Abstract
BACKGROUND
Trauma/hemorrhagic shock is one of the major consequences of battlefield injury as well as civilian trauma. FTY720 (sphingosine-1 phosphate agonist) has the capability to decrease the activity of the innate and adaptive immune systems and, at the same time, maintain endothelial cell barrier function and vascular homeostasis during stress. For this reason, we hypothesize that FTY720, as part of resuscitation therapy, would limit T/HS induced multiple organ dysfunction syndrome (MODS) in a rodent trauma-hemorrhagic shock (T/HS) model.
METHODS
Rats subjected to trauma/sham-shock (T/SS) or T/HS (30 mm Hg × 90 min), were administered FTY720 (1 mg/kg) post-T/HS during volume resuscitation. Lung injury (permeability to Evans Blue dye), PMN priming (respiratory burst activity), and RBC rigidity were measured. In addition, lymph duct cannulated rats were used to quantify the effect of FTY720 on gut injury (permeability and morphology) and the biologic activity of T/HS vs. T/SS lymph on PMN-RB and RBC deformability.
RESULTS
T/HS-induced increased lung permeability, PMN priming and RBC rigidity were all abrogated by FTY720. The systemic protective effect of FTY720 was only partially at the gut level, since FTY720 did not prevent T/HS-induced gut injury (morphology or permeability,) however, it did abrogate T/HS lymph-induced increased respiratory burst and RBC rigidity.
CONCLUSION
FTY720 limited T/HS-induced MODS (lung injury, red cell injury, and neutrophil priming) as well as T/HS lymph bioactivity, although it did not limit gut injury.
Keywords: acute lung injury, neutrophil activation, red blood cell rigidity, lymph
INTRODUCTION
Trauma hemorrhagic shock (T/HS) is a major consequence of battlefield injury as well as civilian trauma. It has several peaks of mortality, one of which is due to the initial extent of blood loss and inability to adequately resuscitate individuals (1). The second peak is related to head injury. The third peak is related to the development of multiple organ failure, which is a consequence of the injury shock state. Our work focusing on the pathogenesis of multiple organ failure indicates that many components of the early post-trauma-hemorrhagic shock (T/HS)-induced multiple organ dysfunction syndrome (MODS) are related to gut injury and lipid and protein factors exiting the stressed gut via the intestinal lymphatics (2,3,4). Since the mechanism by which these gut-derived factors appear to cause acute organ dysfunction involves the induction of an acute immune-inflammatory state, the goal of this study was to study a novel potential mechanism-based agent for preventing or limiting the development of MODS after traumatic shock. This potential therapeutic agent, FTY720 (Fingolimod), is a sphingosine-1 phosphate agonist and was chosen due to its multiple potentially-protective physiologic activities. Specifically, FTY720 has the unique ability to limit the activation of the innate and adaptive immune systems after stress and injury while maintaining endothelial cell barrier function and vascular homeostasis (5). As described by Garris et al, sphingosine-1-phosphate (S1P) is a lipid second messenger that signals via five G protein-coupled receptors. This receptor signaling is associated with a wide variety of physiologic processes, such as vascular development, central nervous system homeostasis, and lymphocyte biology, particularly their recirculation and determination of T cell phenotype (6). Hence, FTY720 has the potential to limit tissue damage and excessive systemic inflammation and thereby reduce the development or magnitude of trauma-induced MODS. Further support for this study of FTY720 are based on preclinical studies documenting that FTY720 and/or S1P administration can attenuate ischemia-reperfusion injuries to the liver as well as other organs (7) and is protective in a model of endotoxin-induced lung injury (8). Additionally, a recently published study by Hawksworth, documented that FTY720 improved survival time and limited immune and inflammatory changes in a porcine hemorrhagic shock model (9). Importantly, FTY720 could be rapidly transitioned from preclinical to clinical studies, since it is currently approved for human use in the treatment of multiple sclerosis (10) and has been used safely in several Phase I, II and III studies in other patient populations, including transplant recipients (11).
MATERIALS AND METHODS
Animals
Male Sprague-Dawley rats (Charles River Laboratories, Wilmington, Mass) weighing 350g to 400g were housed under barrier-sustained conditions and kept at 25°C with 12 hour light/dark cycles. They were provided with chow and water ad libitum. All animals were maintained according to the Guide for the Care and Use of Laboratory Animals. In addition, all animal protocols were approved by the New Jersey Medical School Animal Care Committee.
Trauma-Hemorrhagic Shock
Rats were anesthetized with intraperitoneal pentobarbital (50mg/kg). Using aseptic techniques, a midline laparotomy was made and the intestines were eviscerated for 15 minutes. After this time, the intestines were placed back into the abdominal cavity and the laparotomy incision was closed using 4-0 silk suture. The right external jugular vein was dissected out and cannulated using silastic PE-50 tubing for blood withdrawal. In the same way, the right femoral artery was cannulated using polyethylene PE-50 catheter for blood pressure monitoring. Trauma sham shock (T/SS) animals were prepared as previously stated, but no blood was removed. For the trauma-hemorrhagic shock rats (T/HS), blood was withdrawn into a heparinized syringe through the jugular vein line. The mean arterial blood pressure was reduced to 30 mm Hg and maintained at this level for 90 minutes by reinfusing or withdrawing blood as needed (12). After shock was complete, the rats received 1mL/kg of either FTY720 (1mg/kg in normal saline; NS) or vehicle over 3 minutes. After this was complete, the T/HS rats were resuscitated with 3 times the amount of shed blood in lactated ringers (LR) at the rate of 1mL/min and observed for 3 hours. The mean arterial pressure returned toward a normal level within a few minutes after the rats were volume resuscitated.
Experimental Design
The primary hypothesis being tested is that FTY720, through its immuno-inflammatory and vascular effects will limit T/HS-induced systemic inflammation and MODS when administered as part of the shock-resuscitation regimen. The secondary hypothesis is that this systemic protective effect of FTY720 would be associated with a reduction in the biologic activity of mesenteric lymph and less gut injury after T/HS.
To test the hypothesis that T/HS-induced MODS is prevented by the use of FTY720, four groups of rats were studied. These include two non-shock control groups of rats subjected to trauma-sham shock (T/SS) receiving either vehicle or FTY720 (1mg/kg) as well as two groups of T/HS rats, one of which received vehicle and the other FTY720. The FTY720 or vehicle was administered at the end of the shock period during the first 5 minutes of volume resuscitation and thus utilized a post-treatment strategy. Based on our earlier results showing that acute lung and gut injury as well as neutrophil activation and RBC dysfunction are present as early as 3 hrs after the end of the resuscitation period (12), these four groups of rats were sacrificed at this time period. Lung injury was quantified using Evans blue dye to measure lung permeability. Gut injury was assessed morphologically based on histologic criteria. (13) In a second group of rats, gut barrier function was measured physiologically, using a fluorescent-labeled dextran intestinal permeability probe. (17) In addition, neutrophil priming, as reflected by an increased respiratory burst, was measured as was Red blood cell deformability and function, since increased RBC rigidity after T/HS has been associated with impaired microcirculatory blood flow (14). Both samples were obtained using fresh heparinized blood. Lastly, due to the potential effects of FTY720 on lymphocyte sequestration, a complete blood count (CBC) was also obtained using 25μL of fresh heparinized blood in the Hema True blood analyzer (HESKA, Loveland, CO).
