 | 59% | <0.01 |
| Medical records | 3 | 0.19 | 0.07 to 0.52 | 16% | ||
| Self-report | 1 | 0.09 | 0.05 to 0.17 | – | ||
| Duration–response | <2–3 years | 3 | 1.09 | 0.47 to 2.56 | 48 | 0.15 |
| >2–3 years | 3 | 0.45 | 0.19 to 1.06 | 44 |
BO, Barrett’s oesophagus; HGD, Barrett’s oesophagus with high-grade dysplasia; OAC, oesophageal adenocarcinoma; PPI, proton pump inhibitor.
The independent protective association between PPIs and risk of OAC and/or BO-HGD persisted, even on restricting analysis to studies, which adjusted for the concomitant use of NSAIDs/aspirin/statins (n=2 studies; adjusted OR 0.44; 95% CI 0.24–0.83).10,11 On the others, analysis of the remaining five studies which did not adjust for concomitant use of NSAIDs/aspirin/statins revealed a similar protective association between PPIs and risk of OAC and/or BO-HGD (adjusted OR 0.23; 95% CI 0.06 to 0.89). In three studies, which accounted for the presence of erosive esophagitis or reflux symptoms, use of PPI was still protective against OAC (adjusted OR 0.15; 95% CI 0.04 to 0.55). To assess whether any one study had a dominant effect on the summary OR, each study was excluded and its effect on the main summary estimate was evaluated. No study markedly affected the summary estimate or p value for heterogeneity among the other summary estimates, and the pooled point estimate remained statistically significant (range 0.22–0.41), with the corresponding 95% CI bounds remaining below 1.
Duration–response relationship
The presence of a duration–response relationship was examined in five studies, of which four studies observed a greater protective effect with longer duration of PPI use.10,11,18–20 For meta-analysis, we used data from three studies that were conducive for pooling since they divided exposure time intervals as short term (<2–3 years) and long term (>2–3 years);10,11,20 one of the other studies used a 6-month cutoff18 and another assessed PPI exposure in terms of delay in starting it after BO diagnosis (eventually, all patients were placed on PPIs in this cohort).19 Based on these three studies, long-term exposure to PPIs (>2–3 years) was associated with a greater protective effect on OAC and/or BO-HGD risk (adjusted OR 0.45; 95% CI 0.19 to 1.06) whereas short-term exposure (<2–3 years) was not significantly associated with OAC and/or BO-HGD (adjusted OR 1.09; 95% CI 0.47 to 2.56).
There were insufficient data to allow pooling based on type, dose or frequency of use of PPIs. One study reported that the protective association of PPIs was seen at all doses, with all types and regardless of whether they were used once a day or twice a day.10 In the same study, however, the importance of adherence to PPI for the chemopreventive effect was highlighted—compared with patients who filled <90% of their PPI prescription, patients with BO who filled >90% of their PPI prescription had a significantly greater reduction in risk of progressing to OAC and/or HGD (adjusted HR 0.24; 95% CI 0.08 to 0.71).
Number needed to treat
The observed cumulative incidence rates of OAC and/or BO-HGD in patients with BO overall, non-dysplastic BO and BO-LGD are 10.2, 6.8 and 18.3 per 1000 patient years, respectively.22,28,29 Using a 67% summary risk reduction of OAC and/or BO-HGD with PPI use in patients with BO, derived from cohort studies (with low heterogeneity), we estimate the number needed to treat with PPIs to prevent one case of OAC or BO-HGD in these patients at 147, 220 and 82, respectively.
