To The Editor
Psoriasis is generally considered to be an immunologically-initiated disorder, which shares certain common susceptibility loci with autoimmune diseases (Zhang, 2012). Yet, both clinical experience (Gottlieb et al.,1990; Griffiths et al., 1995; Volden et al., 2001;) and recent molecular studies (Bergboer et al., 2012; Kim et al., 2011; Mischke et al., 1996; Vermeij et al., 2011) support an emerging concept that psoriasis could be ‘driven’ by a primary defect in epidermal permeability barrier function. Clinicians know well that psoriasis predictably flares during winter months (Park and Youn, 1998; Kwon et al., 2012), when the barrier is under additional stress due to low stratum corneum hydration, which accelerates transepidermal water loss (TEWL) rates (Lin, 2009; Muizzuddin et al., 2013). They also appreciate that sites vulnerable to epidermal trauma, such as the extensors of the extremities and the scalp, are preferentially involved in psoriasis. The Koebner phenomenon offers an additional, eloquent example of how psoriasis can be provoked by external perturbations. Finally, improvement of epidermal permeability barrier function by occlusion alone often alleviates psoriasis (e.g., Friedman, 1987).
Among psoriasis susceptibility genes, PSORS4 is located on chromosome 1q21, within the epidermal differentiation complex, which encodes numerous proteins required for epidermal differentiation and the formation of the cornified envelope (Mischke et al., 1996), a structure that is critical for the permeability barrier (Vermeij et al., 2011). Additionally, deletion of differentiation-related proteins, such as keratin1, whose levels are reduced in psoriasis (Thewes et al., 1991; Bata-Csörgö and Szell, 2012), not only compromises the permeability barrier, but also leads to upregulation of inflammatory genes and altered cytokine production in a pattern that resembles psoriasis (Roth, et al. 2012). Finally, in experimental models, disruption of the epidermal permeability barrier in otherwise normal skin stimulates epidermal hyperproliferation (Man et al., 2008; Prokch et al., 1991), cytokine production (Denda et al., 1996; Wood et al., 1996), downstream inflammatory cell infiltration (Lin et al., 2013; Proksch et al., 1996), and epidermal vascular endothelial growth factor production, leading to dermal capillary proliferation (Elias et al., 2008), all of which are prominent features of psoriasis.
Although prior studies have demonstrated phenotype-dependent abnormalities in basal permeability barrier function in psoriatic lesions (Ghadially et al., 1996), the uninvolved skin in psoriasis reportedly displays normal TEWL levels (Takahashi et al., 2014). Yet, expression levels of filaggrin, a protein of known importance for the barrier (Scharschmidt et al., 2009; Irvine et al., 2011), and loricrin reportedly are lower than normal in uninvolved skin sites of psoriasis (Kim et al., 2011). Because alterations in the epidermal differentiation should predict an abnormality in barrier function, we hypothesized that if abnormal epidermal function plays a role in the pathogenesis of psoriasis, then epidermal function should be abnormal in uninvolved psoriatic skin. Hence, we assessed changes in epidermal function in the uninvolved and involved skin of a large cohort of Chinese patients, with either stable or progressive psoriasis.
44 patients with psoriasis vulgaris and 64 normal controls were enrolled (Table 1). All participants were given written informed consent. This study was carried out according to the Helsinki Declaration Principles and to protocol approved by the Human Research Subcommittee, Dalian Skin Disease Hospital. Stable psoriasis was defined as no recent development of new lesions, as well as no recent expansion of pre-existing lesions, while progressive psoriasis was defined as recent or ongoing development of new lesions and prominent inflammation, often accompanied by pruritus. None of the psoriatic patients had other skin or systemic disorders, and controls had no history of psoriasis, other skin disorders, or systemic conditions that could affect stratum corneum (SC) biophysical properties. No topical medications were used by patients for ≥ one week prior to study; no skin care products were applied to measured sites for ≥ 24 hour prior to measurements; and measured sites were not washed with either soaps or surfactants for ≥ 2 hours prior to study.
