Abstract
Hepatic ischemia-reperfusion (IR) results in progressive injury, initiated by oxidative stress during ischemia and compounded by cytokine-mediated inflammation during reperfusion. Recovery requires strict regulation of these events. Recombinant human erythropoietin (rhEPO) is thought to mitigate hepatocellular IR injury by altering the non-parenchymal liver microenvironment. This study sought to identify additional mechanisms whereby rhEPO is protective after liver IR injury.
Methods
Mice were treated with rhEPO (4units/gm SQ) at the onset of partial liver ischemia and assessed for transaminase and histologic injury at intervals after reperfusion. Induction of cytokines, activation of signal transducers and activators of transcription (STATs), suppressors of cytokine signaling (Socs1, Socs3, Cis), caspase-3 activation, and heme oxygenase-1 (HO-1) expression were assessed in post-ischemic liver. Effects of rhEPO stimulation were further characterized in whole liver lysates from mice undergoing rhEPO injection alone and in cultured AML-12 hepatocytes.
Results
rhEPO treatment at the onset of severe (90min) hepatic IR confirmed commensurate biochemical and histological protection without affecting tissue cytokine levels. Although Socs3 and STAT5 activation were induced in normal liver after in vivo rhEPO injection, this treatment did not augment expression beyond that seen with IR alone, and neither was induced in cultured hepatocytes treated with rhEPO. rhEPO inhibited caspase-3 activation in non-parenchymal cells, while hepatocellular HO-1 was rapidly induced both in vivo and in vitro with rhEPO treatment.
Conclusion
These data suggest HO-1 as a potent mechanism of rhEPO-mediated protection after liver IR, which involves both direct hepatocellular and non-parenchymal mechanisms.
Keywords: hepatocyte, oxidative stress, inflammation, liver injury, JAK-STAT signaling
INTRODUCTION
Ischemia-reperfusion (IR) injury is the primary driver of acute liver dysfunction after elective liver resection and prolonged hemorrhagic shock. The initial hypoperfusion phase of any IR injury, including liver IR, is characterized by tissue hypoxia and cellular oxidative stress[1]. Reperfusion initiates a complex chain of cytokine-mediated events that culminate in a distinct, neutrophil-mediated “reperfusion injury”. If unchecked, this second phase of injury is dominated by tissue necrosis and sets the stage for a systemic response and remote organ failure, particularly when the primary ischemic injury is severe. Despite advances in critical care, a failing liver is difficult to support, short of transplantation. While strategies to improve outcomes in the acute setting have aimed at blocking individual components of reperfusion injury, to date none have translated to clinically relevant therapies. Aside from limiting the period of ischemia, or pre-conditioning with brief periods of portal occlusion[2], few strategies have focused on mitigating the oxidative stress phase of injury to improve tolerance of acute IR.
Erythropoietin (EPO) is a glycoprotein hormone vital to the differentiation of committed erythroid progenitor cells. A variety of non-hematopoietic properties of EPO have also been identified, suggesting additional potential clinical applications. Exogenous recombinant human (rh) EPO has been shown to be protective after ischemia in a variety of tissues, including brain, heart, kidney and liver[3, 4]. This protection has been observed in animal models utilizing both pre- and post-injury treatment strategies, and has generally been attributed to induction of anti-apoptotic mechanisms such as Bcl2 and Bclx. In addition, as is the case for a number of inflammatory mediators associated with acute hepatic IR injury, EPO signals through the signal transducer and activator of transcription (STAT) signaling pathway, and has been shown to regulate inflammatory cytokine function through induced expression of suppressor of cytokine signaling proteins (SOCS) in hematopoietic cell lines[5]. It may therefore be postulated that rhEPO mitigates IR injury through both anti-apoptotic and anti-inflammatory mechanisms.
The hepatocellular protective affect of rhEPO treatment in liver IR has been reported as being virtually entirely indirect, through effects on non-parenchymal cells (NPCs)[6]. We present studies of rhEPO protection after hepatic IR injury that challenge this belief. Post-ischemia treatment with rhEPO resulted in sustained protection after IR. Although rhEPO induces SOCS3 and STAT3 anti-inflammatory mechanisms in both injured and control whole livers, this induction did not occur in cultured hepatocytes treated with rhEPO, and thus may require NPCs. By contrast, heme oxygenase-1 (HO-1), which is known to serve both anti-inflammatory and anti-apoptotic functions in the face of liver injury, is induced by rhEPO in both whole liver and in cultured hepatocytes. These data indicate that rhEPO protection after hepatic IR is affected through a spectrum of both parenchymal (i.e. hepatocytic) and NPC mechanisms.
MATERIALS and METHODS
Mice
All animal protocols were approved by the Institutional Animal Care and Use Committees of the Veterans Affairs and the University of Washington. C57/Bl6 male mice, free of Helicobacter species and 6–8 weeks of age were purchased (Jackson Labs, Maine) and allowed to acclimate to their environment for 7 days prior to utilization in experiments. All animals were housed in a specific pathogen free facility, fed standard rodent chow with water ad libitum, and subjected to 12h day/night light cycles.
Surgical preparation and sampling
Non-fasted mice underwent in situ partial liver ischemia followed by reperfusion (8 per group) under isoflurane anesthesia between 8AM and noon. Through a midline incision, an atraumatic vascular clip was applied to the porta hepatis, such that the cephalad (median and left) lobes were ischemic while the posterior (right) and caudate lobes remained continuously perfused as previously described [7, 8]. Immediately after vascular clip placement, rhEPO (4units/gm in PBS, Amgen: commercial stock of 2000 units/ml in solution containing 0.11mg citric acid, 0.5mg human albumin, 1.3mg sodium citrate, 8.2mg sodium chloride, 1% benzoyl alcohol preservative per ml) or an equivalent volume of PBS was subcutaneously (SQ) administered. Animals remained under anesthesia on a heating pad at 39°C with the abdomen temporarily closed to minimize fluid losses during the period of ischemia. After 90min of ischemia, the clip was removed and reperfusion was visually confirmed. The abdomen was closed after infiltration of the wound with 0.25% marcaine and the recovered animals were allowed ad libitum access to food and water. Animals undergoing laparotomy without vascular occlusion for similar periods of anesthesia served as sham-operated controls. All animals demonstrated normal activity and eating patterns by 24h.
Cohorts of animals were euthanized 0.5, 1, 2, 4, or 24h after reperfusion. At the time of CO2 inhalation euthanasia, serum was collected by cardiac puncture and stored at −80° for serum aspartate aminotransferase (AST) and alanine transaminase (ALT) kinetic assay analysis (Sigma-Aldrich, St. Louis, MO). Liver from sham, ischemic and continuously perfused lobes was preserved in 10% formalin for histology or snap frozen in liquid nitrogen and stored at −80°C for future RNA and protein analyses. An additional cohort was treated with SQ rhEPO (no surgery) and euthanized 30, 60, 90, 210, or 330 minutes after injection to determine the kinetic affects of rhEPO injection on normal liver (4 mice per time point). These post-injection time points were chosen to correspond to major time points in the IR protocol −90min, severe ischemia time; 210min, 2h reperfusion; 330min, 4h reperfusion.
