Abstract
Objective
To determine the interaction between a high fructose diet and PA levels on postprandial lipidemia and inflammation in normal weight, recreationally active individuals.
METHODS
Twenty-two men and women (age: 21.2 ± 0.6 yrs; BMI = 22.5 ± 0.6 kg/m2) consumed an additional 75 g of fructose for 14 days on two separate occasions: high physical activity (~12,500 steps/day: FR+Active) and low PA (~ 4,500 steps/day; FR+Inactive). A fructose-rich test meal was given prior to and at the end of each intervention. Blood was sampled at baseline and for 6 h after the meal for triglycerides (TG), very-low density lipoproteins (VLDL), total cholesterol (TC), glucose, insulin, tumor necrosis factor-α (TNF-α), interleukin 6 (IL-6) and c-reactive protein (CRP).
RESULTS
Log transformed TG AUC significantly increased from pre (10.1 ± 0.1 mg/dL x min for 6 h) to post (10.3 ± 0.08 mg/dL x min for 6 h; p = 0.04) in the FR+Inactive intervention with an 88% increase in Δpeak[TG] (p=0.009) and an 84% increase in Δpeak[VLDL] (p=0.002). Δpeak[IL-6] also increased by 116% after FR+Inactive intervention (p=0.009). Insulin tAUC significantly decreased after FR+Active intervention (p=0.04) with no change in AUC after the FR+Inactive intervention. No changes were observed in glucose, TNF-α and CRP concentrations (p>0.05).
CONCLUSIONS
Low physical activity during a period of high fructose intake augments fructose-induced postprandial lipidemia and inflammation while high PA minimizes these fructose-induced metabolic disturbances. Even within a young healthy population, maintenance of high PA (>12500 steps/day) decreases susceptibility to cardiovascular risk factors associated with elevated fructose consumption.
Keywords: lipidemia, inflammation, exercise, carbohydrates
Introduction
There is a growing body of evidence that a high dietary intake of fructose is associated with the development of metabolic risk factors including insulin resistance (6), fatty liver (25), hyperlipidemia (23,24), hypertension (7), and reduced lipid oxidation (1). Specifically, a study by Stanhope et al (33) demonstrated an ad libitum diet consisting of 25% of calories from fructose induced hepatic insulin resistance, increased de novo lipogenesis (DNL) and increased visceral adiposity in obese adults within only 10 weeks.
The proposed metabolic dysregulation associated with high fructose consumption relates to the unique metabolism of fructose. Fructose is almost exclusively phosphorylated to fructose-1-phosphate in an ATP-dependent process catalyzed by fructokinase in hepatic cells. This results in a rapid and relatively unregulated production of triose-phosphates which may subsequently be converted to pyruvate for conversion to lactate or acetyl CoA (34). Large concentrations of acetyl CoA can stimulate hepatic de novo lipogenesis (DNL) and simultaneously inhibit hepatic lipid oxidation resulting in fatty acid re-esterification and VLDL-triglyceride synthesis (31). The consequence of the elevation in VLDL-triglyceride synthesis is an increased supply of TG to the adipose tissue and ectopic tissues such as muscle and liver, increasing the size of TG depots. This leads to impaired insulin signaling, dyslipidemia, and low-grade inflammation (12).
Despite the mounting evidence demonstrating adverse adaptations to a high fructose diet, these studies often use unrealistically high levels of fructose, some in excess of 25% of total energy intake (1,33), which may not be clinically relevent. Specifically, Stanhope et al (33) used an additional 25% of a fructose drink to an ad libitum diet. Moreover, research from Abdel-Sayed et al (1) used an additional 234g of fructose per day for 7 days on healthy young males. Both of these aforementioned studies are well above the average intake of ~70g of added sugar in the form of fructose. Moreover, there are no studies to date researching the metabolic effects of increased physical activity during a high fructose diet. Physical activity is a lifestyle modification often implemented to decrease risk factors associated with metabolic syndrome and may be a clinically relevent tool to use to combat the deleterious affects of a high fructose diet. A previous review by Bassuk et al (5) determined through various epidemiological studies that physcially active individuals are at a 30-50% lower risk of developing type 2 diabetes and heart disease due to a possible reduction in body weight, insulin resistance, hypertension, athrogenic dyslipidemia, and inflammation, all previously mentioned risk factors of a high fructose diet. Hence, public health initiatives should focus on increased physical activity in conjunction with a diet low in fructose to attenuate metabolic syndrome risk factors.
Moreover, physical inactivity is associated with low-grade inflammation as is evidenced by increased concentrations of pro-inflammatory markers such as monocyte chemo-attractant protein-1 (MCP-1) and interleukin-6 (IL-6) (15). Since fructose consumption is independently associated with increased low-grade inflammatory markers (10) and increased rates of DNL (1, 7, 24, 25, 33), we expect previously reported deleterious health outcomes from chronic fructose consumption to be confounded by physical inactivity.
Our aim was to test the hypothesis that a diet rich in fructose would increase postprandial lipidemia to a greater extent when coupled with physical inactivity, independent of energy intake. Secondly, that after ingesting a high fructose diet for two weeks, pro-inflammatory markers associated with elevated lipid production would be elevated and these effects would be ameliorated with high physical activity.