Histamine-receptor antagonist use and risk of advanced neoplasia in patients with BO
Two studies reported on the association between H2RA use and the risk of OAC in patients with BO (156 cases of OAC in 1352 patients with BO; 25.4% were on H2RAs).10,20 None of the studies individually observed a protective association. However, in one study all but one H2RA user were also on PPIs concomitantly and hence it was not possible to adequately assess the independent association between H2RA use and risk of advanced neoplasia in patients with BO10 ;in this study, H2RA use was not significantly associated with decreased risk of progression to advanced neoplasia (adjusted HR 0.83; 95% CI 0.11 to 6.03). In the other study, only unadjusted analysis on the association between H2RA use and risk of OAC was presented (unadjusted OR 1.17; 95% CI 0.78 to 1.76).20 Given these limitations, a meta-analysis on the association between H2RAs and risk of OAC and/or BO-HGD in patients with BO was not performed.
DISCUSSION
In this systematic review of all published studies in 2813 patients with non-dysplastic BO (or BO-LGD), of whom 317 progressed to OAC and/or BO-HGD, we observed that PPI use was associated with a 71% risk reduction in progression to OAC and/or BO-HGD in a duration-dependent manner—PPI use >2–3 years after BO diagnosis was associated with a lower risk of OAC and/or BO-HGD, whereas PPI use for <2–3 years was not associated with a protective effect. This association was consistent and stable in cohort studies, which are at low risk of recall bias. In addition, we observed an association between PPI and risk of OAC and/or BO-HGD in patients with BO, independent of the presence of erosive esophagitis or reflux symptoms, and independent of the concomitant use of NSAIDs/aspirin and statins, albeit in a small number of studies. However, there is limited information in only two studies on the association between H2RA use and risk of OAC and/or BO-HGD in patients with BO, and a meaningful conclusion on their potential chemopreventive effect could not be drawn.
Given the high incidence and mortality from OAC, chemo-preventive agents are highly desirable.3–5 Our findings of an estimated 71% risk reduction in OAC and/or BO-HGD are similar to those observed in a previous small limited systematic review of three of these studies, published as a letter to the editor.30 However, in that review there was no quantitative synthesis, assessment of quality of included studies, and no subgroup or sensitivity analyses. Aspirin and statins have also been proposed as effective agents associated with a lower risk of OAC and/or BO-HGD in patients with BO, but the effect size is moderate, with a 32–41% estimated risk reduction.31,32
Antineoplastic effect of PPI therapy
The primary hypothesised chemopreventive mechanism of PPIs is by decreasing intra-oesophageal acid and bile exposure, thus promoting oesophageal mucosal healing. Intermittent exposure to acid has been shown to induce epithelial proliferation in vivo and in vitro.33 Acid and bile exposure have also been shown to upregulate cyclooxygenase-2 (COX-2) expression in BO, which has been implicated in oesophageal carcinogenesis.34,35 Complete, but not partial, acid suppression by PPIs over 6 months as measured by 24 h pH monitoring, decreases markers of epithelial proliferation and increases cell differentiation markers in patients with BO.36 PPIs also decrease duodeno-gastroesophageal reflux, protecting the metaplastic epithelium from bile acid induced injury.7 PPIs also have anti-inflammatory properties independent of their acid-suppressive effects, and this may also contribute to their chemopreventive effect against OAC.37 In addition, long-term aggressive PPI therapy induces the formation of oesophageal squamous islands in patients with BO7,38 and may decrease the length of the BO segment, though this association is inconsistent.7,10,39 However, clinical studies have been inconclusive on whether PPI causes regression of established dysplasia in patients with BO, although these studies have typically involved only a short duration of PPI therapy (<6 months), have enrolled small numbers of patients with dysplasia, and have had short-term follow-up to allow estimation of progression to OAC and/or BO-HGD.7 A recent phase II chemoprevention trial demonstrated that a combination of high-dose aspirin (325 mg/day) with esomeprazole decreases levels of prostaglandin E2, a marker of resistance to apoptosis, increased angiogenesis and enhanced invasion in BO mucosa.6
There has been a theoretical concern that prolonged acid suppressive therapy with PPIs can induce hypergastrinemia, which may induce proliferation, COX-2 upregulation, potentiating oesophageal carcinogenesis.8,40 However, Obszynska and colleagues demonstrated that, though gastrin enhances epithelial restitution in Barrett’s mucosa, it does not promote proliferation and expansion of Barrett’s segments during long-term PPI treatment.41,42 Hence, there is no evidence from preclinical and clinical studies that prolonged therapy with PPIs promotes oesophageal carcinogenesis, and this was also borne out in our review.