Cultured Hepatocyte studies
AML-12 cells were plated and cultured as described[9], and treated with rhEPO (2U/mL) after overnight serum starvation. Triplicate samples were harvested in Triton X lysis buffer at the indicated time points and subjected to immunoblotting as described below.
RNA Preparation and Real Time Polymerase Chain Reaction (RT-PCR) analysis
Total RNA was prepared from frozen liver tissue using Trizol/chloroform extraction according to the manufacturer’s recommendations (Invitrogen, Carlsbad, CA). Total RNA purity and concentration were determined using a spectrophotometer (BioRad. Hercules, CA). Wavelength ratios (260:280) between 1.7 and 2.0 indicated acceptable RNA quality. To confirm RNA integrity, 1µg RNA samples were electrophoresed on 1% agarose/ethidium bromide gels.
DNase purified total RNA from ischemic, perfused and sham treated liver was reverse transcribed into cDNA (Invitrogen) and subjected to quantitative RT-PCR, utilizing FAM-labeled primers for Socs1, Socs3, and Cis on ABI Prism® 7000 instrumentation, with reagents and software from Applied Biosystems (Foster City, CA). Delta delta Ct (ΔΔCt) values were calculated by subtracting Ct values for the gene of interest from Ct values for β-actin (housekeeping gene) and then subtracting the ΔCt value obtained for each gene from non-operated control mice. Fold change was calculated by normalizing all values to those of non-operated wild-type mice.
Protein isolation, Caspase analysis and Immunoblotting
Ischemic liver tissue samples were homogenized in Triton-X lysis buffer containing protease inhibitors as previously described [10]. Protein concentrations were determined using Bradford analysis with bovine serum albumin as a standard. Caspase 3 activity in liver homogenates was determined using a fluorogenic substrate as previously described [11]. Normalized lysate aliquots were stored in sample buffer at −80°C for future immunoblot analyses.
Tissue or cell lysate samples (30–50µg) diluted in sample buffer were subjected to SDS-PAGE and transferred to PVDF membranes (Amersham Biosciences, Piscataway, NJ). Immunoblotting was performed using primary antibodies to phospho-STAT-3, total STAT-3, phospho-STAT5, total STAT-5, β-actin (all Cell Signaling, Danvers, MA), or HO-1 (Assay Designs). Membranes were incubated for 1h at room temperature in appropriate horseradish peroxidase secondary antibody and signals detected using ECL Western Blotting Detection Reagent (Amersham). Autoradiographic film representing each phospho- and total STAT-3 blot was scanned and NIH Image analysis (version X; National Institutes of Health) was used to quantify the density of the appropriate bands.
Luminex®protein analysis
Whole liver lysates were tested for TNFα, IL-1β, IL-4, IL-6, and IL-10 by the laboratory of James Lederer, PhD (Brigham and Women’s Medical Center, Boston, Massachusetts) on a custom-made Luminex multiplexcytokine detection bead assay platform using a Luminex 200 instrument (Luminex Corporation, Austin, TX). These Luminex bead multiplexcytokine assays have a detection sensitivity range of 5–25,000 pg/ml and were performed using 200µl of cleared sample. Results were calculated using StarStation 3.0 Software (Applied Cytometry, Sheffield, UK).
Histologic scoring and Immunhistochemistry
Formalin-fixed samples from the right, middle and left lobes of the ischemic liver were analyzed for injury severity by a Comparative Medicine pathologist, who was blinded to treatment groups. Samples were scored based on calculation of the area occupied by necrotic tissue in the following manner: images of all histologic sections of liver in perfused and control groups were captured using a Nikon Digital Sight D5-Fi1 camera and Nikon 80i microscope; surface areas occupied by the entire liver and individual or confluent necrotic areas within the liver were each digitally outlined using the area function of NIS-Elements Software (Nikon Instruments). The percentage of each liver section occupied by necrotic tissues was calculated using this data, and a a severity score ranging from 0 (normal) to 4+ (15% or greater of combined liver sectional area affected) was constructed. Immunohistochemistry (IHC) for cleaved caspase 3 and HO-1 was performed using standard protocols and primary antibodies from Biocare Medical (cleaved caspase 3, cat # CP 229B) and Assay Designs (HO-1, cat # SPA-895).
Statistical analysis
Data are expressed as means ± SEM. Two-way ANOVA and verified by Bonferroni-Dunn post hoc testing was performed using GraphPad Prism 4.00 for Windows (SanDiego, CA). Values for each ischemic condition at intervals after reperfusion were compared, with significant differences accepted for P values ≤0.05.
RESULTS
Erythropoietin is protective in hepatic IR
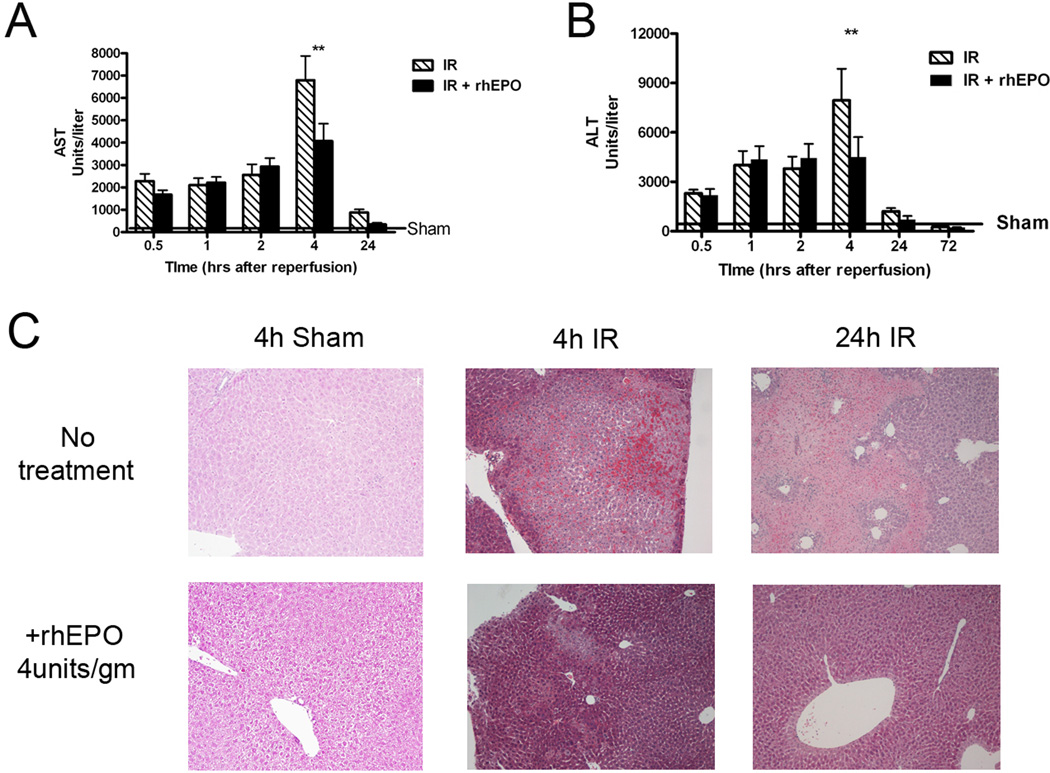
rhEPO treatment prior to the onset of ischemia results in hepatic protection after mild or moderate IR[12, 13]. More severe degrees of ischemia followed by reperfusion have not been investigated. We confirmed rhEPO protection against IR injury by performing partial liver IR as described[14], with or without administration of SQ rhEPO at the time of ischemia. rhEPO treatment significantly decreased AST (Figure 1A) and ALT levels (1B) four hours after severe (90 min) IR. Liver lobes subjected to 90min of ischemia showed extensive areas of coagulative necrosis with hemorrhage and neutrophil infiltration, but rhEPO treatment at the onset of ischemia resulted in a comparatively less severe injury at both 4 and 24h reperfusion (representative sections shown in Figure 1C). Calculation of the percentage of necrotic parenchyma in sections of the right, middle, and left ischemic lobes from treated and untreated animals confirmed that rhEPO treatment decreased the severity of histologic injury by approximately 60%. Liver from perfused and sham treated lobes had normal histology with or without rhEPO treatment (data not shown). The mean severity score in untreated mice was 3.25+(8/8 section affected) compared to a mean score of 1.7+ (7/10 sections affected) for those mice receiving rhEPO treatment. These data support the hypothesis that rhEPO-mediated hepatocellular protection after IR occurs early after reperfusion.
