Abstract
Ullrich congenital muscular dystrophy (UCMD) is an autosomal recessive disorder characterized by generalized muscular weakness, contractures of multiple joints, and distal hyperextensibility. Homozygous and compound heterozygous mutations of COL6A2 on chromosome 21q22 have recently been shown to cause UCMD. We performed a genomewide screening with microsatellite markers in a consanguineous family with three sibs affected with UCMD. Linkage of the disease to chromosome 2q37 was found in this family and in two others. We analyzed COL6A3, which encodes the α3 chain of collagen VI, and identified one homozygous mutation per family. In family I, the three sibs carried an A→G transition in the splice-donor site of intron 29 (6930+5A→G), leading to the skipping of exon 29, a partial reduction of collagen VI in muscle biopsy, and an intermediate phenotype. In family II, the patient had an unusual mild phenotype, despite a nonsense mutation, R465X, in exon 5. Analysis of the patient’s COL6A3 transcripts showed the presence of various mRNA species—one of which lacked several exons, including the exon containing the nonsense mutation. The deleted splice variant encodes collagen molecules that have a shorter N-terminal domain but that may assemble with other chains and retain a functional role. This could explain the mild phenotype of the patient who was still ambulant at age 18 years and who showed an unusual combination of hyperlaxity and finger contractures. In family III, the patient had a nonsense mutation, R2342X, causing absence of collagen VI in muscle and fibroblasts, and a severe phenotype, as has been described in patients with UCMD. Mutations in COL6A3 are described in UCMD for the first time and illustrate the wide spectrum of phenotypes which can be caused by collagen VI deficiency.
Introduction
Congenital muscular dystrophies (CMDs) constitute a group of disorders characterized by early-onset muscular weakness and joint contractures, as well as dystrophic features identified by the morphological analysis of skeletal muscle. A subgroup of CMDs with merosin deficiency displays a fairly uniform clinical phenotype, usually including severe muscular weakness and always associated with brain white-matter changes. Patients with CMD who did not have merosin deficiency (i.e., who had merosin-positive CMDs) constitute a heterogeneous group showing a broad clinical spectrum with variations in distribution of the muscle involvement, degree of severity, progression, and other accompanying features (Voit 2001). A peculiar form of CMD was first reported, under the name of “congenital atonic-sclerotic muscular dystrophy,” by Otto Ullrich in 1930, in two boys presenting with an unusual combination of marked distal joint looseness (atonic) and contracture of proximal joints (sclerotic), with generalized muscle wasting and weakness since birth (CMD) and normal intelligence (Ullrich 1930). Other cases sharing a similar clinical picture confirmed the existence of this condition, which was named “Ullrich disease” (see Nonaka et al. 1981; Voit 1998). An autosomal recessive mode of inheritance was determined in familial cases of the disease (Nonaka et al. 1981). The progression of muscle involvement is slow, and the severity of this condition varies from mild to severe, with loss of walking (Ricci et al. 1988; De Paillette et al. 1989).
The involvement of extracellular matrix proteins in the pathogenesis of CMD was first suggested by different authors and later was demonstrated immunologically and genetically (Tomé 1999). Mutations in the laminin α2 chain (merosin), one of the three chains forming laminin-2 and laminin-4, a major component of the extracellular matrix, cause merosin-deficient CMD (Helbling-Leclerc et al. 1995). Recently, after an initial report on collagen VI deficiency in the muscle-biopsy samples of two Japanese patients with Ullrich disease (Higuchi et al. 2001b), mutations of COL6A2, the gene encoding the α2 chain of collagen VI, were identified in three Italian patients and one Japanese patient with the disease known as “Ullrich scleroatonic muscular dystrophy” or Ullrich CMD (UCMD [MIM 254090]) (Camecho Vanegas et al. 2001; Higuchi et al. 2001a). Mutations in this gene (Jöbsis et al. 1996), as well as in COL6A1 and COL6A3 (encoding the α1 and α3 chains of collagen VI), cause an autosomal dominant disorder, Bethlem myopathy (MIM 158810) (Pepe et al. 1999a; Sasaki et al. 2000).
By genomewide analysis, we studied a consanguineous family in which three patients had merosin-positive CMD and distal hyperlaxity, and we mapped the disease to a locus in 2q37 by homozygosity mapping. Linkage was confirmed in two other consanguineous families, with a maximum LOD score of 4.28 at a recombination fraction of θ = 0.00. We analyzed a candidate gene located in the region, COL6A3, and identified three homozygous mutations (two nonsense mutations and a splice-site mutation) in these three families.
Patients and Methods
Patients
We studied 5 patients and 11 unaffected relatives from three consanguineous families in which CMD was associated with distal laxity (fig. 1). They provided written informed consent to participate in our study. CMD diagnosis was based on the European Neuromuscular Center workshop consensus criteria: onset of symptoms at birth, diffuse muscle weakness, proximal contractures, and muscle-biopsy samples showing fibers of variable size and an increase in endo- and perimysial connective tissue (Dubowitz 1997). Immunocytochemically, the expression of laminin α2 chain and dystrophin-associated glycoproteins was normal. Family I (patients 1–3) originated from Morocco, family II (patient 4) from Italy, and family III (patient 5) from Turkey. The parents in each of the three families were first cousins.
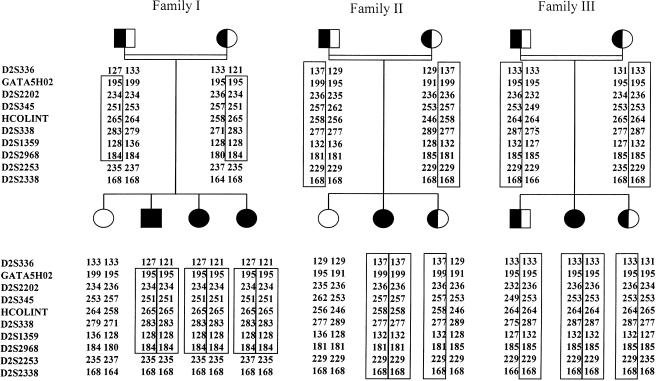
Figure 1.
Pedigrees of three families with CMD, with distal hyperlaxity, showing linkage to chromosome 2q37. Disease-bearing haplotypes are boxed for each family. Affected individuals are represented by blackened symbols, heterozygous carriers of the disease haplotype by half-blackened symbols, and unaffected noncarrier individuals by unblackened symbols. The order of the markers was obtained from the databases of the Whitehead Institute/MIT Center for Genome Research, the National Center for Biotechnology Information (Genemap 99), and the Marshfield Center for Medical Genetics. HCOLINT is a COL6A3 intronic CA-repeat microsatellite (Pan et al. 1998b).
Genotyping
Genomic DNA was extracted from peripheral lymphocytes by standard procedures. Linkage to known CMD loci (LAMA2, RSMD1, MEB, and MDC1B) was excluded by genotypic analysis of polymorphic markers (Guicheney et al. 1998; Cormand et al. 1999; Moghadaszadeh et al. 1999; Brockington et al. 2000). We performed a whole-genome linkage analysis of 280 microsatellite markers, as described elsewhere (Vicart et al. 1998). Additional polymorphic markers were used for fine mapping of potential loci. Forward primers were labeled with 6-Fam, Hex, or Ned fluorochromes. The fluorescent PCR products were separated and detected on 4.25% acrylamide gels with an ABI 377 DNA sequencer, and alleles were assigned with Genotyper software version 2.5 (Applied Biosystems).
