Abstract
Filopodia are cellular protrusions that have been implicated in many types of mechanosensory activities. Morphogens are signaling proteins that regulate the patterned development of embryos and tissues. Both have long histories that date to the beginnings of cell and developmental biology in the early 20th century, but recent findings tie specialized filopodia called cytonemes to morphogen movement and morphogen signaling. This review explores the conceptual and experimental background for a model of paracrine signaling in which the exchange of morphogens between cells is directed to sites where cytonemes directly link cells that produce morphogens to cells that receive and respond to them.
Keywords: morphogen, cytoneme, developmental organizer, signaling center
Experimental manipulations have taught us that cells in animal embryos do not develop autonomously as they progress through undifferentiated, pre-terminal states. Instead, cells grow, take on fates and assume roles under the influence of the environment in the embryo. Much remains to be learned about the cues that inform cells where they are and what they should do. This review focuses on one aspect of the informational molecules that impart positional information - what we know and how we conceptualize the mechanisms that distribute them to their target fields. Recently published experiments suggest that some of the core tenets that have long influenced this field require reevaluation. I begin with a brief description of the general attributes of the contexts and systems within which the cues move and operate.
Signaling centers and morphogen gradients
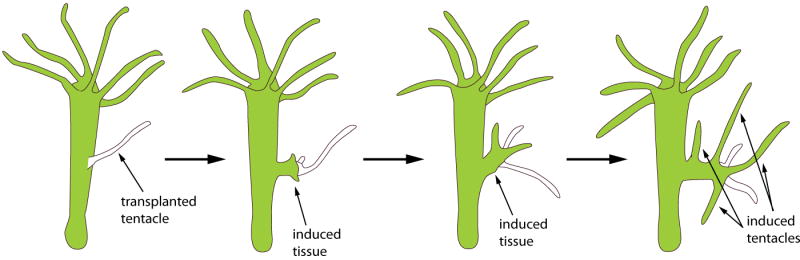
Perhaps the first experimental evidence for signaling centers whose specialized cells produce the cues that induce their neighbors to adopt specific fates came from the work of E.N. Browne, a student of E.B. Wilson 1, 2. Working with Hydra viridis, Browne discovered that transplantation of tentacle and peristome base material into the middle of the body of a host hydra induced a new head composed of both graft and host material (Figure 1). Browne’s publication did not comment on the significance of this finding, but subsequent studies by Mangold and Spemann demonstrated a similar type of inductive capacity in the gastrula of newts. Transplantation of the dorsal blastopore lip of a Triton cristatus grastrula into virtually any site in a Triton taeniatus host induced a second embryo to develop that was composed of both donor and host cells, and these investigators developed the concept of an “organizing center” to describe the special function of dorsal lip cells 3. The “inducer” molecule responsible for organizer activity proved to be elusive, and its identification resisted significant and determined effort by many labs for many years. Nevertheless, studies of other animals and other organ systems revealed the apparent universality of the organizer concept – that the developmental fields that constitute these organ systems are regulated by local signaling centers.
Figure 1. Developmental induction revealed by grafting experiments in Hydra.
When grafted onto the middle of the body of a healthy green hydra, transplantation of a white tentacle with peristome tissue at its base induces the host to produce ectopic tentacles. (adapted from 1)
In the absence of an identified “inducer”, the mathematician A. Turing proposed the term morphogen to describe a hypothetical diffusible molecule that specifies cell fate in a concentration-dependent fashion, and Turing developed mathematics for a reaction-diffusion system capable of forming a stable concentration gradient from an initially homogeneous equilibrium 4. This hypothesis ascribed two properties to the inducer: the ability to elicit concentration-dependent responses from target cells, and a mode of dispersal – diffusion - that forms a concentration gradient of the morphogen. These two aspects of morphogen inducers are functionally independent and will be considered separately.
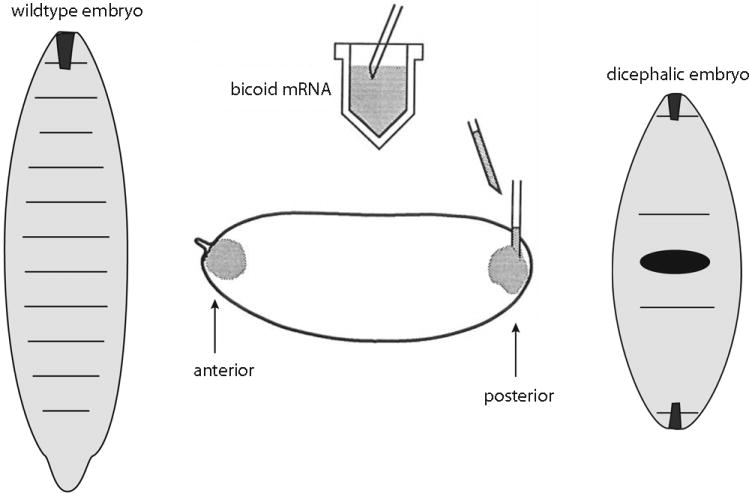
Sixty-plus years after the Mangold and Spemann publication, identification of a morphogen with the properties expected of an inducer emerged from genetic studies of Drosophila embryogenesis (reviewed in 5). Nüsslein-Volhard’s lab showed that mutants defective for the bicoid (bcd) gene, which encodes a homeodomain protein that can regulate transcription, fail to develop embryos with anterior structures. In normal syncytial blastoderm embryos, Bcd protein distributes in a monotonic concentration gradient with highest levels at the anterior pole, and evidence of several types indicates that Bcd levels determine position-specific expression of target genes in the anterior embryo and along the A–P axis. The position at which target genes are expressed changes in response to experimentally engineered decreases or increases in Bcd levels; the expression of reporter genes constructed with varied numbers of Bcd binding sites show similar behaviors; and most tellingly, perhaps, experiments conceptually similar to the earlier Hydra and amphibian ones show that transplantation of Bcd mRNA to the posterior region of host embryos induces ectopic anterior structures with the most anterior elements arising from the regions closest to the site of injection (Figure 2). Demonstration that the anterior morphogen of the Drosophila embryo is a protein was a watershed event that fully validated the concept of signaling centers and gradients. The discovery and characterization of several other morphogen gradient systems has followed.
Figure 2. Embryonic induction by Drosophila bicoid mRNA.
bicoid mRNA is localized to the anterior pole in normal, wildtype Drosophila embryos, but injection of bicoid mRNA into the posterior pole induces the embryo to develop with head and thoracic structures closest to the site of injection. (adapted from 128)
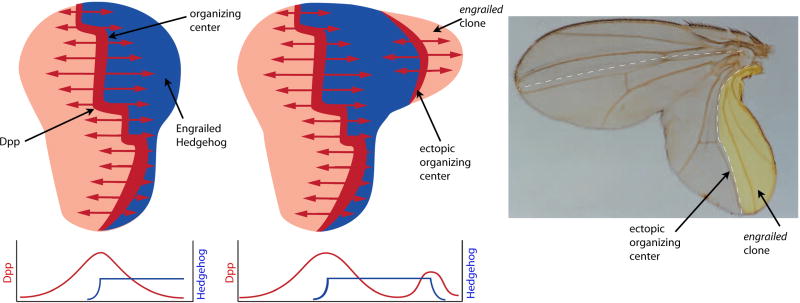
In the Drosophila wing imaginal disc, for example, an organizing (signaling) center produces and disseminates the TGF-β family member Decapentaplegic (Dpp) (Figure 3). This signaling center is a stripe 3–4 cells wide that is situated just to the anterior side of the A/P compartment border and runs the length of the disc’s A/P axis. Dpp disperses from the signaling center across the disc and regulates target genes at both close range in Dpp-expressing cells as well as at long range in cells as far as 150 μm away at the flanks of the disc. Under conditions in which Dpp:GFP is over-expressed in the Dpp signaling center, GFP fluorescence spreads outward toward the flanks of the disc forming monotonic concentration gradients to either side 6. Ectopic expression of Dpp, either induced by an ectopic compartment border (Figure 3) 7 or in somatic clones that make Dpp in certain regions of the disc under Gal4-UAS control 8, elicits responses that are consistent with Dpp’s role as a morphogen: wing duplications grow that are composed of both cells whose genetic constitution changed as well as neighboring cells that were induced to adopt new fates and patterns. The morphology and patterns of the duplicated wings are remarkably similar to normal wings (Figure 3).
Figure 3. Induction in the Drosophila wing imaginal disc.
The normal, wildtype wing disc (left) has an organizing center that expresses Dpp (red) in response to signaling by Hh (blue), which originates in the Posterior (P) compartment. Levels of Dpp and Hh across the disc are depicted in the graph below. A disc (middle) with a clone in the P compartment that is deficient for engrailed function induces an ectopic organizing center and a duplication that includes both engrailed mutant and normal cells. Micrograph of wing with induced duplication and induced ectopic organizing center (right). (adapted from 7)
Hedgehog (Hh), Wingless (Wg), epidermal growth factor (EGF) and fibroblast growth factor (FGF) are other proteins that have been shown in various contexts to emanate from localized sources and to elicit distinct responses at various distances from their source. The list of these six morphogens - Bcd, Dpp, Hh, Wg, EGF and FGF - is evidence of the rapid progress that has been made in recent years. But this list is also notable for its apparent exclusivity – various combinations of the Dpp, Hh, Wg, EGF and FGF proteins regulate the development of tissues and organs in all vertebrate and invertebrate systems that have been studied, suggesting that these proteins may represent a universal vocabulary that mediates positional information in metazoans.
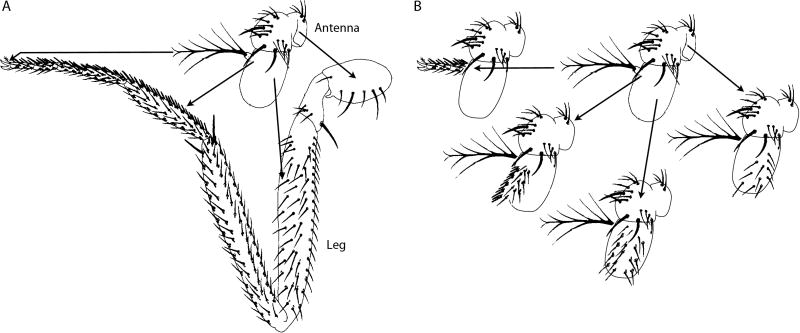
The idea that patterning systems in different organs and body structures are functionally homologous is not new. In 1971, a description of partial homeotic transformations induced in a Drosophila Antennapedia mutant (AntpR) suggested that the patterning systems in Drosophila legs and antennae are functionally related 9. The specific antennal regions that were transformed to leg structures differed in individual AntpR mutant flies, but point-to-point mapping revealed that the type of leg structure in any given region of the antenna of a particular fly corresponded precisely to the homologous position of the antenna cells that had transformed (Figure 4). This apparent homology can now be understood in terms of the morphogens that pattern both organs. Although the two organs analyzed in the study of AntpR are closely related developmentally and their inter-conversion was brought about by a single genetic change, the widespread role of the known morphogens suggests that most systems responsible for defining position and establishing patterns share components and mechanisms.
Figure 4. Homeotic transformation of the Drosophila antenna.
The antenna to leg transformation in AntpR mutants is incompletely penetrant such that individual flies have mosaics patches of transformed tissue. There is a precise correspondence between the location of the transformed patch and the type of leg structure in antenna-leg chimeras. (adapted from 9)
Commonality of patterning mechanisms between organs of an animal and between distantly related animals has interesting implications; one that is relevant to this discussion is the manner by which morphogens disseminate. If every organ and developmental field in an animal is regulated by local organizing centers, and if all cells determine their position in their respective field by responding to a shared and limited set of morphogens, then the mechanism that distributes these positional cues must also constrain them 10. Dispersal must not only deliver the cues in a manner that informs the receiving cells of each cue’s identity and quantity, but dispersion must also ensure that the cells respond only to the outputs of the relevant local organizing centers.