Figure 1.
Erythropoietin (rhEPO) is protective against liver ischemia reperfusion (IR) injury. A, B. Serum aspartate aminotransferase (AST, A) and alanine aminotransferase (ALT, B) levels were significantly decreased 4 hours after reperfusion when rhEPO was given at the time of reperfusion. ** = p<0.01, rhEPO treated vs. untreated. C. Hematoxylin & eosin staining of livers 4 and 24 hours after IR with or without rhEPO demonstrates a significant decrease in necrosis in rhEPO-treated livers. Sham treated and untreated sections are shown as controls; representative sections are at 10×.
rhEPO injection induces hepatic JAK-STAT and SOCS signaling mechanisms
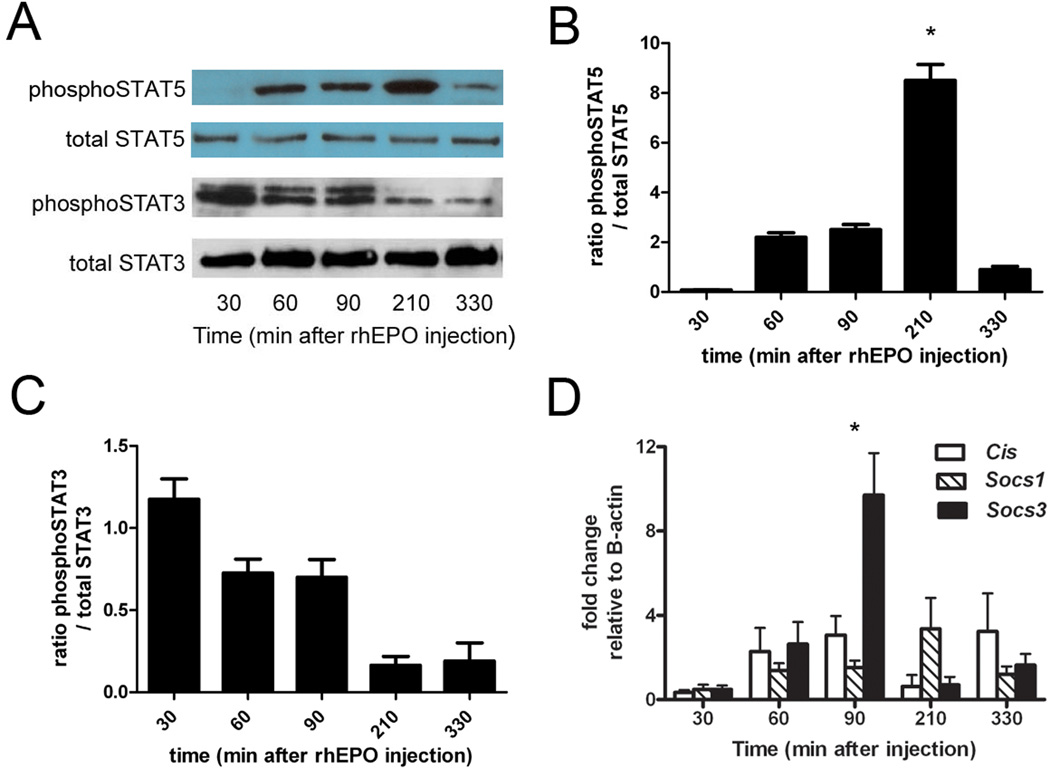
EPO is known to activate JAK-STAT signaling and induce Socs expression in hematopoietic cells[15], suggesting a mechanism for modulation of activated inflammation after reperfusion. We first examined these pathways after rhEPO injections alone (without IR) to determine whether these mechanisms are induced in whole liver and thus may contribute to rhEPO-induced protection after IR. Using time points similar to harvest points after reperfusion in our IR protocol, we characterized the kinetics of rhEPO-induced whole liver activation of phosphoSTAT5, which peaked at 210 minutes (Figure 2A), and phosphoSTAT3, which was not significantly induced. Densitometric analyses confirmed statistically significant activation of STAT5 (2B), but not in STAT3 (2C). We then assayed expression of Cis, Socs1, and Socs3 over the same time course of rhEPO treatment. Induction of Socs3 mRNA predominated, peaking within 90min and declining to near basal levels over the ensuing 4h (Figure 2D). Socs1 induction was comparatively delayed and Cis induction appeared to be biphasic, with an early peak at 90min and a second at 330min.
Figure 2.