Subjects and Methods
Twenty-two healthy male (n=11) and female subjects (n=11) between the ages of 18-25 years old were recruited from the Syracuse University community. Subjects had to be recreationally active, as determined by a physical activity questionnaire (3-4 days/week of moderate to vigorous activity for at least 20-60min/day), with a body mass index (BMI) < 27 kg/m2 (mean: 22.5 ± 1.6 kg/m2). All subjects completed an informed consent form, approved by the Syracuse University Institutional Review Board prior to participating in this study. Exclusion criteria included the use of lipid and/or glucose-lowering medications or other medications that may affect glucose and lipid metabolism (e.g. antidepressants, oral contraceptives, etc.), chronic NSAID usage (>2 x/week), daily antioxidant supplementation, orthopedic limitations to walking, type 2 diabetes or glucose intolerance, overt cardiovascular disease, hypertension, and/or an abnormal lipid profile. Subjects were excluded if they were currently ingesting more than one high fructose drink/day (>20 grams). Women started the intervention period within the first 7 days of the menstrual cycle to minimize the potential effects of estrogen on glucose/insulin concentration (13).
Intervention
Initially, subjects participated in a one–week control period to determine their normal physical activity (Table 1) and dietary habits. The two interventions were separated by a two-week wash-out period and participants were randomly allocated to each intervention using a counter-balanced, cross-over experimental design. During each intervention period, the subjects’ usual ad libitum diet was supplemented with an additional 75 g of fructose per day. The first intervention involved two-weeks of high physical activity levels (>12,500 steps; FR+Active). The second intervention involved low physical activity (<4500 steps; FR+Inactive). A study-day with a fructose -rich meal was given at the beginning and at the end of each 14-day intervention as described below.
Table 1.
Subject characteristics
| Males (n=11) | Females (n=11) | |
|---|---|---|
| Age (yr) | 20.8 ± 0.7 | 21.5 ± 0.9 |
| Weight (kg) | 76.5 ± 4.1* | 58.4 ± 1.9 |
| Height (cm) | 178.6 ± 2.3 | 167.3 ± 2.3 |
| BMI (kg/m2) | 23.9 ± 0.9* | 21.1 ± 0.5 |
| Body fat (%) | 13.8 ± 2.5* | 23.6 ± 0.6 |
| VO2 peak (ml/kg/min) | 46.7 ± 1.2 | 42.0 ± 1.2 |
| Baseline Steps | 8,752 ± 513 | 9,100 ± 215 |
| Active Intervention (steps/day) | 13,959 ± 719† | 12,584 ± 413 |
| Inactive Intervention (steps/day) | 4291 ± 160 | 4155 ± 245 |
Values are mean ± standard deviations. BMI: Body Mass Index; VO2max: Maximum aerobic capacity.
P<0.05 for differences between genders based on ANOVA.
P<0.05 differences between intervention steps based on ANOVA.
Pre-screening Visit
All subjects completed a medical history, physical activity and sedentary behavior questionnaire prior to the start of the intervention. Following a review of the subject's activity level with the use of a validated questionnaire monitoring physical activity at work, during leisure time and during sport (11), the potential subjects were excluded from participation if their activity level included regular structured exercise more than five times/week or less than 2 times/week. Height and weight were measured, and body composition was assessed by air-displacement plethysmography (BODPOD system, Life Measurement, Inc. Concorde, CA) (30). After anthropometric measurements were completed, subjects performed a graded exercise test on the treadmill using a protocol that has been previously published (14) to determine peak oxygen consumption (VO2 peak).
At the conclusion of the initial visit, the subjects underwent a nutritional consultation with a registered dietician to ensure compliance with the dietary intervention and to estimate normal fructose intake. Subjects were instructed to refrain from ingesting any added sugar such as sweetened beverages, fruit juices, pastries, and cookies aside from the study drinks, during the intervention period. Further, estimates of food intake during the control and intervention period were collected by random 24-hour recall (via telephone) two times per week using the USDA 5-step multiple-pass method (9). The same registered dietician administered the recall to all subjects. Recalls were then analyzed using Diet Analysis Plus (version 7, Thomson Wadsworth, Thomson Corporation, Independence, KY). Following the initial visit, the subjects began a one-week control period at which time they were instructed to maintain normal activities of daily living and dietary habits. During this period, physical activity (steps) was monitored with the use of an Accelerometer (Actigraph® GT3X Activity Monitor, Pensacola, FL), which was later uploaded to the computer for further analysis. Subjects also were given pedometers (Accusplit®, Livermore, Ca) to provide visual feedback regarding daily step count. Subjects were asked to refrain from any additional exercise throughout the study duration.
Visit 1
At the end of the 7-day control period, the subjects arrived to the Human Performance Lab at Syracuse University at 0700 h, following a 12-h fast and no exercise 24 h prior to testing. Subjects had a catheter inserted into the antecubital vein by a registered nurse. Subjects then rested in a supine position for 30 minutes before obtaining two baseline blood samples (10 ml each). After baseline blood samples, a test meal was prepared for subjects. The test meal included the following: 139.5g of Wegmans® large eggs, 65.55g of Thomas Better Start Light® Multi Grain English Muffins, 22.54g of I Can't Believe Its Not Butter® Mediterranean Blend butter and a high fructose corn syrup drink consisting of 20.6 g of Swanson® fructose, 16.9g NOW® Sports Glucose, 1.1 Great Value® Artificial Sweetener, 236.5g Vintage® Sodium Free Carbonated Water (600 kcals, 45% Carbohydrate (25% fructose, 20% Complex), 40% Fat, 15% Protein, 5g of fiber). After the test meal, blood samples were obtained at the following time points: 5, 10, 15, 20, 30, 40, 50, 60, 75, 90, 120, 150, 180, 210, 240, 360 minutes. For the duration of the study day, subjects were instructed to sit in a reclining chair and abstain from any strenuous activity.
