Abstract
Learning to control our movements accompanies neuroplasticity of motor areas of the brain. The mechanisms of neuroplasticity are diverse and produce what is referred to as the motor engram, i.e. the neural trace of the motor memory. Transcranial direct current stimulation (tDCS) alters the neural and behavioral correlates of motor learning, but its precise influence on the motor engram is unknown. In this review, we summarize the effects of tDCS on neural activity and suggest a few key principles: 1) firing rates are increased by anodal polarization and decreased by cathodal polarization, 2) anodal polarization strengthens newly formed associations, and 3) polarization modulates the memory of new/preferred firing patterns. With these principles in mind, we review the effects of tDCS on motor control, motor learning, and clinical applications. The increased spontaneous and evoked firing rates may account for the modulation of dexterity in non-learning tasks by tDCS. The facilitation of new association may account for the effect of tDCS on learning in sequence tasks while the ability of tDCS to strengthen memories of new firing patterns may underlie the effect of tDCS on consolidation of skills. We then describe the mechanisms of neuroplasticity of motor cortical areas and how they might be influenced by tDCS. We end with current challenges for the fields of brain stimulation and motor learning.
Keywords: tDCS, motor cortex, motor control, motor learning
Introduction
The survival of a biological entity depends on its ability to accurately control the motion of its limbs, its head, and its eyes. Our nervous system provides us with the ability to learn this control, and the ability to maintain calibrated and accurate movements despite interactions with a changing body (e.g., fatiguing muscles) and a changing environment (e.g., variable tools and terrains). Motor learning is a general term that corresponds to these abilities: the ability to adapt to a change in the environment by forming an internal model that accurately predicts the sensory consequences of motor commands (termed motor adaptation, Lackner and DiZio, 1994; Shadmehr and Mussa-Ivaldi, 1994; Shadmehr et al., 2010), and the ability to become more skilled at a task by reducing the variability of motor commands and increasing accuracy (termed skill learning, Shmuelof et al., 2012). What is the neural basis of motor learning?
Until recently, the tools available to answer this question in humans were limited. One could study patient populations with focal deficits in the cerebellum (Martin et al., 1996; Smith and Shadmehr, 2005; Donchin et al., 2011; Izawa et al., 2012), or the parietal cortex (Mutha et al., 2011), one could disrupt motor cortex function with transcranial magnetic stimulation (Muellbacher et al., 2002; Richardson et al., 2006; Hadipour-Niktarash et al., 2007; Censor et al., 2010; Orban de Xivry et al., 2011a; Villalta et al., 2013) or one could use functional brain imaging in healthy populations (Shadmehr and Holcomb, 1997; Della-Maggiore et al., 2009; Landi et al., 2011; Hardwick et al., 2012; Lohse et al., 2014). However, in the past decade a non-invasive method of investigation, transcranial direct current stimulation (tDCS; Priori et al., 1998; Nitsche and Paulus, 2000), has become increasingly popular, allowing for electrical modulation of the neural tissue in the living human brain, resulting in the ability to alter function of specific regions, providing possibilities in terms of accelerating learning and/or retention, as well as quantifying the contributions of each brain region to the process of motor learning.
For example, consider a well-studied example of motor adaptation, holding a novel tool and attempting to reach to a target (Shadmehr and Mussa-Ivaldi, 1994). The tool’s dynamics will be unfamiliar to the brain, and the motor commands to the arm will produce a motion that will be different than predicted, resulting in sensory prediction errors. The error also produces learning, as evident by the fact that on the next movement the brain alters the motor commands to partially compensate for the novel dynamics of the tool (Thoroughman and Shadmehr, 2000). With training, some of the modifications to the motor commands become a motor memory (Shadmehr and Holcomb, 1997; Criscimagna-Hemminger and Shadmehr, 2008; Joiner and Smith, 2008). Formation of this motor memory appears dependent on the integrity of the cerebellum (Smith and Shadmehr, 2005; Criscimagna-Hemminger et al., 2010; Izawa et al., 2012; Taig et al., 2012; Gibo et al., 2013), the cerebellar output to the motor cortex via the thalamus (Chen et al., 2006). and the motor cortex (Li et al., 2001; Paz et al., 2003; Richardson et al., 2006; Arce et al., 2010a; Orban de Xivry et al., 2011a, 2013).
Remarkably, this form of motor adaptation in humans can be readily up-regulated or down-regulated by non-invasive stimulation of either the motor cortex or the cerebellum. Transcranial direct current (tDCS) stimulation of the brain, a technique where low current is delivered through the skull via two small electrodes, can indeed alter the excitability of the underlying tissue (Nitsche and Paulus, 2000). When placed on the motor cortex, tDCS can strengthen the motor memory formed during motor adaptation (Hunter et al., 2009) and alter the generalization patterns of the learning (Orban de Xivry et al., 2011b). The same stimulation method can facilitate the rate of motor adaptation if the anode is placed on the cerebellum (Galea et al., 2011; Herzfeld et al., 2014), and can inhibit the rate of motor adaptation if the cathode is placed on the cerebellum (Herzfeld et al. 2014).
With repetition of a learned behavior (Huang et al., 2011), variability of movements declines and speed of execution increases (Shmuelof et al., 2012). With repetition of motor commands the neurons in the motor cortex undergo plasticity, forming new synapses and dendritic spines (Xu et al., 2009; Yang et al., 2009). This reorganization of the motor cortex is part of the Motor Engram, i.e. the neural substrate of the motor memory. Interestingly, tDCS of the motor cortex facilitates the ability to retain motor skills (e.g. Reis et al., 2009).
The effects of tDCS on the Motor Engram are the major topics of this review in which we will attempt to describe the mechanisms of action of tDCS at the neuronal level and then consider the possibility that these mechanisms can explain behavioral effects found in modern tDCS studies and in clinical applications. Finally, we turn to the synaptic events observed during motor learning and their modulation by direct current stimulation. We conclude by laying out limitations of tDCS and consider a few open questions whose answer would push the field forward.
Early use of direct current polarization
Research on application of direct current (DC) polarization of the brain began in the 1950’s with the aim of shifting the “steady potential” of the brain. In early experiments (Bishop and O’Leary, 1950), DC polarization was applied in vivo on the dorsal nucleus of the lateral geniculate of the cat. One electrode was placed on the top surface and the other on the bottom. In this setup, all the neurons in the middle layer of the nucleus that were perpendicular to the surface were anodally stimulated in the dendrites while their axon was cathodally stimulated or vice-versa. The experiment in the thalamus, and later in the cerebellum (Chan and Nicholson, 1986; Chan et al., 1988), demonstrated that polarization of the dendrites could alter the response of the neuron to a synaptic input: anodal polarization of the dendrites increased the firing rate of neurons as evoked by a given input, whereas cathodal stimulation decreased this rate.
Later studies investigated the effect of DC polarization at the cellular and behavioral level in vivo. A key experiment was performed by Morrell (1961). In this experiment, small polarizing electrodes were placed subdurally on the surface of the cortex while the reference electrode was attached to the mouth or the ears of the animal. DC polarization of the motor cortex of the rabbit or cat did not produce a movement. However, it facilitated production of movements in response to a startling sensory stimulus (flash of light or sound). This behavioral effect of anodal polarization was termed “dominant focus of excitation” (Morrell, 1961). In addition, there was a residual low-voltage EMG activity in the limb related to the region of M1 that was stimulated.
Morrell made several observations from which we draw three principles. As we will see, these principles may account for most of the behavioral effects reported since the modern era of tDCS started.
First principle: firing rates are increased by anodal polarization and decreased by cathodal polarization
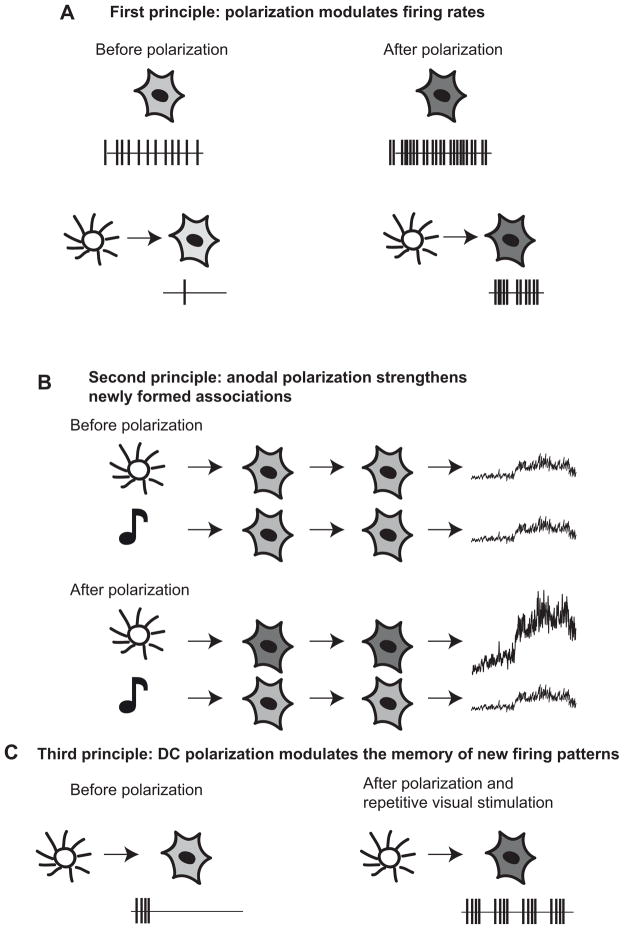
The enhanced behavioral response to the startling stimulus was paralleled at the neural level in the motor cortex (Morrell, 1961): neurons that did not fire in response to the stimulus before anodal polarization started to fire in response to the same stimulus (Fig. 1A). That is, anodal polarization of the cortex increased both the spontaneous and the evoked discharge rates (see also Creutzfeldt et al., 1962; Brazovskaya et al., 1972). Therefore, anodal polarization of the motor areas sensitized the animal to the stimulus that was presented during polarization.
Figure 1.
Illustration of the three main principles of the action of tDCS on neural activity and motor behavior. In all panels, light and dark gray neurons are associated with slightly active and very active neurons. Sun and music notes are associated with visual and auditory stimulus, respectively. In panels A and C, vertical black traces represent action potentials over time. In panel B, black traces on the far right schematically illustrate the degree of muscle activity. In panel A, dark-gray neurons representing polarized neurons exhibit higher spontaneous (top) or evoked (bottom) discharge rate. In panel B, before polarization, neither a flash of light nor a tone elicited a movement when presented (top). After a period of polarization during which a flash of light was repeatedly presented, the light now elicited a motor response while the tone did not. In panel C, a flash of light did not elicit a response in a neuron of the visual cortex. After polarization of the visual cortex and 3Hz visual stimulation, a single flash of light elicited a 3Hz response in the neurons.
Second principle: anodal polarization strengthens newly formed associations
As summarized by Sokolov (Sokolov, 1977), the results above suggest that polarization had the ability to “couple the conditioned (tone) and unconditioned (polarization) inputs” at the neural level (Fig. 1B). This after-effect was specific to the stimulus used during training. The neural response outlasted the polarization interval by 20 minutes (Morrell, 1961). For example, if sounds were used to elicit movements during DC polarization, a sound was still able to elicit a movement up to 20min later. Critically, during the same period, a flash of light was not able to elicit such a movement if it was not used to condition the motor response. This effect of modality suggests that anodal polarization specifically stabilizes newly formed associations in the cerebral cortex. Similar after-effects were observed in another study (Bindman et al., 1962) where an electrode was placed at the surface of the sensory cortex and another electrode was placed on a distal muscle. They observed an increase in the spontaneous and evoked electrical activity of neurons compared to pre-polarization levels during and for several hours after 5 minutes of anodal polarization.
In both studies (Morrell, 1961; Bindman et al., 1962; see also Landau et al., 1964), the neural effect was limited to the area surrounding the polarizing electrode and decreased with the distance between the neuron and this electrode (Morrell, 1961) or was restricted to the stimulated cortex and not found in the thalamus where evoked and spontaneous firing rates did not change (Bindman et al., 1962).
Third principle: DC polarization modulates the memory of new/preferred firing patterns
Lastly, anodal polarization facilitated the formation of a memory of an input pattern (Morrell, 1961). For instance, when a flash of light was repeatedly presented (3Hz) during anodal polarization of the visual cortex, it made visual cortex neurons fire repetitively at this frequency. At the end of the polarization period, a single flash of light entrained the same neuron to fire again at 3Hz (Fig. 1C). The propensity of neurons to fire at 3Hz after a single flash of light decreased over the next 20 minutes following the stimulation period. Therefore, anodal polarization conferred upon these neurons the property of retaining a pattern of discharge for a short period of time.
In summary, spontaneous and evoked firing rates are increase by anodal polarization and decreased by cathodal polarization. Anodal polarization reinforces the coupling between the conditioned and unconditioned inputs at the neural level. That is, learning is facilitated by anodal DC polarization (Sokolov, 1977). Finally, DC polarization modulates the memory of new/preferred firing patterns.
Application of the three principles of polarization
Effect of tDCS on motor control and motor learning in healthy participants
In the absence of any learning requirements, humans are able to perform accurate movements that are under control of an extensive network of brain areas (Shadmehr and Krakauer, 2008). The motor cortex is at the heart of this network as its dynamics sculpts motor behaviors (Churchland et al., 2010, 2012; Shenoy et al., 2013). Therefore, it is not surprising that modulation of neuronal firing by motor cortex polarization (first principle) directly influences simple motor behaviors in the absence of any learning or adaptation. In this context, increases in M1 neuronal firing rates induced by anodal tDCS increases the maximum voluntary force that the subjects can produce (Tanaka et al., 2009; Salimpour and Shadmehr, 2014) when the reference electrode is supra-orbital (cephalic montage) but not when this reference electrode was placed on the shoulder (Cogiamanian et al., 2007; Lampropoulou and Nowicky, 2013). In addition, anodal tDCS of M1 enhances dexterity such as measured by the Purdue Pegboard (PPT) or the Jebsen-Taylor (JTT) tests (Antal et al., 2004; Boggio et al., 2006; Hummel et al., 2010; Williams et al., 2010; Matsuo et al., 2011; Sohn et al., 2012; Kidgell et al., 2013; Bastani and Jaberzadeh, 2014; Convento et al., 2014). The improvement in motor function is even more marked for fine motor skills compared to grosser skills (Hummel et al., 2010). In contrast, cathodal stimulation of M1 can improve the specificity of muscle activation in the ipsilateral arm (McCambridge et al., 2011). These effects could likely be attributed to the effect of tDCS on spontaneous firing rate. Given that motor cortex polarization also modulates the evoked neuronal population response, it is expected that reaction time to a sensory stimulus would be modulated by polarization. However, the data on reaction time are mixed. Some studies reported a reduction in reaction time with anodal polarization of M1 (Elbert et al., 1981) and an increase in reaction time with cathodal polarization (Leite et al., 2011). However, in the absence of learning, most studies reported no modulation of reaction time with brain polarization (Kuo et al., 2008; Tanaka et al., 2009; Stagg et al., 2011b). In summary, it is suggested that the first principle of tDCS accounts for the effect of tDCS on motor behavior that do not involve learning.
