Abstract
Background
Patients’ adherence with post-transplant immunosuppression is known to impact renal transplant outcomes.
Methods
Prospectively, individual medication adherence patterns in 195 kidney transplant recipients were quantified with electronic medication monitors. Monitored drugs were mycophenolate mofetil, sirolimus, or azathioprine. Monitoring began at hospital discharge and continued an average of 15(± 8) months. Patient follow-up for clinical outcomes averaged 8(± 3) years. Each month's adherence percentage was calculated as the sum of daily adherence percents, divided by the number of evaluable days.
Results
During the first 3 months post-transplant, patients (n=44) with declining medication adherence, defined as dropping by ≥7% (equal to missing 2 days) between months 1 and 2, later experienced lower mean medication adherence for months 6-12, 73% versus 92% respectively (p<.0001). Compared to patients with stable adherence, they also had more frequent (p=.034) and earlier (p=.065) acute rejection episodes. This was additionally associated with more frequent (p=.017) and earlier (p=.046) death-censored graft loss.
In addition daily medication adherence, expressed as the percentage of doses taken, decreased as the number of prescribed daily doses increased. During the first 3 months post-transplant, adherence with 4 doses/day averaged 84%, compared to 91% for patients on twice daily dosing (p=.024) and 93.5% for medications prescribed once daily (p=.008).
Conclusions
Early declining medication nonadherence is associated with adverse clinical outcomes. This pattern is detectable during the first 2 post-transplant months. Early detection of nonadherence provides opportunities to target interventions toward patients at the highest risk for adverse behaviors and events.
Keywords: drug monitoring, immunosuppression, transplantation, medication adherence.
Introduction
Renal transplantation is the optimal therapy for many patients with end-stage renal disease. Currently, except for identical twins, long-term successful transplantation requires life-long, daily immunosuppression. Surprisingly a significant number of transplant recipients fail to consistently follow their prescribed immunosuppressive regimen. This medication nonadherence (med-NA) ranges from accidental and rare, to complete cessation of a drug. Although definitions of med-NA vary somewhat, individual studies (1-4), database reviews (5) and meta-analyses (6,7) have all demonstrated substantial med-NA rates following renal transplantation. Indeed med-NA rates in renal transplant recipients are higher than those for any other solid organ transplant (6). Post-transplant med-NA has clearly been shown to be a critical factor associated with increased rates of graft dysfunction and loss (1-3,7).
Despite the obvious importance of med-NA (8,9), there are only a few studies of post-transplant med-NA with the more potent, contemporary immunosuppressive drugs (5). We showed in a previous study of once-daily azathioprine (Aza) adherence, that there was a significant association of early, declining compliance with increased rates of acute rejection and death-censored graft loss (1). These early-declining compliance (“drop2”) patients were those with at least 2 more days of missed doses in month 2 compared to month 1 after transplantation, i.e. adherence dropped by at least 2 days from month 1 to month 2. In the present study we report prospective electronic monitoring of contemporary immunosuppression confirming our earlier observations and demonstrating that the drop2 patients remain at increased risk for adverse outcomes, even when prescribed more potent medications.
Results
From August 1998 through August 2006, 1802 patients received kidney or kidney-pancreas transplants at the University of Minnesota Medical Center-Fairview. Of these, 868 (48.2%) were eligible, contacted and invited to participate in this drug-monitoring study; 452 patients (52.1%) consented to participate. Study patients were given an electronic medication event monitoring system cap (MEMS cap; AARDEX Group Ltd, 1950 Sion, Switzerland) to record adherence with one of their immunosuppressive medications beginning at discharge from their hospitalization for renal transplant.
By study design, prospective medication adherence monitoring was planned to extend to at least one year. One hundred ninety five patients (43%) provided data for all or part of the first study year, 192 patients had evaluable data for the first 3 consecutive mos. after hospital discharge. Of these, 125 were prescribed twice-daily mycophenolate mofetil (MMF), 17 Aza and 28 sirolimus (Rapa) patients were prescribed their medication once daily. Of the 195 patients, 153 (78.5%) completed electronic monitoring through the end of their first post-transplant year. The mean MEMS cap record length was 15.8(± 7.8) mos. Follow-up for clinical outcomes averaged 7.9(±3) years. Outcome data are available for 166 (85%) patients at 5 yrs. post-transplant and 96 patients at post-transplant year 8.