Topologies of developmental fields
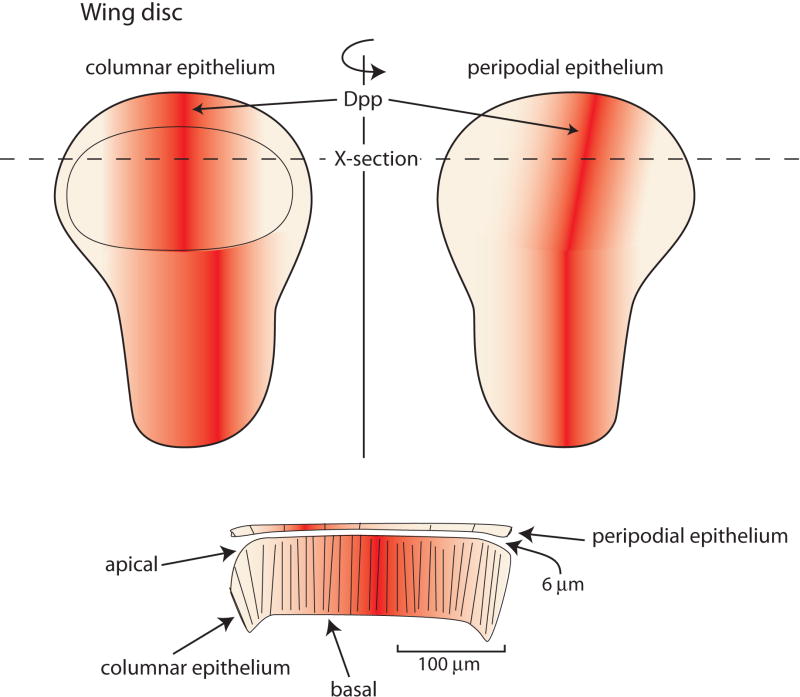
Two aspects of developmental fields that set restrictive parameters for the mechanisms that disperse morphogens are the relative proximity of cells to a signaling center and the field’s physical shape. The importance and significance of proximity and shape are evident in the topography of the Drosophila wing disc and its A/P organizer, but the issues are pertinent to other (and perhaps all) signaling contexts. The third instar wing disc is a flattened sac with two closely apposed epithelial sheets composed of cells that have markedly different shapes. On one side are highly columnar cells (height, 30–45 μm; diameter, 0.5–2.2 μm), each of which has an apical surface that faces a lumen and a basal surface that is juxtaposed to a basal lamina/extra cellular matrix. On the other side of the lumen, which is ≤6 μm wide in the area of the wing primordium, the peripodial layer is comprised of ellipsoidal squamous cells (height, 5 μm; diameters, 15–22 μm and 45–55 μm). The cells of the A/P organizer form a stripe on both the columnar and peripodial layers that connects at a point on the most ventral edge of the disc. Although the stripe is continuous, the stripe’s columnar cells in the late third instar disc are not directly across the disc lumen from its peripodial cells (Figure 5). As a consequence, many cells of both the columnar and peripodial epithelia are farther from their respective A/P organizer (>100 μm) than they are from the A/P organizer center of the apposing epithelial sheet. Therefore, the mechanisms that disperse morphogen proteins to set up the A/P organizers (e.g. Hh), as well as the Dpp morphogen protein that emanates from the A/P organizer must be constrained or targeted so they signal only to the appropriate developmental field.
Figure 5. A/P organizing centers of the wing disc columnar and peripodial epithelia.
Frontal (top) and transverse (bottom) depictions of a wing disc and the distribution of Dpp from the organizing centers of each layer. The disc is a flattened sac and the Dpp organizing centers are not juxtaposed.
Hh, the signaling protein that sets up the wing disc A/P organizer, is produced by the posterior compartment cells of both the columnar and peripodial layers. It moves across the A/P compartment borders in each layer, and in the columnar layer forms a short concentration gradient in nearby A compartment cells. Hh induces A/P organizers in both layers. Although there have been reports suggesting that Hh signals apically 11, 12, the best current evidence indicates that Hh is not released apically from producing cells in the P compartment, but moves basolaterally to receiving cells 13, 14. Thus, Hh does not apparently access the opposing layer. Experimental conditions have been described that result in cross-lumenal Hh signaling by peripodial cells – for instance, over-expression in peripodial cells of Hh-N, a mutant form of Hh that lacks the cholesterol that is normally covalently bound to it’s C-terminus. Hh-N exits these cells, though not apparently by the normal mechanism that releases lipidated Hh 15, and Hh-N that is freed of its normal constraints activates Hh target gene expression in apposing cells of the columnar layer, leading to abnormalities 12. The key point here is not the particular conditions that led to cross-lumenal signaling but the fact that under normal conditions the disc system limits signaling in each layer.
In contrast to Hh, Dpp moves apically between columnar cells 16–19, and cross-lumenal signaling might compromise the integrity of the Dpp gradients of the two layers. Wing disc cells in the A and P compartments of both the columnar and peripodial layers are sensitive to Dpp and the observed patterns of target gene expression are not consistent with signaling between the two layers. Yet, in the 3rd instar wing disc, Dpp apparently moves >100 μm across the plane of each epithelium from the A/P organizers to the flanks but does not signal even the short (~6 μm) distance across the lumen to the apposing columnar layer. Supporting evidence is provided by expression patterns in normal discs as well as the apparent lack of signaling between layers in discs in which peripodial cells over-express Dpp 20.
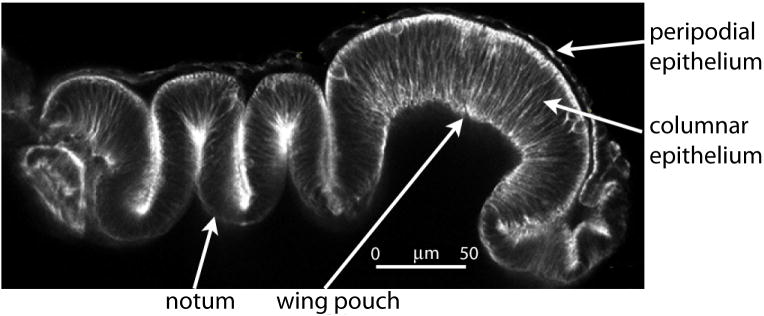
The shape of the disc epithelia is a second important consideration. Although the cells that make up the epithelia are arranged as single-layered sheets, and although Dpp signaling, for example, extends across the disc, the epithelium has deep folds and the wing pouch region is concave (Figure 6). For many cells, the shortest distance to the cells that express Dpp is less than any path that follows the plane of the epithelium. The idea that distance across an epithelium is encoded by the relative concentration of a morphogen demands that concentration decline with distance along the plane of the epithelium, and the mechanism that disperses Dpp must therefore ensure that the contours of morphogen gradients are not distorted by folds or other non-linear topological features.
Figure 6. Topography of the 3rd instar wing disc.
This transverse optical section shows the highly folded topography of the wing disc that juxtaposes cells in space that are far apart within the columnar epithelial layer 129.
Diffusion models for cell-cell signaling
Diffusion is a mode of dispersing signals that can be described as “random and indirect” - it is a mechanism that involves export and release of signals into extracellular fluids for transport to distant locations. The distinguishing feature is that released and disseminated signals influence the activity of distant cells indirectly because the producing and receiving cells do not contact each other. Endocrine cells are an example. They secrete protein hormones into the bloodstream to modulate growth or activity of distant organs. Dissemination of endocrine-derived signaling proteins is essentially unconstrained, and in a human, for example, these proteins can distribute throughout the vasculature in less than a minute. The level of released signaling proteins in the bloodstream is a function of rate of secretion by the endocrine cells and the processes of uptake and degradation. Importantly, target cells respond to levels of signaling proteins without regard to the source.
Long distance signaling appears to attract inflammatory cells of the immune system to sites of injury and infection. Relevant chemoattractants include reactive oxygen species 21 and chemokines, and in cell culture, neutrophils move up chemokine concentration gradients that change only 1–2% across the diameter of the cell 22. Although soluble concentration gradients of chemokines have not been directly observed in vivo, it is assumed that chemokines disperse in a diffusion-limited fashion to form soluble gradients whose high-points define destination sites for cells that respond to both absolute and temporal differences. This mode of chemokine signaling is also indirect – the communicating cells are not in contact. Nevertheless, direct contact-dependent signaling may play roles in chemokine signaling as well. Chemokines also exist in surface-immobilized forms that contribute to migration behaviors 23, 24, and neutrophils and other types of blood cells make cellular extensions that appear to make long distance cell-cell contacts 25, 26. The relative roles and importance of the soluble and tethered chemokines has not been resolved.
Diffusion is a mechanism that has also been proposed to move morphogen proteins from producing to target cells. Diffusing morphogen might generate a concentration gradient whose contours are determined by the properties of the morphogen, by the medium in which the morphogen moves and by the interactions that the morphogen has with its environment. Parameters that describe such morphogen movement were initially analyzed by Crick 27, who broached the idea that the processes that generate morphogen gradients are governed by the chemistry of their components and therefore can be described mathematically. Crick calculated the time needed to set up gradients in embryonic fields and concluded that within the known size ranges of embryos, aqueous diffusion may generate steady concentration gradients in the time intervals of early animal development. His calculations were for small molecules that have a molecular weight range of 300 – 500 daltons and that diffuse freely in and out of cells, in cytoplasm and between cells.
The morphogen activities in the systems that are now characterized have been identified with signaling proteins, not small organic molecules, and contemporary analyses have examined how such morphogen proteins might disperse to generate concentration gradients that form if they are secreted to disperse broadly in extracellular fluid. The approach inaugurated by Crick has been extended to these systems by numerous types of mathematical treatments. These investigations have explored the parameters that must be fulfilled in order to accurately model the experimentally-determined properties of signaling proteins (reviewed in 28, 29). They consider features such as the size and shape of morphogen proteins, interactions of the morphogens with nonreceptor surfaces, kinetics of morphogen dissemination, volumes and distances that morphogens traverse, the paths they can take, and the nature and binding characteristics of their receptors. The numerical solutions show that not only are the distances between source cell and most remote target cell covered in the time frame that the biology requires, but morphogen movement is likely to be so rapid that barriers and efficient removal systems (“sinks”) must exist to sculpt the observed concentration gradients. Thus, these studies show that elegant mathematical models based on diffusion can describe observed morphogen behaviors, and experimental observations of morphogen kinetics that conform to such calculations have been interpreted to indicate diffusive movement 30–34.
However, correlation is not proof of mechanism and does not distinguish between mechanisms, and these conclusions are based on a number of unproven assumptions. For example, the calculations require a value for the path a morphogen can follow, but whereas the total volume of a tissue can be calculated from direct measurements, the extracellular volume is unknown. For purposes of calculations, values have been estimated as fraction of tissue volume not accounted for from cells from electron micrographs, but we lack ways to measure them directly. Similarly, values for geometric tortuosity (increases in diffusive path lengths as a result of physical obstacles), for viscosity of intercellular fluid or for reversible interactions with immobilized molecules cannot be tested. Most importantly, perhaps, all diffusive models assume that morphogen protein disseminates in extracellular fluid after release from producing cells, but as described below, experimental proof that morphogen proteins move long distances while untethered to a cell is difficult to obtain.
