Abstract
Introduction
The Personal Calorie Monitor (PCM) is a portable direct calorimeter that estimates energy expenditure (EE) from measured heat flux (i.e. the sum of conductive, convective, radiative, and evaporative).
Purpose
The primary aim of this study was to compare EE estimated from measures of heat flux to indirect calorimetry in a thermoneutral environment (26°C). A secondary aim was to determine if exposure to ambient temperature below thermoneutral (19°C) influences the accuracy of the PCM.
Methods
34 Adults (mean±SD, age = 28±5 y, body mass index = 22.9±2.6 kg.m2) were studied for 5 h in a whole-room indirect calorimeter (IC) in thermoneutral and cool conditions. Participants wore the PCM on their upper arm and completed two, 20-minute treadmill-walking bouts (0% grade, 3 mph). The remaining time was spent sedentary (e.g., watching television, using a computer).
Results
In thermoneutral, EE (mean (95% CI)) measured by IC and PCM was 560.0 (526.5, 593.5) and 623.3 (535.5, 711.1) kcals, respectively. In cool, EE measured by IC and PCM was 572.5 (540.9, 604.0) and 745.5 (668.1, 822.8) kcals, respectively. Under thermoneutral conditions, mean PCM minute-by-minute EE tracked closely with IC, resulting in a small, non-significant bias (63 kcals (−5.8, 132.4)). During cool conditions, mean PCM minute-by-minute EE did not track IC, resulting in a large bias (173.0 (93.9, 252.1)) (p<0.001).
Conclusion
This study demonstrated the validity of using measured heat flux to estimate EE. However, accuracy may be impaired in cool conditions, possibly due to excess heat loss from the exposed limbs.
Keywords: Physical activity monitor, indirect calorimetry, energy expenditure, heat flux
Introduction
Obesity and obesity-related comorbidities have reached epidemic proportions in most western societies. In recent years significant efforts have been made to identify the genetic, behavioral and environmental causes of obesity and to develop successful prevention and treatment strategies. Fundamental to this effort is understanding the association between energy expenditure (EE) and obesity. However, accurately measuring EE in free-living humans remains a challenge to clinicians and applied researchers. Accurate, reliable and low cost approaches to measuring EE are needed to improve clinical outcomes (e.g. weight management) and to meet public health research objectives (22).
In laboratory settings, EE can be accurately measured via both direct and indirect calorimetry. Direct calorimetry is the measurement of heat produced by the body. Because all of the body’s metabolic processes produce heat, the quantity of heat lost is proportional to EE when core body temperature is maintained. Indirect calorimetry is based on respiratory gas exchange and the premise that all energy releasing reactions in the body require oxygen. Thus, EE is proportional to oxygen consumption and can be calculated from an equation based on oxygen consumed (VO2) and carbon dioxide produced (VCO2) (26). Whole room calorimeters or “metabolic chambers” are sealed rooms that accurately measure EE via either direct or indirect calorimetry. Although these methods allow detailed measurement of EE for periods lasting from several hours up to several days, the confined space may limit human movement and is not representative of typical free-living environments. Portable indirect calorimeters (often worn as a backpack) that use a facemask or mouthpiece to capture VO2 and VCO2 can also be used to accurately measure EE (17). This allows research participants to be more mobile and to perform activities in a variety of settings (e.g. participants’ homes or workplaces). However, issues with comfort and restricted eating and speaking make these methods impractical for more than a few hours.
The gold standard for measuring free-living EE is the doubly labeled water (DLW) method (18). This method is based on the principles of indirect calorimetry and involves ingesting a dose of stable isotopes 2H2O and H2 18O (19,20). The difference in elimination rates of these isotopes is proportional to the magnitude of metabolic CO2 production, which is then used to calculate O2 consumption and EE (19,20). The DLW approach is ideal for free-living settings because it does not require any type of restrictive device and it yields accurate measurements of total EE over periods of several days to a few weeks (17). However, DLW is limited in that it does not provide important information about the pattern of EE, such as time spent in physical activity intensity categories. Moreover, the high cost associated with the isotopes and sample analysis prohibits the widespread use of DLW in many clinical and research settings (20).
There have been attempts to develop body worn calorimeters based on both indirect and direct calorimetry. Portable indirect calorimeters require the use of a mask to collect expired gases, and the instruments are typically carried by the subject in a backpack (17), limiting the ability to use these devices for long-term free-living assessments. There have also been attempts to develop body worn direct calorimeters that measure heat flux from the skin’s surface. Early attempts were based on exchange of body heat to garments worn on the body that contained chilled water (24). These instruments were cumbersome, requiring external water chilling equipment, and limited by the fact that the suits themselves disrupted normal heat flux by occluding the skin. This latter limitation can be overcome by employing the use of heat flow gages (HFG). HFGs measure the difference in heat flow across an insulator; the voltage output is proportional to heat flux in units of energy per time per area, which can then be used to estimate total body heat flux. Attempts to incorporate HFGs into a body worn system to measure free-living EE were largely unsuccessful (2,6), in part due to their inability to capture heat flux from evaporation.
Heat produced by the body is exchanged with the environment via four different routes; 1) convection, the exchange of heat between the body and air or water molecules moving past the skin; 2) conduction, the exchange of heat between the body and materials in contact with the skin; 3) radiation, the electromagnetic exchange of heat between the body and the environment and 4) evaporation, the exchange of heat from the skin to vaporized sweat. Although HFGs can accurately measure heat flux from convection, conduction, and radiation, they have limited ability to measure evaporative heat flux. Moreover, early HFGs used in human studies tended to be fairly large, in terms of both surface area and thickness, which can alter heat flow from the site of measurement. Recently, a new body worn direct calorimeter has been introduced (Personal Calorie Monitor (PCM); MetaLogics Corporation, Minneapolis, MN). The PCM has been designed to overcome the limitations of previous body worn direct calorimeters. First, the size has been reduced to minimize the potential impact on skin temperature at the measurement site. Second, the PCM incorporates a thin, permeable membrane that allows the transport of perspiration onto the impermeable HFG, permitting the measurement of evaporative heat exchange to be captured.