Skin Fibroblasts
Primary fibroblasts were grown from skin-biopsy samples of patients 4 and 5. Before RNA extraction, confluent cells were treated with 100 μg/ml cycloheximide (Sigma) for 8 h, as described elsewhere (Lamandé et al. 1998a).
RNA Extraction and RT-PCR
Total RNA was extracted from fibroblasts or muscle-biopsy samples by use of RNA-PLUS extraction solution (Q.Biogen). We performed reverse transcription with OligodT for fibroblast RNA and with random hexamers for muscle RNA, using the Superscript kit (Life Technologies). The resulting cDNA was used as a template for PCR with the 15 primer pairs designed by Pan et al. (1998a). RT-PCR products were analyzed by electrophoresis on 2% agarose gel.
Sequencing
RT-PCR products were sequenced on both strands by use of an Applied Biosystems automated sequencer (Perkin Elmer). Sequence changes identified at cDNA level were confirmed on genomic DNA by amplification and direct sequencing of the specific exons. We designed new primers: for exon 5, 5F (5′-GAAGTCAACAAGAGAGACATA-3′) and 5R (5′-TAGAGCAGAGCCCGTGTA-3′); for exon 29, 29F (5′-CAGCGGCAGGGCAGAGAC-3′) and 29R (5′-TCATCAAACGAAAACACTCACTCAA-3′); and, for exon 31, 31F (5′-TTACCCCACGGACCCTCA-34) and 31R (5′-CAAAGCCTCAGCAGTTAATCACA-3′). The sequences of F1 and B5 primers are 5′-TCCTACAACTCATGCCCAGC-3′ and 5′-GTCAGATGACCTTCCTGCGACC-3′, respectively.
SSCP Analysis
SSCP analysis was performed as described elsewhere (Di Blasi et al. 2001). A silver-staining kit (Promega, Silver Sequence, DNA staining reagents) was used to visualize the DNA bands on the gels.
Restriction Analysis
We digested PCR fragments of exon 29 (5 μl) for 1.5 h at 37°C in a final volume of 15 μl with MaeII (1 U, Biolabs) and visualized digested fragments on 2% agarose gels.
Immunohistochemistry
Unfixed frozen sections of muscle tissue from patients and from controls were double labeled with anti-collagen VI antibody (MAB1944 or MAB3303, Chemicon) diluted 1/200, and rat anti-perlecan (Chemicon) diluted 1/100, followed by fluorescein isothiocyanate (FITC)–conjugated anti-mouse (DAKO), and tetramethylrhodamine isothiocyanate–conjugated anti-rat (Sigma). Other extracellular-matrix proteins, such as laminin α2 chain (Chemicon), laminin β1 chain (Chemicon), collagen IV (Sigma), and fibronectin (Sigma) were studied to evaluate the integrity of basal lamina. Primary antibodies were diluted 1/1,000, 1/100, 1/50, and 1/400, respectively, and were revealed with anti-mouse or anti-rabbit FITC-conjugated secondary antibodies (DAKO). All samples were mounted in Pro-long anti-fade reagent (Molecular Probes) and were observed with a Nikon E 600 fluorescence microscope.
Cultured skin fibroblasts from patients and controls were grown to confluence on coverslips and were treated with 0.25 mM ascorbic acid for 5 d, to allow collagen molecules to be hydroxylated and secreted (Chan et al. 1990). Samples were fixed with cold methanol at −20°C for 7 min, were incubated for 1 h at room temperature with anti-collagen VI monoclonal antibodies (MAB3303 and MAB1944, Chemicon) diluted 1/100 with PBS containing 2% BSA and 5% normal goat serum, as well as with FITC-conjugated rabbit anti-mouse antibody (Dako) diluted 1/100. The slides were stained with 1μg/ml 4′,6′-diamidino-2-phenylinodole hydrochloride (DAPI).
Linkage Analysis
Linkage analysis was performed by use of the LINKAGE 5.2 package, under the assumptions of an autosomal recessive inheritance, an equal male and female recombination rate, a disease-gene frequency of 0.0001, and a penetrance of 0.95. LOD scores were calculated with allele frequencies determined in a control population of European origin.
Results
Identification of a New CMD Locus
Two hundred eighty microsatellite markers were genotyped in a consanguineous family with merosin-positive CMD, comprising three affected and one unaffected siblings (family I). Seven markers were found to be homozygous in the three affected siblings (patients 1–3). Four of these regions were excluded after analysis of flanking microsatellite markers; the remaining three corresponded to potential loci on 2q34-35, 2q37, and 14q24. We analyzed the markers corresponding to these three loci in other consanguineous merosin-positive families with CMD who had normal intellectual development and brain MRI. We found two additional families with linkage to the potential locus on chromosome 2q37 (fig. 1).
The patients of the three families presented some similar phenotypic characteristics, especially joint contractures coexisting with distal laxity. Two recombination events between D2S336 and GATA5HO2 and between D2S2968 and D2S2253 in patient 3 defined a 13-cM interval, according to the Marshfield chromosome 2 sex-averaged linkage map (Broman et al. 1998), containing a candidate gene, COL6A3, which encodes the α3 chain of collagen VI (fig. 1). Six markers gave significant positive cumulative LOD scores, the highest ones being 4.28, for D2S338, and 4.00, for HCOLINT and D2S345, at θ=0.00 (table 1). As expected, given the autosomal recessive mode of transmission, all the affected individuals had the two at-risk haplotypes, whereas three unaffected siblings from families II and III had inherited one at-risk haplotype (fig. 1).
Table 1.
Cumulative Two-Point LOD scores for UCMD versus Chromosome 2q37
| LOD Scores at θ = |
|||||||
| Marker | .00 | .01 | .05 | .1 | .2 | .3 | .4 |
| D2S336 | .37 | 1.48 | 1.82 | 1.70 | 1.16 | .57 | .12 |
| GATA5HO2 | 3.13 | 3.05 | 2.73 | 2.34 | 1.58 | .90 | .36 |
| D2S2202 | 3.29 | 3.21 | 2.87 | 2.44 | 1.63 | .92 | .37 |
| D2S345 | 4.00 | 3.90 | 3.53 | 3.06 | 2.13 | 1.25 | .51 |
| HCOLINT | 4.00 | 3.90 | 3.53 | 3.06 | 2.13 | 1.25 | .51 |
| D2S338 | 4.28 | 4.17 | 3.76 | 3.24 | 2.23 | 1.29 | .52 |
| D2S1359 | 3.57 | 3.48 | 3.10 | 2.62 | 1.72 | .95 | .37 |
| D2S2968 | 2.68 | 2.62 | 2.34 | 2.00 | 1.34 | .76 | .30 |
| D2S2253 | −2.29 | 1.21 | 1.59 | 1.50 | 1.04 | .55 | .18 |
| D2S2338 | 2.68 | 2.62 | 2.34 | 2.00 | 1.34 | .76 | .30 |
Phenotypic Features
The patients from the three families shared significant clinical features (table 2), consistent with what is generally referred to as Ullrich disease. Neonatal hypotonia, severe in most cases, was the presenting sign in all patients; in spite of it, no respiratory or feeding problems were ever noticed. Distal hyperlaxity, involving hands and feet, was also present in every case from birth, associated with congenital arthrogryposis in families I and III. Abnormal motor development was constant, ranging from inability to run in patient 4 (the less-severely involved patient) to failure to acquire gait in patient 5; the two eldest siblings in family I were never able to walk without support (calipers or walking frame). Generalized muscle weakness, associated with predominantly distal amyotrophy, became evident in the first years of life. The axial muscles (mainly neck flexors), the pelvic girdle muscles, and the intrinsic muscles in hands and feet were the most severely affected ones, scoring ∼2/5 (MRC scale). Lower limbs tended to be more severely affected than upper limbs; in most cases, hip extensors and abductors were weaker than hip flexors, leading to hip contractures that interfered with standing position. No significant facial weakness was observed. CK levels were normal or moderately raised (up to four times the normal values).