A direct delivery model for morphogen dispersion
Direct delivery of morphogens at points of contact between producing and target cells is conceptually distinct from diffusion-based mechanisms, all of which involve indirect transfer. A direct signaling mechanism, in contrast, releases signals specifically where producing and target cells contact each other and where uptake occurs. In essence, it involves cell-to-cell transfers in a way that is conceptually similar to signaling at neuronal synapses.
Neurons signal across distances and intervening cells by extending processes (axons and dendrites) that terminate at synaptic junctions where neurotransmitters released by presynaptic cells are taken up specifically and exclusively by postsynaptic cells. Neuronal projections can extend long distances and can transmit signals by several different means. One mode employs chemical neurotransmitters that are released by a presynaptic cell at a synapse and that bind to receptors present in the juxtaposed membrane of the postsynaptic cell. These receptors initiate synaptic transmission by generating a wave of depolarization that conveys information from the synapse, but the important point is not the specific mechanism that conveys responses from the synapse, but that neurotransmitters transfer between producing and target cells at cell-cell contacts that are pre-selected, regulated and structured. The cell bodies of the communicating cells may be far apart, but exchanges of signal are focused to a synaptic cleft whose composition and size (approximately 15–20 nm across) are tightly controlled and may define a privileged environment. The process that generates the synapse determines the identities of the communicating cells.
In addition to small molecule neurotransmitters, neuronal signals include neurotrophins that promote survival and morphogenesis. Neurotrophins released from producing cells bind to receptors of target neurons, and are taken up, packaged into endosomes and transported to the neuronal cell body. Two other protein signals with roles at synapses are Hh and Wg, which both play essential roles during neuronal development. Photoreceptor neurons in the developing Drosophila retina synthesize Hh, package it in vesicles, and release it at axonal termini where postsynaptic neurons require Hh for growth and differentiation (Fig. 2) 35, 36. Wg is required at Drosophila glutamatergic neuromuscular junctions to establish normal bouton number and morphology 37. Wg concentrates in vesicles at presynaptic boutons and is secreted and taken up by the postsynaptic cell across the synaptic cleft 38. Thus, it appears that the various types of neuronal signals move from pre- to postsynaptic cell by a similar route.
The direct delivery model of signaling by non-neuronal cells posits that signaling proteins such as Hh, Dpp, EFG, FGF and Wg transfer between these cells by mechanisms that share key features with the one that transfers Wg at neuronal synapses – by release and uptake at organized and regulated sites of cell-cell contact.
Cytonemes: specialized signaling filopodia
Filopodia are thin protrusions that extend from many types of cells. They have been observed in many developmental contexts and have been given many names – microspikes, pseudopods, thin filopodia 39, thick filopodia 40, gliopodia 41, myopodia 42, invadopodia 43, podosomes 44, telopodes 45, tunneling nanotubes 46 and cytonemes 47. Although these organelles have physical properties in common – all are constructed with tight parallel bundles of actin filaments that assemble with actin-related and cytoskeletal proteins – but they are not a constant size. Some have been measured as thin as 0.1 μm in diameter, others 0.3 μm; as short as 2 μm or extending more than 200 μm. And despite the more than one hundred years since filopodia were first observed, their roles remained unproven because it has not been possible to selectively remove or inactivate them without compromising the integrity of the cells that make them. Therefore, the questions remain what exactly the filopodia do and what the significance of the physical features that distinguish them in different cell types might be.
Filopodia have been associated with many processes including cell migration 48–50, cell adhesion 51, chemotaxis 52, force generation 53–55, wound healing 56–58, environmental sensing 26, antigen presentation 59, neuronal growth cone pathfinding 42, 60–68, angiogenesis 69, 70, virus transmission 71 and embryonic development 40, 72, 73. Although there are no experiments that clearly reveal the roles filopodia may have in these processes, circumstantial evidence has suggested two types of functions: as chemosensory “antennae” that probe the microenvironment of a cell, and as tethers that transmit mechanical tension between cells. In systems that support real time observation, some have dynamic and complex behaviors that are thought to reflect a sensory role. The growth cones of developing axons of nerve cells, for example, have many filopodia that grow and retract rapidly, appearing to search the surrounding space for guidance cues. Filopodia extending from the dendrites of neurons have lifetimes that range from minutes to hours and have tips that appear to make transient contacts with axons 74. Some of the contacts are more stable than others, leading to the idea that their behavior reflects an active process that discriminates between possible targets.
Filopodia with similar characteristics have been observed in non-neuronal cells; indeed, descriptions of filopodia made by primary mesenchyme cells of the sea urchin blastula were the first to note their dynamic nature 75. The intriguing behaviors of these filopodia led to the idea that they play active roles as sensors of patterning information. Although direct evidence for the function of these filopodia is still lacking today, data from subsequent studies are consistent with both structural 76 and sensory roles 39. Most interesting and relevant to this discussion are parallels that were drawn between the “thin filopodia” of the primary mesenchyme cells and the filopodia of neuronal growth cones 39. These types of filopodia have similar diameters and extension and retraction rates, and for both, observed responses to perturbations are consistent with anthropomorphic depictions as sensory implements that extend the reach of cells into surrounding space.
Cytonemes were first noted as long, actin-based filopodia that extend from the apical surface of wing imaginal disc cells that express cytoplasmic GFP 47. These fluorescent tendrils were visible where they extend over a non-fluorescent background and the cytoneme name was coined to denote their cytoplasmic content and “thread-like” appearance, and to distinguish them for their specialized role in signaling. They were estimated to be ≤200 nm diameter, and in the wing pouch primordium they orient uniformly toward the anterior/posterior (A/P) compartment boundary. As noted above, cells at the A/P compartment boundary express Dpp, and diffusion models for its dispersion assume that Dpp is secreted and finds receptors on target cells by a random walk either through or around intervening cells. The presence of long filopodia that extend from wing disc “receiving” cells to Dpp-expressing cells at the compartment border (Fig. 7) suggested an alternative possibility – that physical contacts are sites at which Dpp transfers to its targets 47. This mechanism of direct delivery is similar, at least in concept, to neurotransmitter release and uptake.
Figure 7. Hh and Dpp are transported by cytonemes in the wing disc.
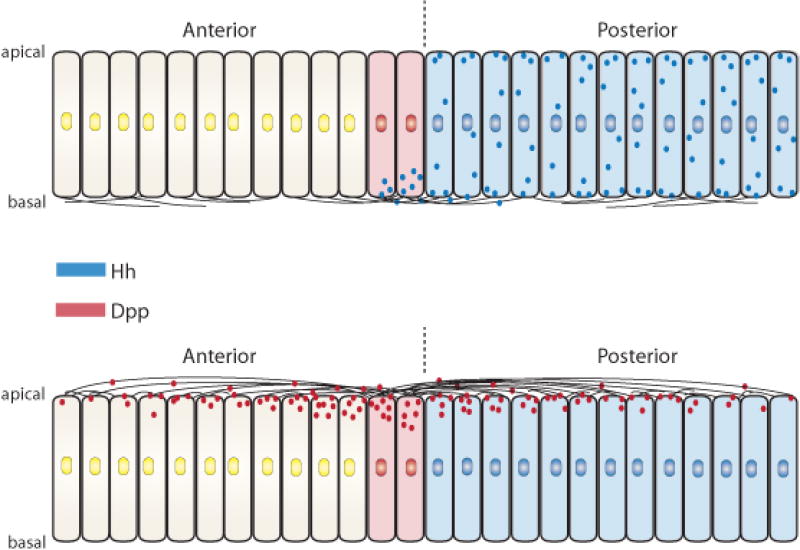
Depictions of transverse sections of the wing disc columnar epithelium showing that (upper drawing) basal cytonemes move Hh (blue) from the cells that make it in the posterior compartment to anterior compartment cells across the compartment border (dashed line) that activate Hh signal transduction and express Dpp (red). Dpp movement from the A/P signaling center is via apical cytonemes (lower drawing).
Defining a cytoneme
As noted above, filopodia have been christened with various names in the many contexts in which they have been studied, and the term cytoneme was coined to denote the filopodia that mediate exchange and transport of signaling proteins. Although the roles of cytonemes in signaling are now established (as described in the following sections), it is not yet clear how many different forms of filopodia exist or in which specific contexts the cytoneme designation is appropriate. Filopodia are associated with many processes, including invasion (invadopodia), force generation, neuronal targeting (growth cone filopodia, dendritic spines, gliopodia and myopodia), cell adhesion (podosomes), antigen presentation, wound healing, virus transfer, Notch and growth factor signaling, and embryonic development. Studies in Drosophila have shown that there are cytonemes that send signaling proteins and cytonemes that receive signaling proteins, and that cytonemes involved in Dpp, Hh, EGF and FGF signaling can be distinguished by composition, location and behavior. We anticipate that as better methods are developed, we will be better equipped to identify filopodia components, to resolve the different types of filopodia, and to learn how composition and structure correlate with function. The many cytoneme subtypes already recognized in Drosophila suggests that these filopodia are a diverse family of organelles, and it may be that the current nomenclature will prove to be inadequate.
Cytonemes of the wing imaginal disc
Cytonemes extend over both the apical and basal surfaces of the polarized columnar epithelial cells in 3rd instar wing discs (Fig. 7). Like many actin-based structures, the apical cytonemes do not survive standard chemical fixation protocols, and even with expression of membrane-tethered GFP, their detection is challenging. Cytonemes can be detected that extend over several adjacent cells along the apical surface of the columnar epithelium, but only with methods that overcome their low fluorescence and rapid quenching, and when discs are flattened to bring the cytoneme shafts into focus. Strict conditions of gentle handling are critical, however, as the cytonemes fragment under excessive force. Short apical cytonemes and basal cytonemes are less challenging to detect, although they are visible only where they extend over non-fluorescent cells.
There are cytonemes on the apical surface that orient toward the middle of the disc from wing pouch cells of both the A and P compartments, and the longest ones reach >80 μm from the far edges of the primordium to the compartment border. They therefore span the entire developmental field of the wing primordium. They appear to track along the surface, following its convoluted contours. On average, they are approximately 20 μm in length, extending across more than ten cells (cell diameters range from 0.5–2.2 μm). Wing pouch cells also extend apical cytonemes toward the dorsal/ventral compartment border; cytonemes of this type are short, have been observed rarely, and have not been characterized. Cells of the notum primordium make apical cytonemes that are approximately 2–13 μm long; their orientations lack apparent directional bias, appearing almost random. Apical cytonemes have not been detected emanating from the cells of the hinge primordium except under conditions that express Dpp ubiquitously 77.
Basal cytonemes in the wing pouch orient laterally, extending either toward the middle of the disc or toward the flanks; there are also basal cytonemes that cross the A/P border from either the A or P side 13, 14, 78. Basal cytoneme lengths range from 4–30 μm, and average 12 μm 79. They are marked and stabilized by over-expressed Ihog (Interference hedgehog) 13, 14. Ihog functions as a Hh co-receptor in Hh receiving cells 80, 81, and although its role in cells that produce Hh have not been defined, under conditions of Ihog over-expression, cytonemes in P compartment cells are stable to chemical fixation, enabling fine structure EM analysis 13.
The Dpp receptor Thickveins (Tkv) concentrates in motile puncta that populate apical cytonemes 77. In wing discs with reduced levels of Dpp (e.g. dppts), cytonemes are abnormal and not uniformly oriented toward the A/P compartment border as they are in normal discs, and in discs with uniform over-expression of Dpp (e.g. hs-dpp), the long cytonemes that orient toward the A/P border in normal discs are absent and only short cytonemes are present. These cytonemes have Tkv-containing puncta and lack apparent directional bias. The changes to the patterns of apical cytonemes appear to be specific to ubiquitous over-expression of Dpp because they were not observed under similar conditions of Hh, FGF or EGF over-expression 79. Thus, the distribution of apical cytonemes in the wing pouch under normal conditions, the localization of Tkv to these cytonemes, and the dependence of the long cytonemes on normal Dpp expression implicate apical cytonemes in Dpp dispersion. Their properties and responses are consistent with the idea that they convey Dpp from the cells that express it at the A/P signaling center to outlying cells of the A and P compartments.