Induction of the STAT-Socs pathway in whole liver after injection of erythropoietin (rhEPO). A. Immunoblotting for phosphoSTAT5 and phosphoSTAT3 whole liver after rhEPO injection. Total STAT5 and total STAT3 are shown as loading controls. B, C. demonstrate densitometry analysis of the immunoblots shown in A, n=3–5 per time point. *=p<0.05 versus untreated. D. RNA was isolated from whole liver after rhEPO injection, and expression of Cis, Socs1, and Socs3 was assessed by qPCR. rhEPO significantly induced Socs3 expression 90 minutes after injection. * = p<0.05
Expression of inflammatory mediators in ischemic liver is unaffected by rhEPO
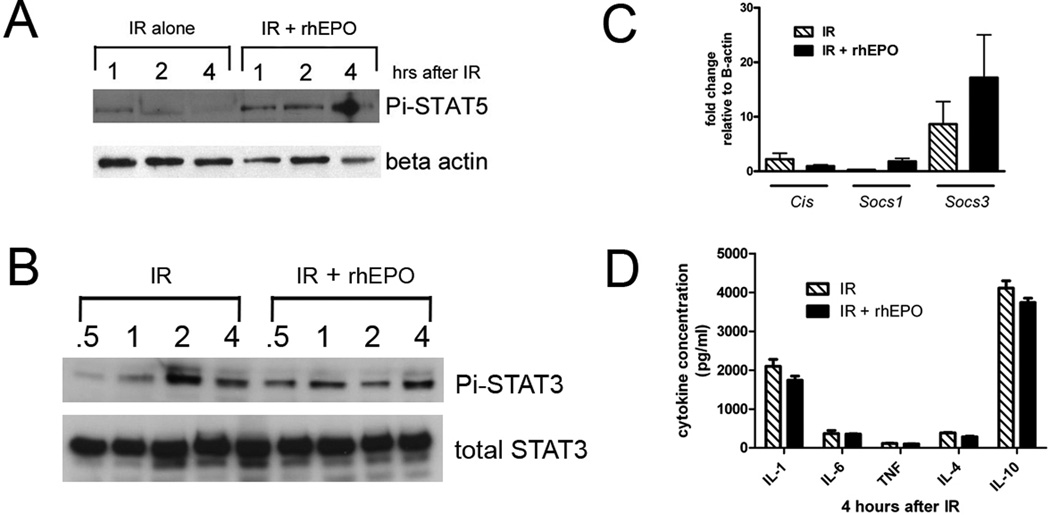
Given our findings that rhEPO injection leads to activation of STAT5 and Socs3, we returned to our murine IR model to determine whether similar mechanisms are involved in protection from IR by rhEPO. Similar to after injection alone, we found that IR + rhEPO lead to increased activation of STAT5 (Figure 3A) but also an earlier activation of STAT3 (Figure 3B) compared with IR alone. Similar to after rhEPO injection alone, Socs3 was the predominant SOCS family member induced after IR with rhEPO treatment (Figure 3C). Given the activation of the STAT-Socs pathway, we investigated whether rhEPO’s protective effects reflected altered inflammatory mediator expression. Interestingly, Luminex® assay of ischemic liver homogenates from rhEPO treated and untreated mice showed similar induction of both pro- and anti-inflammatory cytokines (TNF, IL-1β, IL-6; IL-4, IL-10) both 1h (data not shown) and 4h after reperfusion (Figure 3D). These data indicate that altered cytokine expression is not a primary mechanism underlying rhEPO protection during liver IR.
Figure 3.
STATs are activated in ischemic liver by erythropoietin, but cytokine expression is not affected. Immunoblotting whole liver lysates demonstrates that rhEPO treatment leads to enhanced phosphoSTAT5 (A) and earlier (B) phosphoSTAT3 activation after ischemia-reperfusion (IR). C. qPCR of whole liver lysates shows that Socs3 is the primary family member induced after IR, with or without rhEPO treatment. D. Luminex multiplex cytokine analysis demonstrates that rhEPO treatment does not effect expression of key inflammatory or anti-inflammatory cytokines in liver lysates after IR.
Anti-apoptotic affects of rhEPO after liver IR
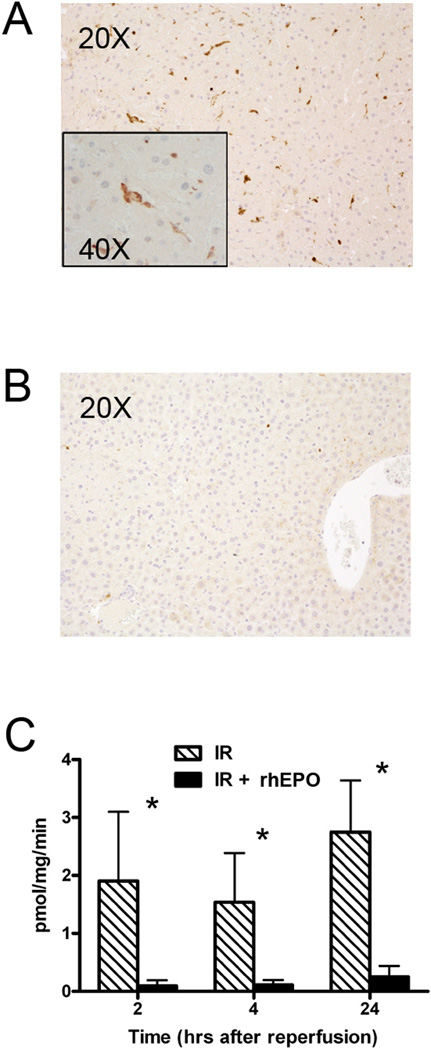
Given that we could not identify significant changes in cytokine expression after IR due to rhEPO treatment, and that our findings of differential activation of STATs and Socs3 were subtle, we investigated effects of rhEPO on alternative protective pathways after IR. Previous investigators have suggested that inhibition of apoptosis within minutes of reperfusion is a dominant mechanism responsible for rhEPO-mediated protection after IR injury[12, 13]. We first performed IHC for activated caspase-3 in post-IR livers. As shown in Figure 4A, caspase-3 activity in untreated mice was most prominent in NPCs immediately adjacent to areas of patchy necrosis. IHC of rhEPO treated ischemic liver demonstrated that this treatment abrogated the apoptotic response (Figure 4B). To better quantify levels of apoptosis, activated caspase-3 levels were assayed using a fluorogenic assay 2, 4 and 24h after reperfusion. As shown in Figure 4C, caspase-3 was activated at multiple time points after 90min IR, and rhEPO treatment lead to a significant reduction in caspase-3 activation throughout the examined period of reperfusion. This suggests that caspase-mediated anti-apoptotic affects of rhEPO are in NPCs at the margins of injury, and that its contribution to parenchymal protection reflects an indirect limitation of local hepatocellular loss.
Figure 4.
