Abstract
Purpose
At present, a human epidermal growth factor receptor 2 (HER2)-based concept of tumor biology has been established, and trastuzumab (Herceptin®; Genentech/Roche, San Francisco, CA, USA), a monoclonal humanized antibody directed against HER2, is a pivotal agent for the management of HER2 positive (HER2+) metastatic breast cancer. It is also known that HER2 has a predictive value in gastric cancer; however, its association with the prognosis of this disease remains uncertain. The purpose of this study was to evaluate both the relationship between HER2 overexpression in the tumors of gastric cancer patients, and the prognosis of these patients who have had curative resection.
Materials and Methods
A total of 139 consecutive patients with gastric cancer who underwent surgery at the Kosin University Gospel Hospital between October 2011 and March 2012 were included in this retrospective study. All tumor samples were examined for HER2 expression by immunohistochemistry. A retrospective review of the medical records was conducted to determine the correlation between the presence of HER2 overexpression and clinicopathological factors.
Results
The HER2+ rate was 15.1%. HER2 overexpression was associated with histological grade (P=0.044) and Lauren classification (P=0.036). There was no significant difference in the 2-year overall survival between HER2+ and HER2- patients (P=0.396). Multivariate analysis showed that HER2 was not an independent prognostic factor.
Conclusions
HER2 overexpression in tumors was associated with histological grade and Lauren classification in gastric cancer patients with curative resection. However, HER2 was not an independent prognostic factor for gastric cancer in our study.
Keywords: Stomach neoplasms, Human epidermal growth factor receptor 2, Prognosis
Introduction
Gastric cancer is one of the most common and aggressive carcinomas worldwide with a high mortality, especially in South Korea. The trastuzumab for gastric cancer (ToGA) trial, has assessed human epidermal growth factor receptor 2 (HER2)-targeting agents for treating advanced gastric cancer;1,2 and HER2 evaluation is an important approach for predicting patient response to HER2-targeting agents. Although many studies have previously evaluated HER2 status in gastric cancer, the patient cohorts and scoring criteria have varied, resulting in discrepancies in HER2 positivity that have ranged from 8.2% to 53.4%.3 The HER2 status of South Korean gastric cancer patients currently remains uncertain. Furthermore, the association between HER2 status and prognosis in gastric cancer patients is even more unclear. The purpose of this study was to evaluate the frequency of HER2+ gastric cancer, by applying the standard scoring criteria in patients with curatively resected gastric cancer; and elucidating the relationships between HER2 expression, prognosis, and clinicopathological features.
Materials and Methods
From October 2011 to March 2012, a total of 139 consecutive cases with gastric cancer treated by curative resection without any preoperative therapy, were retrieved from the Department of Surgery at Kosin University College of Medicine. The medical records and surgical specimens of these patients were retrospectively evaluated after obtaining approval from the Investigational Review Board of the Kosin University Gospel Hospital. The disease stage was determined according to the American Joint Committee on Cancer tumor size, lymph nodes affected, and metastases (TNM) classification.4 One patient who experienced gastric cancer recurrence and 4 patients with synchronous multiple primary cancers (e.g., thyroid cancer and colon cancer) were excluded from the study. The clinicopathological characteristics of each patient were retrieved from hospital information systems, retrospectively. Clinicopathological parameters, including age, gender, tumor location, histological classification, pathological TNM stage, neural invasion, lymphatic invasion, and vascular invasion status were retrieved from medical charts or pathology reports. Histological classification was determined according to Lauren's classification and the World Health Organization (WHO) classification.
1. Immunohistochemistry (IHC)
Tumor tissues were fixed in 10% formalin and embedded in paraffin. Immunohistochemical staining was carried out using anti-HER2/neu (Dako, Glostrup, Denmark) as the primary antibody for c-erbB-2. After using a microtome to cut 4 µm-thick tissue sections, the sections were immersed in xylene solution to remove residual paraffin and hydrated in an alcohol series. Sections were boiled for 5 minutes in citrate buffer (pH 6.0) to retrieve antigenicity and left for 30 minutes at room temperature. After exhausting endogenous peroxidase for 10 minutes with H2O2 in methyl alcohol, sections were washed thrice with phosphate-buffered saline (PBS). Under room temperature conditions, sections were blocked for 30 minutes with blocking solution (Histostain™ kit; Zymed Company, San Francisco, CA, USA), and then incubated with anti-HER2/neu (1 : 200; Dako). After rinsing thrice with PBS, sections were incubated with biotinylated anti-mouse immunoglobulin G (1 : 300; Zymed Company), washed, and incubated with avidin-alkaline phosphatase for 7 minutes at 40℃. Next, sections were visualized with red chromogen at 40℃ and counterstained using the Mayer hematoxylin method, before being mounted and observed under light microscopy.