Strengths and limitations
The strengths of this systematic review include the following: comprehensive and systematic literature search with well defined inclusion criteria, carefully excluding redundant studies; rigorous evaluation of study quality; subgroup and sensitivity analyses to evaluate the stability of findings and identify potential factors responsible for inconsistencies; assessment of duration–response relationship using a fairly stable 2–3-year cutoff from included studies; simultaneous evaluation of unadjusted (based on raw numbers) and adjusted risk estimates, and hence, being able to evaluate the potential influence of measured confounders on the summary estimate; and low likelihood of misclassification bias since the included studies rigorously defined BO, often being reviewed by two or at least one expert gastrointestinal pathologist.
There are several limitations in our study. First, the meta-analysis included only observational studies. No RCTs have been performed to explore this association. Observational studies lack the experimental random allocation of the intervention necessary to test exposure–outcome hypotheses optimally. Since >80% of the patients in the included studies were on PPIs at the time of BO diagnosis (and more patients were eventually prescribed PPIs during follow-up), it is difficult to tease out the exact efficacy of these agents in decreasing neoplastic progression in patients with BO. Despite adjusting for several covariates, it is not possible to eliminate the potential of residual confounding, especially with regard to factors that go into acid-suppressive medication prescription in patients with BO. It is possible that patients with endoscopic findings of severe erosive esophagitis (who are more likely to progress to OAC) were more likely to be prescribed PPIs, thereby spuriously weakening the observed association (confounding by severity of disease). However, it is possible that health-conscious patients with ready access to healthcare may be more likely to be prescribed PPIs, thereby spuriously strengthening the observed association (healthy user bias). Additionally, most of the included studies did not account for several potential confounding factors, including obesity and smoking, which influence exposure and outcome. However, using the adjusted data for any use of PPIs had little effect on the summary OR, suggesting that any difference attributable to using different confounders for adjustment is likely small. Second, considerable heterogeneity was observed in the overall analysis; however, this was primarily attributable to two case–control studies with disparate results—meta-analysis of cohort studies resulted in a consistent result with very low heterogeneity (I2=9%). Although six of the included studies reported a lower risk of OAC and/or BO-HGD with PPI use (four were statistically significant), one large nested case–control study did not observe any association between PPIs and risk of OAC.20 In this study, ~95% of the entire cohort of patients with BO were on PPIs and the majority (>80%) of these included cases and controls had been on PPIs for <2 years. Baseline dysplasia status of control patients with BO was not available. This may have resulted in an inability to identify a significant association between PPIs and OAC risk in this study. Third, we were unable to rule out the presence of a publication bias. With such limited number of studies, statistical testing for publication bias assessment is not recommended.17 We tried to minimise the potential for this by carefully examining published abstracts. Finally, the included studies had inherent limitations. Three of the studies were performed in the military veteran population, which is overwhelmingly male and tends to have higher prevalence of smoking and metabolic syndrome, both of which are associated with greater risk of progression to OAC. Additionally, in our analysis, the majority of patients were older men; our results would require independent validation in the general population, particularly women. Except for one study, there was inadequate information on medication compliance.10 Moreover, there was insufficient information on the efficacy of PPIs in acid suppression. A definite dose of PPI required for chemopreventive effect is unclear; only one study assessed different doses and types of PPIs and did not report significant differences in risk estimates.10