The objective of this study was to determine the validity of using measured heat flux to estimate EE compared to whole room indirect calorimetry (IC). EE was measured concurrently over 5 hours using both the PCM and IC. The primary aim was to test the validity of the PCM in a thermoneutral environment (ambient temperature ~26° C). Because heat flux from the body is influenced by ambient conditions, a secondary aim was to determine if variations in ambient temperature below the thermoneutral zone (~19° C) influence the validity of the PCM.
Methods
Institutional Approval
Procedures followed were in accordance with the ethical standards of the Helsinki Declaration of 1975 as revised in 1983. The Colorado Multiple Institutional Review Board and the Scientific Advisory Board of the Clinical Translational Research Center (CTRC) at the University of Colorado Anschutz Medical Campus approved the study.
Participants
All participants completed an informed consent document approved by The Colorado Multiple Institutional Review Board. Healthy men and women were recruited from Denver, Colorado and surrounding communities. Participants were relatively young (20-45 y), lean (body mass index (BMI) = 19-25 kg.m−2) and non-smokers. Participants were excluded if they had any acute or chronic health conditions (e.g. diabetes, heart disease), reported regular tobacco use within the past 6 months, resting systolic blood pressure > 160 mmHg, resting diastolic blood pressure > 100 mmHg, or reported any contra-indications to exercise (e.g. orthopedic limitations). Additional exclusion criteria for female volunteers were pregnancy or lactation within the past year. After initial screening eligible participants completed a health history and physical examination. The study sample consisted of 15 males (mean±SD; age = 27±6 y, weight = 76.2±8.5 kg, body mass index (BMI) = 23.9±2.2 kg/m2), and 19 females (30±4 y, 63.5±9.8 kg, 22.1±2.6 kg/m2).
Personal Calorie Monitor (PCM)
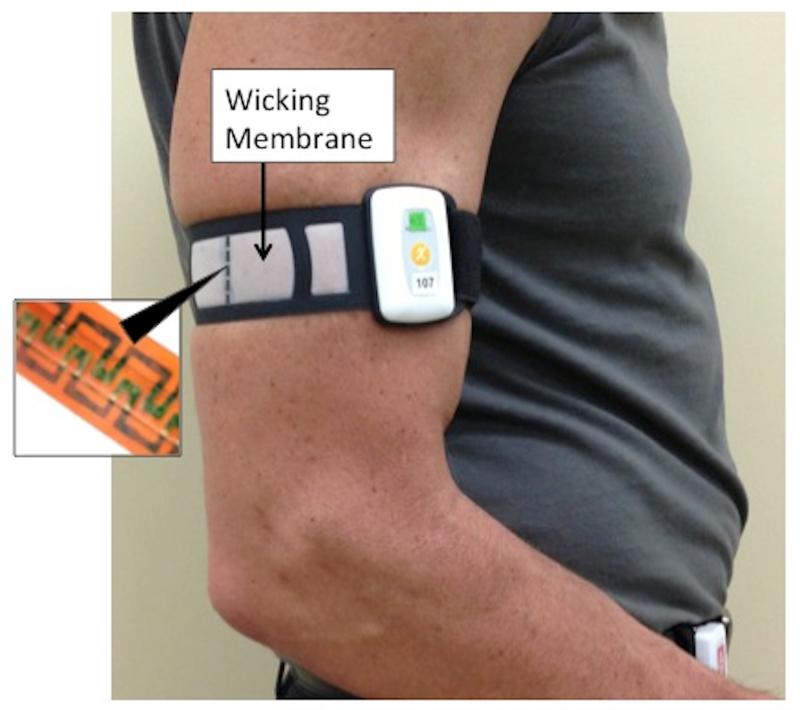
The PCM used in this study is a prototype and is not yet commercially available. It is a small (5 × 3.5 × 1 cm) and lightweight (31 g) device attached to the body, typically the upper arm with a Velcro strap (Figure 1). The device consists of a heat flow sensor connected to a self-contained electronics package powered by a replaceable 3V lithium cell. The output from the heat flow sensor is digitized four times per minute using a 24 bit analog-digital converter and the average for the minute is stored in internal memory. The PCM memory capacity allows EE data to be stored for up to 14 days.
Figure 1.
Image of participant wearing the PCM on upper arm. The small heat flow gage is surrounded by the wicking membrane. Inset shows the serpentine pattern of the small heat flow gage.
Heat flow gauges work by measuring the temperature differential on two sides of an insulator, most often with a pair of thermocouples. The custom heat flow gauge used in the PCM consists of multiple pairs of thin thermocouples that measure the temperature on opposite sides of a polyimide film. The gauge is approximately 25mm in length, 3.5mm in width and 0.2mm thick. Two key design elements allow the custom gauge to accurately capture all forms of heat flow. First, the thermocouple pairs are etched into a U-shaped serpentine pattern (Figure 1 inset), allowing for a dense population of thermocouples within a very small area. The large number of thermocouples ensures adequate signal strength to measure heat flow. Second, the short width and height of the sensor combined with the use of a wicking membrane (Figure 1) prevent the device from occluding the skin surface and changing heat flow in the sensing area. The membrane is positioned over the heat flow gauge to facilitate the migration of perspiration from the skin surface to the top of the heat flow gauge (Figure 2). The membrane mimics the skin’s normal evaporative rate and does not trap moisture, allowing for accurate measurement of evaporative heat loss. One PCM sensor was used throughout the study and this device was calibrated approximately every two months. The PCM sensor is calibrated against an Omega HFS-3, used as a reference heat flow gage (rHFG) by clamping the device under test (DUT) and rHFG between a heat source and heat sink. A set of compliant but minimally compressible layers between the rHFG, DUT and heat source and sink help ensure that the heat flow is uniform across structure, i.e. the DUT and HFG are subjected to the same heat flow. The output of the DUT and reference HFG are captured over a period of rising heat flow and a linear regression calculation is performed to derive the calibration factor. The PCM uses estimated body surface area (DuBois formula) to convert heat flux sensed at the site of the gauge to an estimate of heat flux from the entire body. This information is then used to calculate minute-to-minute EE, assuming heat flux is evenly distributed throughout the body surface.