2. Assessment of HER2 expression
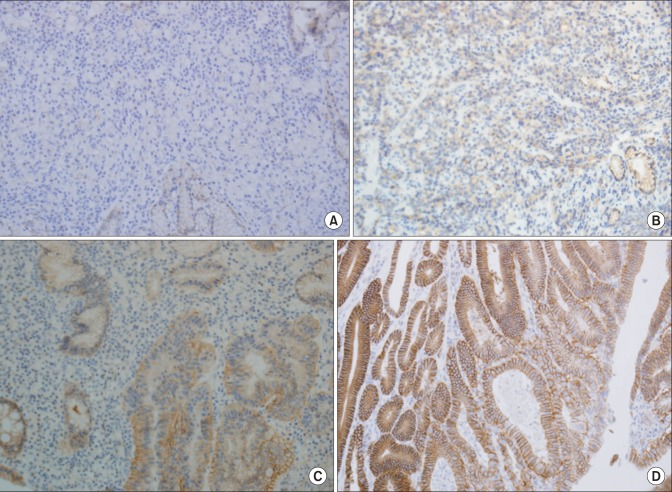
The amended c-erbB-2 scoring system was applied according to location and degree of completion of staining: 0 points: staining of membrane was ≤10%; 1 point: incomplete membrane staining was >10%; 2 points: weak-to-moderate complete staining of the membrane; and 3 points: strong or complete staining of the membrane. An IHC score of 3+, or an IHC score of 2+ and HER2 gene amplification as detected by double-color fluorescent in situ hybridization (FISH), were defined as overexpression of HER2 (Fig. 1).
Fig. 1.
Immunohistochemical analysis of human epidermal growth factor receptor 2 protein expression (×200). (A) Immunostaining shows no staining on tumor cell membrane. (B) Immunostaining shows positive reaction (1+). (C) Immunostaining shows positive reaction (2+). (D) Immunostaining shows positive reaction (3+) with complete or basolateral membraneous staining.
3. Follow-up and statistical analysis
According to the study method, the Institutional Ethics Committee of the Kosin University Gospel Hospital approved the collection of survival information, for the 139 patients. Patients were asked to return for follow-up every 6 months for oncological assessment.
Data analysis was conducted using PASW Statistical Software version 18 (IBM Co., Armonk, NY, USA). The chi-squared test and Kruskal-Wallis test were carried out to compare the distributions of HER2 status and clinicopathological factors. The chi-squared test and the logistic regression test were used to investigate the association between HER2 status and each clinicopathological variable. Survival analysis was carried out using the Kaplan-Meier method and multivariate survival analysis was conducted using COX proportional hazards regression models. All significance tests were two-sided and a P-value of <0.05 was considered statistically significant.
Results
1. Demographic characteristics
Of the 139 cases enrolled in this study, there were 90 men and 49 women, the median age of the patients at diagnosis was 60 years (range 34~85 years), and all patients were Korean. The demographics and tumor-related factors are summarized in Table 1. Seventy-six, 40, and 22 cases had a tumor located in the lower, middle, and upper third of the stomach, respectively. According to the WHO classification standards, 83 patients (59.7%) had well or moderately differentiated tumors and 56 patients (40.3%) had poorly differentiated carcinomas. According to the pathological depth of tumor, 96 patients (69.1%) were pT1a, 17 (12.2%) were pT1b, 0 (0.0%) was pT2, 16 (11.5%) were pT3, and 10 (7.2%) was pT4a. Regarding the tumor stage, 103 (74.1%) were stage I, 19 (13.7%) stage II, and 17 (12.2%) were stage III.
Table 1.
