Abstract
Purpose
Information regarding antimicrobial prophylaxis (AMP) for gastric cancer surgery is limited. The present study investigated the efficacy of single-dose AMP for the prevention of surgical site infection (SSI) in patients undergoing gastrectomy for gastric carcinoma.
Materials and Methods
Between 2011 and 2013, 1,330 gastric carcinoma surgery patients were divided into two AMP administration groups depending on the duration of treatment. Postoperative outcomes including morbidity and SSI were compared between the two groups overall and in matched patients. Risk factors for SSI were analyzed.
Results
The extended group (n=1,129) received AMP until postoperative day 1 and the single-dose group (n=201) received singledose AMP only during an operation. Postoperatively, there were no significant differences between the two groups with respect to overall morbidity, mortality, or length of hospital stay. The SSI rate of the single-dose group was not significantly different from that of the extended group overall (4.5% vs. 5.5%, respectively, P=0.556) or in matched patients (4.5% vs. 4.0%, respectively, P=0.801). There was no increase in the SSI rate of the single-dose group compared to the extended group in subgroups based on different clinicopathological and operative factors. Univariate and multivariate analyses revealed male gender, open surgery, and operating time (≥180 minutes) as independent risk factors for SSI.
Conclusions
Single-dose AMP showed no increase in the postoperative SSI rate compared to postoperative extended use in patients undergoing gastrectomy for gastric carcinoma. The efficacy of single-dose AMP requires further investigation in randomized clinical trials specific to gastric cancer surgery.
Keywords: Antibiotic prophylaxis, Surgical wound infection, Stomach neoplasms
Introduction
Surgical site infection (SSI) is the most common nosocomial infection among surgical patients with an incidence of up to 20% after major abdominal surgeries.1,2 As for gastric cancer surgery, the incidence of SSI is reported to be 5% to 20% depending on the patient population, operation type, and the operational definition of SSI according to previous studies.3,4,5,6 Antimicrobial prophylaxis (AMP) can effectively prevent SSI in gastric cancer surgery; however, the optimum duration of prophylaxis remains uncertain. Although current guidelines commonly recommend single-dose AMP for gastrointestinal surgery,7,8,9,10,11,12 most published studies regarding AMP in abdominal surgeries have focused on either biliary or colorectal surgery as opposed to AMP for gastric cancer surgery.13,14,15
Evidence for the efficacy of single-dose AMP for gastric cancer surgery is very limited. Within the last decade, two small randomized trials have demonstrated the efficacy of single-dose AMP for gastric cancer surgery.16,17 However, prolonged postoperative use of prophylactic antibiotics after gastric cancer surgery remains a common practice in Asia. According to a survey of 14 high-volume centers in Korea and Japan, AMP was administered longer than 24 hours after gastric cancer surgery in as many as 11 institutions.18 Another large Japanese survey of 3,823 surgeons revealed that 56.4% administered prophylactic antibiotics until postoperative day (POD) 3 or 4 after gastrointestinal surgery, while only 2.4% adhered to the recommended use of AMP for 24 hours or less.19 Single-dose AMP has several advantages over prolonged postoperative use, as it minimizes the development of bacterial resistance and antibiotic-related complications. In the present study, we investigated postoperative outcomes including SSI in patients undergoing gastrectomy for gastric carcinoma. Patients were divided according to duration of AMP administration, and the efficacy of single-dose AMP for preventing SSI was evaluated.
Materials and Methods
1. Patients
Using a gastric cancer database at Chonnam National University Hwasun Hospital (CNUHH), we identified 1,433 patients who underwent surgery for gastric carcinoma between 2011 and 2013. Of these patients, those with bypass surgery (n=35), preoperative chemotherapy (n=21), emergency operation prompted by bleeding or perforation (n=9), preoperative antibiotic use (n=14), and incomplete medical records (n=24) were excluded. Hence, 1,330 patients who underwent an elective operation for gastric carcinoma were included. The patients were divided into two groups according to the duration of AMP administration. Patients in the first study period (before October 2012) received AMP until POD 1 (extended group). In this group, cefazolin 1 g was administered just before skin incision and every 12 hours until POD 1. Patients in the second period (after October 2012) received single-dose AMP only during an operation without postoperative use (single-dose group). Postoperative outcomes including morbidity, mortality, length of hospital stay, and SSI were compared between the groups. This study was approved by the Institutional Review Board of Chonnam National University Hwasun Hospital (CNUH 2013-043), which waived the requirement of informed consent from the patients.
2. Operative procedures and perioperative care
Patients underwent radical subtotal or total gastrectomy with lymph node dissection (LND) as described by the Japanese gastric cancer treatment guidelines.20 Laparoscopic gastrectomy was indicated for mucosal or submucosal cancers unsuitable for endoscopic resection. Billroth I gastroduodenostomy was the primary reconstruction procedure for subtotal gastrectomy, and Billroth II and Roux-en-Y gastrojejunostomy were alternatives. After total gastrectomy, Roux-en-Y esophagojejunostomy was performed using a circular stapler in most cases.