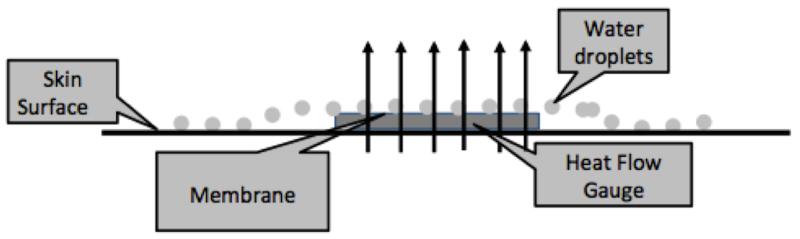
Figure 2.
Schematic of the heat flow gauge used in the PCM. Water droplets from sweat migrate over the membrane of the heat flow gauge. Heat from the water droplets is lost when the water droplets evaporate. The total heat flow across the heat flow gauge (arrows) is the sum of convective, radiative, conductive, and evaporative heat flow.
Description of Room Calorimeter
The calorimeter room is ~3.6 × 3.3 × 2.4 m (length × width × height), with a total volume of ~28.5 m3. Oxygen (O2) and carbon dioxide (CO2) concentrations were measured continuously using a fuel-cell based dual channel O2 analyzer (FC-2 Oxzilla, Sable Systems, International, Las Vegas, NV) and two infrared CO2 analyzers (CA-10 CO2 analyzers, Sable Systems, International., Las Vegas, NV), as previously described (16). O2 consumption (VO2) and CO2 production (VCO2) were calculated in one-minute intervals using the flow rate and the differences in CO2 and O2 concentrations between entering and exiting air, and minute EE was calculated using the equations of Jequier et al. (9). The room contains a regular hospital bed, treadmill, desk, toilet, telephone, TV and DVD player, and a computer with Internet access. The accuracy and precision of the system is tested monthly using propane combustion tests. The average O2 and CO2 recoveries during the period when the study was performed were 98.7 (0.7%) and 99.3 (0.1%) (mean (SD)), respectively. A complete description of the room calorimeter and the technical specifications of the gas exchange system can be found at Melanson et al. (16).
Experimental Design
This was a randomized crossover trial with two temperature conditions; thermoneutral (~26° C) and cool (~19° C). The order of conditions was randomized by participant and individual participants were studied at the same time of day, on separate days.
Experimental Procedures
Experimental procedures were identical for each condition. Conditions were performed following at least a 4-hour fast, and EE was measured simultaneously by the PCM and whole room indirect calorimeter for five hours. Before entering the room calorimeter height and weight were measured and input into the PCM software. Clothing was standardized for each condition; closed toed shoes, socks, jeans and a sleeveless t-shirt.
Once in the calorimeter, participants sat for minutes 1-60, 80-180 and 200-300. During this time participants were seated in a chair and were permitted to read, watch TV, use the computer, talk on the phone etc. Participants were instructed to get up from sitting only to use the restroom. During minutes 60-80 and 180-200 participants walked on a treadmill at 0% grade, 3.0 mph. To confirm compliance, participants were observed using a closed-circuit camera.
Data Cleaning and Statistical Evaluation
The first 10 minutes of each measurement period was eliminated to ensure accurate synchronization of the PCM and IC. Data were log transformed in order to achieve normality for analysis. A Linear mixed model was used to analyze the data with 4 repeated measurements on subjects (2 methods for measuring EE × 2 ambient temperatures). Temperature (thermoneutral vs. cool), measurement methods (PCM or IC) and their interaction were included as fixed effects. Unstructured covariance was assumed within subjects. Mean bias and its 95% CI were calculated to evaluate the difference between PCM and IC. PCM was considered not statistically significantly different from IC if the 95% CI of their ratio spanned 1 (i.e., the bias of the log-transformed measurements spanned zero). The association between PCM EE and IC was then assessed using Pearson correlation and Bland-Altman analysis based on log-transformed data was used to assess agreement. Enhanced Bland-Altman needle plots (7) were used to illustrate the performance of the PCM compared to IC. In these figures, a needle plot is used to clearly show the spread of the data, the regression line, its 95% CI band, and limit of agreement are used to assess the magnitude, homogeneity and direction of the bias, and zero bias line and mean bias line are included as reference lines. To further describe the differences between PCM and IC EE, root mean squared error (rMSE) was calculated and minute-to-minute EE for each of three time segments was compared: 1) rest (10-60 min), 2) exercise 1 plus recovery (61-180 min) and 3) exercise 2 plus recovery (181-300 min). Unless otherwise indicated, data were reported as mean (95% CI) on original EE scale (analysis results obtained using log-transformed data). Because rMSE and minute-to-minute data were for descriptive purposes, they were presented on the original EE scale for ease of interpretation. All the statistical analyses were conducted using R 2.14.1.
