Abstract
Rosacea is a chronic inflammatory skin disease whose pathophysiological mechanism is still unclear. However, it is known that mast cell (MC) numbers is increased in the dermis of rosacea patients. MC proteases not only recruit other immune cells, which amplify the inflammatory response, but also cause vasodilation and angiogenesis. MCs are also one of the primary sources of cathelicidin LL-37 (Cath LL-37), an antimicrobial peptide that has been shown to be an enabler of rosacea pathogenesis. Here, we demonstrate that MCs are key mediators of cathelicidin initiated skin inflammation. Following Cath LL-37 injection into the dermis, MC deficient B6.Cg-KitW-sh/HNihrJaeBsmJ (KitW-sh) mice did not develop rosacea-like features. Conversely, chymase (p<0.001), tryptase and Mmp9 (p<0.01) mRNA levels were significantly higher in C57BL/6 Wild Type (WT) mice. Treating WT mice with a MC stabilizer significantly decreased the expressions of Mmp9 and Cxcl2 (p<0.01). Our data was confirmed on Erythematotelangiectatic rosacea subjects that showed a decrease in MMP activity (p<0.05), after eight weeks of topical cromolyn treatment.
We conclude that MCs play a central role in the development of inflammation subsequent to Cath LL-37 activation and that down regulation of activated MCs may be a therapy for rosacea treatment.
INTRODUCTION
Rosacea is a chronic inflammatory skin disease that affects ~16 million Americans (The National Rosacea Society website). Flares often occur without specific triggers, and when left untreated, can take weeks to subside (Scharschmidt et al., 2010). The formation of an aberrant form of antimicrobial peptide, Cath LL-37, on the surface of the skin, has been previously implicated as a key initiator of rosacea (Yamasaki et al., 2007). Cath LL-37 in the skin is also implicated in a wide range of inflammatory diseases including atopic dermatitis (Ong et al., 2002) and psoriasis (Lande et al., 2007). The active form of cathelicidin LL-37 peptide is not only able to kill microbes, but also modify host immunity and growth responses by promoting leukocyte chemotaxis (De et al., 2000), angiogenesis (Koczulla et al., 2003), and can alter the expression of extracellular matrix components (Park et al., 2009). In rosacea, the formation of an aberrant form of Cath LL-37 on the surface of the skin has been attributed to the overactivity of the kallikrein-related peptidases (KLKs), which are a family of tryptic serine proteases that are responsible for generating Cath LL-37 from its precursor protein, hCAP18, in the epidermis (Yamasaki et al., 2007) (Yamasaki and Gallo, 2008). In an animal model, intradermal injection of Cath LL-37, but not mouse cathelicidin, induced an inflammatory response with rosacea-like features (Yamasaki et al., 2007). Interestingly, sub-antimicrobial doses (40 mg/day) of doxycycline, a proven treatment for human rosacea, directly inhibits matrix metalloproteinases, which in turn, inhibits the activity of KLKs and prevents the activation of cathelicidins in human epidermal keratinocytes (Korting and Schollmann, 2009) (Kanada et al., 2012). Therefore, Cath LL-37 has emerged as a key mediator in the pathogenesis of rosacea.
Many studies have proven that MCs are able to enhance host defense through direct effects on pathogens by initiating the inflammation associated with innate immune responses (Galli et al., 2008). Although MCs can contribute to overall host protection by releasing a variety of molecules, including TNF-α (McLachlan et al., 2008), leukotriene B4 (Malaviya and Abraham, 2000), and cathelicidin AMP (Di Nardo et al., 2003), accumulated studies have also specifically implicated MCs in the development of inflammation in the skin (Kawakami et al., 2009) (Lin et al., 2011) (Thurmond et al., 2008). Most importantly in the context of our study, MCs have been observed in increased numbers in the skin lesions of rosacea patients. Until now, however, there has been no clear proof of the direct connection between MCs and rosacea formation.
In addition to inflammation, neuronal dysregulation is equally important to rosacea pathogenesis. In fact, neuronal dysregulation contributes to the disease via various mechanisms, such as vasomotor instability, release of pro-inflammatory neuropeptides, and neuronal injury (Wang et al., 2008) (Roosterman et al., 2006). MCs can also be activated by neuropeptides to release their components upon cell degranulation. Neuropeptides have also been shown to induce the production of TNF-α, CCL2, CCL5, CXCL9, CXCL10, and CXCL8 in human mast cells (Kulka et al., 2008), which leads to the recruitment of dendritic cells/macrophages (via CCL2), TH1 lymphocytes (via CCL5, CXCL9, or CXCL10) and neutrophils (via CXCL8) (Gangavarapuet al., 2012). In the current study, we show that neuropeptides induce MC enzyme activation and subsequent Cath LL-37 generation from hCAP18. Based on these results, it is likely that MCs are mediators of the stress-response network in skin inflammation (Arck et al., 2006). Considering previous studies of the role of MCs in rosacea, we propose that MCs and MC proteases have critical functions during the development of skin inflammation in rosacea. Furthermore, understanding the in vivo connections between MCs, epidermal keratinocytes and sensory nerves respectively, may lead to a better interpretation of the pathophysiology of rosacea.
Results
MC deficient mice do not develop inflammation following Cath LL-37 injection in the skin
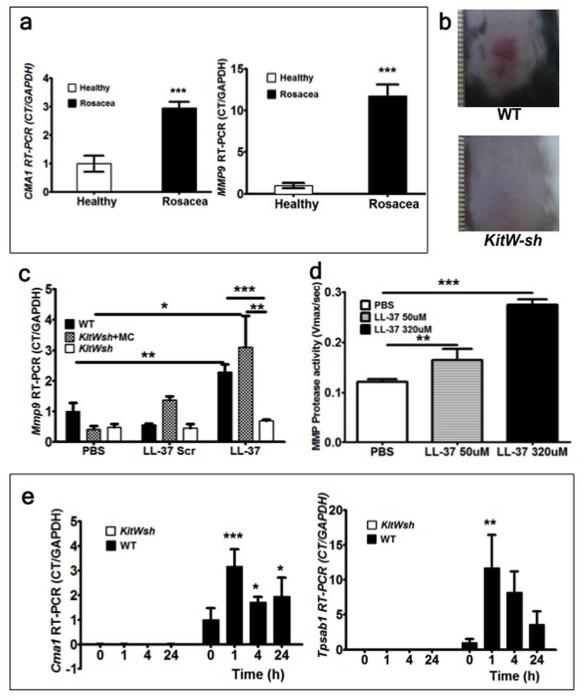
To verify that MC proteases are increased in rosacea skin, biopsies from 6 rosacea patients and 6 healthy control volunteers were collected and CMA1 (chymase gene) and MMP9 (metallo protease 9 gene) mRNA expressions were measured as essential markers of MC presence and activation (Tchougounova et al., 2005). Both CMA1 and MMP9 mRNA levels showed significant increases in rosacea skin (n=6) compared with healthy skin (n=6) (Figure 1a).