Of 195 participants, 44 patients (22.6%) demonstrated adherence declines of 7% or more (equivalent to missing two or more additional days in mo. 2 versus mo. 1; “drop2”). The remaining 151 patients had either stable or improving rates of adherence during their second post-transplant mo. Although the assignment of each patient's immunosuppressive drug protocol was not randomized, there were no significant demographic differences between patient groups stratified by their drug regimens other than donor source and transplant number. Also while nonadherence was higher in patients taking more than one dose daily, the proportion of drop2 patients did not significantly differ by initial dosing regimen (Table 1). The drop2 group had experienced significantly more cases of early (≤90 days) acute rejection. The only demographic factor associated with the drop2 group was being nonwhite, with no other significant differences noted (Table 2).
Table 1.
Demographic characteristics of patients divided by initial drug and dose prescription at hospital discharge.
| All | AZA | RAPA | MMF-2* | MMF-4* | P-value | |
|---|---|---|---|---|---|---|
| N | 195 | 17 | 28 | 128 | 22 | |
| Female | 43% | 59% | 32% | 40% | 59% | .115 |
| Age | 48 ± 14 | 44 ± 11 | 45 ± 14 | 49 ± 14 | 45 ± 13 | .141 |
| Donor: | .024 | |||||
| DD | 44% | 24% | 36% | 48% | 46% | |
| LRD | 36% | 71% | 39% | 29% | 45% | |
| LURD | 20% | 6% | 25% | 23% | 9% | |
| TX number: | .036 | |||||
| 1 | 83% | 65% | 93% | 80% | 100% | |
| 2 | 14% | 24% | 7% | 17% | 0 | |
| 3 | 2% | 12% | 0 | 1.5% | 0 | |
| 4 | 1% | 0 | 0 | 1.5% | 0 | |
| Kidney & Pancreas | 31% | 35% | 32% | 27% | 55% | .072 |
| DM at TX | 47% | 47% | 54% | 41% | 68% | .109 |
| Nonwhite | 7% | 0 | 14% | 5% | 14% | .096 |
| Teenaged | 3% | 0 | 4% | 4% | 0 | .669 |
| Early acute rejection*** | 8% | 6% | 4% | 9% | 5% | .667 |
| Drop2** | 23% | 24% | 25% | 19% | 41% | .144 |
MMF-2 indicates dosing twice daily, and MMF-4 indicates four times a day dosing.
Drop2 indicates subjects whose calculated percentage of adherence declined by a total of 2 or more days during the second monitored mo. compared to the first mo.
Acute rejection during the first 90 days after hospital discharge after transplant.
Values are percent, or mean ± standard deviation.
P-value for comparison between four drug-dose groups by chi-square test or ANOVA F-test.
Table 2.
Demographic characteristics and transplant outcomes – drop2 patients* versus the remaining steady adherence patient group.
| Drop2* (n = 44) | Steady Adherence (n =151) | P-value | |
|---|---|---|---|
| Female | 43% | 42% | .925 |
| Age | 46 ± 14 | 48 ± 14 | .356 |
| Donor type: | .397 | ||
| DD | 50% | 42% | |
| LRD | 36% | 36% | |
| LURD | 14% | 22% | |
| TX number: | .482 | ||
| 1 | 80% | 83% | |
| 2 | 16% | 14% | |
| 3 | 5% | 1% | |
| 4 | 0 | 1% | |
| Kidney & Pancreas | 20% | 34% | .078 |
| Diabetes at TX | 34% | 50% | .057 |
| Nonwhite | 18% | 3% | .002 |
| Teenaged | 2% | 3% | .726 |
| Drug-dose**: | .144 | ||
| AZA | 9% | 9% | |
| RAPA | 16% | 14% | |
| MMF – 2 times daily | 55% | 69% | |
| MMF – 4 times daily | 20% | 9% | |
| Corticosteroids after discharge | 34% | 37% | .716 |
| Initial immunosuppression | .849 | ||
| CSA | 66% | 62% | |
| Tacrolimus | 32% | 35% | |
| Only MMF | 2% | 3% | |
| Early acute rejection (<90d) | 16% | 5% | .020 |
| Transplant outcomes | |||
| Acute rejection a,b | 6.4 ± 1.6 | 2.5 ± .5 | .034 |
| Loss begore death a | 3.7 ± 1.2 | 1.6 ± .4 | .017 |
| Death a | 3.7 ± 1.1 | 2.7 ± .5 | .327 |
Drop2 indicates subjects whose calculated percentage of adherent days declined by a total of 2 or more days during the second monitored mo. compared to the first mo.
Drug-dose is initial drug and dose regimen at the time of hospital discharge.
Values are percent, or mean ± standard deviation, or rate per 100 patient-years ± standard error.
P-value for comparison by chi-square test or t-test.
Rates per 100 patient-years ± standard error.
For acute rejection, rates include repeated occurrences of acute rejection while log-rank test compares product-limit curves to first rejection (see Figure 1A). Acute rejections during the first 90 days after transplant were omitted.