Results
Thermoneutral Condition
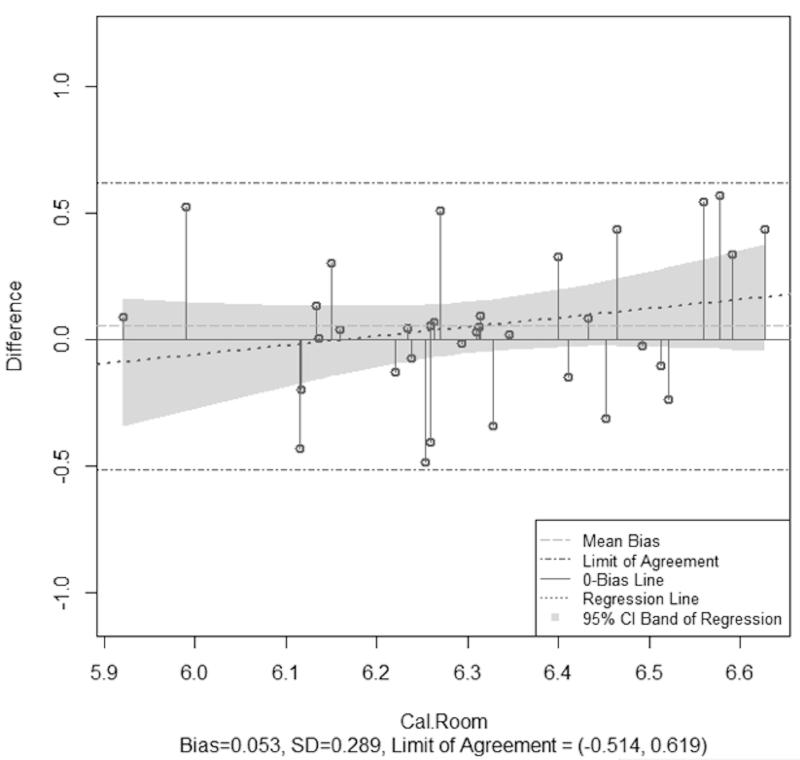
The mean (SD) temperature and humidity during the thermoneutral condition was 26.4 °C (2.9) and 52.3% (18.1), respectively. EE measured by the PCM (623.3 kcal (535.5, 711.1)) did not significantly differ from IC (560.0 kcal (526.5, 595.5)). Analysis on log-transformed data indicated this difference was not statistically significant (p = 0.30). PCM bias was 63.3 kcal (−5.8, 132.4) and rMSE was 205.1 kcals (Table 1). The Pearson correlation between log-scaled PCM EE and IC was 0.64 (p-value < 0.001). In the enhanced Bland-Altman analysis, the regression line showed a slight increasing trend but the zero bias line fell within the 95% CI band, which indicates a non-significant heterogeneous bias (log-scaled bias (95% CI) = 0.05 (−0.05, 0.15) with a narrow limit of agreement (−0.51, 0.62) between log-scaled PCM EE and IC (Figure 3a).
Table 1.
PCM EE compared to whole room indirect calorimetry (mean (95% CI))
| N=34 | IC | PCM | IC | PCM |
|---|---|---|---|---|
|
| ||||
| Warm | Cold | |||
| Kcals | 560.0 (526.5, 593.5) | 623.3 (535.5, 711.1) | 572.5 (540.9, 604.0) | 745.5 (668.1, 822.8) |
| Bias | - | 63.3 (−5.8, 132.4) | - | 173.0 (93.9, 252.1) |
| rMSE | - | 205.1 | - | 282.6 |
| Bias # | - | 0.05 (−0.05, 0.15)+ | - | 0.24 (0.13, 0.34) |
| Pearson R # | - | 0.64* | - | 0.10 |
N = number of observations, rMSE = root mean squared error,
Log transformed.
Not significantly different from IC.
Significant correlations
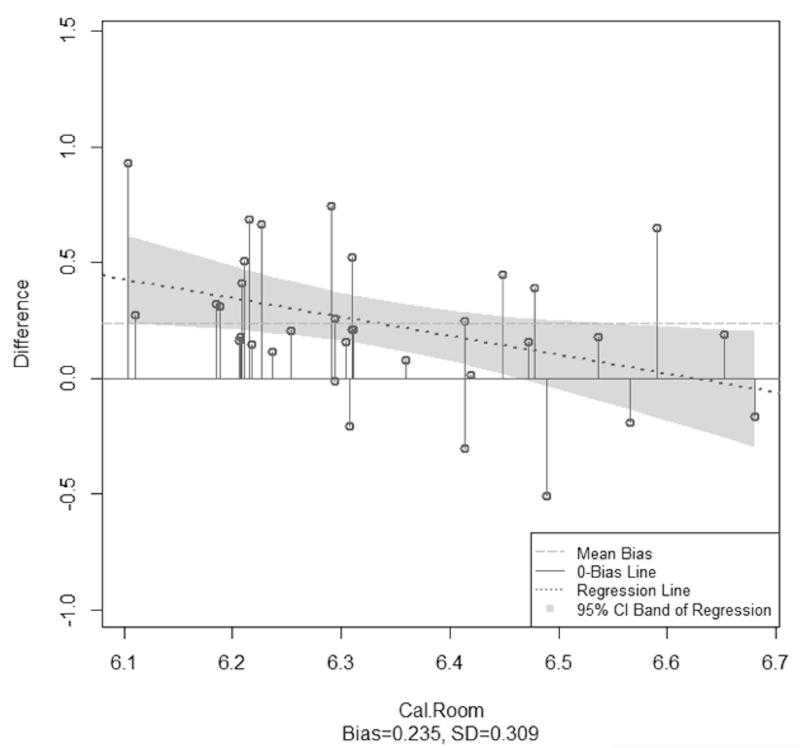
Figure 3.
Enhanced Bland-Altman plots for thermoneutral (a) and cool (b) conditions. Data were not normally distributed and thus are shown on a log scale. The needle plots illustrate the heterogeneous bias for both conditions.