Patients in both groups were managed perioperatively using the same standardized protocol of CNUHH. Neither preoperative mechanical bowel preparations nor nasogastric tubes were used. Briefly, preoperative fasting was avoided until the night before the operation. Intraoperative normothermia was maintained using a warm air blanket. An abdominal drain was not routinely inserted, but was inserted in selected patients. Postoperatively, patients started oral feeding on POD 1 or 2, and restricted intravenous fluid (20~25 ml·kg-1·d-1) was administered for 3 to 4 PODs. Patients were usually discharged from the hospital on POD 6 to 8.
3. Data collection
Using a prospectively constructed database, we retrospectively reviewed patients' baseline demographic features, operative outcomes, pathological reports, and hospital courses, including postoperative complications. Pathological stages were recorded on the basis of the 7th edition of the Union for International Cancer Control Tumor Node Metastasis (TNM) classification.21 Complications or deaths during hospitalization or within POD 1 to 30 were defined as morbidity and mortality. Regarding postoperative complications, those associated with the operative field were considered local, and others were regarded as systemic. The type and severity of each postoperative complication were recorded according to the institutional guidelines of surgical complications after gastric carcinoma.22
SSI was defined and classified on the basis of the National Nosocomial Infections Surveillance system.2 Briefly, an SSI was defined as an infection occurring within 30 days after an operation that appeared to be related to the operative procedure. SSIs were further classified into superficial incisional (i.e., affecting the skin and subcutaneous tissue), deep incisional (i.e., affecting the deeper soft tissues of the incision), and organ/space (i.e., affecting the any part of the anatomy other than the incision). Regarding organ/space SSIs, both primary abdominal infections and secondary infections due to any other reason requiring therapeutic antibiotics or intervention were included.
4. Statistical analyses