Patient demographics and tumor-related factors in 139 patients with curative resection
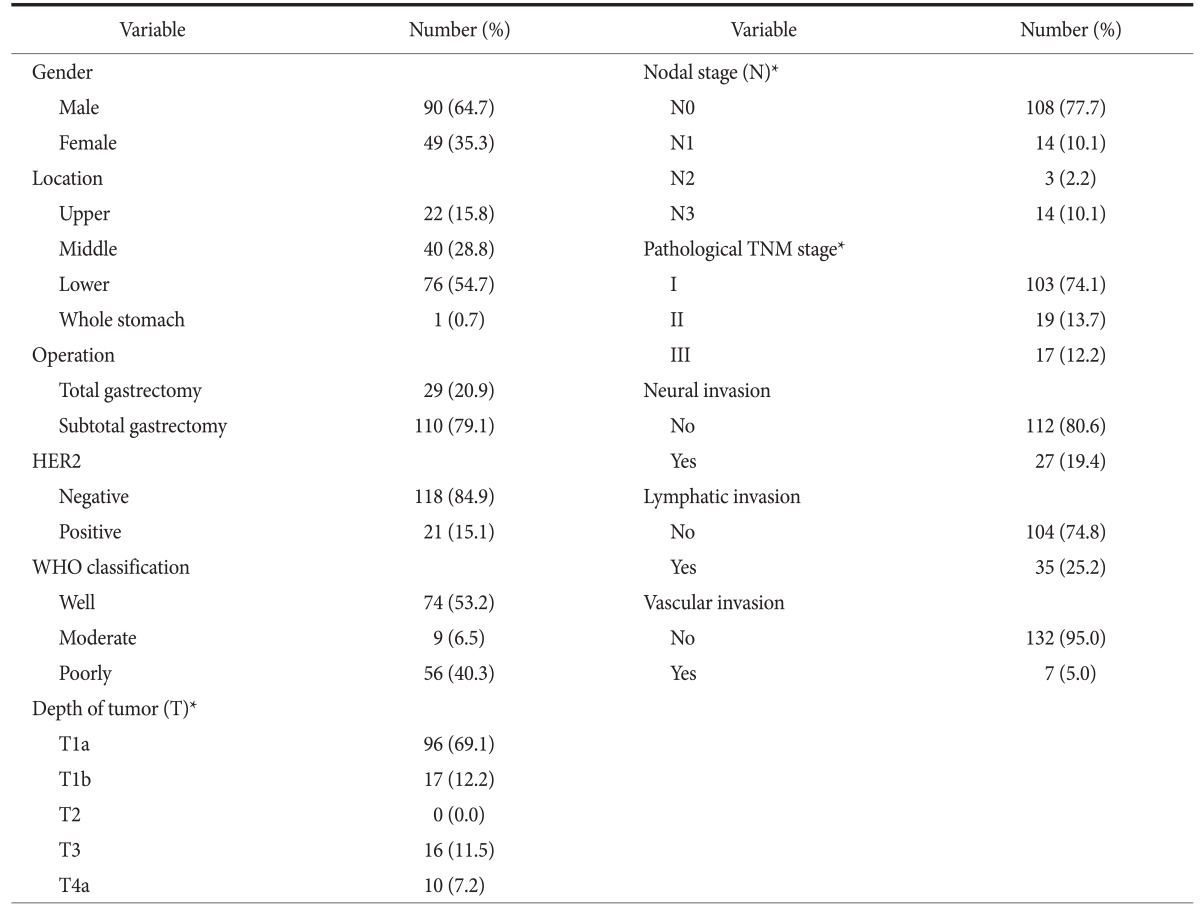
HER2 = human epidermal growth factor receptor 2; WHO = World Health Organization. *Classification according to the standard of TNM stage.
2. Correlation of HER2 status with clinicopathological features
The correlation between HER2 status and patient clinicopathological features is shown in Table 2. HER2 positivity was statistically associated (P=0.044) with histological grade and Lauren classification. HER2 overexpression was more frequently detected in intestinal-type tumors (20.8%) than in the diffuse-, mixed-, or indeterminate-type tumors (9.1%, 6.7%, and 0.0%, respectively). The positivity rate of HER2 was similar between stage I and stage II to III diseases (14.6% and 16.7%, respectively; P=0.789). There were no statistically significant associations with lymph node metastasis, pT stage, or pN stage. The presence of HER2 overexpression in the tumor was not influenced by tumor location or tumor size.
