Abstract
X-linked adrenoleukodystrophy (X-ALD) results from mutations in ABCD1. ABCD1 resides on Xq28 and encodes an integral peroxisomal membrane protein (ALD protein [ALDP]) that is of unknown function and that belongs to the ATP-binding cassette–transporter superfamily. Individuals with ABCD1 mutations accumulate very-long-chain fatty acids (VLCFA) (carbon length >22). Childhood cerebral X-ALD is the most devastating form of the disease. These children have the earliest onset (age 7.2 ± 1.7 years) among the clinical phenotypes for ABCD1 mutations, but onset does not occur at <3 years of age. Individuals with either peroxisomal biogenesis disorders (PBD) or single-enzyme deficiencies (SED) in the peroxisomal β-oxidation pathway—disorders such as acyl CoA oxidase deficiency and bifunctional protein deficiency—also accumulate VLCFA, but they present during the neonatal period. Until now, it has been possible to distinguish unequivocally between individuals with these autosomal recessively inherited syndromes and individuals with ABCD1 mutations, on the basis of the clinical presentation and measurement of other biochemical markers. We have identified three newborn boys who had clinical symptoms and initial biochemical results consistent with PBD or SED. In further study, however, we showed that they lacked ALDP, and we identified deletions that extended into the promoter region of ABCD1 and the neighboring gene, DXS1357E. Mutations in DXS1357E and the ABCD1 promoter region have not been described previously. We propose that the term “contiguous ABCD1 DXS1357E deletion syndrome” (CADDS) be used to identify this new contiguous-gene syndrome. The three patients with CADDS who are described here have important implications for genetic counseling, because individuals with CADDS may previously have been misdiagnosed as having an autosomal recessive PBD or SED
Introduction
X-linked adrenoleukodystrophy (X-ALD [MIM 300100]) is a recessive neurodegenerative disorder that has a hemizygote frequency of 1:21,000 in the United States (Bezman et al. 2001). Mutations in the ALD gene, ABCD1, are associated with a broad spectrum of clinical phenotypes. Childhood cerebral X-ALD has the earliest onset (mean age 7.2 ± 1.7 years) and most devastating effects but does not manifest clinically until age ⩾3 years (Moser et al. 2001). ABCD1 encodes the peroxisomal integral-membrane ALD protein (ALDP) and belongs to the ATP-binding cassette (ABC) transporter superfamily (Mosser et al. 1993). The four known peroxisomal ABC proteins are considered to be half-transporters that probably require dimerization in order to function (Liu et al. 1999; Smith et al. 1999). The ligands transported by these proteins have not been determined, although the role of the nucleotide-binding fold in ATP binding and hydrolysis has been demonstrated in vitro (Roerig et al. 2001). The relationship between mutations in ABCD1 and the reduced capacity of X-ALD fibroblasts to catabolize very-long-chain fatty acids (VLCFA) (carbon length >22) by β-oxidation remains unknown. Although it has been reported that X-ALD peroxisomes have reduced very-long-chain acyl-CoA synthetase activity (Lazo et al. 1988; Wanders et al. 1988), ALDP does not function as a synthetase and is not required for localization of the human enzyme to the peroxisome (Steinberg et al. 1999a). Without exception, however, individuals hemizygous for ABCD1 mutations accumulate VLCFA in plasma (Moser et al. 1999).
Although X-ALD is the most common peroxisomal disorder, other peroxisomal diseases also can be identified on the basis of elevated VLCFA levels in plasma. Individuals with peroxisomal biogenesis disorders (PBD) such as Zellweger syndrome (ZS) or single-enzyme deficiencies (SED) in the peroxisomal β-oxidation pathway—such as acyl-CoA oxidase deficiency (OxD), d-bifunctional protein deficiency (BD), and β-ketothiolase deficiency—all accumulate VLCFA in complex lipid fractions in plasma and other tissues (Gould et al. 2001; Wanders et al. 2001). In spite of the overlap in biochemical phenotype, it has previously been possible to distinguish between these syndromes and those in individuals with ABCD1 mutations, by measurements of other biochemical markers of peroxisomal function and by their clinical presentation. For instance, individuals with PBD fail to assemble mature peroxisomes and thus have deficiencies in multiple peroxisomal pathways, including plasmalogen synthesis, l-pipecolic acid metabolism, branched-chain fatty-acid α-oxidation, and cholesterol metabolism (Gould et al. 2001). Individuals with BD have elevated plasma VLCFA and urinary bile-acid intermediates dihydroxycholestanoic acid (DHCA) and trihydroxycholestanoic acid (THCA), and their cultured fibroblasts have a reduced capacity to α-oxidize phytanic acid (Wanders et al. 2001). In contrast, individuals with X-ALD have both normal levels of other peroxisomal analytes and normal peroxisome structure (Moser et al. 2001), although fibroblasts representing at least two-thirds of nonrecurrent ABCD1 mutations are immunonegative for ALDP (Watkins et al. 1995a; Feigenbaum et al. 1996; Kemp et al. 2001). Newborns with either PBD or SED commonly have congenital abnormalities, failure to thrive, hypotonia, and developmental delay, and many die during the first months to years of life. In contrast, newborns with ABCD1 mutations accumulate VLCFA in the adrenal gland and plasma but appear healthy at birth and thrive postnatally.
The vast majority of individuals with an increase in plasma VLCFA levels who have been identified through diagnostic screening are X-ALD hemi- or heterozygotes (Moser et al. 1999). Nonetheless, the Peroxisomal Diseases Laboratory at the Kennedy Krieger Institute investigates ⩾40 individuals a year who have increased plasma VLCFA and clinical symptoms suggestive of a PBD. Approximately 20% of these individuals have a biochemical phenotype consistent with a β-oxidation SED other than X-ALD (hereafter, “SED” will refer only to OxD and BD) (Moser 1999). We have identified three patients whose early clinical symptoms and initial biochemical results appeared to be consistent with BD or OxD; however, a study of cultured fibroblasts demonstrated that they had defects consistent with an ABCD1 mutation. Elsewhere, we have reported, in abstract form, preliminary investigations of patients 1 and 2 (Corzo et al. 1999; Steinberg et al. 2001). Here we describe the unique clinical features in three patients, document the biochemical evidence for a defect in fibroblast peroxisomal β-oxidation, and describe molecular investigations establishing that these patients have a contiguous deletion spanning the 5′ ends of ABCD1 and DXS1357E.