Data are presented as mean±standard deviation or numbers with percentages. For between-group comparisons, Student's t-test, χ2 test, or Fisher's exact test was used, where appropriate. A binary logistic regression model was used for multivariate analysis of risk factors for SSI. To reduce the impact of heterogeneity in the baseline characteristics on patient outcomes and more accurately compare the SSI rate between groups, patients in the single-dose group were individually matched to patients in the POD 1 group with respect to age, sex, body mass index (BMI), comorbidity, operative procedure, operating time, and TNM stage using the propensity score matching method.23 All statistical analyses were performed using SPSS version 12.0 for Windows (SPSS Inc., Chicago, IL, USA), and the level of significance for all tests was set at P<0.05.
Results
The study subjects consisted of 896 men and 434 women, with a mean age of 61.3±12.1 years. Among all patients, 1,129 and 201 were in the extended and single-dose AMP groups, respectively. The baseline characteristics of the two groups are summarized in Table 1. There were no significant differences between groups with respect to sex, BMI, comorbidity, or curative resection rate. However, the single-dose group was significantly younger than the extended group (57.4 vs. 61.9 years, respectively, P<0.001), and there were more patients with TNM stage III or IV in the extended group (P=0.025). Regarding operative procedures, laparoscopic surgery (71.6% vs. 57.5%, P=0.001) and limited LND (72.1% vs. 46.1%, P<0.001) were performed more frequently in the single-dose group. Operating time was significantly longer in the single-dose group than in the extended group (194 vs. 179 minutes, respectively, P=0.002).
Table 1.
Baseline characteristics
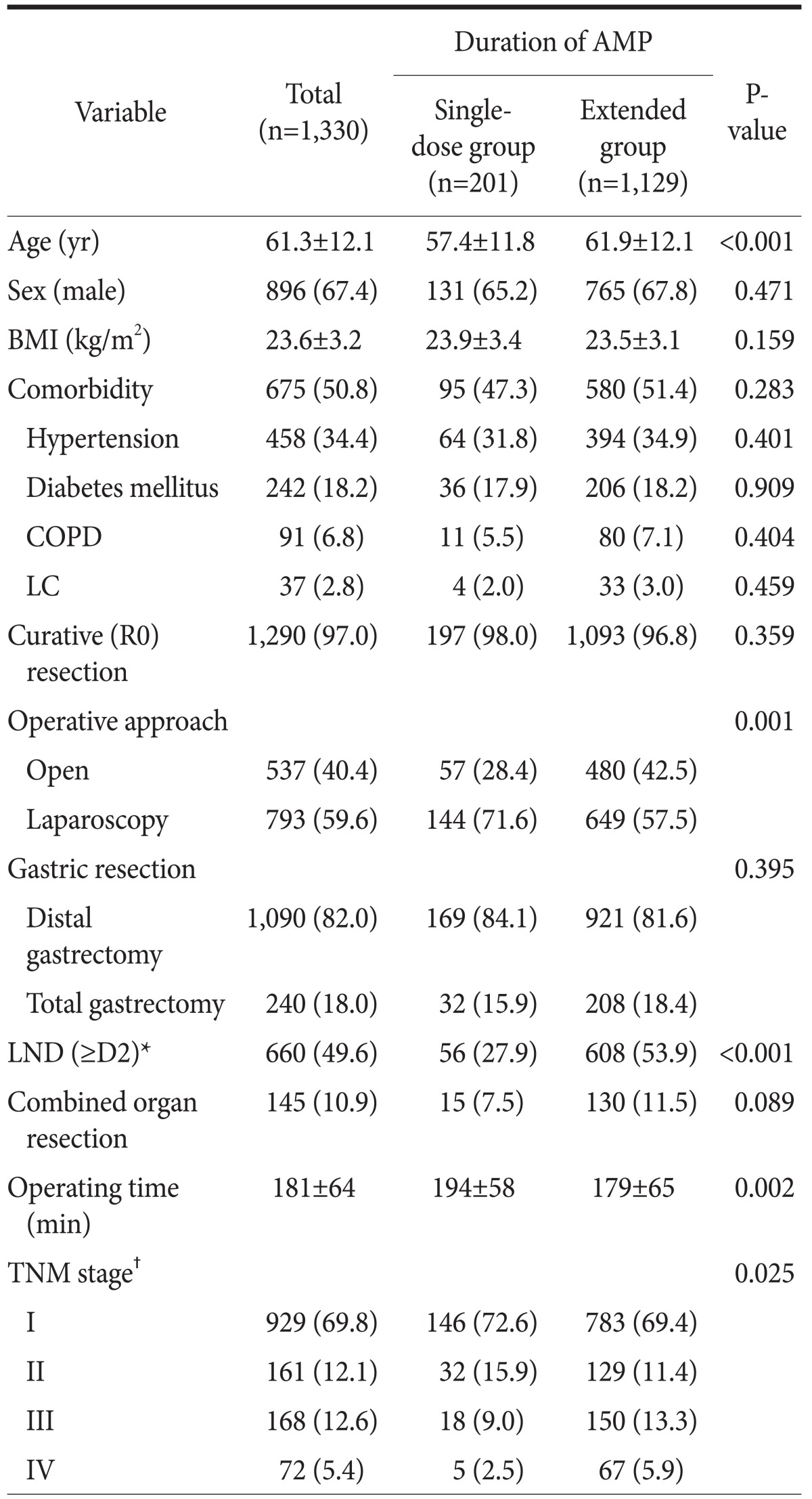
Values are presented as mean±standard deviation or number (%). BMI = body mass index; COPD = chronic obstructive pulmonary disease; LC = liver cirrhosis; LND = lymph node dissection; TNM = Tumor Node Metastasis. *LND according to the Japanese gastric cancer treatment guideline 2010 (ver. 3). †TNM stage was based on the 7th edition of the American Joint Committee on Cancer staging system.
1. Comparison of surgical site infections in the overall patient population
Table 2 shows comparisons of postoperative outcomes, including SSIs, between the two AMP groups in the overall patient population. Postoperatively, there were no significant inter-group differences with respect to length of hospital stay (P=0.204), morbidity (P=0.143), or mortality (P=1.000). Overall, SSIs including intra-abdominal abscess (n=25), wound infection (n=15), anastomosis leakage (n=20), and pancreatic fistula (n=11) developed in 71 (5.3%) patients. The incidences of SSIs in the single-dose and extended AMP groups did not differ significantly (4.5% vs. 5.5%, respectively, P=0.556). Furthermore, the incidence of each infectious complication and the types of SSIs did not differ significantly between the groups.