Figure 1. (a-e) MC proteases and MMP-9 are crucial for rosacea inflammation development.
(a) Human biopsy samples from healthy subjects (n=6) and rosacea subjects (n=6) were assessed for CMA1 and MMP9 mRNA expressions. (b) The back skins of wild type C57BL/6 (WT) and MC-deficient (KitW-sh) mice were injected intradermally with Cath LL-37. Pictures were taken 72 hours after LL-37 injections. (c) RT-PCR of Mmp9 mRNA expression in skin biopsies from WT, WT MC reconstituted KitW-sh (KitWsh) and KitW-sh. Mouse skin was injected with LL-37, LL-37 scrambled peptide (LL-37 Scr) or PBS control (PBS) and harvested after 72 hours of observation. (d) Assessment of MMP protease activity in WT mouse skin following challenge with PBS or Cath LL-37 at 50 and 320 μM. (e) mouse Chymase (Cma1) and mouse Tryptase (Tpsab1) were analyzed by RT-PCR in the skin from WT or KitW-sh mice (KitWsh) at 0, 1, 4, and 24 hrs after Cath LL-37 challenge. All of the experiments were repeated at least three times. Statistics: Mann Whitney test, one-way and two-way ANOVA*p<0.05, **p<0.01, ***p<0.001 (n=3)
Meaning that MCs were abnormally activated and were specifically expressing enzymes involved in Cath LL-37 processing.
To prove that MCs are central to the pathogenesis of rosacea inflammation, we used a well-established mouse model of rosacea-like inflammation (Yamasaki et al., 2007). We injected Cath LL-37 intradermally into MC deficient mice B6.Cg-KitW-sh/HNihrJaeBsmJ (KitW-sh) and compared the resulting inflammation with Wild Type (WT) mice. Our clinical end point observation (at 72hrs) showed, as previously described, inflammation in the skin of WT mice injected with Cath LL-37, whereas KitW-sh mice did not develop any rosacea-like features (Figure 1b). In order to further establish the essential role of MCs in the observed phenotype, we reconstituted the MC deficient mice with wild type MCs and repeated the injections with Cath LL-37. To define the specificity of Cath LL-37 in MC activation, we also included a Cath LL-37 scrambled peptide in the experiments. Our results showed that, following Cath LL-37 challenge, Mmp9 mRNA expression in skin from MC deficient mice was significantly lower than in skin from WT (p<0.01) and WT MC-reconstituted KitW-sh mice (p<0.05). There was no significant difference observed between any of the mouse groups when Cath LL-37 scrambled peptide was used (Figure 1c). We also injected different concentrations (50 μM and 320 μM) of Cath LL-37 peptide into WT mice and demonstrated that Cath LL-37 induced an increase in MMP activity in a dose dependent manner in WT mice (Figure 1d). Furthermore, a time course experiment showed that mRNA of the MC specific proteases chymase and tryptase were expressed immediately after injection of Cath LL-37, while the same enzymes were not detectable in the skin of the MC-deficient mice (Figure 1e).
Mouse MCs (mMCs) release of MMP-9 and IL-6 in response to Cath LL-37
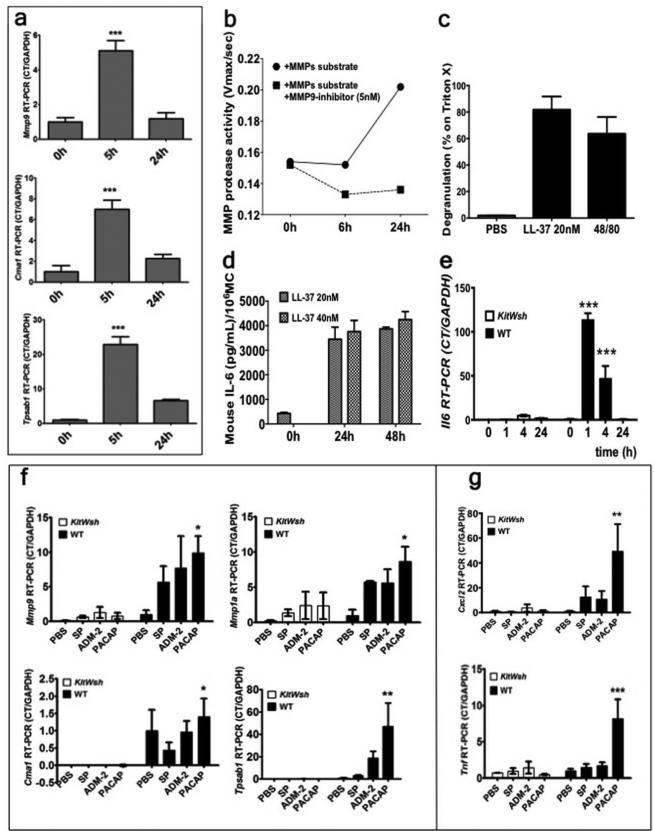
To confirm that MCs are responsive to direct Cath LL-37 stimulation, bone marrow derived mouse MCs (mMCs) were stimulated with different concentrations of Cath LL-37 at different time points. Mmp9, Cma1 (the gene for Chymase) and Tpsab1 (the gene for Tryptase) mRNA expressions were significantly higher at 5 hrs (p<0.001) (Figure 2a), while Mmp-1a, Klk5, and Klk6 were not detectable at 5 hrs (data not shown). There were no differences in MMP9, Cma1 and Tpsab1 mRNA expressions with different Cath LL-37 concentrations at 24 hrs (data not shown). MMP-9 protease activity in the culture medium of MCs stimulated with Cath LL-37 for 24 hrs was confirmed by fluorescence enzymatic activity assay using an MMP specific substrate and an MMP-9 specific inhibitor (Figure 2b). In Figure 2b, the difference between the two curves indicates MMP-9 specific activity. MC degranulation was confirmed by measuring β-hexosaminidase release in Cath LL-37 stimulated MC supernatants. We also found that a very low concentration of Cath LL-37 (20nM) was enough to induce degranulation (Figure 2c). In addition, ELISA detected high levels of secreted IL-6 in mMCs after 24 and 48 hrs of stimulation with different Cath LL-37 concentrations (20 nM and 40 nM) (Figure 2d). IL-6 increase was also confirmed in vivo. In fact, Il6 mRNA expression was also observed in the skin from WT mice, but not in MC deficient mice, following Cath LL-37 challenge (Figure 2e).