A subsequent experiment was done testing a range of doses to determine if lower doses (0.1mg/kg or 0.5 mg/kg) of FTY720 would be as protective as the 1.0 mg/kg dose of FTY720. The same experimental design as outlined above was carried out.
The third set of experiments tested the hypothesis that one of the major mechanisms by which FTY720 prevents/limits T/HS-induced MODS is by limiting T/HS-induced gut injury and dysfunction and consequently the production of biologically-active mesenteric lymph. The same two T/HS and T/SS groups of rats, receiving vehicle or FTY720 (1 mg/kg) described above, in which mesenteric lymph duct catheters were placed, was used in this experiment. Separate aliquots of lymph were collected prior to the induction of T/HS, during the 90 minute shock (sham-shock) period as well as during the first 3 hours after the end of the shock or sham-shock period (post-shock lymph). This 3 hour post-shock lymph collection period was based on our previous studies documenting that the in vivo biologic activity of T/HS lymph is maximal during the first 3 hour post-shock period and is relatively rapidly lost thereafter (15). After the lymph was collected, it was processed, aliquoted and stored at −80°C until tested. Subsequently the ability of the lymph samples (T/HS and T/SS lymph ± FTY720) to activate neutrophils from normal animals as well as to alter RBC physiology was examined by incubating the various lymph samples (T/SS±FTY720 and T/HS±FTY720) with whole blood from naïve male rats. The number of lymph samples from each group ranged from 9–11. We used 3 normal (naïve) rats as blood donors for the in vitro assay with a similar number of lymph samples from each group being tested in each animal. Physiologically relevant concentrations of lymph (5–10% v/v) were tested (12, 16).
Mesenteric Lymph Duct Cannulation
As previously reported (12), the mesenteric lymph duct was catheterized using the following protocol: a midline incision was made and the efferent mesenteric lymphatic vessel was identified (adjacent to the superior mesenteric artery) by reflecting the loops of intestine with moist gauze swabs to the left of the animal. Next, the lymphatic duct was isolated and cannulated with silastic PE-50 tubing. This tubing was secured and brought out through a separate stab incision in the right flank. Lymph was collected on ice in sterile tubes and the sterility of the samples was validated by culturing an aliquot of each collected sample.
Gut Permeability Assay
At the end of the shock resuscitation period, the midline laparotomy incision was released. The cecum was identified and protected with moist gauze. A 12cm segment of terminal ileum was ligated proximally and distally with 4-0 silk tie. An enterotomy was made at both ends. This segment was flushed with 0.9% saline to remove the feces. The enterotomies were excluded and a 10cm segment was ligated. The permeability probe, Fluorescein isothiocyanate–dextran (FD4) was prepared at a concentration of 25mg/mL and a total of 1mL was carefully injected into the 10cm segment to prevent any spillage onto surrounding bowel. The segment was protected from light under a moist gauze and aluminum foil. Blood samples of 1mL were removed from the external jugular catheter prior to and 30 minutes after injection of FD4. As previously reported (17), plasma FD4 measurements were obtained by centrifuging the samples at 10,000rpm for 2 minutes at 4°C and compared with a standard curve of FD4 measured on FLX800 Microplate Fluorescence reader (Biotek) at excitation 485/20, emission 528/20, and a sensitivity of 40.
Lung Permeability Assay
On completion of the FD4 protocol, animals were injected with 1mL of 1% Evans blue dye (EBD) through the external jugular catheter. Blood sample of 1mL was removed 20 minutes after injection of EBD from the inferior vena cava. The animals were then killed and a cardiopulmonectomy was performed. Modified bronchoalveolar lavage (BAL) was performed on the excised lungs using 5mL of normal saline for each of the 2 washes (as previously reported 12). Two small cuts were made in the lower lobes to facilitate fluid removal. The blood samples and BAL fluid were centrifuged at 10,000rpm for 2 minutes at 4°C. The plasma was serially diluted to form a standard curve. The plasma and supernatant fluid were assayed with a spectrophotometer at 620nm for dye concentration and was compared with the standard curve.
Intestinal villous injury
After the rats were sacrificed, a segment of the terminal ileum was excised and fixed in 10% buffered formalin for morphologic analysis as previously described (18). Briefly, once processed, semi-thin sections (2–4μm) were cut and stained with toluidine blue. Five random fields with 100–250 villi from each animal were analyzed in a blinded fashion using light microscopy at x100 magnification. The overall incidence of villous damage was expressed as a percentage where the number of injured villi was divided by the total number of villi counted.
Determination of Neutrophil Respiratory Burst
To assess the in vivo effects of T/HS on PMN priming, heparinized blood samples were obtained from each animal pre shock/sham shock and 3 hours after the end of shock/sham shock for the measurement of neutrophil respiratory burst activity. As previously described (16), the samples were treated with RBC lysis buffer (1mL Lyse Buffer, Sigma, St. Louis, MO) and incubated for 15 minutes at room temperature to remove the RBCs. Next, they were centrifuged at 25°C, washed twice with Hank’s balanced salt solution and the supernatant was discarded. The resulting pellet was then suspended in Hank’s balanced salt solution at a concentration of 4×106 cells/mL. Dihydrorhodamine (15ng/mL) was added to 100μL of the PMN sample and warmed to 37°C. Phorbol myristic acid (0.4μmol/L) was then added to stimulate the cells for 15 minutes at 37°C. The PMN respiratory burst was measured by flow cytometry, where the neutrophils were identified by forward and side scatter analysis. The data is expressed as the mean fluorescence index. To assess the effects of the various lymph samples on PMN priming in vitro, PMN’s isolated from the whole blood of naïve rats were incubated with rat T/SS or T/HS lymph (10% vol/vol) for 5 minutes. Following this incubation period, PMA-induced respiratory burst was measured as described above.
Determination of RBC deformability
Since RBC dysfunction has been shown to impair microvascular blood flow, (14) we assessed the in vivo effects of T/HS on RBC deformability, by analyzing heparinized whole blood that was collected pre shock/sham shock and 3 hours after shock/sham shock. RBC deformability was determined with a laser optical rotational cell analyzer (LORCA, RR Mechatronics, Netherlands) as previously reported (14). An aliquot of RBCs (15μL) containing approximately 30 million cells was suspended in 1mL of 5% polyvinylpyrrolidone (molecular weight 360,000; Sigma, St. Louis, MO) in phosphate-buffered saline at a final viscosity of 30mPa. After gently mixing for 15 minutes at room temperature to assure complete oxygenation of the hemoglobin, cell deformability was determined at 37°C. Cell deformability was assessed by calculating the elongation index (EI) at shear stresses ranging from 0.3Pa to 30Pa. From the shear-stress elongation curve created previously, the data was analyzed using the Lineweaver-Burke analysis to determine the stress required for the erythrocytes to reach 50% of their maximal elongation (KEI). An elevation in KEI reflects a decrease in RBC deformability. To assess the effects of the various lymph samples on RBC deformability in vitro, the whole blood of naïve rats were incubated with rat T/SS or T/HS lymph (10% vol/vol) for 3 hours. Following this incubation period, RBC deformability was measured as described above.