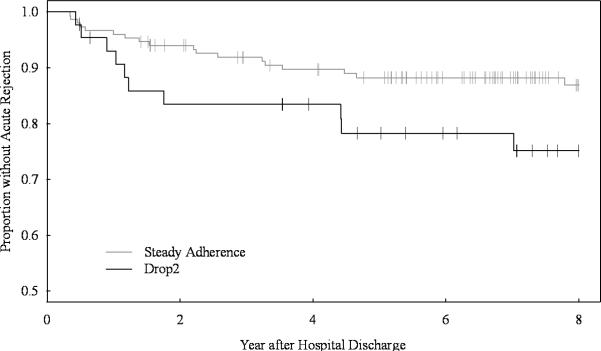
These early adherence patterns persisted. Longer-term follow-up demonstrated that during months 6–12 after transplant, drop2 patients had mean medication adherence rates of 73% ±30, while adherence in the stable group is 93% ±14 (p<.0001). Drop2 patients experienced twice the rate of acute rejection (p=.034) and death-censored graft loss (p=.017) seen in the stable adherence group (Table 1). Drop2 patients’ first rejection event tended to appear sooner (Figure 1A, p=.065) than patients with stable adherence. Similarly, allograft losses also appeared earlier (Figure 1B, p=.046). There were no significant differences in death rates or time to death between drop2 patients and the stably adherent participants. Setting aside the 15 patients who experienced early rejections (7 in drop2 and 8 in the stable adherence group), both rejection (p=.099) and graft loss (p=.050) remained twice as frequent in the drop2 group.
Figure 1A.
Time to first acute rejection beginning 90 days after hospital discharge. Kaplan-Meier curves defining the rejection-free survival of patients with steady or declining (drop2) medication adherence, vertical dashes mark censoring events. The table indicates the number of patients at risk in 2 yr. intervals. There is a trend toward earlier and more frequent rejections in the drop2 group compared to the steadily adhering group (log rank p=.065).
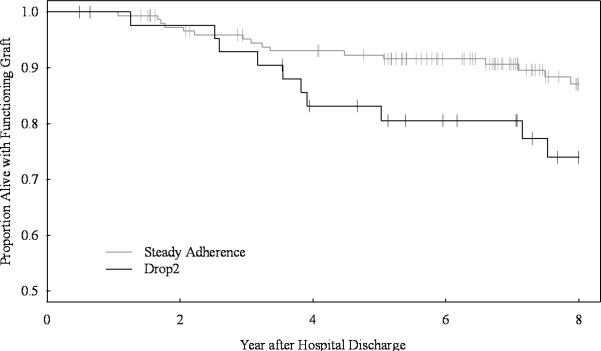
Figure 1B.
Time to death-censored graft loss.
The Kaplan-Meier curves defining the death-censored allograft survival for patients with steady or declining (drop2) medication adherence, vertical dashes mark censoring events. The table indicates the number of patients at risk in 2 yr. intervals. There were more frequent and earlier graft losses in the drop2 group compared with the steadily adhering group (log rank p=.046).
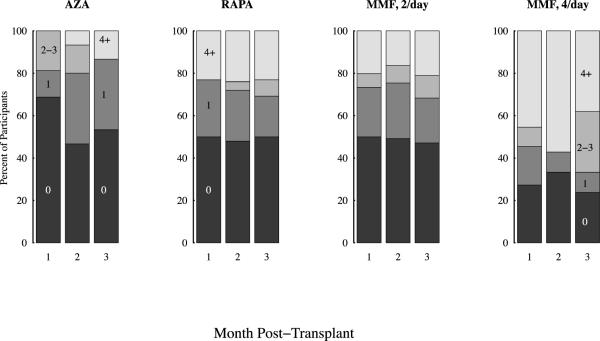
Of the 195 recipients, 45 had their monitored drug (Aza, or Rapa) prescribed as a single daily dose. The remaining 150 patients were initially prescribed MMF at a frequency of twice daily (n=128), or in an empiric effort to minimize side effects, 4 times daily (n=22). Independent of the specific drug monitored, the 3 mo. medication adherence rates varied inversely with the number of daily drug doses prescribed. During the first mo. after discharge, 43% of patients taking single daily doses of a monitored medication missed at least one dose. This percentage increased to 49% during mo. 3. During the same intervals, 73% of patients prescribed 4 doses per day missed at least 1 dose of medication during mo. 1 and 76% missed doses in mo. 3 (Figure 2). During the first 3 mos., patients prescribed single daily doses of medication took a mean of 93.5% of their medication and twice daily doses a mean of 91%. Patients prescribed medication four times per day took 84% of their prescribed doses. Medication adherence rates for once daily (p=.008) and twice daily dosing (p=.024) were significantly better than 4 times per day dosing. Comparing adherence rates, there was no statistically significant difference between once and twice daily dosing.