Figure 2. (a-e) Cath LL-37 induces pro-inflammatory responses.
(a) in vitro mMCs expression of Metallo protease 9 (Mmp9), Chymase (Cma1) and Tryptase (Tpsab1) after Cath LL-37 stimulation. (b) mMC Metallo pretease activity (MMP protease activity) after Cath LL-37 challenge. (c) mMCs degranulation (as β hexosaminidase release) in response to 24 hrs treatment with 20nM LL-37, positive control (48/80) and negative control (PBS). (d) ELISA IL-6 level (as pg/ml per million MCs) in mMC culture, 24 and 48 hours after 20 and 40 nM LL-37 treatment. (e) In vivo Il6 mRNA expression in mouse skin after 0, 1, 4, and 24 hrs of Cath LL-37 challenge in WT and MC deficient mice (KitWsh). (f-g) Neuropeptide-induced pro-inflammatory cytokine expression in skin is decreased in the absence of MCs in KitW-shmice. WT and KitW-sh (KitWsh), mice were injected with PBS or neuropeptide (SP, substance P; ADM-2, adrenomedullin-2; PACAP, pituitary adenylate cyclase-activating peptide) intradermally. (f) mRNA expressions of mouse Chymase (Cma1), mouse Tryptase (Tpsab1), mouse metalloprotease 9 (Mmp9), and metalloprotease 1 (Mmp1a) after NP challenge. (g) mRNA expressions of proinflammatory cytokines (Cxcl2 and Tnf) after NP challenge. Statistics: two-way ANOVA*p<0.05, **p<0.01, ***p<0.001 (n=3) repeated 3 times.
Neuropeptides activate tryptic-MC serine proteases in WT mouse skin
Rosacea flare-ups are frequently initiated by face flushing due to neuronal dysfunction and increased release of pituitary adenylate cyclase-activating peptide (PACAP) (Schwab et al., 2011). To investigate whether neuropeptides (NPs) require MC activation for the development of the inflammatory response in rosacea, 100μL of 1 μM NPs (substance P: SP; adrenomedullin-2: ADM-2; Pituitary adenylate cyclase-activating peptide: PACAP) were injected intradermally into WT and MC deficient mice. After 6 hours from the NP challenge, skin biopsies were taken and processed for mRNA expression analysis. Tryptase mRNA expression was observed to be dramatically increased by PACAP stimulation (p<0.01) in WT mice. Cma1, Tpsab1, Mmp1a and Mmp9 were also significantly increased (p<0.05) in WT mice (Figure 2f) but not as substantially as PACAP. In addition, mRNA expressions of the pro-inflammatory cytokines Cxcl2 (mouse homolog of human IL8) and Tnf (gene for TNF-α) were also significantly increased with PACAP challenge in WT mice, but not in MC deficient mice (p<0.01) (Figure 2g). These results prove that neurovascular alteration and neuropeptide release during a face flushing can be translated to rosacea inflammation in skin through the activation of MC enzymes. Moreover, these same MC enzymes can further increase the levels of free Cath LL-37 and amplify the inflammatory response.
MC stabilizer suppresses the development of rosacea-like inflammation in vivo
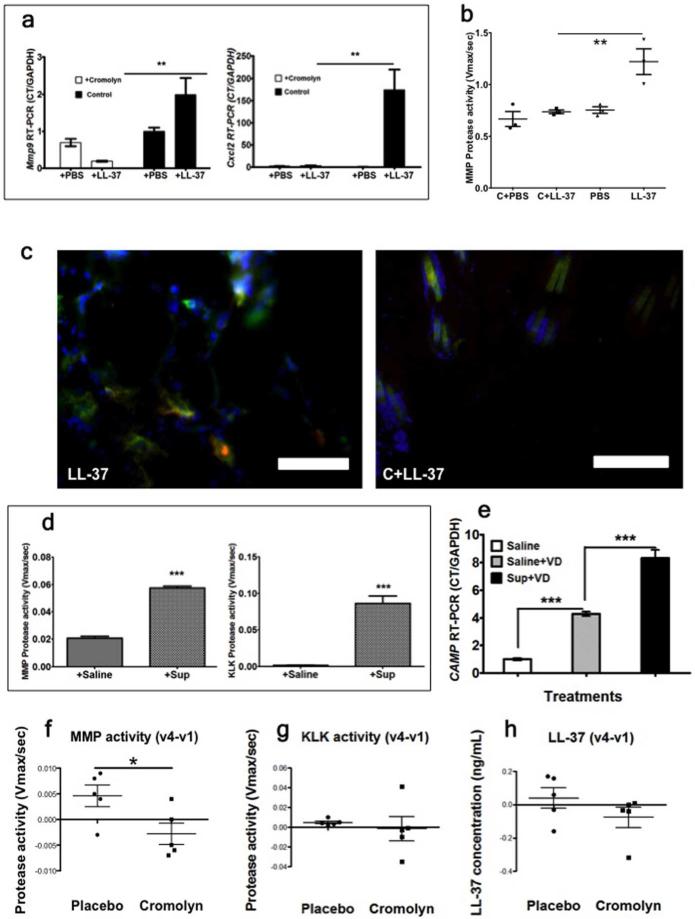
According to the obtained results, MC proteases and MMP-9, which are released from MCs upon stimulation by Cath LL-37, are central in promoting rosacea–like skin inflammation. Therefore, we anticipated that blocking MC degranulation would also prevent the formation of rosacea-like inflammation in the skin. To test this hypothesis, cromolyn sodium, a very well known MC stabilizer, was injected intra peritoneal (I.P.) into WT mice (10mg/kg body weight, per day) for 4 days before Cath LL-37 challenge. 24 hours after the last cromolyn sodium injection, mice were injected with 50 μl of 320 μm Cath LL-37 or PBS, twice a day for 2 days. As expected, skin inflammation did not develop in the mice pretreated with cromolyn. Mmp9 and Cxcl2 expressions were significantly decreased in the cromolyn treated mice (p<0.01) (Figure 3a). Consistent with Mmp9 mRNA expression, MMP activity in the tissue was also dramatically decreased by cromolyn pretreatment (p<0.01) (Figure 3b). In addition, frozen skin sections were stained with anti-MMP-9 and anti-FcεRI antibodies for the detection of MCs. The immunostaining images showed that numerous MMP-9 positive MCs were observed in Cath LL-37 treated mice, but not in cromolyn pretreated Cath LL-37 mice (Figure 3c left panel versus right panel).