Table 2.
Postoperative outcomes including SSIs in the two AMP groups
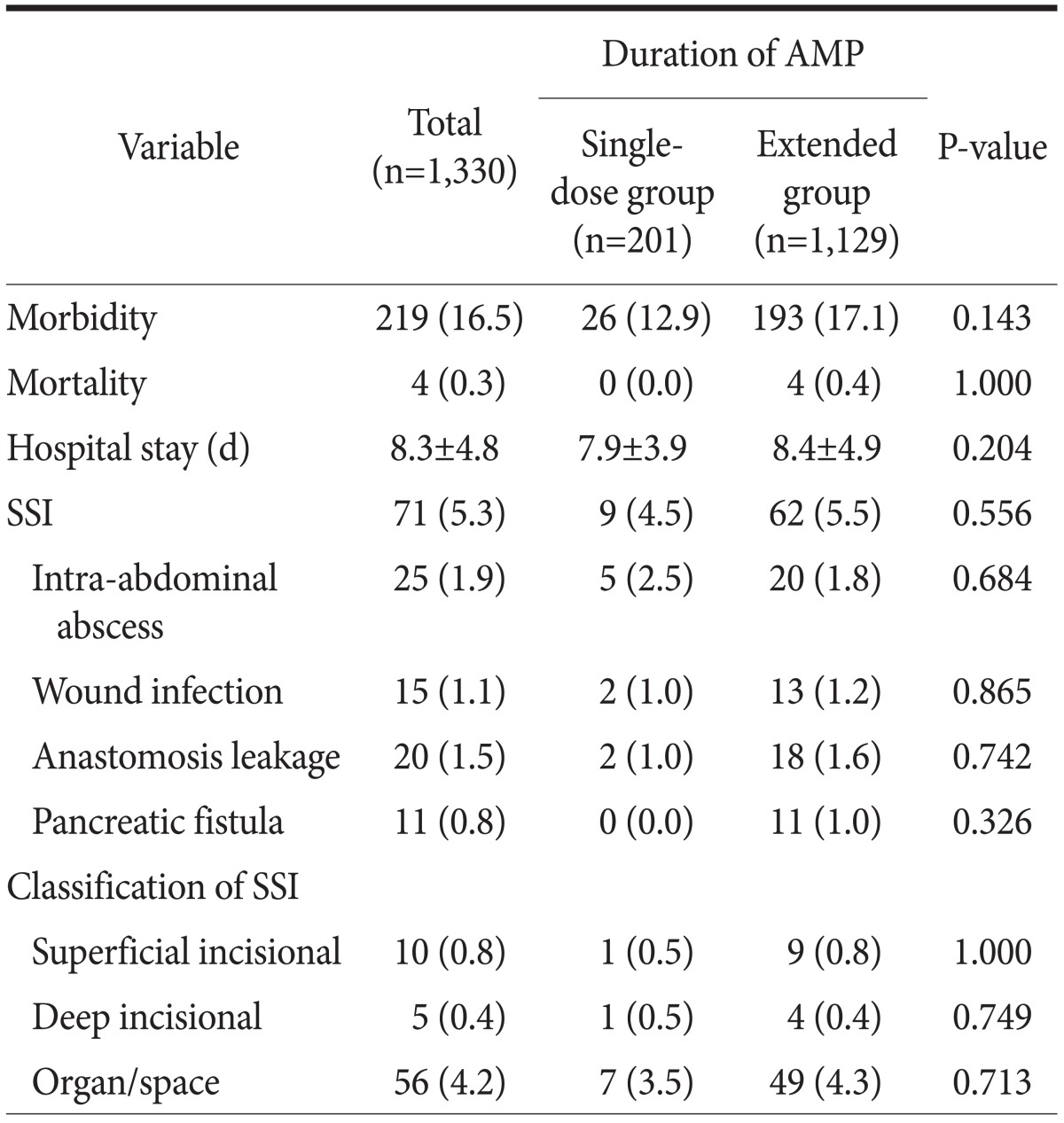
Values are presented as number (%) or mean±standard deviation. SSI = surgical site infection; AMP = antimicrobial prophylaxis.
Table 3 shows the incidences of SSIs in the single-dose and extended groups in the subgroups with different clinicopathological and operative factors. The incidences of SSIs did not differ significantly between the two AMP groups in subgroups based on age, gender, BMI, operative approach, the extent of LND, combined organ resection, operating time, or tumor stage. However, in the subgroup with total gastrectomy, the incidence of SSI was significantly higher in the extended AMP group than in the single-dose group (11.1% vs. 0.0%, P=0.048).
Table 3.
Subgroup analysis of SSIs between the two AMP groups

Values are presented as number or number (%). SSI = surgical site infection; AMP = antimicrobial prophylaxis; BMI = body mass index; LND = lymph node dissection; TNM = Tumor Node Metastasis. *LND according to the Japanese gastric cancer treatment guideline 2010 (ver. 3). †TNM stage was based on the 7th edition of the American Joint Committee on Cancer staging system.
2. Comparison of surgical site infections in matched groups
Because patients in the two AMP groups showed some heterogeneity in their baseline characteristics, we individually matched patients in the single-dose group with patients in the extended group using the propensity score matching method based on clinicopathological and operative factors in order to compare postoperative morbidity and SSI rates. Table 4 shows that matched patients were well balanced with respect to age, sex, BMI, comorbidity, operative procedures, operating time, and TNM stage. In matched samples, patients in the single-dose group showed no increase in the SSI rate compared to patients in the extended AMP group (4.5% vs. 4.0%, respectively, P=0.801). Additionally, there were no significant differences between the single-dose and extended AMP groups with respect to overall morbidity (both 12.9%, P=1.000), mortality (0% vs. 0.5%, respectively, P=1.000), or the length of the hospital stay (7.9 vs. 8.0 days, respectively, P=0.908).
Table 4.