Intervention
The subjects consumed a fructose-rich diet containing 74.9 grams/day of fructose (two 20 oz Lemon Lime WPOP® drinks, Rochester, NY) along with their ad libitum diet during both conditions. The ad libitum diet was chosen on the basis that sugar-sweetened beverages are usually consumed in conjunction with an ad libitum diet (33). Previous research has indicated that within 7 days, metabolic abnormalities can occur with a high fructose diet (1), therefore, a 2-week intervention was chosen to ensure changes occur. The subjects collected their beverages twice weekly from the Human Performance Lab. They were required to return their empty drink bottles to the lab once per week to assess drink compliance and to record step counts. Each subject met with a registered dietician who assisted in maintaining eating habits recording of weight during these weekly visits to the lab.
Visit 2
On the day following the 14-day intervention period, a post intervention test meal was provided using the same procedures as visit one. After visit 2, subjects were instructed to maintain their normal activities of daily living and dietary habits for two weeks. Previous research has indicated that a two-week washout period is adequate to normalize metabolic markers associated with hyperlipidemia (21). Visits 3 and 4 were then completed before and a day after the alternate two-week intervention period respectively, using the same procedures as outlined for visit 1 and 2.
Metabolic Assays
A lipid profile (Cholestech LDX, Biosite International, San Diego, CA) was performed on the samples taken at -5, 0, 60, 120, 180, 240, and 360 min and measured TG, VLDL, total cholesterol, and glucose concentrations. The use of this equipment has previously been validated (27). Additionally, blood samples obtained at -5, 0, 5, 10, 15, 20, 30, 40, 50, 60, 75, 90, 120, 150, 180, 210, 240, 360 min were transferred to BD Vacutainer® Plus Plastic EDTA tubes (Franklin Lakes, NJ), separated by centrifugation, divided into two sets of polypropylene tubes and stored at -80°C for subsequent analysis. Insulin, TNF-α and IL-6 were analyzed using Luminex xMap Technology (Linco Research, St.Charles, MO) on a Luminex 100/200 platform (Luminex Corporation, Austin, TX). All procedures followed the manufacturer's instructions (Millipore, Billerica, MA), with quality controls within expected ranges for each assay (Insulin: Inter-assay coefficient of variation (CV): 5.0%; Intra-assay CV: 4.0%, TNF-α: Inter-assay CV, 9.9%; Intra-assay CV: 10.6%, IL-6: Inter-assay CV: 10.3; Intra-assay CV 11.9%). CRP assays were performed using Quantikine assay kit (R&D Systems, Inc., Minneapolis, MN) [Inter-assay CV: 6.5%; Intra-assay CV: 4.2%]). Insulin sensitivity was calculated by the homeostatic model assessment (HOMA) method as previously described by Levy et al (20) and the quantitative insulin sensitivity check index (QUICKI) (8).
Statistics
All results were reported as mean ± SEM using SPSS 19.0 (Chicago, IL, USA). Descriptive variables (n=22) and dietary analysis were analyzed using a two-way repeated measures analysis of variance to depict differences in pre and post intervention weight, BMI, % body fat and macronutrient consumption. Postprandial responses for all blood variables were determined by calculating total (tAUC) or incremental (iAUC) area under the curve (AUC) (Excel, Microsoft Corporation, Redmond, WA) and absolute change from peak to baseline concentrations (Δpeak) for all variables. A between-subjects analysis was performed on all variables to depict differences in genders. A log transformation (Log10) was used for data that was not normally distributed based on visual appearance of skewed data. Transformations were applied so that the data more closely met the assumptions of normality based on parametric analysis statistical procedures (19). Lipid and inflammatory variables were analyzed using a three-way ANOVA with repeated measures to assess the changes in lipid measures and inflammatory markers over the 6-hour test day: 2 (high vs. low physical activity) x 2 (pre vs. post intervention) x 18 (time points). If a significant interaction was found, differences between timepoints were analyzed using a paired t-test with Bonferroni correction factor. Statistical significance for AUC and Δpeak concentrations was computed using a two-way repeated measures ANOVA (intervention x pre-post). Pearson’s correlation coefficient was computed to determine any correlations between fasting TG and inflammatory variables. A priori significance was set at P<0.05. Sample size was determined by previous research with similar methodology (3).
Results
Subject Characteristics
Table 1 represents the subject characteristics of the study participants. Males were significantly heavier, taller and had a lower body fat percentage than the females (P<0.05). There were no significant differences in weight (Pre: 67.4 ± 9.1 kg, Post: 66.9 ± 10.2 kg), BMI or % body fat after either intervention for both males and females (P<0.05) (data not shown). There were no gender differences in any of the lipid or inflammatory markers; therefore, all subjects were combined for further analysis. No significant differences in fasting metabolic, inflammatory and glucose markers for both pre- and post- FR+Active and FR+ Inactive intervention were observed (data not shown). Moreover, there were no significant correlations between lipid and inflammatory markers (P> 0.05).