Erythropoietin (rhEPO) treatment decreases apoptosis after liver ischemia-reperfusion (IR). A, B. Immunohistochemistry for activated caspase 3 in untreated liver after IR demonstrates immunoreactivity in non-parenchymal cells. B. rhEPO treatment prevents caspase activation after IR, as shown by immunohistochemistry. C. A fluorogenic caspase activity assay performed after IR shows that rhEPO decreases caspase activity in the ischemic liver. * = p<0.05 in treated vs. untreated samples
Protective Mechanisms of rhEPO in hepatocytes in culture
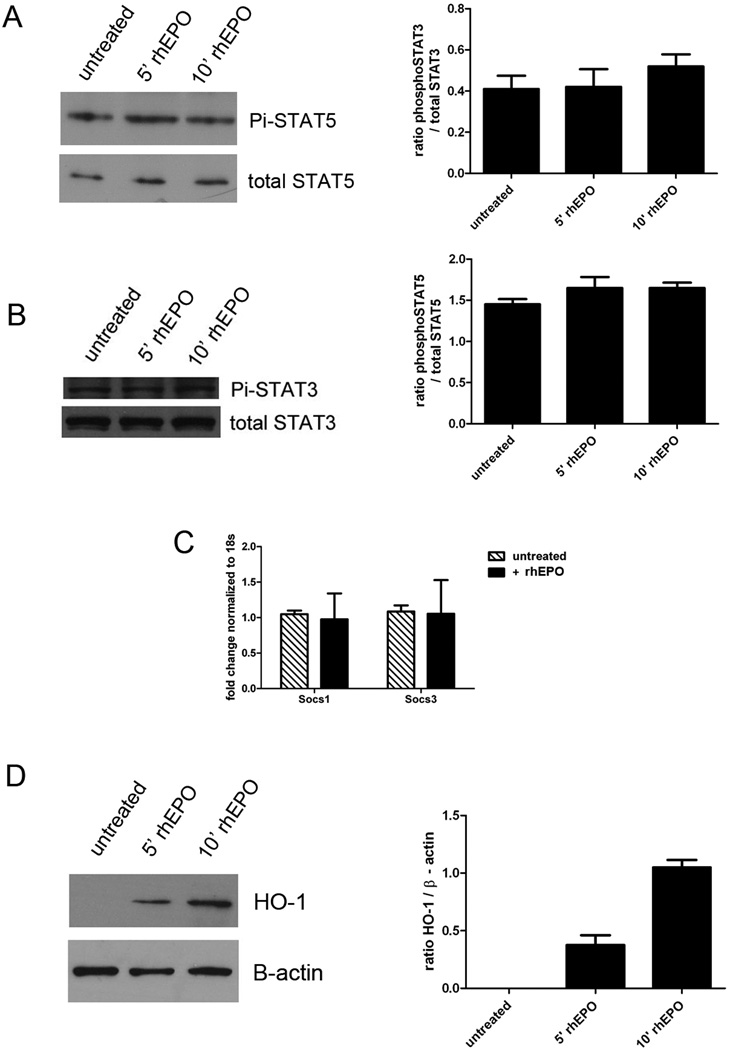
Since both hepatocytes and NPCs utilize STAT5 and SOCS3 in the regulation of cytokine-signaling, EPO-mediated expression could reflect induction in either cellular compartment. Previous authors have reported a lack of JAK-STAT signaling induction by rhEPO in either whole rat liver or isolated hepatocytes, despite the constitutive expression of rhEPO receptor mRNA[6]. However, given our in vivo observations, we reassessed those findings by evaluating effects of rhEPO on cultured hepatocytes. AML12 cells were cultured and stimulated with rhEPO, then assayed for STAT5 (Figure 5A), STAT3 (Figure 5B) and Socs induction (Figure 5C) at various time points thereafter. These pathways indeed were not significantly induced by rhEPO treatment of cultured hepatocytes. These data suggest that the induction of STAT5 and Socs3 by rhEPO that we detected in whole liver is likely to require NPCs in vivo, and that, like caspase-3 limitation of apoptosis, hepatocellular protection via such mechanisms is indirect.
Figure 5.
Induction of protective pathways in isolated hepatocytes. AML12 cells were stimulated with rhEPO and cell lysates were prepared. Immunoblotting revealed that rhEPO does not activate STAT5 (A) or STAT3 (B) in hepatocytes in vitro. Total STAT5 and total STAT3 are shown as loading controls, and densitometry analysis of n = 3–5 samples per time point is shown. C. rhEPO did not induce expression of Socs1 or Socs3 in AML12 cells, as assessed by qPCR. D. rhEPO induces HO-1 expression in hepatocytes in vitro. B-actin is shown as a loading control, and densitometric analysis of n=3–5 samples per time point is shown.
Hepatocellular induction of HO-1 by rhEPO
IR injury and its progression can be curtailed through modulation of hepatocellular oxidative stress. Reduction of oxidative stress through induction of HO-1 has shown significant cytoprotection in models of acute and chronic liver damage, including IR injury[16, 17]. We found that rhEPO treatment of AML12 cells did induce HO-1 expression by immunoblotting, as shown in Figure 5D. This HO-1 induction in isolated hepatocytes provides evidence that even in the absence of ischemic injury, rhEPO is capable of directly stimulating hepatocytes.
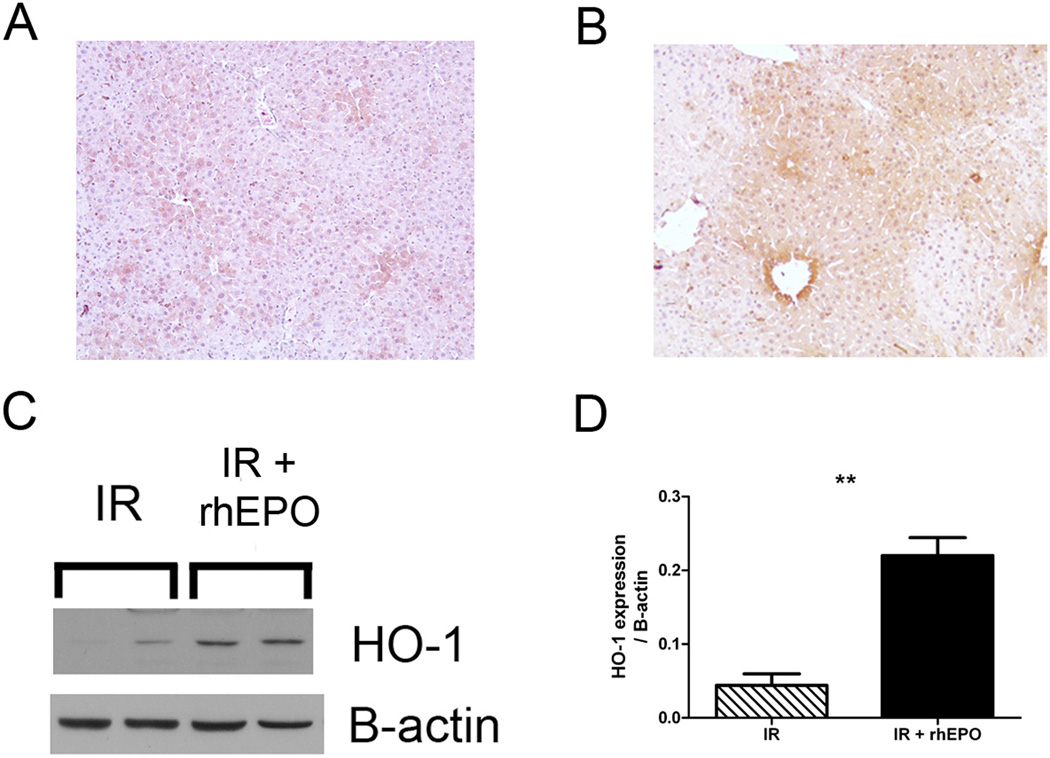
After determining that rhEPO could stimulate HO-1 expression in cultured hepatocytes, we returned to our model of rhEPO-treated, severe hepatic IR. We performed IHC for HO-1 4 hours after IR and confirmed that there is minimal expression of hepatocyte HO-1 in untreated mice undergoing IR (Figure 6A). As shown in Figure 6B, rhEPO treatment at the time of IR injury leads to high levels of HO-1 expression in hepatocytes. Immunoblotting for HO-1 demonstrated that rhEPO induces significant HO-1 expression in lysates from ischemic liver after 90min of ischemia followed by 4h reperfusion (Figure 6C, D). In summary, these data suggest that the abrogation of liver IR by rhEPO treatment is not limited to paracrine effects originating in nonparenchymal cells as has previously been asserted. Rather, direct stimulation of hepatocyte HO-1 provides an additional potent mediator effecting rhEPO-based parechymal protection.