It is possible that the second and third principles can be linked to the two phases of motor learning: respectively learning and consolidation. Most motor learning experiments follow three steps: a baseline period where normal motor behaviors are quantified in order to get some reference data on the behavior at hand. Then, the manipulation of the task is introduced which will induce learning. In the case of motor adaptation paradigms, a perturbation is introduced. During this initial phase, behavior is adapted from trial-to-trial in order to increase the performance at the task (learning phase). Finally, in the last phase, the behavior stabilizes and the learned or adapted motor behavior is stored in memory (consolidation phase). Several mechanisms are thought to drive the two learning phases described above. These mechanisms are either a fast and slow version of the same process (Smith et al., 2006), different components of motor learning altogether (Haith and Krakauer, 2013) or a single component of motor learning (Reis et al., 2009). In addition, motor learning can affect motor behavior in at least three different ways: it can link different action phases (Sailer et al., 2005; Safstrom et al., 2013), it can shift the speed-accuracy trade-off (Reis et al., 2009; Lefebvre et al., 2012a; Shmuelof et al., 2012) or adapt the motor commands to the new environment (Shadmehr et al., 2010; Huang et al., 2011). Clearly, the effect of tDCS on learning depends on the mechanisms that govern it.
Linking of different action phases is particularly important to for sequence learning and for navigating a cursor smoothly. During the learning stage, such linking of action phases has been associated with the formation of new spatiotemporal patterns of neural activity in the motor cortex (Lu and Ashe, 2005; Matsuzaka et al., 2007; Peters et al., 2014). The observation that such formation of new patterns of motor activity is facilitated by tDCS would be accounted for by the second principle. Anodal tDCS of the motor cortex is particularly effective in augmenting learning in sequence learning tasks (Nitsche et al., 2003; Vines et al., 2006, 2008a, 2008b; Kuo et al., 2008; Kang and Paik, 2011; Stagg et al., 2011b; Cuypers et al., 2013; Karok and Witney, 2013; Waters-Metenier et al., 2014). In sequence learning tasks, the benefits of tDCS is restricted to the learning phase (Saucedo Marquez et al., 2013). In contrast, in skill learning tasks where a shift in speed-accuracy trade-off is required for improvements of performance (cursor navigation), tDCS mostly affects the time-dependent consolidation of the skill (Reis et al., 2009, 2013; Fritsch et al., 2010; Saucedo Marquez et al., 2013; Prichard et al., 2014) although one studies also reported online effects for a cursor navigation task (Lefebvre et al., 2012b). Interestingly, for both types of skill learning tasks, tDCS also improves the skilled performance in untrained tasks (Lefebvre et al., 2012b; Waters-Metenier et al., 2014).
Several studies have demonstrated that tDCS of M1 does not alter the rate of motor adaptation (Galea et al., 2011; Orban de Xivry et al., 2011b; Herzfeld et al., 2014). In accordance with the third principle, M1 tDCS may affect the retention of the memories acquired during adaptation training, although this remains controversial. Some studies reported that anodal stimulation of M1 slowed the decay rates of motor output (Hunter et al., 2009; Galea et al., 2011) or improved the spatial generalization of these memories (Orban de Xivry et al., 2011b). However, Herzfeltd et al. (2014) repeatedly measured the decay rates during acquisition with anodal M1 stimulation and observed no significant changes, and also found no effects on retention as measured at 24 hours. However, each of these three studies adopted very different paradigms (number of targets, length of training, etc.) and measured retention differently. Therefore, the effect of tDCS on M1 during motor adaptation tasks remains unclear.
Restoration of motor performance with tDCS
In motor learning tasks, a manipulation (e.g. introduction of a sequence or a perturbation) affects motor performance and the subjects learn to improve their motor performance. As described in the previous section, tDCS facilitates learning in these artificial tasks. However, motor performance can also be affected by aging (Leversen et al., 2012), brain lesion (Coderre et al., 2010; Tyryshkin et al., 2014) or brain disease (Mazzoni et al., 2012) and produces a decrease in motor performance. The potential of tDCS to restore motor performance in these more natural contexts has been investigated in the recent years.
Aging is associated with a decrease in motor performance (Smith et al., 1999; Carmeli et al., 2003) that is accompanied by a larger recruitment of brain areas (Ward, 2003; Heuninckx et al., 2008). Several studies suggest that anodal tDCS can restore normal motor performance in healthy older subjects (Hummel et al., 2010; Parikh and Cole, 2014). In addition, this age-related decline in motor performance is also accompanied by an age-related decline in motor learning/motor adaptation (Seidler, 2007; Lustig et al., 2009). Interestingly, anodal stimulation of M1 is able to compensate this deficit in skill learning (Goodwill et al., 2013; Zimerman et al., 2013) even when tDCS does not modulate the same learning process in younger adults (Zimerman et al., 2013). Cerebellar stimulation is also able to increase the rate of motor adaptation in older subjects (Hardwick and Celnik, 2014).
For stroke patients, the effects of tDCS on motor functions are generally positive (see Bastani and Jaberzadeh, 2012; Kandel et al., 2012; Butler et al., 2013; Marquez et al., 2013 for systematic reviews and meta-analyses). Many studies have found improvements in motor function in stroke patients as measured by clinical scales or the JTT (Fregni et al., 2005; Hummel et al., 2005; Lindenberg et al., 2010; Mahmoudi et al., 2011; Khedr et al., 2013; Lefebvre et al., 2013) and an increase in pinch or grip force and a decrease in reaction times (Hummel et al., 2006; Stagg et al., 2012; Lefebvre et al., 2013). The improvement in reaction time with the ipsilesional stimulation protocol was correlated with the change in movement related activity under the anodal electrode (M1) and in the ipsilesional premotor cortex (Stagg et al., 2012). Finally, in one patient, tDCS had the ability to decrease spasticity of the affected limb and, therefore, to improve motor function (Vandermeeren et al., 2013). A similar decrease in rigidity by tDCS was noted in PD patients (Fregni et al., 2006). The principles of tDCS governing these effects are unclear. Decrease in GABA concentration is probably helpful for motor-related activity in the ipsilesional cortex (Clarkson et al., 2010). An increase in spontaneous/evoked firing rates might also drive some of the effects such as increase in pinch force (Hummel et al., 2006) and the increase in movement-related activity (Stagg et al., 2012). The long-lasting effect of tDCS on motor function is likely due to the after-effect of tDCS and its ability to engrave new firing patterns in memory. Finally, in the same way it improves motor function in these patients, tDCS has also the ability to improve the acquisition and the memory of a new skill as it does in healthy subjects (Madhavan et al., 2011; Lefebvre et al., 2012b; Zimerman et al., 2012).
In contrast, the application of tDCS in Parkinson’s disease (PD) has produced puzzling results which are partly due to the apparent effect of dopaminergic medication on stimulation results. In patients who were taken off their dopamine medication, anodal tDCS of M1 significantly improved clinical scores and performance in a reaction time task (Fregni et al., 2006). Slight improvements were also observed with cathodal stimulation, making the comparisons between anodal and cathodal stimulation sessions often inconclusive (Fregni et al., 2006). In another study (Benninger et al., 2010), patients performed a walking test, and tests of arm function during ‘on’ and ‘off’ medication sessions. The authors found that anodal stimulation decreased walk time (the primary outcome) compared to sham stimulation, but only when tested off medication. In addition, the time to perform sequential arm and hand movements (a measure of bradykinesia) was reduced by anodal stimulation for patients both in the on and off medication states. However, clinical motor symptoms were only slightly reduced by active stimulation. Similar results were obtained in a study where anodal stimulation of M1 improved both the gait and clinical motor symptoms of PD patients in the ‘on’ medication state (Valentino et al., 2014). However, in a recent double blind study of PD patients who were ‘on’ medication, participants were compared to themselves when receiving either anodal or sham stimulation of M1 (Verheyden et al., 2013). The authors found no significant effects of stimulation in all but one measure (time for walking a 10m distance), and noted that this statistical significance may have been due to chance. While all the above-mentioned studies focused on anodal stimulation of the ipsilesional motor cortex, a recent study on rats suggests that cathodal stimulation of the contralesional motor cortex may be beneficial for PD patients. Indeed, cathodal stimulation of the frontal motor areas produced robust and sustained increases in striatal dopamine concentrations, whereas anodal stimulation produced little or no change (Tanaka et al., 2013). This observation raises interesting avenues for improvements of motor functions of PD patients with cathodal tDCS of M1. For instance, in a study of patients with PD ‘on’ medication, Salimpour et al. (2013) found improvements in clinical motor symptoms and in a bimanual isometric force production task with cathodal M1 stimulation.
Because hand dystonia is associated by increased excitability of hand area of the motor cortex, it has been postulated that decreasing this excitability would alleviate the symptoms of dystonia. Given that a reduction of excitability of M1 via inhibitory 1Hz rTMS help focal hand dystonia (Siebner et al., 1999), it was expected that cathodal tDCS of the affected M1 would have the same effect. A recent study that used bi-hemispheric tDCS (cathodal polarization of the affected hemisphere and anodal polarization of the unaffected hemisphere) concurrently to a training procedure reveals that motor function of the dystonic hand was improved after five days of training (Furuya et al., 2014). Such improvement was absent after sham or unilateral stimulation, which is consistent with the absence of effect found in earlier studies with unilateral tDCS montage (Buttkus et al., 2010; Benninger et al., 2011). Furthermore, these results demonstrate the importance of electrode montage for clinical applications of tDCS but require further confirmations.
Alternative models for the effect of tDCS on motor control and motor learning
In the recent years, several alternative models have been proposed to account for the effects of tDCS on cognitive or motor functions. Here, we discuss these alternatives models, what they can and cannot explain. However, it is likely that the actual picture is a mixture of them as they are not completely independent.
Some authors suggest that tDCS mediates motor control/learning process by modulating long-term potentiation (Reis and Fritsch, 2011). Support for this idea stems from the impact of tDCS on motor learning, for which LTP is essential (Rioult-Pedotti et al., 2000) and from detailed neurophysiological study in animals (Fritsch et al., 2010) and in humans (Cantarero et al., 2013a, 2013b). However, the LTP model does not account for the simple effect of tDCS on motor behaviors independently of any learning process, nor does it account for the effect of cathodal stimulation on motor learning or control.
An alternative model, quite opposite to the previous one, might suggest that all the effects of tDCS might be due to the observed decrease in GABA due to anodal tDCS (Stagg et al., 2009). Indeed, it is known that a decrease in GABA is required for long-term potentiation in the motor cortex (Hess et al., 1996). Therefore, such a decrease in GABA concentration would be sufficient to account for most of the effects accounted for by the second principle. However, a decrease in GABA would be associated with less precise movements, hence a decrease in motor function, and not an improvement in motor function such as found in numerous tDCS studies. Indeed, animal studies have shown that polarization (Morrell, 1961) or application of a GABA antagonist (Castro-Alamancos and Borrell, 1993) results in involuntary muscle activity. In addition, decreasing GABA concentration through application of GABAA antagonist reduces the ability to contract muscles individually (Matsumura et al., 1992; Kubota, 1996; Schieber and Poliakov, 1998). In contrast, anodal stimulation of the motor cortex improves individuation of the fingers (Waters-Metenier et al., 2014). This observation is probably related to the importance of inhibition in shaping the output of the M1 (Merchant et al., 2008; Isomura et al., 2009).
Finally, an interesting model that was devised on the effects of tDCS in cognitive tasks suggests that both TMS and tDCS modulate cognitive functions through increasing or decreasing the level of noise in the system (Miniussi et al., 2013). In this model, anodal tDCS injects noise to the neural activity while cathodal tDCS filters out some of the noise. The effect of noise injection on task performance depends on the state of the neural population, the characteristics of the stimulation and the task performed. This state-dependency reflects co-activation of the neuronal population by its input and by polarization. In the motor domain, anodal tDCS of M1 increased motor cortical excitability before but not after motor learning (Cantarero et al., 2013a). However, a bimanual coordination study that investigated how tDCS could affect the contribution of each arm found that tDCS could increase the unimanual maximum voluntary force but tended to reduce the unimanual variability of the force produced during a unimanual isometric force production task (Salimpour and Shadmehr, 2014). There is no explanation for how an increased in noise in the neural population would tend to reduce noise in the motor output. In addition, as acknowledged by the authors (Miniussi et al., 2013), this model can only account for the online effects of tDCS but not for its offline effects.
Effects of tDCS not accounted for by the three principles
Indirect effect of tDCS on the contralateral hemisphere
The effect of tDCS on the ipsilesional motor cortex on motor function has been explored in studies that show that skill learning or hand function can be improved by reducing the excitability of the ipsilateral motor cortex that controls the non-dominant hand (Vines et al., 2006, 2008b) or the contralesional motor cortex of stroke patients (Fregni et al., 2005; Zimerman et al., 2012). The neurophysiological mechanisms underlying this effect are unclear because long periods of cathodal stimulation on one motor cortex does not affect the excitability or inhibition of the other motor cortex (Di Lazzaro et al., 2012).
Common effects of anodal and cathodal polarization
In a few studies (Orban de Xivry et al., 2011b; Stagg et al., 2011b), anodal and cathodal stimulations of the motor cortex were found to have the same effects. In addition, the difference between the effects of anodal and cathodal stimulation on learning was weak (Nitsche et al., 2003). Clearly, none of the three principles could account for effects that are independent of the polarity.
Cerebellar tDCS influences motor learning
Behavioral effects of cerebellar stimulation have been recently uncovered in motor adaptation tasks. In these tasks, anodal polarization of the ipsilateral cerebellum increases the speed of learning of a reaching task (Galea et al., 2011; Hardwick and Celnik, 2014; Herzfeld et al., 2014) or a split-belt treadmill walking adaptation task (Jayaram et al., 2012). Cathodal cerebellar stimulation decreases the speed of learning the reaching task (Herzfeld et al. 2014).