Dietary Analysis
Baseline energy intake for all subjects was 2,701 kcals (data not shown). Energy intake was not significantly different between baseline and either intervention and we observed no change in subject body weight (P>0.05). Likewise there was no difference in the macronutrient composition between the interventions (P>0.05) (Table 2). The subjects consumed an additional 75 g of fructose per day from the drink provided (500 kcal, 0 g of fat, 135 g of carbohydrates, 74.9 g of fructose).
Table 2.
Average daily energy and macronutrient intake prior to the interventions and between the two interventions.
| Baseline | %-Cals | FR+Active Intervention | %-Cals | FR+Inactive Intervention | %-Cals | |
|---|---|---|---|---|---|---|
| Calories (kcal) | 2701 ±101 | 2,654 ± 205 | 2404 ± 197 | |||
| Fat (g) | 75.2 ± 9.1 | 25% | 65.2 ± 5.2 | 22% | 57.8 ± 4.1 | 21% |
| Saturated | 20.1 ± 1.7 | 18.9 ± 0.9 | 14.2 ± 1.5 | |||
| Monounsaturated | 18.2 ± 1.1 | 23.5 ± 0.4 | 11.0 ± 0.9 | |||
| Polyunsaturated | 36.9 ± 5.4 | 30.0 ± 0.6 | 12.6 ± 2.1 | |||
| Cholesterol (mg) | 400.5 ± 24.0 | 595.0 ± 19.9 | 503.0 ± 23.0 | |||
| Protein (g) | 108.0 ± 8.9 | 16% | 116.8 ± 10.8 | 17% | 115.2 ± 14.6 | 19% |
| Carbohydrates (g) | 398.4 ± 31.1 | 59% | 404.7 ± 25.1 | 61% | 360.6 ± 19.0 | 60% |
| Fiber (g) | 28.2 ± 10.2 | 22.1 ± 0.7 | 19.0 ± 1.7 | |||
| Sugar (g) | 99.0 ± 10.1* | 185.0 ± 5.3 | 199.0 ± 10.3 |
Mean ± SEM.
P<0.05
Glucose and Insulin
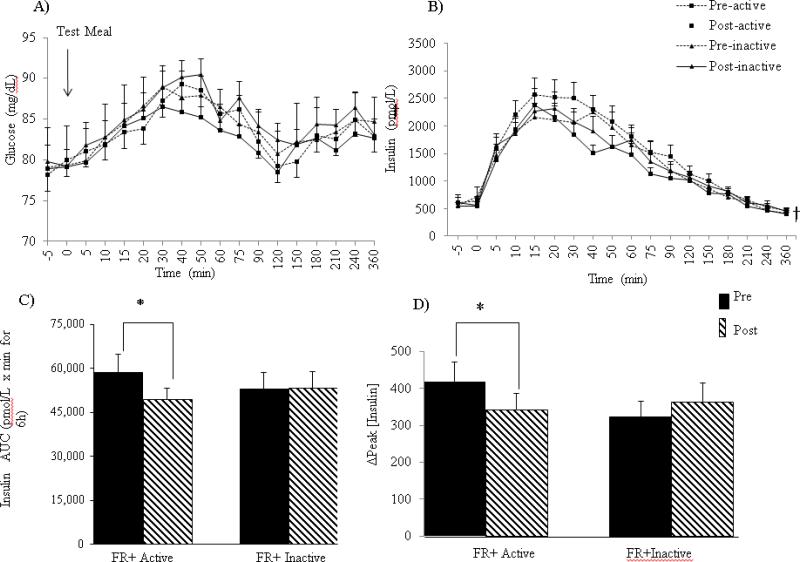
The test meal induced a significant postprandial response in both glucose and insulin concentrations (P< 0.05) (Figure 1 A-B). Glucose tAUC and postprandial Δpeak concentrations were not different after either intervention but a significant intervention x time interaction occurred in insulin concentrations (Figure 1B). Specifically, insulin tAUC for FR+Active intervention decreased from pre to post intervention, whereas, there was no change in insulin tAUC after the inactive intervention (P=0.04) (Figure 1B). These differences in AUC can be accounted for by a 19% lower Δpeak [insulin] response after the FR+Active intervention while the Δpeak [insulin] in the FR+Inactive condition was 21% higher post-intervention (P< 0.01) (Figure 1C).
Figure 1.
(A) Postprandial response to the test meal on glucose concentrations. (B) Postprandial response to the test meal on insulin concentrations (C) insulin tAUC. (D) Δpeak insulin concentrations. Data are expressed as mean ± SEM. *P<0.05 significant intervention x time interaction. † P<.05 for main effect of meal.