Implications for clinical practice
While PPIs are routinely recommended for management of GERD, we believe, based on the results of this systematic review, that in patients with BO with multiple risk factors for progression to OAC and/or BO-HGD (such as long-segment BO, BO-LGD, central adiposity, smoking, advanced age), PPI therapy should be considered for its primary chemopreventive potential. While an exact dose and therapeutic efficacy endpoint are not known, a regular once-daily dose of PPI therapy may be appropriate. There is no clinical evidence supporting the notion that PPI therapy may increase the risk of neoplastic progression in patients with BO. Furthermore, a cost-effectiveness analysis comparing six strategies of chemoprevention in BO (no drugs, generic PPI, brand name PPI (both estimating a 50% risk reduction in neoplastic progression), chemoprevention drug (estimating a 35% reduction in risk), or a combination of generic or brand name PPI with chemoprevention drug) identified use of a generic PPI as the most cost-effective strategy for the prevention of OAC in patients with BO.43 However, given the concerns about side effects and potential long-term risks associated with chronic PPI use, the risks and benefits would need to be carefully discussed with patients.44
CONCLUSIONS
Based on a systematic review and meta-analysis of all existing studies, PPI therapy is associated with a significantly decreased risk of progression to OAC and/or BO-HGD in patients with BO. Long duration (>2–3 year) of PPI use may provide a greater benefit than short duration. The effect of PPI therapy seems to be independent of NSAID/aspirin/statin use or the presence of erosive esophagitis. There is insufficient evidence to assess whether H2RAs have an independent chemopreventive effect in patients with BO.
Supplementary Material
Supplementary Table 1A. Newcastle-Ottawa scale for assessment of quality of included studies – Case-control studies (each asterisk represents if individual criterion within the subsection were fulfilled)
Supplementary Table 1B. Newcastle-Ottawa scale for assessment of quality of included studies – Cohort studies (each asterisk represents if individual criterion within the subsection were fulfilled)
Significance of this study.
What is already known about this subject?
The incidence of oesophageal adenocarcinoma (OAC) is rising and it is associated with high mortality.
Barrett’s oesophagus (BO), which is a result of chronic inflammation of the oesophagus, confers a significant increased risk of OAC.
Acid-suppressive medications, particularly proton pump inhibitors (PPIs), decrease the risk and severity of erosive esophagitis and gastroesophageal reflux disease.
What are the new findings
PPIs are associated with a 71% decrease in the risk of progression to OAC and/or high-grade dysplasia in patients with BO.
This effect of PPIs is typically seen after 2–3 years of use and is independent of the presence of erosive esophagitis and use of other putative chemopreventive agents like aspirin and statins.
There is insufficient literature to assess whether histamine receptor antagonists modify the risk of OAC in patients with BO.
How might it impact on clinical practice in the foreseeable future?
In patients with BO at high risk of progression to OAC, PPI therapy may be considered for its primary chemopreventive potential.
Acknowledgments
We sincerely thank Ms Pat Erwin, Medical Librarian at the Mayo Clinic Library for helping in the literature search for this systematic review and meta-analysis.
Footnotes
Contributors Study concept and design: SS, HES; acquisition of data: SS, SK, PPS; analysis and interpretation of data: SS, SK, PPS, PGI, HES; drafting of the manuscript: SS; critical revision of the manuscript for important intellectual content: SK, PPS, PGI, HES; approval of the final manuscript: SS, SK, PPS, PGI, HES; study supervision: HES.
Competing interests None.
Provenance and peer review Not commissioned; externally peer reviewed.
Data sharing statement Data are available for sharing for scientific purposes.