Table 1.
Characteristics of Subjects
| Stage | Gender | N | Mean Age ± SEM (year) |
|---|---|---|---|
| Psoriasis | |||
| Stable | Male | 15 | 33.07 ± 2.63 |
| Female | 10 | 38.60 ± 2.72 | |
| Progressive | Male | 10 | 36.20 ± 2.80 |
| Female | 9 | 36.33 ± 2.44 | |
| Control | |||
| Normal | Male | 34 | 39.15 ± 0.96 |
| Female | 34 | 38.47 ± 1.36 | |
| Total | 112 | ||
Epidermal functions were evaluated in psoriatic lesions on the extensor forearms and contralateral, uninvolved skin sites, and comparable sites in normal controls. Transepidermal water loss (TEWL) and SC hydration were assessed using probes (TM300 for TEWL and CM825 for hydration, respectively) connected to a MPA5 (C&K, Cologne, Germany). A pH900 pH meter with a flat surface electrode (C&K, Cologne, Germany) was used to measure skin surface pH. For assessment of barrier recovery kinetics, TEWL was measured both immediately after (0 hours) and 3 hours after 6 sequential D-Squame applications, and percent barrier recovery was then calculated. All subjects rested at 20–24°C, at a relative humidity of 50–55%, for at least 30 min before measurements were taken. Since psoriasis often flares during late autumn and winter through early spring, all studies were performed during February and March, 2012, and during October, 2013, representing early spring and autumn seasons in northern China
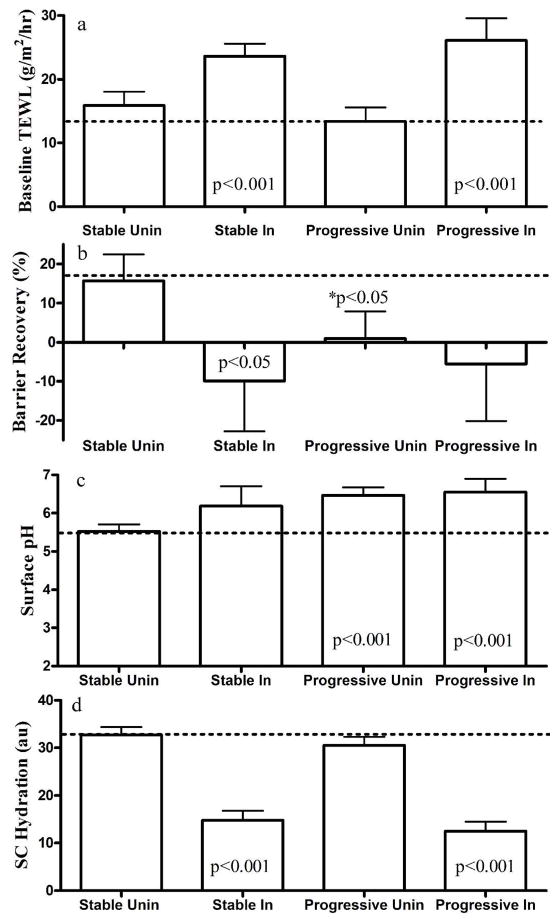
Consistent with prior findings Ghadially et al., 1996, our results showed that both stable and progressive psoriatic lesions displayed significantly higher basal levels of TEWL than the uninvolved skin and skin sites of normal control subjects (Fig. 1a). Barrier recovery was also delayed in the involved skin of both progressive and stable psoriasis (Fig. 1b). Although basal TEWL readings in uninvolved sites of both stable and progressive disease were comparable to those in normal skin, barrier recovery kinetics were significantly delayed in the uninvolved skin sites of patients with progressive psoriasis (Fig. 1b).