RBC-Endothelial cell adhesion assay
To assess the effects of the various lymph samples on RBC adhesion to the endothelium, whole blood of naïve rats were incubated with rat T/SS or T/HS lymph (10% vol/vol) for 3 hours. Following this incubation period, RBC adhesion to an endothelial cell monolayer was measured as follows. Briefly, Human Umbilical Vein Endothelial Cells (HUVECs, LONZA, Walkersville, MD) were seeded onto 35 mm tissue culture plates and used 48 hours after seeding when the endothelial cells had reached confluence. Then, RBCs (60μl of whole blood) samples incubated with the T/HS or T/SS lymph collected from FTY720 or vehicle treated rats were added to the tissue culture plates containing the HUVECs monolayers together with 2ml of Essential Basal Media (EBM, LONZA Walkersville, MD). The plates were gently swirled, then incubated for 5 minutes at room temperature and washed 3 times with 2ml of EBM following which the supernatant was discarded and the plates were placed in an inverted position for 10 seconds. RBC adhesion was quantified by optical microscopy at 40 X magnification (ZEISS microscope). In this assay the number of adherent RBC in 3 randomly selected 40 X fields were counted in a blinded fashion.
Flow cytometry assay for CD36 positive RBC
Since our recent work has documented that increased adhesion of T/HS RBC to the endothelium is mediated to a large extent by increased expression of CD36 (19), the ability of the various lymph samples to increase RBC CD36 expression was quantified. In this assay, whole blood samples from naïve male rats that had been previously incubated with the lymph samples were stained for 30 minutes with CD36 Fluorescein Isothiocyanate (FITC, Invitrogen, Camarillo, CA) or an isotype control antibody (Invitrogen, Camarillo, CA). Following which they were resuspended in 400μl of 1 x PBS and analyzed by a Fluorescence Activated Cell Sorter (FACS-Calibur, Becton-Dickinson, CA). A 488-nm argon laser beam was used for excitation. A two-parameter dot-plot of forward light scatter and side light scatter was set up to include only RBC and to exclude white blood cells and lymphocytes. Green fluorescence of gated 10,000 RBC was then measured using linear amplification. The percentage of CD36 positive FITC RBC was derived by Cell Quest software.
Statistical Analysis
Analysis of variance (ANOVA) with the post hoc Turkey-Kramer multiple comparison test was used for comparison between multiple groups, where as Student t tests were used for comparison between two groups. Results are expressed as mean ± standard deviation. P<0.05 was considered statistically significant.
RESULTS
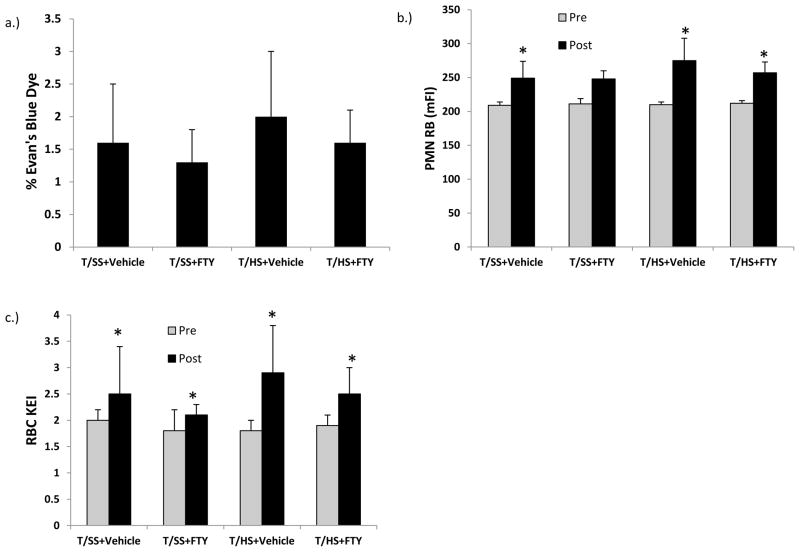
The first major set of key observations was that post-T/HS treatment with FTY720 protected against T/HS-induced a) acute lung injury, b) neutrophil priming, and c) red blood cell dysfunction (Table 1). Specifically, the administration of FTY70 given during the post-T/HS resuscitation period completely prevented the 3 fold increase in T/HS-induced lung permeability. Furthermore, although some degree of increased neutrophil priming was observed in the T/SS groups (Table 1) indicating that the laparotomy and instrumentation led to some neutrophil activation, the magnitude of neutrophil priming was significantly increased in the T/HS plus vehicle group and abrogated by FTY720. However, neutrophil priming remained increased in the T/HS plus FTY720 group as compared to the T/SS group. Like the response observed for neutrophil priming, FTY720 significantly limited T/HS-induced RBC rigidification, but its protective effect was not complete (Table 1).
Table 1.
Effect of FTY720 at a dose of 1 mg/kg on lung injury, neutrophil activation and RBC dysfunction
| Group | Lung - % EB in BALF |
Pre-Shock PMN RB |
Post-Shock PMN RB |
Pre-Shock RBC KEI |
Post-Shock RBC KEI |
|---|---|---|---|---|---|
| T/SS + Vehicle | 1.8 ± 0.8 | 200 ± 12 | 247 ± 8 ** | 1.6 ± 0.1 | 1.7 ± 0.2 |
| T/SS + FTY720 | 1.2 ± 0.4 | 205 ± 8 | 249 ± 14 ** | 1.6 ± 0.2 | 1.7 ± 0.2 |
| T/HS + Vehicle | 5.3 ± 1.6 * | 217 ± 17 | 386 ± 32 **# | 1.5 ± 0.2 | 3.2 ± 0.6 **# |
| T/HS + FTY720 | 1.5 ± 0.6 | 215 ± 12 | 313 ± 12 **+ | 1.6 ± 0.2 | 2.3 ± 0.7 **+ |
Data expressed as Mean ± SD with n=7–9 rats/group.