Table 2.
Correlation of HER2 overexpression with demographics and tumor-related factors
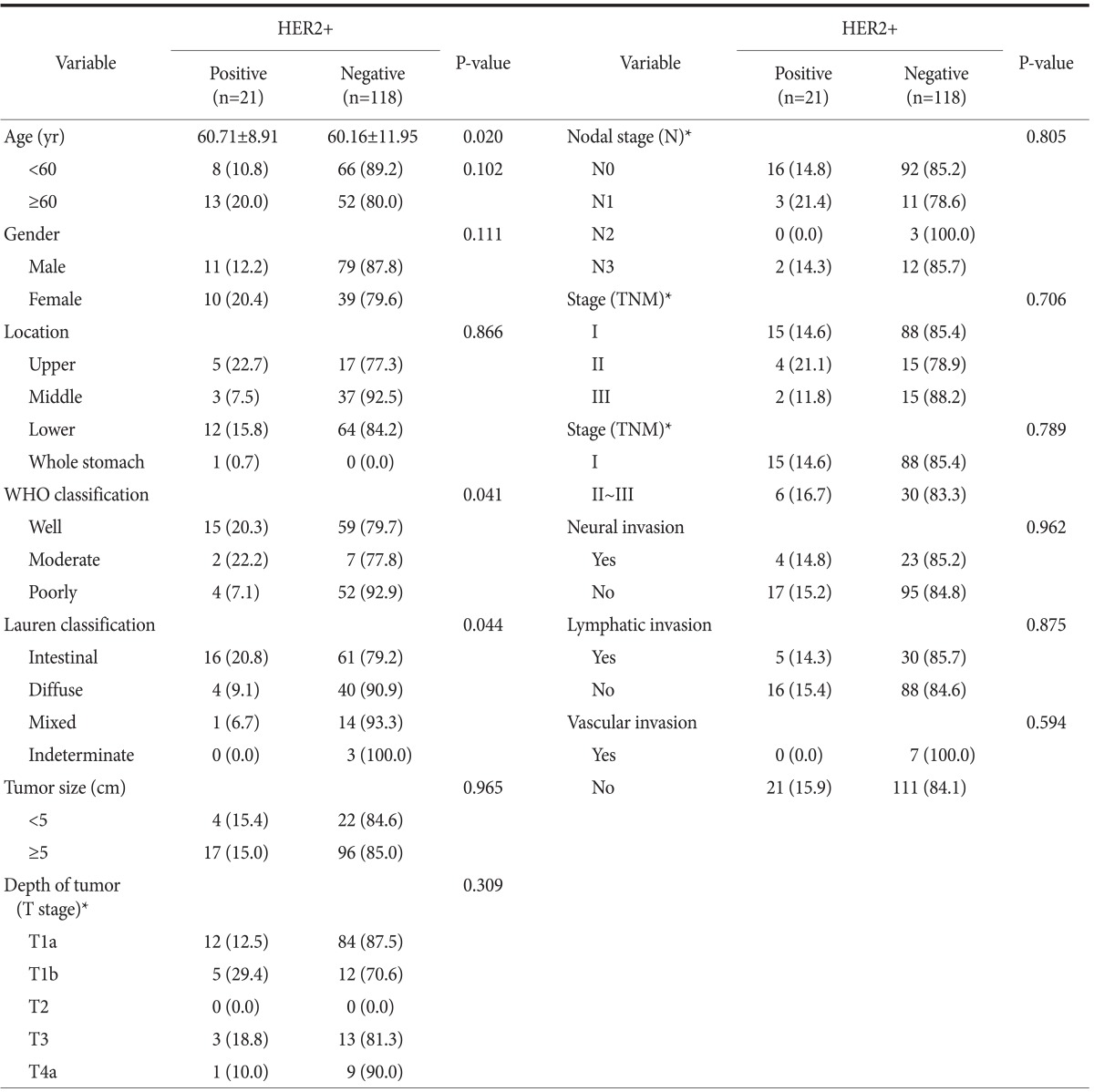
Values are presented as mean±standard deviation or number (%). HER2 = human epidermal growth factor receptor 2. *Classification according to the standard of TNM stage.
