Abstract
Cancer immunotherapy induces a variety of auto-inflammatory responses, including those against the thyroid gland, which can be exploited to predict clinical outcomes. Considering the paucity of information about thyroid autoimmunity in patients receiving cancer vaccines, we designed the present study to assess the development of thyroglobulin antibodies (TgAbs) in patients treated with GVAX and/or ipilimumab and correlated seroconversion with survival.
Using both in house and commercial ELISA assays, we measured TgAbs in patients with pancreatic (No. = 53), prostate (No.= 35), or colon (No.= 8) cancer, before and after treatment with GVAX only (No= 34), GVAX plus ipilimumab (No = 42), or ipilimumab (No =20), and correlated their levels with patient’s survival, disease status, and T cell surface markers. Antibodies to thyroperoxidase, myeloperoxidase, proteinase 3, insulin and actin were also measured.
TgAbs specifically developed after GVAX, independent of the underlying cancer (81% in prostate, 75% colon cancer, and 76% pancreatic cancer) and co-administration of ipilimumab (75% in GVAX only and 78% in GVAX plus ipilimumab). This TgAbs seroconversion could be detected mainly by the in house assay, suggesting that the thyroglobulin epitopes recognized by the antibodies induced by GVAX are different from the epitopes seen in the classic form of Hashimoto thyroiditis. Notably, TgAbs seroconversion was associated with significantly prolonged survival (p=0.01 for pancreas and p= 0.005 for prostate cancer).
In conclusion, GVAX immunotherapy induces the appearance of TgAbs that recognize a unique antigenic repertoire and associate with prolonged survival.
Keywords: Thyroglobulin antibodies, immunotherapy, GVAX, CTLA-4
INTRODUCTION
Cancer immunotherapy has the ultimate goal of boosting the patient’s own immune responses against cancer antigens, as to control and eliminate neoplastic cells as well as prevent recurrences1. One of the approaches used to achieve this goal is to administer allogeneic tumor cells to provide a source of multiple tumor antigens that have been irradiated (to prevent replication) and transfected to express granulocyte-macrophage colony-stimulating factor (GM-CSF, to recruit and activate tumor specific dendritic cells), a cancer vaccine known as GVAX2. Another approach is to block cytotoxic T lymphocyte-associated antigen 4 (CTLA-4), a receptor expressed mainly on T lymphocytes upon activation that ultimately dampens T cell responses. When blocked, CTLA-4 induces a generalized activation and unbridled proliferation of T cells, including not only the clones responding to cancer cells but also those recognizing self-antigens. Ipilimumab (Yervoy®, Bristol-Myers Squibb) is a humanized monoclonal antibody that blocks CTLA-4 by preventing the binding to its natural ligands. Ipilimumab was approved by the FDA in March 2011 for the treatment of advanced melanoma, and is now being tested in several other types of cancer3. The two approaches of GVAX and ipilimumab have been recently combined for the treatment of patients with prostate4 or pancreatic cancer5. In both studies, the combination was safe, tolerable, and associated with improved clinical outcomes and survival.
Cancer immunotherapy is associated with a variety of auto-inflammatory responses, collectively referred to as “immune-related adverse events” (irAEs)6. The most common irAEs are those affecting skin, colon, liver, and pituitary gland7, but many other organs and systems can be involved. For example, the development of autoimmune thyroiditis in this patient population is increasingly reported, with an overall prevalence around 3%. This form of secondary thyroiditis, recently reviewed by Torino et al. 8, can manifest as hypothyroidism or hyperthyroidism, and occur in isolation or associated with other irAEs.
The development of irAEs, being a reflection of the patient’s immune activation, has been used as a tool to predict more favorable clinical outcomes9. For example, in a study of metastatic melanoma patients receiving ipilimumab and a vaccine based on the melanoma associated antigen gp100, irAEs development was associated with durable and favorable clinical responses10. Although these findings await confirmation, they support the notion that autoimmune manifestations are intimately related to anti-tumor activity.
Responses to cancer immunotherapy can be monitored in the clinical laboratory using assays that analyze T cell and/or B cell (antibody) responses. Examples of T cell assays are ELISPOT for mesothelin in pancreatic cancer patients receiving GVAX11, and tetramer staining for the 209–217 peptide from the melanoma gp100 antigen in the context of HLA-A*020112. Antibody responses have been described for melanoma antigens such as NY-ESO-113, prostate antigens like PSMA, and more broadly expressed antigens such as filamin B4. No report to date has analyzed thyroid autoimmunity in cancer patients receiving immunotherapy. Considering that thyroid autoimmunity is the most common autoimmune disease in the general population14, has never been evaluated in this cancer treatment setting, and can be diagnosed relatively well with widely used antibody tests, we designed the present study to assess the prevalence and significance of humoral responses to thyroglobulin in cohorts of cancer patients treated with either GVAX only, GVAX plus ipilimumab, or ipilimumab only.
MATERIALS & METHODS
Patients
The study analyzed 96 patients (82 from the original cohort and 14 from a validation cohort) with pancreatic, prostate, or colon cancer who received GVAX only, GVAX plus ipilimumab, or ipilimumab only (Table 1). Patients were included in the study only if they had sera available from both before and after treatment time points.
Table 1.
Distribution of the 96 patients used in the study according to the type of cohort (original versus validation), adenocarcinoma (colon, pancreas, prostate), and immunotherapy treatment (Tx: GVAX only, GVAX plus ipilimumab, or ipilimumab only). The clinical setting, trial number, and presence or absence of thyroglobulin antibodies at baseline are also shown.
| Type of cohort |
Type of adenocarcinoma |
Type of immunotherapy |
Clinical setting |
Clinical trial number |
Total serum samples |
Total patients |
Patients without Tg Abs at baseline |
Patients who developed TgAbs after Tx |
|---|---|---|---|---|---|---|---|---|
| original | Colon | GVAX only | Metastatic | NCT00656123 | 16 | 8 | 4 | 3 |
| original | Pancreatic | GVAX only | Resected | NCT00389610 | 71 | 12 | 10 | 10 |
| validation | Pancreatic | GVAX only | Resected | NCT00389610 | 48 | 14 | 11 | 6 |
| original | aPancreatic | GVAX + Ipi | Metastatic | NCT00836407 | 74 | 15 | 12 | 9 |
| original | bProstate | GVAX + Ipi | Metastatic | NCT01510288 | 126 | 27 | 26 | 21 |
| original | aPancreatic | Ipi only | Metastatic | NCT00836407 | 47 | 12 | 10 | 0 |
| original | cProstate | Ipi only* | Metastatic | NCT00323882 | 52 | 8 | 8 | 0 |
|
| ||||||||
| 434 | 96 | 81 | 46 | |||||
Patients published by
Le DT et al. in J Immunotherapy 2013;
van den Eertwegh AJ et al. In Lancet Oncology 2012;
Slovin SF et al in Ann Oncol 2013.
Patients who also received radiotherapy.
The GVAX only group included 8 patients with metastatic colon adenocarcinoma, and 26 patients with resected pancreatic adenocarcinoma (12 from the original and 14 from the validation cohort) who had no radiographic evidence of pancreatic cancer disease recurrence (Table 1). The colon GVAX vaccine consisted of two allogeneic colon cancer cell lines (SW620 and SW837, irradiated and mixed in a 1:1 ratio) that were then mixed in a 5:2 ratio with irradiated K562/GM-CSF cells, a chronic myeloid leukemia cell line engineered to secrete GM-CSF. Patients received intravenous low dose cyclophosphamide one day prior to intradermal administration of the colon GVAX vaccine for a total of 4 cycles, 28 days apart. The pancreas GVAX vaccine has been previously described11 and was administered intradermally monthly for three priming vaccinations followed by boost vaccinations every six months.