p < 0.01 vs. all other groups
p <0.05 vs. Pre-Shock group
p < 0.01 vs. all other groups at that time point
p < 0.05 vs. T/SS group at that time point
BALF: Bronchoalveolar lavage fluid
RB: Respiratory burst measured in Mean Fluorescence Intensity
KEI: Kinetic Energy Interactive
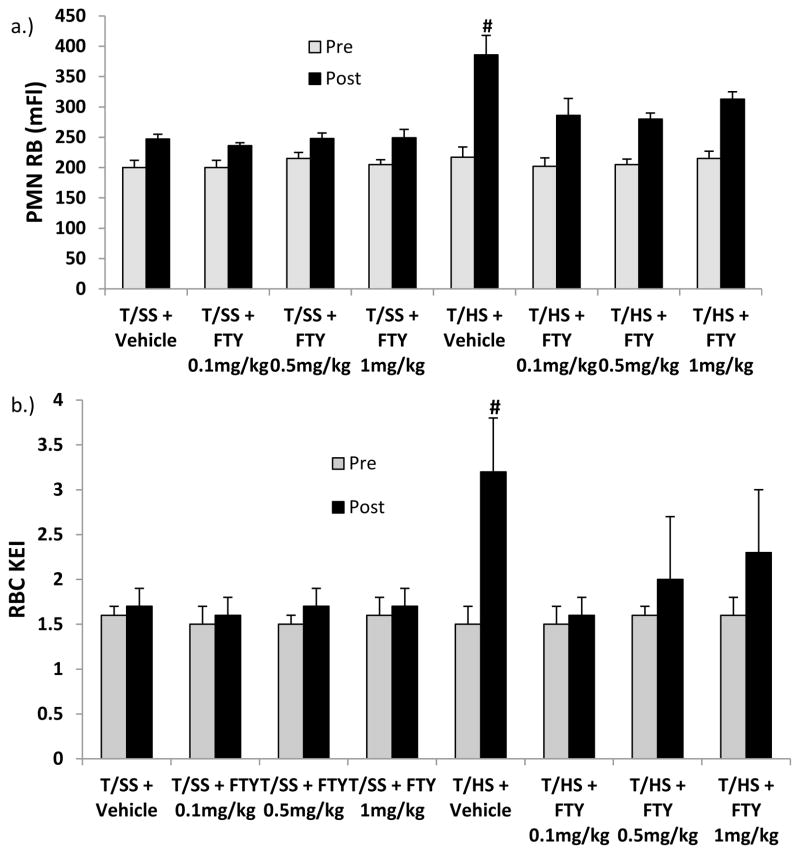
Having shown that FTY720, at a dose of 1mg/kg was effective at limiting T/HS-induced organ and cellular injury, a dose range study of FTY720 was performed using 2 lower doses. The results of this study showed that the beneficial effects of FTY720 observed with the 1 mg/kg dose, also existed when lower doses (0.5mg/kg and 0.1mg/kg) were tested (Figure 1 & 2). Specifically, all of the doses of FTY720 tested were similarly effective in limiting T/HS-induced lung injury, neutrophil priming and RBC dysfunction. A dose range study was also done with T/SS rats but since these values were not different from each other or the T/SS vehicle group.
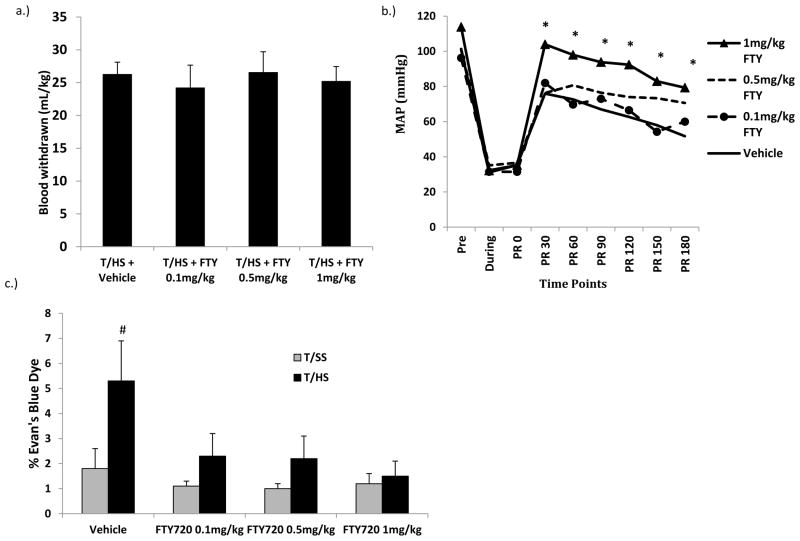
Figure 1.
A) There were no differences in the volume of blood required to be withdrawn to induce and maintain shock. B) The average MAP post resuscitation was significantly higher in the 1mg/kg FTY720 dose group compared with the vehicle group. All FTY720 doses were effective in limiting T/HS-induced C) lung injury
Data expressed as Mean ± SD except for Figure 1b, where the mean value without SD was used for clarity. n=6–9 rats per group; # p<0.05 vs. all other groups; * p<0.05 vs. THS + Vehicle; PR=Post Resuscitation.
Figure 2.
All FTY720 doses were effective in limiting T/HS-induced A) neutrophil priming and B) red cell dysfunction.
Data expressed as Mean ± SD. n=6–9 rats per group; # p<0.05 vs. all other groups
Since FTY720 has vasoactive properties and this could have accounted for part of its protective effects, we measured the post shock hemodynamic response. Additionally, to confirm standardization of the groups, we measured the volume of blood required to be withdrawn to induce and maintain the shock state. There were no differences in the volume of blood removed between the T/HS animals receiving vehicle and the three T/HS groups receiving different doses of FTY720 (Figure 1a). On the other hand, the average mean arterial pressure (MAP) post resuscitation was significantly higher in the rats treated with 1mg/kg of FTY720 compared to the vehicle group. Yet, no difference was seen between the 0.5mg/kg and the 0.1mg/kg FTY720 groups compared with the vehicle group or the 1mg/kg FTY720 group. (Figure 1b) A second way in which FTY720 could have abrogated T/HS-induced lung injury was through its ability to increase leucocyte sequestration thereby decrease circulating leukocyte counts (5, 20). Thus, the pre and post T/HS, (T/SS) neutrophil and lymphocyte counts were quantified (Table 2). For clarity, only the data obtained for the 1.0 mg/kg dose of FTY720 is shown, because similar results were observed in rats receiving the 0.1 and the 0.5 mg/kg doses of FTY720. None of the pre-T/HS PMN values differed between the T/SS and T/HS groups. Although the post-T/HS (T/SS) PMN counts were lower than the pre-T/HS (T/SS) PMN counts, this decrease was not statistically significant. However, there was a significant decrease when the post-shock PMN T/SS+Vehicle and T/SS+FTY groups were compared to the T/HS+FTY group. Similarly, the post-T/HS lymphocyte counts had significantly decreased in all groups. However, although the post-T/HS lymphocyte counts were reduced, no statistical differences were observed between the various T/HS and T/SS groups, suggesting that FTY720’s protective effects were not mediated to any significant extent by lymphocyte sequestration.
Table 2.