Forming memories in the motor cortex
With motor learning, there are changes in the firing rates of M1 neurons and/or the output of M1 neuronal ensembles. This is true for adaptation tasks (Gandolfo et al., 2000; Li et al., 2001; Paz et al., 2003, 2005; Paz and Vaadia, 2004; Arce et al., 2010a, 2010b; Mandelblat-Cerf et al., 2011; Richardson et al., 2012) and in skill learning tasks (Kargo and Nitz, 2003, 2004; Cohen and Nicolelis, 2004; Costa et al., 2004; Lu and Ashe, 2005; Jackson et al., 2006; Matsuzaka et al., 2007; Kilavik et al., 2009; Komiyama et al., 2010; Nazarpour et al., 2012; Huber et al., 2012; Picard et al., 2013; Peters et al., 2014).
Kleim et al. (1998) examined the reorganization of M1 during skill learning in rats. In a reaching task, the animals used their paw to retrieve a single pellet of food through a small aperture (Buitrago et al., 2004). Initially, rats were successful on 10–15% of the attempts. Performance increased during several days of training before plateauing around a success rate of 50–60%. In an acrobatic task (Kleim et al., 1996), rats had to reach a platform through obstacles as fast as possible. With training, performance improved so that trial duration decreased from 15s to 5s. During these tasks, there were long-term potentiation (LTP) and depression (LTD) of synapses in the motor cortex (Rioult-Pedotti et al., 2000; Hodgson et al., 2005), as well as LTP of existing but masked horizontal synapses (Rioult-Pedotti et al., 1998). There are a number of factors that contribute to formation and maintenance of synaptic plasticity in the motor cortex during motor learning (see reviews Luft and Buitrago, 2005; Monfils et al., 2005):
Protein synthesis is important for long-term potentiation (LTP) of synapses (Krug et al., 1984; Teyler and DiScenna, 1987; Grzegorzewska et al., 2004; Hess, 2004; Mei et al., 2011), for acquisition of new motor behavior (Kleim et al., 1996; Luft et al., 2004; Derksen et al., 2007), and for maintenance of the existing motor repertoire (Kleim et al., 2003). It has been found that protein kinase Mzeta secretion (an atypical and autonomously active form of protein kinase C), which is essential for the maintenance of spatial memories (Serrano et al., 2008), is also critical for the maintenance of motor memories (von Kraus et al., 2010). This protein is also necessary and sufficient for LTP maintenance (Ling et al., 2002). BDNF plays a key role in LTP and in motor learning: BDNF concentration is modulated by motor learning in rats (Klintsova et al., 2004) and diminished secretion of BDNF in mice and in humans due to a genetic mutation appears to impair motor learning in a variety of tasks (Kleim et al., 2006; Fritsch et al., 2010; McHughen et al., 2010, 2011).
Neurotransmitter concentrations are critical for synaptic transmission. The modulation of these concentrations can affect motor cortex plasticity, hence learning. This is true for dopamine (Hosp et al., 2009, 2011; Molina-Luna et al., 2009) and for GABA (Jacobs and Donoghue, 1991; Trepel and Racine, 2000).
Motor learning is correlated with the number of new synapses that have been formed in the motor cortex (Xu et al., 2009; Yang et al., 2009; Fu et al., 2012). During early training, many new synapses are formed but some of them are later pruned away (Xu et al., 2009). Synaptogenesis is especially present in neurons that control the trained limb (Wang et al., 2011).
Whether these events occur in parallel, in series or both is not yet known. However, they may interact. For instance, in the hippocampus, LTP can increase the survival rate of new spines (Tanaka et al., 2008; Hill and Zito, 2013) while in the motor cortex, LTP is accompanied by an increase in the number of spines (Ivanco et al., 2000).
The components of the motor engram described here could be influenced by a small current flowing on the surface of the brain such as during tDCS, which is known to modulate motor performance.
The effects of DC polarization on the neurons and on motor memories
The effect of DC polarization on neural firing was analyzed by Bindmann (1964). Using the same electrode to polarize and record neuronal activity (but at different time), these authors found that both the discharge rate and the number of active units were increased by anodal polarization (units that were silent became active due to polarization). Importantly, they noted that evoked potentials did not change immediately. Rather, the effect of DC polarization built up in several minutes before a peak of the effect was reached but this effect persisted once the current was stopped. The effect of polarization continued to increase for the next 15–30 minutes. Finally, as a precursor of transcranial alternate current stimulation (tACS), they observed that intermittent passage of current from one polarity to the other enhanced the effect of polarization.
Further insights were gained by intracellular recordings of motor cortex cells during anodal polarization (Purpura and McMurtry, 1965). Short periods of polarization (max 40s) and weak currents (40–80microA/mm2) modulated the evoked responses of cells measured by electrocorticogram without modifying the membrane potentials. In contrast, larger currents elicited similar change in cortical waves but also in membrane potentials in response to an input (see also Denney and Brookhart, 1962; Voronin, 1968). Strong anodal polarization of the cortical surface depolarized while cathodal polarization hyperpolarized the soma membrane of pyramidal tract cells. This finding was confirmed by more recent in-vitro studies that found that modulation of the neural membrane is time (Bikson et al., 2004) and localization-specific (Bikson et al., 2004; Rahman et al., 2013), is affected by polarization of afferents (Rahman et al., 2013), by cell type and morphology (Radman et al., 2009), and by the orientation of axons (Kabakov et al., 2012). Therefore, neurons in a single cortical column could be affected completely differently by direct current stimulation because of their diverse orientations.
Finally, membrane oscillation and fast pre-potentials appeared during anodal polarization. Interestingly, those two features might be important for learning and memory. Membrane oscillation can improve the precision of an action potential (Gross, 2006; Schaefer et al., 2006). The presence of fast pre-potentials during anodal polarization suggests that surface polarization changes the properties of the dendrites and thus alters how the cells respond to synaptic input. These fast pre-potentials represent a powerful mechanism to boost the output of neocortical neurons in response to given inputs (Crochet et al., 2004). Fast pre-potentials were also observed in another study in the rabbit (Voronin, 1968). In that study, a decrease and an increase in EPSPs was reported with anodal and cathodal polarization, respectively. This surprising finding could be explained by a shift in the membrane potential at rest (increase and decrease for anodal and cathodal stimulation). More action potentials were elicited in response to tone or flash during anodal polarization.
To test the nature of the DC polarization after-effects, Gartside (1968a) cooled the brain to inhibit firing but found that when it warmed up, it went back to the post-polarization level and not to the pre-polarization levels. That is, the after-effect cannot stem from reverberating loops. LTP of synapses might account for this long-lasting effect. Recent studies found that anodal but not cathodal tDCS accompanied by ongoing activity gave rise to LTP of synapses both in motor cortex slices (Fritsch et al., 2010) and in hippocampal slices (Ranieri et al., 2012). Maintenance of long-term potentiation depends on protein synthesis and so does the after-effect of tDCS. Indeed, blocking protein synthesis 30 minutes before anodal polarization abolished the after-effect but not the increase in firing rates during the polarization itself (Gartside, 1968b).
Protein Kinase C is increased in several cortical areas after 3uA 30min anodal polarization (Islam et al., 1994). This increase lasts for 3 hours after polarization and then decreases in 72h. An atypical form of this protein is essential for motor memories (von Kraus et al., 2010). In addition, there is an increase in c-fos expression in the 30 minutes after anodal stimulation (but not 15minutes) in many brain areas but especially surrounding the electrode (Islam et al., 1995). In addition, Ranieri et al. (2012) noted an increase in c-fos with potentiation elicited by anodal current stimulation. These studies suggest that NMDA receptors are involved in the effects of anodal polarization, C-fos is required for the stabilization of LTP in dentate gyrus (Demmer et al., 1993) but not necessary for induction of LTP. C-fos expression is modulated during motor skill learning (Kleim et al., 1996).
Secretion of BDNF depends on a particular gene that has different variants in the population. This gene variant affects how people perform at motor tasks (Kleim et al., 2006; McHughen et al., 2010). It also affects the ability of tDCS to induce long-term potentiation in motor cortex slices (Fritsch et al., 2010) and the ability of tDCS to improve motor learning (Fritsch et al., 2010).
Modulation of the neurotransmitter GABA is critical for motor learning and memory. GABA is decreased during motor learning (Floyer-Lea et al., 2006) and its increase reduces rates of adaptation (Donchin et al., 2002). A decrease in GABA reduces the specificity of neural firing in M1. That is, a neuron that would only fire for a specific input will start firing for various inputs after decrease in GABA concentration (Matsumura et al., 1992). In addition, a decrease in GABA concentration shortly after stroke improves motor recovery in rats (Clarkson et al., 2010). The concentration of this neurotransmitter is modulated during tDCS (Stagg et al., 2009). Both anodal and cathodal stimulation decrease GABA concentration while only cathodal tDCS also decreases glutamate concentration (Stagg et al., 2009). In addition, inter-individual differences in the sensitivity of the GABAergic system to tDCS was an excellent predictor of inter-individual skill learning performance (Stagg et al., 2011a). Adenosine, which is another neurotransmitter and is involved in LTD in cortical areas is modulated during anodal polarization (Hattori et al., 1990) and influences the effect of cathodal polarization (Márquez-Ruiz et al., 2012).
In summary, most of the synaptic events that accompany motor learning (i.e. LTP, protein synthesis and decrease in GABA concentration) are modulated by direct current stimulation in such a way that anodal polarization should facilitate motor learning.
Methodological note
There exists many different tDCS protocols and there is no general consensus about which parameters are optimum. There are three main degrees of freedom: current type, time of stimulation (before or during training) and electrode montage. Electrode size (Nitsche et al., 2007), current density (Galea et al., 2009; Bastani and Jaberzadeh, 2012) and stimulation frequency and duration (Shekhawat et al., 2013; Bastani and Jaberzadeh, 2014) can also modulate the effectiveness of the stimulation.
The impact of current type (continuous, intermittent, alternating or random noise) on motor learning/control has not been investigated in details yet (see next section) although one study suggests that intermittent stimulation was more efficient than continuous current (Bindman et al., 1964).
The time of stimulation (before or during the training) appears critical to determine the the effect of stimulation (Stagg et al., 2011b). Most of the studies on motor learning apply the stimulation during and not before or after training. The effect of tDCS on memory consolidation (stimulation after learning) yielded very mixed results (Rosenkranz et al., 2000; Tecchio et al., 2010).
Finally, the impact of electrode montage has been largely investigated. When the stimulating electrode is place on one motor cortex, the reference electrode is usually placed on the contralateral supraorbital position (supra-orbital or unilateral montage). However, both extra-cephalic reference (ipsilateral arm/shoulder; extracephalic montage) and the contralateral motor cortex (bilateral montage) have been also used. The use of extra-cephalic reference reduces the effectiveness of the stimulation (Bikson et al., 2010; Moliadze et al., 2010a; DaSilva et al., 2011; Schambra et al., 2011). A new electrode montage (HD tDCS; Kuo et al., 2012) has appeared recently but its impact on motor functions has not been tested yet. In healthy subjects, the bilateral montage might be more effective than the supra-orbital montage for augmenting motor learning (Vines et al., 2008a). However, this effect has not been demonstrated for stroke patients (Mahmoudi et al., 2011). For these patients, stimulating the ipsilesional hemisphere with anodal polarization or the contralesional hemisphere with cathodal polarization appears equally effective (Mahmoudi et al., 2011; Stagg et al., 2012). However, in some cases, the electrode montage appears critical to improve motor function in patients. For instance, bi-lateral montage is required to improve the symptoms of hand dystonia (Furuya et al., 2014) as a unilateral montage is not effective (Buttkus et al., 2010; Benninger et al., 2011; Furuya et al., 2014) although these results require further confirmation.
In conclusion, it appears that cephalic montages have a larger effect on motor function than extra-cephalic montage (e.g. Schambra et al., 2011). In addition, stimulating during and not before the performance of a motor task is also more effective (e.g. Stagg et al., 2011b).
Limitations of current tDCS work
Our three principles are directly drawn from the effect of polarization on single neurons activity. However, it is difficult to draw direct inferences from modulation of single neurons by tDCS and modulation of function at the network level. Indeed, recent works (Bikson et al., 2004; Radman et al., 2009) suggest that the direction of the dendrites are particularly important in order to decide the effect of tDCS on neuronal activity. Therefore, it seems that inferring the impact of polarization on motor function from its impact on neuronal activity is tenuous. However, this review suggests that there are impressive similarities between the effects of polarization on function and on single neurons.
Older studies demonstrate that the effect of DC polarization on neurons decreased with distance from the polarizing electrode (Morrell, 1961). Accordingly, behavioral studies demonstrated that anodal tDCS on the leg area of the motor cortex could selectively improve leg motor behavior but not hand motor behavior (Tanaka et al., 2009) and tDCS on the little finger area selectively increased MEPs in the little finger but not in the index finger (Nitsche et al., 2007). Finally, stimulating the motor cortex and not the posterior parietal cortex influences the generalization of force-field adaptation (Orban de Xivry et al., 2011b). These evidences for spatial specificity are confounded by most models that predict current flow from tDCS. In these models, the peak activation is often not localized beneath the electrode (see Fig. 2 of Datta et al., 2012). This feature is very surprising because the anodal tDCS is placed on the motor cortex and not posterior to it in order to obtain an increase in MEPs. Therefore, these models directly contradict data from the above-mentioned studies. There is a need to validate these models with independent data (Caparelli-Daquer et al., 2012; Edwards et al., 2013). This validation should include the direct comparison between different electrode montages (e.g. effect of conventional vs. HD tDCS on MEP size) and the effect of each of these montages on motor function.
Most of the studies discussed in this review focus on the modulation of the motor cortex by anodal stimulation. In contrast, the mechanisms of cathodal stimulation have received much less attention. For instance, in the most cited tDCS studies (Nitsche et al., 2003; Galea and Celnik, 2009; Reis et al., 2009; Fritsch et al., 2010), cathodal stimulation does not modulate the task at hand and is not discussed further. More surprisingly, some studies reported similar effects for anodal and cathodal stimulations of the motor cortex on task modulation (Orban de Xivry et al., 2011b; Stagg et al., 2011b).
The reliability of the effects of tDCS on the different aspects of motor control and motor learning is currently unknown but might not be very high. For instance, many studies do not test adequately for interactions (Nieuwenhuis et al., 2011), do not correct for multiple comparisons (Bennett et al., 2011) and the spectra of p-hacking is well present (Simmons et al., 2011; Murayama et al., 2013). The real questions are: 1) How many of the current results are we able to replicate? Though it is important to bear in mind that non-replication does not equate fraud and that it can happen for any perfectly-fine study (Ioannidis, 2014); and 2) How many studies did not reach statistical significance and were therefore never published (aka the file-drawer problem; Simonsohn et al., 2014) ? These problems impact all fields of neuroscience but solutions have been recently proposed (Lakens and Evers, 2014; Simonsohn et al., 2014). One easy and practical first solution would be to increase the size of the groups as studies with larger number of participants are more immune to some of these weaknesses (Button et al., 2013).
