Abstract
Objective
To assess the efficacy of a simple, goal-directed sepsis treatment protocol for reducing mortality in patients with severe sepsis in Zambia.
Design
Single center non-blinded randomized controlled trial
Setting
Emergency room, ICU, and medical wards of the national referral hospital in Lusaka, Zambia
Patients
112 patients enrolled within 24 hours of admission with severe sepsis, defined as systemic inflammatory response syndrome with suspected infection and organ dysfunction
Interventions
Simplified Severe Sepsis Protocol (SSSP) consisting of up to 4 liters of intravenous fluids within 6 hours, guided by jugular venous pressure assessment, and dopamine and/or blood transfusion in selected patients. Control group was managed as usual care. Blood cultures were collected and early antibiotics administered for both arms.
Measurements and Main Results
Primary outcome was in-hospital all-cause mortality. 109 patients were included in the final analysis. 88 (80.7%) were HIV positive. Pulmonary infections were the most common source of sepsis. In-hospital mortality rate was 64.2% in the intervention group and 60.7% in the control group (RR 1.05, 95%CI:0.79-1.41). Mycobacterium tuberculosis complex was isolated from 31 of 82 (37.8%) HIV positive patients with available mycobacterial blood culture results. SSSP patients received significantly more IV fluids in the first 6 hours (2.7 liters vs. 1.7 liters, p=0.002). The study was stopped early because of high mortality rate among patients with hypoxemic respiratory failure in the intervention arm (8/8, 100%) compared with the control arm [7/10, 70%, RR 1.43 (95%CI:0.95-2.14)].
Conclusion
Factors other than tissue hypoperfusion probably account for much of the end organ dysfunction in African patients with severe sepsis. Studies of fluid-based interventions should utilize inclusion criteria to accurately capture patients with hypovolemia and tissue hypoperfusion who are most likely to benefit from fluids. Exclusion of patients with severe respiratory distress should be considered when ventilator support is not readily available.
Keywords: Africa, sepsis, goal-directed therapy, Zambia, tuberculosis
In the United States, 750,000 people die each year from sepsis.(1) Although available data are limited, the number of sepsis-related deaths is likely much higher in sub-Saharan Africa, where more than half of all deaths are attributed to infections.(2) Cohort studies from the region have found sepsis to be the third leading cause of death among HIV-infected adults, after tuberculosis and cryptococcal meningitis,(3) and an unpublished audit at the University Teaching Hospital in Zambia showed sepsis to be the leading cause of death among hospitalized medical patients. However, optimal management strategies for septic patients in Africa remain controversial.(4-7)
Protocol-based management of sepsis has had wide uptake in North America and Europe.(8,9) Studies of early goal directed therapy have demonstrated that aggressive intravenous (IV) fluid administration, hemodynamic support, and blood transfusion can significantly reduce mortality due to sepsis. Central venous pressure or serum-lactate-guided approaches have generally resulted in patients receiving between 4 and 5 liters of fluid in the first 6 hours of admission.(10,11) In sub-Saharan Africa, however, uptake has been generally non-existent due to resource limitations.(12) Central venous catheters and lactic acid tests are not widely available, and the use of IV fluids for volume resuscitation has been much more conservative than guidelines recommend.(13,14) There are also questions regarding the generalizability of existing evidence to the sub-Saharan African setting, considering the under-representation of resource-limited study sites and HIV/AIDS patients in most sepsis trials.(15) Furthermore, the limited existing evidence from the region is conflicting regarding the potential benefits and harms of aggressive fluid resuscitation.(4,6)
We hypothesized that a novel simplified treatment protocol, based on existing early goal directed therapy protocols, would reduce mortality compared with usual care in African patients with severe sepsis. The simplified severe sepsis protocol (SSSP) intervention consisted of early goal-directed fluid administration, plus dopamine and/or blood transfusion when indicated. Patients in both arms received close nurse monitoring with early blood cultures and antibiotics.
Methods
We conducted a pilot non-blinded randomized controlled trial of patients presenting to the University Teaching Hospital (UTH) in Lusaka, Zambia with severe sepsis between February and July 2012. UTH is the national referral hospital for Zambia and is also a major primary care hospital for the city of Lusaka. Ethical approval was obtained from the University of Zambia Biomedical Research Ethics Committee (UNZA BREC) and the Vanderbilt University Institutional Review Board (IRB), and the study was registered with clinicaltrials.gov (NCT01449916).
Patients
All patients presenting to the emergency room were screened for eligibility. Enrollment occurred 24 hours per day from Monday 7:30am to Friday 1:00pm. Patients were eligible if they were 18 years of age or older and met criteria for severe sepsis upon presentation to the emergency room. Additionally, patients who manifested severe sepsis after arrival to the hospital were eligible if they were still in the emergency room less than 24 hours after presentation and were within 6 hours of first meeting severe sepsis criteria. Severe sepsis was defined as the presence of all 3 of the following: 1) infection suspected by treating doctor, 2) systemic inflammatory response syndrome (SIRS), defined as 2 or more of heart rate > 90/minute, respiratory rate > 20/minute, temperature >=38.0C or <=36.0C, or white blood count > 12,000/cmm or < 4,000/cmm, and 3) 1 or more signs of organ dysfunction. Organ dysfunction criteria were systolic blood pressure < 90 mmHg or mean arterial blood pressure (MAP) < 65 mmHg, altered mentation, creatinine > 1.85 mg/dL, platelet count < 100 × 109/L, respiratory rate > 40/minute, or jaundice. Patients were excluded if they had a gastrointestinal bleed, required immediate surgery, or had suspected congestive heart failure exacerbation or end-stage renal disease. Patients with raised jugular venous pressure (JVP) > 3 cm above the sternal angle, as measured with a level and a ruler, were also excluded. JVP was measured vertically from the sternal angle with the patient positioned between 0 and 45° incline so that the waveform was visible. The normal range for JVP is +1 to +3 cm above the sternal angle, and JVP over +3 cm is a reliable measure of volume overload by physical exam.(16,17) Study doctors and nurses underwent 2 half-day training sessions in assessing JVP, followed by regular periodic bedside assessments by the principal investigator until staff members felt confident with the technique.