Circulating blood leukocyte counts
| Group | Pre-Shock PMN |
Post-Shock PMN |
Pre-Shock lymphocyte | Post-Shock lymphocyte |
|---|---|---|---|---|
| T/SS + Vehicle | 9.3 ± 2.0 | 10.2 ± 4 ^ | 7.1 ± 1.7 | 3.5 ± 1.7 * |
| T/SS + FTY | 7.5 ± 1.5 | 10 ± 3.6 ^ | 5.5 ± 2.8 | 2.8 ± 1.4 * |
| T/HS + Vehicle | 7.9 ± 2.2 | 7.8 ± 2.5 | 6.1 ± 1.6 | 2.8 ± 0.8 * |
| T/HS + FTY | 7.9 ± 1.5 | 5.5 ± 2.6 | 6.1 ± 1.4 | 2.2 ± 0.9 * |
Data expressed as Mean (103cells/microliter) ± SD with n= 6–9/group
p < 0.01 vs. pre-shock control value for that group
p < 0.01 vs. Post-Shock T/HS + FTY PMN Value
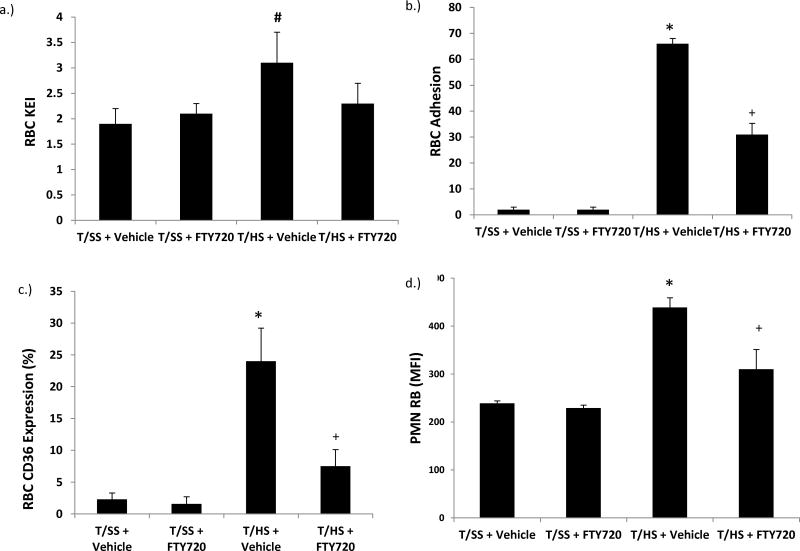
Since early post T/HS-induced lung injury, neutrophil priming and RBC changes have been mechanistically tied to the generation of biologically active lymph exiting the stressed gut, we next tested the hypothesis that FTY720 would limit the biologic effects of T/HS lymph. This was done by incubating whole blood from naïve healthy male rats with lymph (10% v/v) collected from T/SS or T/HS rats that had received vehicle or FTY720 at a dose of 1 mg/kg. As shown in Figure 3a, T/HS lymph decreased the deformability of RBCs as reflected in an elevated KEI. This T/HS lymph effect on RBC deformability was prevented by the administration of FTY720 and the KEI values of the naive RBCs incubated with T/HS lymph from the rats receiving FTY720 was similar to that of the RBCs incubated with T/SS lymph samples (Figure 3a). This ability of T/HS lymph to decrease RBC deformability was also associated with increased adherence to a HUVEC endothelial monolayer (Figure 3b) and surface expression of CD36 (Figure 3c). The T/HS lymph from the FTY720 rats reduced but did not totally prevent these effects on RBC adhesion and CD36 expression (Figure 3). Likewise, the T/HS lymph samples primed normal neutrophils to a greater degree than the T/SS lymph samples (Figure 3d). Treatment with FTY720 significantly reduced the ability of T/HS lymph to prime PMNs, however, FTY720 was not fully protective (Figure 3d). There were no differences between the sham values in the dose range experiments comparing lung injury, neutrophil activation or RBC deformability (Figure 1c & Figure 2). These results suggest that part of FTY720’s systemic protective effects could be mediated through abrogation of the production of toxic intestinal lymph.
Figure 3.
T/HS lymph from FTY720-treated rats manifested less ability to A) increase RBC rigidity, B) increase RCB adhesion to endothelium, C) induce RBC CD36 expression or D) induce neutrophil priming.
Data is expressed as Mean ± SD with lymph samples from 9–11 rats tested in each group. # p<0.05 vs. sham groups; * p<0.05 vs. all other groups; + p<0.05 vs. T/SS+FTY720 group
It is important to stress that the intestinal lymph samples were sterile and were tested after all of the cells had been removed by centrifugation. Cell-free lymph was tested based on our earlier studies showing that lymph’s bioactivities were not related to the cellular components of lymph, but were due to humoral factors contained in lymph (21). However, since FTY720 has been reported to reduce lymphocyte counts and a large number of lymphocytes exit the gut through the lymphatics, it is possible that FTY720 would reduce the lymphocyte count of intestinal lymph. Thus, pre-T/HS (T/SS) and post-T/HS (T/SS) lymphocyte counts were quantified in mesenteric lymph (Table 3). The lymphocyte count decreased from the pre-T/HS or T/SS levels in all the groups except the T/SS plus vehicle group, indicating that both the administration of FTY720 as well as actual T/HS was associated with a decrease in the lymphocyte count of the lymph samples. Thus, consistent with the potential of FTY720 to sequester lymphocytes in lymphoid organs, the lymphocyte count in the T/SS rats receiving FTY720 was significantly reduced as compared to the T/SS rats receiving vehicle.
Table 3.
Lymphocyte cell counts in mesenteric lymph
| Group | Pre Shock -lymphocyte | Post-Shock lymphocyte |
|---|---|---|
| T/SS + Vehicle | 29.0 ± 13.0 | 24.5 ± 14.6 |
| T/SS + FTY | 25.0 ± 11.3 | 11.5 ± 6.3 * # |
| T/HS + Vehicle | 28.6 ± 11.5 | 13.1 ± 6.9 * |
| T/HS + FTY | 20.0 ± 8.3 | 7.5 ± 3.9 * # |
Data expressed as Mean (103cells/microliter) ± SD with n= 6–9/group
p < 0.05 vs. the pre-shock control value for that group.
p < 0.05 vs. T/SS + vehicle at that time point.
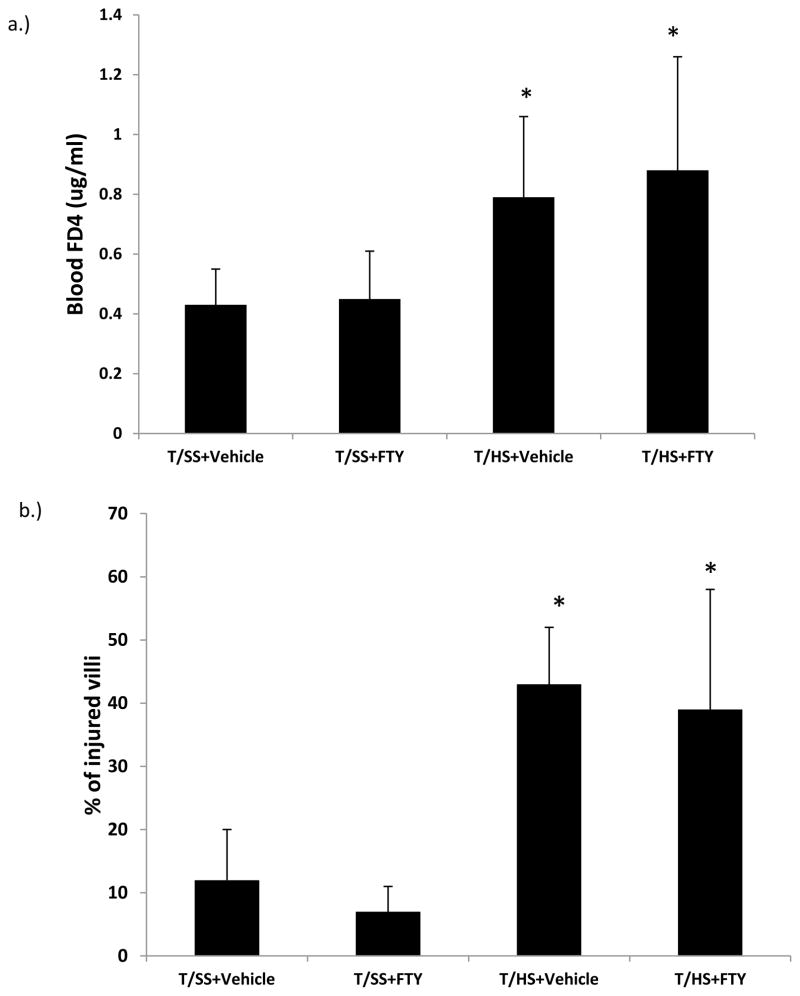
To assess the potential protective effect of FTY720 on T/HS-induced gut injury, we measured gut permeability using the permeability probe FD4, where FD4 is placed in the lumen of the gut and 30 minutes later plasma samples are collected and the blood FD4 concentration is measured. Morphologic studies of the intestinal villi were also performed to complement the physiologic FD4 studies. We found that, in contrast to the protective effects of FTY720 on T/HS lymph bioactivities, the FTY720 did not prevent T/HS-induced increases in gut permeability or limit the extent of morphologic gut injury (Figure 4).