References
- 1.Lagergren J, Lagergren P. Recent developments in esophageal adenocarcinoma. CA Cancer J Clin. 2013;63:232–48. doi: 10.3322/caac.21185. [DOI] [PubMed] [Google Scholar]
- 2.Spechler SJ, Sharma P, Souza RF, et al. American Gastroenterological Association technical review on the management of Barrett’s esophagus. Gastroenterology. 2011;140:e18–52. doi: 10.1053/j.gastro.2011.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jankowski JA, Hawk ET. A methodologic analysis of chemoprevention and cancer prevention strategies for gastrointestinal cancer. Nat Clin Pract Gastroenterol Hepatol. 2006;3:101–11. doi: 10.1038/ncpgasthep0412. [DOI] [PubMed] [Google Scholar]
- 4.Falk GW, Jankowski J. Chemoprevention and Barrett’s esophagus: decisions, decisions. Am J Gastroenterol. 2008;103:2443–5. doi: 10.1111/j.1572-0241.2008.02129.x. [DOI] [PubMed] [Google Scholar]
- 5.Hur C, Broughton DE, Ozanne E, et al. Patient preferences for the chemoprevention of esophageal adenocarcinoma in Barrett’s esophagus. Am J Gastroenterol. 2008;103:2432–42. doi: 10.1111/j.1572-0241.2008.02117.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Falk GW, Buttar NS, Foster NR, et al. A combination of esomeprazole and aspirin reduces tissue concentrations of prostaglandin E(2) in patients with Barrett’s esophagus. Gastroenterology. 2012;143:917–26. doi: 10.1053/j.gastro.2012.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fitzgerald RC, Lascar R, Triadafilopoulos G. Review article: Barrett’s oesophagus, dysplasia and pharmacologic acid suppression. Aliment Pharmacol Ther. 2001;15:269–76. doi: 10.1046/j.1365-2036.2001.00939.x. [DOI] [PubMed] [Google Scholar]
- 8.Abdalla SI, Lao-Sirieix P, Novelli MR, et al. Gastrin-induced cyclooxygenase-2 expression in Barrett’s carcinogenesis. Clin Cancer Res. 2004;10:4784–92. doi: 10.1158/1078-0432.CCR-04-0015. [DOI] [PubMed] [Google Scholar]
- 9.Garcia Rodriguez LA, Lagergren J, Lindblad M. Gastric acid suppression and risk of oesophageal and gastric adenocarcinoma: a nested case control study in the UK. Gut. 2006;55:1538–44. doi: 10.1136/gut.2005.086579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kastelein F, Spaander MC, Steyerberg EW, et al. Proton pump inhibitors reduce the risk of neoplastic progression in patients with Barrett’s esophagus. Clin Gastroenterol Hepatol. 2013;11:382–8. doi: 10.1016/j.cgh.2012.11.014. [DOI] [PubMed] [Google Scholar]
- 11.Nguyen DM, El-Serag HB, Henderson L, et al. Medication usage and the risk of neoplasia in patients with Barrett’s esophagus. Clin Gastroenterol Hepatol. 2009;7:1299–304. doi: 10.1016/j.cgh.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151:264–9. W64. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 13.Wells GA, Shea B, O’Connell D, et al. [accessed 31 Oct 2013];The Newcastle–Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- 14.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 15.Guyatt GH, Oxman AD, Kunz R, et al. GRADE guidelines: 7. Rating the quality of evidence—inconsistency. J Clin Epidemiol. 2011;64:1294–302. doi: 10.1016/j.jclinepi.2011.03.017. [DOI] [PubMed] [Google Scholar]
- 16.Kanwal F, White D. Systematic reviews and meta-analyses. Clinical Gastroenterology and Hepatology Clin Gastroenterol Hepatol. 2012;10:1184–6. doi: 10.1016/j.cgh.2012.09.019. [DOI] [PubMed] [Google Scholar]