Open questions
This review of the literature brings new questions about the modern use of tDCS for experimental or clinical perspectives. Here is a list of a few questions that need to be addressed.
In this review, we postulated that the effect of tDCS on motor control and motor learning could be accounted for by a few principles. However, it is possible that the first principle (modulation of firing rate by polarization) can on its own account for all the effects reported in the studies presented in this review. Different results would argue against such a view. For instance, increase in evoked and spontaneous discharge rate by anodal polarization are present during and after tDCS but the effect of tDCS on learning appears to be different if polarization is delivered during or before motor training (Stagg et al., 2011b). Low frequency rTMS but not cathodal tDCS delivered on the motor cortex can relieve hand dystonia (Siebner et al., 1999; Buttkus et al., 2010; Benninger et al., 2011) while both techniques decrease evoked neural activity (Creutzfeldt et al., 1962; Aydin-Abidin et al., 2006; Allen et al., 2007). Therefore, the causal link between the modulation of firing rates by tDCS (first principles) on one hand and the strengthening of new associations and the modulation of memory (second and third principles) should be explored in the future. Looking at the effects of modulation of neural activity by other means than electrical current (e.g. optogenetics) on motor control and learning should answer this question.
The links between the different models of actions of tDCS should also be investigated into more details. For instance, we currently don’t know whether modulation of motor cortex activity by polarization induced a modulation of GABA concentration or whether the modulation of GABA concentration by polarization is responsible for the change in neuronal activity under the electrode. In addition, high frequency stimulation of the motor cortex, which induces LTP in the motor cortex, leads to an increase in GABA concentration and impairs skilled reaching in mice (Henderson et al., 2012). Similar links should be sought to understand the modes of action of tDCS
Older studies employed different stimulation protocols. Often, the current was pulsatile, i.e. the device was only on for every other 15s time interval. This patterned tDCS appeared to be more effective than a constant current flow (Bindman et al., 1964; Albert, 1966). Such intermittent tDCS has been used on the dorsolateral prefrontal cortex to modulate working memory (Marshall et al., 2005). There is a limited view on the efficacy of different versions of DC polarization (transcranial alternating current stimulation, transcranial random noise stimulation, etc.). One study suggests that tACS and tDCS influence firing rates but that spike timing was only affected by tACS (Reato et al., 2010). Interestingly, transcranial alternate current stimulation modulates motor cortex excitability but not motor performance at SRTT (Moliadze et al., 2010b) while transcranial random noise stimulation affected both (Terney et al., 2008). It is unclear which tDCS protocol (continuous, intermittent, alternating or random noise) is the most effective.
Synaptogenesis is considered a critical component of motor learning (Xu et al., 2009; Yang et al., 2009; Yu and Zuo, 2010; Fu et al., 2012; Peters et al., 2014). It is known that the direction of an electric field can influence the direction of nerve growth (Fox et al., 1984; McCaig and Rajnicek, 1991). However, it is unknown if tDCS directly affects the rate of new synapses or their direction. Alternatively, tDCS could only facilitate long-term potentiation of nascent spines (Hill and Zito, 2013).
Brazovskaya et al. (1972) demonstrated that there was an increase in the number of glial cells near pyramidal neurons after anodal polarization of the cortex. The influence of tDCS on glial cells has been postulated on the basis of theoretical arguments (Ruohonen and Karhu, 2012). How glial cells modulate learning and how their function is modulated by tDCS remains unknown.
While the effect of tDCS on manual dexterity of stroke patients and some specific aspects of their motor function appears to be consistent across laboratory studies (Kandel et al., 2012; Butler et al., 2013; Marquez et al., 2013), evidence for the ability of tDCS to improve activities of the daily living is still weak (Elsner et al., 2013), which questions the clinical relevance of tDCS for stroke rehabilitation. Clearly, large clinical trials are needed to settle this question not only for stroke but for any neurological disorders whose symptoms have been claimed to be alleviated by tDCS (PD, dystonia …).
Conclusion
Electrifying the motor engram has become popular over the last decade and its clinical applications have gained interest. In this review, we highlighted the similarities between the effects of brain polarization on neuronal activity and its effects on motor performance and learning. In addition, we detailed the neural substrate of the motor engram and showed that many of the mechanisms that are implicated in the formation of this engram are modulated by brain polarization. While these mechanisms are also likely responsible for the clinical benefits of tDCS demonstrated in several studies, further research is needed to demonstrate the clinical relevance of the tDCS technique. Further success of the tDCS technique will depend on the ability of scientists to provide reliable studies on the effects of tDCS on motor function in health and disease and to deepen our understanding of the mechanisms of tDCS on the motor engram.
Contributor Information
Jean-Jacques Orban de Xivry, Email: jj.orban@uclouvain.be.
Reza Shadmehr, Email: shadmehr@jhu.edu.
References
- Albert DJ. The effects of polarizing currents on the consolidation of learning. Neuropsychologia. 1966;4:65–77. [Google Scholar]
- Allen Ea, Pasley BN, Duong T, Freeman RD. Transcranial magnetic stimulation elicits coupled neural and hemodynamic consequences. Science (80- ) 2007;317:1918–21. doi: 10.1126/science.1146426. [DOI] [PubMed] [Google Scholar]
- Antal A, Nitsche MA, Kincses TZ, Kruse W, Hoffmann K-P, Paulus W. Facilitation of visuo-motor learning by transcranial direct current stimulation of the motor and extrastriate visual areas in humans. Eur J Neurosci. 2004;19:2888–92. doi: 10.1111/j.1460-9568.2004.03367.x. [DOI] [PubMed] [Google Scholar]
- Arce F, Novick I, Mandelblat-Cerf Y, Israel Z, Ghez C, Vaadia E. Combined adaptiveness of specific motor cortical ensembles underlies learning. J Neurosci. 2010a;30:5415–25. doi: 10.1523/JNEUROSCI.0076-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arce F, Novick I, Mandelblat-Cerf Y, Vaadia E. Neuronal correlates of memory formation in motor cortex after adaptation to force field. J Neurosci. 2010b;30:9189–98. doi: 10.1523/JNEUROSCI.1603-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aydin-Abidin S, Moliadze V, Eysel UT, Funke K. Effects of repetitive TMS on visually evoked potentials and EEG in the anaesthetized cat: dependence on stimulus frequency and train duration. J Physiol. 2006;574:443–55. doi: 10.1113/jphysiol.2006.108464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastani a, Jaberzadeh S. Does anodal transcranial direct current stimulation enhance excitability of the motor cortex and motor function in healthy individuals and subjects with stroke: A systematic review and meta-analysis. Clin Neurophysiol. 2012;123:644–57. doi: 10.1016/j.clinph.2011.08.029. [DOI] [PubMed] [Google Scholar]
- Bastani a, Jaberzadeh S. Within-session repeated a-tDCS: The effects of repetition rate and inter-stimulus interval on corticospinal excitability and motor performance. Clin Neurophysiol. 2014 Jan 29; doi: 10.1016/j.clinph.2014.01.010. [DOI] [PubMed] [Google Scholar]
- Bennett C, Baird A, Miller MB, Wolford GL. Neural correlates of interspecies perspective taking in the post-mortem atlantic salmon: an argument for proper multiple comparisons correction. J Serendipitous. 2011;1:1–5. [Google Scholar]
- Benninger DH, Lomarev M, Lopez G, Pal N, Luckenbaugh Da, Hallett M. Transcranial direct current stimulation for the treatment of focal hand dystonia. Mov Disord. 2011;26:1698–702. doi: 10.1002/mds.23691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benninger DH, Lomarev MP, Lopez G, Wassermann EM, Li X, Considine E, Hallett M. Transcranial direct current stimulation for the treatment of Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2010;81:1105–11. doi: 10.1136/jnnp.2009.202556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikson M, Datta A, Rahman A, Scaturro J. Electrode montages for tDCS and weak transcranial electrical stimulation: Role of “return” electrode’s position and size. Clin Neurophysiol. 2010;121:1976–1978. doi: 10.1016/j.clinph.2010.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikson M, Inoue M, Akiyama H, Deans JK, Fox JE, Miyakawa H, Jefferys JGR. Effects of uniform extracellular DC electric fields on excitability in rat hippocampal slices in vitro. J Physiol. 2004;557:175–90. doi: 10.1113/jphysiol.2003.055772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bindman LJ, Lippold OCJ, Redfearn JWT. Long-lasting Changes in the Level of the Electrical Activity of the Cerebral Cortex produced by Polarizing Currents. Nature. 1962;196:584–585. doi: 10.1038/196584a0. [DOI] [PubMed] [Google Scholar]
- Bindman LJ, Lippold OCJ, Redfearn JWT. The action of brief polarizing currents on the cerebral cortex of the rat (1) during current flow and (2) in the production of long-lasting after-effects. J Physiol. 1964;172:369–82. doi: 10.1113/jphysiol.1964.sp007425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop GH, O’Leary JL. The effects of polarizing currents on cell potentials and their significance in the interpretation of central nervous system activity. Electroencephalogr Clin Neurophysiol. 1950;2:401–16. doi: 10.1016/0013-4694(50)90077-0. [DOI] [PubMed] [Google Scholar]
- Boggio PS, Castro LO, Savagim Ea, Braite R, Cruz VC, Rocha RR, Rigonatti SP, Silva MTa, Fregni F. Enhancement of non-dominant hand motor function by anodal transcranial direct current stimulation. Neurosci Lett. 2006;404:232–6. doi: 10.1016/j.neulet.2006.05.051. [DOI] [PubMed] [Google Scholar]
- Brazovskaya F, Malikova A, Pavlygina R. After-effects of anodal polarization in the cat cerebral cortex. Neurophysiology. 1972;4:194–199. [PubMed] [Google Scholar]
- Buitrago MM, Ringer T, Schulz JB, Dichgans J, Luft AR. Characterization of motor skill and instrumental learning time scales in a skilled reaching task in rat. Behav Brain Res. 2004;155:249–56. doi: 10.1016/j.bbr.2004.04.025. [DOI] [PubMed] [Google Scholar]
- Butler AJ, Shuster M, O’Hara E, Hurley K, Middlebrooks D, Guilkey K. A meta-analysis of the efficacy of anodal transcranial direct current stimulation for upper limb motor recovery in stroke survivors. J hand Ther. 2013;26:162–71. doi: 10.1016/j.jht.2012.07.002. [DOI] [PubMed] [Google Scholar]
- Buttkus F, Weidenmüller M, Schneider S, Jabusch H-C, Nitsche MA, Paulus W, Altenmüller E. Failure of cathodal direct current stimulation to improve fine motor control in musician’s dystonia. Mov Disord. 2010;25:389–94. doi: 10.1002/mds.22938. [DOI] [PubMed] [Google Scholar]
- Button KS, Ioannidis JPa, Mokrysz C, Nosek Ba, Flint J, Robinson ESJ, Munafò MR. Power failure: why small sample size undermines the reliability of neuroscience. Nat Rev Neurosci. 2013;14 doi: 10.1038/nrn3475. [DOI] [PubMed] [Google Scholar]
- Cantarero G, Lloyd a, Celnik Pa. Reversal of Long-Term Potentiation-Like Plasticity Processes after Motor Learning Disrupts Skill Retention. J Neurosci. 2013a;33:12862–12869. doi: 10.1523/JNEUROSCI.1399-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantarero G, Tang B, O’Malley R, Salas R, Celnik Pa. Motor Learning Interference Is Proportional to Occlusion of LTP-Like Plasticity. J Neurosci. 2013b;33:4634–4641. doi: 10.1523/JNEUROSCI.4706-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caparelli-Daquer EM, Zimmermann TJ, Mooshagian E, Parra LC, Rice JK, Datta A, Bikson M, Wassermann EM. A pilot study on effects of 4×1 High-Definition tDCS on motor cortex excitability. Conf Proc IEEE Eng Med Biol Soc. 2012;2012:735–8. doi: 10.1109/EMBC.2012.6346036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmeli E, Patish H, Coleman R. The aging hand. J Gerontol A Biol Sci Med Sci. 2003;58:146–52. doi: 10.1093/gerona/58.2.m146. [DOI] [PubMed] [Google Scholar]
- Castro-Alamancos Ma, Borrell J. Motor activity induced by disinhibition of the primary motor cortex of the rat is blocked by a non-NMDA glutamate receptor antagonist. Neurosci Lett. 1993;150:183–6. doi: 10.1016/0304-3940(93)90531-o. [DOI] [PubMed] [Google Scholar]