Table 2.
Clinical Features of the Patients[Note]
|
Feature in Patient |
|||||
| Characteristic | 1 | 2 | 3 | 4 | 5 |
| Present age (years) | 17 | 15 | 3 | 18 | 12 |
| Neonatal hypotonia | Present | Present | Present | Present | Present |
| Congenital arthrogryposis | Present | Present | Present | − | Present |
| Congenital hip dislocation | − | − | − | − | Present |
| Congenital torticollis | Present | − | − | − | Present |
| Delayed motor milestones | Present | Present | Present | − | Present |
| Age at walking (mo) | 36 | 24 | 26 | 13 | Never |
| Maximal motor capacity | Walking with support | Walking with support | Walking | Walking | Stepping for a short distance |
| Weakness: | |||||
| Facial muscles | − | − | − | − | + |
| Shoulder girdle | + | + | + | + | +++ |
| Pelvic girdle | ++ | ++ | ++ | + | +++ |
| Feet and hands | ++ | ++ | ++ | +++ | +++ |
| Neck flexors | +++ | ++ | + | +++ | ++ |
| Contractures: | |||||
| Spine extensors | − | +/− | − | + | |
| Knees | ++ | + | − | ++ | +++ |
| Hips | ++ | + | ++ (transient) | + | +++ |
| Fingers | − | − | ++ (transient) | ++ | − |
| Ankles | ++ | + | − | +++ | + |
| Distal hyperlaxity | +++ | +++ | ++ | +++ | ++ |
| Scoliosis | − | − | − | − | + |
| Kyphosis | − | − | Transient | − | + |
| Creatine kinase | 3× normal (5 d); normal (11 mo) | Normal | Normal | 4× normal (13 years) | Normal |
| Clinical course | Improving at age 0–12 years; slowly progressive at age >12 years | Improving at age 0–8 years; slowly progressive at age >8 years | Improving | Static | Slowly progressive |
| Intelligence | Normal | Normal | Normal | Borderline | Normal |
| Collagen VI | Reduced | Reduced | − | ||
| Perlecan | Normal | Normal | Normal | ||
Note.— − = absent; + = mild; ++ = moderate; +++ = severe.
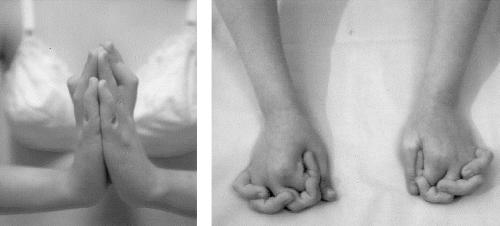
Muscle weakness appeared almost stable in all cases. In the families presenting with congenital arthrogryposis, contractures improved or even disappeared (patient 3) with intense physiotherapy during the first years of life. However, from the age of 8–12 years, contractures evolved and became severe in proximal joints, especially in lower limbs. Ankle and feet hyperlaxity was replaced by ankle contractures and/or equino-varus feet, requiring surgical correction in patient 4 and eventually leading to loss of ambulation in patients 1 and 2, at 15 and 14 years of age, respectively. Finger retractions were noticed only in patient 4, from the age of 13 years (fig. 2). Only patient 5 developed scoliosis and kyphosis.
Figure 2.
The 18-year-old patient (patient 4) shows the unusual combination of striking distal contractures and hyperlaxity. When the wrist is extended, the retraction of finger flexor muscles impedes a complete finger extension, causing the flexion contractures of the fingers, which is regarded as a characteristic “Bethlem” sign. When the wrist is not extended, the finger laxity becomes evident. The patient is able to overlap, cross, or extend fingers beyond the normal range of motion (“Ullrich” sign).
Heart-function tests (electrocardiogram and echocardiography) and brain MRI were performed in at least one patient per family and did not show any abnormality. There was no clear mental retardation in any case, although school performance was poor in patients 1, 4, and 5. No ocular abnormalities were observed.
Examination of other family members, including parents, showed no abnormality except for the youngest heterozygous sib of family III, who showed, at birth, a marked hyperlaxity of hands and feet without muscle weakness. Her early motor and mental development was normal at 1.5 years of age, and hyperlaxity tended to decrease.
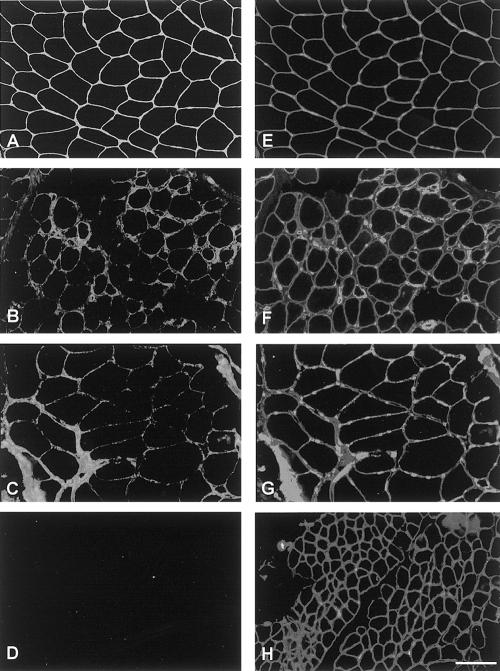
Immunohistochemistry of Collagen VI
Muscle-biopsy samples (from one patient per family) were analyzed for the presence of collagen VI. Results obtained with MAB3303 and MAB1944 were identical. For patients 1 and 4, a mild reduction in collagen VI was observed in the endomysium; however, in the areas showing marked collagen increase, the perimysium and endomysium labeling appeared to be similar to that of controls (fig. 3A–C). In contrast, for patient 5, no immunostaining of collagen VI could be detected (fig. 3D). Labeling with antibody against perlecan showed a normal pattern in all cases examined (fig. 3E–H), as did labeling with antibodies against collagen IV, laminin α2 chain, and fibronectin (data not shown). Laminin β1 chain was moderately reduced in the basal lamina of muscle fibers of patient 4 and was normally expressed in the muscle of patients 1 and 5 (data not shown).
Figure 3.
Double labeling of collagen VI with MAB1944 (A, B, C, and D) and perlecan (E, F, G, and H) in muscle-biopsy samples. Collagen VI is strongly expressed at the basal lamina of muscle fibers and around the vessel in a control subject (A) but appears reduced in patients 1 (B) and 4 (C) and absent in patient 5 (D). Same sections were labeled with anti-perlecan antibody to check the integrity of basal lamina. The labeling was comparable in all samples examined. E, F, G, and H, normal muscle and muscle from patients 1, 4, and 5 respectively (of families I, II, and III, respectively). Bar = 100 μm.