Randomization
Patients were randomly assigned in a 1:1 ratio to either usual care or the simplified severe sepsis protocol (SSSP). Assignments were based on computer-generated permuted blocks of 2, 4, and 6. Randomization assignments were placed in numbered, sealed opaque envelopes, which were opened after written informed consent was obtained. Study staff were not blinded to patient assignments. Emergency room and internal medicine doctors were not informed of patient assignment, although protocol orders were readily visible in the patients’ files.
Interventions
Patients in the usual care group received care as per orders written by the emergency room physicians. Usual care consisted of IV fluids, antibiotics, and occasional use of non-titrated dopamine. A dedicated study nurse monitored all patients in both groups, ensured all orders were carried out, and notified emergency room physicians of critical vital signs or changes in condition. Vital signs were monitored every hour for 6 hours. The SSSP (intervention) group received protocol-based care for the first 6 hours following enrollment. Patients in the SSSP group received an initial 2 liter bolus of normal saline or lactated Ringer’s within 1 hour of assessment. After the initial bolus, an investigator or study nurse re-evaluated the patient’s JVP. If JVP was less than 3 cm above the sternal angle, patients received an additional 2 liters of fluid over 4 hours, for a total of 4 liters in the first 5-6 hours of enrollment. The target of 4 liters was determined based on previous goal-directed therapy studies.(10,11) Fluids were stopped for any patient who developed worsening respiratory signs or symptoms as determined by the study physician or non-study doctors. If mean arterial blood pressure (MAP) was below 65 mmHg after 2 liter fluid bolus, then a dopamine infusion was started at a rate of 10 mcg/kg/min, to be titrated to maintain a MAP>=65 mmHg. SSSP patients with hemoglobin <7g/dL were offered whole blood transfusion. In both groups, blood cultures were drawn and antibiotics were commenced as soon as possible, preferably within one hour of recognition of sepsis. One aerobic blood culture was drawn from each patient and incubated in a Bactec FX (BD Diagnostics, Sparks, MD, USA). In HIV positive patients, an additional mycobacterial blood culture was collected and incubated. Positive mycobacterial cultures were speciated as Mycobacterium tuberculosis complex or non-tuberculosis complex based on MPT64 rapid antigen test (Standard Diagnostics, Seoul, South Korea).(18) Selection of antibiotics, anti-malarials, and tuberculosis therapy was left to the admitting non-study physicians, as was the determination of additional investigations, such as malaria blood slides and cerebral spinal fluid studies. Decisions regarding transfer to intensive care unit (ICU) and initiation of mechanical ventilation or hemodialysis were likewise left to non-study physicians. Patients in both arms could receive empiric whole blood transfusion if ordered by the admitting doctors.
Outcomes
The primary outcome was in-hospital all-cause mortality. Secondary mortality outcomes included 28-day all-cause mortality, in-hospital and 28-day mortalities adjusted for the Simplified Acute Physiology Score-3 (SAPS3), and time to death.(19) Process measures included volume of IV fluids administered in the first 6, 24, and 72 hours, proportion of patients receiving antibiotics within 1 hour and proportion of patients receiving blood transfusion. Patients were monitored for changes in respiratory status, including rise in respiratory rate of 5 or more and decrease in oxyhemoglobin saturation (SpO2) of 3% or more. Reasons for stopping IV fluids were also recorded.
Statistical analysis
Our previous data found a 54.9% inpatient mortality rate in patients admitted to our hospital with severe sepsis.(14) We used this figure as the expected mortality in the control group. Assuming a 5% two-sided type I error rate and 80% power, we estimated that a sample size of 342 patients would be required to detect a 15% absolute reduction in in-hospital mortality.
Baseline characteristics of patients with severe sepsis were compared by intervention using t-test for continuous variables and chi-squared or Fisher’s exact tests for categorical variables. Kaplan-Meier estimates and log-rank test were used to compare survival by intervention up to twenty-eight days following admission. All hypothesis testing was two-sided with a level of significance set at 0.05. We assessed in-hospital mortality for the following pre-specified subgroups: HIV positive vs. negative, MAP≤65 vs. >65 mmHg, RR ≥40 vs. <40, hemoglobin ≥7 vs. <7 g/dL, and above vs. below median score for SAPS3. Post hoc subgroup analyses included patients with hypoxemic respiratory distress, defined as baseline respiratory rate >40/min and SpO2<90%, and patients with tuberculosis. Multivariable log-linear regression was used for subgroup analyses to estimate risk ratios adjusted for baseline SAPS3 score. We tested for interaction effect between subgroup and intervention group on the risk of in-hospital mortality using Breslow-Day test of homogeneity. Linear regression was used to assess changes in fluid administration in the usual care group as a function of date of study initiation. All analyses were intention to treat, using Stata version 12.1 (StataCorp, College Station, TX, USA) and OpenEpi (www.openepi.com) for Breslow-Day calculations.