Material and Methods
Biochemical Analyses
Whole-blood samples were collected in EDTA tubes. Plasma was isolated and used for analysis of VLCFA and l-pipecolic acid, as described elsewhere (Kelley 1991; Moser and Moser 1991). Plasmalogens were measured in washed erythrocyte membranes by capillary gas chromatography (Bjorkhem et al. 1986). Organic acids were extracted from urine, were derivatized, and were analyzed by gas chromatography/mass spectrometry (Tanaka et al. 1980). Cultures were initiated and maintained in Eagle’s minimum essential medium supplemented with 10% fetal calf serum (Moser et al. 1995) and were assayed for VLCFA content, C24:0 β-oxidation, plasmalogen synthesis, phytanic acid oxidation, and catalase solubility, as described elsewhere (Wanders et al. 1984; Roscher et al. 1985; Moser and Moser 1991; Zenger-Hain et al. 1992; Watkins et al. 1995b). Cholesterol-ester fractions were extracted from whole tissue and were analyzed by gas chromatography (Rasmussen et al. 1994). Somatic-cell hybridization was conducted by use of polyethylene glycol as a fusogen and ficoll for gradient separation (Moser et al. 1995). All research activities were approved by the Joint Committee of Clinical Investigation, and parental consents were obtained.
Immunocytochemical Analyses of Cultured Fibroblasts
Cells were cultured on glass coverslips, were fixed with formaldehyde, were permeabilized, and were incubated with primary antibodies and fluorescein- or rhodamine-conjugated secondary antibodies, as described elsewhere (Watkins et al. 1995a).
ABCD1 DNA Analyses
Genomic DNA was isolated from cultured fibroblasts by use of a Puregene kit (Gentra). The 10 exons of ABCD1 were amplified, by PCR, as 10 amplicons and were directly sequenced (Boehm et al. 1999). For Southern blot analysis, 5 μg of genomic DNA was digested with BamHI, separated by agarose-gel electrophoresis, transferred to nitrocellulose membrane, and hybridized by use of a full-length ABCD1 cDNA 32P[dCTP]-labeled probe.
Other DNA Analyses
STS oligonucleotide primers for PCR were synthesized at the DNA Analysis Facility at Johns Hopkins University, on the basis of sequences reported in the STS database at the National Center for Biotechnology Information (dbSTS: database of “Sequence Tagged Sites”). Reaction mixtures containing 0.1 μg of genomic DNA were prepared as described elsewhere (Steinberg et al. 1999b), by use of the Expand High Fidelity system (Roche). The following PCR program was used: 94°C for 4 min (1 cycle); 94°C for 30 s, 66°C for 1 min, and 72°C for 1 min (35 cycles); and 72°C for 10 min (1 cycle). Further analysis of DXS1357E exon 5 was performed with PCR primers CDM1-F (5′-ggtctaactggaagcagtggatgg-3′) and CDM1-R (5′-gagtacgacatcgctccgagaagg-3′) and with sequencing primer CDM1-S (5′-gagcagcaacaccctcctcctcacc-3′).
Results
Clinical Presentation Similar to That of Individuals with a PBD or a SED
Patients 1, 2, and 3 (hereafter denoted by “Pt1,” “Pt2,” and “Pt3,” respectively) were born to healthy women with no known family history of adrenoleukodystrophy or other peroxisomal disease. None of the parental relationships were consanguineous. All three patients were male. Two of the patients were French Canadian, and the third patient was Vietnamese. Table 1 summarizes the clinical features of these cases and compares them to those of individuals with either ZS, BD, OxD, or X-ALD. All boys had profound neonatal hypotonia, subsequent failure to thrive, and cholestatic liver disease. Liver biopsies in all patients showed intracanalicular and ductal cholestasis. Pt2 and Pt3 developed seizures at age 2 mo. Pt1 did not have seizures, but did experience frequent episodes of opisthotonos and bruxism. Provocative adrenal-function testing was not performed on any of the patients. However, at autopsy small, fibrotic adrenal glands (0.5 g, combined) were found in Pt3. Limited gross autopsy findings were available on Pt3 only. The total body weight was low (2.19 kg), and the liver appeared small, fibrotic, and jaundiced. The cut brain revealed white-matter abnormalities. Postmortem magnetic-resonance images, compared with images obtained several months earlier, indicated that myelination had progressed but that overall myelination was significantly delayed. As part of the extensive evaluations of all three patients, VLCFA were measured.
Table 1.
Clinical Features in Pt1, Pt2, and Pt3, Compared to Other Peroxisomal Disorders
|
Other Peroxisomal Disorders |
||||||||
| Feature | Pt1 | Pt2 | Pt3 | All ThreePatients | BD | OxD | ZS | X-ALDa |
| Ethnicity | French Canadian | French Canadian | Vietnamese | |||||
| Age at death | 11 mo | 4 mo | 4 mo | <1 year | 9 mo | 4 years | <1 year | 9.4 years |
| Cause of death | Liver failure and gastrointestinal bleeding | Respiratory failure and gastrointestinal bleeding | Liver and respiratory failure | |||||
| Clinical symptoms:b | ||||||||
| Neonatal hypotonia | + | + | + | + | + | + | + | − |
| Neonatal seizures | −c | At age 2 mo | At age 2 mo | +/− | + | + | + | − |
| Craniofacial dysmorphism | − | − | − | − | + | − | + | − |
| Intrauterine growth retardation | − | − | Severe | +/− | − | − | − | − |
| Liver disease | + | + | + | + | +/− | − | + | − |
| Neonatal cholestasisd | + | +e | +f | + | − | − | +/− | − |
| Cataract | + | − | − | +/− | − | − | + | − |
| Sensorineural deafness | + | + | − | +/− | +/− | +/− | +/− | − |
| Reference | Watkins et al. (1995b) | Watkins et al. (1995b) | Gould et al. (2001) | Moser et al. (2001) | ||||
For childhood cerebral disease; average of 167 patients.