Figure 2.
Sorted by drug and dose schedule the stacked bar graph displays the percentage of patients missing 0, 1, 2-3, and 4 or more doses per month, during the first 3 mos. after transplant. There were 16 patients on once a day azathioprine (Aza), 26 on once a day sirolimus (Rapa), 124 on mycophenolate mofetil (MMF) twice daily, and 22 on MMF 4 times a day. Seven patients were excluded because they either changed drug or dose schedule during the first month or had less than 5 evaluable days in any month.
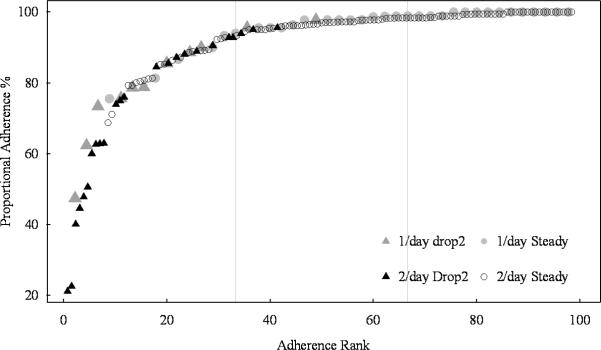
Rank ordering each patient's mean proportional adherence during the first 3 mos., according to prescription of once or more than once daily, produces similar patterns (Figure 3) indicating that at least two thirds of patients in both groups took more than 90% of their medication. Focusing exclusively on patients prescribed MMF twice a day (n=128), the mean inter-dose interval in months 1–3 after transplant, expected to be about 12 hours, was 19 ±13 hours for the 24 drop2 patients and 13 ±6 hours for the 104 stably adherent patients (p = .0014). Longer-term differences in adherence persisted: mean adherence during mos. 6-12 was 63% ±33 in the drop2 group and 92% ±15 in the stable group (p <.0001). On overall followup, drop2 patients experienced 4 times the rate of acute rejection (p=.021) and almost 3 times the rate of death-censored graft loss (p=.012) observed in stable adherence patients (data not shown). Even omitting patients with early rejections (5 patients from the drop2 group and 7 stable patients), the drop2 rates remained more than twice as high as stably adherent patients for both rejection (p=.256) and death-censored graft loss (p=.030).
Figure 3.
Illustrates the two distributions of mean proportional adherence per patient during the first three months for patients taking one versus multiple daily medication doses. Patients taking MMF two or more times a day (n=150) are represented by black symbols, while the 45 patients taking medication once daily (Aza, n=17 or Rapa, n=28) have gray symbols. In each subgroup, drop2 patients are represented by triangles and steadily adhering patients by circular symbols. Vertical lines divide subjects into tertiles. Note that drop2 patients are not limited to the lowest tertile. Note the highly similar distribution curves indicating that the proportional definition of adherence identifies a similar adherence distribution in either single or multiple dose patients.
Discussion
Data in this study highlight two important early patterns in med-NA. First, this prospective patient cohort confirms that med-NA appears early post-transplant and that the pattern of early declining adherence is associated with significantly poorer late allograft outcomes. Second, the complexity (i.e., doses per day) of the immunosuppressant medication regimen directly impacts adherence rates.
Quantitative medication adherence has been reported in a variety of chronic clinical conditions including: seizures (10,11), glaucoma (12,13), HIV (14-17), hypertension (18,19), chronic anticoagulation (20,21), and congestive heart failure (22). Although most studies were of short duration and used differing adherence definitions, they all observed that: 1) med-NA was detectable in each study, and 2) was regularly associated with adverse outcomes. Med-NA occurs commonly in asymptomatic medical conditions requiring chronic medication. In a wide variety of chronic diseases 15-25% of patients have been reported to rapidly reduce or discontinue their prescribed drug shortly after the initial prescription (11,12,17,18,20). Individually, adherence rates vary, perhaps reflecting each patient's perception of the clinical importance of the condition being treated (23) and the anticipated risks associated with missing medication. In this regard, solid organ transplant recipients consistently demonstrate better overall rates of adherence with their medications compared to patients with asymptomatic conditions such as hypercholesterolemia (23) or hypertension (18).
Remarkably even though solid organ transplant recipients are regularly reminded that immunosuppressive NA may result in graft loss or even death, med-NA appears ubiquitous (6). With improving transplant protocols, decreasing rates of early rejection and patient care advances; med-NA has emerged as a critical barrier to achieving optimal long-term transplant outcomes (1-3, 24, 25).