Results
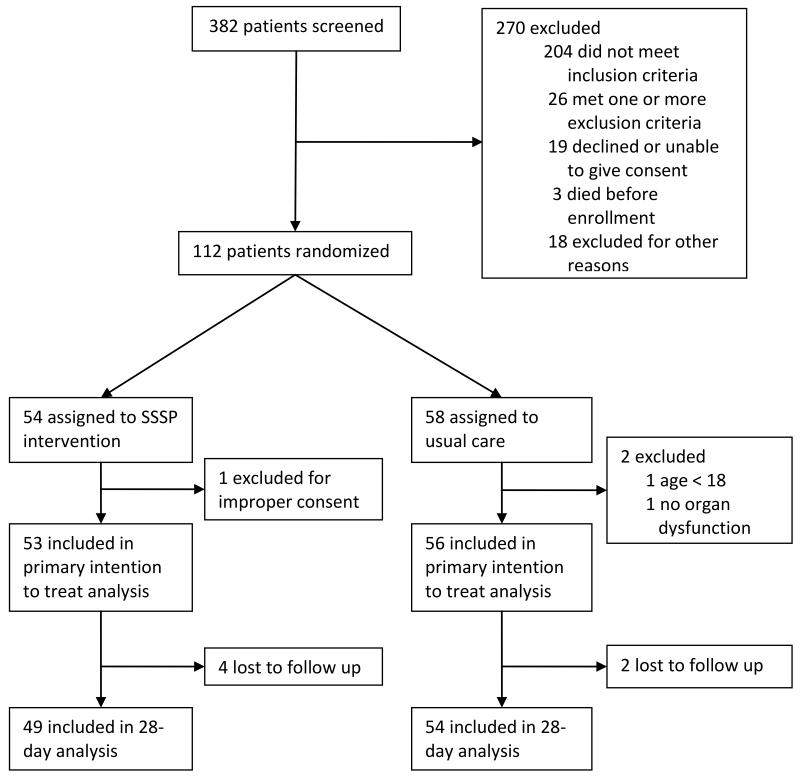
We enrolled 112 patients in the study (Figure 1). Two patients not meeting the eligibility criteria were excluded within hours of enrollment. One patient was excluded within one hour of randomization because informed consent was verbal and not written. The remaining 109 patients were included in intention to treat analysis. In-hospital survival was available for all patients. The 28-day survival outcome was not ascertained in six patients who were lost to follow-up after discharge.
Figure 1. Study flow diagram.
88 patients (80.7%) were HIV positive, with median CD4 count of 49 cells/cmm. Median time from admission to enrollment was 2.5 hours (Interquartile range [IQR] 0.9 – 6.4 hours). In 100 patients for whom JVP was assessable, 88 (88%) had below-normal JVP, including 28 with expiratory JVP at the level of the sternal angle (0cm), and 60 with a JVP that was below the sternal angle and only visible in the supine position. Mean respiratory rate was very high in both the intervention and control groups, 38.2 and 37.7 breaths per minute, respectively. Pulmonary infection, was the most commonly suspected source of sepsis, occurring in 63 of 109 participants (57.8%). 81 patients (74.3%) were non-ambulatory at the time of admission with a median time of 5 days (IQR 3-7) since last walking. Baseline characteristics are summarized in Table 1.
Table 1.
Baseline characteristics of Zambian patients with severe sepsis
| SSSP n=53 |
Control n=56 |
p | |
|---|---|---|---|
| Age, years, mean (SD) | 35.2 (1.3) | 34.8 (1.4) | 0.85 |
|
| |||
| Male, n (%) | 28 (52.8) | 30 (53.6) | 0.94 |
|
| |||
| Admission Vital Signs, mean (SD) | |||
| SBP, mmHg | 102.6 (21.4) | 101.6 (24.2) | 0.83 |
| DBP, mmHg | 62.4 (14.4) | 65.2 (17.2) | 0.35 |
| MAP, mmHg | 75.8 (15.4) | 77.3 (18.8) | 0.64 |
| RR, breaths/min | 38.2 (10.9) | 37.7 (11.2) | 0.81 |
| HR, beats/min | 119.2 (15.8) | 122.9 (22.3) | 0.32 |
| Temp, degrees C | 37.3 (1.5) | 37.9 (1.7) | 0.07 |
|
| |||
| GCS, median (IQR) | 14 (11-15) | 14 (10-15) | 0.76 |
|
| |||
| HIV positive, n (%) | 42 (79.2) | 46 (82.1) | 0.70 |
| CD4 count, median (IQR)+ | 40 (17-107) | 70 (24-109) | 0.41 |
| On ARVs, n (% of HIV positive) | 18 (42.9) | 16 (35.6) | 0.49 |
|
| |||
| Suspected site of infection, n (%) | 0.86 | ||
| Pulmonary | 31 (58.5) | 32 (57.1) | |
| Central nervous system | 19 (35.8) | 15 (26.8) | |
| Abdomen | 3 (5.7) | 4 (7.1) | |
| Other ^ | 6 (11.3) | 7 (12.5) | |
|
| |||
| Chief complaint, n (%) | 0.62 | ||
| Dyspnea | 13 (24.5) | 15 (26.8) | |
| Altered mentation | 9 (17.0) | 10 (17.9) | |
| Cough | 9 (17.0) | 8 (14.3) | |
| Headache | 7 (13.2) | 10 (17.9) | |
| Weakness/fatigue | 10 (18.9) | 4 (7.1) | |
| Abdominal pain | 3 (5.7) | 5 (8.9) | |
| Other ^ | 2 (3.8) | 4 (7.1) | |
|
| |||
|
Duration of chief complaint, days,
median (IQR) |
7 (4-30) | 14 (3-30) | 0.81 |
|
| |||
|
Duration of any symptoms*, days,
median (IQR) |
14 (7-30) | 30 (14-60) | 0.26 |
|
| |||
| SAPS3 score, mean (SD) | 59.0 (1.6) | 56.3(1.5) | 0.23 |
|
| |||
| APACHE II score, mean (SD) | 17.8 (0.8) | 17.9 (0.9) | 0.95 |
SSSP=Simplified Severe Sepsis Protocol, SD=Standard deviation, SBP=Systolic blood pressure, DBP=Diastolic blood pressure, MAP=Mean arterial pressure, RR=Respiratory rate, HR=Heart rate, Temp=Temperature, GCS=Glasgow coma scale, ARVs=Antiretrovirals, SAPS3=Simplified Acute Physiology Score-3
Other chief complaints included fever (3), diarrhea (1), vomiting (1), and seizure (1)
Symptoms include inability to walk or any symptom listed under chief complaint
Management
Treatment of the two groups is summarized in Table 2. Slightly over half of patients received a third-generation cephalosporin, either alone or as part of a combination. Anti-tuberculous therapy was given to 21 (39.6%) SSSP patients and 14 (25.0%) control patients. Median time to first dose of antibiotics was 1.4 hours (IQR 0.5-3.1) from time of admission and 0 hours (IQR −1.3-2.0) from time of enrolment. Patients in the SSSP group received significantly more IV fluids in the first 6 hours compared with control (2.8L vs. 1.6L, p<0.001). 30 of 53 patients (56.6%) in the SSSP group received at least 3L in the first 6 hours, and 14 of 53 (26.4%) received at least 4L. The most common reasons for stopping fluids included tachypnea or decreased SpO2 (in 8 patients), raised JVP (7), lost IV access (2), blood transfusion (5) and poor urine output in suspected oliguric renal failure (1). The SSSP group received more fluid in the first 72 hours, 5.5L vs. 4.3L (p = 0.02). Regressing 6-hour IV fluid administration on date of admission in the control group resulted in no meaningfully different fluid administration over the study period (−0.005/day, 95% CI −0.013–0.003), suggesting that usual practices were not substantially impacted by a Hawthorne-like effect during the study period.