The GVAX plus ipilimumab group consisted of 15 patients with previously treated metastatic pancreatic adenocarcinoma, and 27 patients with castration resistant, metastatic prostate cancer (Table 1). The pancreas cancer patients received GVAX given concurrently with ipilimumab (10 mg/kg). The treatments were administered every 3 weeks for 4 doses, then regularly every 12 weeks for the duration of the study5. The prostate cancer patients received 13 doses of GVAX prostate (one every two weeks) plus 6 doses of ipilimumab (one every month) at a dose comprised between 0.3 mg/kg and 5 mg/kg4. Prostate cancer patients did not receive any boosting treatment dose after the initial 6 months of treatment.
The ipilimumab only group comprised 12 patients with previously treated metastatic pancreatic adenocarcinoma, and 8 patients with castration resistant metastatic prostate cancer (Table 1). The pancreas cancer patients received ipilimumab (10 mg/kg) every 3 weeks for 4 doses, then every 12 weeks for the duration of the study5. The prostate cancer patients received 4 doses of ipilimumab (at a dose between 3 and 10 mg/kg) in combination with radiotherapy15.
Serum samples (No. = 434) were collected at baseline (No. = 96) and at several time points after initiation of immunotherapy (No. = 338), then aliquoted and stored at −80 °C until use.
Detection of serum thyroglobulin antibodies
Thyroglobulin antibodies (TgAbs) were measured using both in house and commercial assays to allow a more comprehensive representation of the epitope repertoire that, given the large size of thyroglobulin, can be altered in commercial preparations whereas is more completely represented in fresh thyroglobulin purifications.
The in house ELISA assay was performed in all 434 serum samples, as previously described16. Briefly, we purified thyroglobulin from a human thyroid gland collected at autopsy using gel filtration chromatography with Sephacryl S-300. After verification of purity and integrity by gel electrophoresis, thyroglobulin was diluted in carbonate/bicarbonate buffer pH 9.6 at a concentration of 2 μg/ml, and then used to coat Immunolon-2 ELISA plates (Thermo Scientific, Rochester, NY), at an amount of 100 ng per well. After blockade of non-specific antibody binding with 5% normal goat serum and proper washing, patient sera were diluted 1:100 in phosphate buffered saline and incubated overnight at 4 °C. Antibody binding was detected by the addition of a goat anti-human IgG conjugated to alkaline phosphatase (Jackson ImmunoResearch Laboratories, PA) for 1 hour at room temperature. Color development was induced by the addition of the pNPP substrate (BioRad) and read at 405 nm in a Emax microplate reader (Molecular Devices, Sunnyvale, CA). Each plate included a standard curve of serial dilutions of a pool of sera with known high WHO units of TgAbs. Using this assay in a cohort of 47 healthy volunteers, we established a value of 145 WHO U/ml as the cutoff above which sera were considered positive for TgAbs. To assess the linearity and detection limit of the in house assay, serum samples from baseline and post treatment time points were tested at 1:25, 1:50, 1:100, and 1:200 dilutions, using thyroglobulin concentrations of 2, 0.2 and 0.02 μg/ml (Supplementary Fig 1).
Two commercial assays, ELISA QUANTA Lite (INOVA Diagnostics, Inc., San Diego, CA) and RIA KRONUS thyroglobulin antibodies (Kronus Inc., Star, ID), were used in a subset of 23 of the 96 patients (24%) to ensure reproducibility of the findings. The 23 patients were randomly selected to represent different cancer types and treatment combinations: resected pancreatic cancer treated with GVAX only (No. = 12), metastatic colon cancer treated with GVAX only (No. = 8), and metastatic pancreatic cancer treated with GVAX plus ipilimumab (No. = 3). Patient sera were analyzed both at baseline (No. = 23) and after treatment (No. = 73) for a total of 96 sera. Commercial assays, performed according to the manufacturer’s recommendations, considering positive values >100 WHO units per ml in the INOVA kit, and >1 unit in the Kronus kit.
To assess the presence of non-immunoglobulin substances in the serum that could interfere with the TgAbs assays, we purified immunoglobulins from 3 patients treated with GVAX by affinity chromatography using protein G columns (Protein G cartridges from Pierce). The purified immunoglobulins were then tested for TgAbs in both the in house and commercial ELISA assays, and results compared with those obtained using whole serum.
Detection of serum antibodies to thyroperoxidase, insulin, myeloperoxidase proteinase 3, actin, and measurement of serum TSH
To evaluate the presence of pre-existing but clinically silent autoimmune thyroid condition, we measured thyroperoxidase antibodies in 58 of the 96 (60%) patients, both at baseline and at several time points after cancer immunotherapy (No = 239 sera), using a commercial ELISA kit (QUANTA Lite from INOVA Diagnostics, Inc., San Diego, CA).
To assess the specificity of autoimmune responses toward the thyroid gland, we measured antibodies against five additional autoantigens, using both commerical and in house assays. Commercial assays included antibodies to insulin (No. = 71 sera, Kronus RIA), myeloperoxidase (No. = 311 sera, QUANTA Lite MPO from INOVA), proteinase 3 (No. = 311 sera, QUANTA Lite PR3 from INOVA), and actin (No.= 82, QUANTA Lite actin IgG from INOVA). In house assays included antibodies to insulin (No. = 71), myeloperoxidase (No.= 88), and actin (No.= 82). Proteins were purchased from Sigma (human insulin 91077C, human myeloperoxidase M6908, and bovine actin A3653), reconsituted in carbonate/bicarbonate buffer and used to coat Immunolon-2 ELISA plates at an amount of 500 ng per well. ELISA was then performed and interpreted as indicated above for the in house thyroglobulin assay.
Serum TSH, the best marker to evaluate thyroid function, was assessed using a automated analyzer and a third generation immuno-chemiluminescent assay, with a normal reference range between 0.45 and 5 mIU/L. TSH was measured in 196 serum samples corresponding to patients treated with GVAX plus ipilimumab or with ipilimumab only. It was not measured in patients treated with GVAX only because the evaluation of thyroid function is not included in current clinical protocols.
Detection of thyroglobulin RNA and protein in pancreatic GVAX cell lines
To determine whether GVAX cancer cell lines ectopically expressed thyroglobulin, we selected, based on availability, the pancreatic cancer cell lines PANC 10.05 and PANC 6.03, and evaluated them by short tandem repeat analysis at the Johns Hopkins Fragment Analysis Facility. These cell lines were found to match Leibniz Institute DSMZ German Collection of Microorganisms and Cell Cultures cell line CRL-2547 and CRL-2550 respectively (EV 1.13 and EV 1.06). The cell lines were then grown in the appropriate culture medium, transfected with GM-CSF or left untreated17, and then used to extract total RNA by TRIzol® (Life Technologies, Grand Island, NY). Total RNA was reverse transcribed using oligo-d(T) primers and iScript cDNA Synthesis Kit (BioRad). The resulting cDNA was amplified by PCR using thyroglobulin primers 5′-GAGCCTACCTCTTCTGGCA-3′ (forward) and 5′-GAGGTCCTCATTCCTCAGCC-3′ (reverse), which yielded an amplicon of 320-bp corresponding to region 2590–2270 on thyroglobulin cDNA. Experimental controls included RNA extracted from human thyroid (positive control) and HEK 293 cells (negative control), as well as amplification of a housekeeping gene (GADPH forward 5′-GAGCCACATCGCTCAGACAC-3′ and reverse 5′-CATGTAGTTGAGGTCAATGAAGG-3′, yielding a 150-bp DNA product), and a RNA sample not incubated with reverse transcriptase. A HEK293 cell line served as negative control, and human thyroid gland as positive control.
We also purified proteins from the same pancreatic cancer cell lines indicated above, separated them by SDS-PAGE on 4-12% gradient gels, and then transferred them to nitrocellulose membranes. After blockade of aspecific binding in 5% nonfat dry milk, membranes were incubated overnight with a commercial antibody specific for thyroglobulin (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, sc-7836 diluted 1:100). Antibody binding was detected by the addition of a secondary anti-human IgG antibody conjugated to horseradish peroxidase, and the signal revealed by a chemiluminescent substrate (Novex® ECL Reagent Kit, Invitrogen). A non-transfected COS-7 cell line served as negative control, and purified human thyroglobulin as positive control.