We previously reported that significant post-transplant med-NA could be detected during the first few weeks after hospital discharge (1, 2). In that analysis of a natural history cohort, a 7% decline (e.g., 2 missed doses over 30 days) in Aza adherence during the second post-transplant mo. identified patients who experienced significantly earlier and more frequent episodes of acute rejection as well as increased rates of allograft loss.
Now analyzing twice daily MMF using a proportional adherence model, the distribution of adherence is virtually identical to that seen with once daily Aza or Rapa (Figure 3) (1). Despite historically lower rejection rates (26), the present prospective study confirms our earlier finding that early declining adherence was associated with significantly more frequent and earlier episodes of rejection (Figure 1A). Using contemporary immunosuppression, acute rejection rates are 250% higher in patients with early declining adherence compared to stably adherent patients, demonstrating that even today's potent immunosuppressive drugs are ineffective at preventing rejection if taken inconsistently. Clearly med-NA will remain a concern during the development and study of future immunosuppressant drugs.
Declining medication adherence is further associated with both earlier and higher rates of death-censored graft loss (Figure 1B; p=.046). The drop2 group exhibits a 200% increase in graft loss when compared to stably adherent allograft recipients at 5 yrs. post-transplant.
Recognition of early (first 2-3 mo.) declining adherence consistently identifies patient groups at risk for early discontinuation or significant med-NA to their therapeutic regimen (9). These dynamic patterns are only demonstrable with quantitative data such as that provided by MEMS technology (11, 22). Clinically this drop2 measure of dynamic declining adherence is available immediately for each patient since it is derived from the patient's own records without reference to any outside group or norm. The pivotal importance of this observation is that early recognition of med-NA permits targeting adherence-promoting interventions to a defined subset of patients at high-risk for adverse behaviors and outcomes. Newer generations of electronic medication monitors provide adherence data in “real time”. Ideally, effective and sustained interventions will provide enduring improvements in adherence and subsequent clinical benefits for both renal transplant recipients and other patient populations (11,13,18, 22).
It has long been recognized that the complexity of a medication regimen affects adherence. Our data demonstrate that post-transplant, the more times per day a patient is expected to take a medication, the more likely they are to miss doses. A previous review of quantitative medication adherence by Claxton and coworkers linked the prescribed number of daily doses to the electronically documented adherence rates in 76 separate studies across diverse medical conditions (27). They demonstrated that on average, a single daily dose yields the highest adherence rate at 79%. More frequent doses resulted in less adherence; twice daily dosing yielded 69%, 3 doses/day produced 65% and with 4 doses/day, adherence declined to 51%. Our patients’ adherence patterns are strikingly similar. However, perhaps due to the importance of a renal transplant, the mean adherence rates are all proportionately higher. Similar to Claxton, et al. our data do not show statistical differences in adherence between once and twice daily dose schedules. Clinically any expected benefit from more frequent medication dosing must be balanced against the likelihood that patients will not take all of the prescribed doses.
Certainly medication costs present yet another barrier to adherence. In this cohort of renal transplants, medication costs were covered by Medicare and supplemented by additional third party insurance. This was critically true during those first 2-3 months post transplant when the drop2 pattern was detected. Unfortunately Medicare prescription coverage abruptly ends 3 years after transplantation and thus becomes an added barrier to individual medication adherence (28) and successful transplantation.
This study has some limitations related to both sampling bias and technology. We could only measure adherence in those patients who consented to be observed. This may limit the generalizability of our findings. But since we may have sampled a group of patients likely biased to be more adherent, med-NA in the entire transplant population is perhaps even more prevalent than we observed. Even after consenting, patients sometimes dropped out or failed to return their monitor cap, further limiting our assessment. Although the MEMS technology is an excellent tool to measure adherence (9), there is no certain proof that a patient removing the monitor cap actually takes the prescribed dose of medication at that time. Also, since all patients were informed that their medication taking was being monitored, this may have masked some early med-NA. Finally the extent to which our renal transplant data accurately characterize adherence for other solid organ transplants including liver or heart is not known (6).
In conclusion, med-NA is a major clinical problem in renal transplantation. We demonstrated that it is possible to prospectively identify patients at increased risk for adverse events including acute rejection and graft loss based on their adherence patterns observed during the first 2-3 mos. post-transplant. The sign of early declining adherence deserves more careful attention since it predicts an increased risk of chronic med-NA as well as later adverse outcomes (2, 24). Also it should now be possible to focus behavioral intervention efforts on these vulnerable patients early, when their med-NA pattern is first recognized. Similarly the observation that medication regimens consisting of more frequent daily doses are less likely to be precisely followed has management implications, since simpler drug regimens (i.e., fewer doses per day) should promote better adherence. The consistency of our findings in 2 prospective renal transplant patient cohorts as well as the findings of other investigators underscore the need for additional research to identify and better understand medication adherence patterns while also developing strategies to improve medication adherence.