Figure 3. (a-c) Cromolyn treatment of the rosacea-mouse model.
Mice were treated with Cromolyn (C) or Cromolyn and LL-37 (C+LL-37). (a) Skin mRNA expression of mouse metalloprotease 9 (Mmp9) and pro-inflammatory cytokine (Cxcl2). (b) Skin Metalloproteases total activity (MMP activity). (c) Mouse skin immunohistochemistry of mMC-MMP9 contents after LL-37 challenge (left image, LL-37) and cromolyn pre-treatment LL-37-challenge (right image, C+LL-37). MMP-9 (green), FcεRI (Red: MC marker) and DAPI (Blue: cell nuclei). Yellow –orange hue indicates MMP9 and mMC co-localization. Yellow orange color is not present in the right image. Scale bar = 50.0μm. (d-e) MC degranulation increases protease activity in NHEK. (d) MMP and KLK protease activities in NHEK after addition of the product of huMC degranulation in saline buffer (+Sup), or saline buffer (+Saline). (e) RT-PCR Cath LL-37 mRNA (CAMP) expression in NHEK cells treated with the product of huMC degranulation in saline buffer and 1,25(OH)2VD3 (Sup+VD), or saline buffer and 1,25(OH)2VD3 (Saline+VD) and saline alone (Saline); (f-h) Cromolyn effect on protease activity of rosacea patients. (f) MMP activity on the skin of patient treated with placebo (n=5) or cromolyn (n=5) (g) KLK activity. (h) LL-37 peptide level. Statistics: ***p<0.001, **p<0.01, *p<0.05
Human MC (huMC) proteases increase keratinocyte expression of LL-37
Because we wanted to understand how human keratinocytes respond to human MCs (huMCs), we examined a co-culture of normal human epidermal keratinocytes (NHEK) cells with supernatant from huMCs, which had been degranulated by compound 48/80. MMP and KLK enzyme activities were significantly increased in NHEK cells when the cells were co-cultured for 24hrs with 100μL of huMC supernatant (p<0.001) (Figure 3d). Since the activation of cathelicidin in keratinocytes requires the active form of vitamin D3, 1,25(OH)2VD3 (Schauber et al., 2007), we also added 1,25(OH)2VD3 in the co-culture model. The Cath LL-37 mRNA (CAMP) level was significantly increased after co-culture with huMC supernatant (p<0.001) (Figure 3e).
Therefore, this confirms that, while LL-37 activates MCs, MC supernatants increase protease production in human skin epidermis, amplifying the inflammation and creating a vicious cycle.
Stabilization of MCs could be a target therapy for erythematotelangiectatic rosacea
To test whether our results were relevant in humans, 10 randomized adults with erythematotelangiectatic rosacea were chosen to apply a solution containing either the MC stabilizer (4% cromolyn sodium) or placebo, topically to their face, twice daily. Six tape strips were obtained from both right and left cheeks, at the time of first visit (v1) and last visit (v4). Facial erythema was clinically assessed by a blind investigator using the Clinician's Erythema Assessment (CEA). None of the subjects presented with papules and pustules; therefore, after eight week (v4), only facial erythema levels decreased in the cromolyn treatment group (Data not shown). MMP activity was significantly decreased in the cromolyn treatment group (Figure 3f) while KLK activity (Figure 3g) and Cath LL-37 protein levels (Figure 3h) only showed a mild decrease.
Discussion
An abnormal increase in the free form of hCAP18 (Cath LL-37) antimicrobial peptide in human skin is crucial for the pathogenesis of rosacea (Yamasaki et al., 2007). MCs are one of the primary sources of cathelicidin in the skin and they are also the main source of enzymes that activate cathelicidin to its active form (Cath LL-37) (Di Nardo et al., 2003). Previous studies have reported that MC activity increases in the skin of rosacea patients (Yamasaki et al., 2007). However, a great number of questions remain regarding the manner in which inflammatory signals are transmitted from the epidermis to vessels and inflammatory cells. In the present study, we found that MC proteases and Mmp9 mRNAs are highly expressed in skin from rosacea patients (Figure 1a). Therefore, we hypothesize that after Cath LL-37 has been released from the epidermis in rosacea skin, it in turn activates MCs to induce inflammation and neutrophil recruitment, which results in more Cath LL-37.
We next demonstrated that Cath LL-37 peptide strongly activates skin MCs to release proteases that are crucial in the development of rosacea-like inflammation in vivo. We clearly showed that in MC-deficient mice, intradermal Cath LL-37 injections did not generate all of the dermal events that are usually found in rosacea, including inflammation and MMP-9 activation. To more strongly support our hypothesis that MCs are crucial in the generation of skin inflammation, we also reconstituted MC deficient mice with WT MCs and showed that the rosacea-like inflammation phenotype reappears. Moreover, we demonstrated the specificity of Cath LL-37 peptide in MC activation by showing that injection of a scrambled peptide did not induce inflammation (Figure 1c). This result is nicely paralleled by our in vitro experiments, where we observed an increase in MMP-9 protease activity in the culture supernatant of Cath LL-37 treated mMCs (Figure 2b). Our hypothesis that MMP-9 is involved in rosacea pathogenesis is supported by the recent discovery that doxycycline, which is an effective therapeutic for improving rosacea symptoms, inhibits MMPs directly (Kanada et al., 2012).