A plus sign (+) denotes presence; a minus sign (−) denotes absence; a plus/minus sign (+/−) denotes that the clinical symptom may or may not be present (and is used only when multiple patients are described).
Frequent episodes of opisthonos and bruxism.
Neonatal cholestasis, documented by liver biopsy.
Increased plasma and urine bile-acid levels and large amounts of several unknown compounds.
Mild increase in cholestanol, which can be related to defects in bile-acid synthesis.
Biochemical Analyses—Results Consistent with a SED of Peroxisomal β-Oxidation
Plasma VLCFA analysis in Pt1, Pt2, and Pt3 showed elevations consistent with a deficiency in peroxisomal β-oxidation (table 2). Erythrocyte plasmalogens and plasma l-pipecolic acid were normal for all three patients (table 2). Epoxydicarboxylic acids and 2-hydroxysebacic acid were not detected in the urine organic-acid profile of Pt3. Cultured fibroblasts from each patient demonstrated VLCFA accumulation and a reduced capacity to β-oxidize the VLCFA C24:0 (table 2). Each cell line had a normal capacity for α-oxidation and plasmalogen synthesis (table 2). Catalase solubility in fractionated cells demonstrated normal peroxisomal localization (table 2) and provided further support for the presence of a SED. THCA and DHCA were not detected in the urine of Pt2 and Pt3. Total bile acids were increased in Pt2, and Pt 3 had a moderate increase in cholestanol. Results of liver biopsies were consistent with cholestasis in all three patients. Peroxisomes were present in liver tissue, as demonstrated by electron microscopy in Pt1 and Pt2.
Table 2.
Peroxisomal Biochemical Profile in Pt1, Pt2, and Pt3[Note]
| Assay | Units | Pt1 | Pt2 | Pt3 | Control | PBD | X-ALDa |
| Blood analytes: | |||||||
| VLCFA | μg/ml, for C26:0 | 2.09 | 2.48 | 2.98 | .22±.08 | 3.31±1.63 | 1.18±.53 |
| μg/ml, for C26:1 | 2.02 | 1.86 | 2.34 | .12±.05 | 1.55±.55 | .19±.05 | |
| C24/C22 | 1.56 | 1.65 | 1.85 | .84±.08 | 1.95±.42 | 1.49±.45 | |
| C26/C22 | .16 | .21 | .18 | .01±.01 | .52±.24 | .07±.04 | |
| Plasmalogensb | C16 DMA/C16 | .054 | .059 | .050 | .051-.090 | .001-.025 | NM |
| C18 DMA/C18 | .14 | .151 | .118 | .137-.255 | .001-.050 | NM | |
| l-Pipecolic acid | Micromolar | 1.2 | .2 | 1.1 | 1.8±.9c | 47±42.1c | NM |
| Fibroblasts: | |||||||
| VLCFA | μg/mg protein, for C26:0 | .335 | .535 | .329 | .07±.04 | .87±.44 | .42±.15 |
| μg/mg protein, for C26:1 | .116 | .216 | .057 | .09±.07 | 1.06±.72 | .17±.1 | |
| C26/C22 | .731 | .828 | .728 | .08±.03 | 1.00±.33 | .69±.19 | |
| C24:0 β-oxidation | nmol/h/mg protein | .229 | .298 | .109 | 1.16±.166 | .10±.05 | .31±.06 |
| Plasmalogen synthesis | 3H/14C | .56 | .93 | .7 | .67±.19 | 9.92±4.4 | NM |
| Phytanic acid oxidation | % of control value | 106 | 99.3 | 123 | 100 | 2.2 | NM |
| Catalase solubility | % Soluble | 18 | 22 | 26 | <25 | >85 | NM |
Note.— For each test and each disease category, the results are for ⩾10 cases—except in the case of β-oxidation, for which the results are from 10 control cell lines, 7 PBD cell lines, and 7 X-ALD cell lines.
NM = not measured routinely.
DMA = dimethylacetal.
Patients were 1–6 mo of age.
Fibroblasts—Immunonegative for Peroxisomal ALD Protein (ALDP)
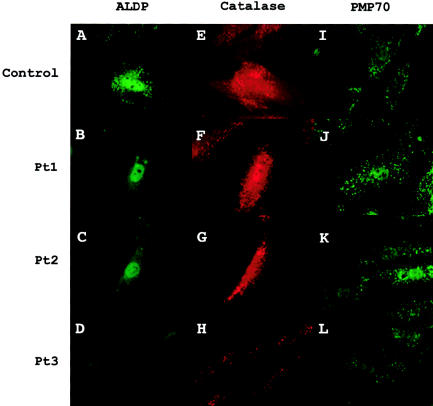
When fibroblasts from these cases were evaluated by immunocytochemistry with an antibody to ALDP, no peroxisomal staining was seen (fig. 1B–D). In contrast, when cells were stained with antibody to the matrix protein catalase, peroxisomes of normal size and number were observed (see fig. 1F–H). Furthermore, peroxisomes of normal size and number were visualized when cells were treated with antibody to the peroxisomal ABC protein PMP70 (fig. 1J–L).
Figure 1.
Peroxisomal proteins in cultured fibroblasts evaluated by immunocytochemical analysis. Fibroblasts were double-labeled for ALDP and catalase and were labeled separately for PMP70. A–D, ALDP, a protein encoded by ABCD1. Pt 1–Pt3 are immunonegative for this protein. Two-thirds of ABCD1 mutations result in immunonegative status (Watkins et al. 1995a; Feigenbaum et al. 1996). E–H, Catalase, a peroxisomal matrix protein. Cells from Pt1, Pt2, and Pt3 and from a control subject have normal peroxisome size and number. In the control-subject panel, the catalase signal colocalizes with that seen for ALDP. I–L, PMP70, a peroxisomal membrane protein that belongs to the same ABC transporter subtype as does ALDP. Compared with those from the control subject, cell lines from Pt1, Pt2, and Pt3 have normal PMP70 localization. All cells were visualized at 1,000× magnification.