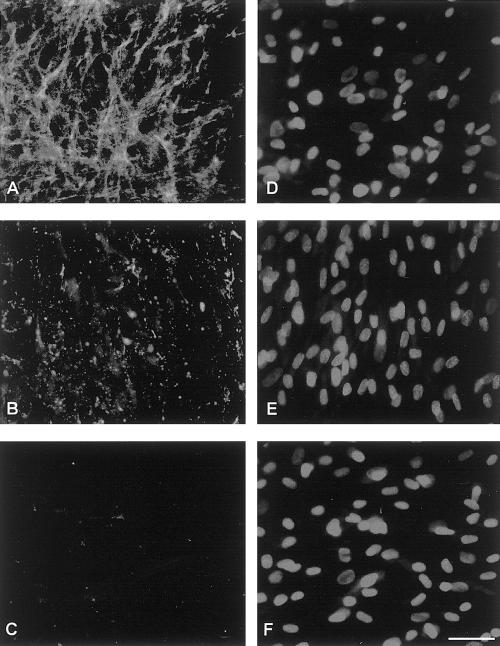
Abnormality of collagen VI was confirmed in patients 4 and 5 by the study of cultured skin fibroblasts (fig. 4). Cultured fibroblasts from patient 4 presented a reduced amount of secreted protein, with a dot-like appearance, when compared with the fine network of the collagen VI deposited by control fibroblasts (fig. 4A and B). Collagen VI was absent in cultured fibroblasts from patient 5, both inside the cells and in the extracellular-matrix (fig. 4C). DAPI staining of the nuclei demonstrated that patients and control fibroblasts had a comparable cell density.
Figure 4.
Immunofluorescence analysis of collagen VI MAB1944 (A, B, and C) and DAPI (D, E, and F) in cultured fibroblasts. Collagen VI in the control subject (A) is highly expressed in the extracellular matrix and develops a fine network. The protein is reduced in amount and is not well organized in fibroblasts of patient 4 (B), whereas it is absent in fibroblasts of patient 5 (C). The DAPI staining shows a comparable density of cells in the three samples: normal control (D), patient 4 (E), and patient 5 (F). Bar = 100 μm.
COL6A3 Genomic Sequence
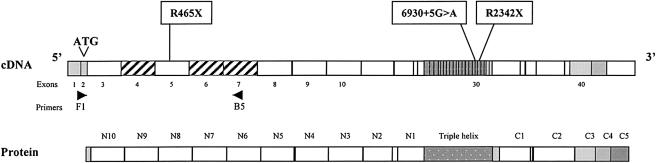
On the basis of the sequences of COL6A3 mRNA (X52022) and several PAC clones (AC025878, AC025933, and AC0259332), we identified and numbered the first 40 COL6A3 exons (fig. 5). The coding sequence begins in exon 2 (ATG), which corresponds to the exon 11 previously defined by Stokes et al. (1991). N-terminal domains, named “N10” to “N2” (Stokes et al. 1991) correspond to exons 3–11, whereas the N1 domain is encoded by exons 12–14 and the triple-helical domain is encoded by exons 15–33.
Figure 5.
Localization of the three mutations. Schematic representation of the 41 first exons COL6A3 cDNA with the corresponding structural domains, as defined by Chu et al. (1990). The primers F1 and B5 are shown (arrows). The three hatched exons (4, 6, and 7) has been reported elsewhere to be spliced in human muscle and fibroblasts.
Mutation Analysis
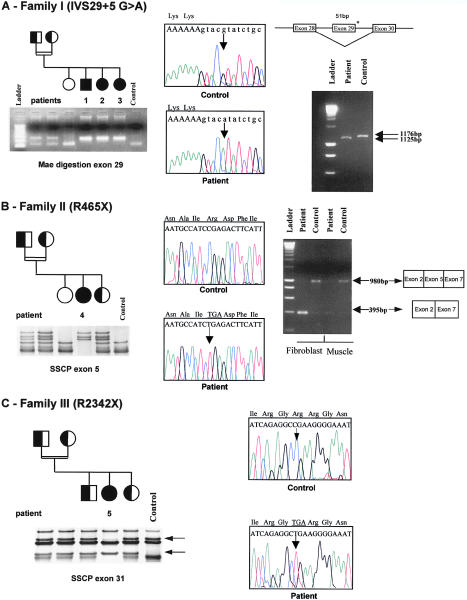
In patient 1 from family I, analysis of muscle mRNA by RT-PCR showed a shorter product for one of the COL6A3 PCR products than was seen in control subjects. Sequence analysis revealed a 51-bp deletion, corresponding to the skipping of exon 29 (fig. 6A). Sequence analysis of this exon with intronic primers revealed a homozygous G→A transversion at the +5 position in the donor-splice site of intron 29, 6930+5G→A. This mutation should induce a homozygous in-frame deletion of 17 amino acids in the triple-helical domain of the α3 chain of collagen VI. The mutation abolishes a MaeII restriction site. Since we could not detect the mutation by PCR-SSCP analysis, we amplified DNA from family members and from 50 unrelated Moroccan control subjects and digested the PCR products by MaeII. No control subject carried the mutation, but the two parents were heterozygous carriers (fig. 6A). These data strongly suggest that this mutation is not a DNA polymorphism, but a splicing mutation leading to the disease.
Figure 6.
Mutation analysis in families I, II, and III. A, Family I. RT-PCR amplification of the coding α3 triple-helical domain of COL6A3 with primers COL6A3-1 et COL6A3-2 showed a smaller fragment, corresponding to a deletion of 51 bp in patient muscle cDNA, compared with control muscle cDNA; this deletion was due to the skipping of exon 29, as is shown by control and patient cDNA sequences (right panel). At the genomic level, an homozygous 6930G→A+5 was found in the donor splice site of intron 29 (middle panel). The mutation cosegregated with the disease in the family, as is shown by RFLP analysis. The 6930G→A substitution abolishes a MaeII site in a 264-bp PCR fragment of exon 29. One band, corresponding to two fragments (130 and 134 bp), was found in control subjects and in the oldest unaffected sibling, whereas one uncut fragment (264 bp) indicated the homozygous mutated alleles. Parents were carriers of a mutant and a normal allele (left panel). B, Family II. Electrophoregram sections showing a homozygous C→T transversion at nucleotide 1393, changing Arg465 (CGA) to a stop codon (TGA) (middle panel). Segregation of the mutation in family II by SSCP analysis (left panel). RT-PCR amplification of patient 5 and control fibroblast and muscle cDNA samples encompassing exons 2–7 of COL6A3, by use of primers F1-B5, showed two main products: one of 980 bp, corresponding to exons 2, 5, and 7, and a smaller one of 395 bp, corresponding to exons 2 and 7. The shorter band was much more abundant in fibroblast and muscle cDNA from patient 5 than in that from control subjects (right panel). Segregation of the mutation in family II, by SSCP analysis, showing abnormal conformers in the homozygous patient and in the three heterozygous family members compared with control subjects (left panel). C, Family III. Electrophoregram sections showing a homozygous C→T transversion at nucleotide 7024 creating a stop codon (right panel). Segregation of the mutation in family III by SSCP analysis showing abnormal conformers in the homozygous patient and in the four heterozygous family members compared with control subjects (left panel).