Any signal (especially from neurogenic factors) that induces the activation of MCs results in the amplification of the inflammatory response, thus we propose that MCs are the missing link between flaring and inflammation in rosacea. Tryptase activates PAR-2 on nerve endings and keratinocytes, which in turn causes the release of neuropeptides (Steinhoff et al., 1999). On the other hand, activated PAR-2 stimulates MC mediator release (e.g. histamine release) during cutaneous inflammation (Aubdool and Brain, 2011). Transient receptor potential vanilloid 1 (TRPV1) and ankyrin 1 (TRPA1), which are highly expressed on primary sensory neuron endings, are also strongly present in rosacea skin (Aubdool and Brain, 2011). Activated PAR-2 enhances these receptors in the vascular endothelial cells and immune cells (Aubdool and Brain, 2011), explaining how MCs might be linked to rosacea flashing and erythema. PACAP is a neuropeptide that has been shown to be one of the main mediators in the response to psychological stress and is abundantly expressed in rosacea-affected skin (Schwab et al., 2011). It has been reported that PACAP is not only able to induce neuronal inflammation, but also edema and flushing in human skin (Roosterman et al., 2006). Our in vivo experiments have proven that intradermal injections of PACAP significantly induce the expression of the MC proteases, Mmp1a and Mmp9 in WT mice, but not in MC deficient mice (Figure 2f). Moreover, dramatically increased expressions of the pro-inflammatory cytokines Tnf and Cxcl2 were observed in skin after PACAP challenge (Figure 2g). Though it is well known that neuronal activation results in the release of MC-derived histamine and leukotriene, our study also confirms that PACAP increases the expressions of Mmp1 and Mmp9, which are activators of hCAP18 processing to Cath LL-37. In our last experiment, we also proved that activated MCs, release enzymes that increase LL-37 expression in keratinocytes. Therefore, we have proven a link between PACAP and Cath LL-37. Consequently, these peptides work synergistically in the development of rosacea symptoms in human skin.
Since MC activation plays an important role in rosacea inflammation, we examined whether the stabilization of MCs can directly reduce skin inflammation in WT mice. In our research, mice injected I.P. with cromolyn did not develop rosacea-like skin inflammation after Cath LL-37 challenge. Moreover, MMP activity, Mmp9 and Cxcl2 mRNA expressions and MMP-9/MC immunostaining in the skin were significantly decreased compared with mice not treated with cromolyn. Cxcl2 is the mouse homolog of human IL8 and is crucial for neutrophil recruitment in vivo.
Few case reports have cited MC stabilizers as a potential treatment for rosacea and Morbus Morbihan (J F Mayberry et al., 1975), (Hu et al., 2012); however, we have now determined that MC stabilizers actually block MC induced inflammation and have the potential to be developed as therapeutics for the treatment of rosacea. Skin MCs are localized near keratinocytes and sensory nerve endings in the epidermis and dermis, therefore, MC proteases (chymase and tryptase), histamine, MMP-9 and pro-inflammatory cytokines amplify the progression of skin inflammation, tissue remodeling and angiogenesis (Coussens et al., 2000). Chymase, a MC specific protease, can not only activate pro-MMP-9 and other MMP cascades (Tchougounova et al., 2005), but can also inhibit enzyme degradation by TIMP-1, further stabilizing MMP-9. From our observations of the mouse model of rosacea, we have proven that pro-inflammatory peptides generated from epidermal keratinocytes and sensory nerve endings, activate dermal MCs, that in turn amplify skin inflammation and angiogenesis via their secretion of proteases, MMP-9, and pro-inflammatory cytokines. In addition, our in vitro data of NHEK cells strongly support the hypothesis that protease generated from MCs will induce dermal inflammation and increase the production of enzymes from the epidermal layer able to generate LL-37, with the result of creating a pro-inflammatory loop (Figure 3d and e). Release of MC proteases activate MMP-9 and KLKs in NHEK, which results in the generation of additional active Cath LL-37 peptide.
In conclusion, rosacea features cannot be generated in mouse skin in the absence of MCs or when MCs have been pharmacologically stabilized. LL-37 and neuropeptides activate MCs; activated huMC, in turn, increase NHK expression and processing of LL-37, making huMCs central in amplifying rosacea inflammation and symptoms, especially erythema, flushing and telangiectasia. Moreover, our preliminary results from a limited clinical trial, supports the hypothesis that MC stabilizers can change metalloproteinase activity in rosacea in humans. Based on these observations, MC stabilizers should be considered as a future therapy in the treatment and prevention of rosacea symptoms.
MATERIALS AND METHODS
Reagents
Mca-Lys-Pro-Leu-Gly-Leu-Dpa-Ala-Arg-NH2 fluorogenic substrate and 6-Amidino-2-naphthy-4-guanidinobenzoate dimethanesulfonate were purchased from Enzo Life Sciences (Plymouth Meeting, PA). Cromolyn sodium and substance P fragment were purchased from Sigma-Aldrich (St Louis, MO). Adrenomedullin-2 and PACAP were purchased from AnaSpec (Fremont, CA). 4-methylumbelliferyl-2-acetamide-2-deoxy-b-D-glucopyranoside was purchased from Calbiochem (EMD Millipore; Billerica, MA). MMP-9 specific inhibitor I, chymotryptase-specific fluorogenic substrate, and Pierce BCA protein assay kit were purchased from Thermo Fisher Scientific (Chicago, IL).
Human specimens
All the procedures involving human subjects were approved by the University of California San Diego Institutional Review Board under IRB number 071032 in accordance with adherence to the Helsinki Guidelines and written informed patient consent was obtained. 2mm skin biopsies were taken from the faces of healthy and rosacea subjects, as well as tape strips from the face of erythematotelangiectatic rosacea subjects in the dermatology clinic at the University of California, San Diego. Ten randomized adults with erythematotelangiectatic rosacea were applied a water-based solution containing either 4% cromolyn sodium or placebo, topically to their face twice a day, for 8 weeks. A clinical evaluation was performed at each visit. A total of six tape strips were obtained from right and left cheek at each of the first visit and the final visit. Facial erythema was clinically assessed by a blind investigator using the Clinician's Erythema Assessment (CEA), which grades erythema on a five point scale: 0 = none (no redness), 1 = mild (slight pinkness), 2 = moderate (definite redness), 3 = significant (marked erythema), 4 = severe (fiery redness). At each visit, the CEA was performed on five different facial regions: right cheek, left cheek, nose, chin and glabella. The subject's final CEA score for each visit was calculated by summing the CEA score of each of the five facial regions for that visit.