Table 2.
Treatments administered and adverse events
| SSSP n=53 |
Control n=56 |
p | |
|---|---|---|---|
| Initial antibiotic regimens a | -- | ||
| 3rd gen cephalosporin b | 22 (41.5) | 20 (35.7) | |
| 3rd gen cephalosporin combination c | 5 (9.4) | 10 (17.9) | |
| Penicillin G | 4 (7.5) | 4 (7.2) | |
| Penicillin G + chloramphenicol | 6 (11.3)d | 7 (12.5) | |
| Other Penicillin G combination c | 6 (11.3) | 3 (5.4) | |
| Ciprofloxacin +/− metronidazole | 5 (9.4) | 3 (5.4) | |
| Other e | 4 (7.5) | 7 (12.5) | |
| Not documented | 1 (1.9) | 2 (3.6) | |
|
| |||
| Co-trimoxazole f | 6 (11.3) | 8 (14.3) | 0.64 |
|
| |||
| Antituberculous therapy | 0.087 | ||
| Started at admission or before | 8 (15.1) | 9 (16.1) | |
| Started after admission | 13 (24.5) | 5 (8.9) | |
|
| |||
| Median time to antibiotics, hrs (IQR) g | |||
| from admission | 1.5 (0.5-3.7) | 1.3 (0.4-3.0) | 0.42 |
| from study enrollment | −0.7 (−2.5-0.5) | 0 (−1.5-0.9) | 0.19 |
|
| |||
| Received ≥3L fluid in 6 hours, n (%) | 30 (56.6) | 11 (20.0) | <0.001 |
|
| |||
| Fluids administered, L, mean (SD) h | |||
| In first 6 hours | 2.9 (1.0) | 1.6 (1.1) | <0.001 |
| In first 24 hours | 3.9 (1.3) | 3.0 (2.1) | 0.02 |
| In first 72 hours | 5.6 (2.3) | 4.3 (2.9) | 0.02 |
|
| |||
| Received blood transfusion, n (%) | 16 (30.2) | 11 (19.6) | 0.20 |
|
| |||
| Median time to transfusion, hrs (IQR) g | 16.0 (7.3-32.9) | 5.5 (0-13.8) | 0.17 |
|
| |||
| Received dopamine, n (%) | 1 (1.8) | 3 (5.7) | 0.28 |
|
| |||
| Stopped IV fluids early, n (%) | 25 (49.0) | -- | -- |
|
| |||
| Reasons for stopping: | |||
| Raised JVP | 7 (13.2) | ||
| Respiratory changes | 8 (15.1) | ||
| Lost IV access | 2 (3.8) | ||
| Transfuse blood | 5 (9.4) | ||
| Oliguria | 1 (1.9) | ||
| Other | 2 (3.8) | ||
|
| |||
|
Increase in RR or decrease in SpO2 in
first 6 hours, n (%) i |
18 (34.0) | 16 (28.6) | 0.54 |
Excludes anti-tuberculous therapy or co-trimoxazole therapy, listed separately
Includes 3 patients who received 3rd gen cephalosporin plus crystalline penicillin
Includes combinations with ciprofloxacin, erythromycin, metronidazole, and/or cloxacillin
Includes 2 patients who received Crystalline penicillin + chloramphenicol + metronidazole
Cloxacillin+metronidazole (1), chloramphenicol (1), erythromycin (3), cotrimoxazole mono-threrapy (2), antituberculous therapy only (4)
Includes prophylaxis or treatment doses, used with one of the above combinations in 12 patients; 2 patients received only co-trimoxazole
Missing data on time to blood transfusion in 2 transfused patients and antibiotics start time in 12 patients
Missing data for fluids in first 6 hours (1 patient), 24 hours (8 patients), and 72 hours (15 patients)
Respiratory changes included respiratory rate increase of 5 breaths/min or more or decrease in oxyhemoglobin saturation of 3% or more
SSSP=Simplified Severe Sepsis Protocol, IQR=Interquartile range, SD=Standard deviation, L=Liters, JVP=Jugular venous pressure, IV=Intravenous
Only 3 patients in the SSSP group and 1 patient in the control group received dopamine. 16 patients (30.2%) in the intervention group and 11 (19.6%) control patients received blood transfusion (p=0.20). Median number of units transfused was identical between the two groups (2 units). The majority of patients received antibiotics within one hour of enrollment, although antibiotics start time was missing for 12 patients. Only 2 patients – one in each group – were treated in the ICU. For both patients, the indication for ICU transfer was mechanical ventilation. The local conventions for ICU use are described in the Discussion section.