3. Correlation of HER2 status with survival
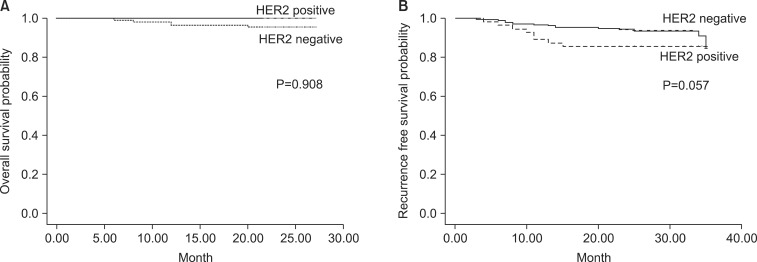
A total of 139 patients were successfully followed for survival analysis. The median follow-up time was 23.5 months (range: 3~27 months), and 4 patients (2.9%) died of cancer, while the remaining 135 patients (97.1%) were still alive at the end of the study period. The 2-year overall survival (OS) rates were 76.2% and 61% in HER2+ and HER2- patients, respectively (P=0.225). The mean survival time was 24.5 months (range: 22~27 months) in HER2+ patients, compared with 23.7 months (range: 6~27 months) in HER2- patients. Tumor location, Lauren classification, stage, grade, gender, HER2 status, lymph node metastasis, lymphatic invasion, and vascular invasion were included in the univariate and multivariate analyses. TNM stage, N stage, lymphatic invasion, and vascular invasion were identified as independent risk factors for OS (P=0.012, 0.008, 0.005, and 0.016, respectively); however, there was no statistically significant association with OS on multivariate regression analysis. HER2 positivity was not found to be an independent prognostic factor for gastric cancer (Fig. 2).
Fig. 2.
Kaplan-Meier survival analysis. (A) Overall survival curves of 139 gastric cancer patients according to human epidermal growth factor receptor 2 (HER2) detection (P=0.908). (B) Recurrence-free survival (P=0.057).
Discussion
The c-erbB-2 gene is a proto-oncogene located on chromosome 17. It expresses HER2/neu protein, one of the epithelial growth factor receptor families, and has tyrosine kinase activity, which mediates cancer proliferation.5 Since the overexpression of HER2 in gastric cancer was first published in 1986,6 many studies have reported the frequency of HER2 positivity in gastric cancer patients from various regions throughout the world. According to a review by Jørgensen and Hersom,3 the positivity rate ranged from 4% to 53% by IHC alone and from 9% to 18% when in situ hybridization (ISH) was included. Additionally, Sheng et al.7 reported a HER2+ rate of 13%, as detected by both IHC and ISH. In the present study, the rate of HER2 positivity was estimated to be 15.1%. Recently, in an open-label international phase 3 randomized controlled trial (ToGA) undertaken in 122 centers in 24 countries, patients with advanced gastric or gastro-esophageal junction carcinomas were studied in order to verify if their tumors showed overexpression of HER2 protein by IHC or gene amplification by FISH.1 In the ToGA study, the HER2+ ratio was higher in tumors at the gastroesophageal junction than that in gastric cancer (33.2% vs. 20.9%). It has been shown that the addition of trastuzumab to chemotherapy improved survival in patients with advanced gastric or gastro-esophageal junction carcinomas compared with chemotherapy alone.1,8,9,10 Of the 21 HER2+ tumors in the present study, 16 were intestinal-type and 4 were diffuse-type according to Lauren's classification. These data are consistent with previous reports that the intestinal-type showed a higher rate of HER2 positivity than the diffuse/mixed-type.8,11,12,13,14,15,16 A strong correlation between HER2 positivity and intestinal histological type is also supported by our finding. HER2 positivity was correlated with moderate- to well-differentiated histology, but not associated with lymph node metastasis, pT, pN, or pM staging.