Mouse experiments
B6.Cg-KitW-sh/HNihrJaeBsmJ MC deficient mice were bred at our facility. Institutional Animal Care and Use Committee (IACUC) of UC San Diego approved all animal experiments. B6.Cg-KitW-sh/HNihrJaeBsmJ (KitW-sh) the W-sash (Wsh) inversion mutation have MC deficiency, but lack anemia and sterility. Adult KitW-sh mice had a profound deficiency in MCs in all tissues examined, but normal levels of major classes of other differentiated lymphoid cells. MC deficient mice and wild type mice (C57BL/6) were utilized for these experiments. WT and MC deficient mice were shaved 24 hrs before intradermal injection with 50 μl of 50 μM or 320 μM of Cath LL-37 or filtered PBS (n=3), twice a day for 2days. Skin biopsies were taken after 72 hours of observation and tissues were immersed immediately into RNA stabilization reagent (RNAlater; QIAGEN science, Germantown, MD). Total RNA was extracted and purified by RNeasy Mini Kit (QIAGEN science). mRNA expressions of proteases and cytokines were assessed by RT-PCR. For the time course observation of chymase and tryptase mRNAs, skin biopsies of WT and MC deficient mice were taken at 1, 4, and 24 hrs after Cath LL-37 injection and analyzed by RT-PCR.
For the neuropeptide challenge experiment, 0.1 μM neuropeptide or PBS was injected into WT mice and MC deficient mice intradermally. Skin biopsies were taken 6 hrs after injection and analyzed by RT-PCR.
For the MC stabilizer experiment: Cromolyn sodium (10mg/kg body weight, per day) (Sigma-Aldrich, St Louis, MO) was diluted in filtered PBS and injected I.P. into WT mice for 4 days. After 4 days of treatment, 50 μl of 320 μM of Cath LL-37 or PBS were injected intradermally twice a day for 2 days while continuing daily cromolyn treatment. 72 hrs after the beginning of the Cath LL-37 injections, skin biopsies were taken and analyzed for mRNA expression and protease activity.
MCs
Primary MCs were generated from mouse bone marrow and cultured in RPMI 1640 medium (Invitrogen) supplemented with 10% inactivated FBS (Thermo Fisher Scientific, Chicago, IL), 25 mM HEPES (pH 7.4), 4 mM L-glutamine, 0.1 mM nonessential amino acids, 1 mM sodium pyruvate, 50 mM 2-ME, 100 IU/ml penicillin, and 100 mg/ml streptomycin, recombinant murine IL-3 (R&D Systems, Minneapolis, MN) and recombinant murine stem cell factor (R&D Systems). MMCs derived from bone marrow cells that are cultured with SCF and IL-3 become mature in vitro. After 4 weeks, MCs were consistently generated, as confirmed by the expression of CD117 (c-Kit) and FcεRI. Cell maturation was confirmed by metachromatic staining with toluidine blue. The purity of MCs was greater than 98%. MCs were cultured in Stemline® II Hematopoietic Stem Cell Expansion Medium (Sigma-Aldrich).
BMMC reconstitution in KitW-sh mice
4×106 cells of C57BL/6 mature BMMCs were diluted in filtered PBS and injected into the shaved back skin of 6 week old C57BL/6-KitW-sh mice (400 μl; 8 × 50 μl injections) intradermally. After 6 weeks, MCs were confirmed as resident cells in skin by toluidine blue staining.
Measurement of protease activity
Total-MMP activities were determined in 0.1 M Tris-HCl (pH 7.5) containing 150 mM NaCl, 10 mM CaCl2, 0.1 mM ZnCl2, 0.05% (v/v) Brij35, 0.1% (w/v) PEG6000. Protein extracts were normalized with a BCA assay kit. Total-MMP activity was determined with total-MMP fluorogenic substrate (5 μM): Mca-Lys-Pro-Leu-Gly-Leu-Dpa-Ala-Arg-NH2 (Enzo Life Sciences), in protein extracts, incubated for 2 hrs at 37°C, and subsequently measuring activity (Vmax/sec) at a fluorescence excitation wavelength 328 nm and an emission wavelength of 400 nm in a fluorescence plate reader (Gemini EM microplate spectrofluorometer; Molecular Devices, Sunnyvale, CA). To determine MMP-9 activity, MMP-9 specific inhibitor I (Thermo Fisher Scientific, Chicago, IL) was added to protein extracts. Chymase and tryptase activities were assayed in 0.1 mM Tris-HCl (pH 8.0), 200 mM NaCl. Chymase activity was monitored by adding chymotryptase-specific fluorogenic substrate (Thermo Fisher Scientific) (5 μM) to protein extracts, incubating for two hours at 37 °C and subsequently measuring activity (Vmax/sec) at a fluorescence excitation wavelength of 380 nm and an emission wavelength of 460 nm. Tryptase activity was determined by adding serine protease-specific fluorogenic substrate (Boc-Phe-Ser-Arg-AMC, Bachem Biosceince Inc.) (5 μM) with/without ABESF (Sigma) to protein extracts, incubating for two hours at 37°C, and subsequently measuring relative fluorescence units (RFU) at a fluorescence excitation wavelength of 354 nm and an emission wavelength of 422 nm. Proteins in skin tissues or cells were normalized with the Pierce BCA protein assay kit (Thermo Fisher Scientific).
Semi quantitative real-time-PCR
cDNA was synthesized from RNA using the iScript cDNA Synthesis Kit (BioRad, Hercules, CA) according to the manufacturer's protocol. Synthesized cDNA was normalized using a NanoDrop spectrophotometer (Thermo Fisher Scientific Inc.). TaqMan Gene Expression Assays (Applied Biosystems ABI, Foster City, CA) were used to analyze the expressions of human MMP9, human CMA1, human CAMP, mouse Mmp9, mouse Mmp1a, mouse Klk5, mouse Klk6, mouse Tpsab1, mouse Cma1, mouse Cxcl2 and mouse Tnf as described by the manufacturer's instructions (Applied Biosystems; ABI, Foster City, CA). GAPDH and Gapdh mRNA were used as an internal control to validate RNA for each sample. mRNA expression was calculated as the relative expression to GAPDH or Gapdh mRNA, and all data are presented as fold change of each control.