- 17.Sterne JA, Egger M, Smith GD. Systematic reviews in health care: investigating and dealing with publication and other biases in meta-analysis. BMJ. 2001;323:101–5. doi: 10.1136/bmj.323.7304.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Jonge PJF, Steyerberg EW, Kuipers EJ, et al. Risk factors for the development of esophageal adenocarcinoma in Barrett’s esophagus. Am J Gastroenterol. 2006;101:1421–9. doi: 10.1111/j.1572-0241.2006.00626.x. [DOI] [PubMed] [Google Scholar]
- 19.Hillman LC, Chiragakis L, Shadbolt B, et al. Proton-pump inhibitor therapy and the development of dysplasia in patients with Barrett’s oesophagus. Med J Aust. 2004;180:387–91. doi: 10.5694/j.1326-5377.2004.tb05991.x. [DOI] [PubMed] [Google Scholar]
- 20.Nguyen DM, Richardson P, El-Serag HB. Medications (NSAIDs, statins, proton pump inhibitors) and the risk of esophageal adenocarcinoma in patients with Barrett’s esophagus. Gastroenterology. 2010;138:2260–6. doi: 10.1053/j.gastro.2010.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Altawil JIB, Jinjuvadia R, Antaki F, et al. Can progression to dysplasia in Barrett’s esophagus be prevented by proton pump inhibitors? Am J Gastroenterol. 2011;106:S31. [Google Scholar]
- 22.Jung KW, Talley NJ, Romero Y, et al. Epidemiology and natural history of intestinal metaplasia of the gastroesophageal junction and Barrett’s esophagus: a population-based study. Am J Gastroenterol. 2011;106:1447–55. doi: 10.1038/ajg.2011.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.El-Serag HB, Aguirre TV, Davis S, et al. Proton pump inhibitors are associated with reduced incidence of dysplasia in Barrett’s esophagus. Am J Gastroenterol. 2004;99:1877–83. doi: 10.1111/j.1572-0241.2004.30228.x. [DOI] [PubMed] [Google Scholar]
- 24.Sikkema M, Looman CWN, Steyerberg EW, et al. Predictors for neoplastic progression in patients with Barrett’s esophagus: a prospective cohort study. Am J Gastroenterol. 2011;106:1231–8. doi: 10.1038/ajg.2011.153. [DOI] [PubMed] [Google Scholar]
- 25.Altawil J, Irwin B, Jinjuvadia R, et al. Chemoprevention of Barrett’s esophagus in patients with acid reflux. Gastroenterology. 2011;140:S258. [Google Scholar]
- 26.Gatenby PA, Ramus JR, Caygill CP, et al. Treatment modality and risk of development of dysplasia and adenocarcinoma in columnar-lined esophagus. Dis Esophagus. 2009;22:133–42. doi: 10.1111/j.1442-2050.2008.00886.x. [DOI] [PubMed] [Google Scholar]
- 27.Hillman LC, Chiragakis L, Shadbolt B, et al. Effect of proton pump inhibitors on markers of risk for high-grade dysplasia and oesophageal cancer in Barrett’s oesophagus. Aliment Pharmacol Ther. 2008;27:321–6. doi: 10.1111/j.1365-2036.2007.03579.x. [DOI] [PubMed] [Google Scholar]
- 28.Sikkema M, de Jonge PJ, Steyerberg EW, et al. Risk of esophageal adenocarcinoma and mortality in patients with Barrett’s esophagus: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2010;8:235–44. doi: 10.1016/j.cgh.2009.10.010. [DOI] [PubMed] [Google Scholar]
- 29.Wani S, Falk GW, Post J, et al. Risk factors for progression of low-grade dysplasia in patients with Barrett’s esophagus. Gastroenterology. 2011;141:1179–86. doi: 10.1053/j.gastro.2011.06.055. [DOI] [PubMed] [Google Scholar]
- 30.Islami F, Kamangar F, Boffetta P. Use of proton pump inhibitors and risk of progression of Barrett’s esophagus to neoplastic lesions. Am J Gastroenterol. 2009;104:2646–8. doi: 10.1038/ajg.2009.369. [DOI] [PubMed] [Google Scholar]