Subset profiling of CD4 and CD8 T cells
The prostate cancer patients treated with GVAX plus ipilimumab have been previously characterized for the expression of peripheral blood T cell markers, identifying the CD4+/CTLA-4+ subpopulation as the strongest predictor of survival18. Using the same dataset we assessed by cluster analysis whether serum thyroglobulin antibodies associated with T cell parameters, using the statistical tools described by Santegoets et al18.
Statistical analysis
The study analyzed two main outcomes: the levels of TgAbs among time points, types of immunotherapy, and types of cancer, and the association between TgAbs development and overall survival.
Differences in the levels of TgAbs were assessed by longitudinal data regression analysis with generalized estimating equation. Here, repeated measures for each patient are clustered and the status or changing value of the covariates at each time point was accounted for.
Association between TgAbs development and survival (expressed in months after the first dose of immunotherapy) was assessed separately for pancreatic cancer patients receiving GVAX only (No.= 26), pancreatic cancer patients treated with GVAX plus ipilimumab (No.= 15), and prostate cancer patients treated with GVAX plus ipilimumab (No.= 27). The survival analysis was also performed in these three groups after exclusion of the 9 patients who had positive TgAbs at baseline (Table 1). Survival analysis was not done in the two patient groups that received ipilimumab only since they did not develop TgAbs, and in the colon cancer patients receiving GVAX only because the sample size was too small and the majority of them (5 of 8, 62.5%) were still alive. Differences in survival between patients who developed TgAbs and those who did not were assessed by log-rank testing.
Ancillary outcomes were the comparisons of antiboby to myeloperoxidase, insulin and actin measured by commercial and in house assays.
P values ≤ 0.05 were considered statistically significant. All analyses were performed using Stata 13 (Stata Corporation, College Station, TX).
RESULTS
TgAbs develop after GVAX immunotherapy, either when administered alone or in combination with ipilimumab
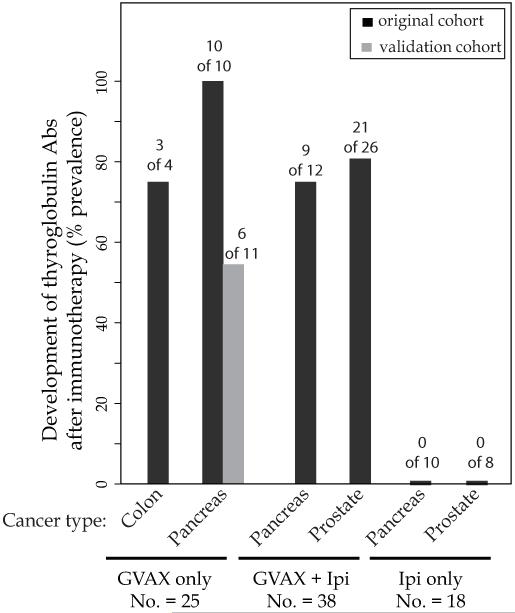
TgAbs developed after cancer immunotherapy in the majority of patients who did not have them at baseline (46 of 81, 57%, Table 1). This seroconversion depended upon the use of GVAX. TgAbs, in fact, appeared in 3 of 4 (75%) GVAX colon cancer patients, 16 of 21 (76%) GVAX pancreas cancer patients (10 of 10 in the original cohort and 6 of 11 in the validation cohort), 9 of 12 (75%) GVAX plus ipilimumab pancreas cancer patients, and 21 of 26 (81%) GVAX plus ipilimumab prostate cancer patients (Figure 1). On the contrary, none of the patients receiving ipilimumab only, either with pancreatic cancer (0 of 10) or prostate cancer (0 of 8), developed TgAbs (Figure 1).
Figure 1.
Development of thyroglobulin antibodies (% prevalence) in 81 (original and validation cohort) cancer patients after treatment with GVAX only (No. = 25), GVAX plus ipilimumab (No. = 38), or ipilimumab only (No. = 18).
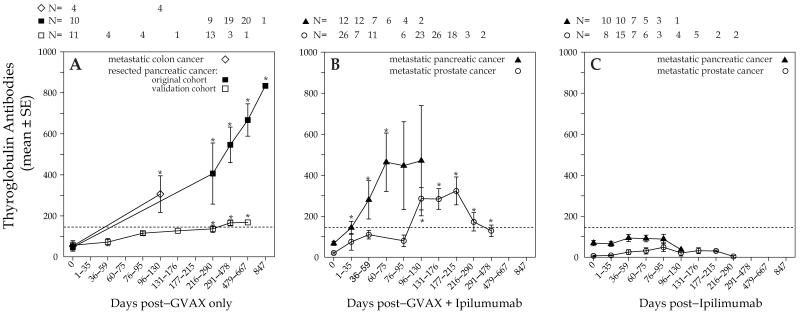
Circulating TgAbs became detectable approximately one month after the first GVAX administration and remained elevated thereafter, although at different levels related to the different protocol regimens. In particular, TgAbs increased progressively in patients with resected pancreatic cancer receiving GVAX only, becoming significantly higher than baseline at 216-290 days, the mean WHO units was 406 (p=0.04 versus baseline) (Figure 2A, closed squares). Thereafter, the mean WHO units for TgAbs were 546 at 291-478 days (p< 0.001 versus baseline); 667 at 479-667 days (p< 0.001), and 834 at 847 days (p< 0.001, Figure 2A, closed squares). A similar trend was observed in patients with metastatic colon cancer: mean WHO units of 306 at 96-130 days (p= 0.03 versus baseline, Figure 2A, open diamonds). In the validation cohort TgAbs also increased significantly over time, reaching the treshold for positivity at 216-290 days (Figure 2A open squares) and further increasing at 291-478 days, with mean values of 166 (p=0.004 versus baseline) and 168 (p=0.001 versus baseline), respectively.
Figure 2.
Levels of thyroglobulin antibodies over time in 81 cancer patients after treatment with either GVAX only (panel A), GVAX plus ipilimumab (panel B), or ipilimumab only (panel C). Panel B, prostate cancer patients did not receive any boosting dose of GVAX + ipilimumab after the initial 180 days. The symbol * indicates p<0.05 over baseline.
In the GVAX plus ipilimumab combination treatment, patients with metastatic pancreatic cancer developed TgAbs that began to rise above baseline on days 1-35 and reached a zenith of 460 WHO units at 60-75 days (Figure 2B, closed triangles). Similarly, in patients with metastatic prostate cancer TgAbs rose progressively, reaching a 15-fold increase (323 versus 21 mean WHO units, Figure 2B, open circles) at days 177-215 after immunotherapy initiation. TgAbs then decreased, still remaining significantly higher than baseline (Figure 2B, open circles). This late decrease is likely explained by the fact that these patients did not receive additional GVAX boosting after the initial 6 months of treatment, but were monitored for disease progression and survival.
TgAbs remained negative at all time points in patients treated with ipilimumab only (Figure 2C).
Baseline serum TSH levels did not differ among the four treatment groups where this analyte was assessed (Supplementary Table 1), and did not change significantly after immunotherapy (Supplementary Table 1).
Overall, our results show that TgAbs develop after immunotherapy only when GVAX is administered, independently of the underlying cancer type.