Figure 4.
FTY720 did not prevent T/HS-induced increases in A) gut permeability or limit the extent of B) morphologic gut injury. Data is expressed as Mean ± SD with n=6 rats per group; * p<0.05 vs. the respective T/SS group.
Since previous studies document that mesenteric lymph diversion or lymph duct ligation limits T/HS-induced lung injury, PMN priming and RBC injury (2, 19), we also measured these variables in the FTY720 and vehicle-treated groups that had systemic lymph diversion via the implanted catheters to compare the protective effects of FTY720 to that of lymph diversion. This experiment is based on the concept that the injured gut produces toxic lymph which enters the systemic circulation and can lead to end organ failure. If the toxic lymph is prevented from reaching systemic circulation (lymph catheter), end organ failure is prevented. (13) As predicted both the T/HS-vehicle and T/HS-FTY720 groups did not manifest acute lung injury when lymph was prevented from entering the systemic circulation (Figure 5a). Likewise there was no difference in the post-shock neutrophil respiratory burst priming or RBC deformability between the groups. However, the manipulations associated with the laparotomy, lymph collection and other instrumentation was associated with increased PMN priming and decreased RBC deformability of the post-shock values compared to the animals’ pre-shock values (Figure 5). As in the T/HS and T/SS groups that did not undergo lymph diversion, the post-T/SS and T/HS blood lymphocyte counts decreased similarly among the groups, while the neutrophil counts did not decrease (Table 4).
Figure 5.
A) Both the T/HS + Vehicle and T/HS + FTY720 groups did not manifest acute lung injury when lymph was prevented from entering the systemic circulation. B) Likewise there was no difference in the post shock neutrophil respiratory burst priming or C) RBC deformability between the groups. Data is expressed as Mean ± SD with n=9–10 rats per group; * p<0.05 vs. respective Pre-operative group.
Table 4.
Circulating leukocyte blood counts in animals with lymph duct diversion
| Group | Pre-Shock PMN |
Post-Shock PMN |
Pre-Shock lymphocyte | Post-Shock lymphocyte |
|---|---|---|---|---|
| T/SS + Vehicle | 7.4 ± 1.5 | 8.7 ± 3.4 | 5.8 ± 1.0 | 3.3 ± 1.4 * |
| T/SS + FTY | 8.0 ± 4.4 | 9.3 ± 3.2 | 6.0 ± 3.3 | 2.9 ± 1.3 * |
| T/HS + Vehicle | 7.7 ± 2.3 | 7.8 ± 3.0 | 5.9 ± 1.6 | 2.9 ± 1.4 * |
| T/HS + FTY | 7.1 ± 1.9 | 6.8 ± 2.6 | 5.5 ± 1.5 | 2.0 ± 0.8 * |
Data expressed as Mean (10 cells/microliter) ± SD with n= 6–9/group
p < 0.01 vs. pre-shock control value for that group
DISCUSSION
The major observation of this study is that FTY720 administered post-shock as part of the fluid resuscitation therapy is able to abrogate T/HS-induced acute lung injury, neutrophil priming and RBC dysfunction as well as abrogate the deleterious biologic activity of mesenteric lymph. These results are encouraging since post-injury therapy with FTY720 was able to prevent lung injury and neutrophil priming, which are two of the major consequences of trauma-hemorrhage that have been associated with increased morbidity and mortality (22, 23). This failure of FTY720 to completely abrogate the effects of T/HS on RBC function and neutrophil priming shows that its protective effects were not complete. Two possibilities are that this is the result of involvement of other pathways, or higher drug doses may have been necessary. A limitation of this study is that neither of these possibilities were investigated. Another potential limitation to this work is that we did not perform a mortality study to investigate whether FTY720 prevents mortality as well as morbidity. However, the fact that the protective effect of FTY720 was apparent over a range of doses and at levels as low as 0.1 mg/kg indicates that the drug is effective at doses not that much higher than those that have been safely administered to patients which range from a total dose of 0.5 mg to 1.25 mg (24).
Due to its broad spectrum of biologic activities, there are several potential mechanisms by which FTY720 could have limited T/HS-induced acute lung injury (5). Specifically, FTY720 has been shown to reduce lymphocyte cell trafficking, induce lymphopenia, limit immune and inflammatory cell activation, maintain vascular homeostasis and limit tissue injury in several ischemia-reperfusion models (5,7,8). These biologic activities of FTY720 appear to overlap with the processes involved in the pathogenesis of acute lung injury. That is, dysregulation of vasomotor tone, immune-inflammatory cell trafficking and activation as well as gut injury have been documented to play a role in several models of acute lung injury (2, 25), while RBC dysfunction has been shown to impair microvascular blood flow (14, 26). Thus, we measured the effect of FTY720 on the number of circulating leukocytes, the amount of withdrawn blood required to induce shock, the post-shock hemodynamic response as well as the magnitude of gut injury, the biologic activity of mesenteric lymph, neutrophil priming and a panel of RBC parameters.
The circulating lymphocyte counts as well as the lymphocyte count in the intestinal lymph samples were measured, because there is increasing evidence that lymphocytes are rapidly activated in an alloantigen-independent manner in conditions associated with an ischemia-reperfusion injury (27, 28). Additionally, recent studies have shown that activated T lymphocytes participate in ischemia-reperfusion-induced tissue injury as well as neutrophils (29, 30). In fact, there is evidence that activated T cells may even be involved in coordinating the innate neutrophil response following ischemia-reperfusion (31). Although the administration of FTY720 was associated with a decrease in circulating blood lymphocytes, this decrease was similar to the vehicle treated animals suggesting that post-T/HS lymphocyte sequestration or lymphopenia is likely not involved in the protective effect of FTY720. However, FTY720 may have produced alterations in lymphocyte subpopulations which could have had protective effects. Since lymphocyte subpopulations measurements were not made, this possibility cannot be excluded. Better preservation of post shock blood pressure could have been one mechanism by which FTY720 was beneficial based on the observation that the post shock MAP was better preserved in the T/HS group receiving 1mg/kg of FTY720 compared to the vehicle group. On the other hand, the 0.1mg/kg and 0.5mg/kg doses of FTY720, which were also systemically protective, did not show better preservation of post shock MAP. Thus, although FTY720 at 1mg/kg preserved the post shock MAP, suggesting that its systemic protective effects were associated with an improved post shock hemodynamic response, the fact that the other doses of FTY720 did not improve the post shock MAP, yet still limited organ injury and systemic neutrophil activation suggests that the hemodynamic effects of FTY720 cannot fully explain its systemic protective effects.