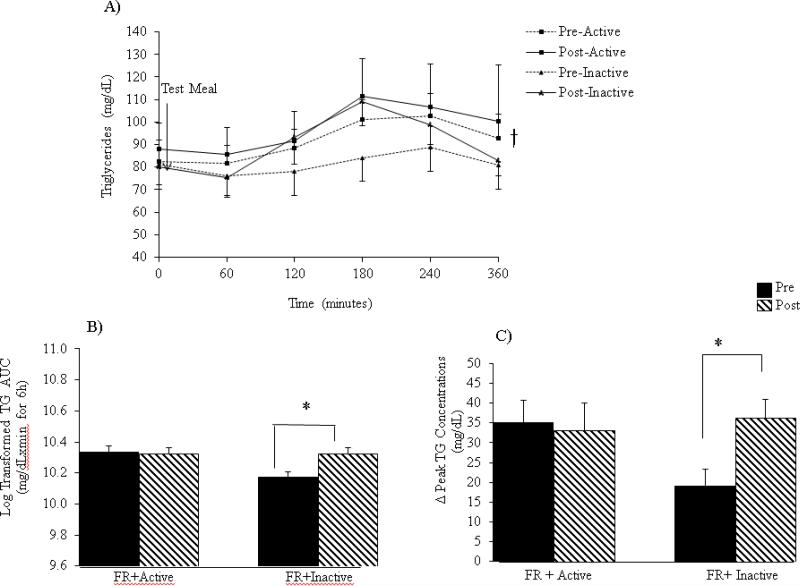
Triglycerides (Figure 2)
Figure 2.
(A) Postprandial effects of the test meal on triglyceride concentrations. (B) Triglyceride tAUC during the 6-hour test visits. (C) Change in triglyceride concentrations from baseline to peak levels. Data are expressed as mean ± SEM. *P< 0.05 intervention x time interaction. †P< 0.05 for main effect of meal.
Triglyceride concentrations significantly increased in response to the test meal under both interventions (P< 0.01) (Figure 2A). After a log transformation, TG tAUC concentrations significantly increased from pre- to post-FR+Inactive intervention (P= 0.04); whereas there was no change from pre-to-post FR+Active intervention (Figure 2B). Similarly, Δpeak [TG] increased by 88% as a result of the FR+Inactive intervention but decreased by 5% (P<0.01) with the FR+Active intervention (Figure 2C).
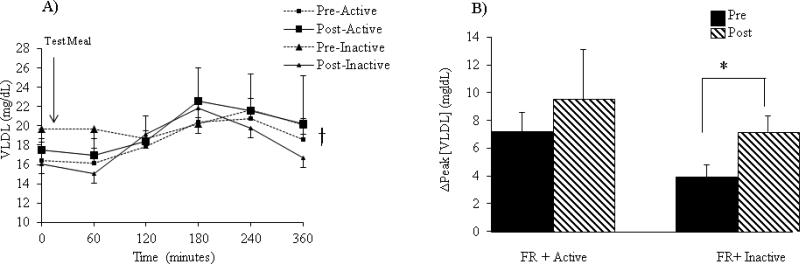
Very-Low Density Lipoproteins (VLDL) (Figure 3A-B):
Figure 3.
(A) Postprandial effects of the test meal on VLDL concentrations. (B) Change in VLDL concentrations from baseline to peak levels. Data are expressed as mean ± SEM. *P<0.05 intervention x time interaction. †P< 0.05 main effect of meal.
Figure 3A represents the 6 h postprandial response of VLDL concentrations after a test meal. Both pre- and post- FR+Active and FR+Inactive interventions demonstrated a significant main effect across time for VLDL concentrations at which point concentrations peaked at 3 h (P=0.03). The Δpeak [VLDL] induced a significant intervention x time interaction such that the difference from pre to post FR+Inactive intervention was significantly larger than the difference from pre to post FR+Active intervention. The inactivity induced an 84% increase in Δpeak [VLDL] whereas only a 33% increase following the FR+Active intervention was observed (P= 0.009) (Figure 3B).
Cholesterol
There was a significant main effect across time following the meal for total cholesterol (P=0.03), but tAUC and Δpeak [cholesterol] was not significantly different between interventions (data not shown).
Inflammatory Markers
In response to the fructose test meal, a significant meal effect was observed for TNF-α concentrations such that the test meal resulted in an increase in TNF-α concentrations over the course of the 6 h (P=0.02), however, there were no changes in CRP concentrations over time (P>0.05) (data not shown). The FR+Active and FR+Inactive interventions did not result in any significant differences in TNF-α or CRP tAUC, iAUC or Δpeak concentrations (P>0.05).
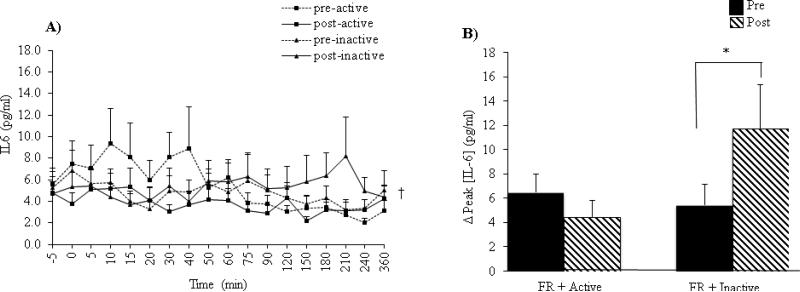
In response to the test meal, IL-6 concentration showed a significant main effect of time (P=0.02) (Figure 4A). Furthermore, Δpeak [IL-6] demonstrated a significant intervention x time interaction such that Δpeak IL-6 concentrations decreased by 30% following the FR + Active intervention while they increased by 116% following the FR+ Inactive intervention from pre to post intervention (P=0.048) (Figure 4B).
Figure 4.