In family II, a homozygous C→T transition was found at position 1393 in the COL6A3 coding sequence, changing an arginine 465 (CGA) to the stop codon TGA. This 465R→X nonsense mutation was predicted to cause a small truncated protein with only the two first N-terminal domains. This mutation was detected in patient fibroblast and muscle cDNA, as well as in genomic DNA in exon 5. It was transmitted by the parents and was not found in 100 unrelated Italian control subjects by SSCP analysis (fig. 6B). Since the patient presented only a partial deficiency in collagen VI, a skipping of exon 5, which consists of a multiple of 3 (585 bp), could explain the mild phenotype. As shown in figure 6B, different patterns were observed in patient and control cDNA amplified by F1-B5. In control fibroblasts and control muscle, a main band of 980 bp comprises exons 2, 5, and 7. In contrast, the main band in patient fibroblasts was of 395 bp and comprised only exons 2 and 7, suggesting the skipping of exons 3, 4, 5, and 6. This band was also clearly detected by PCR in cDNA from patient muscle.
In family III, we identified a homozygous C→T transition at position 7024 in exon 31 encoding the alpha triple-helical domain. It changed codon 2342, coding for an arginine (CGA), to a stop codon, TGA, which should result in a truncated protein lacking the whole C-terminal domain. This mutation in exon 31 was not detected in 100 unrelated Turkish control subjects by SSCP analysis (fig. 6C).
Discussion
We identified a locus on chromosome 2q37 in three families with merosin-positive CMD with distal hyperlaxity. In this locus, COL6A3, the gene encoding the α3 chain of collagen VI, was a candidate gene for this condition, since a partial or a complete deficiency in collagen VI was found in the muscle-biopsy samples of patients from these families. In addition, one of our patients (patient 4) had both distal hyperlaxity and finger contractures, the latter of which is known to be a typical feature of Bethlem myopathy (Merlini et al. 1994), which is caused by mutations in the three chains of collagen VI (Jöbsis et al. 1996). Reports concerning the identification of collagen VI deficiency (Higuchi et al. 2001b) and homozygous mutations of COL6A2 in Ullrich’s disease (Camecho Vanegas et al. 2001; Higuchi et al. 2001a) strengthened that possibility. As a result, we screened COL6A3 coding sequence and identified homozygous mutations.
Collagen VI is a major cell-adhesive protein that forms a microfibrillar network in the extracellular matrices of a wide variety of tissues, including skeletal muscle, skin, and cartilage (Keene et al. 1988; Bonaldo et al. 1998). Collagen VI is composed of three different polypeptide chains (α1, α2, and α3) assembled intracellularly to form triple-helical monomers (Timpl and Chu 1994). Monomers further associate into dimers by antiparallel overlapping alignment, and subsequently associate into tetramers by lateral association (Furthmayr et al. 1983; Odermatt et al. 1983; Chu et al. 1988). These molecules are stabilized by disulfide bonds. Multimer assembly is essential for secretion and the formation of stable collagen monomers (Lamandé et al. 1998b). All three chains comprise a central triple-helical domain of 335 or 336 amino acids with repeating Gly-X-Y sequences, flanked by N-and C-terminal globular domains (Timpl and Chu 1994). The N globular domain is composed of ∼200 consecutive amino acid–residue repeats, each of which forms a globular subdomain. The α3 chain is the largest, with 10 residue motifs (N10–N1), whereas, in both α1 and α2 chains, only one (N1) exists. The subdomains in N and C-terminal regions show homology to the type A domains of the von Willebrand factor, fibronectin type III domains, and Kunitz-type protein inhibitors (Bonaldo and Colombatti 1989; Chu et al. 1990).
In our series, three children from family I were hypotonic at birth, and one of them presented with arthrogryposis multiplex congenita. Clinical status improved, and they achieved ambulation but had difficulties running and climbing stairs, because of weakness and contractures, as generally occurs in patients with CMD. A distal hyperlaxity of the hands, feet, and arms was noticed early in all three children. No finger contracture was detected. In this family, we found a homozygous deletion of 51 nucleotides at the transcript level by analysis of the muscle specimen from one of the affected children. A homozygous point mutation in the donor splice site of COL6A3 intron 29, identified in the three affected children, resulted in skipping of exon 29 and in-frame deletion of 17 amino acids in the triple helical domain. In 85% of the primate genes, a G is found in the donor site at +5 position, and, if an A or T change occurs, it generally induces skipping of the exon (Nakai and Sakamoto 1994). This is what we observed, even if a very small amount of primary transcript with the mutation undergoes correct splicing. No specific function has been assigned to the deleted region. It does not include cysteine residue and does not affect multimeric assembly and secretion. Expression of partially functional proteins due to in-frame deletions has also been reported to cause mild phenotypes in patients with merosin-negative CMD (Allamand et al. 1997; Di Blasi et al. 2001).
In families II and III, we identified two homozygous nonsense mutations having very different functional and pathophysiological consequences. In family II, the patient presented with the mildest phenotype of the five patients reported in this article. At the age of 18 years, he was still ambulant but had some contractures. He had the rare association of marked distal hyperlaxity and finger contractures, as is shown in figure 2. In this patient, we identified a nonsense mutation in the exon 5 coding N8 globular domain of α3 chain. Although the transition of an arginine residue to a stop codon was predicted to truncate 2,712 amino acids, the immunohistochemical analysis of both muscle-biopsy samples and fibroblasts showed only slightly reduced collagen VI expression. The mild clinical phenotype of the patient was also inconsistent with the presence of a nonsense mutation. It has been shown that common alternative splicing or aberrant splicing induced by a nonsense mutation could moderate the consequence of the nonsense mutation by skipping of the exon (Valentine 1998). Alternative splicing of exons 3, 4, 6, and 7 of COL6A3 have been reported in various human tissues, including skeletal muscle and fibroblasts from healthy individuals (Stokes et al. 1991; Zanussi et al. 1992). In constrast, splicing of exon 5 had not been observed before in human tissue. We showed splicing of exons 3, 4, 5, and 6 in the fibroblast and muscle of both the patients and the control subjects (fig. 6B). Because removal of the exons did not cause a frameshift in the coding sequence, the alternatively spliced mRNA may produce a functional protein. In an expression study, an α3 chain cDNA construct lacking domains N10–N7 was shown to assemble and incorporate into the extracellular matrix (Lamandé et al. 1998b). The maintenance of this functional collagen VI isoform may explain the mild phenotype in the patient and the absence of clinical signs in the heterozygous carriers.
Patient 4 (family III), who presented with the most severe phenotype, had a nonsense mutation in exon 31 coding for the distal part of the triple helical domain. The protein could not be detected by immunocytochemical analysis of muscle biopsy and fibroblasts, either in the intracellular or extracellular compartment (fig. 3). The truncated part included the C-terminal globular domain, which interacts with the triple-helical domain of another monomer in the stabilization of collagen VI dimers. Expression of the α3 chain is essential for the formation of functional collagen VI molecules and their secretion (Chu et al. 1988; Lamandé et al. 1998b). When the α3 chain is absent, α1 and α2 chains cannot form helical assemblies and are unstable. The absence of immunostaining with the two monoclonal antibodies directed against the α3 chain could be due to the truncation of the epitope or/and more probably to the instability of the RNA or the synthesized truncated collagen VI, as shown elsewhere (Lamandé et al. 1999). The parents were both heterozygous carriers of the nonsense mutation, but they did not present any symptoms. A marked distal laxity was noticed at examination in their youngest child, also a heterozygous carrier, but he did not develop weakness or hypotonia.