TgAbs development or enhancement is associated with improved overall survival in patients receiving GVAX only or GVAX plus ipilimumab
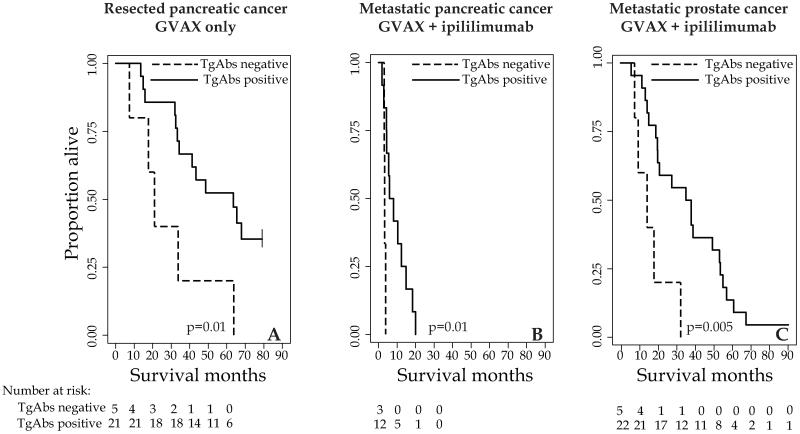
We next analyzed the association between TgAbs development and survival (months after the first treatment) in patients receiving either GVAX only or GVAX plus ipilimumab. In both pancreatic and prostate cancer, patients who developed TgAbs at any of the time points (Figure 3, solid lines) lived longer than those who did not (Figure 3, dashed lines). In particular, the median overall survival in patients with or without TgAbs seroconversion was 63 versus 21 months in the resected pancreatic cancer group receiving GVAX only (Figure 3A, p= 0.01), 7 versus 3.3 months in the metastatic pancreatic cancer group receiving GVAX plus ipilimumab (Figure 3B, p= 0.01), and 41 versus 15 months in the in the metastatic prostate cancer group receiving GVAX plus ipilimumab (Figure 3C, p= 0.005).
Figure 3.
Survival curves comparing presence (solid line) or absence (dashed line) of thyroglobulin antibody seroconversion in patients with resected pancreatic cancer receiving GVAX only (panel A), metastatic pancreatic cancer patients receiving GVAX plus ipilimumab (panel B), or metastatic prostate cancer patients receiving GVAX plus ipilimumab.
The symbol | indicates censoring
Exclusion of the patients with TgAbs positive at baseline did not change the overall findings. The median overall survival in patients with or without TgAbs seroconversion was 65 versus 21 months in the resected pancreatic cancer group receiving GVAX only (p= 0.009, data not shown), 5.9 versus 3.2 months in the metastatic pancreatic cancer group receiving GVAX plus ipilimumab (p= 0.05, data not shown), and 37 versus 15 months in the metastatic prostate cancer group receiving GVAX plus ipilimumab (p= 0.007, data not shown).
TgAbs seroconversion did not associate with specific T cell differentiation/activation (Supplementary Figure 2), such as the CD4+/CTL-4+ T cell subset that have been previously associated with survival in the prostate GVAX/ipilimumab-treated patients18, indicating that these two immunological events are independently regulated. In addition, we found no relationship between clinical response and TgAbs titers: the development of TgAbs showed similar trends in patients with progressive, stable disease or partial response in the prostate cancer group treated with GVAX plus ipilimumab (Supplementary Figure 3).
Detection of TgAbs seroconversion is assay dependent
The measurement of TgAbs, thus far carried out using the in house ELISA assay, was also performed using two commercial assays (Quanta Lite ELISA from INOVA and KRONUS RIA).
The in house assay performed similarly to the commercial ELISA assay when using a set of positive controls with an established clinical diagnosis of Hashimoto thyroiditis (No. = 20) and a set of healthy controls (No. = 47). The two assays, in fact, showed similar sensitivity (95% for both assays) and specificity (93 and 91%, respectively), yielding areas under the ROC curve of 0.985 and 0.992, respectively (Supplementary Figure 4). The in house ELISA also yielded similar intra- and inter-assay variability to those reported for the commercial ELISA and RIA assays: 6%, 9%, 12% intra-assay and 2%, 2%, 4% inter-assay variability using a panel of negative, moderate positive, and strong positive controls (data not shown).
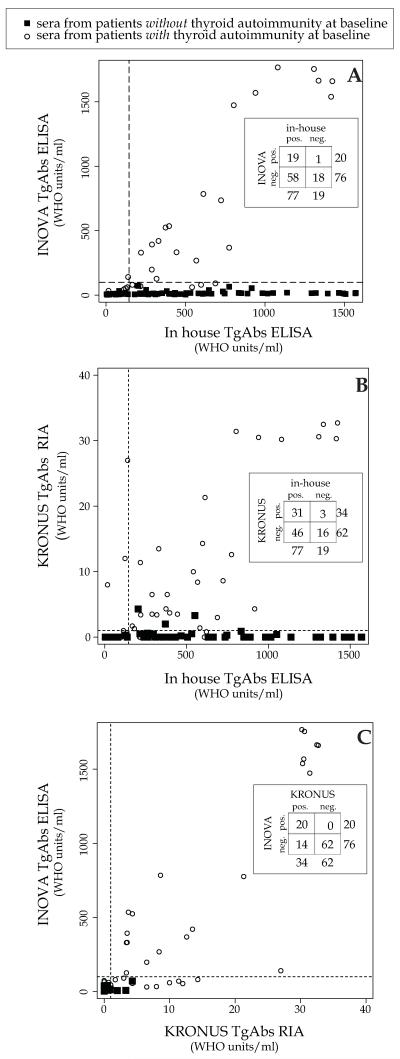
The assays, however, varied greatly when used in a randomly selected subset of 23 patients before (No. 23 sera) and at several time points (No.= 73 sera) after GVAX administration. A good proportion of the sera, 59 of the total 96, were discordant because because positive in the in house but negative in the commercial assay (No. 58), or viceversa (No. = 1, Figure 4A, inset). The remaining 37 of the total 96 sera (38%) were concordant because either positive (No. = 19) or negative (No. = 18) in both assays (Figure 4A, inset). A similar low concordance was obtained when compared in house assay to the commercial RIA: 31 sera were positive and 16 negative in both assays (Figure 4B, inset), yielding a percent agreement of 49% (47 of 96). Concordance was higher but not perfect (82 of 96, 85%) even when the two commercial assays were compared (Figure 4C, inset).
Figure 4.
Comparison of thyroglobulin antibodies between in house and INOVA TgAbs ELISA assay (panel A), between in house and KRONUS RIA (panel B) and INOVA TgAbs ELISA and KRONUS RIA (panel C) in a subset of 96 sera obtained from cancer patients receiving immunotherapy. Open circles indicate sera from patients who already had thyroid autoimmunity at baseline, whereas closed squares indicate those without.
Two by two table has been inserted in each panel to indicate concordant and discordant samples.
To provide an explanation for this incomplete concordance, we took into account the presence of pre-existing, albeit clinically latent, thyroid autoimmunity at baseline, as assessed by the presence of positive thyroperoxidase antibodies. Interestingly, TgAbs were detected equally well by all three assays in patients with pre-existing thyroid autoimmunity (Figure 4A, B, and C, open circles). In contrast, TgAbs were detected mainly by the in house assay (Figure 4A and B, filled squares in the lower right quadrant) when patients developed them after GVAX treatment. These findings suggest that GVAX induces antibodies against region(s) of thyroglobulin different from the one(s) recognized by patients with an established clinical diagnosis of Hashimoto thyroiditis.
To assess whether the negative TgAbs values obtained with the commercial assays were caused by interfering substances present in the sera of patients receiving GVAX, we purified immunoglobulins G from 6 serum samples that had yielded positive (No. = 3) or negative (No. = 3) results in the in house assay and all negative results in the commercial ELISA assay. Using the in house assay, the purified immunoglobulins gave nearly identical results to those obtained with whole serum (Supplementary Figure 5A). Using the commercial ELISA assay, all purified samples remained negative as they were with whole serum (Supplementary Figure 5B), thus ruling out assay interference.