(A) Postprandial effects of the test meal on IL-6 concentrations. (B) Change in IL-6 concentrations from baseline to peak levels. Data are expressed as mean ± SEM. *P<0.05 intervention x time interaction. †P< 0.05 meal main effect
Discussion
The purpose of the current study was to determine whether manipulation of physical activity levels would alter individual susceptibility for metabolic risk factors associated with consumption of a moderate dose of excess fructose (~75 g/day) over a two-week period, independent of energy intake. This study demonstrated in young healthy individuals, that consumption of an additional 75g of fructose per day in conjunction with physical inactivity (~4200 steps/day) resulted in increased postprandial lipidemia and precursors to low-grade inflammation. These results were not observed when fructose was consumed during the two-week high physical activity intervention (~13000 steps/day); suggesting that the higher level of physical activity conferred protection against the metabolic stress of the additional fructose.
Previous research has identified that a diet high in fructose induces hepatic DNL, causing an increase in plasma TG and VLDL concentrations (1, 29, 33). In addition, although not studied in this particular study, physical inactivity may reduce LPL activity resulting in reduced rates of fatty acid utilization and oxidation in peripheral tissues (35). Therefore, we speculated that the increased rates of DNL associated with high fructose consumption seen previously (33), coupled with the reduction in LPL activity with sedentary behavior (35) and thus, reduced clearance rates of blood-lipids, likely explains the increases observed in TG and VLDL concentrations. In agreement with previous research (16, 35), postprandial TG and VLDL concentrations in this study were elevated by 88% and 84% from baseline respectively in the FR + Inactive intervention, however no changes were observed in the active intervention.
Although glucose concentration was unaffected by the additional fructose intake during either two-week intervention, insulin concentration was 19% lower during the FR+Active intervention when compared to the FR+Inactive intervention. This is consistent with previous research showing decreased plasma insulin concentrations with physical activity (22). Regular physical activity has beneficial effects on insulin sensitivity by enhancing insulin signaling, glucose transport and substrate metabolism in muscles (22). Therefore, despite the fact that a high fructose diet increases postprandial plasma lipids, which may alter the insulin-signaling cascade, increased physical activity seems to offset these deleterious consequences, mostly likely by altering intracellular substrate utilization.
Our data show a 116% increase in postprandial-induced peak IL-6 concentration in response to the FR+Inactive intervention (Figure 4), which was not observed in response to the FR+Active intervention. Though the current study did not find changes in CRP and TNF-α (Table 2), the changes in IL-6 are in line with those of Hojbjerre et al (17) who demonstrated an association between physical inactivity (without dietary modification) and elevated IL-6 concentrations. In the present study, the low physical activity (~50% reduction in steps/day) during the two weeks seemed sufficient to increase postprandial IL-6 systemic concentration. In contrast, we found a 30% decrease in IL-6 levels following physical activity (increasing steps/day by ~50%). Physical activity is a known protector against increases in inflammatory markers (18). The acute increases in IL-6 levels that occur after exercise stimulate the release of many anti-inflammatory cytokines, particularly from skeletal muscle, which may cause a long-term effect of attenuating low-grade inflammation and chronic IL-6 release (18, 28). As stated in previous research, physical inactivity increases IL-6 concentrations which is known to reduce the expression of insulin substrate receptor-1 and GLUT 4 receptors in adipocytes as well as decrease insulin-stimulated glucose transport, resulting in insulin resistance and glucose intolerance (18). Based on previous studies and results from the current study, it is possible to speculate that a longer duration of physical inactivity, in conjunction with a diet high in fructose, could lead to deleterious effects of insulin signaling, as a consequence of chronically elevated IL-6 concentrations (3).
Although pro-inflammatory markers are often up-regulated with physical inactivity (32), the addition of physical activity did not cause any change in TNF-α concentrations. These results were not unexpected as research has suggested that TNF-α concentrations are not affected by exercise (17). Moreover, two weeks of a moderately high fructose diet may not be long enough to induce an up-regulation of TNF-α.
In conclusion, in a population of young, healthy individuals, being physically inactive (~4200 steps/day) while consuming an addition of 75 grams of fructose resulted in increased postprandial lipidemia and signs of potential low-grade inflammation, independent of energy intake, in as few as two weeks. However, increased physical activity levels (~13000 steps/day) seems to protect against these adverse changes. Thus low physical activity may increase susceptibility to both metabolic and cardiovascular risk factors within just two-weeks, in a non-clinical population. While future research in clinical populations and additional dietary modifications should be explored in combination with other lifestyle factors, it becomes evident that basic advice concerning increased exercise needs to continue in the clinical setting.
Acknowledgments
Sources of support: This study was supported by a NIH R21DK084467-01 grant and the Syracuse University School of Education Research and Creative Grant.
Footnotes
Conflict of Interest: All authors read and approved the final manuscript. None of the authors declared a conflict of interest.
Author Responsibilities and Conflict of Interest: The author's responsibilities were as follows- AJB: conducted the main part of the intervention, interpreted the data and wrote the manuscript; JR: was the RD on the study and conducted all dietary assessments and analysis; LW: assisted with data interpretation and writing of the manuscript; TJF: assisted with data interpretation and writing of manuscript; JAK: assisted with data analysis, interpretation and writing of manuscript. All authors read and approved the final manuscript. None of the authors declared a conflict of interest. Results of the present study do not constitute endorsement by ACSM.