Figure 1. Comparison of SC Biophysical Properties among Normal, Involved and Uninvolved Psoriatic Skin Sites.
a & b depicts basal TEWL and barrier recovery rates, respectively. c & d presents skin surface pH and SC hydration, respectively. Results are expressed as mean ± SEM in comparison with normal skin, as shown by the horizontal dotted lines. GraphPad Prism 4 software (GraphPad Software, Inc., La Jolla, CA, USA) was used for all statistical analysis. Dunnett’s Multiple Comparison Test was used to determine the difference between normal, and psoriasis involved and uninvolved skin, except in figure 1b where the differences between normal and progressive uninvolved skin were determined with unpaired T test. P<0.05 was considered to a statistically significant difference. Significant differences are shown in the figures and numbers of subjects are detailed in Table 1.
Previous studies have demonstrated that elevations in SC pH influence permeability barrier homeostasis (Hachem et al., 2005; Mauro et al., 1998); and conversely, that barrier disruption increases SC pH (Man et al., 2009). Hence, we next measured changes in surface pH in normal and psoriatic patients. As seen in Figure 1c, the surface pH of both involved and uninvolved skin sites in progressive psoriasis was significantly higher than in normal controls (p<0.001), and an apparent elevation in SC pH was also observed in stable psoriatic lesions, though the results did not achieve statistical significance. In contrast, the uninvolved skin of stable psoriasis displayed a normal surface pH (Figure 1c).
Because SC hydration is a critical regulator of epidermal proliferation and cutaneous inflammation (Denda et al., 1998; Denda, 2000), we next assessed SC hydration in involved and uninvolved psoriatic skin sites. SC hydration markedly declined in involved skin of both progressive and stable psoriasis, but hydration levels were normal in uninvolved skin sites of both psoriatic cohorts (Figure 1d).
Recently, the central role of epidermal permeability barrier abnormalities in the pathogenesis of inflammatory dermatoses has attracted increased attention. Previous studies have shown that sustained defect in permeability barrier function predisposes skin to the development of epidermal hyperplasia and inflammation, resulting in part from the stimulation of a cytokine cascade that recruits a downstream inflammatory cell infiltration (Proksch et al., 1996; Elias et al., 1999; Elias and Wakefield, 2011). Because not only involved, but also the uninvolved skin sites of patients with progressive disease display altered epidermal function, the results presented here are consistent with an emerging concept that an underlying abnormality in epidermal function initiates, triggers or exacerbates psoriasis, together supporting an ‘outside-to-inside’ concept of psoriasis pathogenesis (Elias et al., 1999). The fact that stable uninvolved psoriatic skin sites does not display demonstrable defect in epidermal function, can be explained by the reestablishment of a steady-state, where epidermal function has largely been normalized, coupled with reduced exposure to external stressors that otherwise place additional stress on the barrier. Nonetheless, it should be noted that the uninvolved skin of psoriasis could still display subtle abnormalities that are not detectable by the biophysical technique utilized here. For example, we have shown accelerated movement of the water-soluble, electron-dense tracer, lanthanum nitrate, in some situations where TEWL levels otherwise appeared normal (e.g., Scharschmidt et al., 2009).
A defective permeability barrier not only stimulates epidermal proliferation (Proksch et al., 1991), but it also increases pro-inflammatory cytokine expression, as well as increasing Langerhans cell and mast cell infiltration (Nickoloff and Naidu 1994; Proksch et al., 1996; Wood et al., 1992 & 1996; Lin et al., 2013). Moreover, the abnormalities in surface pH in the uninvolved skin of progressive psoriasis could further predispose to disease expression in psoriasis as follows (Supplemental Figure 1): An elevation in pH inevitably activates serine proteases (kallikreins) in the outer epidermis, which in turn degrade lipid processing enzymes leading to abnormal permeability barrier homeostasis (Hachem et al., 2005), and catalyze pro-IL-1 beta to active IL-1 beta (Nylander-Lundqvist E and Egelrud T, 1997), initiating the cytokine cascade. Finally, reduced SC hydration, which often parallels abnormalities in barrier function, alone places further stress on the barrier, evidenced by the development of epidermal proliferation and the initiation of cutaneous inflammation in experimental animals exposed to low ambient humidity (Denda, 2000; Ashida et al., 2001; Ashida and Denda, 2003). Coupling the present with prior findings, we hypothesize that psoriasis is characterized by a failure to complete barrier repair due to primary abnormalities in epidermal structural proteins, analogous to atopic dermatitis, and further aggravated by exogenous stress to barrier (Supplemental Figure 1). Our findings further suggest that approaches that normalize barrier function could prove valuable for the prevention and/or treatment of psoriasis.