- Censor N, Dimyan MA, Cohen LG. Modification of Existing Human Motor Memories Is Enabled by Primary Cortical Processing during Memory Reactivation. Curr Biol. 2010;20:1545–9. doi: 10.1016/j.cub.2010.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan CY, Hounsgaard J, Nicholson C. Effects of electric fields on transmembrane potential and excitability of turtle cerebellar Purkinje cells in vitro. J Physiol. 1988;402:751–71. doi: 10.1113/jphysiol.1988.sp017232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan CY, Nicholson C. Modulation by applied electric fields of Purkinje and stellate cell activity in the isolated turtle cerebellum. J Physiol. 1986;371:89–114. doi: 10.1113/jphysiol.1986.sp015963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Hua SE, Smith MA, Lenz Fa, Shadmehr R. Effects of human cerebellar thalamus disruption on adaptive control of reaching. Cereb Cortex. 2006;16:1462–73. doi: 10.1093/cercor/bhj087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchland MM, Cunningham JP, Kaufman MT, Foster JD, Nuyujukian P, Ryu SI, Shenoy KV. Neural population dynamics during reaching. Nature. 2012;487:51–6. doi: 10.1038/nature11129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchland MM, Cunningham JP, Kaufman MT, Ryu SI, Shenoy KV. Cortical Preparatory Activity: Representation of Movement or First Cog in a Dynamical Machine? Neuron. 2010;68:387–400. doi: 10.1016/j.neuron.2010.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson AN, Huang BS, MacIsaac SE, Mody I, Carmichael ST. Reducing excessive GABA-mediated tonic inhibition promotes functional recovery after stroke. Nature. 2010;468:305–309. doi: 10.1038/nature09511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coderre AM, Zeid AA, Dukelow SP, Demmer MJ, Moore KD, Demers MJ, Bretzke H, Herter TM, Glasgow JI, Norman KE, Bagg SD, Scott SH. Assessment of upper-limb sensorimotor function of subacute stroke patients using visually guided reaching. Neurorehabil Neural Repair. 2010;24:528–41. doi: 10.1177/1545968309356091. [DOI] [PubMed] [Google Scholar]
- Cogiamanian F, Marceglia S, Ardolino G, Barbieri S, Priori A. Improved isometric force endurance after transcranial direct current stimulation over the human motor cortical areas. Eur J Neurosci. 2007;26:242–9. doi: 10.1111/j.1460-9568.2007.05633.x. [DOI] [PubMed] [Google Scholar]
- Cohen D, Nicolelis MAL. Reduction of single-neuron firing uncertainty by cortical ensembles during motor skill learning. J Neurosci. 2004;24:3574–82. doi: 10.1523/JNEUROSCI.5361-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Convento S, Bolognini N, Fusaro M, Lollo F, Vallar G. Neuromodulation of parietal and motor activity affects motor planning and execution. Cortex. 2014 Mar; doi: 10.1016/j.cortex.2014.03.006. [DOI] [PubMed] [Google Scholar]
- Costa RM, Cohen D, Nicolelis MAL. Differential Corticostriatal Plasticity during Fast and Slow Motor Skill Learning in Mice. Curr Biol. 2004;14:1124–1134. doi: 10.1016/j.cub.2004.06.053. [DOI] [PubMed] [Google Scholar]
- Creutzfeldt O, Fromm G, Kapp H. Influence of transcortical dc currents on cortical neuronal activity. Exp Neurol. 1962;452:436–452. doi: 10.1016/0014-4886(62)90056-0. [DOI] [PubMed] [Google Scholar]
- Criscimagna-Hemminger SE, Bastian AJ, Shadmehr R. Size of Error Affects Cerebellar Contributions to Motor Learning. J Neurophysiol. 2010;103:2275–2284. doi: 10.1152/jn.00822.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criscimagna-Hemminger SE, Shadmehr R. Consolidation patterns of human motor memory. J Neurosci. 2008;28:9610–8. doi: 10.1523/JNEUROSCI.3071-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crochet S, Fuentealba P, Timofeev I, Steriade M. Selective amplification of neocortical neuronal output by fast prepotentials in vivo. Cereb Cortex. 2004;14:1110–21. doi: 10.1093/cercor/bhh071. [DOI] [PubMed] [Google Scholar]
- Cuypers K, Leenus DJF, van den Berg FE, Nitsche Ma, Thijs H, Wenderoth N, Meesen RLJ. Is Motor Learning Mediated by tDCS Intensity? PLoS One. 2013;8:e67344. doi: 10.1371/journal.pone.0067344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DaSilva AF, Volz MS, Bikson M, Fregni F. Electrode positioning and montage in transcranial direct current stimulation. J Vis Exp. 2011:1–9. doi: 10.3791/2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta A, Truong D, Minhas P, Parra LC, Bikson M. Inter-Individual Variation during Transcranial Direct Current Stimulation and Normalization of Dose Using MRI-Derived Computational Models. Front Psychiatry. 2012;3:1–8. doi: 10.3389/fpsyt.2012.00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Della-Maggiore V, Scholz J, Johansen-Berg H, Paus T. The rate of visuomotor adaptation correlates with cerebellar white-matter microstructure. Hum Brain Mapp. 2009;30:4048–53. doi: 10.1002/hbm.20828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demmer J, Dragunow M, Lawlor Pa, Mason SE, Leah JD, Abraham WC, Tate WP. Differential expression of immediate early genes after hippocampal long-term potentiation in awake rats. Brain Res Mol Brain Res. 1993;17:279–86. doi: 10.1016/0169-328x(93)90012-e. [DOI] [PubMed] [Google Scholar]
- Denney D, Brookhart JM. The effects of applied polarization on evoked electro-cortical waves in the cat. Electroencephalogr Clin Neurophysiol. 1962;14:885–97. doi: 10.1016/0013-4694(62)90139-6. [DOI] [PubMed] [Google Scholar]
- Derksen MJ, Ward NL, Hartle KD, Ivanco TL. MAP2 and synaptophysin protein expression following motor learning suggests dynamic regulation and distinct alterations coinciding with synaptogenesis. Neurobiol Learn Mem. 2007;87:404–15. doi: 10.1016/j.nlm.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Donchin O, Rabe K, Diedrichsen J, Lally N, Schoch B, Gizewski ER, Timmann D. Cerebellar regions involved in adaptation to force field and visuomotor perturbation. J Neurophysiol. 2011 Oct 5; doi: 10.1152/jn.00007.2011. [DOI] [PubMed] [Google Scholar]
- Donchin O, Sawaki L, Madupu G, Cohen LG, Shadmehr R. Mechanisms influencing acquisition and recall of motor memories. J Neurophysiol. 2002;88:2114–23. doi: 10.1152/jn.2002.88.4.2114. [DOI] [PubMed] [Google Scholar]
- Edwards D, Cortes M, Datta A, Minhas P, Wassermann EM, Bikson M. Physiological and modeling evidence for focal transcranial electrical brain stimulation in humans: a basis for high-definition tDCS. Neuroimage. 2013;74:266–75. doi: 10.1016/j.neuroimage.2013.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbert T, Lutzenberger W, Rockstroh B, Birbaumer N. The influence of low-level transcortical DC-currents on response speed in humans. Int J Neurosci. 1981;14:101–14. doi: 10.3109/00207458108985821. [DOI] [PubMed] [Google Scholar]
- Elsner B, Kugler J, Pohl M, Mehrholz J. Transcranial direct current stimulation (tDCS) for improving function and activities of daily living in patients after stroke. Cochrane database Syst Rev. 2013;11:CD009645. doi: 10.1002/14651858.CD009645.pub2. [DOI] [PubMed] [Google Scholar]
- Floyer-Lea A, Wylezinska M, Kincses TZ, Matthews PM. Rapid modulation of GABA concentration in human sensorimotor cortex during motor learning. J Neurophysiol. 2006;95:1639–44. doi: 10.1152/jn.00346.2005. [DOI] [PubMed] [Google Scholar]
- Fox GQ, Kötting D, Richardson GP. Investigations into a bioelectric component of synaptogenesis. Brain Res. 1984;311:31–7. doi: 10.1016/0006-8993(84)91395-7. [DOI] [PubMed] [Google Scholar]
- Fregni F, Boggio PS, Mansur CG, Wagner T, Ferreira MJL, Lima MC, Rigonatti SP, Marcolin MA, Freedman SD, Nitsche MA, Pascual-Leone A. Transcranial direct current stimulation of the unaffected hemisphere in stroke patients. Neuroreport. 2005;16:1551–5. doi: 10.1097/01.wnr.0000177010.44602.5e. [DOI] [PubMed] [Google Scholar]
- Fregni F, Boggio PS, Santos MC, Lima M, Vieira AL, Rigonatti SP, Silva MTa, Barbosa ER, Nitsche MA, Pascual-Leone A. Noninvasive cortical stimulation with transcranial direct current stimulation in Parkinson’s disease. Mov Disord. 2006;21:1693–1702. doi: 10.1002/mds.21012. [DOI] [PubMed] [Google Scholar]
- Fritsch B, Reis J, Martinowich K, Schambra HM, Ji Y, Cohen LG, Lu B. Direct Current Stimulation Promotes BDNF-Dependent Synaptic Plasticity: Potential Implications for Motor Learning. Neuron. 2010;66:198–204. doi: 10.1016/j.neuron.2010.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu M, Yu X, Lu J, Zuo Y. Repetitive motor learning induces coordinated formation of clustered dendritic spines in vivo. Nature. 1:2012. doi: 10.1038/nature10844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuya S, Nitsche MA, Paulus W, Altenmüller E. Surmounting retraining limits in musicians’ dystonia by transcranial stimulation. Ann Neurol. 2014 Apr 7; doi: 10.1002/ana.24151. [DOI] [PubMed] [Google Scholar]
- Galea JM, Celnik Pa. Brain polarization enhances the formation and retention of motor memories. J Neurophysiol. 2009;102:294–301. doi: 10.1152/jn.00184.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galea JM, Jayaram G, Ajagbe L, Celnik Pa. Modulation of cerebellar excitability by polarity-specific noninvasive direct current stimulation. J Neurosci. 2009;29:9115–22. doi: 10.1523/JNEUROSCI.2184-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galea JM, Vazquez A, Pasricha N, Orban de Xivry J-J, Celnik Pa. Dissociating the roles of the cerebellum and motor cortex during adaptive learning: the motor cortex retains what the cerebellum learns. Cereb Cortex. 2011;21:1761–70. doi: 10.1093/cercor/bhq246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandolfo F, Li C, Benda BJ, Padoa-Schioppa C, Bizzi E. Cortical correlates of learning in monkeys adapting to a new dynamical environment. Proc Natl Acad Sci U S A. 2000;97:2259–63. doi: 10.1073/pnas.040567097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gartside IB. Mechanisms of sustained increases of firing rate of neurons in the rat cerebral cortex after polarization: reverberating circuits or modification of synaptic conductance? Nature. 1968a;220:382–3. doi: 10.1038/220382a0. [DOI] [PubMed] [Google Scholar]
- Gartside IB. Mechanisms of sustained increases of firing rate of neurones in the rat cerebral cortex after polarization: role of protein synthesis. Nature. 1968b;220:382–3. doi: 10.1038/220383a0. [DOI] [PubMed] [Google Scholar]
- Gibo TL, Criscimagna-Hemminger SE, Okamura AM, Bastian AJ. Cerebellar motor learning: Are environment dynamics more important than error size? J Neurophysiol. 2013 Apr 17; doi: 10.1152/jn.00745.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwill AM, Reynolds J, Daly RM, Kidgell DJ. Formation of cortical plasticity in older adults following tDCS and motor training. Front Aging Neurosci. 2013;5:1–9. doi: 10.3389/fnagi.2013.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross L. Membrane oscillations keep neurons on the right track. PLoS Biol. 2006;4:e191. doi: 10.1371/journal.pbio.0040191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grzegorzewska M, Przybylo M, Litynska A, Hess G. Chemically-induced long-term potentiation in rat motor cortex involves activation of extracellular signal-regulated kinase cascade. Brain Res. 2004;1021:192–9. doi: 10.1016/j.brainres.2004.06.049. [DOI] [PubMed] [Google Scholar]
- Hadipour-Niktarash A, Lee CK, Desmond JE, Shadmehr R. Impairment of retention but not acquisition of a visuomotor skill through time-dependent disruption of primary motor cortex. J Neurosci. 2007;27:13413–9. doi: 10.1523/JNEUROSCI.2570-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haith AM, Krakauer JW. Model-based and model-free mechanisms of human motor learning. Adv Exp Med Biol. 2013;782:1–21. doi: 10.1007/978-1-4614-5465-6_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardwick RM, Celnik Pa. Cerebellar direct current stimulation enhances motor learning in older adults. Neurobiol Aging. 2014 Apr; doi: 10.1016/j.neurobiolaging.2014.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardwick RM, Rottschy C, Miall RC, Eickhoff SB. A quantitative meta-analysis and review of motor learning in the human brain. Neuroimage. 2012 Nov 27; doi: 10.1016/j.neuroimage.2012.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori Y, Moriwaki A, Hori Y. Biphasic effects of polarizing current on adenosine-sensitive generation of cyclic AMP in rat cerebral cortex. Neurosci Lett. 1990;116:320–4. doi: 10.1016/0304-3940(90)90094-p. [DOI] [PubMed] [Google Scholar]
- Henderson aK, Pittman QJ, Teskey GC. High frequency stimulation alters motor maps, impairs skilled reaching performance and is accompanied by an upregulation of specific GABA, glutamate and NMDA receptor subunits. Neuroscience. 2012;215:98–113. doi: 10.1016/j.neuroscience.2012.04.040. [DOI] [PubMed] [Google Scholar]
- Herzfeld DJ, Pastor D, Haith AM, Rossetti Y, Shadmehr R, O’Shea J. Contributions of the cerebellum and the motor cortex to acquisition and retention of motor memories. Neuroimage. 2014 May; doi: 10.1016/j.neuroimage.2014.04.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess G, Aizenman CD, Donoghue JP. Conditions for the induction of long-term potentiation in layer II/III horizontal connections of the rat motor cortex. J Neurophysiol. 1996;75:1765–78. doi: 10.1152/jn.1996.75.5.1765. [DOI] [PubMed] [Google Scholar]
- Hess G. Synaptic plasticity of local connections in rat motor cortex. Acta Neurobiol Exp (Wars) 2004;64:271–6. doi: 10.55782/ane-2004-1511. [DOI] [PubMed] [Google Scholar]
- Heuninckx S, Wenderoth N, Swinnen SP. Systems neuroplasticity in the aging brain: recruiting additional neural resources for successful motor performance in elderly persons. J Neurosci. 2008;28:91–9. doi: 10.1523/JNEUROSCI.3300-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill TC, Zito K. LTP-Induced Long-Term Stabilization of Individual Nascent Dendritic Spines. J Neurosci. 2013;33:678–686. doi: 10.1523/JNEUROSCI.1404-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgson Ra, Ji Z, Standish S, Boyd-Hodgson TE, Henderson aK, Racine RJ. Training-induced and electrically induced potentiation in the neocortex. Neurobiol Learn Mem. 2005;83:22–32. doi: 10.1016/j.nlm.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Hosp Ja, Pekanovic a, Rioult-Pedotti M-S, Luft AR. Dopaminergic Projections from Midbrain to Primary Motor Cortex Mediate Motor Skill Learning. J Neurosci. 2011;31:2481–2487. doi: 10.1523/JNEUROSCI.5411-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosp JA, Molina-Luna K, Atiemo CO, Hertler B, Luft AR. Dopaminergic modulation of motor maps in rat motor cortex: an in vivo study. Neuroscience. 2009;159:692–700. doi: 10.1016/j.neuroscience.2008.12.056. [DOI] [PubMed] [Google Scholar]