Failure of Complementation Studies with X-ALD Fibroblasts to Correct the Defect
Somatic-cell hybridization studies were performed with an ALDP immunonegative cell line derived from an individual with X-ALD and the ABCD1 mutation Nt1876G→A (A626T). Cells from Pt1 and Pt2 were fused with the X-ALD cells and then were separated into two fractions, one enriched with multinuclear cells and the other containing mainly cocultivated mononuclear cells. A cross between the X-ALD and a PBD cell line was used as a positive control. The ability to β-oxidize lignoceric acid (i.e., C24:0) was used as a marker for correction. C24:0 β-oxidation was restored to normal control levels in the cross with the PBD cell line, but cells from Pt1 and Pt2 did not complement the known X-ALD cell line (table 3).
Table 3.
Complementation Analysis
| Source | C24:0 β-Oxidation in MulinuclearCells/Mononuclear Cells [Ratio](nmol/h/mg protein) |
| Cell line 1/cell line 2:a | |
| X-ALD/PBD | 1.338/.214 [6.3] |
| X-ALD/Pt1 | .329/.361 [0.9] |
| X-ALD/Pt2 | .372/.418 [0.9] |
| Control (not fused) | NA/1.267b |
Cell line 1 is immunonegative for ALDP and is derived from an individual with X-ALD who had classic childhood cerebral onset and ABCD1 mutation Nt1876G→A. PBD = cell line from an individual with ZS who had deficiencies in multiple peroxisomal pathways and lacked peroxisomes.
NA = not applicable.
DNA Sequencing and Southern Blot Analysis—Identification of Large ABCD1 Deletions
To determine whether patients had a mutation in the ABCD1 gene, the 10 exons were amplified and directly sequenced. We were unable to amplify product for exons 1–10 of Pt1 and Pt3 or for exons 1–5 of Pt2. No sequence variation was detected in exons 6–10 of Pt2. Since we were able to amplify other gene targets from the purified DNA for Pt1 and Pt3, the failed ABCD1 reactions suggested the presence of a large gene deletion.
Southern blot analysis was performed as described above (see the Material and Methods section), by use of a full-length ABCD1 cDNA probe. This region of Xq28 has undergone interchromosomal duplication several times (Eichler et al. 1997). Loci sharing high sequence identity to the 3′ end of ABCD1 are found on 2p11, 10p11, 16p11, and 22q11. To discriminate Xq28 genomic DNA from the autosomal partial homologs, validation studies were performed by use of the cell line AHA11a, a mouse/human somatic hybrid harboring the human X chromosome (Dorman et al. 1978; Smith et al. 1999). AHA11a DNA had three bands that corresponded to the three expected fragment sizes (10, 8.3, and 5.6 kb, respectively) for BamHI digestion of human Xq28 and one band that appeared in the wild-type mouse alone (fig. 2B). In addition to the three bands corresponding to ABCD1, the lane for the human control subject contained two bands that represent cross-reactivity of the cDNA probe to autosomal homolog DNA fragments. Pt1 and Pt3 lacked the three ABCD1 bands corresponding to all 10 exons (fig. 2B). In contrast, Pt2 lacked bands corresponding to exons 1–6 but retained the 8.3-kb band representing exons 6–10 (fig. 2B). The two bands that represent DNA fragments from autosomal partial homologs were present in all three affected patients and serve as an internal control for the method.
Figure 2.
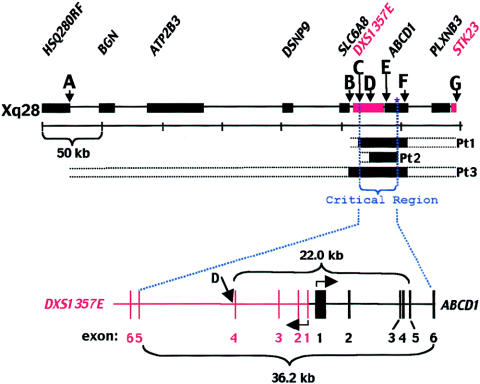
Large ABCD1 deletions that extend into the coding region of DXS1357E, in Pt1, Pt2, and Pt3. A, ABCD1, which spans ∼24 kb and has 10 exons (Sarde et al. 1994). Genomic DNA BamHI digestion was predicted to yield three ABCD1 fragments from Xq28. B, Results of Southern blot validation studies and analyses of patients. Validation studies were conducted to identify genomic fragments containing DNA from autosomal homologs (Eichler et al. 1997; Smith et al. 1999) that might cross-react with the full-length ABCD1 cDNA probe. DNA from wild-type mouse, from a mouse cell line harboring one human X chromosome (AHA11a [Dorman et al. 1978]), and from a human control subject (Ctl) were analyzed simultaneously. Four bands appeared for the AHA11a DNA, one corresponding to the band from wild-type mouse and three matching the predicted sizes (i.e., 10, 8.3, and 5.6 kb) of fragments for ABCD1 on Xq28 and aligning with bands in Ctl. The two additional bands in Ctl correspond to autosomal homologs that cross-react with the cDNA probe, since they did not appear in the mouse hybrid cell line harboring the human X chromosome. Pt1 and Pt3 lack the three ABCD1 bands corresponding to all 10 exons. In contrast, Pt2 lacks bands corresponding to exons 1–6 but retains the 8.3-kb band representing exons 6–10. Ex = exons. C, DNA map for Xq28, showing the location of four STS markers (gels A, B, D, and G), in relation to ABCD1 and surrounding genes (se the Entrez Nucleotide Web site of the National Center for Biotechnology Information [accession number U52111]). In addition, the location of ABCD1 exons 2 and 10 (gels E and F, respectively), the boundary between ABCD1 intron 5 and exon 6 (denoted by the asterisk [*]), and DXS1357E exon 5 (gel C) are shown. DXS1357E shares a CpG island and is in a head-to-head orientation with ABCD1 (Mosser et al. 1994). D, PCR primers for the markers shown were used for analysis in Pt1, Pt2, and Pt3 and in a male control subject (Ctl). These results are summarized in figure 3. E, Amplification of template for Xq28 and 16P11. Because of sequence homology between Xq28 and 16p11, the primers for reaction C (DXS1357E exon 5) amplify both templates. Compared with DNA from the control subject and Pt2, that from Pt1 and Pt3 yielded a small amount of product. When the amplicon from Pt1 and Pt3 was sequenced by use of primer CDM-1S (see the “Material and Methods” section), a 16p11-specific sequence was revealed, whereas the products from Pt2 and the control subject had DNA sequence specific to Xq28. Sequence specific to 16p11 was detectable only in the absence of the corresponding Xq28 region.