Our patients constituted a relatively homogeneous clinical group on the basis of the main features—namely, early onset of the disease, distal hyperlaxity, proximal contractures, and marked involvement of flexor neck muscles. However, the severity of the disease varied. We observed the most severe phenotype in the patient from family III with no collagen expression. Indeed, a high proportion of patients with typical Ullrich disease never walk or become ambulant but lose gait after some years (Nonaka et al. 1981). Among the reported patients with a known mutation leading to an absence of collagen VI, one of the main features was the development of a marked respiratory insufficiency, leading to tracheotomy in the four oldest patients, at the ages of 7, 8, 14, and 17 years, respectively (Camecho Vanegas et al. 2001; Higuchi et al. 2001a). In contrast, our patients with mildly reduced collagen VI expression presented with a milder phenotype and no respiratory insufficiency, suggesting a correlation between the degree of collagen VI reduction and the severity of the disease. The existence of mutations leading to protein deficiency supported such correlation. However, the phenotypical consequence of mutations in the COL6A3 gene may be modulated by the functional properties of collagen VI or by alternative splicing. Mutations interacting with intracellular assembly may interrupt secretion of collagen VI, leading to functional haploinsufficiency of collagen VI in the extracellular matrix (Lamandé et al. 1999). On the other hand, aberrant exon skipping may suppress the effect of mutation, as is seen in the patient from family II.
It is noteworthy that none of the heterozygous carriers of a UCMD-causing mutation in COL6A3 or COL6A2 was affected or even presented with weakness at adulthood. In contrast, in Bethlem myopathy, heterozygous carriers of mutations in any of the genes encoding an α chain, develop an early-onset, slowly progressive limb-girdle weakness with contractures. The mutations so far identified in Bethlem myopathy and UCMD are different. In Bethlem myopathy, seven different mutations were reported. Four are missense mutations: three of them are glycine substitutions within the triple-helical domain of one of the three chains that interrupt the collagen Gly-X-Y amino acid repeat sequence (Jöbsis et al. 1996; Pepe et al. 1999a), whereas the other one leads to a glycine-to-glutamic-acid change within the N2 repeat of the α3 chain (Pan et al. 1998a). Two other mutations are splice-site mutations in COL6A1 inducing an in-frame deletion of 18 amino acids in triple helical domain of the α1 chain (Lamandé et al. 1999; Pepe et al. 1999b). Finally, another mutation was reported to induce a 1-bp deletion (979−1G→A) and a premature termination of the protein (Lamandé et al. 1998a). It is noteworthy that the phenotype associated with this latter mutation in COL6A1 is at variance with the phenotype of the heterozygous carriers of a COL6A2 or COL6A3 UCMD mutation leading to premature termination codon, who are all healthy.
In Bethlem myopathy, like in other collagenopathies, mutant chains may assemble with normal chains, disrupting the stability of the monomers, dimers, or tetramers, and may lead to increased intracellular degradation and poor collagen secretion. In relation to the mutation, a proportion of mutant molecules could not be degraded but were secreted and incorporated into the extracellular matrix, where the presence of even a small number of abnormal molecules can exert a dominant negative effect, thus disturbing the entire matrix architecture and no longer adequately anchoring the muscle cell to the surrounding connective tissue. It is now known that, in most patients with Bethlem myopathy, no reduction in collagen VI can be detected in muscle-biopsy samples, which suggests that the mechanisms are multiple and are more complex than a haploinsufficiency of collagen VI, as has been suggested for one patient (Lamandé et al. 1998a).
The mechanisms causing the dystrophic changes in Ullrich congenital muscular dystrophy are still speculative, although the major role of collagen VI is proven. Extracellular matrix has been shown to be essential for the formation of myotubes and expression of later muscle-specific gene products during skeletal-muscle-cell differentiation (Melo et al. 1996). Collagen VI differs from other members of the large family of collagens by its unique crosslinking properties; it interacts with numerous extracellular matrix components (Kuo et al. 1997; Tillet et al. 1997)—such as collagen IV, perlecan, fibronectin, and fibrillar collagen I/III—that are indirectly linked to the cell surface. Expression of collagen IV, perlecan, and fibronectin was normal in all the muscle-biopsy samples that we studied. Laminins were also normally expressed, except for the laminin β1 chain, which appeared moderately reduced only in the muscle fibers of the oldest patient (patient 4); a similar age-related alteration has been previously reported in three patients affected by Bethlem myopathy (Merlini et al. 1999). However, a reduced expression of laminin β1 cannot be considered specific to collagen VI-related pathologies, since it has been described in other muscle diseases (Yamada et al. 1995).
On the other hand, it has been recently demonstrated that absence of collagen VI modifies the fibronectin network architecture in fibroblast cultures (Sabatelli et al. 2001) and markedly reduces cell proliferation (Atkinson et al. 1996). In addition, collagen VI prevents apoptosis in serum-starved fibroblasts, possibly through activation of tyrosine phosphorylation of focal adhesion proteins, such as FAK (Ruhl et al. 1999). These complex mechanisms affecting differentiation, regeneration, and apoptosis may also apply in muscle and may be involved in the pathological pathways caused by collagen VI deficiency.
The functional effect of the two mutations causing a partial collagen VI deficiency might be related to disruption of specific interactions. Indeed, most proteins interact with collagen VI through its helical domain. The deletion found in the distal part of the helix, induced by 6930+5G→A, may thus affect some of these specific interactions. In contrast, the R465X mutation, which induces an in-frame deletion in the N-terminal globular domain, should not disturb interactions with the helical domain, but might abolish the specific interaction of terminal N9-N2 domains with heparin and hyaluronic acid (Specks et al. 1992).
In conclusion, in this study, we report the first homozygous mutations in COL6A3 leading to complete or partial collagen VI deficiency associated with severe or milder phenotypes of UCMD. These results suggest that a significant proportion of patients with still-unclassified merosine-positive CMD—particularly if they show distal hyperlaxity—may present a partial deficiency in collagen VI. Immunocytochemical analysis of muscle-biopsy samples or skin fibroblasts and identification of the genetic defects should allow a better understanding of these diseases.
Acknowledgments
The authors wish to thank all the patients and family members who participated in this study, as well as E. Mattioli for technical assistance in cell culture, Dr. C. Hu for analysis of COL6A3 genomic structure, Dr. F. Rivier for providing a muscle biopsy, and the Association Française contre les Myopathies (AFM) Banque de Tissus pour la Recherche for providing human tissues. The authors are grateful to F. Tomé for his continuous support and critical reading of the manuscript. This work was supported by funds from the INSERM (French INSERM/AFM Research network on rare disorders), the AFM, and the European Commission (contract N°- QLG1-1999-00870). V.A. and A.F. were the recipients of fellowships from the Fondation pour la Recherche Médicale and the Fondation Electricité-Santé, respectively.