Comparison of SSI in matched patients*
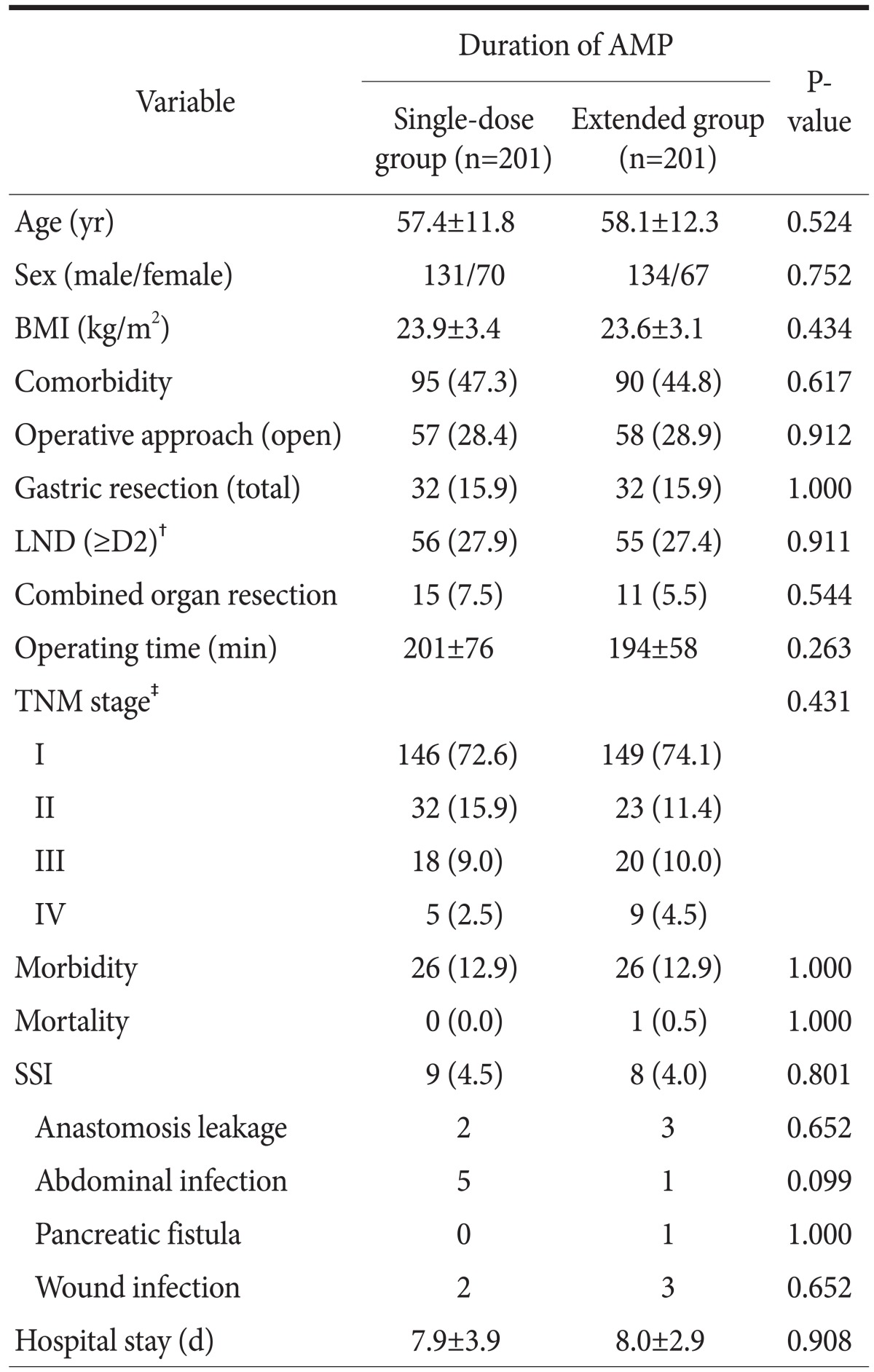
Values are presented as mean±standard deviation, number, or number (%). SSI = surgical site infection; AMP = antimicrobial prophylaxis; BMI = body mass index; LND = lymph node dissection; TNM = Tumor Node Metastasis. *Patients were matched with respect to age, sex, BMI, comorbidity, operative procedures, operating time, and TNM stage using the propensity score matching method. †LND according to the Japanese gastric cancer treatment guideline 2010 (ver. 3). ‡TNM stage was based on the 7th edition of the American Joint Committee on Cancer staging system.
3. Risk factors for surgical site infection
The risk factors for SSIs determined in the overall patient population by univariate and multivariate analyses are summarized in Table 5. In the univariate analysis, age, BMI, comorbidity, operative approach, extent of gastric resection and LND, operating time, combined organ resection, TNM stage, and the duration of AMP administration were compared between the two groups. Male sex, open surgery, total gastrectomy, longer operating time, and combined organ resection were significantly associated with SSIs after gastrectomy. Multivariate analysis of these factors revealed that male gender (odds ratio [OR]: 1.87, 95% confidence interval [CI]: 1.01~3.49), open gastrectomy (OR: 1.73, 95% CI: 1.02~2.95), and operating time (≥180 minutes, OR: 2.46, 95%: CI 1.42~4.26) were independent risk factors for SSIs after gastrectomy for gastric carcinoma.
Table 5.
Univariate and multivariate analyses of risk factors for SSI
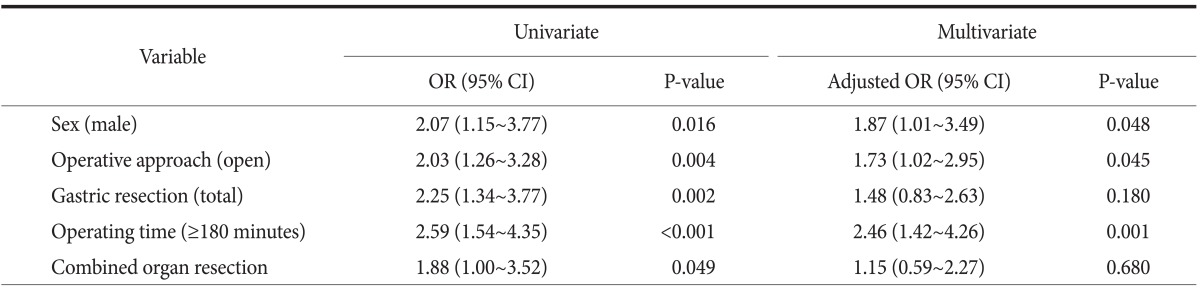
SSI = surgical site infection; OR = odds ratio; CI = confidence interval.