In breast cancer, genomic amplification and overexpression of the HER2 gene were also associated with poor outcomes, higher mortality, and increased higher recurrence and metastasis.17,18,19 However, the association between HER2 status and prognosis in gastric cancer remains controversial, and a correlation between HER2 amplification or overexpression and favorable survival has only been shown in a few studies.20,21,22 Some studies have indicated that HER2 overexpression is strongly associated with differentiated or intestinal-type gastric cancers, which generally have a better prognosis than undifferentiated or diffuse-type cancers. This may be the major source of controversies surrounding the prognostic value of HER2 overexpression. Most published studies assessing this association have shown a poor prognosis in HER2+ gastric cancers, and although reported rates of HER2 overexpression seem to be very variable, there is a general agreement about a higher HER2 positivity in gastro-esophageal junction cancer (24%~35%) than in gastric carcinoma (9.5%~21%).8,23,24 In our study, no significant difference in the 2-year OS was found between HER2+ and HER2- patients. Moreover, HER2 positivity was not an independent prognostic factor by multivariate analyses.
In summary, we assessed the HER2 status in 139 samples from consecutive surgical cases of gastric cancer. The total HER2+ rate was 15.1%; and although HER2 status was found to be associated with histological grade and Lauren classification, it was not a prognostic marker for gastric cancer based on the results of our study. During the study, the Herceptin® experimental group size (n=1) was smaller than the control group and the due to the short-term follow-up of this group, statistical verification could not be accomplished. To produce successful results, long-term follow-up should be done in the future. We recognize that this study has some limitations. First, the study's follow-up-period was too short, and second, there was only one patient who used Herceptin®. For the above reasons, there were limitations in analyzing the expression of HER2 and additional therapies such as chemotherapies and Herceptin®. To overcome these limitations, we think that a further randomized control study design in a large population is necessary.
In summary, we assessed the HER2 status in 139 samples of consecutive surgical cases of gastric cancer. The total HER2+ rate was 15.1%. HER2 overexpression was correlated with histological grade and Lauren classification; however, HER2 was not a prognostic marker for gastric cancer based on the results of our study.
References
- 1.Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, et al. ToGA Trial Investigators. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastrooesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–697. doi: 10.1016/S0140-6736(10)61121-X. [DOI] [PubMed] [Google Scholar]
- 2.National Cancer Institute. SEER Cancer Statistics Review, 1975-2006 [Internet] Bethesda (MD): National Cancer Institute; 2009. [cited 2010 Mar 5]. Available from: http://www.joplink.net/prev/201003/ref/16-001.html. [Google Scholar]
- 3.Jørgensen JT, Hersom M. HER2 as a Prognostic marker in gastric cancer: a systematic analysis of data from the literature. J Cancer. 2012;3:137–144. doi: 10.7150/jca.4090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471–1474. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 5.Schechter AL, Stern DF, Vaidyanathan L, Decker SJ, Drebin JA, Greene MI, et al. The neu oncogene: an erb-B-related gene encoding a 185,000-Mr tumour antigen. Nature. 1984;312:513–516. doi: 10.1038/312513a0. [DOI] [PubMed] [Google Scholar]
- 6.Sakai K, Mori S, Kawamoto T, Taniguchi S, Kobori O, Morioka Y, et al. Expression of epidermal growth factor receptors on normal human gastric epithelia and gastric carcinomas. J Natl Cancer Inst. 1986;77:1047–1052. [PubMed] [Google Scholar]
- 7.Sheng WQ, Huang D, Ying JM, Lu N, Wu HM, Liu YH, et al. HER2 status in gastric cancers: a retrospective analysis from four Chinese representative clinical centers and assessment of its prognostic significance. Ann Oncol. 2013;24:2360–2364. doi: 10.1093/annonc/mdt232. [DOI] [PubMed] [Google Scholar]