Basal cytonemes have a different role – trafficking Hh. Hh is present and can be seen moving along basal cytonemes, but Hh does not localize to them immediately following its synthesis (reviewed in 82). Rather, it arrives at basal cytonemes via a roundabout intracellular pathway that was discovered by the Guerrero lab 14 (and reviewed in15). This pathway “recycles” Hh, placing newly synthesized Hh in the apical membrane prior to capture in endocytic vesicles that move it to the basolateral compartment. Export to A compartment cells is either along basal cytonemes that extend from Hh-producing cells in the P compartment 13, 14, or along basal A compartment cytonemes that extend from Hh-receiving cells 13, 78, 83. These A compartment basal cytonemes are required for the gradient of Hh on the anterior side of the compartment border and for normal signaling 13, 78.
Tracheal cytonemes
The wing disc in the 3rd instar larva attaches to a branch of the tracheal system (the transverse connective), forming a functional association that enables wing disc-produced signals to regulate tracheal development locally 84, 85. In response to FGF (Branchless/FGF) produced by the wing disc, a tube grows out from the transverse connective approximately 10–16 hours into the 3rd instar period 86. This tube is called the Air Sac Primordium (ASP) because it matures to form the dorsal air sacs of the adult fly. The 3rd instar ASP takes up FGF and Dpp from the disc, requiring both to develop normally 87. Uptake is mediated by cytonemes that directly contact signal-producing disc cells 87. These contacts can be marked with GRASP fluorescence (GFP Reconstitution Across Synaptic Partner), a method that was developed to image membrane contacts at neuronal synapses 88, 89. Dpp and FGF uptake is by one of two types of ASP cytonemes – cytonemes that are populated with motile puncta containing Tkv and that contact Dpp-expressing disc cells, or that are populated with motile puncta containing the FGF receptor (FGFR) and that contact FGF-expressing disc cells 79. Tkv-containing cytonemes that contact Dpp-expressing disc cells take up Dpp, but FGFR-containing cytonemes that contact FGF-expressing disc cells do not take up Dpp 87. Cytonemes containing both receptors have not been observed.
The ASP is a powerful system for studies of cytoneme-mediated paracrine signaling, in part because the methods to prepare samples for imaging are less demanding than the methods required to image apical disc cytonemes, and because there are genetic tools that can be used to target the ASP independently of the wing disc. By taking advantage of these attributes, it was shown that ASPs with defective cytonemes do not activate Dpp or FGF signal transduction and are unable to take up Dpp from discs that express Dpp 87. This study established that signaling from the disc to the ASP requires ASP cytonemes and that normal disc cells that produce Dpp normally do not signal to ASP cells that do not contact the disc with cytonemes. Dpp signaling is therefore contact-dependent.
Cytonemes in other contexts
Cytonemes have been characterized most extensively in the wing disc and ASP, two epithelial tissues of the 3rd instar larva. These cytonemes were studied in unfixed specimens in which fluorescent tags marked either the cytoneme membrane (e.g., CD8:GFP, CD8:Cherry or Cherry-CAAX) or selected cytoneme components (e.g., receptors (Tkv:GFP/Cherry, FGFR:GFP/Cherry), an actin binding protein (Diaphanous:GFP (Dia:GFP)), cell adhesion proteins (Neuroglian:GFP (Nrg:GFP), Capricious:GFP (Caps:GFP)) 77, 79, 87. The low fluorescence conferred by these tags in the thin, ~200 nm diameter cytonemes sets strict signal to noise limits for detection. The wing disc and ASP satisfy these conditions: they have low background fluorescence, and fluorescent tags that are expressed in subsets of cells are visible in cytonemes that extend over non-fluorescent cells. Cytonemes have also been observed in other contexts that meet these parameters.
The 3rd instar eye imaginal disc has low background fluorescence similar to the wing disc, and cytonemes marked with CD8:GFP have been observed extending from the apical surface of some columnar cells 79. The columnar layer of the 3rd instar eye disc is subdivided by a morphogenetic furrow (MF) that passes from anterior to posterior. Whereas cells in the wake of the MF organize into ommatidial clusters and divide one time or not at all, cells posterior to the MF divide logarithmically. No long apical cytonemes have been observed emanating from cells anterior to the MF, but posterior to the MF, cells extend two types of cytonemes – either orienting toward the MF or toward the centrally located “equator” that is perpendicular to the MF. The EGF receptor concentrates in motile puncta in the MF-directed cytonemes but is not present in the cytonemes directed to the equator. In the presence of dominant-negative EGFR, the long MF-directed cytonemes were absent, but with uniform, over-expressed EGF ligand, the only eye disc cytonemes detected are short and lack a directional bias. These characteristics are consistent with the idea that the MF-directed cytonemes mediate EGF signaling; cells posterior to the MF require EGFR for proliferation 90, 91.
The cells that generate the abdomen of the adult fly are another context in which cytonemes have been characterized. Whereas the integument of the fly head and thoracic segments are produced by imaginal discs that grow in the embryo and larva, the abdominal integument is made by small nests of histoblasts that initiate mitotic cycling only after the larval periods conclude. Each hemi-segment in the dorsal abdomen has an anterior and posterior histoblast nest, and the nests initiate rapid divisions at metamorphosis to populate the A and P compartments of the adult abdomen, respectively. Hh expressed in cells from the P compartment nests signals to cells in the adjacent A compartment nest, and because the histoblasts are situated immediately underneath the pupal case, the process can be imaged live through a “window” cut out of the pupal cuticle 92, 93. P compartment histoblasts extend basal cytonemes that appear to ferry Hh to adjacent A compartment cells 13. These cytonemes are dynamic, with lifetimes averaging ~11 minutes, growing and retracting at rates (~5 μm/minute) similar to filopodia in other contexts 39, 94. They are up to 40 μm long (~9 cell diameters) and their presence and length distribution correlates with the dispersion of Hh and with Hh signaling in the A compartment cells. These cytonemes contain Hh and Ihog.
There are numerous other contexts in which signaling-associated filopodia appear to orient with respect to the local signaling landscape. Such filopodia appear to mediate Hh transport in Drosophila ovary germline stem cells 95 and larval lymph glands 96, 97, to mediate Notch-dependent lateral inhibition in the wing disc 98, and to deliver EGF in the leg disc 99. They may be involved in dorsal closure 73, development of neuromuscular junctions 42, 66 and the CNS 41. In spider embryos, filopodia have been associated with Dpp signaling 100, and in earwigs with the ovarian stem cell niche 101. In vertebrate systems, they have been observed in embryos 39, 72, 75, 102, cultured cells 44, 103–106, telocytes 45, mast cells 107, B lymphocytes 108, neutrophils 109, zebrafish melanophores 110, 111, and in the chick limb bud 112.
Cytoneme attributes
Cytonemes are complex and diverse organelles whose diaphanous nature has impeded efforts to understand their structure and function. However, recent improvements in fluorescent protein design, light microscopy, and techniques for genetic manipulation have made it possible to glean aspects of their appearance, behavior and role in systems that are favorably endowed for optical and genetic analysis. The following summarizes these attributes.
Cytoneme structure and composition
Cytonemes are predominantly linear, with diameters estimated at 100–200 nm and lengths between 2–150 μm 13, 47, 79, 112. The cytoneme core is composed of actin filaments that can be marked with actin:fluorescent protein (FP) chimeras 13, 77, 85 and with actin-binding protein:FPs (e.g. moesin:FP 13, 113 and Dia:FP 87). Dia is a member of the formin family 114, proteins that are involved in actin polymerization and that associate with the growing end of actin filaments. A constitutively active form of Dia linked to FP 115 concentrates at the tips of ASP cytonemes and cytonemes do not extend normally in the absence of dia function 87. The Rho family member Vav localizes to wing disc basal cytonemes 77, and Capping protein, SCAR and Pico are actin-binding proteins that have been implicated in cytoneme function by genetic loss-of-function studies 13; they presumably also locate to cytonemes.
Other constituents include signaling protein receptors (e.g. Tkv 77, 79, Btl 79, 85, Ptc 78, FGFR 104), signaling proteins (e.g. Dpp 79, Hh 13, 14, 83), and components of signaling pathways (e.g. Ihog, Brother of Ihog (Boi), DmWif, Dallylike (Dlp), Dispatched (Disp) 13, 83, and Delta 98). Two features of these constituents are noteworthy. First, expression of FP-linked Tkv, Btl, Ptc, Dpp, Hh, Ihog, Boi, and Dlp mark motile puncta in cytonemes. The size and appearance of the puncta in the Hh-containing basal cytonemes of the wing disc suggest that they may be exovesicles 13. The identity and nature of the puncta in cytonemes that receive signaling proteins is not known, but they move at speeds similar to rates of myosin-linked actin motors 78, and their motility and composition suggest that they may be vehicles that transport signaling proteins between a cytoneme tip and the cell body. Second, cytonemes do not all have the same constituents. For instance, the wing disc’s apical cytonemes contain components of the Dpp pathway (i.e. Tkv) 77, the basal cytonemes contain components of the Hh pathway (i.e. Hh, Ihog, Dally, Dlp, Shf/DmWif1, Disp and Ptc) 13, 14, 78, 83, and in the ASP, cytonemes contain either Btl or Tkv, but not both 79. It may be that every signaling pathway has a dedicated set of cytonemes that mediate trafficking between specific signaling cells.
Cytoneme tips
The appearance and physical characteristics of cytoneme tips suggest that they are specialized regions. Whereas the shafts of cytonemes marked with either membrane-tethered FP or constituent protein:FP have a uniform diameter when viewed with fluorescence optics, cytoneme tips are brighter and appear to be wider 13, 85, 87. The tips of ASP cytonemes concentrate over-expressed DiaAct:GFP, Nrg:GFP and Caps:GFP, and the only cytonemes that take up Dpp are those whose tips contact disc cells 87. GRASP fluorescence at cytoneme tips indicates that these contacts are relatively stable (GFP folding is not instantaneous) and close (the GRASP constructs extend approximately 15 nm from the juxtaposed cells) 87. The idea that cytoneme tips are sites where signals transfer between cells is also supported by the fact that cytonemes that lack normal Caps function do not make GRASP-marked contacts and do not take up signaling proteins, and by the absence of signaling in the cells that have contact-defective cytonemes 78, 87. Tips of the wing disc basal cytonemes implicated in Hh signaling also appear to be directly involved with the transfer of signals between cells. Vesicles that may carry Hh emanate from the tips of these cytonemes 13.
Cytoneme plasticity
The cardinal feature of cytonemes is their presence linking cells that produce signaling proteins to cells that receive them. There are many examples. Cells in the wing blade primordium direct Tkv-containing cytonemes toward the Dpp-producing cells at the A/P compartment border 77. At the late 3rd instar stage, cells in the medial part of the ASP direct Tkv-containing cytonemes toward nearby Dpp-producing cells of the wing disc 79. Cells at the tip of the ASP direct Btl-containing cytonemes toward wing disc cells that express FGF 79, 85. Cells in the eye disc direct EGFR-containing cytonemes toward EGF-producing cells of the MF 77. Hh-producing cells in the wing disc and in posterior histoblast nest project cytonemes to anterior Hh-receiving cells 13, 14, 83. Cytonemes implicated in Hh signaling extend between Hh-producing cap cells and Hh-dependent escort cells in the ovarian germ cell niche 95. And there are more examples in the Drosophila leg 99 and wing discs 98, 116, 117, chick limb bud 112, and zebrafish vasculature 69.