The fact that FTY720 treatment limited neutrophil priming is of potential clinical relevance since primed neutrophils have been tightly associated with the development of acute lung injury in both preclinical and clinical studies (23, 32). This suggests that one of the potential mechanisms by which FTY720 may have exerted its beneficial effects was through its ability to limit neutrophil activation. RBC changes induced by T/HS were measured based on an increasing number of studies documenting that RBC rigidification and increased RBC adhesion to the endothelium can directly impair microvascular blood flow and hence lead to organ injury (14, 26). Since FTY720 limited T/HS-induced RBC rigidification, adhesion to the endothelium and upregulation of surface CD36 expression, a second mechanism by which FTY720 could have exerted its protective effect is by limiting these T/HS-induced RBC changes. This possibility is supported by the fact that RBCs contain S1P receptors and are one of the principle cells involved in S1P regulation (5). Since activated neutrophils have been documented to bind to the endothelium and cause a capillary leak syndrome as well as contribute to the no reflow phenomenon after shock (23,32,33), it is possible that both RBCs and neutrophils could have contributed to post T/HS microvascular dysfunction and thereby potentiated acute lung injury. More work will be required to better understand these observations. Nonetheless, these studies complement the work by Hawksworth et al. (9) showing that FTY720 improves survival time in swine and found that this beneficial effect of FTY720 was associated with central lymphocyte sequestration.
Our results that FTY720 did not protected the gut from T/HS-induced injury, although it abrogated the biologic activity of the mesenteric lymph, was surprising and unexpected based on our previous work showing that loss of lymph bioactivity was consistently associated with abrogation of gut injury in this model system (2). We have no explanation for this observation, although one possibility could be that FTY720 blocked the process of toxic lymph production by the injured gut. Another possibility is that the differences between the gut and lung responses to FTY720 reflects differences in S1P receptors, since S1P-1 and S1P-2 receptors dominate in the gut (34) while S1P-1, S1P-2, & S1P-3 receptors dominate in the lung (35).
In summary, the post-treatment administration of FTY720 successfully prevented T/HS-induced lung injury, neutrophil priming and RBC phenotypic and functional changes. Thus, FTY720 shows promise as a potential therapeutic agent based on the current work, the work of Hawksworth (9) in a swine hemorrhagic shock model, and a number of preclinical studies documenting that FTY720 successfully limited ischemia-reperfusion mediated organ injury. The potential clinical importance of these encouraging preclinical studies are strengthened by the safety profile of FTY720 and its FDA approval for use in a number of clinical conditions.
Acknowledgments
Supported by Department of Defense Grant W81XWH-12-1-0091 (EAD) and T32 069330 (JAB, JYS)
References
- 1.Minei JP, Cuschieri J, Sperry J, Moore EE, West MA, Harbrecht BG, O’Keefe GE, Cohen MJ, Moldawer LL, Tompkins RG, Maier RV. Inflammation and the Host Response to Injury Collaborative Research Program. The changing pattern and implications of multiple organ failure after blunt injury with hemorrhagic shock. Critical Care Med. 2012;40:1129–1135. doi: 10.1097/CCM.0b013e3182376e9f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Deitch EA, Xu DZ, Lu Q. Gut lymph hypothesis of early shock and trauma-induced multiple organ dysfunction syndrome: A new look at gut origin sepsis. J Organ Dysfunct. 2006;2:70–9. [Google Scholar]
- 3.Kaiser VL, Sifri ZC, Dikdan GS, Berezina T, Zaets S, Lu Q, Xu DZ, Deitch EA. Trauma-hemorrhagic shock mesenteric lymph from rat contains a modified form of albumin that is implicated in endothelial cell toxicity. Shock. 2005;23:417–425. doi: 10.1097/01.shk.0000160524.14235.6c. [DOI] [PubMed] [Google Scholar]
- 4.Qin X, Dong W, Sharpe SM, Sheth SU, Palange DC, Rider T, Jandacek R, Tso P, Deitch EA. Role of lipase-generated free fatty acids in converting mesenteric lymph from a noncytotoxic to a cytotoxic fluid. Am J Physiol Gastrointest Liver Physiol. 2012;303:G696–G978. doi: 10.1152/ajpgi.00290.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brinkmann V. Sphingosine 1-phosphate receptors in health and disease: Mechanistic insights from gene deletion studies to reverse pharmacology. Pharmacol Therap. 2007;11:84–105. doi: 10.1016/j.pharmthera.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 6.Sphingosine-1-phosphate Receptor 1 (S1P1) Signaling in T cells: Trafficking and Beyond. Garris CS, Blaho VA, Hla T, Han MH. Immunology. 2014 Mar 5; doi: 10.1111/imm.12272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Man K, Ng KT, Lee TK, Lo CM, Sun CK, Li XL, Zhao Y, Ho JW, Fan ST. FTY720 attenuates hepatic ischemia-reperfusion injury in normal and cirrhotic livers. Am J Transplant. 2005;5:40–49. doi: 10.1111/j.1600-6143.2004.00642.x. [DOI] [PubMed] [Google Scholar]
- 8.Peng X, Hassoun PM, Sammani S, McVerry BJ, Burne MJ, Rabb H, Pearse D, Tuder RM, Garcia JG. Protective effects of sphingosine 1-phosphate in murine endotoxin-induced inflammatory lung injury. Am J Respir Crit Care Med. 2004;169:1245–1251. doi: 10.1164/rccm.200309-1258OC. [DOI] [PubMed] [Google Scholar]
- 9.Hawksworth JS, Graybill JC, Brown TS, Wallace SM, Davis TA, Tadaki DK, Elster EA. Lymphocyte modulation with FYT720 improves hemorrhagic shock survival in swine. PLoS One. 2012;7(4):e34224. doi: 10.1371/journal.pone.0034224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kappos L, Radue EW, O’Connor P, Polman C, Hohlfeld R, Calabresi P, Selmaj K, Agoropoulou C, Leyk M, Zhang-Auberson L, Burtin P FREEDOMS Study Group. A placebo-controlled trial of oral fingolimod in relapsing multiple sclerosis. N Engl J Med. 2010;362:387–401. doi: 10.1056/NEJMoa0909494. [DOI] [PubMed] [Google Scholar]
- 11.Zhang Z, Schluesener HJ. FYT720: A most promising immunosuppressant modulating immune cell functions. Mini Rev Med Chem. 2007;7:845–850. doi: 10.2174/138955707781387948. [DOI] [PubMed] [Google Scholar]