Figure 6.
HO-1 is induced in ischemic liver by rhEPO treatment. A, B. Immunohistochemistry for HO-1 was performed after ischemia-reperfusion (IR) with (B) or without (A) erythropoietin (rhEPO) treatment, and demonstrates that hepatocellular expression of HO-1 after rhEPO treatment. C. rhEPO treatment induces HO-1 expression in whole liver, 4 hours after IR. Β-actin is shown as a loading control. D. Densitometric analysis of immunoblots from n=4–6 mice per time point. **=p<0.01 treated vs. untreated.
DISCUSSION
While inflammation is a normal response to acute injury and crucial to injury resolution, it can also lead to unintended consequences. The events leading to injury following liver IR have been well described [18], and include the generation of reactive oxygen species during portal vascular occlusion and activation of the complement cascade. Activated Kupffer cells produce a spectrum of inflammatory cytokines, including IL-1β, IL-10, and TNFα, which activate adjacent hepatocytes and endothelial cells. Activated hepatocytes in turn amplify the initial Kupffer cell response by expressing and releasing additional mediators (e.g. IL-6) to perpetuate injury through activation of neighboring cells. Neutrophils recruited during reperfusion adhere to the sinusoidal endothelium, contributing to microcirculatory vasoconstriction and setting the stage for injury progression to tissue necrosis. Control of injury and return to homeostasis hinge on a dynamic process of continuous, subtle re-balancing of inflammatory signals across the hepatic microenvironment. Given the complexity of this system, the entire spectrum of inflammatory responses associated with IR must be highly regulated. Successful therapeutic strategies will need to impact more than one aspect of injury.
EPO is a glycoprotein hormone vital for the differentiation of committed erythroid progenitor cells. The recombinant human formulation has been used safely for many years as an effective means of recovering red cell mass, but non-hematopoietic functions of rhEPO have become increasing apparent. Corwin and colleagues reported that early treatment with rhEPO provides a survival advantage for trauma patients, independent of hematopoietic impact [19]. Studies in laboratory animal models of injury, including ours, further support this observed effect on outcome.
Our data offer further insight into rhEPO-mediated hepatic IR protection on several levels. We have shown that rhEPO-mediated protection is effective when treatment occurs at the onset reperfusion, when the primary effects of the ischemic injury phase are established. Alterations in cytokine expression have long been assumed to be a primary mechanism contributing to IR protection. We have shown that this is not the case for early reperfusion injury protection mediated through rhEPO. Our focus in this study was to explore early mechanisms that might further enhance our understand of how rhEPO might be exploited in the management of IR injury
Erythropoietin and apoptosis
To date, the protective non-hematopoietic effects of rhEPO in pre- and/or post-injury treatment models of IR in a variety of tissues [3, 4] have been largely attributed to up-regulation of anti-apoptotic genes [20–22]. Pre-treatment with rhEPO has been shown to ameliorate hepatic IR injury; inhibition of apoptosis has been cited as a primary mechanism of this protection[23]. Warm hepatic IR leads to necrotic cell death (oncosis), and often occurs within minutes of reperfusion. Since the endogenous tissue receptor for EPO is up-regulated by hypoxia[20–22], circulating rhEPO might be expected to have a rapid and intense effect on ischemic liver injury soon after reperfusion.
Although studies have suggested that apoptosis contributes to the early evolution IR injury, strict interpretation of TUNEL assays leads to the conclusion that no more than 2% of the liver cells at risk are undergoing apoptosis[24]. Nevertheless, caspase dependent signaling pathways have been proposed as potential targets of rhEPO protection in IR [25, 26]. Although our data confirm that rhEPO significantly diminishes caspase activity initiated by IR, IHC localizes this alteration to NPCs (likely Kupffer cells), rather than hepatocytes. Given the relatively small number of Kupffer cells in the liver parenchyma, this cellular location would account for the low-level total caspase activation in our IR controls. These data indicate that rhEPO’s primary anti-apoptotic affects after liver IR are centered in NPCs. We thus questioned whether additional mechanisms might be contributing to its protection in hepatic IR.
Erythropoietin signaling in liver
Like other inflammatory mediators associated with hepatic IR, rhEPO signals through the JAK-STAT pathway, predominantly through STAT 5, but has been shown to induce SOCS proteins in erythroid cell lines [5]. Induction of these potent regulators of inflammation might be expected to contribute to tissue protection through mitigation of the cytokine-mediated reperfusion injury. We have previously shown that expression levels of Socs1 and Socs3, signaling through STAT3, parallel the severity of hepatic IR injury [14]. Since induction of mechanisms that regulate cytokine signaling is integral to the response to IR, these mechanisms might be treatment targets in the management of acute IR injury and recovery. The contribution of Socs gene induction to rhEPO-mediated protection after hepatic IR has not previously been explored.
Several models of IR have utilized rhEPO pre-treatment strategies and our data suggest that rhEPO has an effect on liver parenchyma in the absence of a hypoxic injury. We have shown that Socs genes are induced in normal liver within 2h of rhEPO injection and that Socs3 is the dominant family member. In order to determine whether STAT/Socs activation is in NPCs or hepatocytes, we stimulated cultured hepatocytes with rhEPO and found that it failed to induce expression of either STAT3 or STAT5 in these cells (Figure 5A,B). These findings affirm that the contribution of JAK-STAT signaling associated with rhEPO-mediated protection in hepatic IR is through NPC.
We hypothesized that early NPC STAT3 activation by rhEPO would rapidly induce sufficient Socs3 in whole liver to interrupt cytokine-mediated inflammation and inhibit reperfusion injury, even if rhEPO was injected after the onset of ischemia. While rhEPO did induce early STAT3 phosphorylation in ischemic liver after IR when compared with untreated mice, circulating and whole liver lysate levels of a spectrum of pro-and anti-inflammatory mediators were unaffected by rhEPO treatment. These data suggest that induction of JAK-STAT regulatory mechanisms may not be the dominant feature of rhEPO-mediated protection after severe IR.