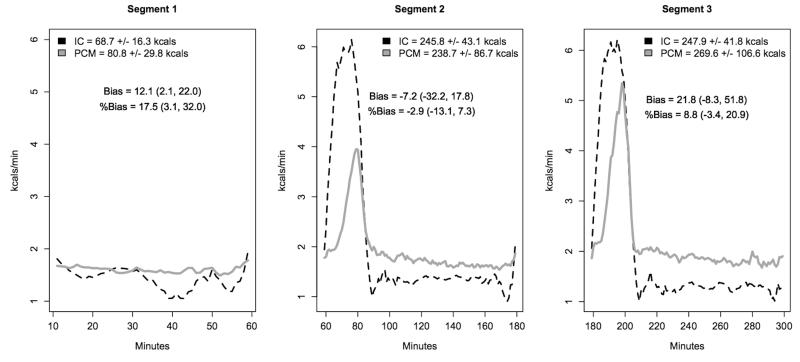
When averaged across all participants, PCM minute-to-minute EE tracked closely, but were slightly delayed compared to IC. To illustrate this, Figure 4a shows minute-to-minute EE (on original scale) over the 5 h measurement period partitioned in three segments, 1) rest (10-60 min), 2) exercise 1 plus recovery (61-180 min) and 3) exercise 2 plus recovery (181-300 min). Segments 2 and 3 clearly illustrate the delayed response of the PCM when exercise was initiated and ended. At the start of both exercise bouts, PCM EE rose very slightly, briefly leveled off, rose substantially for the remainder of exercise and then remained slightly elevated once exercise had ended. During recover from exercise, PCM EE remained slightly elevated compared to pre-exercise EE. Despite this lag, PCM EE was very close to IC for each segment (segment 1 bias = 12.1 kcals (1.7, 22.4), segment 2 bias = −7.2 kcals (−33.1, 18.8), segment 3 bias = 21.8 kcals (−9.4, 52.9)).
Figure 4.
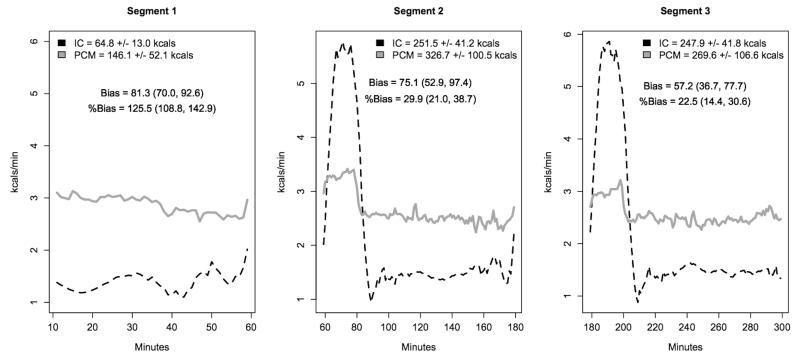
Mean minute-by-minute energy expenditure measured by IC and PCM in thermoneutral (a) and cool (b) conditions. The 5 h measurement period is partitioned into three segments (1) rest (10-60 min), 2) exercise 1 plus recovery (61-180 min) and 3) exercise 2 plus recovery (181-300 min). PCM bias was calculated for each segment.
Cool Condition
The mean (SD) temperature and humidity during the cool condition was 19.1 °C (6.8) and 40.0% (11.2), respectively. EE measured by the PCM (745.5 kcals (668.1, 822.8)) was significantly different than IC (572.5 kcals (540.9, 604.0)) (Table 1). PCM bias was 173.0 kcals (93.9, 252.1) and rMSE was 282.6 kcals. Analysis on log-transformed data indicated this was statistically significant difference (p < 0.001). Pearson correlation between log-scaled PCM EE and IC was 0.10 (p-value=0.59). In the enhanced Bland-Altman analysis, the regression line had a negative slope and the zero bias line intersected with the 95% CI band, which revealed a significant heterogeneous bias (log-scaled bias (95% CI) = 0.24 (0.13, 0.34)). Limits of agreement were not calculated because there was a significant difference in PCM EE and IC and therefore agreement need not be assessed (23).
During the cool condition, PCM minute-to-minute EE (on original scale) did not track well with IC; PCM EE was higher than IC at rest and lower during the two walking bouts (Figure 4b). When partitioned by segments (1) rest (10-60 min), 2) exercise 1 plus recovery (61-180 min) and 3) exercise 2 plus recovery (181-300 min)), PCM EE was significantly greater than IC for each segment. However, PCM EE moved toward IC as the measurement period progressed (bias segment 1 = 81.3 (64.4, 98.2), bias segment 2 = 75.1 (41.9, 108.4), bias segment 3 = 57.2 (26.7, 87.7)).
Discussion
The Personal Calorie Monitor (PCM; MetaLogics Corporation, Minneapolis, MN) is a new, body worn device that estimates EE based on measured heat flux. The small, custom design of the PCM allows it to be worn comfortably and inconspicuously for extended periods in free-living settings. In the current study, the PCM was worn on the upper arm and 5 h EE was compared to IC. The primary finding of this study was that by using all forms of heat flux to estimate EE, the PCM accurately measures total EE over 5 h in thermoneutral conditions. However, when subjects are exposed to cool conditions for several hours (with their upper limbs exposed), the heat flux based measurement overestimates EE compared to indirect calorimetry.