Tissue growth and morphogenesis changes spatial relationships between signal producing and signal receiving cells, and if cytonemes mediate signal exchange, the distributions of cytonemes are expected to change accordingly. Cytoneme plasticity has been observed in contexts in which signaling protein is expressed ectopically – novel cytonemes project to the cells at the ectopic sites 77, 79, 85. It is also evident in the ovarian germ cell niche where cytonemes changed in conditions of reduced Hh signaling 95. Perhaps the most striking evidence is provided by real-time imaging of the developing zebrafish vascular endothelium 69 and Drosophila histoblast nests 13. Characterization of the histoblast cytonemes showed that the area over which they distribute correlates precisely with location of cells that activate Hh signal transduction, even as the number of signaling cells changes and the distance between Hh producing and receiving cells increases 13. Thus, although cytonemes present specialized tips at long distance that make stable contacts with target cells, the dynamic nature of cytonemes accommodates changes in the signaling landscape, indicating that the functional associations they make are temporally regulated.
Morphogen release and dispersion
Morphogens influence cells that are far from the cells that produce them, and the idea that they move long distances to engage the cells they regulate has strong experimental support. For example, Dpp regulates target genes in wing disc cells that are distant from cells that express Dpp (reviewed in 118), and the Dpp receptor is required in the responding cells 119. In discs with Dpp:GFP over-expressed in the normal domain of Dpp expression, GFP fluorescence encompasses much of the territory that is regulated by Dpp 6, 17. Despite such evidence, the state of Dpp that is in transit has been difficult to define. Although biochemical (e.g. sensitivity to protease digestion and surface biotinylation 6), histochemical 18 and immunohistochemical 17 assays show that a portion of the Dpp is extracellular, the results do not distinguish whether the Dpp is in extracellular space, in or on a cytoneme, or attached and exposed at the extracellular surface of a cell. The resolution of these methods is not sufficient to differentiate between tethered and untethered forms because the intercellular distances are small and cytonemes appear to track along cell surfaces.
Routes of morphogen dispersion have also been studied by comparing steady state distributions of Hh 11 and of Dpp signaling 33 in normal and genetically altered wing discs. However, there are many steps involved in release, movement, uptake, and response, and because the relative rates of these processes are unknown and we do not know which are rate-limiting, models based on steady state distributions are inconclusive.
Morphogens have been characterized in cultured cell systems to investigate their subcellular localization and secretion. Although there are many reports of morphogen accumulation in culture medium, the constitutive, unregulated secretion in these cell culture systems may be attributable to the artificial conditions of ex vivo culture. Paracrine signals are inactive unless released, and it may be that cell culture systems lack the subcellular organization and processes that orchestrate and control secretion. This line of reasoning suggests that the presence of morphogen protein in culture medium should not infer in vivo behavior.
Under conditions in which cytonemes and signaling proteins were simultaneously marked and imaged in intact organs, all signaling protein that could be detected between the cell body of a producing cell and a receiving cell was in or on a cytoneme 87. Although marked protein may appear to be traveling untethered through extracellular space under conditions in which cytonemes are not marked, the results with simultaneous marking are unambiguous and show that it is not. It is possible that the available imaging methods lack sufficient sensitivity to detect all the forms of in transit signaling proteins, but the experiments that have been carried out identify only cytoneme-associated protein that is in transit. The additional finding that cytoneme-mediated movement and uptake are required for signaling shows that if signaling proteins disperse independently of cytonemes, they do not signal 87.
Morphogens without cytonemes?
If there is strong evidence for cytoneme-mediated, direct signaling in Drosophila imaginal discs and histoblasts, the question arises whether there is compelling evidence for morphogen distribution by diffusion in other contexts. Many gradient systems have been characterized for which extracellular diffusion would appear to be an obvious and simple mechanism to disperse the relevant signaling proteins. These include Nodal and Lefty gradients in the zebrafish embryo 31, the Hh and Dpp gradients in Drosophila that were described above, as well as several different morphogen gradient systems in the early Drosophila embryo. Two of the gradient systems in the Drosophila embryo will be briefly reviewed here; the issues are relevant to the other contexts.
The physical properties of the early Drosophila embryo may allow for constrained diffusion of morphogens (discussed in 10). Internally, the embryo is a syncytium during the period when, for example, the Bicoid (Bcd) protein distributes in a monotonic concentration gradient and directs rostrocaudal axial patterning. Although Bcd may be restricted to the cytoplasm of the embryo in which it is made, its movement within this single cell may be unrestricted because there are no cell membranes that compartmentalize the embryo. The spatial distribution of Bcd appears to be a product of two processes: one that disperses bcd mRNA and may use microtubule-based motors 120, and another that moves Bcd protein in the embryo 121. It is not known whether Bcd protein movement is active or passive, but an active process would presumably also be conducted by molecular motors. Although there is no evidence for the route that Bcd might take, it is difficult to conceive of a role for cytonemes.
A different gradient system that does not involve Bcd sets up the dorsoventral axis of the early Drosophila embryo. The dorsoventral gradient is manifested in the nuclear localization of the Dorsal (Dl) protein, which is seen to be greatest at the ventral midline and to decline dorsally 122, 123. Nuclear localization is triggered by activation of the Toll receptor whose ligand, Spätzle (Spz), is generated by an extracellular proteolytic cascade 124, 125. Although neither the spatial distributions of active Spz or activated Toll have been observed directly, the assumption that they reflect the contours of the Dorsal nuclear gradient seems reasonable. The key question, however, is how the distribution of active Spz is generated. The extracellular space that surrounds the embryo is a narrow rim of fluid that is bounded on the outside by the waxy vitelline membrane and on the inside by the plasma membrane of the embryo. Both membranes should be impermeable to proteins and are therefore likely to constrain protein constituents in the perivitelline fluid. The current model for the dorsoventral gradient posits that prior to fertilization, Spz distributes uniformly in the perivitelline fluid, and that post-fertilization, processing specifically at the ventral midline generates active Spz that diffuses dorsally to form a spatial concentration gradient. Elegant molecular mechanisms that may generate the sharp gradients from initially broad distributions have been proposed 126, but all current models are based on the unproven assumption that Spz is free to diffuse within the perivitelline fluid. Now that many signaling systems have been shown to be contact-dependent 13, 78, 83, 87, 104, 111, 112, it is imperative to define the state of dispersing morphogens, despite the apparent simplicity of an extracellular diffusion mechanism.
Synaptic signaling
The attributes of cytonemes and the cellular behaviors they imply may be novel for epithelial cells, but they are not unique - neurons also have these properties. In essence, cytonemes are asymmetric extensions that send or receive signals, akin to axons and dendrites, the asymmetric extensions of neurons that send or receive signals. There is evidence for cross-talk between neurons and potential target cells during development 66, and neuronal connectivity exhibits plasticity, both during development for the elaboration of neuronal networks and in the course of the functional lifetime of the neuron. Many neurons are specific for a particular neurotransmitter and neurons package neurotransmitters, both chemical and protein, into membrane-bound vesicles, and segregate proteins to axons and dendrites (as do polarized epithelial cells). The point is that the remarkable properties of cytonemes have been recognized previously in neurons, so the cell biology that underlies cytoneme function is not likely to be novel. Indeed, it seems reasonable to recast the argument and to suggest that not only is the capacity to send and receive signals via cellular extensions not unique to neurons, but this capacity is an attribute of many, or perhaps most (or all) cells. Direct signaling at a distance via cellular extensions must have predated the invention of a neuron, and the underlying cell biology must have existed prior to their evolution. In this context, neurons can be considered to have elaborated upon pre-existing signaling functionalities for specialized purposes.
Given the functional homologies between cytonemes and neuronal extensions, it becomes important to know how extensively attributes are shared between these organelles. For example, the strength and presence of neuronal connections are activity-dependent in many contexts; if the stability of cytonemes is similarly determined by signal traffic, then similar mechanisms may explain their capacity to respond to changes in the landscape of signaling protein sources and perhaps to generate concentration gradients of signaling proteins. In other words, we would like to understand the extent to which the properties of neuronal extensions are shared with cytonemes, and whether neuronal attributes such as potentiation and desensitization underlie the observed patterns and functions of cytonemes.
Direct signaling is a mechanism of movement that can deliver signaling proteins over long distance and simultaneously limit them to selected destinations. By analogy, exogenous application of neurotransmitters can evoke physiologically relevant responses from specimens containing neurons, but in vivo, neurotransmitters are released only in regulated settings so that their effects are spatially limited and precisely directed. The direct model for long distance signaling by the Hh, Dpp, EFG and FGF signaling systems provides a similar degree of targeting specificity.
Synaptic signaling also confers exquisite quantitative control, as both neurotransmitter release and uptake can be precisely regulated. Neurotransmitters are packaged in synaptic vesicles, and neurotransmitter release from these quantized packets is accomplished by controlling exocytosis and fusion of the vesicles with the presynaptic membrane. Uptake of neurotransmitter that is released into the synaptic cleft is also regulated - by the environment of the synaptic cleft and by receptors in the postsynaptic membrane. It is tempting to speculate that direct delivery of the morphogen signaling proteins that control growth and patterning may have similar features. For example, Hh and EGF are lipid-modified proteins, and their tight association with membranes tethers them to the cells that make them until they are released. In wing disc cells, Hh is packaged into endocytic vesicles for transport that move along cytonemes that contact Hh-receiving cells 13, 14. It is therefore possible that the morphogen signaling systems may have the organizational attributes for quantized release and uptake at synaptic contacts.
Pathfinding
The mechanism by which a cytoneme finds its target is not known. Two plausible models are homing to a target by following an attractant gradient, or random searching by rapid extension and retraction. Observations to date do not distinguish between these alternatives, but imaging in sea urchin 40, Drosophila 13, 47 and chick 112 systems shows that cytonemes actively and rapidly extend and retract. Moreover, the plasticity of cytonemes in response to changes in the landscape of signaling protein sources suggests that historical associations do not fix either cytoneme orientation or contact. These properties are consistent with a random search mechanism and with the possibility that cytoneme orientation may not be dependent upon extracellular attractants. The results of the GRASP studies that identify cytoneme synapses suggest that functional contacts may be stabilized and therefore that the apparent biased orientations of cytonemes may reflect the steady state rather than guided or informed directionality. If the formation of a functional synapse stabilizes both signal-receiving and signal-delivering cytonemes, the pathfinding process may be similar for both types.
Concluding remarks
At this point we know that there are several types of cytonemes, that signaling protein receptors are present and move along cytonemes, that cytonemes have orientations and are at sites that correlate with signaling, that cytonemes make direct contact with their target cells, and that cytonemes can ferry signaling proteins between cells. There is much that we don’t know. We don’t understand how cytonemes locate the cells they contact, where or how signaling protein taken up by a cytoneme is internalized, how concentration gradients are generated or where signal transduction is initiated in the receiving cell. Nevertheless, the properties of cytonemes – their presence at sites of signaling, their plasticity, their trafficking of signal proteins and signal protein receptors, and their specificity – have fundamental implications for the ways cells communicate.
Diffusion as a mechanism of dispersion has been central to the concept of inducers and morphogens and has been explored extensively since Turing first developed mathematics for a reaction-diffusion process that could distribute them in concentration gradients. Despite many years of experiments and theoretical treatments 30, 32, 34, there is no direct proof for extracellular diffusion of morphogen signaling proteins. By contrast, evidence for direct delivery has now been obtained for Wg at the neuromuscular junction 38, for Hh in synaptic termini of photoreceptor neurons 36, wing disc cells 13, 78 and abdominal histoblasts 13, and for Dpp in the ASP 87. Moreover, the studies of signaling in the abdominal histoblasts and wing disc show that formation of the Hh gradient is dependent on cytoneme-mediated transport and uptake 13, 78. And in the zebrafish, interactions between pigment cells that generate the longitudinal stripes, though consistent with Turing’s mathematical model 127, are in fact dependent on cellular projections and direct cell-cell contacts 111. It remains for future work to determine if direct delivery is the sole mechanism that moves signaling proteins between cells.