Materials and Methods
Medication adherence in outpatients after renal transplant was monitored using an electronic medication event monitoring system (MEMS; AARDEX Group Ltd, 1950 Sion, Switzerland), to quantify adherence. Recipients were eligible for this study if they were discharged with a functioning renal allograft, were able to speak and read English, and were directly responsible for taking their own medication. All patients received initial induction therapy with an anti-lymphocyte antibody. For this study, the choice of immunosuppressive medications was not randomly assigned, but based on the clinical assessment of each patient. Most adult patients were treated with rapid discontinuation of all corticosteroids (29) and received either cyclosporine or tacrolimus (Table 2). The monitored drug was either MMF, Rapa, or Aza.
Details of the medication monitoring protocol have been previously published (1,2). Briefly, each time the monitor cap was removed from the medication vial the date and time of that event was recorded in the cap memory and presumed to represent a medication dose taken. To minimize confusion about dose times, each patient's daily medication record began at 3:00 AM and ended the next day at 2:59 AM. Beginning the first day after the initial hospital discharge, each monitored day was evaluated for medication adherence. Using proprietary software, continuous dosing records were compiled for each patient and analyzed.
Every patient's chart was reviewed and all hospitalizations, drug dose or schedule changes were noted. When a medication was temporarily discontinued, the absence of a cap opening on that day was considered “adherent”. When a patient was hospitalized or the cap data were not available for technical reasons, those days were considered as “missing”, all other days were evaluable. No data are missing due to cap technical failures. Proportional adherence was expressed as the proportion or percentage of prescribed doses taken each day. Thus for once daily dosing, each day was either 100% or 0% adherent. For a drug prescribed twice daily, each day could be 100%, 50%, or 0% adherent, based on taking 2, 1 or no doses respectively. The individual's monthly adherence percentage was calculated as the sum of daily adherence percents, divided by the number of evaluable days. The number of “missed dose days” in a month was the number of evaluable days minus the sum of daily adherence percents. The drop2 subgroup patients (1,2) were those with month 2 “missed dose days” at least 2 days larger than in month 1. For each “month” of 30 days, drop2 corresponds to an increase in monthly percent med-NA (≥6.7% from mo. 1 to mo. 2).
All patients were followed for the clear clinical end points of acute rejection, allograft survival, and death through December 1, 2011. By design (1,2) early acute rejection (≤90 days) was analyzed separately to evaluate its impact on later outcomes. Acute rejection was diagnosed in kidney biopsy or nephrectomy specimens (1). When a tissue diagnosis was not available, the clinical diagnosis of acute rejection was based on an otherwise unexplained elevation of creatinine, coupled with appropriate physical signs (including fever, hypertension, or oliguria) resulting in the clinical decision to treat the patient for acute rejection. Renal transplants were considered lost when patients received a new transplant or returned to regular dialysis. We compared the rates of these outcomes for drop2 patients versus all the remaining patients with more stable adherence. We also determined the overall rates of adherence during the first three mos. as a function of the monitored drug and its daily dosage schedule.
Statistics
Demographic factors and outcomes were compared using chi-square or Fisher's exact test; continuous demographic variables were compared with analysis of variance. Event rates were compared using Poisson regression that can accommodate repeated occurrences of acute rejection, and Kaplan-Meier estimates of time to event were compared with the log-rank test. Values reported are percents or mean ± standard deviation. Computations were performed using SAS Version 9.3 (SAS Institute, Inc. Cary, NC). Figures were drawn in R (R Foundation for Statistical Computing, 2012, http://www.R-project.org).
The University of Minnesota Institutional Review Board approved this study (#9611M11943) and reviews it annually. Participants were specifically informed that their medication taking behavior was being monitored from the beginning of the study.
Acknowledgments
This work was supported by grants from the NIH, DK-13083 and the Minnesota Medical Foundation. The authors of this manuscript have no conflicts of interest to disclose.
The authors gratefully acknowledge encouragement, support and critical review from Drs. Arthur Matas and Michael Mauer. Also deeply appreciated are the daily efforts and perseverance of research coordinators; Nancy Flaherty, Christine Jacox, Trudy Strand, Judith Graziano, and Linda Kruse.
Support:
The authors all received salary support as faculty at the University of Minnesota, and from a grant: NIH - DK-13083.