A novel feature of the PCM is its ability to measure evaporative heat loss. As homeotherms, humans maintain a nearly constant core temperature (37°C) by balancing the generation of heat (from metabolic processes) with the loss of heat through evaporation, convection, radiation, and conduction. In most situations, conduction does not contribute substantially to total heat flux. During exercise, or in warm ambient conditions, convection and radiation processes do not sufficiently transfer heat from the body and thus evaporative heat loss must be initiated to maintain core temperature. Evaporative heat loss occurs both sensibly (e.g. sweating) and insensibly (without obvious sweating) and can provide several fold greater heat loss than convection and radiation combined. Similar devices that measure heat flux but are not sensitive to evaporative heat loss report an underestimation of EE during exercise. For example, the SenseWear Pro ArmbandTM (Body Media, Pittsburgh, PA) incorporates a heat flow sensor and other sensors (e.g., accelerometer) to estimate energy expenditure. Convective heat flux is measured using dual thermistors on the skin and airside of an internal “heat pipe”, and galvanic skin resistance, which is proportional to perspiration, is used as an index of evaporative heat loss. Although the SenseWear performs well in most situations (21), there is evidence it underestimates EE during exercise, particularly during high intensity exercise (4,8,11). It has been speculated that these errors are due to the inability to accurately capture evaporative heat flux during vigorous intensity exercise (4). In the current study, the exercise performed produced noticeable perspiration in most subjects under thermoneutral conditions. The lack of cumulative difference in EE under thermoneutral conditions suggests that the PCM accurately captured evaporative heat loss. However, our conclusion is limited by the fact that we did not directly measure evaporative heat loss, and that the amount of perspiration induced during this moderate intensity activity was rather modest. To more rigorously assess the ability of the PCM to measure evaporative heat flux, future studies will incorporate direct measurements of evaporative heat loss and more intense exercise bouts to promote higher rates of sweating.
In thermoneutral conditions, minute-by-minute EE based on heat flux measurements tracked well with IC. During exercise, the increase in EE measured by the PCM was slightly delayed compared to IC (Figure 4a). The delay is likely due to the fact that heat exchange occurred more slowly than respiratory gas exchange (25). As seen in Figure 4a, IC detected a rapid increase in EE at the onset of each walking bout (minutes 61 and 181). However, because heat produced during activity was slowly dissipated, the PCM did not detect a rise in EE until a few minutes later. During the second exercise bout the PCM appears to have detected the rise in EE more quickly than during the first exercise bout, resulting in a higher peak EE (4 and 5.5 kcals.min−1 for bouts one and two, respectively). This is likely due to a shortened time to onset of vasodilation and sweating with successive exercise bouts, as has previously been demonstrated (10,13). During the post-exercise recovery period, EE measured by IC returned rapidly towards baseline, but PCM EE based on heat flux measurements returned more slowly, and remained elevated longer than IC. This is likely due to the heat loss mechanisms initiated during exercise (including evaporation) remaining elevated until heat produced during the exercise bout was dissipated and heat balance was achieved (25). Thus, when EE was summarized for each 20-minute bout of walking and the succeeding 100 minutes of resting recovery, we saw that despite differences in minute-by-minute values, the difference in total EE was small (−7.2 kcals (−32.2, 17.8) and 21.8 kcals (−8.3, 51.8), respectively). Because metabolic heat production is balanced against 24 h heat loss periods (25), it is likely that cumulative heat loss will be proportional to EE over longer periods of time. Thus, we hypothesize that PCM EE will even more closely match IC over longer periods of time, e.g., 24 h.
Although the PCM performed well in thermoneutral conditions, the PCM overestimated EE when subjects were exposed to cool temperatures for several hours. This significant overestimation of EE is likely explained by the fact that during rest periods, heat was being lost from the body, particularly the exposed arms, to the cool environment. In the current study, clothing was standardized across conditions (closed toed walking shoes, socks, undergarments, jeans and a sleeveless t-shirt) and although not specifically measured, participants reported being uncomfortably cold throughout the measurement period suggesting they were experiencing thermal imbalance (i.e. losing more heat than they were generating). The observed heat flow declined during the period, which may represent the body’s attempt to achieve thermal equilibrium. These observations suggest this was an extreme condition that participants would not self-select and if given the choice would have added layers of clothing to help maintain thermo-comfort. It’s possible that with more temperature appropriate clothing (e.g. long sleeve shirt or sweatshirt) the PCM would not overestimate EE as much as observed in the current study. It is also possible that if the PCM were worn at an alternative site that better reflects core temperature (e.g. torso), or if multiple PCM’s were worn, the effect of temperature would be minimized. The PCM measures heat flux at a single site and assumes this is uniform for the entire body surface area. This is a potential limitation of the PCM, however we chose to test a single PCM worn on the upper arm because past experience suggests that over longer measurement periods participant compliance declines with multiple devices and that participants prefer to wear devices on their arms/wrists. Future work will test the effect of alternative anatomical sites and clothing on the validity of the PCM.
Despite overestimating cumulative EE over a 5 h measurement period, PCM minute-to-minute EE moved toward IC over time, with PCM bias being reduced from segment 1 to segment 3; bias segment 1 = 81.3 (70.0, 92.6), bias segment 2 = 75.1 (52.9, 97.4), bias segment 3 = 57.2 (36.7, 77.7) (Figure 4b). This was likely due to some of the metabolic heat produced during exercise being retained to help preserve core temperature, and a subsequent decrease in the rate of heat loss. Figure 4b shows that during the exercise bouts, PCM minute-by-minute EE was much lower than IC, suggesting heat retention. During the two post-exercise recovery periods, PCM EE declined, but remained somewhat elevated as metabolic heat production drops, but the heat produced during the previous exercise bout continued to be dissipated. The slope of the PCM line however appears to decrease, suggesting a decrease in the rate of heat loss after each successive exercise bout.
If no exercise was performed and subjects remained at rest in the cool condition, we expect that minute-by-minute PCM EE would have eventually matched or even be lower than IC, depending on the duration of exposure. As stated above, this is based on the premise that heat production and heat loss will balance over time. However in the current study the rest periods were not long enough to confirm this, as the exercise bouts altered the pattern of heat loss. Again, because heat production and heat loss are balanced over time, we hypothesize errors caused by ambient temperature and/or clothing may be reduced over longer measurement periods. Nonetheless, this study provides evidence that PCM EE is influenced by ambient conditions.