Acknowledgments
I thank Sougata Roy and reviewers for suggestions, and acknowledge the National Institutes of Health (grants GM030637 and GM105987) for support.
References
- 1.Browne NE. The production of new hydranths in Hydra by the insertion of small grafts. J Exp Zool. 1909;7:1–23. [Google Scholar]
- 2.Lenhoff HM. Ethel Browne, Has Spemann, and the DIscovery of the Organizer Phenomenon. Biol Bull. 1991;181:72–80. doi: 10.2307/1542490. [DOI] [PubMed] [Google Scholar]
- 3.Spemann H, Mangold H. Über Weckung organisatorischer Fähigkeiten durch Verplanzung in organisatorische Umgebung. Roux’s Arch. 1924;109:557–577. doi: 10.1007/BF02079696. [DOI] [PubMed] [Google Scholar]
- 4.Turing AM. The chemical basis of morphogenesis. Phil Tran R Soc Lond. 1952;237:37–72. [Google Scholar]
- 5.St Johnston D, Nusslein-Volhard C. The origin of pattern and polarity in the Drosophila embryo. Cell. 1992;68:201–219. doi: 10.1016/0092-8674(92)90466-p. [DOI] [PubMed] [Google Scholar]
- 6.Teleman AA, Cohen SM. Dpp gradient formation in the Drosophila wing imaginal disc. Cell. 2000;103:971–980. doi: 10.1016/s0092-8674(00)00199-9. [DOI] [PubMed] [Google Scholar]
- 7.Tabata T, Schwartz C, Gustavson E, Ali Z, Kornberg TB. Creating a Drosophila wing de novo, the role of engrailed, and the compartment border hypothesis. Development. 1995;121:3359–3369. doi: 10.1242/dev.121.10.3359. [DOI] [PubMed] [Google Scholar]
- 8.Zecca M, Basler K, Struhl G. Sequential organizing activities of engrailed, hedgehog and decapentaplegic in the Drosophila wing. Development. 1995;8:2265–2278. doi: 10.1242/dev.121.8.2265. [DOI] [PubMed] [Google Scholar]
- 9.Postlethwait JH, Schneiderman HA. Pattern formation and determination in the antenna of the homoeotic mutant Antennapedia of Drosophila melanogaster. Dev Biol. 1971;25:606–640. doi: 10.1016/0012-1606(71)90008-x. [DOI] [PubMed] [Google Scholar]
- 10.Kornberg TB, Guha A. Understanding morphogen gradients: a problem of dispersion and containment. Curr Opin Genet Dev. 2007;17:264–271. doi: 10.1016/j.gde.2007.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ayers KL, Gallet A, Staccini-Lavenant L, Therond PP. The long-range activity of Hedgehog is regulated in the apical extracellular space by the glypican Dally and the hydrolase Notum. Dev Cell. 2010;18:605–620. doi: 10.1016/j.devcel.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 12.Gallet A, Ruel L, Staccini-Lavenant L, Therond PP. Cholesterol modification is necessary for controlled planar long-range activity of Hedgehog in Drosophila epithelia. Development. 2006;133:407–418. doi: 10.1242/dev.02212. [DOI] [PubMed] [Google Scholar]
- 13.Bischoff M, Gradilla AC, Seijo I, Andres G, Rodriguez-Navas C, Gonzalez-Mendez L, Guerrero I. Cytonemes are required for the establishment of a normal Hedgehog morphogen gradient in Drosophila epithelia. Nat Cell Biol. 2013;15:1269–1281. doi: 10.1038/ncb2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Callejo A, Bilioni A, Mollica E, Gorfinkiel N, Andres G, Ibanez C, Torroja C, Doglio L, Sierra J, Guerrero I. Dispatched mediates Hedgehog basolateral release to form the long-range morphogenetic gradient in the Drosophila wing disk epithelium. Proc Natl Acad Sci U S A. 2011;108:12591–12598. doi: 10.1073/pnas.1106881108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kornberg TB. Barcoding Hedgehog for intracellular transport. Sci Signal. 2011;4:pe44. doi: 10.1126/scisignal.2002447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bokel C, Schwabedissen A, Entchev E, Renaud O, Gonzalez-Gaitan M. Sara endosomes and the maintenance of Dpp signaling levels across mitosis. Science. 2006;314:1135–1139. doi: 10.1126/science.1132524. [DOI] [PubMed] [Google Scholar]
- 17.Entchev EV, Schwabedissen A, Gonzalez-Gaitan M. Gradient formation of the TGF-beta homolog Dpp. Cell. 2000;103:981–991. doi: 10.1016/s0092-8674(00)00200-2. [DOI] [PubMed] [Google Scholar]
- 18.Gibson MC, Lehman DA, Schubiger G. Lumenal transmission of decapentaplegic in Drosophila imaginal discs. Dev Cell. 2002;3:451–460. doi: 10.1016/s1534-5807(02)00264-2. [DOI] [PubMed] [Google Scholar]
- 19.Szuperak M, Salah S, Meyer EJ, Nagarajan U, Ikmi A, Gibson MC. Feedback regulation of Drosophila BMP signaling by the novel extracellular protein larval translucida. Development. 2011;138:715–724. doi: 10.1242/dev.059477. [DOI] [PubMed] [Google Scholar]
- 20.Pallavi SK, Shashidhara LS. Signaling interactions between squamous and columnar epithelia of the Drosophila wing disc. J Cell Sci. 2005;118:3363–3370. doi: 10.1242/jcs.02464. [DOI] [PubMed] [Google Scholar]
- 21.Niethammer P, Grabher C, Look AT, Mitchison TJ. A tissue-scale gradient of hydrogen peroxide mediates rapid wound detection in zebrafish. Nature. 2009;459:996–999. doi: 10.1038/nature08119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Herzmark P, Campbell K, Wang F, Wong K, El-Samad H, Groisman A, Bourne HR. Bound attractant at the leading vs. the trailing edge determines chemotactic prowess. Proc Natl Acad Sci U S A. 2007;104:13349–13354. doi: 10.1073/pnas.0705889104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Patel DD, Koopmann W, Imai T, Whichard LP, Yoshie O, Krangel MS. Chemokines have diverse abilities to form solid phase gradients. Clin Immunol. 2001;99:43–52. doi: 10.1006/clim.2000.4997. [DOI] [PubMed] [Google Scholar]
- 24.Schumann K, Lammermann T, Bruckner M, Legler DF, Polleux J, Spatz JP, Schuler G, Forster R, Lutz MB, Sorokin L, et al. Immobilized chemokine fields and soluble chemokine gradients cooperatively shape migration patterns of dendritic cells. Immunity. 2010;32:703–713. doi: 10.1016/j.immuni.2010.04.017. [DOI] [PubMed] [Google Scholar]
- 25.Galkina SI, Fedorova NV, Stadnichuk VI, Sud’ina GF. Membrane tubulovesicular extensions (cytonemes): Secretory and adhesive cellular organelles. Cell Adhesion & Migration. 2006;7:174–186. doi: 10.4161/cam.23130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Galkina SI, Romanova JM, Stadnichuk VI, Molotkovsky JG, Sud’ina GF, Klein T. Nitric oxide-induced membrane tubulovesicular extensions (cytonemes) of human neutrophils catch and hold Salmonella enterica serovar Typhimurium at a distance from the cell surface. FEMS Immunol Med Microbiol. 2009;56:162–171. doi: 10.1111/j.1574-695X.2009.00560.x. [DOI] [PubMed] [Google Scholar]
- 27.Crick F. Diffusion in embryogenesis. Nature. 1970;225:420–422. doi: 10.1038/225420a0. [DOI] [PubMed] [Google Scholar]
- 28.Meinhardt H. Models for the generation and interpretation of gradients. Cold Spring Harb Perspect Biol. 2009;1:a001362. doi: 10.1101/cshperspect.a001362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nahmad M, Lander AD. Spatiotemporal mechanisms of morphogen gradient interpretation. Curr Opin Genet Dev. 2011;21:726–731. doi: 10.1016/j.gde.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lander AD, Nie Q, Wan FY. Do morphogen gradients arise by diffusion? Dev Cell. 2002;2:785–796. doi: 10.1016/s1534-5807(02)00179-x. [DOI] [PubMed] [Google Scholar]
- 31.Muller P, Rogers KW, Jordan BM, Lee JS, Robson D, Ramanathan S, Schier AF. Differential diffusivity of Nodal and Lefty underlies a reaction-diffusion patterning system. Science. 2012;336:721–724. doi: 10.1126/science.1221920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saha K, Schaffer DV. Signal dynamics in Sonic hedgehog tissue patterning. Development. 2006;133:889–900. doi: 10.1242/dev.02254. [DOI] [PubMed] [Google Scholar]
- 33.Schwank G, Dalessi S, Yang SF, Yagi R, de Lachapelle AM, Affolter M, Bergmann S, Basler K. Formation of the long range dpp morphogen gradient. PLoS Biol. 2011;9:e1001111. doi: 10.1371/journal.pbio.1001111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zeng X, Goetz JA, Suber LM, Scott WJ, Jr, Schreiner CM, Robbins DJ. A freely diffusible form of Sonic hedgehog mediates long-range signalling. Nature. 2001;411:716–720. doi: 10.1038/35079648. [DOI] [PubMed] [Google Scholar]
- 35.Chu T, Chiu M, Zhang E, Kunes S. A C-terminal motif targets Hedgehog to axons, coordinating assembly of the Drosophila eye and brain. Dev Cell. 2006;10:635–646. doi: 10.1016/j.devcel.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 36.Huang Z, Kunes S. Hedgehog, transmitted along retinal axons, triggers neurogenesis in the developing visual centers of the Drosophila brain. Cell. 1996;86:411–422. doi: 10.1016/s0092-8674(00)80114-2. [DOI] [PubMed] [Google Scholar]
- 37.Speese SD, Budnik V. Wnts: up-and-coming at the synapse. Trends Neurosci. 2007;30:268–275. doi: 10.1016/j.tins.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Korkut C, Ataman B, Ramachandran P, Ashley J, Barria R, Gherbesi N, Budnik V. Trans-synaptic transmission of vesicular Wnt signals through Evi/Wntless. Cell. 2009;139:393–404. doi: 10.1016/j.cell.2009.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miller J, Fraser SE, McClay D. Dynamics of thin filopodia during sea urchin gastrulation. Development. 1995;121:2501–2511. doi: 10.1242/dev.121.8.2501. [DOI] [PubMed] [Google Scholar]
- 40.McClay DR. The role of thin filopodia in motility and morphogenesis. Exp Cell Res. 1999;253:296–301. doi: 10.1006/excr.1999.4723. [DOI] [PubMed] [Google Scholar]
- 41.Vasenkova I, Luginbuhl D, Chiba A. Gliopodia extend the range of direct glia-neuron communication during the CNS development in Drosophila. Mol Cell Neurosci. 2006;31:123–130. doi: 10.1016/j.mcn.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 42.Ritzenthaler S, Suzuki E, Chiba A. Postsynaptic filopodia in muscle cells interact with innervating motoneuron axons. Nat Neurosci. 2000;3:1012–1017. doi: 10.1038/79833. [DOI] [PubMed] [Google Scholar]
- 43.Chen WT. Proteolytic activity of specialized surface protrusions formed at rosette contact sites of transformed cells. J Exp Zool. 1989;251:167–185. doi: 10.1002/jez.1402510206. [DOI] [PubMed] [Google Scholar]
- 44.Tarone G, Cirillo D, Giancotti FG, Comoglio PM, Marchisio PC. Rous sarcoma virus-transformed fibroblasts adhere primarily at discrete protrusions of the ventral membrane called podosomes. Exp Cell Res. 1985;159:141–157. doi: 10.1016/s0014-4827(85)80044-6. [DOI] [PubMed] [Google Scholar]