Clinical outcomes
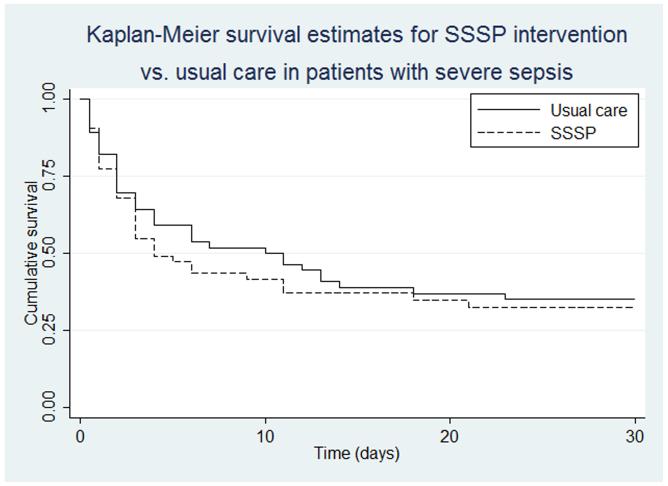
Overall, 68 (62.4%) patients died prior to discharge. In-hospital mortality was not significantly different between the two groups. Thirty-four of 53 (64.2%) in the intervention group died in hospital compared with 34 of 56 (60.7%) in controls (RR 1.05, 95% CI 0.79-1.41, SAPS3-adjusted risk ratio 1.01, 95% CI 0.76-1.33). 28-day mortality was 71.4% in the intervention group compared with 66.7% for controls [RR 1.07 (0.83-1.39), ARR 1.03 (0.81-1.32)]. We failed to detect differences between intervention and control for the pre-specified subgroups (Table 3). Because categorizing continuous predictors into intervals may lead to loss of power, models were re-run with continuous exposures (i.e, not categorized into a priori subgroups) with similar results (not shown). Median survival was 4 days in the SSSP group versus 9 days in the control group, but the IQR was 2 to 28 days in both groups and Kaplan-Meier estimates showed little difference (log-rank p=0.57)(Fig. 2).
Table 3.
Relative risk of in-hospital death in patients managed with Simplified Severe Sepsis Protocol (SSSP) vs. usual care: Subgroup Analysis#
| Subgroups | SSSP* | Control* | RR (95% CI) | p-value# |
|---|---|---|---|---|
| HIV positive | 29/42 (69.0) | 29/46 (63.0) | 1.10 (0.81-1.48) | 0.70 |
| HIV negative | 5/11 (45.5) | 5/10 (50.0) | 0.91 (0.37-2.22) | |
|
| ||||
| MAP>=65 mmHg | 26/39 (66.7) | 28/46 (60.9) | 1.10 (0.79-1.51) | 0.72 |
| MAP <65 mmHg | 8/14 (57.1) | 6/10 (60.0) | 0.95 (0.48-1.88) | |
|
| ||||
| Respiratory rate>40 ** | 16/20 (80.0) | 15/23 (65.2) | 1.23 (0.85-1.78) | 0.37 |
| Respiratory rate<=40 | 18/33 (54.5) | 18/32 (56.3) | 0.97 (0.63-1.50) | |
|
| ||||
| Hb<7 *** | 8/13 (61.5) | 5/8 (62.5) | 0.98 (0.50-1.96) | 0.83 |
| Hb>=7 | 20/33 (60.6) | 22/39 (56.4) | 1.07 (0.73-1.59) | |
|
| ||||
| SAPS3 >= median | 22/28 (78.6) | 20/29 (69.0) | 1.14 (0.83-1.56) | 0.52 |
| SAPS3 < median | 12/25 (48.0) | 14/27 (51.9) | 0.93 (0.54-1.60) | |
|
| ||||
| Confirmed TB | 10/15 (66.7) | 12/16 (75.0) | 0.89 (0.56-1.40) | 0.39 |
| No confirmed TB | 24/38 (63.2) | 22/40 (55.0) | 1.15 (0.79-1.66) | |
|
| ||||
|
Hypoxemic respiratory
distress † |
8/8 (100.0) | 7/10 (70.0) | 1.43 (0.95-2.14) | 0.17 |
|
No hypoxemic
respiratory distress |
26/45 (57.8) | 27/46 (58.7) | 0.98 (0.70-1.39) | |
|
| ||||
| Overall | 34/53 (64.2) | 34/56 (60.7) | 1.06 (0.79-1.41) | |
All subgroups were prespecified, except tuberculosis and hypoxemic respiratory distress
Percent mortality in parentheses
missing respiratory rate in 1 participant
missing Hb in 18 participants
Hypoxemic respiratory distress defined as RR>40 and oxyhemoglobin saturation < 90%
Test for interaction of risk ratio over subgroups.
SSSP=Simplified Severe Sepsis Protocol, MAP=Mean arterial pressure, Hb=Hemoglobin, SAPS3=Simplified Acute Physiology Score-3
Figure 2. Kaplan-Meier survival estimates for SSSP intervention vs. usual care in patients with severe sepsis.
Bacteriological findings
Twenty-six patients (23.9%) had positive aerobic blood cultures. The most common organisms were Staphylococcus aureus (7) and Streptococcus pneumoniae and Salmonella typhi (3 each). Mycobacterial blood cultures were collected from HIV positive patients only, with 31 of 82 (37.8%) demonstrating tuberculosis mycobacteremia. Two patients had co-infection with TB and S. aureus. The other 29 patients with TB bacteremia had no other identifiable etiology for their sepsis. Among 46 patients with CD4 count less than 75 cells/cmm, 22 (47.8%) had positive TB blood cultures. Four patients, all in the control group, had evidence of Cryptococcus neoformans in the cerebrospinal fluid. Two patients, one in each group, had blood-slide-positive malaria.
Decision to Stop Study
The study was stopped early by the investigators prior to the scheduled interim analysis, in communication with UNZA BREC and Vanderbilt IRB, due to the observation that patients with hypoxemic respiratory distress at baseline might be at increased risk from the intervention. Because only 2 of 109 patients were transferred to ICU for mechanical ventilation, the investigators initiated an unscheduled analysis of participants with baseline respiratory rate above 40/min and oxygen saturation < 90%. In this group, 15 of 18 (83.3%) died during hospitalization, including 8 of 8 (100%) in the intervention group and 7 of 10 in the controls (70%, p=0.09). Based on these findings, the decision was made to stop the study.
Discussion
In this pilot randomized controlled trial in Lusaka, Zambia, a novel goal-directed therapy protocol consisting of early aggressive IV fluids, with dopamine and blood transfusion in selected patients, was not effective in reducing in-hospital mortality compared with usual care. The study was stopped early due to observations that patients with severe respiratory distress were unlikely to benefit from the intervention, and were at potential risk of harm. The study intentionally used broad inclusion criteria, defining severe sepsis as probable infection with SIRS and organ dysfunction. End-organ dysfunction was likely unrelated to tissue hypoperfusion in a significant proportion of patients. Patients with confusion due to meningitis or respiratory distress due to pulmonary inflammation have other mechanisms of organ damage that might be worsened with aggressive fluid administration.