Supplementary Material
Acknowledgments
This work was supported in part by National Institutes of Health grants AR 19098 and the Medical Research Service, Department of Veterans Affairs Medical Center.
Footnotes
All authors declare no conflicts of interest
References
- Ashida Y, Denda M. Dry environment increases mast cell number and histamine content in dermis in hairless mice. Br J Dermatol. 2003;149:240–7. doi: 10.1046/j.1365-2133.2003.05408.x. [DOI] [PubMed] [Google Scholar]
- Ashida Y, Ogo M, Denda M. Epidermal interleukin-1 alpha generation is amplified at low humidity: implications for the pathogenesis of inflammatory dermatoses. Br J Dermatol. 2001;144:238–43. doi: 10.1046/j.1365-2133.2001.04007.x. [DOI] [PubMed] [Google Scholar]
- Bata-Csörgö Z, Szell M. The Psoriatic Keratinocytes. Expert Rev Dermatol. 2012;7:473–481. [Google Scholar]
- Bergboer JG, Zeeuwen PL, Schalkwijk J. Genetics of psoriasis: evidence for epistatic interaction between skin barrier abnormalities and immune deviation. J Invest Dermatol. 2012;132:2320–1. doi: 10.1038/jid.2012.167. [DOI] [PubMed] [Google Scholar]
- Denda M. Influence of dry environment on epidermal function. J Dermatol Sci. 2000;24:S22–8. doi: 10.1016/s0923-1811(00)00137-7. [DOI] [PubMed] [Google Scholar]
- Denda M, Sato J, Tsuchiya T, et al. Low humidity stimulates epidermal DNA synthesis and amplifies the hyperproliferative response to barrier disruption: implication for seasonal exacerbations of inflammatory dermatoses. J Invest Dermatol. 1998;111:873–8. doi: 10.1046/j.1523-1747.1998.00364.x. [DOI] [PubMed] [Google Scholar]
- Denda M, Wood LC, Emami S, et al. The epidermal hyperplasia associated with repeated barrier disruption by acetone treatment or tape stripping cannot be attributed to increased water loss. Arch Dermatol Res. 1996;288:230–8. doi: 10.1007/BF02530090. [DOI] [PubMed] [Google Scholar]
- Elias PM, Arbiser J, Brown BE, et al. Epidermal vascular endothelial growth factor production is required for permeability barrier homeostasis, dermal angiogenesis, and the development of epidermal hyperplasia: implications for the pathogenesis of psoriasis. Am J Pathol. 2008;173:689–99. doi: 10.2353/ajpath.2008.080088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias PM, Wakefield JS. Therapeutic implications of a barrier-based pathogenesis of atopic dermatitis. Clin Rev Allergy Immunol. 2011;41:282–95. doi: 10.1007/s12016-010-8231-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias PM, Wood LC, Feingold KR. Epidermal pathogenesis of inflammatory dermatoses. Am J Contact Dermat. 1999;10:119–26. [PubMed] [Google Scholar]
- Friedman SJ. Management of psoriasis vulgaris with a hydrocolloid occlusive dressing. Arch Dermatol. 1987;123:1046–52. [PubMed] [Google Scholar]
- Ghadially R, Reed JT, Elias PM. Stratum Corneum Structure and Function Correlates with Phenotype in Psoriasis. J Invest Dermatol. 1996;107:558–564. doi: 10.1111/1523-1747.ep12582813. [DOI] [PubMed] [Google Scholar]
- Gottlieb AB, Staiano-Coico L, Cohen SR, et al. Occlusive hydrocolloid dressings decrease keratinocyte population growth fraction and clinical scale and skin thickness in active psoriatic plaques. J Dermatol Sci. 1990;1:93–6. doi: 10.1016/0923-1811(90)90221-x. [DOI] [PubMed] [Google Scholar]