- 31.Liao LM, Vaughan TL, Corley DA, et al. Nonsteroidal anti-inflammatory drug use reduces risk of adenocarcinomas of the esophagus and esophagogastric junction in a pooled analysis. Gastroenterology. 2012;142:442–52. doi: 10.1053/j.gastro.2011.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Singh S, Singh AG, Singh PP, et al. Statins are associated with reduced risk of esophageal cancer, particularly in patients with Barrett’s esophagus: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2013;11:620–9. doi: 10.1016/j.cgh.2012.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fitzgerald RC. Barrett’s oesophagus and oesophageal adenocarcinoma: how does acid interfere with cell proliferation and differentiation? Gut. 2005;54(Suppl 1):i21–6. doi: 10.1136/gut.2004.041558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shirvani VN, Ouatu-Lascar R, Kaur BS, et al. Cyclooxygenase 2 expression in Barrett’s esophagus and adenocarcinoma: ex vivo induction by bile salts and acid exposure. Gastroenterology. 2000;118:487–96. doi: 10.1016/s0016-5085(00)70254-x. [DOI] [PubMed] [Google Scholar]
- 35.Jurgens S, Meyer F, Spechler SJ, et al. The role of bile acids in the neoplastic progression of Barrett’s esophagus—a short representative overview. Z Gastroenterol. 2012;50:1028–34. doi: 10.1055/s-0032-1312922. [DOI] [PubMed] [Google Scholar]
- 36.Ouatu-Lascar R, Fitzgerald RC, Triadafilopoulos G. Differentiation and proliferation in Barrett’s esophagus and the effects of acid suppression. Gastroenterology. 1999;117:327–35. doi: 10.1053/gast.1999.0029900327. [DOI] [PubMed] [Google Scholar]
- 37.Kedika RR, Souza RF, Spechler SJ. Potential anti-inflammatory effects of proton pump inhibitors: a review and discussion of the clinical implications. Dig Dis Sci. 2009;54:2312–17. doi: 10.1007/s10620-009-0951-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cooper BT, Chapman W, Neumann CS, et al. Continuous treatment of Barrett’s oesophagus patients with proton pump inhibitors up to 13 years: observations on regression and cancer incidence. Aliment Pharmacol Ther. 2006;23:727–33. doi: 10.1111/j.1365-2036.2006.02825.x. [DOI] [PubMed] [Google Scholar]
- 39.Win LL, Yuan Y, Hunt RH. Effect of proton pump inhibitors (PPIs) treatment on the length of Barrett’s esophagus: a systematic review of cohort studies. Gastroenterology. 2011;140:S219. [Google Scholar]
- 40.Haigh CR, Attwood SE, Thompson DG, et al. Gastrin induces proliferation in Barrett’s metaplasia through activation of the CCK2 receptor. Gastroenterology. 2003;124:615–25. doi: 10.1053/gast.2003.50091. [DOI] [PubMed] [Google Scholar]
- 41.Kuipers EJ. Barrett’s oesophagus, proton pump inhibitors and gastrin: the fog is clearing. Gut. 2010;59:148–9. doi: 10.1136/gut.2009.191403. [DOI] [PubMed] [Google Scholar]
- 42.Obszynska JA, Atherfold PA, Nanji M, et al. Long-term proton pump induced hypergastrinaemia does induce lineage-specific restitution but not clonal expansion in benign Barrett’s oesophagus in vivo. Gut. 2010;59:156–63. doi: 10.1136/gut.2009.186775. [DOI] [PubMed] [Google Scholar]
- 43.Sharaiha RZ, Wang YC, Neugut AI, et al. Cost-effectiveness of chemoprevention with and without proton pump inhibitors in Barrett’s esophagus. Gastroenterology. 2011;140:S204–5. [Google Scholar]
- 44.Ali T, Roberts DN, Tierney WM. Long-term safety concerns with proton pump inhibitors. Am J Med. 2009;122:896–903. doi: 10.1016/j.amjmed.2009.04.014. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1A. Newcastle-Ottawa scale for assessment of quality of included studies – Case-control studies (each asterisk represents if individual criterion within the subsection were fulfilled)
Supplementary Table 1B. Newcastle-Ottawa scale for assessment of quality of included studies – Cohort studies (each asterisk represents if individual criterion within the subsection were fulfilled)