Previous studies from sub-Saharan Africa have shown both harm and benefit with fluid-based interventions. The FEAST trial in Kenya, Uganda, and Tanzania found increased mortality from fluid boluses in children with severe febrile illness.(4) On the other hand, a before-after study in Ugandan adults, by the PRISM-U group demonstrated a 12.7% absolute reduction in 30-day mortality.(6) There were several key differences between our study and PRISM-U. Their study enrolled only patients with low or low-normal blood pressures. Our median systolic blood pressure of 100 mmHg was considerably higher than the median of 81-85 mmHg in PRISM-U. The control group in the PRISM-U study received a median of only 500 mL of fluids in the first 6 hours and 1 liter in the first 24 hours. In contrast, median fluid administration in the control arm of the SSSP study was 1.6 liters in 6 hours and 3.0 liters in the first 24 hours. It is likely that the observational “before” arm of PRISM-U received less nursing attention than the “after” intervention arm. In resource limited settings, low volumes of fluid administration may be the result of doctors’ orders or inadequate nurse staffing. In the SSSP study both the intervention and control group received one-to-one care in the first 6 hours from a dedicated study nurse. Furthermore, the two groups received equal care and attention except for the use of the SSSP protocol to direct fluid, dopamine, and transfusion administration.
Our intervention was similar in many respects to the protocol-based standard therapy arm in the recently reported PROCESS study, based in the United States.(20) Their protocol-based standard therapy also called for 2L initial fluid bolus within 1 hour, noninvasive monitoring, jugular venous distension and respiratory monitoring for fluid overload, blood pressure triggers for vasopressor initiation, and blood transfusion for severe anemia (<7.5g/dL in PROCESS; <7.0 g/dL in SSSP). Neither study demonstrated a significant mortality difference between the protocolized groups and usual care.
At 62%, our mortality rate was higher than mortality rates seen in PRISM-U and PROCESS. ICU utilization in our study was extremely low. The decision to transfer patients to the ICU was left to non-study physicians in order to limit bias in this non-blinded study. The study hospital, UTH, has only 10 ICU beds for a 1500-bed hospital with catchment population of 13 million, and the majority of ICU beds are usually occupied by surgical patients. The bias of medical staff to transfer to ICU is generally in favor of patients with easily reversible conditions, such as hydrostatic pulmonary edema, status epilepticus, and severe malaria, and against patients with chronic wasting from HIV and/or TB. The low rate of ICU transfer was an important factor in the decision to stop this study early, as appropriate ventilatory support could not be guaranteed in patients with pre-existing or fluid-induced severe respiratory distress.
Compared with a previous study of septic patients with and without organ dysfunction at our hospital, our study patients had higher mortality rates (62% vs. 40%) and baseline respiratory rates (mean RR 38/min vs. 28/min), and pulmonary source of infection was more common (58% vs. 25%).(14) These differences could be attributable to methodologic differences, particularly the SSSP organ failure inclusion criteria, which included respiratory rate > 40/min, and to a high proportion of unspecified (32.3%) infections in the observational study. Of note, the usual care arm of SSSP received more fluids at 6 hours (median of 1.6L vs. 1L) and 24 hours (median of 3.0L vs. 1L) than previously observed. Although usual care did not change appreciably from the beginning to the end of this study, we cannot rule out the possibility that the pre-study training may have impacted usual prescribing practices. We think it is more likely, though, that dedicated study nurses led to more consistent implementation of doctors’ orders compared with pre-study periods. However, any study such as ours that compares a new intervention to usual care runs the risk of influencing the usual care control arm towards imitation of an as-yet unproven intervention.
There were several important lessons learned in conducting this study. First and foremost, the use of simplified inclusion criteria that only loosely reflect the pathophysiology of interest should be avoided. As with the FEAST study, SSSP sought to identify hypoperfused severely septic patients without utilizing costly testing such as lactic acid measurement. The intention was to use inclusion criteria that could be generalizable to clinical use in the most resource-limited settings. In FEAST, the majority (70%) of patients qualified as hypoperfused based on severe tachycardia, a nonspecific measure that could reflect hypovolemia, hypoxemia, anaemia, high fever, or other distress. Our study made a faulty assumption that organ dysfunction alone was indicative of tissue hypoperfusion in a septic population with a high prevalence of volume depletion. In retrospect, our criteria failed to adequately consider the direct tissue damage attributable to inflammation of the lungs and/or brain. Design of future sepsis studies involving IV fluids should consider more reliable measures of hypoperfusion or fluid responsiveness.
Another important lesson learned regarded the management of patients with respiratory distress. In the absence of available ventilator support, caution must be exercised when administering IV fluid boluses to such patients. Where ventilator support is unavailable, the decision to include patients with moderate to severe respiratory distress should be scrutinized prior to any study of IV fluid intervention. FEAST and SSSP included severe respiratory distress and severe tachypnea, respectively, as organ dysfunction inclusion criteria, so it shouldn’t have been surprising that 83% of participants in FEAST had respiratory distress and 39% of SSSP participants had respiratory rate above 40. Interestingly, the median respiratory rates in SSSP (38/min) were nearly identical to those seen in PRISM-U (36-38/min). However, despite similar volumes of fluid, more patients in SSSP developed worsening respiratory signs. Adjunctive therapies, such as non-invasive positive pressure ventilation (NIV), might be considered for patients with respiratory distress, and studies into the efficacy of NIV are warranted in this setting.