Abbreviations
- Aza
Azathioprine
- drop2
Drop 2
- med-NA
Medication nonadherence
- MEMS
Medication Event Monitoring System
- MMF
Mycophenolate mofetil
- Rapa
Sirolimus
Footnotes
Contributions:
Doctor Nevins developed the study concept, assisted with data interpretation, and wrote the primary manuscript.
Doctor Robiner assisted with the study design and execution, and revised the manuscript.
Doctor Thomas performed all the statistical data analyses, prepared the figures and tables, and revised the manuscript.
The authors have no conflicts of interest to disclose.
References
- 1.Nevins TE, Kruse L, Skeans MA, Thomas W. The natural history of azathioprine compliance after renal transplantation. Kidney Int. 2001;60(4):1565–1570. doi: 10.1046/j.1523-1755.2001.00961.x. [DOI] [PubMed] [Google Scholar]
- 2.Nevins TE, Thomas W. Quantitative patterns of azathioprine adherence after renal transplantation. Transplantation. 2009;87(5):711–718. doi: 10.1097/TP.0b013e318195c3d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gaston RS, Hudson SL, Ward M, Jones P, Macon R. Late renal allograft loss: noncompliance masquerading as chronic rejection. Transplant Proc. 1999;31(4A):21S–23S. doi: 10.1016/s0041-1345(99)00118-9. [DOI] [PubMed] [Google Scholar]
- 4.Butler JA, Peveler RC, Roderick P, Horne R, Mason JC. Measuring compliance with drug regimens after renal transplantation: comparison of self-report and clinician rating with electronic monitoring. Transplantation. 2004;77(5):786–789. doi: 10.1097/01.tp.0000110412.20050.36. [DOI] [PubMed] [Google Scholar]
- 5.Pinsky BW, Takemoto SK, Lentine KL, Burroughs TE, Schnitzler MA, Salvalaggio PR. Transplant outcomes and economic costs associated with patient noncompliance to immunosuppression. Am J Trans. 2009;9:2597–2606. doi: 10.1111/j.1600-6143.2009.02798.x. [DOI] [PubMed] [Google Scholar]
- 6.Dew MA, DiMartini AF, De Vito Dabbs A, et al. Rates and risk factors for nonadherence to the medical regimen after adult solid organ transplantation. Transplantation. 2007;83(7):858–873. doi: 10.1097/01.tp.0000258599.65257.a6. [DOI] [PubMed] [Google Scholar]
- 7.Butler JA, Roderick P, Mullee M, Mason JC, Peveler RC. Frequency and impact of nonadherence to immunosuppressants after renal transplantation: a systematic review. Transplantation. 2004;77(5):769–776. doi: 10.1097/01.tp.0000110408.83054.88. [DOI] [PubMed] [Google Scholar]
- 8.Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353(5):487–497. doi: 10.1056/NEJMra050100. [DOI] [PubMed] [Google Scholar]
- 9.Blaschke TF, Osterberg L, Vrijens B, Urquhart J. Adherence to medications: insights arising from studies on the unreliable link between prescribed and actual drug dosing histories. Annu Rev Pharmacol Toxicol. 2012;52:275–301. doi: 10.1146/annurev-pharmtox-011711-113247. [DOI] [PubMed] [Google Scholar]
- 10.Jones RM, Butler JA, Thomas VA, Peveler RC, Prevett M. Adherence to treatment in patients with epilepsy: associations with seizure control and illness beliefs. Seizure. 2006;(7):504–508. doi: 10.1016/j.seizure.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 11.Modi AC, Rausch JR, Glauser TA. Patterns of nonadherence to antiepileptic drug therapy in children with newly diagnosed epilepsy. JAMA. 2011;305(16):1669–1676. doi: 10.1001/jama.2011.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Okeke CO, Quigley HA, Jampel HD, et al. Adherence with topical glaucoma medication monitored electronically the Travatan Dosing Aid study. Ophthalmology. 2009;116(2):191–199. doi: 10.1016/j.ophtha.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 13.Okeke CO, Quigley HA, Jampel HD, et al. Interventions improve poor adherence with once daily glaucoma medications in electronically monitored patients. Ophthalmology. 2009;116(12):2286–2293. doi: 10.1016/j.ophtha.2009.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vrijens B, Goetghebeur E, de Klerk E, Rode R, Mayer S, Urquhart J. Modeling the association between adherence and viral load in HIV-infected patients. Stat Med. 2005;24(17):2719–2731. doi: 10.1002/sim.2130. [DOI] [PubMed] [Google Scholar]