Carrier Status of the Mothers
The mother of Pt1 had a second pregnancy with a male fetus. Prenatal diagnosis of cultured amniocytes indicated that the fetus had a defect in peroxisomal fatty-acid metabolism (table 4), and the pregnancy was terminated (Moser and Moser 1999). VLCFA comprised 36.6% of the total fatty acids in the cholesterol-ester fraction extracted from fetal adrenal, compared with the normal control mean of 1.02% (n=4) (Moser et al. 1982). Thus, the mother of Pt1 is the only obligate heterozygote among these three women. Plasma and fibroblast VLCFA were normal in all three mothers. The discriminant function for females, on the basis of three plasma measures (Moser et al. 1999), was 4.6–4.8 for these mothers, compared with a median normal value of ∼5 (n=11,800) and obligate-heterozygote level of ∼9 (n=281). In fibroblasts immunohistochemically stained for ALDP, a normal peroxisomal pattern was visualized in 75%, 94%, and >99% of cells from the mothers of Pt1, Pt2, and Pt3, respectively (not shown). Southern blot analysis of the mothers of Pt1 and Pt2 confirmed that, on one allele, they have the same deletion as is seen in their sons (results not shown). In contrast, Southern blot analysis of the mother of Pt3 showed that she is not a carrier of the ABCD1 deletion detected in her son.
Table 4.
Prenatal Diagnosis in the Mother of Pt1
| Source of Amniocytes | C26:0(μg/mg protein) | C26:0/C22:0 |
| Male fetus and mother of Pt1 | .43 | .64 |
| Unaffected male subjects (n=127)a | .10 ± .07 | .17 ± .10 |
| X-ALD hemizygotes (n=45)a | .47 ± .17 | 1.06 ± .48 |
Source.—Moser and Moser (1999).
Further Molecular Studies−Indications That Xq28 Deletions Extend Beyond ABCD1
To determine whether the deletion in these three patients extended beyond the 5′ region of ABCD1, two STS markers upstream from the ABCD1-gene promoter were selected for PCR analysis (fig. 2C). These markers are associated with two genes that are immediately upstream of ABCD1. Marker stSG39985 is in intron 4 and very near exon 4 of DXS1357E, a gene that is in a head-to-head orientation with ABCD1 (Mosser et al. 1994). The stSG4965 marker lies at the extreme 3′ end of SLC6A8, a gene that encodes a creatine transporter. Mutations in this gene cause a creatine-deficiency syndrome that impairs neurological function (Salomons et al. 2001). The marker in the middle of DXS1357E failed to amplify in all three patients (fig. 2D) and indicated that these deletions extend beyond exon 1 and the promoter of ABCD1 and into the neighboring, DXS1357E gene.
To verify and further delineate the deletions in this region, DXS1357E exon 5 was selected for PCR amplification (figs. 2D and 3). A 26.5-kb duplication from Xq28 that includes the coding regions of DXS1357E and SLC6A8 is located on 16p11 (Eichler et al. 1996). The DXS1357E homolog on 16p11 appears to be a pseudogene (Eichler et al. 1997), but the 16p11 paralog to SLC6A8 encodes a testes-specific transcript, and the gene is named “SLC6A10” (Iyer et al. 1996). DXS1357E exon 5 on Xq28 has 93% sequence identity to the 16p11 paralog. The degree of sequence identity between the PCR primers for the DXS1357E exon 5 reaction and exon 5 of both Xq28 and 16p11 suggests that both regions would be amplified if both were present, although the primers were designed to favor the Xq28 sequence. The exon 5 reaction yielded an intense band of the expected size for the control subject and for Pt2 and yielded a barely detectable amplicon for Pt1 and Pt3. Results from sequencing initiated by primer CDM-1S (see the “Material and Methods” section, above) are shown across a region that clearly distinguishes between 16p11 and Xq28 (fig. 2E). The exon 5 product in Pt1 and Pt3 had a 16p11-specific sequence, whereas Pt2 and the control amplicons were Xq28 specific. The PCR primers and reaction conditions favored the amplification of the Xq28 DNA to such an extent that the 16p11 sequence was detectable only when the homologous Xq28 region was deleted.
Figure 3.
CADDS critical region, which lies between DXS1357E exon 5 and ABCD1 exon 6. The minimum and maximum extent of Xq28 deletion, on the basis of PCR and Southern blot analyses, is shown for each patient. The black segments denote DNA demonstrated to be absent. The horizontal dotted lines extending from the ends of each box denote regions that have yet to be excluded. An enlargement of the critical region that is missing in all three patients is shown. The smallest deletion occurs in Pt2 and spans 22.0 ⩾ 36.2 kb.
DXS1357E exon 5 was present in Pt2 and indicated that the 5′ breakpoint for this deletion resides between exons 4 and 5. Since the 3′ breakpoint resides between ABCD1 exons 5 and 6, these studies predict, overall, that the deletion in Pt2 spans 22.0⩾36.2 kb (fig. 3). This deletion is the smallest in the three patients investigated and suggests that the critical region responsible for this disorder is between DXS1357E exon 5 and ABCD1 exon 6.
Discussion
The three male patients reported here were referred to the Peroxisomal Diseases Laboratory for evaluation of an inborn error of peroxisomal function. All three newborns were profoundly hypotonic and developmentally delayed, failed to thrive, and had cholestatic liver disease. The atrophied adrenal glands in Pt3 and the high level of VLCFA in adrenals from the fetus from the mother of Pt1 suggest that these patients may also have had adrenal insufficiency (Govaerts et al. 1984; Moser et al. 2001). Testing these patients to rule out either a PBD such as ZS or a related SED was appropriate (Moser and Raymond 1998). Each patient had both an accumulation of VLCFA in plasma and fibroblasts and reduced peroxisomal β-oxidation rates in fibroblasts, but other peroxisomal biochemical pathways were normal, and matrix-protein import remained intact. The biochemical profile in blood, urine, and cultured fibroblasts from Pt1, Pt2, and Pt3 supported the diagnosis of an isolated deficiency in peroxisomal fatty-acid metabolism.