GVAX immunotherapy induces antibodies to other autoantigens but less frequently and at lower titers than TgAbs
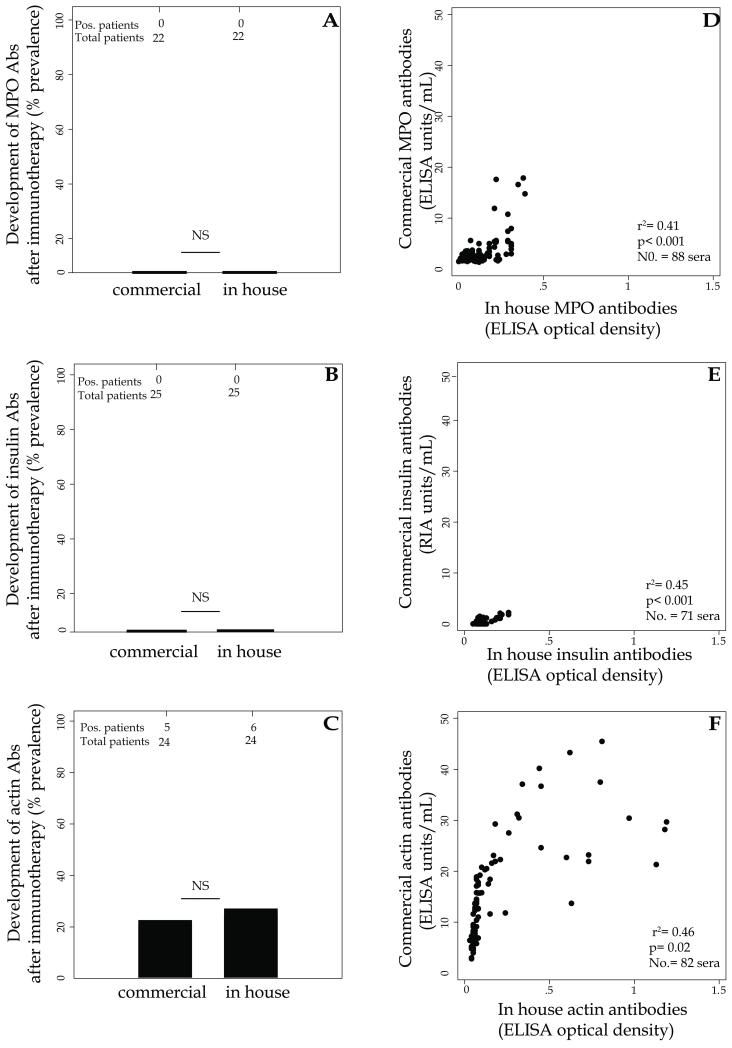
To assess whether the antibody response observed after GVAX immunotherapy was generalizable or rather specific to the thyroglobulin antigen, we measured the presence and development of antibodies to five additional autoantigens, representative of both systemic and organ-specific autoimmunity: thyroperoxidase (TPO), proteinase-3 (PR-3), myeloperoxidase (MPO), insulin, and actin, using both commercially available assays and in house ELISA assays. For PR-3 (Supplementary Table 2), MPO (Supplementary Table 2 and Figure 5A), and insulin (Supplementary Table 2 and Figure 5B), no autoantibodies were found before treatment and no serconversion was observed upon cancer immunotherapy (Supplementary Table 2). For TPO, 11 of the 58 patients tested (19%) were already positive before immunotherapy but their TPO titer did not change significantly after treatment (data not shown). The remaining 47 were negative before treatment and remained negative thereafter (Supplementary Table 2). For actin (Figure 5C), autoantibodies were present at baseline in 3 of 26 tested patients (12%), increased in these 3 patients after GVAX, and developed in 2 additional patients (Figure 5 C, left bar).
Figure 5.
Development of myeloperoxidase (MPO) (panel A), insulin (panel B) and actin (panel C) antibodies in cancer patients receiving immunotherapy.
Comparison between commercial and in house assays for the detection of MPO (panel D), insulin (panel E) and actin (panel F) antibodies.
To evaluate whether these results depended upon the detection method, as presented above for the TgAbs, we compared commercial to in house assay for MPO, insulin, and actin antibodies. In all 3 cases, no differences were found in the development frequenceis (Figure 5, A, B, abd C), and significant correlation was observed between commercial and in house assays (Figure 5, D, E, abd F).
Overall, these results indicate that the increase in antibody titers induced by GVAX is preferentially directed toward thyroglobulin but not restricted to it.
DISCUSSION
This study reports that TgAbs develop upon GVAX administration and are associated with improved overall survival.
Using patients with different cancer types and exposed to different immunotherapy regimens, we showed that GVAX was required for TgAbs induction. The rationale of GVAX is to induce at the site of injection a potent immune response against tumor (as well as self) antigens, which then amplifies to target the patient’s own cancer (as well as self) antigens. Local inflammation can be achieved by forcing the injected cancer cells to express a cytokine, such as GM-CSF, that is capable of recruiting and activating dendritic cells19. It is interesting to speculate on the mechanisms underlying the development of TgAbs upon GVAX administration, in particular referring to multiple tumor antigens and GM-CSF.
Being composed of whole cancer cells, GVAX theoretically challenges the patient’s immune system with multiple tumor antigens, rather than a single one. Cancer cells are known to express a variety of tumor antigens that can be recognized by the patient’s T and B cells. Some are proteins normally expressed by the cell from which the cancer originates, such as tyrosinase for melanoma20. Others are proteins abnormally modified at the post-translational level, such as MUC-1 for breast cancer21, or proteins typically expressed only in male germ cells, such as the NY-ESO-1 in melanoma22. Additional tumor antigens are proteins not existing in the human genome, such as the oncoviral protein HPV E6 in cervical carcinoma23, or the chimeric BCR/ABL fusion protein in 25. In keeping with this ectopic scenario, we assessed whether the GVAX pancreatic cancer cell lines used in this study expressed thyroglobulin, but found no evidence of it, either at the RNA (Supplementary Figure 6A) or protein level (Supplementary Figure 6B). Although these results render the hypothesis of molecular mimicry unlikely, the negative findings could be related to the methodology used. For example, mass spectrometry recently revealed that peptides from thyroid-restricted antigens such as thyroglobulin and thyrotropin receptor were present in a patient with synovial sarcoma receiving NY-ESO-1 vaccine26.
GM-CSF, naturally produced by macrophages and T cells, stimulates the growth and differentiation of myelomonocytic lineage cells, especially dendritic cells. It thus acts as an adjuvant and is capable of eliciting immune responses directed not only toward tumor but also non-cancerous self-antigens. Previous clinical studies have shown that periodical intravenous injections of GM-CSF to patients with breast cancer or sarcomas induced the appearance of TgAbs in 1 of 25 patients (4%) and primary hypothyroidism in 2 of 25 (8%)27, suggesting that broad activation of dendritic cells by GM-CSF is capable of targeting the thyroid gland. Similar findings were described in a thyroid patient with acute myeloid leukemia28, as well as in patients with other autoimmune diseases29. Considering that GM-CSF produced by GVAX cells can be detected in the circulation up to 4 days after the injection30, we postulate that GM-CSF plays a major role in the observed development of TgAbs. These findings are consistent with studies showing the development of thyroid autoimmunity secondary to injection of other cytokines, such as interleukin-231 or interferon α–2b32.
Thyroid autoimmunity can be assessed by measuring serum autoantibodies to thyroperoxidase and thyroglobulin, traditionally requested as a pair to confirm a diagnostic suspicion of autoimmune thyroiditis. Since TgAbs are found not only in autoimmune thyroiditis but also in other thyroid diseases and even in healthy controls (albeit at lower frequencies), investigators have long hypothesized that antibodies in different diseases are directed toward different epitopes on the same antigen. We previously showed that TgAbs in patients with overt Hashimoto thyroiditis have a unique recognition profile, as assessed by competitive inhibition of a panel of monoclonal antibodies raised against human thyroglobulin33. Similarly, Latrofa and colleagues reported that TgAbs found in autoimmune thyroiditis bind to different regions than those found in multinodular goiter or differentiated thyroid cancer34. Our study supports the notion that TgAbs recognize different epitopes in different disease states.