- 45.Popescu LM, Faussone-Pellegrini MS. TELOCYTES - a case of serendipity: the winding way from Interstitial Cells of Cajal (ICC), via Interstitial Cajal-Like Cells (ICLC) to TELOCYTES. J Cell Mol Med. 2010;14:729–740. doi: 10.1111/j.1582-4934.2010.01059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rustom A, Saffrich R, Markovic I, Walther P, Gerdes HH. Nanotubular highways for intercellular organelle transport. Science. 2004;303:1007–1010. doi: 10.1126/science.1093133. [DOI] [PubMed] [Google Scholar]
- 47.Ramírez-Weber FA, Kornberg TB. Cytonemes: cellular processes that project to the principal signaling center in Drosophila imaginal discs. Cell. 1999;97:599–607. doi: 10.1016/s0092-8674(00)80771-0. [DOI] [PubMed] [Google Scholar]
- 48.Fulga TA, Rorth P. Invasive cell migration is initiated by guided growth of long cellular extensions. Nature cell biology. 2002;4:715–719. doi: 10.1038/ncb848. [DOI] [PubMed] [Google Scholar]
- 49.Izzard CS. Contractile filopodia and in vivo cell movement in the tunic of the ascidian, Botryllus schlosseri. J Cell Sci. 1974;15:513–535. doi: 10.1242/jcs.15.3.513. [DOI] [PubMed] [Google Scholar]
- 50.Ridley AJ, Schwartz MA, Burridge K, Firtel RA, Ginsberg MH, Borisy G, Parsons JT, Horwitz AR. Cell migration: integrating signals from front to back. Science. 2003;302:1704–1709. doi: 10.1126/science.1092053. [DOI] [PubMed] [Google Scholar]
- 51.Vasioukhin V, Bauer C, Yin M, Fuchs E. Directed actin polymerization is the driving force for epithelial cell-cell adhesion. Cell. 2000;100:209–219. doi: 10.1016/s0092-8674(00)81559-7. [DOI] [PubMed] [Google Scholar]
- 52.Han YH, Chung CY, Wessels D, Stephens S, Titus MA, Soll DR, Firtel RA. Requirement of a vasodilator-stimulated phosphoprotein family member for cell adhesion, the formation of filopodia, and chemotaxis in dictyostelium. J Biol Chem. 2002;277:49877–49887. doi: 10.1074/jbc.M209107200. [DOI] [PubMed] [Google Scholar]
- 53.Kress H, Stelzer EH, Holzer D, Buss F, Griffiths G, Rohrbach A. Filopodia act as phagocytic tentacles and pull with discrete steps and a load-dependent velocity. Proc Natl Acad Sci U S A. 2007;104:11633–11638. doi: 10.1073/pnas.0702449104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Locke M. The very rapid induction of filopodia in insect cells. Tissue Cell. 1987;19:301–318. doi: 10.1016/0040-8166(87)90014-0. [DOI] [PubMed] [Google Scholar]
- 55.Sheetz MP, Wayne DB, Pearlman AL. Extension of filopodia by motor-dependent actin assembly. Cell Motil Cytoskeleton. 1992;22:160–169. doi: 10.1002/cm.970220303. [DOI] [PubMed] [Google Scholar]
- 56.Crosson CE, Klyce SD, Beuerman RW. Epithelial wound closure in the rabbit cornea. A biphasic process. Invest Ophthalmol Vis Sci. 1986;27:464–473. [PubMed] [Google Scholar]
- 57.Edwards KA, Demsky M, Montague RA, Weymouth N, Kiehart DP. GFP-moesin illuminates actin cytoskeleton dynamics in living tissue and demonstrates cell shape changes during morphogenesis in Drosophila. Dev Biol. 1997;191:103–117. doi: 10.1006/dbio.1997.8707. [DOI] [PubMed] [Google Scholar]
- 58.Wood W, Jacinto A, Grose R, Woolner S, Gale J, Wilson C, Martin P. Wound healing recapitulates morphogenesis in Drosophila embryos. Nat Cell Biol. 2002;4:907–912. doi: 10.1038/ncb875. [DOI] [PubMed] [Google Scholar]
- 59.Raghunathan A, Sivakamasundari R, Wolenski J, Poddar R, Weissman SM. Functional analysis of B144/LST1: a gene in the tumor necrosis factor cluster that induces formation of long filopodia in eukaryotic cells. Exp Cell Res. 2001;268:230–244. doi: 10.1006/excr.2001.5290. [DOI] [PubMed] [Google Scholar]
- 60.Bentley D, Toroian-Raymond A. Disoriented pathfinding by pioneer neurone growth cones deprived of filopodia by cytochalasin treatment. Nature. 1986;323:712–715. doi: 10.1038/323712a0. [DOI] [PubMed] [Google Scholar]
- 61.Dickson BJ. Molecular mechanisms of axon guidance. Science (New York, NY) 2002;298:1959–1964. doi: 10.1126/science.1072165. [DOI] [PubMed] [Google Scholar]
- 62.Goodman CS. Mechanisms and molecules that control growth cone guidance. Annu Rev Neurosci. 1996;19:341–377. doi: 10.1146/annurev.ne.19.030196.002013. [DOI] [PubMed] [Google Scholar]
- 63.Heidemann SR, Lamoureux P, Buxbaum RE. Growth cone behavior and production of traction force. J Cell Biol. 1990;111:1949–1957. doi: 10.1083/jcb.111.5.1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kater SB, Rehder V. The sensory-motor role of growth cone filopodia. Curr Opin Neurobiol. 1995;5:68–74. doi: 10.1016/0959-4388(95)80089-1. [DOI] [PubMed] [Google Scholar]
- 65.Mooseker MS, Tilney LG. Organization of an actin filament-membrane complex. Filament polarity and membrane attachment in the microvilli of intestinal epithelial cells. J Cell Biol. 1975;67:725–743. doi: 10.1083/jcb.67.3.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ritzenthaler S, Chiba A. Myopodia (postsynaptic filopodia) participate in synaptic target recognition. J Neurobiol. 2003;55:31–40. doi: 10.1002/neu.10180. [DOI] [PubMed] [Google Scholar]
- 67.Yuste R, Bonhoeffer T. Genesis of dendritic spines: insights from ultrastructural and imaging studies. Nat Rev Neurosci. 2004;5:24–34. doi: 10.1038/nrn1300. [DOI] [PubMed] [Google Scholar]
- 68.Zheng JQ, Wan JJ, Poo MM. Essential role of filopodia in chemotropic turning of nerve growth cone induced by a glutamate gradient. J Neurosci. 1996;16:1140–1149. doi: 10.1523/JNEUROSCI.16-03-01140.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gerhardt H, Golding M, Fruttiger M, Ruhrberg C, Lundkvist A, Abramsson A, Jeltsch M, Mitchell C, Alitalo K, Shima D, et al. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J Cell Biol. 2003;161:1163–1177. doi: 10.1083/jcb.200302047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lawson ND, Weinstein BM. In vivo imaging of embryonic vascular development using transgenic zebrafish. Dev Biol. 2002;248:307–318. doi: 10.1006/dbio.2002.0711. [DOI] [PubMed] [Google Scholar]
- 71.Lehmann MJ, Sherer NM, Marks CB, Pypaert M, Mothes W. Actin- and myosin-driven movement of viruses along filopodia precedes their entry into cells. J Cell Biol. 2005;170:317–325. doi: 10.1083/jcb.200503059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Danilchik M, Williams M, Brown E. Blastocoel-spanning filopodia in cleavage-stage Xenopus laevis: Potential roles in morphogen distribution and detection. Dev Biol. 2013;382:70–81. doi: 10.1016/j.ydbio.2013.07.024. [DOI] [PubMed] [Google Scholar]
- 73.Jacinto A, Wood W, Balayo T, Turmaine M, Martinez-Arias A, Martin P. Dynamic actin-based epithelial adhesion and cell matching during Drosophila dorsal closure. Curr Biol. 2000;10:1420–1426. doi: 10.1016/s0960-9822(00)00796-x. [DOI] [PubMed] [Google Scholar]
- 74.Lohmann C, Bonhoeffer T. A role for local calcium signaling in rapid synaptic partner selection by dendritic filopodia. Neuron. 2008;59:253–260. doi: 10.1016/j.neuron.2008.05.025. [DOI] [PubMed] [Google Scholar]
- 75.Gustafson T, Wolpert L. Studies on the cellular basis of morphogenesis in the sea urchin embryo. Gastrulation in vegetalized larvae. Experimental Cell Research. 1961;22:437–449. doi: 10.1016/0014-4827(61)90120-3. [DOI] [PubMed] [Google Scholar]
- 76.Hardin J, Cheng LY. The Mechanisms and Mechanics of Archenteron ELongation during Sea Urchin Gastrulation. Dev Biol. 1986;115:490–501. [Google Scholar]
- 77.Hsiung F, Ramírez-Weber FA, Iwaki DD, Kornberg TB. Dependence of Drosophila wing imaginal disc cytonemes on Decapentaplegic. Nature. 2005;437:560–563. doi: 10.1038/nature03951. [DOI] [PubMed] [Google Scholar]
- 78.Chen W, Kornberg TB. Dispersion via cytonemes: how the Hedgehog gradient forms in the Drosopohila wing imaginal disc. 55th Annual Drosophila Research Conference; San Diego, CA. 2014. [Google Scholar]
- 79.Roy S, Hsiung F, Kornberg TB. Specificity of Drosophila cytonemes for distinct signaling pathways. Science. 2011;332:354–358. doi: 10.1126/science.1198949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yao S, Lum L, Beachy P. The ihog cell-surface proteins bind Hedgehog and mediate pathway activation. Cell. 2006;125:343–357. doi: 10.1016/j.cell.2006.02.040. [DOI] [PubMed] [Google Scholar]
- 81.Zheng X, Mann RK, Sever N, Beachy PA. Genetic and biochemical definition of the Hedgehog receptor. Genes Dev. 2010;24:57–71. doi: 10.1101/gad.1870310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Guerrero I, Kornberg TB. Hedgehog and its circuitous journey from producing to target cells. Semin Cell Dev Biol. 2014 doi: 10.1016/j.semcdb.2014.06.016. [DOI] [PubMed] [Google Scholar]
- 83.Bilioni A, Sanchez-Hernandez D, Callejo A, Gradilla AC, Ibanez C, Mollica E, Carmen Rodriguez-Navas M, Simon E, Guerrero I. Balancing hedgehog, a retention and release equilibrium given by Dally, Ihog, Boi and shifted/dWif. Dev Biol. 2012 doi: 10.1016/j.ydbio.2012.12.013. [DOI] [PubMed] [Google Scholar]
- 84.Guha A, Lin L, Kornberg TB. Regulation of Drosophila matrix metalloprotease Mmp2 is essential for wing imaginal disc:trachea association and air sac tubulogenesis. Dev Biol. 2009;335:317–326. doi: 10.1016/j.ydbio.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sato M, Kornberg TB. FGF is an essential mitogen and chemoattractant for the air sacs of the drosophila tracheal system. Dev Cell. 2002;3:195–207. doi: 10.1016/s1534-5807(02)00202-2. [DOI] [PubMed] [Google Scholar]
- 86.Guha A, Kornberg TB. Tracheal branch repopulation precedes induction of the Drosophila dorsal air sac primordium. Dev Biol. 2005;287:192–200. doi: 10.1016/j.ydbio.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 87.Roy S, Huang H, Liu S, Kornberg TB. Cytoneme-mediated contact-dependent transport of the Drosophila decapentaplegic signaling protein. Science. 2014;343:1244624. doi: 10.1126/science.1244624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Feinberg EH, Vanhoven MK, Bendesky A, Wang G, Fetter RD, Shen K, Bargmann CI. GFP Reconstitution Across Synaptic Partners (GRASP) defines cell contacts and synapses in living nervous systems. Neuron. 2008;57:353–363. doi: 10.1016/j.neuron.2007.11.030. [DOI] [PubMed] [Google Scholar]