Erythropoietin, HO-1 and Liver Injury
Formation of reactive oxygen species and oxidative stress are central to the injury invoked by hepatic IR[1, 27]. The heme oxygenase system and its role in the control of oxidative stress and subsequent inflammation has been the focus of considerable interest for more than a decade. HO-1 in particular has been identified as a potent defense mechanism, induced by a varied of insults including hypoxia, endotoxin, cytokines, heat shock, and shear stress in a variety of tissues and disease states(Wunder and Potter, 2003). HO-1 is a highly conserved molecule; its loss is characterized by growth retardation, susceptibility to oxidative stress, and chronic inflammation, particularly in liver and kidney[17]. Chronic overexpression in the liver, with its attendant impaired apoptosis, has been associated with the development of hepatocellular carcinoma. Interestingly, Geuken et al have reported a range of HO-1 induction in human livers from brain-dead multi-organ donors. They found that those with initially low HO-1 expression showed significantly greater HO-1 induction during reperfusion, less injury, and improved overall function when compared with grafts expressing high levels of HO-1 prior to IR[28]. This suggests that while the capacity for HO-1 induction in liver may be limited, a regimen that offers a controlled microenvironmental shift in HO-1 expression could offer a key to protection from hepatic injury.
The two isozymes of the heme oxygenase system, HO-1 and HO-2, show distinct topographical patterns of expression in normal and stressed liver[29]. HO-2 is most abundant within hepatocytes, sinusoidal endothelial cells and hepatic stellate (Ito cells), while Kupffer cells express both isoforms. In addition to redox pathways (Keap1/Nrf2, transcription repressor Bach1, AP-1 and NF-κB), multiple cell signaling pathways contribute to HO-1 gene regulation, including the omnipresent p38 MAPK, P13K/Akt, TLR-4, IL-10 and JAK-STAT pathways[30]. HO-1 is induced in stressed hepatocytes as a defense mechanism, but the majority of treatment strategies involving HO-1 have targeted Kupffer cell signaling, assuming hepatocyte protection to be the result of paracrine effects.
Luo et al previously described an association between rhEPO protection and HO-1 induction in whole rat liver after a moderate liver IR (45min) with 24 hr rhEPO pre-treatment[31]. Our data build on these earlier observations, showing that protection by rhEPO is sustained in the face of a much more severe IR injury (90min), even when reEPO treatment is initiated at the time of reperfusion. We have shown that rhEPOs is a strong inducer of hepatocytes HO-1, particularly at the interface between necrotic and normal parenchyma after severe IR injury. We have further shown rhEPO is capable of direct hepatocyte stimulation, a finding that is contrary to prior assertions. We believe that these data, taken as a whole, show that the effects of a single, post-ischemia administration of rhEPO on liver are multifaceted, directly inducing both parenchymal and NPC anti-oxidative and anti-inflammatory pathways.
Although we have shown a strong induction of HO-1 in cultured hepatocytes and documented its enhanced expression in tissue, we have not performed the studies that would be necessary to confirm that HO-1 is the sole or even primary mechanism underlying rhEPO-based liver IR protection. Possible areas for further exploration might include functional silencing of HO-1 with zinc protoporphyrin IX or hepatocyte specific knock-down of Ho1 gene expression. Microarray analysis would offer an additional approach to investigate whether other proteins may also be important to the evolution of IR injury protection with rhEPO treatment. Such studies are the subject of ongoing investigation.
CONCLUSION
A number of strategies have been proposed to reduce the risk of postoperative dysfunction in marginal livers that are more vulnerable to IR injury[1, 27]. Therapeutic interventions that target multiple pathways will likely hold the greatest promise. Properly applied, such strategies would also expand the potential donor pool for transplantation. It is clear that the actions of EPO extend well beyond erythropoiesis. This study emphasizes the spectrum of effects mitigated by the use of rhEPO as a therapy in hepatic IR. The inclusion of rhEPO as a component of post-reperfusion support offers a safe and effective means of targeting multiple pathways inherent to liver recovery.
Acknowlegments
James Lederer, PhD and his laboratory at Brigham and Women’s Medical Center, Boston, Massachusetts for Luminex analysis.
FUNDING SUPPORT
LA Langdale: VA Merit Review – 20559
JS Campbell: NIH CA-127228
KJ Riehle: Herbert Coe Foundation, American College of Surgeons Louis C. Argenta Fellowship, American Surgical Association Foundation
ABBREVIATIONS
- IR
ischemia-reperfusion
- HO-1
heme oxygenase-1
- rhEPO
recombinant human erythropoietin
- SOCS1, 3, and Cis
suppressors of cytokine signaling
- STAT3, STAT5
signal transducer and activators of transcription
- ALT
alanine transaminase
- RT-PCR
Real Time Polymerase Chain Reaction
- NPC
Non-parenchymal cell
- IHC
Immunohistochemistry
REFERENCES
- 1.Jaeschke H, Woolbright B. Current strategies to minimize hepatic ischemia-reperfusion injury by targeting reactive oxygen species. Transplantation Reviews. 2012;26:103–114. doi: 10.1016/j.trre.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Teoh N, Farrell G. Hepatic ischemia reperfusion injury: Pathogenic mechanisms and basis for hepatoprotection. J Gastro Hepatol. 2003;18:891–902. doi: 10.1046/j.1440-1746.2003.03056.x. [DOI] [PubMed] [Google Scholar]
- 3.Brines M, Cerami A. Discovering erythropoietin's extra-hematopoietic functions: Biology and clinical promise. Kidney Intern. 2006;70:246–250. doi: 10.1038/sj.ki.5001546. [DOI] [PubMed] [Google Scholar]
- 4.Ghezzi P, Brines M. Erythropoietin as an anti-apoptotic, tissue-protective cytokine. Cell Death Diff. 2004;11:S37–S44. doi: 10.1038/sj.cdd.4401450. [DOI] [PubMed] [Google Scholar]
- 5.Jegalian A, Wu H. Differential roles of SOCS family members in EpoR signal transduction. J Interferon Cytokine Res. 2002;22:853–860. doi: 10.1089/107999002760274863. [DOI] [PubMed] [Google Scholar]