The study also emphasizes that assays used to detect TgAbs are highly discordant35-38. Using an experimental model induced by the administration of GVAX, we were able to show that, in patients who did not have thyroid autoimmunity at baseline, TgAbs developed after GVAX and could be detected mainly by an assay that employs an intact form of human thyroglobulin. Given that the in house assay performed similarly to the commercial assays when used for patients with established thyroid autoimmunity, it is reasonable to assume that the TgAbs induced by GVAX recognize epitope(s) on thyroglobulin that are not found on the thyroglobulin used in the commercial assays. More broadly, our results suggest that in this human model of thyroiditis initiation there is a unique pattern of epitope recognition, which could be exploited for clinical purposes. Once thyroiditis is established, either in a clinically overt or latent fashion, the immune recognition spreads to other more numerous and dominant epitopes, so that the type of assay used for the detection of TgAbs has minimal influence. In support of this hypothesis are the findings that the assays for TgAbs were in agreement when testing patients with positive thyroperoxidase antibodies.
An additional finding of this study was the association between TgAbs seroconversion and prolonged survival. The development of autoimmune phenomena in cancer patients receiving immunotherapy has been associated with better outcomes8,39,40, for example the appearance of vitiligo in melanoma patients who were vaccinated with their own tumor cells transduced with GM-CSF41, overall emphasizing the importance of a robust immune activation for cancer control. More recently, antibodies to prostate specific membrane antigen, measured in a subset of our patient population, were associated with improved overall survival4. Similarly, the appearance of thyroid autoimmunity in patients with melanoma or renal cell carcinoma treated with interferon α–2b or interleukin-2 has been correlated with longer relapse-free survival and overall survival8,42. This study reveals the utility of measuring TgAbs in the clinical setting as an additional tool to predict survival benefit.
In conclusion, we report that GVAX induces the appearance of TgAbs that recognize unique antigenic epitopes, likely a fingerprint of the initiation stages of thyroiditis, and that this seroconversion is associated with improved overall survival.
Supplementary Material
Supplementary Figure 1. Linearity and detection limit of in house ELISA assay for detecting antibodies to thyroglobulin (Tg).
Supplementary Figure 2: Cluster analysis to correlate the expression of peripheral blood T cell markers to development of thyroglobulin antibodies (TgAbs).
Supplementary Figure 3: Relationship between thyroglobulin antibodies titer and clinical response (progressive disease, stable disease or partial response) in patients wicth metastatic prostate cancer receiving GVAX plus ipilimumab.
The symbol * indicates that this analysis was performed on patients with metastatic prostate cancer treated with GVAX plus ipilimumab
Supplementary Figure 4: ROC curves comparing in house and commercial (QuantaLite INOVA TgAbs ELISA assay) ELISA assays to detect thyroglobulin antibodies (TgAbs).
Supplementary Figure 5: Detection of thyroglobulin antibodies (TgAbs) by whole serum or purified immunoglobulins in the two ELISA assays. In the in house assay (panel A), 6 samples yielded similar TgAb results when using whole serum or immunoglobulins. In the commercial assay (panel B), all 6 sera remained negative, independent of the use of whole serum or purified immunoglobulins.
Supplementary Figure 6: Evaluation of ectopic expression of thyroglobulin by the pancreatic cancer cell lines used for the GVAX vaccine. A) RNA level: reverse transcriptase PCR using PANC 10.05 and PANC 6.03 cell lines transfected or not with granulocyte-macrophage colony-stimulating factor (GM-CSF). B) Protein level: immunoblotting using the PANC 10.05 cell line transfected or not with GM-CSF (identical results were obtained with the PANC 6.03 cell line, data not shown).
Novelty & Impact Statements.
We report for the first time the development of thyroglobulin antibodies in cancer patients treated with GVAX, either administered alone or in association with ipilimumab. This seroconversion can be used in the clinical setting as a predictive tool since associates with prolonged survival.
ACKNOWLEDGMENTS
The study was supported by patient donations to the autoimmune hypophysitis center directed by PC, and in part by NIH grant DK080351 to PC. Additional support is indicated below.
Alessandra De Remigis was supported in part by Università degli Studi “G.D’Annunzio”,Chieti, Italy.
Tanja de Gruijl and Alfons van den Eertwegh: Prostate Cancer Foundation (PCF) competitive research award 2004 and the Dutch Cancer Society (KFW; VU 2006-3697)
Shey-Cherng Tzou was supported in part by grant from the National Science Council, Taipei, Taiwan (NSC 101-2320-B-009 -005 and NSC 102-2628-B-009 -002 -MY2).
Shintaro Iwama was supported in part by a fellowship from the Manpei Suzuki Diabetes Foundation.
Lei Zheng: NIH K23 CA148964-01, an American Society of Clinical Oncology Young Investigator Award, The Viragh Family Foundation, and the NCI SPORE in Gastrointestinal Cancers P50 CA062924.
Dung Le: NIH/R21 (CA1266058), NIH/GI SPORE (2P50 CA062924), and The Viragh Family Foundation.
Elizabeth Jaffee: NIH/GI SPORE (2P50 CA062924), and The Viragh Family Foundation.
Daniel Laheru: NIH/R21 (CA1266058), NIH/GI SPORE (2P50 CA062924), and The Viragh Family Foundation.
We thank Anita Stam and Sara Solt for technical assistance and sample handling and SuFey Ong for technical support in reverse transciptase PCR experiments.