- Huang VS, Haith AM, Mazzoni P, Krakauer JW. Rethinking motor learning and savings in adaptation paradigms: model-free memory for successful actions combines with internal models. Neuron. 2011;70:787–801. doi: 10.1016/j.neuron.2011.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber D, Gutnisky Da, Peron S, O’Connor DH, Wiegert JS, Tian L, Oertner TG, Looger LL, Svoboda K. Multiple dynamic representations in the motor cortex during sensorimotor learning. Nature. 2012;484:473–478. doi: 10.1038/nature11039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hummel FC, Celnik Pa, Giraux P, Floel A, Wu W-H, Gerloff C, Cohen LG. Effects of non-invasive cortical stimulation on skilled motor function in chronic stroke. Brain. 2005;128:490–9. doi: 10.1093/brain/awh369. [DOI] [PubMed] [Google Scholar]
- Hummel FC, Heise K, Celnik Pa, Floel A, Gerloff C, Cohen LG. Facilitating skilled right hand motor function in older subjects by anodal polarization over the left primary motor cortex. Neurobiol Aging. 2010;31:2160–8. doi: 10.1016/j.neurobiolaging.2008.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hummel FC, Voller B, Celnik Pa, Floel A, Giraux P, Gerloff C, Cohen LG. Effects of brain polarization on reaction times and pinch force in chronic stroke. BMC Neurosci. 2006;7:73. doi: 10.1186/1471-2202-7-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T, Sacco P, Nitsche MA, Turner DL. Modulation of internal model formation during force field-induced motor learning by anodal transcranial direct current stimulation of primary motor cortex. J Physiol. 2009;587:2949–61. doi: 10.1113/jphysiol.2009.169284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ioannidis JPa. Errors (my very own) and the fearful uncertainty of numbers. Eur J Clin Invest. 2014 Apr 30; doi: 10.1111/eci.12277. [DOI] [PubMed] [Google Scholar]
- Islam N, Moriwaki A, Hattori Y, Hayashi Y, Lu YF, Hori Y. c-Fos expression mediated by N-methyl-D-aspartate receptors following anodal polarization in the rat brain. Exp Neurol. 1995;133:25–31. doi: 10.1006/exnr.1995.1004. [DOI] [PubMed] [Google Scholar]
- Islam N, Moriwaki A, Hattori Y, Hori Y. Anodal polarization induces protein kinase C γ (PKCγ)-like immunoreactivity in the rat cerebral cortex. Neurosci Res. 1994;21:169–172. doi: 10.1016/0168-0102(94)90159-7. [DOI] [PubMed] [Google Scholar]
- Isomura Y, Harukuni R, Takekawa T, Aizawa H, Fukai T. Microcircuitry coordination of cortical motor information in self-initiation of voluntary movements. Nat Neurosci. 2009;12:1586–93. doi: 10.1038/nn.2431. [DOI] [PubMed] [Google Scholar]
- Ivanco T, Racine R, Kolb B. Morphology of layer III pyramidal neurons is altered following induction of LTP in sensorimotor cortex of the freely moving rat. Synapse. 2000;22:16–22. doi: 10.1002/(SICI)1098-2396(200007)37:1<16::AID-SYN2>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Izawa J, Criscimagna-Hemminger SE, Shadmehr R. Cerebellar Contributions to Reach Adaptation and Learning Sensory Consequences of Action. J Neurosci. 2012;32:4230–4239. doi: 10.1523/JNEUROSCI.6353-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson A, Mavoori J, Fetz EE. Long-term motor cortex plasticity induced by an electronic neural implant. Nature. 2006;444:56–60. doi: 10.1038/nature05226. [DOI] [PubMed] [Google Scholar]
- Jacobs K, Donoghue JP. Reshaping the cortical motor map by unmasking latent intracortical connections. Science (80- ) 1991;251:944–947. doi: 10.1126/science.2000496. [DOI] [PubMed] [Google Scholar]
- Jayaram G, Tang B, Pallegadda R, Vasudevan EVL, Celnik Pa, Bastian AJ. Modulating Locomotor Adaptation with Cerebellar Stimulation. J Neurophysiol. 2012 Feb 29; doi: 10.1152/jn.00645.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joiner WM, Smith MA. Long-term retention explained by a model of short-term learning in the adaptive control of reaching. J Neurophysiol. 2008;100:2948–55. doi: 10.1152/jn.90706.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabakov AY, Muller Pa, Pascual-Leone A, Jensen FE, Rotenberg A. Contribution of axonal orientation to pathway-dependent modulation of excitatory transmission by direct current stimulation in isolated rat hippocampus. J Neurophysiol. 2012;107:1881–9. doi: 10.1152/jn.00715.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel M, Beis J-M, Le Chapelain L, Guesdon H, Paysant J. Non-invasive cerebral stimulation for the upper limb rehabilitation after stroke: a review. Ann Phys Rehabil Med. 2012;55:657–80. doi: 10.1016/j.rehab.2012.09.001. [DOI] [PubMed] [Google Scholar]
- Kang EK, Paik N-J. Effect of a tDCS electrode montage on implicit motor sequence learning in healthy subjects. Exp Transl Stroke Med. 2011;3:4. doi: 10.1186/2040-7378-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kargo WJ, Nitz Da. Early skill learning is expressed through selection and tuning of cortically represented muscle synergies. J Neurosci. 2003;23:11255–69. doi: 10.1523/JNEUROSCI.23-35-11255.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kargo WJ, Nitz DA. Improvements in the signal-to-noise ratio of motor cortex cells distinguish early versus late phases of motor skill learning. J Neurosci. 2004;24:5560–9. doi: 10.1523/JNEUROSCI.0562-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karok S, Witney AG. Enhanced motor learning following task-concurrent dual transcranial direct current stimulation. PLoS One. 2013;8:e85693. doi: 10.1371/journal.pone.0085693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khedr EM, Shawky Oa, El-Hammady DH, Rothwell JC, Darwish ES, Mostafa OM, Tohamy AM. Effect of Anodal Versus Cathodal Transcranial Direct Current Stimulation on Stroke Rehabilitation: A Pilot Randomized Controlled Trial. Neurorehabil Neural Repair. 2013 Apr 22; doi: 10.1177/1545968313484808. [DOI] [PubMed] [Google Scholar]
- Kidgell DJ, Goodwill AM, Frazer AK, Daly RM. Induction of cortical plasticity and improved motor performance following unilateral and bilateral transcranial direct current stimulation of the primary motor cortex. BMC Neurosci. 2013;14:64. doi: 10.1186/1471-2202-14-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilavik BE, Roux S, Ponce-Alvarez A, Confais J, Grün S, Riehle A. Long-term modifications in motor cortical dynamics induced by intensive practice. J Neurosci. 2009;29:12653–63. doi: 10.1523/JNEUROSCI.1554-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleim Ja, Chan S, Pringle E, Schallert K, Procaccio V, Jimenez R, Cramer SC. BDNF val66met polymorphism is associated with modified experience-dependent plasticity in human motor cortex. Nat Neurosci. 2006;9:735–7. doi: 10.1038/nn1699. [DOI] [PubMed] [Google Scholar]
- Kleim JA, Barbay S, Nudo RJ. Functional reorganization of the rat motor cortex following motor skill learning. J Neurophysiol. 1998;80:3321–5. doi: 10.1152/jn.1998.80.6.3321. [DOI] [PubMed] [Google Scholar]
- Kleim JA, Bruneau R, Calder K, Pocock D, VandenBerg PM, MacDonald E, Monfils M-H, Sutherland RJ, Nader K. Functional organization of adult motor cortex is dependent upon continued protein synthesis. Neuron. 2003;40:167–176. doi: 10.1016/s0896-6273(03)00592-0. [DOI] [PubMed] [Google Scholar]
- Kleim JA, Lussnig E, Schwarz ER, Comery TA, Greenough WT. Synaptogenesis and Fos expression in the motor cortex of the adult rat after motor skill learning. J Neurosci. 1996;16:4529–4535. doi: 10.1523/JNEUROSCI.16-14-04529.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klintsova AY, Dickson E, Yoshida R, Greenough WT. Altered expression of BDNF and its high-affinity receptor TrkB in response to complex motor learning and moderate exercise. Brain Res. 2004;1028:92–104. doi: 10.1016/j.brainres.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Komiyama T, Sato TR, O’Connor DH, Zhang Y-X, Huber D, Hooks BM, Gabitto M, Svoboda K. Learning-related fine-scale specificity imaged in motor cortex circuits of behaving mice. Nature. 2010;464:1182–6. doi: 10.1038/nature08897. [DOI] [PubMed] [Google Scholar]
- Von Kraus LM, Sacktor TC, Francis JT. Erasing sensorimotor memories via PKMzeta inhibition. PLoS One. 2010;5:e11125. doi: 10.1371/journal.pone.0011125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krug M, Lössner B, Ott T. Anisomycin blocks the late phase of long-term potentiation in the dentate gyrus of freely moving rats. Brain Res Bull. 1984;13:39–42. doi: 10.1016/0361-9230(84)90005-4. [DOI] [PubMed] [Google Scholar]
- Kubota K. Motor cortical muscimol injection disrupts forelimb movement in freely moving monkeys. Neuroreport. doi: 10.1097/00001756-199610020-00020. [DOI] [PubMed] [Google Scholar]
- Kuo H-I, Bikson M, Datta A, Minhas P, Paulus W, Kuo M-F, Nitsche Ma. Comparing cortical plasticity induced by conventional and high-definition 4 × 1 ring tDCS: A neurophysiological study. Brain Stimul. 2012 Oct 13; doi: 10.1016/j.brs.2012.09.010. [DOI] [PubMed] [Google Scholar]
- Kuo M-F, Unger M, Liebetanz D, Lang N, Tergau F, Paulus W, Nitsche MA. Limited impact of homeostatic plasticity on motor learning in humans. Neuropsychologia. 2008;46:2122–8. doi: 10.1016/j.neuropsychologia.2008.02.023. [DOI] [PubMed] [Google Scholar]
- Lackner J, DiZio P. Rapid adaptation to Coriolis force perturbations of arm trajectory. J Neurophysiol. 1994;72:299. doi: 10.1152/jn.1994.72.1.299. [DOI] [PubMed] [Google Scholar]
- Lakens D, Evers ERK. Sailing From the Seas of Chaos Into the Corridor of Stability: Practical Recommendations to Increase the Informational Value of Studies. Perspect Psychol Sci. 2014;9:278–292. doi: 10.1177/1745691614528520. [DOI] [PubMed] [Google Scholar]
- Lampropoulou SI, Nowicky AV. The effect of transcranial direct current stimulation on perception of effort in an isolated isometric elbow flexion task. Motor Control. 2013;17:412–26. doi: 10.1123/mcj.17.4.412. [DOI] [PubMed] [Google Scholar]
- Landau WM, Bishop GH, Clare MH. Analysis of the Form and Distribution of Evoked Cortical Potentials Under the Influence of Polarizing Currents. J Neurophysiol. 1964;27:788–813. doi: 10.1152/jn.1964.27.5.788. [DOI] [PubMed] [Google Scholar]
- Landi SM, Baguear F, Della-Maggiore V. One Week of Motor Adaptation Induces Structural Changes in Primary Motor Cortex That Predict Long-Term Memory One Year Later. J Neurosci. 2011;31:11808–11813. doi: 10.1523/JNEUROSCI.2253-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lazzaro V, Manganelli F, Dileone M, Notturno F, Esposito M, Capasso M, Dubbioso R, Pace M, Ranieri F, Minicuci G, Santoro L, Uncini A. The effects of prolonged cathodal direct current stimulation on the excitatory and inhibitory circuits of the ipsilateral and contralateral motor cortex. J Neural Transm. 2012;119:1499–506. doi: 10.1007/s00702-012-0845-4. [DOI] [PubMed] [Google Scholar]
- Lefebvre S, Dricot L, Gradkowski W, Laloux P, Vandermeeren Y. Brain activations underlying different patterns of performance improvement during early motor skill learning. Neuroimage. 2012a;62:290–9. doi: 10.1016/j.neuroimage.2012.04.052. [DOI] [PubMed] [Google Scholar]
- Lefebvre S, Laloux P, Peeters A, Desfontaines P, Jamart J, Vandermeeren Y. Dual-tDCS Enhances Online Motor Skill Learning and Long-Term Retention in Chronic Stroke Patients. Front Hum Neurosci. 2012b;6:343. doi: 10.3389/fnhum.2012.00343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre S, Thonnard J-L, Laloux P, Peeters A, Jamart J, Vandermeeren Y. Single Session of Dual-tDCS Transiently Improves Precision Grip and Dexterity of the Paretic Hand After Stroke. Neurorehabil Neural Repair. 2013 Mar 13; doi: 10.1177/1545968313478485. [DOI] [PubMed] [Google Scholar]
- Leite J, Carvalho S, Fregni F, Gonçalves ÓF. Task-specific effects of tDCS-induced cortical excitability changes on cognitive and motor sequence set shifting performance. PLoS One. 2011;6:e24140. doi: 10.1371/journal.pone.0024140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leversen JSR, Haga M, Sigmundsson H. From Children to Adults: Motor Performance across the Life-Span. PLoS One. 2012;7:e38830. doi: 10.1371/journal.pone.0038830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CS, Padoa-Schioppa C, Bizzi E. Neuronal correlates of motor performance and motor learning in the primary motor cortex of monkeys adapting to an external force field. Neuron. 2001;30:593–607. doi: 10.1016/s0896-6273(01)00301-4. [DOI] [PubMed] [Google Scholar]
- Lindenberg R, Renga V, Zhu LL, Nair D, Schlaug G. Bihemispheric brain stimulation facilitates motor recovery in chronic stroke patients. Neurology. 2010;75:2176–84. doi: 10.1212/WNL.0b013e318202013a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling DSF, Benardo LS, Serrano Pa, Blace N, Kelly MT, Crary JF, Sacktor TC. Protein kinase Mzeta is necessary and sufficient for LTP maintenance. Nat Neurosci. 2002;5:295–6. doi: 10.1038/nn829. [DOI] [PubMed] [Google Scholar]
- Lohse KR, Wadden K, Boyd La, Hodges NJ. Motor skill acquisition across short and long time scales: A meta-analysis of neuroimaging data. Neuropsychologia. 2014 May 12; doi: 10.1016/j.neuropsychologia.2014.05.001. [DOI] [PubMed] [Google Scholar]
- Lu X, Ashe J. Anticipatory activity in primary motor cortex codes memorized movement sequences. Neuron. 2005;45:967–73. doi: 10.1016/j.neuron.2005.01.036. [DOI] [PubMed] [Google Scholar]
- Luft AR, Buitrago MM, Ringer T, Dichgans J, Schulz JB. Motor skill learning depends on protein synthesis in motor cortex after training. J Neurosci. 2004;24:6515–20. doi: 10.1523/JNEUROSCI.1034-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luft AR, Buitrago MM. Stages of motor skill learning. Mol Neurobiol. 2005;32:205–16. doi: 10.1385/MN:32:3:205. [DOI] [PubMed] [Google Scholar]