Discussion
There is very limited information available regarding the efficacy of single-dose AMP for gastric cancer surgery. Furthermore, in spite of the recommendation for a single dose AMP in current guidelines, postoperative extended use of AMP is still prevalent after gastric cancer surgery, especially in Korea and Japan.18,19 Despite the inherent limitations of the retrospective study design, the present study demonstrated that single-dose AMP is as effective as extended postoperative use for preventing SSIs after radical gastrectomy for gastric carcinoma. In addition, comparing SSIs in matched patients by the propensity score method also showed that single-dose AMP did not increase the risk of SSI as compared to extended postoperative use. The results of the present study are consistent with the results of previous studies that demonstrated the efficacy of single-dose AMP for gastric cancer surgery.16,17 Collectively, these results suggest that AMP should be administered only during the operation for patients undergoing gastric cancer surgery.
Several guidelines present evidence for- and the practice of AMP in different types of operative procedures.7,8,9,10,11,12 As for gastrointestinal surgeries, a single dose of a first-generation cephalosporin (i.e., cefazolin) is commonly recommended in the guidelines. However, this recommendation is limited by the relatively small number of clinical trials evaluating AMP for gastrointestinal surgeries. Additionally, these trials considered a broad range of gastrointestinal surgeries from simple gastrostomy to complex pancreaticoduodenectomy, making it difficult to apply study findings specifically to gastric cancer surgeries. Furthermore, some regional differences are apparent in the recommendations pertaining to AMP for gastrointestinal surgeries. In the guidelines of the Japanese Association for Infectious Diseases, it is recommended that AMP can be extended for up to 1 to 3 days after gastrointestinal surgery due to the high degree of heterogeneity in surgical practices between Eastern and Western countries, such as a high prevalence of drain use and extensive LND.16 The optimum duration of AMP for gastric cancer surgery remains to be elucidated, and more evidence supporting the efficacy of single-dose AMP for gastric cancer surgery is required.
Single-dose AMP has been shown to be as effective as its extended use for gastric cancer surgery in small retrospective studies.24,25 Imamura et al.3 conducted a phase II trial to investigate the efficacy of single-dose AMP after gastric cancer surgery in 2006. In their study, the SSI rate of the single-dose group was not higher than that of the historic control group. Based on this result, a phase III trial comparing single-dose vs. extended postoperative use of AMP was subsequently conducted and demonstrated the non-inferiority of single-dose AMP (relative risk: 0.51, 95% CI: 0.22~1.16) for preventing SSIs in patients undergoing distal gastrectomy for gastric carcinoma.16 In another study conducted by Mohri et al.,17 501 patients with distal or total gastrectomy were randomized into single- and multiple-dose AMP groups. Results from that study revealed that the incidence of SSI did not differ significantly between the groups (9.5% vs. 8.6%, respectively, P=0.752). Collectively, the results of these studies provide growing evidence supporting single-dose AMP for gastric cancer surgery that are expected to contribute to improvements in quality of care for gastric cancer patients.
Identifying risk factors for SSIs is useful because it may allow for targeted preventative measures. In the present study, male gender, open surgery, and longer operating time were independent risk factors for SSIs after radical gastrectomy. Consistent with these results, previous studies have reported that advanced age, male sex, being overweight, malnutrition, comorbidity, and total gastrectomy were associated with SSIs after gastrectomy.26,27 Therefore, patients with these risk factors should be more carefully monitored for the development of SSIs during the postoperative period. Additionally, strict adherence to infection prevention measures, such as aseptic surgical techniques, wound protection, maintenance of intraoperative normothermia, or avoidance of fluid overload, should be emphasized for these patient groups.28
The question remains as to whether more intensive AMP might be required for invasive procedures, such as open gastrectomy, total gastrectomy, D2 LND, or combined organ resection. Therefore, SSIs were also compared between the single-dose and extended AMP groups according to the types of operative procedures. Subgroup analyses in the present study showed that extended postoperative AMP did not decrease the risk of SSI as compared to the single-dose group in patients undergoing open gastrectomy, total gastrectomy, D2 LND, or combined organ resection. This suggests that single-dose AMP can be safely administered regardless of the extent of the gastric cancer surgery.
Although the patients in this study were assigned to different AMP regimens according to when they were treated, the two study groups exhibited some differences in baseline characteristics including age, operative procedures, and tumor stage. The propensity score matching method is useful in this circumstance in order to reduce the impact of heterogeneity on the estimation of causal treatment effects in the observational data.23 To balance these differences between the two groups, patients were individually matched based on clinicopathological and operative factors, and operative outcomes and SSI were compared between the matched patient groups. The SSI rate was still not significantly different between the matched patients in the single-dose and extended groups (4.5% vs. 4.0%, respectively, P=0.801).