MC degranulation assay
Degranulation percentage was assessed by measuring the activity of β-hexosaminidase in the supernatants of 1×105 MCs in 200 ml Tyrode's buffer incubated for 1 hr with 0 or 20 nM of Cath LL-37, 1% Triton X-100 and 10 μg/mL compound 48/80 (Sigma-Aldrich, St Louis, MO) as a positive control. For each sample assayed, supernatant aliquots (25 μL) were mixed with substrate solution (50 μL), which consisted of 10 mM 4-methylumbelliferyl-2-acetamide-2-deoxy-b-D-glucopyranoside (Calbiochem; EMD Millipore, Billerica, MA) in 0.1 M sodium citrate buffer (pH 4.5), and were incubated for 30 min at 37°C. The reaction was then stopped by adding 50 μL of 0.4 M glycine (pH 10.7). The reaction mixtures were excited at 365 nm and measured at 460 nm in a fluorescence plate reader (Gemini EM microplate spectrofluorometer; Molecular Devices, Sunnyvale, CA). To determine the total cellular content of this enzyme, an equivalent number of cells were lysed with 1% Triton X-100 and 10 μg/mL compound 48/80 (Sigma-Aldrich). Release of β-hexosaminidase was calculated as the percentage of the total enzyme content.
Cath LL-37 stimulation of mMCs
mMCs were challenged in vitro with 10ug/mL Cath LL-37. The cells were harvested at 0, 5 and 24 hours and mRNA was extracted and assessed by RT-PCR for mouse metallo protease 9 (Mmp9), mouse Chymase (Cma1) and mouse Tryptase (Tpsab1) expression. mMC Metalloprotease activity was assessed at 6 and 24 hours after Cath LL-37 challenge as previously described. mMC degranulation in response to LL-37 was assessed at 24 hours by β-hexosaminidase release as described; positive control (48/80) and negative control (PBS) were added. For IL-6 level evalulation, mMcs were treated with 20 and 40nM of LL-37 for 24 and 48 hours. ELISA (ELISA MAX™ Deluxe; BioLegend, San Diego, CA) was utilized to determine mouse IL-6 in all supernatants of cell culture medium according to the manufacturer's instructions. All supernatant samples were normalized by Pierce BCA protein assay kit (Thermo Fisher Scientific) to total protein content of the sample.
MC supernant–NHEK stimulation
2 days after confluence in Epilife medium (Cascade biologic) with 0.06mM CaCl2, NHEK cells were stimulated with supernatant from degranulated human cord blood-derived MCs in saline buffer, or saline buffer alone. MMP and KLK protease activities in the NHEK were evaluated after 24 hours. RT-PCR was evaluated after 6 hours from the MCs supernatant addition. For RTPCR Cath LL-37 mRNA (CAMP) expression, 100nM 1,25(OH)2VD3, was added. All of the experiments were repeated at least three times
Fluorescence immunohistochemistry
Mouse skin frozen sections were fixed with acetone, washed in 1X PBS, blocked with 5% goat serum/1% BSA for 30 minutes, and incubated with anti-MMP-9 polyclonal antibody (ABBIOTEC, San Diego, CA) as a primary antibody (diluted 1:200). FITC-conjugated goat antibody to rabbit IgG (Molecular Probes, Grand Island, NY) was used as a secondary antibody (diluted 1:1000) and was incubated for 40 minutes. For FcεRI detection, we used anti-mouse FcεRI PE (eBioscience, San Diego, CA). We mounted sections in ProLong Anti-Fade reagent (Molecular Probes) with 4’, 6-diamidino-2-phenylindole (DAPI). After overnight incubation at 4°C in the dark, images were obtained using a Zeiss LSM510 laser scanning confocal microscope coupled with an Axiovert 100 inverted stage microscope.
Statistical analyses
The data are presented as means ±SEM. To determine the significance between two or more groups, one-way and two-way ANOVA or the two-tailed t test was used and analyzed by GraphPad Prism4 (GraphPad Software, Inc.). For all statistical tests, p<0.05 was considered statistically significant.
ACKNOWLEDGMENTS
We thank Dr. S. Brian Jiang (Division of Dermatology, Department of Medicine, UC San Diego) for providing healthy skin specimens and Dr. Amy Sullivan for helping in revising the manuscript.
This work was supported by: NIH grant R01 AI093957-01 and by the National Rosacea Society Grant
Abbreviations
- AMP
antimicrobial peptide
- KLK
kallikrein
- MMP
matrix metalloproteinase
- MC
mast cells
- NP
neuropeptide
Footnotes
CONFLICT OF INTEREST
The authors state no conflict of interest.
REFERENCES
- Amalinei C, Caruntu ID, Giusca SE, et al. Matrix metalloproteinases involvement in pathologic conditions. Rom J Morphol Embryol. 2010;51:215–228. [PubMed] [Google Scholar]
- Arck PC, Slominski A, Theoharides TC, et al. Neuroimmunology of stress: skin takes center stage. J Invest Dermatol. 2006;126:1697–1704. doi: 10.1038/sj.jid.5700104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubdool AA, Brain SD. Neurovascular aspects of skin neurogenic inflammation. J Investig Dermatol Symp Proc. 2011;15:33–39. doi: 10.1038/jidsymp.2011.8. [DOI] [PubMed] [Google Scholar]
- Coussens LM, Tinkle CL, Hanahan D, et al. MMP-9 supplied by bone marrow-derived cells contributes to skin carcinogenesis. Cell. 2000;103:481–490. doi: 10.1016/s0092-8674(00)00139-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Y, Chen Q, Schmidt AP, et al. LL-37, the neutrophil granule- and epithelial cell-derived cathelicidin, utilizes formyl peptide receptor-like 1 (FPRL1) as a receptor to chemoattract human peripheral blood neutrophils, monocytes, and T cells. J Exp Med. 2000;192:1069–1074. doi: 10.1084/jem.192.7.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Nardo A, Vitiello A, Gallo RL. Cutting edge: mast cell antimicrobial activity is mediated by expression of cathelicidin antimicrobial peptide. J Immunol. 2003;170:2274–2278. doi: 10.4049/jimmunol.170.5.2274. [DOI] [PubMed] [Google Scholar]