Abbreviations
- GM-CSF
granulocyte-macrophage colony-stimulating factor
- GVAX
vaccine made of a tumor cell type transfected with GM-CSF
- CTLA-4
cytotoxic T lymphocyte antigen 4
- TgAbs
thyroglobulin antibodies
REFERENCES
- 1.Cha E, Fong L. Immunotherapy for prostate cancer: biology and therapeutic approaches. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29:3677–85. doi: 10.1200/JCO.2010.34.5025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dranoff G, Jaffee E, Lazenby A, Golumbek P, Levitsky H, Brose K, Jackson V, Hamada H, Pardoll D, Mulligan RC. Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity. Proceedings of the National Academy of Sciences of the United States of America. 1993;90:3539–43. doi: 10.1073/pnas.90.8.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Callahan MK, Wolchok JD. At the Bedside: CTLA-4- and PD-1-blocking antibodies in cancer immunotherapy. Journal of leukocyte biology. 2013;94:41–53. doi: 10.1189/jlb.1212631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van den Eertwegh AJ, Versluis J, van den Berg HP, Santegoets SJ, van Moorselaar RJ, van der Sluis TM, Gall HE, Harding TC, Jooss K, Lowy I, Pinedo HM, Scheper RJ, et al. Combined immunotherapy with granulocyte-macrophage colony-stimulating factortransduced allogeneic prostate cancer cells and ipilimumab in patients with metastatic castration-resistant prostate cancer: a phase 1 dose-escalation trial. The lancet oncology. 2012;13:509–17. doi: 10.1016/S1470-2045(12)70007-4. [DOI] [PubMed] [Google Scholar]
- 5.Le DT, Lutz E, Uram JN, Sugar EA, Onners B, Solt S, Zheng L, Diaz LA, Jr., Donehower RC, Jaffee EM, Laheru DA. Evaluation of Ipilimumab in Combination With Allogeneic Pancreatic Tumor Cells Transfected With a GM-CSF Gene in Previously Treated Pancreatic Cancer. Journal of immunotherapy (Hagerstown, Md : 1997) 2013;36:382–9. doi: 10.1097/CJI.0b013e31829fb7a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weber JS, Kahler KC, Hauschild A. Management of immune-related adverse events and kinetics of response with ipilimumab. J Clin Oncol. 2012;30:2691–7. doi: 10.1200/JCO.2012.41.6750. [DOI] [PubMed] [Google Scholar]
- 7.Corsello SM, Barnabei A, Marchetti P, De Vecchis L, Salvatori R, Torino F. Endocrine side effects induced by immune checkpoint inhibitors. The Journal of clinical endocrinology and metabolism. 2013;98:1361–75. doi: 10.1210/jc.2012-4075. [DOI] [PubMed] [Google Scholar]
- 8.Agueros M, Campanero MA, lrache JM. Simultaneous quantification of different cyclodextrins and Gantrez by HPLC with evaporative light scattering detection. Journal of pharmaceutical and biomedical analysis. 2005;39:495–502. doi: 10.1016/j.jpba.2005.04.034. [DOI] [PubMed] [Google Scholar]
- 9.Weber J, Thompson JA, Hamid O, Minor D, Amin A, Ron I, Ridolfi R, Assi H, Maraveyas A, Berman D, Siegel J, O’Day SJ. A randomized, double-blind, placebo-controlled, phase II study comparing the tolerability and efficacy of ipilimumab administered with or without prophylactic budesonide in patients with unresectable stage III or IV melanoma. Clinical cancer research : an official journal of the American Association for Cancer Research. 2009;15:5591–8. doi: 10.1158/1078-0432.CCR-09-1024. [DOI] [PubMed] [Google Scholar]
- 10.Downey SG, Klapper JA, Smith FO, Yang JC, Sherry RM, Royal RE, Kammula US, Hughes MS, Allen TE, Levy CL, Yellin M, Nichol G, et al. Prognostic factors related to clinical response in patients with metastatic melanoma treated by CTL-associated antigen-4 blockade. Clinical cancer research : an official journal of the American Association for Cancer Research. 2007;13:6681–8. doi: 10.1158/1078-0432.CCR-07-0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lutz E, Yeo CJ, Lillemoe KD, Biedrzycki B, Kobrin B, Herman J, Sugar E, Piantadosi S, Cameron JL, Solt S, Onners B, Tartakovsky I, et al. A lethally irradiated allogeneic granulocyte-macrophage colony stimulating factor-secreting tumor vaccine for pancreatic adenocarcinoma. A Phase II trial of safety, efficacy, and immune activation. Ann Surg. 2011;253:328–35. doi: 10.1097/SLA.0b013e3181fd271c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yuan J, Ginsberg B, Page D, Li Y, Rasalan T, Gallardo HF, Xu Y, Adams S, Bhardwaj N, Busam K, Old LJ, Allison JP, et al. CTLA-4 blockade increases antigen-specific CD8(+) T cells in prevaccinated patients with melanoma: three cases. Cancer immunology, immunotherapy : CII. 2011;60:1137–46. doi: 10.1007/s00262-011-1011-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yuan J, Adamow M, Ginsberg BA, Rasalan TS, Ritter E, Gallardo HF, Xu Y, Pogoriler E, Terzulli SL, Kuk D, Panageas KS, Ritter G, et al. Integrated NY-ESO-1 antibody and CD8+ T-cell responses correlate with clinical benefit in advanced melanoma patients treated with ipilimumab. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:16723–8. doi: 10.1073/pnas.1110814108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McGrogan A, Seaman HE, Wright JW, de Vries CS. The incidence of autoimmune thyroid disease: a systematic review of the literature. Clinical endocrinology. 2008;69:687–96. doi: 10.1111/j.1365-2265.2008.03338.x. [DOI] [PubMed] [Google Scholar]
- 15.Batt DG, Petraitis JJ, Sherk SR, Copeland RA, Dowling RL, Taylor TL, Jones EA, Magolda RL, Jaffee BD. Heteroatom- and carbon-linked biphenyl analogs of Brequinar as immunosuppressive agents. Bioorganic & medicinal chemistry letters. 1998;8:1745–50. doi: 10.1016/s0960-894x(98)00308-4. [DOI] [PubMed] [Google Scholar]
- 16.Kimura H, Kimura M, Tzou SC, Chen YC, Suzuki K, Rose NR, Caturegli P. Expression of class II major histocompatibility complex molecules on thyrocytes does not cause spontaneous thyroiditis but mildly increases its severity after immunization. Endocrinology. 2005;146:1154–62. doi: 10.1210/en.2004-1165. [DOI] [PubMed] [Google Scholar]
- 17.Jaffee EM, Schutte M, Gossett J, Morsberger LA, Adler AJ, Thomas M, Greten TF, Hruban RH, Yeo CJ, Griffin CA. Development and characterization of a cytokine-secreting pancreatic adenocarcinoma vaccine from primary tumors for use in clinical trials. The cancer journal from Scientific American. 1998;4:194–203. [PubMed] [Google Scholar]
- 18.Santegoets SJ, Stam AG, Lougheed SM, Gall H, Scholten PE, Reijm M, Jooss K, Sacks N, Hege K, Lowy I, Cuillerot JM, von Blomberg BM, et al. T cell profiling reveals high CD4+CTLA-4 + T cell frequency as dominant predictor for survival after prostate GVAX/ipilimumab treatment. Cancer immunology, immunotherapy : CII. 2013;62:245–56. doi: 10.1007/s00262-012-1330-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pitts WJ, Jetter JW, Pinto DJ, Orwat MJ, Batt DG, Sherk SR, Petraitis JJ, Jacobson IC, Copeland RA, Dowling RL, Jaffee BD, Gardner TL, et al. Structure-activity relationships (SAR) of some tetracyclic heterocycles related to the immunosuppressive agent Brequinar Sodium. Bioorganic & medicinal chemistry letters. 1998;8:307–12. doi: 10.1016/s0960-894x(98)00010-9. [DOI] [PubMed] [Google Scholar]
- 20.Greten TF, Slansky JE, Kubota R, Soldan SS, Jaffee EM, Leist TP, Pardoll DM, Jacobson S, Schneck JP. Direct visualization of antigen-specific T cells: HTLV-1 Tax11-19-specific CD8(+) T cells are activated in peripheral blood and accumulate in cerebrospinal fluid from HAM/TSP patients. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:7568–73. doi: 10.1073/pnas.95.13.7568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Herbstman CH, Jaffee JB, Tuman KJ, Newman LM. An in vivo evaluation of four spinal needles used for the combined spinal-epidural technique. Anesthesia and analgesia. 1998;86:520–2. doi: 10.1097/00000539-199803000-00013. [DOI] [PubMed] [Google Scholar]
- 22.Jaffee EM, Abrams R, Cameron J, Donehower R, Duerr M, Gossett J, Greten TF, Grochow L, Hruban R, Kern S, Lillemoe KD, O’Reilly S, et al. A phase I clinical trial of lethally irradiated allogeneic pancreatic tumor cells transfected with the GM-CSF gene for the treatment of pancreatic adenocarcinoma. Human gene therapy. 1998;9:1951–71. doi: 10.1089/hum.1998.9.13-1951. [DOI] [PubMed] [Google Scholar]