References
- 1.Abdel-Sayed A, Binnert C, Le KA, Bortolotti M, Schneiter P, Tappy L. A high-fructose diet impairs basal and stress-mediated lipid metabolism in healthy male subjects. Br J Nutr. 2008:1–7. doi: 10.1017/S000711450789547X. [DOI] [PubMed] [Google Scholar]
- 2.Abdullah MM, Riediger NN, et al. Effects of long-term consumption of a high-fructose diet on conventional cardiovascular risk factors in Sprague-Dawley rats. Mol.Cell.Biochem. 2009;327:1–2. 247–256. doi: 10.1007/s11010-009-0063-z. [DOI] [PubMed] [Google Scholar]
- 3.Aeberli I, Gerber PA, Hochuli M, et al. Low to moderate sugar-sweetened beverage consumption impairs glucose and lipid metabolism and promotes inflammation in healthy young men: A randomized controlled trial. Am J Clin Nutr. 2011;94(2):479–485. doi: 10.3945/ajcn.111.013540. [DOI] [PubMed] [Google Scholar]
- 4.Aeberli I, Gerber PA, Hochuli M, et al. Low to moderate sugar-sweetened beverage consumption impairs glucose and lipid metabolism and promotes inflammation in healthy young men: A randomized controlled trial. Am J Clin Nutr. 2011;94(2):479–485. doi: 10.3945/ajcn.111.013540. [DOI] [PubMed] [Google Scholar]
- 5.Bassuk SS, Manson JE. Epidemiological evidence for the role of physical activity in reducing risk of type 2 diabetes and cardiovascular disease. J Appl Physiol. 2005 Sep;99(3):1193–204. doi: 10.1152/japplphysiol.00160.2005. [DOI] [PubMed] [Google Scholar]
- 6.Bey L, Hamilton MT. Suppression of skeletal muscle lipoprotein lipase activity during physical inactivity: A molecular reason to maintain daily low-intensity activity. J Physiol. 2003;551(Pt 2):673–682. doi: 10.1113/jphysiol.2003.045591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown CM, Dulloo AG, Yepuri G, Montani JP. Fructose ingestion acutely elevates blood pressure in healthy young humans. Am J Physiol Regul Integr Comp Physiol. 2008;294(3):R730–7. doi: 10.1152/ajpregu.00680.2007. [DOI] [PubMed] [Google Scholar]
- 8.Chen H, Sullivan G, Yue LQ, Katz A, Quon MJ. QUICKI is a useful index of insulin sensitivity in subjects with hypertension. Am J Physiol Endocrinol Metab. 2003;284(4):E804–12. doi: 10.1152/ajpendo.00330.2002. [DOI] [PubMed] [Google Scholar]
- 9.Conway J, Ingwersen L, Vinyard B, Moshfegh A. Effectiveness of the US department of agriculture 5-step multiple-pass method in assessing food intake in obese and nonobese women. Am J Clin Nutr. 2003;77(5):1171. doi: 10.1093/ajcn/77.5.1171. [DOI] [PubMed] [Google Scholar]
- 10.Cox CL, Stanhope KL, Schwarz JM, et al. Circulating concentrations of monocyte chemo-attractant protein-1, plasminogen activator inhibitor-1, and soluble leukocyte adhesion molecule-1 in overweight/obese men and women consuming fructose- or glucose-sweetened beverages for 10 weeks. J Clin Endocrinol Metab. 2011;96(12):E2034–8. doi: 10.1210/jc.2011-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Craig CL, Marshall AL, Sjo MS, Bauman AE, Booth ML, Ainsworth BA, Pratt, Ekelund U, Yngve A, Sallis JF, Oja P. International Physical Activity Questionnaire: 12-Country Reliability and Validity. Med. Sci. Sports Exerc. 2003;35(8):1381–1395. doi: 10.1249/01.MSS.0000078924.61453.FB. Med Sci Sport Exer. 2003. [DOI] [PubMed] [Google Scholar]
- 12.Dekker MJ, Su Q, Baker C, Rutledge AC, Adeli K. Fructose: A highly lipogenic nutrient implicated in insulin resistance, hepatic steatosis, and the metabolic syndrome. Am J Physiol Endocrinol Metab. 2010;299(5):E685–94. doi: 10.1152/ajpendo.00283.2010. [DOI] [PubMed] [Google Scholar]
- 13.Escalante Pulido JM, Alpizar Salazar M. Changes in insulin sensitivity, secretion and glucose effectiveness during menstrual cycle. Arch Med Res. 1999;30(1):19–22. doi: 10.1016/s0188-0128(98)00008-6. [DOI] [PubMed] [Google Scholar]
- 14.Franklin RM, Baynard T, Weinstock RS, et al. Autonomic responses to physiological stressors in women with type 2 diabetes. Clin Auton Res. 2008;18(2):66–73. doi: 10.1007/s10286-008-0461-4. [DOI] [PubMed] [Google Scholar]
- 15.Hamer M. The relative influences of fitness and fatness on inflammatory factors. Preventive Medicine. 2007;44:3–11. doi: 10.1016/j.ypmed.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 16.Hamilton MT, Areiqat E, Hamilton DG, Bey L. Plasma triglyceride metabolism in humans and rats during aging and physical inactivity. Int J Sport Nutr Exerc Metab. 2001;11(Suppl):S97–104. doi: 10.1123/ijsnem.11.s1.s97. [DOI] [PubMed] [Google Scholar]