In thermoneutral and cool, the rMSE was considerably large and the 95% CI of the bias was relatively wide (Table 1), indicating a large spread of individual data points and low precision. Studies validating other methods (e.g. accelerometers, DLW, SenseWear) report similar findings (8,12,14,15,21). Imprecise measurement tools have low reliability and low sensitivity to change (e.g. pre and post an intervention), resulting in decreased statistical power and larger sample size requirements. More work is needed to understand how the inter-individual variability in thermoregulatory responses influences the precision of the PCM.
This study confirms the potential of the PCM to be a valuable tool in both clinical and research settings. Current approaches for estimating EE in free-living individuals are limited by cost, accuracy and lack of sensitivity to specific characteristics of activity EE (e.g. time spent in physical activity intensity categories). Accelerometer sensors are popular devices to objectively estimate EE because of their minimal subject and researcher burden, versatility, and relative cost efficiency. The use of accelerometers is based on the premise that acceleration can be related to physical activity EE. While accelerometers are very useful to objectively summarize how much an individual moves and accurately estimate features of activity that are important for health (e.g. time spent in moderate-to-vigorous intensity activity), validation studies indicate accelerometers are limited in their ability to precisely estimate EE. Specifically, accelerometers cannot estimate resting metabolic rate (3), are relatively insensitive to sedentary behaviors (12), and tend to underestimate EE for high intensity activity (1,14). Further, accelerometer performance has been shown to decline considerably when tested in free-living environments (15). Laboratory calibration studies use simultaneous recordings of accelerometer output and respiratory gas exchange (measured via indirect calorimetry) to model the relationship between acceleration and EE. In these studies, a limited number of activities are performed, usually consisting of locomotion activities performed on a treadmill, activities of daily living (e.g. common household chores), and sporting activities. However, in free-living settings activity types are limitless and there is a large degree of inter-individual variability in the way specific activities are performed. Thus when laboratory methods are applied in free-living settings accelerometer performance declines considerably. Because the PCM estimates EE based on the principles of direct calorimetry and because it does not rely on laboratory calibration to model the relationship between specific activities and EE, it has the potential to greatly improve estimates of free-living EE across a broad range of activity types and intensities. This however was not specifically tested in the current study and future studies are needed for such verification.
This study had several limitations. First, PCM EE was compared to IC over a relatively short period of time (5 h). As stated above, subjects may not have achieved heat balance during the relatively short measurement period, particularly during the cool condition. Second, this study was performed in a whole room indirect calorimeter, limiting the range of activities that could be performed. Future work using DLW as a criterion will facilitate the validation of the PCM during a range of activity types and intensities in free-living settings. Third, we did not directly measure evaporative sweat loss in this study. Future work will address this limitation, which will help elucidate the effect of incorporating evaporative heat loss in the estimate of EE. Future work is also needed to test how variations in clothing, alternative body placements of the PCM (e.g. thigh), as well as studying how factors such as adiposity and age influence the performance of the PCM.
In conclusion, the PCM is a small, portable direct calorimeter that accurately estimated EE in thermoneutral conditions. However, ambient temperatures below the thermoneutral zone affected the accuracy of the PCM, possibly due to excess heat loss from exposed limbs. Nonetheless, this study demonstrated the potential of using measured heat flux to estimate EE and the potential of the PCM to be a valuable tool in research and clinical weight loss settings.
Acknowledgements
We thank the volunteers, as well as the Nursing, Clinical Lab, and Bionutrition Staffs of the University of Colorado CTRC.
This research was supported by a grant to Dr. Melanson (R01 DK091287), The Colorado Clinical and Translational Science Institute (UL1 TR000154), and the University of Colorado Nutrition and Obesity Research Center (P30 DK048520)
Funding This research was supported by a grant to Dr. Melanson (R01 DK091287), The Colorado Clinical and Translational Science Institute (UL1 TR000154), and the University of Colorado Nutrition and Obesity Research Center (P30 DK048520). The authors have no conflicts of interest to report.
Footnotes
Conflict of Interest The results of the present study do not constitute endorsement by ACSM. The authors have no conflicts of interest to report.