- Galli SJ, Grimbaldeston M, Tsai M. Immunomodulatory mast cells: negative, as well as positive, regulators of immunity. Nat Rev Immunol. 2008;8:478–486. doi: 10.1038/nri2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gangavarapu P, Rajagopalan L, Kolli D, et al. The monomer-dimer equilibrium and glycosaminoglycan interactions of chemokine CXCL8 regulate tissue-specific neutrophil recruitment. J Leukoc Biol. 2012;91:259–265. doi: 10.1189/jlb.0511239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu SW, Robinson M, Meehan SA, et al. Morbihan disease. Dermatol Online J. 2012;18:27. [PubMed] [Google Scholar]
- Kanada KN, Nakatsuji T, Gallo RL. Doxycycline indirectly inhibits proteolytic activation of tryptic kallikrein-related peptidases and activation of cathelicidin. J Invest Dermatol. 2012;132:1435–1442. doi: 10.1038/jid.2012.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami T, Ando T, Kimura M, et al. Mast cells in atopic dermatitis. Curr Opin Immunol. 2009;21:666–678. doi: 10.1016/j.coi.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koczulla R, von Degenfeld G, Kupatt C, et al. An angiogenic role for the human peptide antibiotic LL-37/hCAP-18. J Clin Invest. 2003;111:1665–1672. doi: 10.1172/JCI17545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korting HC, Schollmann C. Tetracycline actions relevant to rosacea treatment. Skin Pharmacol Physiol. 2009;22:287–294. doi: 10.1159/000235550. [DOI] [PubMed] [Google Scholar]
- Kulka M, Sheen CH, Tancowny BP, et al. Neuropeptides activate human mast cell degranulation and chemokine production. Immunology. 2008;123:398–410. doi: 10.1111/j.1365-2567.2007.02705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lande R, Gregorio J, Facchinetti V, et al. Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature. 2007;449:564–569. doi: 10.1038/nature06116. [DOI] [PubMed] [Google Scholar]
- Lin L, Bankaitis E, Heimbach L, et al. Dual targets for mouse mast cell protease-4 in mediating tissue damage in experimental bullous pemphigoid. J Biol Chem. 2011;286:37358–37367. doi: 10.1074/jbc.M111.272401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malaviya R, Abraham SN. Role of mast cell leukotrienes in neutrophil recruitment and bacterial clearance in infectious peritonitis. J Leukoc Biol. 2000;67:841–846. doi: 10.1002/jlb.67.6.841. [DOI] [PubMed] [Google Scholar]
- McLachlan JB, Shelburne CP, Hart JP, et al. Mast cell activators: a new class of highly effective vaccine adjuvants. Nat Med. 2008;14:536–541. doi: 10.1038/nm1757. [DOI] [PubMed] [Google Scholar]
- Nagaoka I, Tamura H, Hirata M. An antimicrobial cathelicidin peptide, human CAP18/LL-37, suppresses neutrophil apoptosis via the activation of formyl-peptide receptor-like 1 and P2X7. J Immunol. 2006;176:3044–3052. doi: 10.4049/jimmunol.176.5.3044. [DOI] [PubMed] [Google Scholar]
- Ong PY, Ohtake T, Brandt C, et al. Endogenous antimicrobial peptides and skin infections in atopic dermatitis. N Engl J Med. 2002;347:1151–1160. doi: 10.1056/NEJMoa021481. [DOI] [PubMed] [Google Scholar]
- Park HJ, Cho DH, Kim HJ, et al. Collagen synthesis is suppressed in dermal fibroblasts by the human antimicrobial peptide LL-37. J Invest Dermatol. 2009;129:843–850. doi: 10.1038/jid.2008.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roosterman D, Goerge T, Schneider SW, et al. Neuronal control of skin function: the skin as a neuroimmunoendocrine organ. Physiol Rev. 2006;86:1309–1379. doi: 10.1152/physrev.00026.2005. [DOI] [PubMed] [Google Scholar]
- Scharschmidt TC, Yost JM, Truong SV, et al. Neurogenic rosacea: a distinct clinical subtype requiring a modified approach to treatment. Arch Dermatol. 2010;147:123–126. doi: 10.1001/archdermatol.2010.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauber J, Dorschner RA, Coda AB, et al. Injury enhances TLR2 function and antimicrobial peptide expression through a vitamin D-dependent mechanism. J Clin Invest. 2007;117:803–811. doi: 10.1172/JCI30142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab VD, Sulk M, Seeliger S, et al. Neurovascular and neuroimmune aspects in the pathophysiology of rosacea. J Investig Dermatol Symp Proc. 2011;15:53–62. doi: 10.1038/jidsymp.2011.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhoff M, Corvera CU, Thoma MS, et al. Proteinase-activated receptor-2 in human skin: tissue distribution and activation of keratinocytes by mast cell tryptase. Exp Dermatol. 1999;8:282–294. doi: 10.1111/j.1600-0625.1999.tb00383.x. [DOI] [PubMed] [Google Scholar]
- Subramanian H, Gupta K, Guo Q, et al. Mas-related gene X2 (MrgX2) is a novel G protein-coupled receptor for the antimicrobial peptide LL-37 in human mast cells: resistance to receptor phosphorylation, desensitization, and internalization. J Biol Chem. 2011;286:44739–44749. doi: 10.1074/jbc.M111.277152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchougounova E, Lundequist A, Fajardo I, et al. A key role for mast cell chymase in the activation of pro-matrix metalloprotease-9 and pro-matrix metalloprotease-2. J Biol Chem. 2005;280:9291–9296. doi: 10.1074/jbc.M410396200. [DOI] [PubMed] [Google Scholar]
- The National Rosacea Society http://www.rosacea.org/
- Thurmond RL, Gelfand EW, Dunford PJ. The role of histamine H1 and H4 receptors in allergic inflammation: the search for new antihistamines. Nat Rev Drug Discov. 2008;7:41–53. doi: 10.1038/nrd2465. [DOI] [PubMed] [Google Scholar]
- Wang J, Li R, Guo C, Fournier C, et al. The influence of fractionation on cell survival and premature differentiation after carbon ion irradiation. J Radiat Res. 2008;49:391–398. doi: 10.1269/jrr.08012. [DOI] [PubMed] [Google Scholar]
- Yamasaki K, Di Nardo A, Bardan A, et al. Increased serine protease activity and cathelicidin promotes skin inflammation in rosacea. Nat Med. 2007;13:975–980. doi: 10.1038/nm1616. [DOI] [PubMed] [Google Scholar]
- Yamasaki K, Gallo RL. Antimicrobial peptides in human skin disease. Eur J Dermatol. 2008;18:11–21. doi: 10.1684/ejd.2008.0304. [DOI] [PMC free article] [PubMed] [Google Scholar]