The present study had some limitations, including an analysis inherently limited by possible selection bias and low generalizability due to the retrospective, single center study design. Based on the results of the present study, a multi-institutional clinical trial investigating the efficacy of single-dose AMP for gastric cancer surgery is planned. In addition, there were relatively fewer patients in the single-dose group as compared to the extended group, which may have undermined the comparability of the two groups. Lastly, patients in each group underwent gastrectomy during a different period. Therefore, advances in surgical techniques and instruments, as well as increased experience, may have affected the study results such that the rate of SSIs in the single-dose group was similar to that of the extended group.
In conclusion, the present study demonstrated the efficacy of single-dose AMP for preventing SSIs after radical gastrectomy for gastric carcinoma. These results confirm and extend findings from previous research supporting single-dose AMP for gastric cancer surgery, and highlight that the current practice of prolonged postoperative use of AMP in Asia is not evidence-based. Finally, the efficacy of single-dose AMP needs be investigated further, specifically in regard to gastric cancer surgery.
Acknowledgments
We sincerely appreciate our clinical nurses, So-Young Bae and Bo-Kyung Kim, for their dedication to collecting and organizing the data. We also acknowledge Dr. Mi-Ran Jung and Han-Soo Kim for their invaluable assistance with data analysis and interpretation.
References
- 1.Burke JP. Infection control - a problem for patient safety. N Engl J Med. 2003;348:651–656. doi: 10.1056/NEJMhpr020557. [DOI] [PubMed] [Google Scholar]
- 2.National Nosocomial Infections Surveillance System. National Nosocomial Infections Surveillance (NNIS) System Report, data summary from January 1992 through June 2004, issued October 2004. Am J Infect Control. 2004;32:470–485. doi: 10.1016/S0196655304005425. [DOI] [PubMed] [Google Scholar]
- 3.Imamura H, Furukawa H, Iijima S, Sugihara S, Tsujinaka T, Tsukuma H, et al. Multicenter phase II study of antimicrobial prophylaxis in low-risk patients undergoing distal gastrectomy for gastric cancer. Gastric Cancer. 2006;9:32–35. doi: 10.1007/s10120-005-0354-3. [DOI] [PubMed] [Google Scholar]
- 4.Imai E, Ueda M, Kanao K, Miyaki K, Kubota T, Kitajima M. Surgical site infection surveillance after open gastrectomy and risk factors for surgical site infection. J Infect Chemother. 2005;11:141–145. doi: 10.1007/s10156-005-0379-x. [DOI] [PubMed] [Google Scholar]
- 5.Migita K, Takayama T, Matsumoto S, Wakatsuki K, Enomoto K, Tanaka T, et al. Risk factors for surgical site infections after elective gastrectomy. J Gastrointest Surg. 2012;16:1107–1115. doi: 10.1007/s11605-012-1838-1. [DOI] [PubMed] [Google Scholar]
- 6.Ozalp N, Zülfikaroğlu B, Göçmen E, Acar A, Ekiz I, Koç M, et al. Risk factors for surgical site infection after gastrectomy with D2 lymphadenectomy. Surg Today. 2009;39:1013–1015. doi: 10.1007/s00595-008-3984-3. [DOI] [PubMed] [Google Scholar]
- 7.Bratzler DW, Dellinger EP, Olsen KM, Perl TM, Auwaerter PG, Bolon MK, et al. American Society of Health-System Pharmacists; Infectious Disease Society of America; Surgical Infection Society; Society for Healthcare Epidemiology of America. Clinical practice guidelines for antimicrobial prophylaxis in surgery. Am J Health Syst Pharm. 2013;70:195–283. doi: 10.2146/ajhp120568. [DOI] [PubMed] [Google Scholar]
- 8.Dellinger EP, Gross PA, Barrett TL, Krause PJ, Martone WJ, McGowan JE, Jr, et al. Quality standard for antimicrobial prophylaxis in surgical procedures. Infectious Diseases Society of America. Clin Infect Dis. 1994;18:422–427. doi: 10.1093/clinids/18.3.422. [DOI] [PubMed] [Google Scholar]
- 9.Gilbert DN, Moellering RC, Sande MA, editors. The Sanford Guide to Antimicrobial Therapy. 33rd ed. Hyde Park, VT: Antimicrobial Therapy; 2003. pp. 123–124. [Google Scholar]
- 10.Mangram AJ, Horan TC, Pearson ML, Silver LC, Jarvis WR. Guideline for prevention of surgical site infection, 1999. Hospital Infection Control Practices Advisory Committee. Infect Control Hosp Epidemiol. 1999;20:250–278. doi: 10.1086/501620. [DOI] [PubMed] [Google Scholar]
- 11.Page CP, Bohnen JM, Fletcher JR, McManus AT, Solomkin JS, Wittmann DH. Antimicrobial prophylaxis for surgical wounds. Guidelines for clinical care. Arch Surg. 1993;128:79–88. doi: 10.1001/archsurg.1993.01420130087014. [DOI] [PubMed] [Google Scholar]