The neonatal onset of disease in Pt1, Pt2, and Pt3 appeared, on a clinical basis, to exclude X-ALD or a mutation in ABCD1, because 2.75 years is the earliest age at onset of neurological symptoms that has been observed in >2,000 documented patients with X-ALD (H.W.M., unpublished data). Furthermore, cholestatic liver disease has never been described in X-ALD. In contrast to these differences, the biochemical abnormalities in the newborns in the present study more closely resembled those in X-ALD. The bile-acid intermediates DHCA and THCA, which are increased in BD (Natowicz et al. 1996), were not detected in the urine of Pt2 and Pt3. The epoxydicarboxylic acids frequently observed in PBD and SED urine (Yamaguchi et al. 2001) were absent in Pt3. All three patients had normal phytanic acid oxidation, which is diminished in BD and, to a lesser extent, in OxD (A.B.M., unpublished data). Unlike the usual pattern in X-ALD, plasma levels of C26:1 were increased in the patients reported here, a difference that may be due to liver disease.
The absence of immunocytochemical staining for ALDP in fibroblasts from Pt1, Pt2, and Pt3 provided the first clue that there was a primary defect in ABCD1. PMP70 and catalase localized to the peroxisome in each cell line, establishing normal peroxisome assembly. The normal localization of PMP70 argues against a unique generalized assembly defect that would affect the routing of multiple peroxisomal ABC proteins to the membrane.
Mutation identification in the three patients was hampered initially by a failure to amplify all or most of the exons. Southern blot analysis demonstrated that these patients had large deletions that encompassed exons 1–10 of Pt1 and Pt3 and exons 1–5 of Pt2. Two-hundred forty-six nonrecurrent ABCD1 mutations have been reported (Kemp et al. 2001), only 4.5% of which represent deletions encompassing at least one entire exon. There has been a single report of a partial deletion involving exon 1 (Koike et al. 1995). This deletion eliminated 0.5 kb of sequence located near the 3′ end of the >1,286-nt exon. The individual presented at age 19 years, with cerebellar and brain-stem signs and thus is phenotypically and genotypically distinct from our three patients. Although mutations in exon 1 occur at the same per-nucleotide rate as in other exons (X-linked Adrenoleukodystrophy Database), deletions involving exon 1 are rare, and, to our knowledge, a complete deletion of exon 1 has not previously been reported.
PCR analysis with STS markers established that the first four exons of DXS1357E were absent in Pt1, Pt2, and Pt3 (fig. 3), indicating that the ABCD1 deletions extended beyond exon 1 and the promoter region. The extent of the deletions was further supported by PCR analyses, which demonstrated absence of DXS1357E exon 5 in Pt1 and Pt3. These three cases are the first described with ABCD1 deletions that extend beyond the initiation codon. Pt2 had normal sequence for ABCD1 exons 6–10, indicating that the genomic DNA downstream of ABCD1 exon 5 is unlikely to be involved in the pathogenetic mechanism. The smaller deletion size in Pt2 suggests that the critical region for this novel phenotype is restricted to a 36.2-kb span residing between DXS1357E exon 5 and ABCD1 exon 6 (fig. 3). Mutation of DXS1357E has not been reported previously in association with human disease, but its partial deletion may be implicated in the new phenotype. Its 1.5-kb transcript is reportedly ubiquitously expressed (Mosser et al. 1994). The translated protein is a B-cell antigen receptor–associated protein, BAP31, that heterodimerizes with BAP29 (Adachi et al. 1996). Extensive studies of the expression and function of this protein have not been reported in the literature.
Deletions involving the 5′ ends of both ABCD1 and DXS1357E suggest that this neonatal phenotype is due to a contiguous-gene defect. We propose using the term “contiguous ABCD1 DXS1357E deletion syndrome” (CADDS) to describe this disorder. We strongly advise against use of the term “neonatal X-ALD,” to avoid further confusion between the terms “X-ALD” and “neonatal adrenoleukodystrophy,” a PBD. In addition, the primary cause of neurological impairment in CADDS most likely is not a leukodystrophy. The brain of Pt3, for example, appeared to have delayed myelination. Further studies to delineate the breakpoints of these deletions and to explore their impact on gene expression are in progress and may shed some light on either the ABCD1 promoter or the function of ALDP. Nevertheless, the elimination of the promoter regions of both DXS1357E and ABCD1 provides a putative mechanism for the new phenotype. The early presentation and phenotypic similarity of these three patients, which are distinct from those of all previously described individuals with ABCD1 mutations, strongly suggest that DXS1357E or another nearby gene regulated by this region contributes to this neonatal phenotype.
There is no direct correlation between ABCD1 mutations and clinical phenotype. The childhood- and adult-onset forms are frequently found in the same kindred (Moser et al. 2001). Although there is evidence for an autosomal modifier gene (Smith et al. 1991; Maestri and Beaty 1992), so far this has not been identified. Recently, O’Neill et al. (2001) reported a kindred in which there is a point mutation in the ABCD1 initiator methionine and in which all affected members have adrenomyeloneuropathy.
The three patients hemizygous for this 5′ ABCD1 deletion are relatively uniform in their clinical phenotype. They all had neonatal cholestasis and profound hypotonia and died at age <1 year. Nevertheless, at this time we cannot rule out the possibility of a genotypic and phenotypic spectrum that might include children who have less neurological or liver impairment (or both) in the neonatal period. Likewise, there may be heterozygous girls with an unfavorable X-inactivation pattern who are less severely affected than are the hemizygotes. The origin and the precise mechanism of the deletion in these three unrelated patients likely vary, because the preliminary data indicate that the breakpoints are different for all three.