- Lustig C, Shah P, Seidler RD, Reuter-Lorenz Pa. Aging, training, and the brain: a review and future directions. Neuropsychol Rev. 2009;19:504–22. doi: 10.1007/s11065-009-9119-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madhavan S, Weber Ka, Stinear JW. Non-invasive brain stimulation enhances fine motor control of the hemiparetic ankle: implications for rehabilitation. Exp Brain Res. 2011;209:9–17. doi: 10.1007/s00221-010-2511-0. [DOI] [PubMed] [Google Scholar]
- Mahmoudi H, Borhani Haghighi A, Petramfar P, Jahanshahi S, Salehi Z, Fregni F. Transcranial direct current stimulation: electrode montage in stroke. Disabil Rehabil. 2011;33:1383–8. doi: 10.3109/09638288.2010.532283. [DOI] [PubMed] [Google Scholar]
- Mandelblat-Cerf Y, Novick I, Paz R, Link Y, Freeman S, Vaadia E. The Neuronal Basis of Long-Term Sensorimotor Learning. J Neurosci. 2011;31:300–313. doi: 10.1523/JNEUROSCI.4055-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquez J, van Vliet P, McElduff P, Lagopoulos J, Parsons M. Transcranial direct current stimulation (tDCS): Does it have merit in stroke rehabilitation? A systematic review. Int J Stroke. 2013:1–11. doi: 10.1111/ijs.12169. [DOI] [PubMed] [Google Scholar]
- Márquez-Ruiz J, Leal-Campanario R, Sánchez-Campusano R, Molaee-Ardekani B, Wendling F, Miranda PC, Ruffini G, Gruart A, Delgado-García JM. Transcranial direct-current stimulation modulates synaptic mechanisms involved in associative learning in behaving rabbits. Proc Natl Acad Sci U S A. 2012;109:6710–5. doi: 10.1073/pnas.1121147109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall L, Mölle M, Siebner HR, Born J. Bifrontal transcranial direct current stimulation slows reaction time in a working memory task. BMC Neurosci. 2005;6:23. doi: 10.1186/1471-2202-6-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin Ta, Keating JG, Goodkin HP, Bastian AJ, Thach WT. Throwing while looking through prisms. I. Focal olivocerebellar lesions impair adaptation. Brain. 1996;119(Pt 4):1183–98. doi: 10.1093/brain/119.4.1183. [DOI] [PubMed] [Google Scholar]
- Matsumura M, Sawaguchi T, Kubota K. GABAergic inhibition of neuronal activity in the primate motor and premotor cortex during voluntary movement. J Neurophysiol. 1992;68:692–702. doi: 10.1152/jn.1992.68.3.692. [DOI] [PubMed] [Google Scholar]
- Matsuo A, Maeoka H, Hiyamizu M, Shomoto K, Morioka S, Seki K. Enhancement of precise hand movement by transcranial direct current stimulation. Neuroreport. 2011;22:78–82. doi: 10.1097/WNR.0b013e32834298b3. [DOI] [PubMed] [Google Scholar]
- Matsuzaka Y, Picard N, Strick PL. Skill representation in the primary motor cortex after long-term practice. J Neurophysiol. 2007;97:1819–32. doi: 10.1152/jn.00784.2006. [DOI] [PubMed] [Google Scholar]
- Mazzoni P, Shabbott Ba, Cortés JC. Motor control abnormalities in Parkinson’s disease. Cold Spring Harb Perspect Med. 2012;2:a009282. doi: 10.1101/cshperspect.a009282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaig CD, Rajnicek AM. Electrical fields, nerve growth and nerve regeneration. Exp Physiol. 1991;76:473–94. doi: 10.1113/expphysiol.1991.sp003514. [DOI] [PubMed] [Google Scholar]
- McCambridge AB, Bradnam LV, Stinear CM, Byblow WD. Cathodal transcranial direct current stimulation of the primary motor cortex improves selective muscle activation in the ipsilateral arm. J Neurophysiol. 2011 Apr 20; doi: 10.1152/jn.00171.2011. [DOI] [PubMed] [Google Scholar]
- McHughen Sa, Pearson-Fuhrhop K, Ngo VK, Cramer SC. Intense training overcomes effects of the Val66Met BDNF polymorphism on short-term plasticity. Exp brain Res. 2011;213:415–22. doi: 10.1007/s00221-011-2791-z. [DOI] [PubMed] [Google Scholar]
- McHughen Sa, Rodriguez PF, Kleim JA, Kleim ED, Crespo LM, Procaccio V, Cramer SC. BDNF val66met polymorphism influences motor system function in the human brain. Cereb Cortex. 2010;20:1254–62. doi: 10.1093/cercor/bhp189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei F, Nagappan G, Ke Y, Sacktor TC, Lu B. BDNF facilitates L-LTP maintenance in the absence of protein synthesis through PKMζ. PLoS One. 2011;6:e21568. doi: 10.1371/journal.pone.0021568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchant H, Naselaris T, Georgopoulos AP. Dynamic sculpting of directional tuning in the primate motor cortex during three-dimensional reaching. J Neurosci. 2008;28:9164–72. doi: 10.1523/JNEUROSCI.1898-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miniussi C, Harris Ja, Ruzzoli M. Modelling non-invasive brain stimulation in cognitive neuroscience. Neurosci Biobehav Rev. 2013;37:1702–12. doi: 10.1016/j.neubiorev.2013.06.014. [DOI] [PubMed] [Google Scholar]
- Moliadze V, Antal A, Paulus W. Electrode-distance dependent after-effects of transcranial direct and random noise stimulation with extracephalic reference electrodes. Clin Neurophysiol. 2010a;121:2165–2171. doi: 10.1016/j.clinph.2010.04.033. [DOI] [PubMed] [Google Scholar]
- Moliadze V, Antal A, Paulus W. Boosting brain excitability by transcranial high frequency stimulation in the ripple range. J Physiol. 2010b;588:4891–904. doi: 10.1113/jphysiol.2010.196998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina-Luna K, Pekanovic A, Röhrich S, Hertler B, Schubring-Giese M, Rioult-Pedotti M-S, Luft AR. Dopamine in motor cortex is necessary for skill learning and synaptic plasticity. PLoS One. 2009;4:e7082. doi: 10.1371/journal.pone.0007082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monfils M-H, Plautz EJ, Kleim Ja. In search of the motor engram: motor map plasticity as a mechanism for encoding motor experience. Neurosci. 2005;11:471–83. doi: 10.1177/1073858405278015. [DOI] [PubMed] [Google Scholar]
- Morrell F. Effect of anodal polarization on the firing pattern of single cortical cells. Ann N Y Acad Sci. 1961;92:860–876. doi: 10.1111/j.1749-6632.1961.tb40962.x. [DOI] [PubMed] [Google Scholar]
- Muellbacher W, Ziemann U, Wissel J, Dang N, Kofler M, Facchini S, Boroojerdi B, Poewe W, Hallett M. Early consolidation in human primary motor cortex. Nature. 2002;415:640–644. doi: 10.1038/nature712. [DOI] [PubMed] [Google Scholar]
- Murayama K, Pekrun R, Fiedler K. Research Practices That Can Prevent an Inflation of False-Positive Rates. Personal Soc Psychol Rev. 2013 Aug 21; doi: 10.1177/1088868313496330. [DOI] [PubMed] [Google Scholar]
- Mutha PK, Sainburg RL, Haaland KY. Critical neural substrates for correcting unexpected trajectory errors and learning from them. Brain. 2011 Nov 10; doi: 10.1093/brain/awr275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazarpour K, Barnard a, Jackson a. Flexible Cortical Control of Task-Specific Muscle Synergies. J Neurosci. 2012;32:12349–12360. doi: 10.1523/JNEUROSCI.5481-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwenhuis S, Forstmann BU, Wagenmakers E. Erroneous analyses of interactions in neuroscience: a problem of significance. Nat Neurosci. 2011;14:1105–1107. doi: 10.1038/nn.2886. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Doemkes S, Karakose T, Antal A, Liebetanz D, Lang N, Tergau F, Paulus W, Karaköse T. Shaping the effects of transcranial direct current stimulation of the human motor cortex. J Neurophysiol. 2007;97:3109. doi: 10.1152/jn.01312.2006. [DOI] [PubMed] [Google Scholar]
- Nitsche MA, Paulus W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol. 2000;527:633–639. doi: 10.1111/j.1469-7793.2000.t01-1-00633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitsche MA, Schauenburg A, Lang N, Liebetanz D, Exner C, Paulus W, Tergau F. Facilitation of implicit motor learning by weak transcranial direct current stimulation of the primary motor cortex in the human. J Cogn Neurosci. 2003;15:619–26. doi: 10.1162/089892903321662994. [DOI] [PubMed] [Google Scholar]
- Orban de Xivry J-J, Ahmadi-Pajouh MA, Harran MD, Salimpour Y, Shadmehr R. Changes in corticospinal excitability during reach adaptation in force fields. J Neurophysiol. 2013;109:124–36. doi: 10.1152/jn.00785.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orban de Xivry J-J, Criscimagna-Hemminger SE, Shadmehr R. Contributions of the Motor Cortex to Adaptive Control of Reaching Depend on the Perturbation Schedule. Cereb Cortex. 2011a;21:1475–1484. doi: 10.1093/cercor/bhq192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orban de Xivry J-J, Marko MK, Pekny SE, Pastor D, Izawa J, Celnik Pa, Shadmehr R. Stimulation of the Human Motor Cortex Alters Generalization Patterns of Motor Learning. J Neurosci. 2011b;31:7102–7110. doi: 10.1523/JNEUROSCI.0273-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parikh PJ, Cole KJ. Effects of transcranial direct current stimulation in combination with motor practice on dexterous grasping and manipulation in healthy older adults. Physiol Rep. 2014;2:n/a–n/a. doi: 10.1002/phy2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paz R, Boraud T, Natan C, Bergman H, Vaadia E. Preparatory activity in motor cortex reflects learning of local visuomotor skills. Nat Neurosci. 2003;6:882–90. doi: 10.1038/nn1097. [DOI] [PubMed] [Google Scholar]
- Paz R, Natan C, Boraud T, Bergman H, Vaadia E. Emerging patterns of neuronal responses in supplementary and primary motor areas during sensorimotor adaptation. J Neurosci. 2005;25:10941–51. doi: 10.1523/JNEUROSCI.0164-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paz R, Vaadia E. Specificity of sensorimotor learning and the neural code: neuronal representations in the primary motor cortex. J Physiol Paris. 2004;98:331–48. doi: 10.1016/j.jphysparis.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Peters AJ, Chen SX, Komiyama T. Emergence of reproducible spatiotemporal activity during motor learning. Nature. 2014 May 4; doi: 10.1038/nature13235. [DOI] [PubMed] [Google Scholar]
- Picard N, Matsuzaka Y, Strick PL. Extended practice of a motor skill is associated with reduced metabolic activity in M1. Nat Neurosci. 2013 doi: 10.1038/nn.3477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prichard G, Weiller C, Fritsch B, Reis J. Effects of different electrical brain stimulation protocols on subcomponents of motor skill learning. Brain Stimul. 2014 Apr; doi: 10.1016/j.brs.2014.04.005. [DOI] [PubMed] [Google Scholar]
- Priori A, Berardelli A, Rona S, Accornero N, Manfredi M. Polarization of the human motor cortex through the scalp. Neuroreport. 1998;9:2257–60. doi: 10.1097/00001756-199807130-00020. [DOI] [PubMed] [Google Scholar]
- Purpura DP, McMurtry JG. Intracellular activities and evoked potential changes during polarization of motor cortex. J Neurophysiol. 1965;28:166–85. doi: 10.1152/jn.1965.28.1.166. [DOI] [PubMed] [Google Scholar]
- Radman T, Ramos RL, Brumberg JC, Bikson M. Role of cortical cell type and morphology in subthreshold and suprathreshold uniform electric field stimulation in vitro. Brain Stimul. 2009;2:215–228.e3. doi: 10.1016/j.brs.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman A, Reato D, Arlotti M, Gasca F, Datta A, Parra LC, Bikson M. Cellular Effects of Acute Direct Current Stimulation: Somatic and Synaptic Terminal Effects. J Physiol. 2013 Apr 22; doi: 10.1113/jphysiol.2012.247171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranieri F, Podda MV, Riccardi E, Frisullo G, Dileone M, Profice P, Pilato F, Di Lazzaro V, Grassi C. Modulation of LTP at rat hippocampal CA3-CA1 synapses by direct current stimulation. J Neurophysiol. 2012;107:1868–80. doi: 10.1152/jn.00319.2011. [DOI] [PubMed] [Google Scholar]
- Reato D, Rahman A, Bikson M, Parra LC. Low-intensity electrical stimulation affects network dynamics by modulating population rate and spike timing. J Neurosci. 2010;30:15067–79. doi: 10.1523/JNEUROSCI.2059-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis J, Fischer JT, Prichard G, Weiller C, Cohen LG, Fritsch B. Time- but Not Sleep-Dependent Consolidation of tDCS-Enhanced Visuomotor Skills. Cereb Cortex. 2013 Aug 19; doi: 10.1093/cercor/bht208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis J, Fritsch B. Modulation of motor performance and motor learning by transcranial direct current stimulation. Curr Opin Neurol. 2011;24:590–6. doi: 10.1097/WCO.0b013e32834c3db0. [DOI] [PubMed] [Google Scholar]
- Reis J, Schambra HM, Cohen LG, Buch ER, Fritsch B, Zarahn E, Celnik Pa, Krakauer JW. Noninvasive cortical stimulation enhances motor skill acquisition over multiple days through an effect on consolidation. Proc Natl Acad Sci U S A. 2009;106:1590–5. doi: 10.1073/pnas.0805413106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson AG, Borghi T, Bizzi E. Activity of the same motor cortex neurons during repeated experience with perturbed movement dynamics. J Neurophysiol. 2012;107:3144–54. doi: 10.1152/jn.00477.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson AG, Overduin Sa, Valero-Cabré A, Padoa-Schioppa C, Pascual-Leone A, Bizzi E, Press DZ. Disruption of primary motor cortex before learning impairs memory of movement dynamics. J Neurosci. 2006;26:12466–70. doi: 10.1523/JNEUROSCI.1139-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rioult-Pedotti M-S, Friedman D, Donoghue JP. Learning-induced LTP in neocortex. Science (80- ) 2000;290:533–6. doi: 10.1126/science.290.5491.533. [DOI] [PubMed] [Google Scholar]
- Rioult-Pedotti M-S, Friedman D, Hess G, Donoghue JP. Strengthening of horizontal cortical connections following skill learning. Nat Neurosci. 1998;1:230–4. doi: 10.1038/678. [DOI] [PubMed] [Google Scholar]