References
- 1.Crouter SE, Churilla JR, Bassett DR., Jr. Estimating energy expenditure using accelerometers. Eur J Appl Physiol. 2006;98(6):601–12. doi: 10.1007/s00421-006-0307-5. [DOI] [PubMed] [Google Scholar]
- 2.Danielsson U. Convective heat transfer measured directly with a heat flux sensor. J Appl Physiol. 1990;68:1275–81. doi: 10.1152/jappl.1990.68.3.1275. [DOI] [PubMed] [Google Scholar]
- 3.Dellava JE, Hoffman DJ. Validity of resting energy expenditure estimated by an activity monitor compared to indirect calorimetry. Br J Nutr. 2009;102:155–9. doi: 10.1017/S0007114508143537. [DOI] [PubMed] [Google Scholar]
- 4.Drenowatz C, Eisenmann JC. Validation of the SenseWear Armband at high intensity exercise. Eur J Appl Physiol. 2011;111:883–7. doi: 10.1007/s00421-010-1695-0. [DOI] [PubMed] [Google Scholar]
- 5.DuBois D, DuBois EF. A formula to estimate the approximate surface area if height and weight be known. Arch Intern Medicine. 1916;17:863–71. [Google Scholar]
- 6.English MJ, Farmer C, Scott WA. Heat Loss in Exposed Volunteers. J Trauma. 1990;30:422–5. [PubMed] [Google Scholar]
- 7.Fernandez R, Fernandez G. Validating the Bland-Altman Method of Agreement. In: Conference W, editor. Western Users of SAS Software. Long Beach, CA: California: [Cited 2013 October 1]. 2012. 2012. http://www.wuss.org/proceedings09/09WUSSProceedings/papers/pos/POSFernandez.pdf. [Google Scholar]
- 8.Jakicic JM, Marcus M, Gallagher KI, Randall C, Thomas E, Goss FL, Robertson RJ. Evaluation of the SenseWear Pro Armband to assess energy expenditure during exercise. Med Sci Sports Exerc. 2004;36(5):897–904. doi: 10.1249/01.mss.0000126805.32659.43. [DOI] [PubMed] [Google Scholar]
- 9.Jequier E, Schutz Y. Long-term measurements of energy expenditure in humans using a respiration chamber. Am J Clin Nutr. 1983;38:989–98. doi: 10.1093/ajcn/38.6.989. [DOI] [PubMed] [Google Scholar]
- 10.Kenny GP, Dorman LE, Webb P, Ducharme MB, Gagnon D, Reardon FD, Hardcastle SG, JAY O. Heat Balance and Cumulative Heat Storage during Intermittent Bouts of Exercise. Med. Sci. Sports Exerc. 2009;41(3):588–96. doi: 10.1249/MSS.0b013e31818c97a9. [DOI] [PubMed] [Google Scholar]
- 11.Koehler K, Braun H, De Marees M, Fusch G, Fusch C, Schaenzer W. Assessing energy expenditure in male endurance athletes: Validity of the SenseWear Armband. Med Sci Sports Exerc. 2011;43(7):1328–33. doi: 10.1249/MSS.0b013e31820750f5. [DOI] [PubMed] [Google Scholar]
- 12.Kozey-Keadle S, Libertine A, Lyden K, Staudenmayer J, Freedson PS. Validation of wearable monitors for assessing sedentary behavior. Med Sci Sports Exerc. 2011;43(8):1561–7. doi: 10.1249/MSS.0b013e31820ce174. [DOI] [PubMed] [Google Scholar]
- 13.Kruk B, Szczypaczewska M, Opaszowski B, Kaciuba-Uściłko H, Nazar K. Thermoregulatory and metabolic responses to repeated bouts of prolonged cycle-ergometer exercise in man. Acta Physiol Pol. 1990;41(7):22–31. [PubMed] [Google Scholar]
- 14.Lyden K, Kozey SL, Staudenmeyer JW, Freedson PS. A comprehensive evaluation of commonly used accelerometer energy expenditure and MET prediction equations. Eur J Appl Physiol. 2011;111(2):187–201. doi: 10.1007/s00421-010-1639-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lyden K, Kozey SL, Staudenmeyer JW, Freedson PS. A Method to Estimate Free-Living Active and Sedentary Behavior from an Accelerometer. Med Sci Sports Exerc. 2014;46(2):386–97. doi: 10.1249/MSS.0b013e3182a42a2d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Melanson EL, Ingebrigtsen JP, Bergouignan A, Ohkawara K, Kohrt WM, Lighton JRB. A new approach for flow-through respirometry measurements in humans. Am J Physiol Regul Integr Comp Physiol. 2010;298:R1571–R9. doi: 10.1152/ajpregu.00055.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Perret C, Mueller G. Validation of a new portable ergospirometric device (Oxycon Mobile) during exercise. Int J Sports Med. 2006;27(5):363–367. doi: 10.1055/s-2005-865666. [DOI] [PubMed] [Google Scholar]
- 18.Schoeller DA. Energy expenditure from doubly labeled water: some fundamental considerations in humans. Am J Clin Nutr. 1983;38(6):999–1005. doi: 10.1093/ajcn/38.6.999. [DOI] [PubMed] [Google Scholar]
- 19.Schoeller DA, Ravussin D, Schutz Y, Acheson KJ, Baertschi P, Jequier E. Energy expenditure by doubly labeled water: validation in humans and proposed calculation. Am J Physiol. 1986;250(5 Pt 2):R823–30. doi: 10.1152/ajpregu.1986.250.5.R823. [DOI] [PubMed] [Google Scholar]
- 20.Schoeller DA, Taylor PB, Shay K. Analytic requirements for the doubly labeled water method. Obes Res. 1995;3(Suppl 1):15–20. doi: 10.1002/j.1550-8528.1995.tb00003.x. [DOI] [PubMed] [Google Scholar]
- 21.St-Onge M, Mignault D, Allison DB, Rabasa-Lhoret R. Evaluation of a portable device to measure daily energy expenditure in free-living adults. Am J Clin Nutr. 2007;85:742–9. doi: 10.1093/ajcn/85.3.742. [DOI] [PubMed] [Google Scholar]
- 22.Troiano RP. A timely meeting: objective measurement of physical activity. Med Sci Sports Exerc. 2005;37(11 Suppl):S487–9. doi: 10.1249/01.mss.0000185473.32846.c3. [DOI] [PubMed] [Google Scholar]
- 23.van Hedel HJ, Wirz M, Dietz V. Assessing Walking Ability in Subjects With Spinal Cord Injury: Validity and Reliability of 3 Walking Tests. Arch Phys Med Rehabil. 2005;86:190–6. doi: 10.1016/j.apmr.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 24.Webb P, Annis JF, Troutman SJ. Human calorimetry with a water-cooled garment. J Appl Physiol. 1972;32(3):412–8. doi: 10.1152/jappl.1972.32.3.412. [DOI] [PubMed] [Google Scholar]
- 25.Webb P. Daily activity and body temperature. Eur J Appl Physiol Occup Physiol. 1993;66(2):174–7. doi: 10.1007/BF01427059. [DOI] [PubMed] [Google Scholar]
- 26.Weir JB de. New methods for calculating metabolic rate with special reference to protein metabolism. J Physiol. 1949;109:1–9. doi: 10.1113/jphysiol.1949.sp004363. [DOI] [PMC free article] [PubMed] [Google Scholar]