- 8.Moelans CB, Milne AN, Morsink FH, Offerhaus GJ, van Diest PJ. Low frequency of HER2 amplification and overexpression in early onset gastric cancer. Cell Oncol (Dordr) 2011;34:89–95. doi: 10.1007/s13402-011-0021-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levine AJ. p53, the cellular gatekeeper for growth and division. Cell. 1997;88:323–331. doi: 10.1016/s0092-8674(00)81871-1. [DOI] [PubMed] [Google Scholar]
- 10.Oren M. Lonely no more: p53 finds its kin in a tumor suppressor haven. Cell. 1997;90:829–832. doi: 10.1016/s0092-8674(00)80347-5. [DOI] [PubMed] [Google Scholar]
- 11.Kim MA, Jung EJ, Lee HS, Lee HE, Jeon YK, Yang HK, et al. Evaluation of HER-2 gene status in gastric carcinoma using immunohistochemistry, fluorescence in situ hybridization, and real-time quantitative polymerase chain reaction. Hum Pathol. 2007;38:1386–1393. doi: 10.1016/j.humpath.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 12.Marx AH, Tharun L, Muth J, Dancau AM, Simon R, Yekebas E, et al. HER-2 amplification is highly homogenous in gastric cancer. Hum Pathol. 2009;40:769–777. doi: 10.1016/j.humpath.2008.11.014. [DOI] [PubMed] [Google Scholar]
- 13.Kunz PL, Mojtahed A, Fisher GA, Ford JM, Chang DT, Balise RR, et al. HER2 expression in gastric and gastroesophageal junction adenocarcinoma in a US population: clinicopathologic analysis with proposed approach to HER2 assessment. Appl Immunohistochem Mol Morphol. 2012;20:13–24. doi: 10.1097/PAI.0b013e31821c821c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yan B, Yau EX, Bte Omar SS, Ong CW, Pang B, Yeoh KG, et al. A study of HER2 gene amplification and protein expression in gastric cancer. J Clin Pathol. 2010;63:839–842. doi: 10.1136/jcp.2010.076570. [DOI] [PubMed] [Google Scholar]
- 15.Im SA, Kim JW, Kim JS, Kim MA, Jordan B, Pickl M, et al. Clinicopathologic characteristics of patients with stage III/IV (M(0)) advanced gastric cancer, according to HER2 status assessed by immunohistochemistry and fluorescence in situ hybridization. Diagn Mol Pathol. 2011;20:94–100. doi: 10.1097/PDM.0b013e3181fc02b7. [DOI] [PubMed] [Google Scholar]
- 16.Boers JE, Meeuwissen H, Methorst N. HER2 status in gastrooesophageal adenocarcinomas assessed by two rabbit monoclonal antibodies (SP3 and 4B5) and two in situ hybridization methods (FISH and SISH) Histopathology. 2011;58:383–394. doi: 10.1111/j.1365-2559.2011.03760.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Toikkanen S, Helin H, Isola J, Joensuu H. Prognostic significance of HER-2 oncoprotein expression in breast cancer: a 30-year follow-up. J Clin Oncol. 1992;10:1044–1048. doi: 10.1200/JCO.1992.10.7.1044. [DOI] [PubMed] [Google Scholar]
- 18.Seshadri R, Horsfall DJ, Firgaira F, McCaul K, Setlur V, Chalmers AH, et al. The South Australian Breast Cancer Study Group. The relative prognostic significance of total cathepsin D and HER-2/neu oncogene amplification in breast cancer. Int J Cancer. 1994;56:61–65. doi: 10.1002/ijc.2910560112. [DOI] [PubMed] [Google Scholar]
- 19.Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 20.Nakajima M, Sawada H, Yamada Y, Watanabe A, Tatsumi M, Yamashita J, et al. The prognostic significance of amplification and overexpression of c-met and c-erb B-2 in human gastric carcinomas. Cancer. 1999;85:1894–1902. doi: 10.1002/(sici)1097-0142(19990501)85:9<1894::aid-cncr3>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 21.Ross JS, McKenna BJ. The HER-2/neu oncogene in tumors of the gastrointestinal tract. Cancer Invest. 2001;19:554–568. doi: 10.1081/cnv-100103852. [DOI] [PubMed] [Google Scholar]
- 22.Latif Z, Watters AD, Bartlett JM, Underwood MA, Aitchison M. Gene amplification and overexpression of HER2 in renal cell carcinoma. BJU Int. 2002;89:5–9. [PubMed] [Google Scholar]
- 23.Tanner M, Hollmén M, Junttila TT, Kapanen AI, Tommola S, Soini Y, et al. Amplification of HER-2 in gastric carcinoma: association with Topoisomerase IIalpha gene amplification, intestinal type, poor prognosis and sensitivity to trastuzumab. Ann Oncol. 2005;16:273–278. doi: 10.1093/annonc/mdi064. [DOI] [PubMed] [Google Scholar]
- 24.Hede K. Gastric cancer: trastuzumab trial results spur search for other targets. J Natl Cancer Inst. 2009;101:1306–1307. doi: 10.1093/jnci/djp341. [DOI] [PubMed] [Google Scholar]