- Griffiths CE, Tranfaglia MG, Kang S. Prolonged occlusion in the treatment of psoriasis: a clinical and immunohistologic study. J Am Acad Dermatol. 1995;32:618–22. doi: 10.1016/0190-9622(95)90347-x. [DOI] [PubMed] [Google Scholar]
- Hachem JP, Man MQ, Crumrine D, et al. Sustained serine proteases activity by prolonged increase in pH leads to degradation of lipid processing enzymes and profound alterations of barrier function and stratum corneum integrity. J Invest Dermatol. 2005;125:510–20. doi: 10.1111/j.0022-202X.2005.23838.x. [DOI] [PubMed] [Google Scholar]
- Hong KK, Cho HR, Ju WC, et al. A study on altered expression of serine palmitoyltransferase and ceramidase in psoriatic skin lesion. J Korean Med Sci. 2007;22:862–7. doi: 10.3346/jkms.2007.22.5.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine AD, McLean WH, Leung DY. Filaggrin mutations associated with skin and allergic diseases. 2011;365:1315–27. doi: 10.1056/NEJMra1011040. [DOI] [PubMed] [Google Scholar]
- Kim BE, Howell MD, Guttman-Yassky E, et al. TNF-α downregulates filaggrin and loricrin through c-Jun N-terminal kinase: role for TNF-α antagonists to improve skin barrier. J Invest Dermatol. 2011;131:1272–9. doi: 10.1038/jid.2011.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon HH, Na SJ, Jo SJ, et al. Epidemiology and clinical features of pediatric psoriasis in tertiary referral psoriasis clinic. J Dermatol. 2012;39:260–4. doi: 10.1111/j.1346-8138.2011.01452.x. [DOI] [PubMed] [Google Scholar]
- Lin TK, Man MQ, Santiago JL, et al. Topical antihistamines display potent anti-inflammatory activity linked in part to enhanced permeability barrier function. J Invest Dermatol. 2013;133:469–78. doi: 10.1038/jid.2012.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin ZX. Study on epidermal permeability barrier function and its associated factors in 160 normal Chinese. Master’s degree theme, Fudan University. 2009 http://cdmd.cnki.com.cn/Article/CDMD-10246-2009184487.htm.
- Man MQ, Shi Y, Man M, et al. Chinese herbal medicine (Tuhuai extract) exhibits topical anti-proliferative and anti-inflammatory activity in murine disease models. Exp Dermatol. 2008;17:681–7. doi: 10.1111/j.1600-0625.2007.00690.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Man WY, Liu ZL, Elias PM, et al. Kinetic study on epidermal pH homeostasis. J Clin Dermatol. 2009;38:152–153. [Google Scholar]
- Mauro T, Holleran WM, Grayson S, et al. Barrier recovery is impeded at neutral pH, independent of ionic effects: implications for extracellular lipid processing. Arch Dermatol Res. 1998;290:215–22. doi: 10.1007/s004030050293. [DOI] [PubMed] [Google Scholar]
- Mischke D, Korge BP, Marenholz I, et al. Genes encoding structural proteins of epidermal cornification and S100 calcium-binding proteins form a gene complex (“epidermal differentiation complex”) on human chromosome 1q21. J Invest Dermatol. 1996;106:989–92. doi: 10.1111/1523-1747.ep12338501. [DOI] [PubMed] [Google Scholar]
- Muizzuddin N, Ingrassia M, Marenus KD, et al. Effect of seasonal and geographical differences on skin and effect of treatment with an osmoprotectant: Sorbitol. J Cosmet Sci. 2013;64:165–74. [PubMed] [Google Scholar]
- Nakajima K, Terao M, Takaishi M, et al. Barrier abnormality due to ceramide deficiency leads to psoriasiform inflammation in a mouse model. J Invest Dermatol. 2013;133:2555–65. doi: 10.1038/jid.2013.199. [DOI] [PubMed] [Google Scholar]