Electronic-Database Information
Accession numbers and the URL for data in this article are as follows:
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for UCMD [MIM 254090] and Bethlem myopathy [MIM 158810])
References
- Allamand V, Sunada Y, Salih MAM, Straub V, Ozo CO, Al-Turaiki HS, Akbar M, Kolo T, Colognato H, Zhang Z, Sorokin L, Yurchenco PD, Tryggvason K, Campbell KP (1997) Mild congenital muscular dystrophy in two patients with an internally deleted laminin α2-chain. Hum Mol Genet 6:747–752 [DOI] [PubMed] [Google Scholar]
- Atkinson JC, Ruhl M, Becker J, Ackermann R, Schuppan D (1996) Collagen VI regulates normal and transformed mesenchymal cell proliferation in vitro. Exp Cell Res 228:283–291 [DOI] [PubMed] [Google Scholar]
- Bonaldo P, Braghetta P, Zanetti M, Piccolo S, Volpin D, Bressan GM (1998) Collagen VI deficiency induces early onset myopathy in the mouse: an animal model for Bethlem myopathy. Hum Mol Genet 7:2135–2140 [DOI] [PubMed] [Google Scholar]
- Bonaldo P, Colombatti A (1989) The carboxyl terminus of the chicken alpha 3 chain of collagen VI is a unique mosaic structure with glycoprotein Ib-like, fibronectin type III, and Kunitz modules. J Biol Chem 264:20235–20239 [PubMed] [Google Scholar]
- Brockington M, Sewry CA, Herrman R, Naom I, Dearlove A, Rhodes M, Topaloglu H, Dubowitz V, Voit T, Muntoni F (2000) Assignment of a form of congenital muscular dystrophy with secondary merosin deficiency to chromosome 1q42. Am J Hum Genet 66:428–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broman KW, Murray JC, Sheffield VC, White RL, Weber JL (1998) Comprehensive human genetic maps: individual and sex-specific variation in recombination. Am J Hum Genet 63:861–869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camecho Vanegas OC, Bertini E, Zhang RZ, Petrini S, Minosse C, Sabatelli P, Giusti B, Chu ML, Pepe G (2001) Ullrich scleroatonic muscular dystrophy is caused by recessive mutations in collagen type VI. Proc Natl Acad Sci USA 98:7516–7521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan D, Lamande SR, Cole WG, Bateman JF (1990) Regulation of procollagen synthesis and processing during ascorbate-induced extracellular matrix accumulation in vitro. Biochem J 269:175–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu ML, Conway D, Pan TC, Baldwin C, Mann K, Deutzmann R, Timpl R (1988) Amino acid sequence of the triple-helical domain of human collagen type VI. J Biol Chem 263:18601–18606 [PubMed] [Google Scholar]
- Chu ML, Zhang RZ, Pan TC, Stokes D, Conway D, Kuo HJ, Glanville R, Mayer U, Mann K, Deutzmann R, Timpl R (1990) Mosaic structure of globular domains in the human type VI collagen α3 chain: similarity to von Willebrand factor, fibronectin, actin, salivary proteins and aprotinin type protease inhibitors. EMBO J 9:385–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cormand B, Avela K, Pihko H, Santavuori P, Talim B, Topaloglu H, de la Chapelle A, Lehesjoki AE (1999) Assignment of the muscle-eye-brain disease gene to 1p32-34 by linkage analysis and homozygosity mapping. Am J Hum Genet 64:126–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Paillette L, Aicardi J, Goutieres F (1989) Ullrich's congenital atonic sclerotic muscular dystrophy: a case report. J Neurol 236:108–110 [DOI] [PubMed] [Google Scholar]
- Di Blasi C, He Y, Morandi L, Cornelio F, Guicheney P, Mora M (2001) Mild muscular dystrophy due to a nonsense mutation in the LAMA2 gene resulting in exon skipping. Brain 124:698–704 [DOI] [PubMed] [Google Scholar]
- Dubowitz V (1997) 50th ENMC International workshop: congenital muscular dystrophy, 28 February 1997 to 2 March 1997, Naarden, The Netherlands. Neuromusc Disord 7:539–547 [PubMed] [Google Scholar]
- Furthmayr H, Wiedemann H, Timpl R, Odermatt E, Engel J (1983) Electron-microscopical approach to a structural model of intima collagen. Biochem J 211:303–311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guicheney P, Vignier N, Zhang X, He Y, Cruaud C, Frey V, Helbling-Leclerc A, Richard P, Estournet B, Merlini L, Topaloglu H, Mora M, Harpey JP, Haenggeli CA, Barois A, Hainque B, Schwartz K, Tome FM, Fardeau M, Tryggvason K (1998) PCR based mutation screening of the laminin alpha2 chain gene (LAMA2): application to prenatal diagnosis and search for founder effects in congenital muscular dystrophy. J Med Genet 35:211–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helbling-Leclerc A, Zhang X, Topaloglu H, Cruaud C, Tesson F, Weissenbach J, Tomé FMS, Schwartz K, Fardeau M, Tryggvason K, Guicheney P (1995) Mutations in the laminin α2-chain gene (LAMA2) cause merosin-deficient congenital muscular dystrophy. Nat Genet 11:216–218 [DOI] [PubMed] [Google Scholar]
- Higuchi I, Shiraishi T, Hashiguchi T, Suehara M, Niiyama T, Nakagawa M, Arimura K, Maruyama I, Osame M (2001a) Frameshift mutation in the collagen VI gene causes Ullrich's disease. Ann Neurol 50:261–265 [DOI] [PubMed] [Google Scholar]
- Higuchi I, Suehara M, Iwaki H, Nakagawa M, Arimura K, Osame M (2001b) Collagen VI deficiency in Ullrich's disease. Ann Neurol 49:544 [PubMed] [Google Scholar]
- Jöbsis GJ, Keizers H, Vreijling JP, de Visser M, Speer MC, Wolterman RA, Baas F, Bolhuis PA (1996) Type VI collagen mutations in Bethlem myopathy, an autosomal dominant myopathy with contractures. Nat Genet 14:113–115 [DOI] [PubMed] [Google Scholar]
- Keene DR, Engvall E, Glanville RW (1988) Ultrastructure of type VI collagen in human skin and cartilage suggests an anchoring function for this filamentous network. J Cell Biol 107:1995–2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo HJ, Maslen CL, Keene DR, Glanville RW (1997) Type VI collagen anchors endothelial basement membranes by interacting with type IV collagen. J Biol Chem 272:26522–26529 [DOI] [PubMed] [Google Scholar]
- Lamandé SR, Bateman JF, Hutchison W, McKinlay Gardner RJ, Bower SP, Byrne E, Dahl HH (1998a) Reduced collagen VI causes Bethlem myopathy: a heterozygous COL6A1 nonsense mutation results in mRNA decay and functional haploinsufficiency. Hum Mol Genet 7:981–989 [DOI] [PubMed] [Google Scholar]
- Lamandé SR, Shields KA, Kornberg AJ, Shield LK, Bateman JF (1999) Bethlem myopathy and engineered collagen VI triple helical deletions prevent intracellular multimer assembly and protein secretion. J Biol Chem 274:21817–21822 [DOI] [PubMed] [Google Scholar]
- Lamandé SR, Sigalas E, Pan TC, Chu ML, Dziadek M, Timpl R, Bateman JF (1998b) The role of the α3(VI) chain in collagen VI assembly. Expression of an α3(VI) chain lacking N-terminal modules N10-N7 restores collagen VI assembly, secretion, and matrix deposition in an α3(VI)-deficient cell line. J Biol Chem 273:7423–7430 [DOI] [PubMed] [Google Scholar]