- 17.Hojbjerre L, Sonne MP, Alibegovic AC, et al. Impact of physical inactivity on adipose tissue low-grade inflammation in first-degree relatives of type 2 diabetic patients. Diabetes Care. 2011;34(10):2265–2272. doi: 10.2337/dc11-0631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: Direct role in obesity-linked insulin resistance. Science. 1993;259(5091):87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 19.Howell DC. Statistical methods for psychology. 7th ed. Thomson Wadsworth; Belmont, CA: 2009. p. 630. [Google Scholar]
- 20.Levy JC, Matthews DR, Hermans MP. Correct homeostasis model assessment (HOMA) evaluation uses the computer program. Diabetes Care. 1998;21(12):2191–2192. doi: 10.2337/diacare.21.12.2191. [DOI] [PubMed] [Google Scholar]
- 21.Malerbi DA, Paiva ES, Duarte AL, Wajchenberg BL. Metabolic effects of dietary sucrose and fructose in type II diabetic subjects. Diabetes Care. 1996;19(11):1249–1256. doi: 10.2337/diacare.19.11.1249. [DOI] [PubMed] [Google Scholar]
- 22.Mamo JC, Hirano T, James L, Szeto L, Steiner G. Partial characterization of the fructose-induced defect in very-low-density lipoprotein triglyceride metabolism. Metabolism. 1991;40(9):888–893. doi: 10.1016/0026-0495(91)90061-z. [DOI] [PubMed] [Google Scholar]
- 23.McKeown NM, Meigs JB, Liu S, Saltzman E, Wilson PW, Jacques PF. Carbohydrate nutrition, insulin resistance, and the prevalence of the metabolic syndrome in the Framingham offspring cohort. Diabetes Care. 2004;27(2):538–546. doi: 10.2337/diacare.27.2.538. [DOI] [PubMed] [Google Scholar]
- 24.Miller A, Adeli K. Dietary fructose and the metabolic syndrome. Curr Opin Gastroenterol. 2008;24(2):204–209. doi: 10.1097/MOG.0b013e3282f3f4c4. [DOI] [PubMed] [Google Scholar]
- 25.Nakagawa T, Hu H, Zharikov S, et al. A causal role for uric acid in fructose-induced metabolic syndrome. Am J Physiol Renal Physiol. 2006;290(3):F625–31. doi: 10.1152/ajprenal.00140.2005. [DOI] [PubMed] [Google Scholar]
- 26.Nakanishi N, Okamoto M, Yoshida H, Matsuo Y, Suzuki K, Tatara K. Serum uric acid and risk for development of hypertension and impaired fasting glucose or type II diabetes in japanese male office workers. Eur J Epidemiol. 2003;18(6):523–530. doi: 10.1023/a:1024600905574. [DOI] [PubMed] [Google Scholar]
- 27.Parikh P, Mochari H, Mosca L. Clinical utility of a fingerstick technology to identify individuals with abnormal blood lipids and high-sensitivity C-reactive protein levels. Am J Health Promot. 2009;23(4):279–282. doi: 10.4278/ajhp.071221140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pil-Byung C, Shin-Hwan Y, Il-Gyu K, et al. Effects of exercise program on appetite-regulating hormones, inflammatory mediators, lipid profiles, and body composition in healthy men. J Sports Med Phys Fitness. 2011;51(4):654–663. [PubMed] [Google Scholar]
- 29.Raben A, Moller BK, Flint A, et al. Increased postprandial glycaemia, insulinemia, and lipidemia after 10 weeks' sucrose-rich diet compared to an artificially sweetened diet: A randomized controlled trial. Food Nutr Res. 2011;55:10. doi: 10.3402/fnr.v55i0.5961. Epub 2011 Jul 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ritz P, Salle A, Audran M, Rohmer V. Comparison of different methods to assess body composition of weight loss in obese and diabetic patients. Diabetes Res Clin Pract. 2007;77(3):405–411. doi: 10.1016/j.diabres.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 31.Rizkalla SW. Health implications of fructose consumption: A review of recent data. Nutr Metab (Lond) 2010;7:82. doi: 10.1186/1743-7075-7-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rockl KS, Witczak CA, Goodyear LJ. Signaling mechanisms in skeletal muscle: Acute responses and chronic adaptations to exercise. IUBMB Life. 2008;60(3):145–153. doi: 10.1002/iub.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stanhope KL, Schwarz JM, Keim NL, et al. Consuming fructose-sweetened, not glucose-sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans. J Clin Invest. May. 2009;119(5):1322–34. doi: 10.1172/JCI37385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tappy L, Le KA. Metabolic effects of fructose and the worldwide increase in obesity. Physiol.Rev. 2010;90:1, 23–46. doi: 10.1152/physrev.00019.2009. [DOI] [PubMed] [Google Scholar]
- 35.Zderic TW, Hamilton MT. Physical inactivity amplifies the sensitivity of skeletal muscle to the lipid-induced down-regulation of lipoprotein lipase activity. J Appl Physiol. 2006;100(1):249–257. doi: 10.1152/japplphysiol.00925.2005. [DOI] [PubMed] [Google Scholar]