- 12.Deitch EA, Adams C, Lu Q, Xu DZ. A time course study of the protective effect of mesenteric lymph duct ligation on hemorrhagic shock-induced pulmonary injury and the toxic effects of lymph from shocked rats on endothelial cell monolayer permeability. Surgery. 2001;129:39–47. doi: 10.1067/msy.2001.109119. [DOI] [PubMed] [Google Scholar]
- 13.Baker JW, Deitch EA, Li M, Berg RD, Specian RD. Hemorrhagic Shock Induces Bacterial Translocation from the Gut. J Trauma. 1988;28:896–906. doi: 10.1097/00005373-198807000-00002. [DOI] [PubMed] [Google Scholar]
- 14.Machiedo GW, Zaets SB, Berenzina TL, Xu DZ, Feketova E, Spolarics Z, Deitch EA. Trauma-hemorrhage shock-induced red blood cell damage leads to decreased microcirculatory blood flow. Crit Care Med. 2009;37:1000–1010. doi: 10.1097/CCM.0b013e3181962d39. [DOI] [PubMed] [Google Scholar]
- 15.Senthil M, Watkins A, Barlos D, Xu DZ, Lu Q, Abungu B, Caputo FJ, Feinman R, Deitch EA. Intravenous injection of trauma-hemorrhagic shock mesenteric lymph causes lung injury that is dependent upon activation of the inducible nitric oxide synthase pathway. Ann Surg. 246:822–830. doi: 10.1097/SLA.0b013e3180caa3af. [DOI] [PubMed] [Google Scholar]
- 16.Deitch EA, Ananthakrishnan P, Cohen DB, Xu DZ, Feketeova E, Hauser CJ. Neutrophil activation is modulated by sex hormones after trauma-hemorrhagic shock and burn injuries. Am J Physiol Heart Circ Physiol. 2006;291:H1456–H1465. doi: 10.1152/ajpheart.00694.2005. [DOI] [PubMed] [Google Scholar]
- 17.Rupani B, Caputo FJ, Watkins AC, Vega D, Magnotti LJ, Lu Q, Xu DZ, Deitch EA. Relationship between disruption of the unstirred mucus layer and intestinal restitution in loss of gut barrier function after trauma hemorrhagic shock. Surgery. 2007;141:481–489. doi: 10.1016/j.surg.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 18.Caputo F, Rupani B, Watkins A, Barlos D, Vega D, Senthil M, Deitch EA. Pancreatic duct ligation abrogates the trauma hemorrhage-induced gut barrier failure and the subsequent production of biologically active intestinal lymph. Shock. 2007;28:441–446. doi: 10.1097/shk.0b013e31804858f2. [DOI] [PubMed] [Google Scholar]
- 19.Deitch EA, Condon M, Feketeova E, Machiedo GW, Mason L, Vinulan GM, Alli VA, Neal MD, Tomaio JN, Fishman JE, Duran WN, Spolarics Z. Trauma-Hemorrhagic Shock Induces a CD36-Dependent RBC Endothelial-Adhesive Phenotype. Crit Care Med. 2013 Dec; doi: 10.1097/CCM.0000000000000119. [DOI] [PubMed] [Google Scholar]
- 20.Brinkmann V, Cyster JG, Hla T. FTY720: sphingosine 1-phosphate receptor-1 in the control of lymphocyte egress and endothelial barrier function. Am J Transplant. 2004;(7):1019–1025. doi: 10.1111/j.1600-6143.2004.00476.x. [DOI] [PubMed] [Google Scholar]
- 21.Adams CA, Jr, Xu DZ, Lu Q, Deitch EA. Factors larger than 100 kd in post-hemorrhagic shock mesenteric lymph are toxic for endothelial cells. Surgery. 2001;129:351–363. doi: 10.1067/msy.2001.111698. [DOI] [PubMed] [Google Scholar]
- 22.ARDSNet. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acture respiratory distress syndrome. The Acute Respiratory Distress Syndrome Network. N Engl J Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 23.Abraham E. Neutrophils and acute lung injury. Crit Care Med. 2003;31(4 Suppl):S195–S199. doi: 10.1097/01.CCM.0000057843.47705.E8. [DOI] [PubMed] [Google Scholar]
- 24.Mehling M, Johnson TA, Antel J, Kappos L, Bar-Or A. Clinical immunology of sphingosine-1- phosphate receptor modulator fingolimod (FYT720) in multiple sclerosis. Neurology. 2011;76:S20–S27. doi: 10.1212/WNL.0b013e31820db341. [DOI] [PubMed] [Google Scholar]
- 25.Brinkman V, Baumruker T. Pulmonary and vascular pharmacology of sphingosine-1-phosphate. Curr Opinions Pharmacol. 2006;6:244–250. doi: 10.1016/j.coph.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 26.Telen MJ. Red blood cell surface adhesion molecules: Their possible roles in normal human physiology and diseases. Semin Hematol. 2000;37:130–142. doi: 10.1016/s0037-1963(00)90038-6. [DOI] [PubMed] [Google Scholar]
- 27.Huang Y, Rabb H, Womer KL. Ischemia-reperfusion and immediate T cell responses. Cell Immunol. 2007;248:4–11. doi: 10.1016/j.cellimm.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kunisawa J, Kurashima Y, Higuchi M, Gohda M, Ishikawa I, Ogahara I, Kim N, Shimizu M, Kiyono H. Sphingosine 1-phosphate dependence in the regulation of lymphocyte trafficking to the gut epithelium. J Exp Med. 2007;204:2335–48. doi: 10.1084/jem.20062446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Man K, Ng KT, Lee TK, Lo CM, Sun CK, Li XL, Zhao Y, Ho JW, Fan ST. FTY720 attenuates hepatic ischemia-reperfusion injury in normal and cirrhotic livers. Am J Transplant. 2005;5:40–49. doi: 10.1111/j.1600-6143.2004.00642.x. [DOI] [PubMed] [Google Scholar]
- 30.Ysebaert DK, De Greef KE, De Beuf A, Van Rompay AR, Vercauteren S, Persy VP, De Broe ME. T cells as mediators in renal ischemia/reperfusion injury. Kidney Int. 2004;66:491–496. doi: 10.1111/j.1523-1755.2004.761_4.x. [DOI] [PubMed] [Google Scholar]
- 31.Horie Y, Wolf R, Chervenak RP, Jennings SR, Granger DN. T-lymphocytes contribute to hepatic leukostasis and hypoxic stress induced by gut ischemia-reperfusion. Microcirculation. 1999;6:267–280. [PubMed] [Google Scholar]
- 32.Belizaire RM, Prakash PS, Richter JR, Robinson BR, Edwards MJ, Caldwell CC, Lentsch AB, Pritts TA. Microparticles from stored red blood cells activate neutrophils and cause lung injury after hemorrhage and resuscitation. J Am Coll Surg. 2012;214:648–655. doi: 10.1016/j.jamcollsurg.2011.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marcus BC, Wyble CW, Hynes KL, Gewertz BL. Cytokine-induced increases in endothelial permeability occur after adhesion molecule expression. Surgery. 1996;120:411–6. doi: 10.1016/s0039-6060(96)80317-5. [DOI] [PubMed] [Google Scholar]
- 34.Hu W, Huang J, Mahavadi S, Li F, Murthy KS. S1P1 & S1P2; Lentiviral siRNA silencing of sphingosine-1-phosphate receptors S1P1 and S1P2 in smooth muscle. Biochem Biophys Res Commun. 2006 May;343:1038–44. doi: 10.1016/j.bbrc.2006.03.079. Epub Mar 22, 2006. [DOI] [PubMed] [Google Scholar]
- 35.Natarajan V, Dudek SM, Jacobson JR, Moreno-Vinasco L, Huang LS, Abassi T, Mathew B, Zhao Y, Wang L, Bittman R, Weichselbaum R, Berdyshev E, Garcia JG. S1P1-3; Sphingosine-1-phosphate, FTY720, and sphingosine-1-phosphate receptors in the pathobiology of acute lung injury. Am J Respir Cell Mol Biol. 2013 Jul;49:6–17. doi: 10.1165/rcmb.2012-0411TR. [DOI] [PMC free article] [PubMed] [Google Scholar]