Our study raises the question of the role of hyper-acute interventions in the management of chronic or sub-acute processes. Unlike in North America and Europe, where sepsis is typically an acute process, sepsis in sub-Saharan Africa frequently results from sub-acute or chronic infection. Over 80% of patients were HIV infected, and the majority had symptoms for at least 2 weeks preceding admission. The leading etiology of sepsis in our study was tuberculosis, with other concomitant pathogens very rarely isolated. Similar prevalence of tuberculous bloodstream infections has been shown in Malawi, Tanzania, and Uganda.(13,21-24) This varies greatly from the literature in high-resource settings, where gram positive and gram negative bacteria typically account for over 60% of confirmed etiologies.(25,26) Our findings suggest that disseminated tuberculosis infection should be strongly suspected in patients presenting with severe sepsis in sub-Saharan Africa, especially in persons infected with HIV. Because the septic process in these patients may be sub-acute or chronic, it is reasonable to expect that acute time-dependent interventions like early goal-directed therapy may have limited impact.
The question of an optimal fluid target for patients with sepsis and tissue hypoperfusion in sub-Saharan Africa remains unanswered. The PRISM-U study demonstrated that a “usual care” consisting of less than 1L of fluids for septic patients with low to low-normal blood pressures was not sufficient. A post hoc observational analysis of PRISM-U suggested that the worst prognosis was in patients receiving <=1 L of fluids in the first 6 hours, and the best prognosis was in patients who received >1 to 2.5L of fluid in the first 6 hours, with similar adjusted outcomes for those who received > 3.5L. The mean 6-hour fluid intake in our study was 1.7L in the control group compared with 2.7L in the intervention group. We set a cut-off of 4 L of fluid in the first 6 hours and we used JVP, rather than blood pressure, as the guide to stopping fluids. We intentionally selected JVP because blood pressure may frequently normalize prior to full volume resuscitation. However we recognize the difficulty in standardizing JVP measurements, particularly among nursing staff and less experienced doctors. Beyond blood pressure and JVP, other methods, such as fluid responsiveness using straight leg raise, warrant investigation in this setting.
Other questions remain with regards to optimal treatment of patients with severe sepsis in sub-Saharan Africa. We must recognize that the sepsis syndrome is a heterogeneous collection of infectious conditions, each with unique physiologic characteristics. While some septic patients may benefit from early fluid administration, in the absence of mechanical ventilation those with severe respiratory distress clearly do not. Delays in appropriate anti-tuberculous therapy likely contribute to poor outcomes,(27) magnifying the importance of clinical algorithms or point-of-care diagnostics to detect TB earlier.(28) A study is ongoing to determine the impact of a urine lipoarabinomannan assay for detection of TB in HIV positive hospitalized patients (Clinicaltrials.gov identifier: NCT01770730). Scalable interventions, such as non-invasive positive pressure ventilation, could be studied as potential therapy for those patients who present with sepsis and severe respiratory distress.
In conclusion, this randomized controlled trial of early goal-directed fluid administration, dopamine, and blood transfusion for Zambian patients with severe sepsis was stopped early due to possible increased risk among patients with hypoxemic respiratory distress. While the question of optimal fluid administration remains unanswered, any future studies of fluid interventions should carefully consider inclusion criteria to identify patients most likely to benefit from IV fluids, and should consider excluding patients with severe respiratory distress when mechanical ventilation is not available.
Acknowledgments
Medical Officers: Dr. Grant Swisher, Boston University Medical Center Medical students: Brian Heiniger, Vanderbilt University Nurses: Emmanuel Chibwe, Peter Nyauma, Joe Musonda, Mary Kaonga, and Jibe Milimo, University Teaching Hospital
Laboratory staff: Eugene Silomba, AIDSRelief Zambia
This work was supported by the National Institutes of Health Office of the Director and Fogarty International Center through the International Clinical Research Fellows Program at Vanderbilt University (R24 TW007988).
Footnotes
Work was performed at the University Teaching Hospital and the University of Zambia, School of Medicine in Lusaka, Zambia.
No reprints are requested.
Copyright form disclosures:
Dr. Andrews received support for article research from NIH. His institution received grant support and support for travel from the National Institutes of Health/Fogarty International Center. Dr. Muchemwa received grant support and support for travel from the National Institutes of Health/Fogarty International Center, received grant support from NIH, and received support for article research from NIH. Dr. Heimburger received support for travel from and served as board member for Dannon Research Institute and he received book royalties from Elsevier. His institution received grant support from the National Institutes of Health/Fogarty International Center. Dr. Bernard received support for article research from NIH. His institution received grant support (Drug supplies for ARDSnet SAILS trial). The remaining authors have disclosed that they do not have any potential conflicts of interest.