These three cases highlight the importance of careful investigation of any individual presenting, during the neonatal period, with a SED affecting VLCFA β-oxidation. Although clinical findings in the patients reported here resemble PBD, they differ profoundly with respect to genetic counseling, because their mode of inheritance is X-linked recessive and not autosomal recessive as in PBD. Pedigree analysis may help in the identification of families that demonstrate an X-linked pattern of inheritance, but, in the three families that we thus far have identified, the only symptomatic individual was the index case. Although two of the mothers are carriers, they may have mutations that arose from the paternal gamete. Since both BD and OxD are autosomal recessive disorders, at-risk families do not become aware of their risk until the first affected child is born. Prenatal diagnosis is available for these couples, but, because of the low carrier frequency, further counseling for the extended family is generally not required. In families with a history of an X-linked disorder, it is crucial to determine the origin of the mutation, so that appropriate counseling can be offered to those at risk. If our three patients had been left with the label of having a SED other than X-ALD, then appropriate counseling could not be offered.
Acknowledgments
We would like to thank Dr. Gerardo Jimenez-Sanchez for PMP70 antibody; Drs. Richard Kelley and Lisa Kratz for plasma cholesterol quantitation; Drs. Kenneth Setchell and Gerald Salen for bile-acid analysis; Dr. Antonio R. Perez for electron microscopy of liver on Pt 2; Dr. George Thomas for urine organic acid analysis; Dr. David Valle for thoughtful discussion; and Sheila Foreman, Anita Liu, Surinder Khangoora, and Anisa Chaudhry for their technical expertise in the laboratory. This work was supported in part by U.S. Public Health Service grants HD 10981 and RR 00052.
Electronic-Database Information
Accession numbers and URLs for data in this article are as follows:
- dbSTS: database of “Sequence Tagged Sites,” http://www.ncbi.nlm.nih.gov/dbSTS/ (for markers stSG4965 and stSG39985)
- Entrez Nucleotide, http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?db=Nucleotide (for Xq28 DNA sequence surrounding ABCD1, including DXS1357E, SLC6A8, and all other genes shown in [accession number U52111])
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for X-ALD [MIM 300100])
- X-linked Adrenoleukodystrophy Database, http://www.x-ald.nl
References
- Adachi T, Schamel WWA, Kim KM, Watanabe T, Becker B, Nielsen PJ, Reth M (1996) The specificity of association of the IgD molecule with the accessory proteins BAP31/BAP29 lies in the IgD transmembrane sequence. EMBO J 15:1534–1541 [PMC free article] [PubMed] [Google Scholar]
- Bezman L, Moser AB, Raymond GV, Rinaldo P, Watkins PA, Smith KD, Kass NE, Moser HW (2001) Adrenoleukodystrophy: incidence, new mutation rate, and results of extended family screening. Ann Neurol 49:512–517 [PubMed] [Google Scholar]
- Bjorkhem I, Sisfontes L, Bostrom B, Kase BF, Blomstrand R (1986) Simple diagnosis of the Zellweger syndrome by gas-liquid chromatography of dimethylacetals. J Lipid Res 27:786–791 [PubMed] [Google Scholar]
- Boehm CD, Cutting GR, Lachtermacher MB, Moser HW, Chong SS (1999) Accurate DNA-based diagnostic and carrier testing for X-linked adrenoleukodystrophy. Mol Genet Metab 66:128–136 [DOI] [PubMed] [Google Scholar]
- Corzo D, Cox G, Hobbs N, Quackenbush EJ, Moser A, Moser H (1999) A new phenotypic variant of X-linked adrenoleukodystrophy (ALD) presenting in the newborn period. Am J Hum Genet Suppl 65:A1295 [Google Scholar]
- Dorman B, Shimizu N, Ruddle FH (1978) Genetic analysis of the human cell surface: antigenic marker for the human X chromosome in human-mouse hybrids. Proc Natl Acad Sci USA 75:2363–2367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichler EE, Budarf ML, Rocchi M, Deaven LL, Doggett NA, Baldini A, Nelson DL, Mohrenweiser HW (1997) Interchromosomal duplications of the adrenoleukodystrophy locus: a phenomenon of pericentromeric plasticity. Hum Mol Genet 6:991–1002 [DOI] [PubMed] [Google Scholar]
- Eichler EE, Lu F, Shen Y, Antonacci R, Jurecic V, Doggett NA, Moyzis RK, Baldini A, Gibbs RA, Nelson DL (1996) Duplication of a gene-rich cluster between 16p11.1 and Xq28: a novel pericentromeric-directed mechanism for paralogous genome evolution. Hum Mol Genet 5:899–912 [DOI] [PubMed] [Google Scholar]
- Feigenbaum V, Lombard-Platet G, Guidoux S, Sarde CO, Mandel JL, Aubourg P (1996) Mutational and protein analysis of patients and heterozygous women with X-linked adrenoleukodystrophy. Am J Hum Genet 58:1135–1144 [PMC free article] [PubMed] [Google Scholar]
- Gould SJ, Raymond GV, Valle D (2001) The peroxisome biogenesis disorders. In: Scriver CR, Beaudet AL, Sly WS, Valle D (eds) The metabolic and molecular bases of inherited diseases, 8th ed. McGraw-Hill, New York, pp 3181–3217 [Google Scholar]
- Govaerts L, Monnens L, Melis T, Trijbels F (1984) Disturbed adrenocortical function in cerebro-hepato-renal syndrome of Zellweger. Eur J Pediatr 143:10–12 [DOI] [PubMed] [Google Scholar]
- Iyer GS, Krahe R, Goodwin LA, Doggett NA, Siciliano MJ, Funanage VL, Proujansky R (1996) Identification of a testis-expressed creatine transporter gene at 16p11.2 and confirmation of the X-linked locus to Xq28. Genomics 34:143–146 [DOI] [PubMed] [Google Scholar]