- 89.Gordon MD, Scott K. Motor control in a Drosophila taste circuit. Neuron. 2009;61:373–384. doi: 10.1016/j.neuron.2008.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Dominguez M, Wasserman JD, Freeman M. Multiple functions of the EGF receptor in Drosophila eye development. Curr Biol. 1998;8:1039–1048. doi: 10.1016/s0960-9822(98)70441-5. [DOI] [PubMed] [Google Scholar]
- 91.Xu T, Rubin GM. Analysis of genetic mosaics in developing and adult Drosophila tissues. Development. 1993;117:1223–1237. doi: 10.1242/dev.117.4.1223. [DOI] [PubMed] [Google Scholar]
- 92.Bischoff M, Cseresnyes Z. Cell rearrangements, cell divisions and cell death in a migrating epithelial sheet in the abdomen of Drosophila. Development. 2009;136:2403–2411. doi: 10.1242/dev.035410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ninov N, Martin-Blanco E. Live imaging of epidermal morphogenesis during the development of the adult abdominal epidermis of Drosophila. Nat Protoc. 2007;2:3074–3080. doi: 10.1038/nprot.2007.417. [DOI] [PubMed] [Google Scholar]
- 94.Mallavarapu A, Mitchison T. Regulated actin cytoskeleton assembly at filopodium tips controls their extension and retraction. J Cell Biol. 1999;146:1097–1106. doi: 10.1083/jcb.146.5.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rojas-Rios P, Guerrero I, Gonzalez-Reyes A. Cytoneme-mediated delivery of hedgehog regulates the expression of bone morphogenetic proteins to maintain germline stem cells in Drosophila. PLoS Biol. 2012;10:e1001298. doi: 10.1371/journal.pbio.1001298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Krzemien J, Dubois L, Makki R, Meister M, Vincent A, Crozatier M. Control of blood cell homeostasis in Drosophila larvae by the posterior signalling centre. Nature. 2007;446:325–328. doi: 10.1038/nature05650. [DOI] [PubMed] [Google Scholar]
- 97.Mandal L, Martinez-Agosto JA, Evans CJ, Hartenstein V, Banerjee U. A Hedgehog- and Antennapedia-dependent niche maintains Drosophila haematopoietic precursors. Nature. 2007;446:320–324. doi: 10.1038/nature05585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cohen M, Georgiou M, Stevenson NL, Miodownik M, Baum B. Dynamic filopodia transmit intermittent Delta-Notch signaling to drive pattern refinement during lateral inhibition. Dev Cell. 2010;19:78–89. doi: 10.1016/j.devcel.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 99.Peng Y, Han C, Axelrod JD. Planar polarized protrusions break the symmetry of EGFR signaling during Drosophila bract cell fate induction. Dev Cell. 2012;23:507–518. doi: 10.1016/j.devcel.2012.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Akiyama-Oda Y, Oda H. Early patterning of the spider embryo: a cluster of mesenchymal cells at the cumulus produces Dpp signals received by germ disc epithelial cells. Development. 2003;130:1735–1747. doi: 10.1242/dev.00390. [DOI] [PubMed] [Google Scholar]
- 101.Tworzydlo W, Kloc M, Bilinski SM. Female germline stem cell niches of earwigs are structurally simple and different from those of Drosophila melanogaster. J Morphol. 2009;271:634–640. doi: 10.1002/jmor.10824. [DOI] [PubMed] [Google Scholar]
- 102.Fierro-Gonzalez JC, White MD, Silva JC, Plachta N. Cadherin-dependent filopodia control preimplantation embryo compaction. Nat Cell Biol. 2013;15:1424–1433. doi: 10.1038/ncb2875. [DOI] [PubMed] [Google Scholar]
- 103.Ayala I, Baldassarre M, Caldieri G, Buccione R. Invadopodia: a guided tour. Eur J Cell Biol. 2006;85:159–164. doi: 10.1016/j.ejcb.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 104.Koizumi K, Takano K, Kaneyasu A, Watanabe-Takano H, Tokuda E, Abe T, Watanabe N, Takenawa T, Endo T. RhoD activated by fibroblast growth factor induces cytoneme-like cellular protrusions through mDia3C. Mol Biol Cell. 2012;23:4647–4661. doi: 10.1091/mbc.E12-04-0315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lidke DS, Lidke KA, Rieger B, Jovin TM, Arndt-Jovin DJ. Reaching out for signals: filopodia sense EGF and respond by directed retrograde transport of activated receptors. J Cell Biol. 2005;170:619–626. doi: 10.1083/jcb.200503140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sherer NM, Lehmann MJ, Jimenez-Soto LF, Horensavitz C, Pypaert M, Mothes W. Retroviruses can establish filopodial bridges for efficient cell-to-cell transmission. Nat Cell Biol. 2007;9:310–315. doi: 10.1038/ncb1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Fifadara NH, Beer F, Ono S, Ono SJ. Interaction between activated chemokine receptor 1 and FcepsilonRI at membrane rafts promotes communication and F-actin-rich cytoneme extensions between mast cells. Int Immunol. 2010;22:113–128. doi: 10.1093/intimm/dxp118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gupta N, DeFranco AL. Visualizing lipid raft dynamics and early signaling events during antigen receptor-mediated B-lymphocyte activation. Mol Biol Cell. 2003;14:432–444. doi: 10.1091/mbc.02-05-0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Galkina SI, Molotkovsky JG, Ullrich V, Sud’ina GF. Scanning electron microscopy study of neutrophil membrane tubulovesicular extensions (cytonemes) and their role in anchoring, aggregation and phagocytosis. The effect of nitric oxide. Exp Cell Res. 2005;304:620–629. doi: 10.1016/j.yexcr.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 110.Hamada H, Watanabe M, Lau HE, Nishida T, Hasegawa T, Parichy DM, Kondo S. Involvement of Delta/Notch signaling in zebrafish adult pigment stripe patterning. Development. 2013 doi: 10.1242/dev.099804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hamada H, Watanabe M, Lau HE, Nishida T, Hasegawa T, Parichy DM, Kondo S. Involvement of Delta/Notch signaling in zebrafish adult pigment stripe patterning. Development. 2014;141:318–324. doi: 10.1242/dev.099804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sanders TA, Llagostera E, Barna M. Specialized filopodia direct long-range transport of SHH during vertebrate tissue patterning. Nature. 2013;497:628–632. doi: 10.1038/nature12157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Cabernard C, Affolter M. Distinct roles for two receptor tyrosine kinases in epithelial branching morphogenesis in Drosophila. Dev Cell. 2005;9:831–842. doi: 10.1016/j.devcel.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 114.Castrillon DH, Wasserman SA. Diaphanous is required for cytokinesis in Drosophila and shares domains of similarity with the products of the limb deformity gene. Development. 1994;120:3367–3377. doi: 10.1242/dev.120.12.3367. [DOI] [PubMed] [Google Scholar]
- 115.Rousso T, Shewan AM, Mostov KE, Schejter ED, Shilo BZ. Apical targeting of the formin Diaphanous in Drosophila tubular epithelia. Elife. 2013;2:e00666. doi: 10.7554/eLife.00666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Milan M, Cohen SM. Notch signaling: filopodia dynamics confer robustness. Current biology: CB. 2010;20:R802–804. doi: 10.1016/j.cub.2010.07.030. [DOI] [PubMed] [Google Scholar]
- 117.Renaud O, Simpson P. scabrous modifies epithelial cell adhesion and extends the range of lateral signalling during development of the spaced bristle pattern in Drosophila. Dev Biol. 2001;240:361–376. doi: 10.1006/dbio.2001.0482. [DOI] [PubMed] [Google Scholar]
- 118.Podos SD, Ferguson EL. Morphogen gradients: new insights from DPP. Trends Genet. 1999;15:396–402. doi: 10.1016/s0168-9525(99)01854-5. [DOI] [PubMed] [Google Scholar]
- 119.Burke R, Basler K. Dpp receptors are autonomously required for cell proliferation in the entire developing Drosophila wing. Development. 1996;122:2261–2269. doi: 10.1242/dev.122.7.2261. [DOI] [PubMed] [Google Scholar]
- 120.Spirov A, Fahmy K, Schneider M, Frei E, Noll M, Baumgartner S. Formation of the bicoid morphogen gradient: an mRNA gradient dictates the protein gradient. Development. 2009;136:605–614. doi: 10.1242/dev.031195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Little SC, Tkacik G, Kneeland TB, Wieschaus EF, Gregor T. The formation of the Bicoid morphogen gradient requires protein movement from anteriorly localized mRNA. PLoS Biol. 2011;9:e1000596. doi: 10.1371/journal.pbio.1000596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Roth S, Stein D, Nusslein-Volhard C. A gradient of nuclear localization of the dorsal protein determines dorsoventral pattern in the Drosophila embryo. Cell. 1989;59:1189–1202. doi: 10.1016/0092-8674(89)90774-5. [DOI] [PubMed] [Google Scholar]
- 123.Steward R, Zusman SB, Huang LH, Schedl P. The dorsal protein is distributed in a gradient in early Drosophila embryos. Cell. 1988;55:487–495. doi: 10.1016/0092-8674(88)90035-9. [DOI] [PubMed] [Google Scholar]
- 124.Morisato D, Anderson KV. The spatzle gene encodes a component of the extracellular signaling pathway establishing the dorsal-ventral pattern of the Drosophila embryo. Cell. 1994;76:677–688. doi: 10.1016/0092-8674(94)90507-x. [DOI] [PubMed] [Google Scholar]
- 125.Schneider DS, Jin Y, Morisato D, Anderson KV. A processed form of the Spatzle protein defines dorsal-ventral polarity in the Drosophila embryo. Development. 1994;120:1243–1250. doi: 10.1242/dev.120.5.1243. [DOI] [PubMed] [Google Scholar]
- 126.Haskel-Ittah M, Ben-Zvi D, Branski-Arieli M, Schejter ED, Shilo BZ, Barkai N. Self-organized shuttling: generating sharp dorsoventral polarity in the early Drosophila embryo. Cell. 2012;150:1016–1028. doi: 10.1016/j.cell.2012.06.044. [DOI] [PubMed] [Google Scholar]
- 127.Nakamasu A, Takahashi G, Kanbe A, Kondo S. Interactions between zebrafish pigment cells responsible for the generation of Turing patterns. Proc Natl Acad Sci U S A. 2009;106:8429–8434. doi: 10.1073/pnas.0808622106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Driever W, Siegel V, Nusslein-Volhard C. Autonomous determination of anterior structures in the early Drosophila embryo by the bicoid morphogen. Development. 1990;109:811–820. doi: 10.1242/dev.109.4.811. [DOI] [PubMed] [Google Scholar]
- 129.Meyer EJ, Ikmi A, Gibson MC. Interkinetic nuclear migration is a broadly conserved feature of cell division in pseudostratified epithelia. Curr Biol. 2011;21:485–491. doi: 10.1016/j.cub.2011.02.002. [DOI] [PubMed] [Google Scholar]