- 15.Podsadecki TJ, Vrijens BC, Tousset EP, Rode RA, Hanna GJ. Decreased adherence to antiretroviral therapy observed prior to transient human immunodeficiency virus type 1 viremia. J Infect Dis. 2007;196(12):1773–1778. doi: 10.1086/523704. [DOI] [PubMed] [Google Scholar]
- 16.Podsadecki TJ, Vrijens BC, Tousset EP, Rode RA, Hanna GJ. “White coat compliance” limits the reliability of therapeutic drug monitoring in HIV-1-infected patients. HIV Clin Trials. 2008;9(4):238–246. doi: 10.1310/hct0904-238. [DOI] [PubMed] [Google Scholar]
- 17.Cooper V, Horne R, Gellaitry G, et al. The impact of once-nightly versus twice-daily dosing and baseline beliefs about HAART on adherence to efavirenz-based HAART over 48 weeks: the NOCTE study. J Acquir Immune Defic Syndr. 2010;53(3):369–377. doi: 10.1097/QAI.0b013e3181ccb762. [DOI] [PubMed] [Google Scholar]
- 18.Vrijens B, Vincze G, Kristanto P, Urquhart J, Burnier M. Adherence to prescribed antihypertensive drug treatments: longitudinal study of electronically compiled dosing histories. BMJ. 2008;336(7653):1114–1117. doi: 10.1136/bmj.39553.670231.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dusing R, Handrock R, Klebs S, Tousset E, Vrijens B. Impact of supportive measures on drug adherence in patients with essential hypertension treated with valsartan: the randomized, open-label, parallel group study VALIDATE. J Hypertens. 2009;27(4):894–901. doi: 10.1097/HJH.0b013e328323f9be. [DOI] [PubMed] [Google Scholar]
- 20.Kimmel SE, Chen Z, Price M, et al. The influence of patient adherence on anticoagulation control with warfarin: results from the International Normalized Ratio Adherence and Genetics (IN-RANGE) Study. Arch Intern Med. 2007;167(3):229–235. doi: 10.1001/archinte.167.3.229. [DOI] [PubMed] [Google Scholar]
- 21.Parker CS, Chen Z, Price M, et al. Adherence to warfarin assessed by electronic pill caps, clinician assessment, and patient reports: results from the IN-RANGE study. J Gen Intern Med. 2007;22(9):1254–1259. doi: 10.1007/s11606-007-0233-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Riegel B, Lee CS, Ratcliffe SJ, et al. Predictors of Objectively Measured Medication Nonadherence in Adults With Heart Failure. Circ Heart Fail. 2012;5:430–436. doi: 10.1161/CIRCHEARTFAILURE.111.965152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jackevicius CA, Mamdani M, Tu JV. Adherence With Statin Therapy in Elderly Patients With and Without Acute Coronary Syndromes. JAMA. 2002;288(4):462–467. doi: 10.1001/jama.288.4.462. [DOI] [PubMed] [Google Scholar]
- 24.Sellarés J, de Freitasa DG, Mengel M, et al. Understanding the Causes of Kidney Transplant Failure: The Dominant Role of Antibody-Mediated Rejection and Nonadherence. Am J Trans. 2012;12:388–399. doi: 10.1111/j.1600-6143.2011.03840.x. [DOI] [PubMed] [Google Scholar]
- 25.Wiebe C, Gibson IW, Blydt-Hansen TD, et al. Evolution and Clinical Pathologic Correlations of De Novo Donor-Specific HLA Antibody Post Kidney Transplant. Am J Transplant. 2012;12:1157–1167. doi: 10.1111/j.1600-6143.2012.04013.x. [DOI] [PubMed] [Google Scholar]
- 26.Meier-Kriesche HU, Schold JD, Srinivas TR, Kaplan B. Lack of improvement in renal allograft survival despite a marked decrease in acute rejection rates over the most recent era. Am J Transplant. 2004;4(3):378–383. doi: 10.1111/j.1600-6143.2004.00332.x. [DOI] [PubMed] [Google Scholar]
- 27.Claxton AJ, Cramer J, Pierce C. A systematic review of the associations between dose regimens and medication compliance. Clin Ther. 2001;23(8):1296–1310. doi: 10.1016/s0149-2918(01)80109-0. [DOI] [PubMed] [Google Scholar]
- 28.Gill JS, Tonelli M. Penny Wise, Pound Foolish? Coverage Limits on Immunosuppression after Kidney Transplantation. N Engl J Med. 2012;366(7):586–589. doi: 10.1056/NEJMp1114394. [DOI] [PubMed] [Google Scholar]
- 29.Rizzari MD, Suszynski TM, Gillingham KJ, et al. Ten-Year Outcome after Rapid Discontinuation of Prednisone in Adult Primary Kidney Transplantation. Clin J Am Soc Nephrol. 2012;7:494–503. doi: 10.2215/CJN.08630811. [DOI] [PMC free article] [PubMed] [Google Scholar]