- Nickoloff BJ, Naidu Y. Perturbation of epidermal barrier function correlates with initiation of cytokine cascade in human skin. J Am Acad Dermatol. 1994;30:535–46. doi: 10.1016/s0190-9622(94)70059-1. [DOI] [PubMed] [Google Scholar]
- Nylander-Lundqvist E, Egelrud T. Formation of active IL-1 beta from pro-IL-1 beta catalyzed by stratum corneum chymotryptic enzyme in vitro. Acta Derm Venereol. 1997;77:203–6. doi: 10.2340/0001555577203206. [DOI] [PubMed] [Google Scholar]
- Park BS, Youn JI. Factors influencing psoriasis: an analysis based upon the extent of involvement and clinical type. J Dermatol. 1998;25:97–102. doi: 10.1111/j.1346-8138.1998.tb02357.x. [DOI] [PubMed] [Google Scholar]
- Proksch E, Brasch J, Sterry W. Integrity of the permeability barrier regulates epidermal Langerhans cell density. Br J Dermatol. 1996;134:630–8. [PubMed] [Google Scholar]
- Proksch E, Feingold KR, Man MQ, et al. Barrier function regulates epidermal DNA synthesis. J Clin Invest. 1991;87:1668–73. doi: 10.1172/JCI115183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth W, Kumar V, Beer HD, et al. Keratin 1 maintains skin integrity and participates in an inflammatory network in skin through interleukin-18. J Cell Sci. 2012;125:5269–5279. doi: 10.1242/jcs.116574. [DOI] [PubMed] [Google Scholar]
- Scharschmidt TC, Man MQ, Hatano Y, et al. Filaggrin deficiency confers a paracellular barrier abnormality that reduces inflammatory thresholds to irritants and haptens. J Allergy Clin Immunol. 2009;124:496–506. doi: 10.1016/j.jaci.2009.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H, Tsuji H, Minami-Hori M, et al. Defective barrier function accompanied by structural changes of psoriatic stratum corneum. J Dermatol. 2014;41:144–8. doi: 10.1111/1346-8138.12393. [DOI] [PubMed] [Google Scholar]
- Thewes M, Stadler R, Korge B, et al. Normal psoriatic epidermis expression of hyperproliferation-associated keratins. Arch Dermatol Res. 1991;283:465–71. doi: 10.1007/BF00371784. [DOI] [PubMed] [Google Scholar]
- Vermeij WP, Alia A, Backendorf C. ROS quenching potential of the epidermal cornified cell envelope. J Invest Dermatol. 2011;131:1435–41. doi: 10.1038/jid.2010.433. [DOI] [PubMed] [Google Scholar]
- Volden G, Kragballe K, van de Kerkhof PCM, et al. Remission and relapse of chronic plaque psoriasis treated once a week with clobetasol propionate occluded with a hydrocolloid dressing versus twice daily treatment with clobetasol propionate alone. J Dermatol Treat. 2001;12:141–4. doi: 10.1080/09546630152607862. [DOI] [PubMed] [Google Scholar]
- Wood LC, Elias PM, Calhoun C, et al. Barrier disruption stimulates interleukin-1 alpha expression and release from a pre-formed pool in murine epidermis. J Invest Dermatol. 1996;106:397–403. doi: 10.1111/1523-1747.ep12343392. [DOI] [PubMed] [Google Scholar]
- Wood LC, Jackson SM, Elias PM, et al. Cutaneous barrier perturbation stimulates cytokine production in the epidermis of mice. J Clin Invest. 1992;90(2):482–7. doi: 10.1172/JCI115884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X. Genome-wide association study of skin complex diseases. J Dermatol Sci. 2012;66:89–97. doi: 10.1016/j.jdermsci.2012.02.017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.