References
- 1.Angus DC, Linde-Zwirble WT, Lidicker J, et al. Epidemiology of severe sepsis in the United States: Analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Mathers CD, Boerma T, Ma Fat D. Global and regional causes of death. Br Med Bull. 2009;92:7–32. doi: 10.1093/bmb/ldp028. [DOI] [PubMed] [Google Scholar]
- 3.Lawn SD, Harries AD, Anglaret X, et al. Early mortality among adults accessing antiretroviral treatment programmes in sub-Saharan Africa. AIDS. 2008;22:1897–1908. doi: 10.1097/QAD.0b013e32830007cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maitland K, Kiguli S, Opoka RO, et al. FEAST Trial Group Mortality after fluid bolus in African children with severe infection. N Engl J Med. 2011;364:2483–2495. doi: 10.1056/NEJMoa1101549. [DOI] [PubMed] [Google Scholar]
- 5.Myburgh JA. Fluid resuscitation in acute illness--time to reappraise the basics. N Engl J Med. 2011;364:2543–2544. doi: 10.1056/NEJMe1105490. [DOI] [PubMed] [Google Scholar]
- 6.Jacob ST, Banura P, Baeten JM, et al. The impact of early monitored management on survival in hospitalized adult Ugandan patients with severe sepsis: A prospective intervention study. Crit Care Med. 2012;40:2050–2058. doi: 10.1097/CCM.0b013e31824e65d7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee BW. Improving sepsis care in resource limited settings. Crit Care Med. 2012;40:2234–2235. doi: 10.1097/CCM.0b013e3182515068. [DOI] [PubMed] [Google Scholar]
- 8.Dellinger RP, Levy MM, Rhodes A, et al. Surviving Sepsis Campaign: International guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med. 2013;41:580–637. doi: 10.1097/CCM.0b013e31827e83af. [DOI] [PubMed] [Google Scholar]
- 9.Levy MM, Dellinger RP, Townsend SR, et al. The Surviving Sepsis Campaign: Results of an international guideline based performance improvement program targeting severe sepsis. Crit Care Med. 2010;38:367–374. doi: 10.1097/CCM.0b013e3181cb0cdc. [DOI] [PubMed] [Google Scholar]
- 10.Rivers E, Nguyen B, Havstad S, et al. Early Goal-Directed Therapy Collaborative Group: Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368–1377. doi: 10.1056/NEJMoa010307. [DOI] [PubMed] [Google Scholar]
- 11.Jones Alan E, Shapiro Nathan I, Trzeciak Stephen, et al. Lactate Clearance vs Central Venous Oxygen Saturation as Goals of Early Sepsis Therapy: A Randomized Clinical Trial. JAMA. 2010;303:739–746. doi: 10.1001/jama.2010.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baelani I, Jochberger S, Laimer T, et al. Availability of critical care resources to treat patients with severe sepsis or septic shock in Africa: a self-reported, continent-wide survey of anaesthesia providers. Crit Care. 2011;15:R10. doi: 10.1186/cc9410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jacob ST, Moore CC, Banura P, et al. Promoting Resource-Limited Interventions for Sepsis Management in Uganda (PRISM-U) Study Group: Severe sepsis in two Ugandan hospitals: A prospective observational study of management and outcomes in a predominantly HIV-1 infected population. PLoS ONE. 2009;4:e7782. doi: 10.1371/journal.pone.0007782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chimese SM, Andrews B, Lakhi S. The etiology and outcome of adult patients presenting with sepsis to the University Teaching Hospital, Lusaka, Zambia. Medical Journal of Zambia. 2012;39:19–22. [PMC free article] [PubMed] [Google Scholar]
- 15.Andrews B. Surviving Sepsis in High HIV Prevalence Settings. Medical Journal of Zambia. 2010;37:104–110. [PMC free article] [PubMed] [Google Scholar]
- 16.Applefeld MM. The Jugular Venous Pressure and Pulse Contour. In: Walker HK, Hall WD, Hurst JW, editors. Clinical Methods: The History, Physical, and Laboratory Examinations. 3rd edition. Boston, Butterworths: 1990. pp. 107–111. [PubMed] [Google Scholar]
- 17.McGee SR. Physical examination of venous pressure: A critical review. Am Heart J. 1998;136:10–18. doi: 10.1016/s0002-8703(98)70175-9. [DOI] [PubMed] [Google Scholar]
- 18.Yin X, Zheng L, Lin L, et al. Commercial MPT64-based tests for rapid identification of Mycobacterium tuberculosis complex: A meta-analysis. J Infect. 2013 doi: 10.1016/j.jinf.2013.06.009. pii: S0163-4453(13)00156-4: doi: 10:1016/j:jinf:2013:06:009: [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 19.Moreno RP, Metnitz PG, Almeida E, et al. SAPS 3--From evaluation of the patient to evaluation of the intensive care unit: Part 2: Development of a prognostic model for hospital mortality at ICU admission. Intensive Care Med. 2005;31:1345–55. doi: 10.1007/s00134-005-2763-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.The ProCESS Investigators [Accessed March 31, 2014];A randomized trial of protocol-based care for early septic shock. N Engl J Med. DOI: 10.1056/NEJMoa1401602. Supplementary material available at: http://www.nejm.org/action/showSupplements?doi=10.1056%2FNEJMoa1401602&viewType=Popup&viewClass=Suppl.
- 21.Peters RPH, Ziljstra EE, Schijffelen MJ, et al. A prospective study of bloodstream infections as cause of fever in Malawi: Clinical predictors and implications for management. Trop Med Int Health. 2004;9:928–934. doi: 10.1111/j.1365-3156.2004.01288.x. [DOI] [PubMed] [Google Scholar]
- 22.Archibald LK, McDonald LC, Nwanyanwu O, et al. A hospital-based prevalence survey of bloodstream infections in febrile patients in Malawi: Implications for diagnosis and therapy. J Infect Dis. 2000;181:1414–1420. doi: 10.1086/315367. [DOI] [PubMed] [Google Scholar]
- 23.Archibald LK, den Dulk MO, Pallangyo KJ, et al. Fatal mycobacterium tuberculosis bloodstream infections in febrile hospitalized adults in Dar es Salaam, Tanzania. Clin Infect Dis. 1998;26:290–296. doi: 10.1086/516297. [DOI] [PubMed] [Google Scholar]
- 24.Ssali F, Kamya M, Wabwire-Mangen F, et al. A prospective study of community-acquired bloodstream infections among febrile adults admitted to Mulago Hospital in Kampala, Uganda. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;19:484–489. doi: 10.1097/00042560-199812150-00007. [DOI] [PubMed] [Google Scholar]
- 25.Bernard GR, Vincent JL, Laterre PF, et al. Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med. 2001;344:699–709. doi: 10.1056/NEJM200103083441001. [DOI] [PubMed] [Google Scholar]
- 26.Kumar A, Roberts D, Wood KE, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med. 2006;34:1589–96. doi: 10.1097/01.CCM.0000217961.75225.E9. [DOI] [PubMed] [Google Scholar]
- 27.Kethireddy S, Light RB, Mirzanejad Y, et al. Mycobacterium tuberculosis septic shock. Chest. 2013;144:474–482. doi: 10.1378/chest.12-1286. [DOI] [PubMed] [Google Scholar]
- 28.Jacob ST, Pavlinac PB, Nakiyingi L, et al. Mycobacterium tuberculosis bacteremia in a cohort of HIV-infected patients hospitalized with severe sepsis in Uganda-high frequency, low clinical sand derivation of a clinical prediction score. PLoS One. 2013;8:e70305. doi: 10.1371/journal.pone.0070305. doi:10.1371/journal.pone.0070305. [DOI] [PMC free article] [PubMed] [Google Scholar]