- Rosenkranz K, Nitsche MA, Tergau F, Paulus W. Diminution of training-induced transient motor cortex plasticity by weak transcranial direct current stimulation in the human. Neurosci Lett. 2000;296:61–3. doi: 10.1016/s0304-3940(00)01621-9. [DOI] [PubMed] [Google Scholar]
- Ruohonen J, Karhu J. tDCS possibly stimulates glial cells. Clin Neurophysiol. 2012;123:2006–2009. doi: 10.1016/j.clinph.2012.02.082. [DOI] [PubMed] [Google Scholar]
- Safstrom D, Flanagan JR, Johansson RS. Skill Learning Involves Optimizing the Linking of Action Phases. J Neurophysiol. 2013 Jun 5; doi: 10.1152/jn.00019.2013. [DOI] [PubMed] [Google Scholar]
- Sailer U, Flanagan JR, Johansson RS. Eye-hand coordination during learning of a novel visuomotor task. J Neurosci. 2005;25:8833–42. doi: 10.1523/JNEUROSCI.2658-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salimpour Y, Mari Z, Shadmehr R. Motor costs in Parkinson’s disease. In. Translational and computational motor control. 2013 [Google Scholar]
- Salimpour Y, Shadmehr R. Motor Costs and the Coordination of the Two Arms. J Neurosci. 2014;34:1806–1818. doi: 10.1523/JNEUROSCI.3095-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saucedo Marquez CM, Zhang X, Swinnen SP, Meesen R, Wenderoth N. Task-specific effect of transcranial direct current stimulation on motor learning. Front Hum Neurosci. 2013;7:333. doi: 10.3389/fnhum.2013.00333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer AT, Angelo K, Spors H, Margrie TW. Neuronal oscillations enhance stimulus discrimination by ensuring action potential precision. PLoS Biol. 2006;4:e163. doi: 10.1371/journal.pbio.0040163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schambra HM, Abe M, Luckenbaugh Da, Reis J, Krakauer JW, Cohen LG. Probing for hemispheric specialization for motor skill learning: a transcranial direct current stimulation study. J Neurophysiol. 2011;106:652–61. doi: 10.1152/jn.00210.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schieber MH, Poliakov aV. Partial inactivation of the primary motor cortex hand area: effects on individuated finger movements. J Neurosci. 1998;18:9038–54. doi: 10.1523/JNEUROSCI.18-21-09038.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidler RD. Aging affects motor learning but not savings at transfer of learning. Learn Mem. 2007;14:17–21. doi: 10.1101/lm.394707. [DOI] [PubMed] [Google Scholar]
- Serrano P, Friedman EL, Kenney J, Taubenfeld SM, Zimmerman JM, Hanna J, Alberini CM, Kelley AE, Maren S, Rudy JW, Yin JCP, Sacktor TC, Fenton Aa. PKMzeta maintains spatial, instrumental, and classically conditioned long-term memories. PLoS Biol. 2008;6:2698–706. doi: 10.1371/journal.pbio.0060318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadmehr R, Holcomb H. Neural correlates of motor memory consolidation. Science (80- ) 1997;277:821–5. doi: 10.1126/science.277.5327.821. [DOI] [PubMed] [Google Scholar]
- Shadmehr R, Krakauer JW. A computational neuroanatomy for motor control. Exp Brain Res. 2008;185:359–81. doi: 10.1007/s00221-008-1280-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadmehr R, Mussa-Ivaldi Fa. Adaptive representation of dynamics during learning of a motor task. J Neurosci. 1994;14:3208–24. doi: 10.1523/JNEUROSCI.14-05-03208.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shadmehr R, Smith MA, Krakauer JW. Error correction, sensory prediction, and adaptation in motor control. Annu Rev Neurosci. 2010;33:89–108. doi: 10.1146/annurev-neuro-060909-153135. [DOI] [PubMed] [Google Scholar]
- Shekhawat GS, Stinear CM, Searchfield GD. Transcranial direct current stimulation intensity and duration effects on tinnitus suppression. Neurorehabil Neural Repair. 2013;27:164–72. doi: 10.1177/1545968312459908. [DOI] [PubMed] [Google Scholar]
- Shenoy KV, Sahani M, Churchland MM. Cortical Control of Arm Movements: A Dynamical Systems Perspective. Annu Rev Neurosci. 2013 doi: 10.1146/annurev-neuro-062111-150509. [DOI] [PubMed] [Google Scholar]
- Shmuelof L, Krakauer JW, Mazzoni P. How is a motor skill learned? Change and invariance at the levels of task success and trajectory control. J Neurophysiol. 2012:052804. doi: 10.1152/jn.00856.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebner HR, Tormos JM, Ceballos-Baumann AO, Auer C, Catala MD, Conrad B, Pascual-Leone A. Low-frequency repetitive transcranial magnetic stimulation of the motor cortex in writer’s cramp. Neurology. 1999;52:529–37. doi: 10.1212/wnl.52.3.529. [DOI] [PubMed] [Google Scholar]
- Simmons JP, Nelson LD, Simonsohn U. False-positive psychology: undisclosed flexibility in data collection and analysis allows presenting anything as significant. Psychol Sci. 2011;22:1359–66. doi: 10.1177/0956797611417632. [DOI] [PubMed] [Google Scholar]
- Simonsohn U, Nelson LD, Simmons JP. P-curve: a key to the file-drawer. J Exp Psychol Gen. 2014;143:534–47. doi: 10.1037/a0033242. [DOI] [PubMed] [Google Scholar]
- Smith CD, Umberger GH, Manning EL, Slevin JT, Wekstein DR, Schmitt Fa, Markesbery WR, Zhang Z, Gerhardt Ga, Kryscio RJ, Gash DM. Critical decline in fine motor hand movements in human aging. Neurology. 1999;53:1458–1458. doi: 10.1212/wnl.53.7.1458. [DOI] [PubMed] [Google Scholar]
- Smith MA, Ghazizadeh A, Shadmehr R. Interacting adaptive processes with different timescales underlie short-term motor learning. PLoS Biol. 2006;4:e179. doi: 10.1371/journal.pbio.0040179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Shadmehr R. Intact ability to learn internal models of arm dynamics in Huntington’s disease but not cerebellar degeneration. J Neurophysiol. 2005;93:2809–21. doi: 10.1152/jn.00943.2004. [DOI] [PubMed] [Google Scholar]
- Sohn MK, Kim BO, Song HT. Effect of Stimulation Polarity of Transcranial Direct Current Stimulation on Non-dominant Hand Function. Ann Rehabil Med. 2012;36:1–7. doi: 10.5535/arm.2012.36.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokolov EN. Brain Functions: Neuronal Mechanisms of Learning and Memory. Annu Rev Psychol. 1977;28:85–112. [Google Scholar]
- Stagg CJ, Bachtiar V, Johansen-Berg H. The Role of GABA in Human Motor Learning. Curr Biol. 2011a;21:480–484. doi: 10.1016/j.cub.2011.01.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagg CJ, Bachtiar V, O’Shea J, Allman C, Bosnell RA, Kischka U, Matthews PM, Johansen-Berg H. Cortical activation changes underlying stimulation-induced behavioural gains in chronic stroke. Brain. 2012;135:276–84. doi: 10.1093/brain/awr313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagg CJ, Best JG, Stephenson MC, O’Shea J, Wylezinska M, Kincses TZ, Morris PG, Matthews PM, Johansen-Berg H. Polarity-sensitive modulation of cortical neurotransmitters by transcranial stimulation. J Neurosci. 2009;29:5202–6. doi: 10.1523/JNEUROSCI.4432-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagg CJ, Jayaram G, Pastor D, Kincses ZT, Matthews PM, Johansen-Berg H. Polarity and timing-dependent effects of transcranial direct current stimulation in explicit motor learning. Neuropsychologia. 2011b;49:800–4. doi: 10.1016/j.neuropsychologia.2011.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taig E, Kuper M, Theysohn N, Timmann D, Donchin O. Deficient Use of Visual Information in Estimating Hand Position in Cerebellar Patients. J Neurosci. 2012;32:16274–16284. doi: 10.1523/JNEUROSCI.1153-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka J-I, Horiike Y, Matsuzaki M, Miyazaki T, Ellis-Davies GCR, Kasai H. Protein synthesis and neurotrophin-dependent structural plasticity of single dendritic spines. Science (80- ) 2008;319:1683–7. doi: 10.1126/science.1152864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka S, Hanakawa T, Honda M, Watanabe K. Enhancement of pinch force in the lower leg by anodal transcranial direct current stimulation. Exp Brain Res. 2009;196:459–65. doi: 10.1007/s00221-009-1863-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T, Takano Y, Tanaka S, Hironaka N, Kobayashi K, Hanakawa T, Watanabe K, Honda M. Transcranial direct-current stimulation increases extracellular dopamine levels in the rat striatum. Front Syst Neurosci. 2013;7:6. doi: 10.3389/fnsys.2013.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tecchio F, Zappasodi F, Assenza G, Tombini M, Vollaro S, Barbati G, Rossini PM. Anodal Transcranial Direct Current Stimulation Enhances Procedural Consolidation. J Neurophysiol. 2010;104:1134–40. doi: 10.1152/jn.00661.2009. [DOI] [PubMed] [Google Scholar]
- Terney D, Chaieb L, Moliadze V, Antal A, Paulus W. Increasing human brain excitability by transcranial high-frequency random noise stimulation. J Neurosci. 2008;28:14147–55. doi: 10.1523/JNEUROSCI.4248-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teyler TJ, DiScenna P. Long-term potentiation. Annu Rev Neurosci. 1987;10:131–61. doi: 10.1146/annurev.ne.10.030187.001023. [DOI] [PubMed] [Google Scholar]
- Thoroughman Ka, Shadmehr R. Learning of action through adaptive combination of motor primitives. Nature. 2000;407:742–7. doi: 10.1038/35037588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trepel C, Racine R. GABAergic modulation of neocortical long-term potentiation in the freely moving rat. Synapse. 2000;128:120–128. doi: 10.1002/(SICI)1098-2396(200002)35:2<120::AID-SYN4>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Tyryshkin K, Coderre AM, Glasgow JI, Herter TM, Bagg SD, Dukelow SP, Scott SH. A robotic object hitting task to quantify sensorimotor impairments in participants with stroke. J Neuroeng Rehabil. 2014;11:47. doi: 10.1186/1743-0003-11-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentino F, Cosentino G, Brighina F, Pozzi NG, Sandrini G, Fierro B, Savettieri G, D’Amelio M, Pacchetti C. Transcranial direct current stimulation for treatment of freezing of gait: A Cross-over study. Mov Disord. 2014;00:1–5. doi: 10.1002/mds.25897. [DOI] [PubMed] [Google Scholar]
- Vandermeeren Y, Lefebvre S, Desfontaines P, Laloux P. Could dual-hemisphere transcranial direct current stimulation (tDCS) reduce spasticity after stroke? Acta Neurol Belg. 2013;113:87–9. doi: 10.1007/s13760-012-0163-5. [DOI] [PubMed] [Google Scholar]
- Verheyden G, Purdey J, Burnett M, Cole J, Ashburn A. Immediate effect of transcranial direct current stimulation on postural stability and functional mobility in Parkinson’s disease. Mov Disord. 2013;28:2040–1. doi: 10.1002/mds.25640. [DOI] [PubMed] [Google Scholar]
- Villalta JI, Landi SM, Fló A, Della-Maggiore V. Extinction Interferes with the Retrieval of Visuomotor Memories Through a Mechanism Involving the Sensorimotor Cortex. Cereb Cortex. 2013 Dec 19; doi: 10.1093/cercor/bht346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vines BW, Cerruti C, Schlaug G. Dual-hemisphere tDCS facilitates greater improvements for healthy subjects’ non-dominant hand compared to uni-hemisphere stimulation. BMC Neurosci. 2008a;9:103. doi: 10.1186/1471-2202-9-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vines BW, Nair DG, Schlaug G. Contralateral and ipsilateral motor effects after transcranial direct current stimulation. Neuroreport. 2006;17:671–674. doi: 10.1097/00001756-200604240-00023. [DOI] [PubMed] [Google Scholar]
- Vines BW, Nair DG, Schlaug G. Modulating activity in the motor cortex affects performance for the two hands differently depending upon which hemisphere is stimulated. Eur J Neurosci. 2008b;28:1667–73. doi: 10.1111/j.1460-9568.2008.06459.x. [DOI] [PubMed] [Google Scholar]
- Voronin L. Action of surface polarization on intracellular unit activity in the motor cortex of waking rabbits. Neurosci Behav Physiol. 1968;18:691–698. [Google Scholar]
- Wang L, Conner JM, Rickert J, Tuszynski MH. Structural plasticity within highly specific neuronal populations identifies a unique parcellation of motor learning in the adult brain. Proc Natl Acad Sci U S A. 2011;108:1–6. doi: 10.1073/pnas.1014335108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward NS. Age-related changes in the neural correlates of motor performance. Brain. 2003;126:873–888. doi: 10.1093/brain/awg071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters-Metenier S, Husain M, Wiestler T, Diedrichsen J. Bihemispheric transcranial direct current stimulation enhances effector-independent representations of motor synergy and sequence learning. J Neurosci. 2014;34:1037–50. doi: 10.1523/JNEUROSCI.2282-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JA, Pascual-Leone A, Fregni F. Interhemispheric modulation induced by cortical stimulation and motor training. Phys Ther. 2010;90:398–410. doi: 10.2522/ptj.20090075. [DOI] [PubMed] [Google Scholar]
- Xu T, Yu X, Perlik AJ, Tobin WF, Zweig JA, Tennant Ka, Jones T, Zuo Y. Rapid formation and selective stabilization of synapses for enduring motor memories. Nature. 2009;462:915–9. doi: 10.1038/nature08389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang G, Pan F, Gan W-B. Stably maintained dendritic spines are associated with lifelong memories. Nature. 2009;462:920–4. doi: 10.1038/nature08577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, Zuo Y. Spine plasticity in the motor cortex. Curr Opin Neurobiol. 2010;21:169–174. doi: 10.1016/j.conb.2010.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimerman M, Heise KF, Hoppe J, Cohen LG, Gerloff C, Hummel FC. Modulation of training by single-session transcranial direct current stimulation to the intact motor cortex enhances motor skill acquisition of the paretic hand. Stroke. 2012;43:2185–91. doi: 10.1161/STROKEAHA.111.645382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimerman M, Nitsch M, Giraux P, Gerloff C, Cohen LG, Hummel FC. Neuroenhancement of the aging brain: restoring skill acquisition in old subjects. Ann Neurol. 2013;73:10–5. doi: 10.1002/ana.23761. [DOI] [PMC free article] [PubMed] [Google Scholar]