- 23.Zhu D, Stumpf CR, Krahn JM, Wickens M, Hall TM. A 5′ cytosine binding pocket in Puf3p specifies regulation of mitochondrial mRNAs. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:20192–7. doi: 10.1073/pnas.0812079106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garcia-Diaz M, Bebenek K, Larrea AA, Havener JM, Perera L, Krahn JM, Pedersen LC, Ramsden DA, Kunkel TA. Template strand scrunching during DNA gap repair synthesis by human polymerase lambda. Nature structural & molecular biology. 2009;16:967–72. doi: 10.1038/nsmb.1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoekman K, Lowik CW, vd Ruit M, Bijvoet OL, Verheijen JH, Papapoulos SE. Regulation of the production of plasminogen activators by bone resorption enhancing and inhibiting factors in three types of osteoblast-like cells. Bone and mineral. 1991;14:189–204. doi: 10.1016/0169-6009(91)90022-r. [DOI] [PubMed] [Google Scholar]
- 26.Vita R, Guarneri F, Agah R, Benvenga S. Autoimmune Thyroid Disease Elicited by NY-ESO-1 Vaccination. Thyroid : official journal of the American Thyroid Association. 2013 doi: 10.1089/thy.2013.0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoekman K, von Blomberg-van der Flier BM, Wagstaff J, Drexhage HA, Pinedo HM. Reversible thyroid dysfunction during treatment with GM-CSF. Lancet. 1991;338:541–2. doi: 10.1016/0140-6736(91)91103-2. [DOI] [PubMed] [Google Scholar]
- 28.Hansen PB, Johnsen HE, Hippe E. Autoimmune hypothyroidism and granulocytemacrophage colony-stimulating factor. European journal of haematology. 1993;50:183–4. doi: 10.1111/j.1600-0609.1993.tb00090.x. [DOI] [PubMed] [Google Scholar]
- 29.Di Giacomo A. The EFIS-EJI Ruggero Ceppellini Advanced School of Immunology Innate Immunity 2012: from evolution to revolution. European journal of immunology. 2013;43:13–4. doi: 10.1002/eji.201370017. [DOI] [PubMed] [Google Scholar]
- 30.Jaffee EM, Hruban RH, Biedrzycki B, Laheru D, Schepers K, Sauter PR, Goemann M, Coleman J, Grochow L, Donehower RC, Lillemoe KD, O’Reilly S, et al. Novel allogeneic granulocyte-macrophage colony-stimulating factor-secreting tumor vaccine for pancreatic cancer: a phase I trial of safety and immune activation. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2001;19:145–56. doi: 10.1200/JCO.2001.19.1.145. [DOI] [PubMed] [Google Scholar]
- 31.Weijl NI, Van der Harst D, Brand A, Kooy Y, Van Luxemburg S, Schroder J, Lentjes E, Van Rood JJ, Cleton FJ, Osanto S. Hypothyroidism during immunotherapy with interleukin-2 is associated with antithyroid antibodies and response to treatment. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1993;11:1376–83. doi: 10.1200/JCO.1993.11.7.1376. [DOI] [PubMed] [Google Scholar]
- 32.Schwartzentruber DJ, White DE, Zweig MH, Weintraub BD, Rosenberg SA. Thyroid dysfunction associated with immunotherapy for patients with cancer. Cancer. 1991;68:2384–90. doi: 10.1002/1097-0142(19911201)68:11<2384::aid-cncr2820681109>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 33.Caturegli P, Mariotti S, Kuppers RC, Burek CL, Pinchera A, Rose NR. Epitopes on thyroglobulin: a study of patients with thyroid disease. Autoimmunity. 1994;18:41–9. doi: 10.3109/08916939409014678. [DOI] [PubMed] [Google Scholar]
- 34.Latrofa F, Ricci D, Grasso L, Vitti P, Masserini L, Basolo F, Ugolini C, Mascia G, Lucacchini A, Pinchera A. Characterization of thyroglobulin epitopes in patients with autoimmune and non-autoimmune thyroid diseases using recombinant human monoclonal thyroglobulin autoantibodies. The Journal of clinical endocrinology and metabolism. 2008;93:591–6. doi: 10.1210/jc.2007-1199. [DOI] [PubMed] [Google Scholar]
- 35.Krahn J, Dembinski T. Thyroglobulin and anti-thyroglobulin assays in thyroid cancer monitoring. Clinical biochemistry. 2009;42:416–9. doi: 10.1016/j.clinbiochem.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 36.Latrofa F, Ricci D, Montanelli L, Rocchi R, Piaggi P, Sisti E, Grasso L, Basolo F, Ugolini C, Pinchera A, Vitti P. Thyroglobulin autoantibodies in patients with papillary thyroid carcinoma: comparison of different assays and evaluation of causes of discrepancies. The Journal of clinical endocrinology and metabolism. 2012;97:3974–82. doi: 10.1210/jc.2012-2406. [DOI] [PubMed] [Google Scholar]
- 37.Taylor KP, Parkington D, Bradbury S, Simpson HL, Jefferies SJ, Halsall DJ. Concordance between thyroglobulin antibody assays. Annals of clinical biochemistry. 2011;48:367–9. doi: 10.1258/acb.2011.010248. [DOI] [PubMed] [Google Scholar]
- 38.Tozzoli R, Bizzaro N, Tonutti E, Pradella M, Manoni F, Vilalta D, Bassetti D, Piazza A, Rizzotti P. Immunoassay of anti-thyroid autoantibodies: high analytical variability in second generation methods. Clinical chemistry and laboratory medicine : CCLM / FESCC. 2002;40:568–73. doi: 10.1515/CCLM.2002.098. [DOI] [PubMed] [Google Scholar]
- 39.Blake KJ, Myette G, Jack J. The products of ribbon and raw are necessary for proper cell shape and cellular localization of nonmuscle myosin in Drosophila. Developmental biology. 1998;203:177–88. doi: 10.1006/dbio.1998.9036. [DOI] [PubMed] [Google Scholar]
- 40.Vaughn DD, Jabra AA, Fishman EK. Pancreatic disease in children and young adults: evaluation with CT. Radiographics : a review publication of the Radiological Society of North America, Inc. 1998;18:1171–87. doi: 10.1148/radiographics.18.5.9747614. [DOI] [PubMed] [Google Scholar]
- 41.Luiten RM, Kueter EW, Mooi W, Gallee MP, Rankin EM, Gerritsen WR, Clift SM, Nooijen WJ, Weder P, van de Kasteele WF, Sein J, van den Berk PC, et al. Immunogenicity, including vitiligo, and feasibility of vaccination with autologous GM-CSF-transduced tumor cells in metastatic melanoma patients. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2005;23:8978–91. doi: 10.1200/JCO.2005.01.6816. [DOI] [PubMed] [Google Scholar]
- 42.Gogas H, Ioannovich J, Dafni U, Stavropoulou-Giokas C, Frangia K, Tsoutsos D, Panagiotou P, Polyzos A, Papadopoulos O, Stratigos A, Markopoulos C, Bafaloukos D, et al. Prognostic significance of autoimmunity during treatment of melanoma with interferon. The New England journal of medicine. 2006;354:709–18. doi: 10.1056/NEJMoa053007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Linearity and detection limit of in house ELISA assay for detecting antibodies to thyroglobulin (Tg).
Supplementary Figure 2: Cluster analysis to correlate the expression of peripheral blood T cell markers to development of thyroglobulin antibodies (TgAbs).
Supplementary Figure 3: Relationship between thyroglobulin antibodies titer and clinical response (progressive disease, stable disease or partial response) in patients wicth metastatic prostate cancer receiving GVAX plus ipilimumab.
The symbol * indicates that this analysis was performed on patients with metastatic prostate cancer treated with GVAX plus ipilimumab
Supplementary Figure 4: ROC curves comparing in house and commercial (QuantaLite INOVA TgAbs ELISA assay) ELISA assays to detect thyroglobulin antibodies (TgAbs).
Supplementary Figure 5: Detection of thyroglobulin antibodies (TgAbs) by whole serum or purified immunoglobulins in the two ELISA assays. In the in house assay (panel A), 6 samples yielded similar TgAb results when using whole serum or immunoglobulins. In the commercial assay (panel B), all 6 sera remained negative, independent of the use of whole serum or purified immunoglobulins.
Supplementary Figure 6: Evaluation of ectopic expression of thyroglobulin by the pancreatic cancer cell lines used for the GVAX vaccine. A) RNA level: reverse transcriptase PCR using PANC 10.05 and PANC 6.03 cell lines transfected or not with granulocyte-macrophage colony-stimulating factor (GM-CSF). B) Protein level: immunoblotting using the PANC 10.05 cell line transfected or not with GM-CSF (identical results were obtained with the PANC 6.03 cell line, data not shown).