- 12.Van Eyk N, van Schalkwyk J Infectious Diseases Committee. Antibiotic prophylaxis in gynaecologic procedures. J Obstet Gynaecol Can. 2012;34:382–391. doi: 10.1016/S1701-2163(16)35222-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Choudhary A, Bechtold ML, Puli SR, Othman MO, Roy PK. Role of prophylactic antibiotics in laparoscopic cholecystectomy: a meta-analysis. J Gastrointest Surg. 2008;12:1847–1853. doi: 10.1007/s11605-008-0681-x. discussion 1853. [DOI] [PubMed] [Google Scholar]
- 14.McDonald M, Grabsch E, Marshall C, Forbes A. Single- versus multiple-dose antimicrobial prophylaxis for major surgery: a systematic review. Aust N Z J Surg. 1998;68:388–396. doi: 10.1111/j.1445-2197.1998.tb04785.x. [DOI] [PubMed] [Google Scholar]
- 15.Song F, Glenny AM. Antimicrobial prophylaxis in colorectal surgery: a systematic review of randomized controlled trials. Br J Surg. 1998;85:1232–1241. doi: 10.1046/j.1365-2168.1998.00883.x. [DOI] [PubMed] [Google Scholar]
- 16.Imamura H, Kurokawa Y, Tsujinaka T, Inoue K, Kimura Y, Iijima S, et al. Intraoperative versus extended antimicrobial prophylaxis after gastric cancer surgery: a phase 3, open-label, randomised controlled, non-inferiority trial. Lancet Infect Dis. 2012;12:381–387. doi: 10.1016/S1473-3099(11)70370-X. [DOI] [PubMed] [Google Scholar]
- 17.Mohri Y, Tonouchi H, Kobayashi M, Nakai K, Kusunoki M Mie Surgical Infection Research Group. Randomized clinical trial of single- versus multiple-dose antimicrobial prophylaxis in gastric cancer surgery. Br J Surg. 2007;94:683–688. doi: 10.1002/bjs.5837. [DOI] [PubMed] [Google Scholar]
- 18.Ahn HS, Yook JH, Park CH, Park YK, Yu W, Lee MS, et al. General perioperative management of gastric cancer patients at high-volume centers. Gastric Cancer. 2011;14:178–182. doi: 10.1007/s10120-011-0012-x. [DOI] [PubMed] [Google Scholar]
- 19.Sumiyama Y, Takesue Y. Current status of prophylactic antibiotic therapy for prevention of postoperative infections after gastrointestinal surgery: a questionnaire covering 3,823 surgeons. Jpn J Chemother. 2004;52:474–485. [Google Scholar]
- 20.Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3) Gastric Cancer. 2011;14:113–123. doi: 10.1007/s10120-011-0042-4. [DOI] [PubMed] [Google Scholar]
- 21.Sobin L, Gospodarowicz M, Wittekind C, editors. International Union Against Cancer (UICC) TNM classification of Malignant Tumours. 7th ed. New York: Wiley; 2009. [Google Scholar]
- 22.Jung MR, Park YK, Seon JW, Kim KY, Cheong O, Ryu SY. Definition and classification of complications of gastrectomy for gastric cancer based on the accordion severity grading system. World J Surg. 2012;36:2400–2411. doi: 10.1007/s00268-012-1693-y. [DOI] [PubMed] [Google Scholar]
- 23.Newgard CD, Hedges JR, Arthur M, Mullins RJ. Advanced statistics: the propensity score--a method for estimating treatment effect in observational research. Acad Emerg Med. 2004;11:953–961. doi: 10.1197/j.aem.2004.02.530. [DOI] [PubMed] [Google Scholar]
- 24.Wang F, Chen XZ, Liu J, Yang K, Zhang B, Chen ZX, et al. Short-term versus long-term administration of single prophylactic antibiotic in elective gastric tumor surgery. Hepatogastroenterology. 2012;59:1784–1788. doi: 10.5754/hge11784. [DOI] [PubMed] [Google Scholar]
- 25.Suehiro T, Hirashita T, Araki S, Matsumata T, Tsutsumi S, Mochiki E, et al. Prolonged antibiotic prophylaxis longer than 24 hours does not decrease surgical site infection after elective gastric and colorectal surgery. Hepatogastroenterology. 2008;55:1636–1639. [PubMed] [Google Scholar]
- 26.Hirao M, Tsujinaka T, Imamura H, Kurokawa Y, Inoue K, Kimura Y, et al. Osaka Gastrointestinal Cancer Chemotherapy Study Group (OGSG) Overweight is a risk factor for surgical site infection following distal gastrectomy for gastric cancer. Gastric Cancer. 2013;16:239–244. doi: 10.1007/s10120-012-0174-1. [DOI] [PubMed] [Google Scholar]
- 27.Utsumi M, Shimizu J, Miyamoto A, Umeshita K, Kobayashi T, Monden M, et al. Age as an independent risk factor for surgical site infections in a large gastrointestinal surgery cohort in Japan. J Hosp Infect. 2010;75:183–187. doi: 10.1016/j.jhin.2010.01.021. [DOI] [PubMed] [Google Scholar]
- 28.Sessler DI, Akça O. Nonpharmacological prevention of surgical wound infections. Clin Infect Dis. 2002;35:1397–1404. doi: 10.1086/344275. [DOI] [PubMed] [Google Scholar]