- 6.Bramey T, Freitag P, Fandrey J, Rauen U, Pamp K, Erhard J, Frede S, deGroot H, Petrat F. No evidence for protective erythropoietin alpha signaling in rat hepatocytes. BMC Gastroenterol. 2009;21(9):26. doi: 10.1186/1471-230X-9-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Langdale L, Wilson L, Jurkovich G, Liggitt H. Effects of immunomodulation with interferon-γ on hepatic ischemia-reperfusion injury. Shock. 1999;11(5):356–361. doi: 10.1097/00024382-199905000-00009. [DOI] [PubMed] [Google Scholar]
- 8.Langdale L, Kajikawa O, Frevert C, Liggitt H. Sustained tolerance to lipopolysaccharide after liver ischemia-reperfusion injury. Shock. 2003;19(6):553–558. doi: 10.1097/01.shk.0000055238.25446.64. [DOI] [PubMed] [Google Scholar]
- 9.Wu J, Merlino G, Fausto N. Establishment and characterization of differentiated, non-transformed hepatocyte cell lines derived from mice transgenic for transforming growth factor alpha. Proc. Nat. Acad. Sci. 1994;91:674–678. doi: 10.1073/pnas.91.2.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Campbell J, Prichard L, Schaper F, Schmitz J, Stephenson-Famy A, Rosenfeld M, Argast G, Heinrich P, Fausto N. Expression of suppressors of cytokine signaling during liver regeneration. J. Clin. Invest. 2001;107:1285–1292. doi: 10.1172/JCI11867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chaisson M, Brooling J, Ladiges W, Tsai S, Fausto N. Hepatocyte-specific inhibition of NF-κB leads to apoptosis after TNF treatment, but not after partial hepatectomy. J. Clin. Invest. 2002;110:193–202. doi: 10.1172/JCI15295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schmeding M, MNeumann U, Boas-Knoop S, Spinelli A, Neuhaus P. Erythropoietin reduces ischemia-reperfusion injury in the rat liver. Eur Surg Res. 2007;39(3):189–197. doi: 10.1159/000101009. [DOI] [PubMed] [Google Scholar]
- 13.Sepodes B, Maio R, Pinto R, Sharples E, Oliveira P, McDonald M, Yagoob M, Thiemermann C, Mota-Filipe H. Recombinant human erythropoietin protects the liver from hepatic ischemia-reperfusion injury in the rate. Transpl Int. 2006;19(11):919–926. doi: 10.1111/j.1432-2277.2006.00366.x. [DOI] [PubMed] [Google Scholar]
- 14.Langdale L, Hoagland V, Benz W, Riehle K, Campbell J, Liggitt H, Fausto N. Suppressor of cytokine signaling expression with increasing severity of murine hepatic ischemia-reperfusion injury. J. Hepatology. 2008;49(2):198–206. doi: 10.1016/j.jhep.2008.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sarna M, Ingley E, Busfield S, Cull V, Lepere W, McCarthy D, Wright M, Palmer G, Chappell D, Syer M, Klinken S, Tilbrook P. Differential regulation of SOCS genes in normal and transformed erythroid cells. Oncogene. 2003;22:3221–3230. doi: 10.1038/sj.onc.1206381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sass G, Barikbin R, Tiegs G. The multiple functions of heme oxygenase-1 in the liver. Z Gastrolenterol. 2012;50(1):34–40. doi: 10.1055/s-0031-1282046. [DOI] [PubMed] [Google Scholar]
- 17.Wunder, Potter CR. The heme oxygenase system: Its role in liver inflammations. Current Drug Targets - Cardiovas., Haemat. Dis. 2003;3(3):199–208. doi: 10.2174/1568006033481410. [DOI] [PubMed] [Google Scholar]
- 18.Jaeschke H, Lemasters J. Apoptosis versus oncotic necrosis in hepatic ischemia/reperfusion injury. Gastroenterology. 2003;125:1246–1257. doi: 10.1016/s0016-5085(03)01209-5. [DOI] [PubMed] [Google Scholar]
- 19.Corwin H, Gettinger A, Fabian T, May A, Pearl R, Heard S, An R, Bowers P, Burton P, Klausner M, Corwin M. Efficacy and safety of Epoetin Alfa in critically ill patients. N Engl J Med. 2007;357(10):965–976. doi: 10.1056/NEJMoa071533. [DOI] [PubMed] [Google Scholar]
- 20.Paschos N, Lykissas MG, Beris AE. The role of erythropoietin as an inhibitor of tissue ischemia. Int J Biol Sci. 2008;4(3):161–168. doi: 10.7150/ijbs.4.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ratcliffe PJ, Ebert BL, Firth JD, Gleadle JM, Maxwell PH, Nagao M, O'Rourke JF, Pugh CW, Wood SM. Oxygen regulated gene expression: erythropoietin as a model system. Kidney Int. 1997 Feb;51(2):514–526. doi: 10.1038/ki.1997.72. [DOI] [PubMed] [Google Scholar]
- 22.Wu H, Klingmuller U, Besmer P, Lodish HF. Interaction of the erythropoietin and stem-cell-factor receptors. Nature. 1995 Sep 21;377(6546):242–246. doi: 10.1038/377242a0. [DOI] [PubMed] [Google Scholar]
- 23.Shawsky H, Younan S, Rashed L, Shoukry H. Effect of recombinant erythropoietin on ischemia-reperfusion-induced apoptosis in rat liver. J Physiol Biochem. 2012;68(1):19–28. doi: 10.1007/s13105-011-0114-2. [DOI] [PubMed] [Google Scholar]
- 24.Gujral J, Bucci T, Farhood A, Jaeschke H. Mechanism of cell death during hepatic ischemia-reperfusion in rats: apoptosis or necrosis? Hepatology. 2001;33:397–405. doi: 10.1053/jhep.2001.22002. [DOI] [PubMed] [Google Scholar]
- 25.Faubel S, Edelstein C. Caspases as drug targets in ischemic organ injury. Curr Drug Targets Immune Endocr Metabol Disord. 2005;5(3):269–287. doi: 10.2174/1568008054863754. [DOI] [PubMed] [Google Scholar]
- 26.Malhi H, Gores G, Lemasters J. Apoptosis and necrosis in the liver: a tale of two deaths? Hepatology. 2006;43(2) Suppl 1:S31–S44. doi: 10.1002/hep.21062. [DOI] [PubMed] [Google Scholar]
- 27.Elias-Miro M, Jimenex-Castro M, Rodes J, Peralta C. Current knowledge on oxidative stress in hepatic ischemia/reperfusion. Free Radical Research. 2013;47(8):555–568. doi: 10.3109/10715762.2013.811721. [DOI] [PubMed] [Google Scholar]
- 28.Geuken E, Buis C, Visser D, Blokzijl H, Moshage H, Nemes B, Leuvenink H, de Jong K, Petters P, Slooff M, Porte R. Expression of heme oxygenase-1 in human livers before transplantation correlates with graft injury and function after transplantation. Am J Transplant. 2005;5:1875–1885. doi: 10.1111/j.1600-6143.2005.00960.x. [DOI] [PubMed] [Google Scholar]
- 29.Bauer I, Wanner G, Rensing H, Alte C, Miescher E, Wolf B, Pannen B, MG C, Bauer M. Expression pattern of heme oxygenase isoenzymes 1 and 2 in normal and stress-exposed rat liver. Hepatology. 1998;27:829–838. doi: 10.1002/hep.510270327. [DOI] [PubMed] [Google Scholar]
- 30.Paine A, Eiz-Vesper B, Blasczyk R, Immenschuh S. Signaling to heme oxygenase-1 and its anti-inflammatory therapeutic potential. Biochemical Pharmacology. 2010;80:1985–1903. doi: 10.1016/j.bcp.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 31.Luo Y, Li Z, Liu L, Dong G. Pretreatment with erythropoietin reduces hepatic ischemia-reperfusion injury. Hepatobiliary Pancreat Dis Int. 2009;8(3):294–299. [PubMed] [Google Scholar]