- Melo F, Carey D, Brandon E (1996) Extracellular matrix is required for skeletal muscle differentiation but not myogenin expression. J Cell Biochem 62:227–239 [DOI] [PubMed] [Google Scholar]
- Merlini L, Morandi L, Granata C, Ballestrazzi A (1994) Bethlem myopathy: early-onset benign autosomal dominant myopathy with contractures. Description of two new families. Neuromuscul Disord 4:503–511 [DOI] [PubMed] [Google Scholar]
- Merlini L, Villanova M, Sabatelli P, Malandrini A, Maraldi NM (1999) Decreased expression of laminin β1 in chromosome 21-linked Bethlem myopathy. Neuromuscul Disord 9:326–329 [DOI] [PubMed] [Google Scholar]
- Moghadaszadeh B, Topaloglu H, Merlini L, Muntoni F, Estournet B, Sewry C, Naom I, Barois A, Fardeau M, Tomé FMS, Guicheney P (1999) Genetic heterogeneity of congenital muscular dystrophy with rigid spine syndrome. Neuromusc Disord 9:376–382 [DOI] [PubMed] [Google Scholar]
- Nakai K, Sakamoto H (1994) Construction of a novel database containing aberrant splicing mutations of mammalian genes. Gene 141:171–177 [DOI] [PubMed] [Google Scholar]
- Nonaka I, Une Y, Ishihara T, Miyoshino S, Nakashima T, Sugita H (1981) A clinical and histological study of Ullrich's disease (congenital atonic-sclerotic muscular dystrophy). Neuropediatrics 12:197–208 [DOI] [PubMed] [Google Scholar]
- Odermatt E, Risteli J, van Delden V, Timpl R (1983) Structural diversity and domain composition of a unique collagenous fragment (intima collagen) obtained from human placenta. Biochem J 211:295–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan TC, Zhang RZ, Pericak-Vance MA, Tandan R, Fries T, Stajich JM, Viles K, Vance JM, Chu ML, Speer MC (1998a) Missense mutation in a von Willebrand factor type A domain of the α3(VI) collagen gene (COL6A3) in a family with Bethlem myopathy. Hum Mol Genet 7:807–812 [DOI] [PubMed] [Google Scholar]
- Pan TC, Zhang RZ, Speer MC, Chu ML (1998b) CA repeat polymorphism of the COL6A3 gene on chromosome 2q37. Hum Hered 48:235–236 [DOI] [PubMed] [Google Scholar]
- Pepe G, Bertini E, Giusti B, Brunelli T, Comeglio P, Saitta B, Merlini L, Chu ML, Federici G, Abbate R (1999a) A novel de novo mutation in the triple helix of the COL6A3 gene in a two-generation Italian family affected by Bethlem myopathy: a diagnostic approach in the mutations' screening of type VI collagen. Neuromuscul Disord 9:264–271 [DOI] [PubMed] [Google Scholar]
- Pepe G, Giusti B, Bertini E, Brunelli T, Saitta B, Comeglio P, Bolognese A, Merlini L, Federici G, Abbate R, Chu ML (1999b) A heterozygous splice site mutation in COL6A1 leading to an in-frame deletion of the α1(VI) collagen chain in an Italian family affected by Bethlem myopathy. Biochem Biophys Res Commun 258:802–807 [DOI] [PubMed] [Google Scholar]
- Ricci E, Bertini E, Boldrini R, Sabatelli M, Servidei S, Mazziotta MR, Bosman C, Tonali P (1988) Late onset scleroatonic familial myopathy (Ullrich disease): a study of two sibs. Am J Med Genet 31:933–942 [DOI] [PubMed] [Google Scholar]
- Ruhl M, Johannsen M, Atkinson J, Manski D, Sahin E, Somasundaram R, Riecken EO, Schuppan D (1999) Soluble collagen VI induces tyrosine phosphorylation of paxillin and focal adhesion kinase and activates the MAP kinase erk2 in fibroblasts. Exp Cell Res 250:548–557 [DOI] [PubMed] [Google Scholar]
- Sabatelli P, Bonaldo P, Lattanzi G, Braghetta P, Bergamin N, Capanni C, Mattioli E, Columbaro M, Ognibene A, Pepe G, Bertini E, L M, Maraldia NM, Squarzoni S (2001) Collagen VI deficiency affects the organization of fibronectin in the extracellular matrix of cultured fibroblasts. Matrix Biology 20:475–486 [DOI] [PubMed] [Google Scholar]
- Sasaki T, Hohenester E, Zhang RZ, Gotta S, Speer MC, Tandan R, Timpl R, Chu ML (2000) A Bethlem myopathy Gly to Glu mutation in the von Willebrand factor A domain N2 of the collagen α3(VI) chain interferes with protein folding. FASEB J 14:761–768 [DOI] [PubMed] [Google Scholar]
- Specks U, Mayer U, Nischt R, Spissinger T, Mann K, Timpl R, Engel J, Chu ML (1992) Structure of recombinant N-terminal globule of type VI collagen alpha 3 chain and its binding to heparin and hyaluronan. EMBO J 11:4281–4290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes DG, Saitta B, Timpl R, Chu ML (1991) Human α3(VI) collagen gene. Characterization of exons coding for the amino-terminal globular domain and alternative splicing in normal and tumor cells. J Biol Chem 266:8626–8633 [PubMed] [Google Scholar]
- Tillet E, Ruggiero F, Nishiyama A, Stallcup WB (1997) The membrane-spanning proteoglycan NG2 binds to collagen V and VI through the central nonglobular domain of its core protein. J Biol Chem 272:10769–10776 [DOI] [PubMed] [Google Scholar]
- Timpl R, Chu M-L (1994) Microfibrillar collagen type VI. In: Yurchenco PD, Birk DE, Mecham RP (eds) Extracellular matrix assembly and structure. Academic Press, Orlando, FL, pp 207–242 [Google Scholar]
- Tomé FMS (1999) The saga of congenital muscular dystrophy. Neuropediatrics 30:55–65 [DOI] [PubMed] [Google Scholar]
- Ullrich O (1930) Kongenitale, atonisch-sklerotische Muskeldystrophie, ein weiteres Typus der heredodegenerativen Erkrankungen des neuromuskulären Systems. Z Gesamte Neurol Psychiat 126:171–201 [Google Scholar]
- Valentine CR (1998) The association of non sense codons with exon skipping. Mutation Research 411:87–117 [DOI] [PubMed] [Google Scholar]
- Vicart P, Caron A, Guicheney P, Li Z, Prevost MC, Faure A, Chateau D, Chapon F, Tome F, Dupret JM, Paulin D, Fardeau M (1998) A missense mutation in the aB-crystallin chaperone gene causes a desmin-related myopathy. Nat Genet 20:92–95 [DOI] [PubMed] [Google Scholar]
- Voit T (1998) Congenital muscular dystrophies: 1997 update. Brain Dev 20:65–74 [DOI] [PubMed] [Google Scholar]
- ——— (2001) Congenital muscular dystrophies. In: Karpati G, Hilton-Jones D, Griggs RC (eds) Disorders of voluntary muscle. 7th ed. Cambridge University Press, pp 503–524 [Google Scholar]
- Yamada H, Tomé FMS, Higuchi I, Kawai H, Azibi K, Chaouch M, Roberds SL, Tanaka T, Fujita S, Mitsui T, Fukunaga H, Miyoshi K, Osame M, Fardeau M, Kaplan JC, Shimizu T, Campbell KP, Matsumura K (1995) Laminin abnormality in severe childhood autosomal recessive muscular dystrophy. Laboratory Investigation 72:715–722 [PubMed] [Google Scholar]
- Zanussi S, Doliana R, Segat D, Bonaldo P, Colombatti A (1992) The human type VI collagen gene. mRNA and protein variants of the alpha 3 chain generated by alternative splicing of an additional 5-end exon. J Biol Chem 267:24082–24089 [PubMed] [Google Scholar]