- Kelley RI (1991) Quantification of pipecolic acid in plasma and urine by isotope-dilution gas chromatography/mass spectrometry. In: Hommes FA (ed) Techniques in diagnostic human biochemical genetics. Wiley-Liss, New York, pp 205–218 [Google Scholar]
- Kemp S, Pujol A, Waterham HR, van Geel BM, Boehm CD, Raymond GV, Cutting GR, Wanders RJ, Moser HW (2001) ABCD1 mutations and the X-linked adrenoleukodystrophy mutation database: role in diagnosis and clinical correlations. Hum Mutat 18:499–515 [DOI] [PubMed] [Google Scholar]
- Koike R, Onodera O, Tabe H, Keneko K, Miyatake T, Iwasaki S, Nakano M, Shizuma N, Ikeguchi K, Nishizawa M, Mosser J, Sarde CO, Tsuji S (1995) Partial deletions of putative adrenoleukodystrophy (ALD) gene in Japanese ALD patients. Hum Mutat 6:263–267 [DOI] [PubMed] [Google Scholar]
- Lazo O, Contreras M, Hashmi M, Stanley W, Irazu C, Singh I (1988) Peroxisomal lignoceroyl-CoA ligase deficiency in childhood adrenoleukodystrophy and adrenomyeloneuropathy. Proc Natl Acad Sci USA 85:7647–7651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu LX, Janvier K, Berteaux-Lecellier V, Cartier N, Benarous R, Aubourg P (1999) Homo- and heterodimerization of peroxisomal ATP-binding cassette half-transporters. J Biol Chem 274:32738–32743 [DOI] [PubMed] [Google Scholar]
- Maestri NE, Beaty TH (1992) Predictions of a 2-locus model for disease heterogeneity: application to adrenoleukodystrophy. Am J Med Genet 44:576–582 [DOI] [PubMed] [Google Scholar]
- Moser AB, Kreiter N, Bezman L, Lu S, Raymond GV, Naidu S, Moser HW (1999) Plasma very long chain fatty acids in 3,000 peroxisome disease patients and 29,000 controls. Ann Neurol 45:100–110 [DOI] [PubMed] [Google Scholar]
- Moser AB, Moser HW (1999) The prenatal diagnosis of X-linked adrenoleukodystrophy. Prenat Diagn 19:46–48 [DOI] [PubMed] [Google Scholar]
- Moser AB, Rasmussen M, Naidu S, Watkins PA, McGuinness M, Hajra AK, Chen G, Raymond G, Liu A, Gordon D, Garnaas K, Walton DS, Skjeldal OH, Guggenheim MA, Jackson LG, Elias ER, Moser HW (1995) Phenotype of patients with peroxisomal disorders subdivided into sixteen complementation groups. J Pediatr 127:13–22 [DOI] [PubMed] [Google Scholar]
- Moser HW (1999) Genotype-phenotype correlations in disorders of peroxisome biogenesis. Mol Genet Metab 68:316–327 [DOI] [PubMed] [Google Scholar]
- Moser HW, Moser AB (1991) Measurement of saturated very long chain fatty acids in plasma. In: Hommes FA (ed) Techniques in diagnostic human biochemical genetics. Wiley-Liss, New York, pp 177–191 [Google Scholar]
- Moser HW, Moser AB, Powers JM, Nitowsky HM, Schaumburg HH, Norum RA, Migeon BR (1982) The prenatal diagnosis of adrenoleukodystrophy: demonstration of increased hexacosanoic acid levels in cultured amniocytes and fetal adrenal gland. Pediatr Res 16:172–175 [DOI] [PubMed] [Google Scholar]
- Moser HW, Raymond GV (1998) Genetic peroxisomal disorders: why, when and how to test. Ann Neurol 44:713–715 [DOI] [PubMed] [Google Scholar]
- Moser HW, Smith KD Watkins PA Powers J Moser AB (2001) X-linked adrenoleukodystrophy. In: Scriver CR, Beaudet AL, Sly WS, Valle D (eds) The metabolic and molecular bases of inherited diseases, 8th edition. McGraw-Hill, New York, pp 3257–3301 [Google Scholar]
- Mosser J, Douar AM, Sarde CO, Kioschis P, Feil R, Moser H, Poustka AM, Mandel JL, Aubourg P (1993) Putative X-linked adrenoleukodystrophy gene shares unexpected homology with ABC transporters. Nature 361:726–730 [DOI] [PubMed] [Google Scholar]
- Mosser J, Sarde CO, Vicaire S, Yates JRW, Mandel JL (1994) A new human gene (DXS1357E) with ubiquitous expression, located in Xq28 adjacent to the adrenoleukodystrophy gene. Genomics 22:469–471 [DOI] [PubMed] [Google Scholar]
- Natowicz MR, Evans JE, Kelley RI, Moser AB, Watkins PA, Moser HW (1996) Urinary bile acids and peroxisomal bifunctional enzyme deficiency. Am J Med Genet 63:356–362 [DOI] [PubMed] [Google Scholar]
- O’Neill GN, Aoki M, Brown RH (2001) ABCD1 translator-initiator mutation demonstrates genotype-phenotype correlation for AMN. Neurology 57:1956–1962 [DOI] [PubMed] [Google Scholar]
- Rasmussen M, Moser AB, Borel J, Khangoora S, Moser HW (1994) Brain, liver, and adipose tissue erucic and very long chain fatty acid levels in adrenoleukodystrophy patients treated with glyceryl trierucate and trioleate oils (Lorenzo's oil). Neurochem Res 19:1073–1082 [DOI] [PubMed] [Google Scholar]
- Roerig P, Mayerhofer P, Holzinger A, Gartner J (2001) Characterization and functional analysis of the nucleotide binding fold in human peroxisomal ATP binding cassette transporters. FEBS Lett 492:66–72 [DOI] [PubMed] [Google Scholar]
- Roscher A, Molzer B, Bernheimer H, Stockler S, Mutz I, Paltauf F (1985) The cerebrohepatorenal (Zellweger) syndrome: an improved method for the biochemical diagnosis and its potential value for prenatal detection. Pediatr Res 19:930–933 [DOI] [PubMed] [Google Scholar]
- Salomons GS, van Dooren SJ, Verhoeven NM, Cecil KM, Ball WS, Degrauw TJ, Jakobs C (2001) X-linked creatine-transporter gene (SLC6A8) defect: a new creatine-deficiency syndrome. Am J Hum Genet 68:1497–1500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarde CO, Mosser J, Kioschis P, Kretz C, Vicaire S, Aubourg P, Poustka A, Mandel JL (1994) Genomic organization of the adrenoleukodystrophy gene. Genomics 22:13–20 [DOI] [PubMed] [Google Scholar]
- Smith KD, Kemp S, Braiterman LT, Lu J-F, Wei H-M, Geraghty M, Stetten G, Bergin JS, Pevsner J, Watkins PA (1999) X-linked adrenoleukodystrophy: genes, mutations, and phenotypes. Neurochem Res 24:521–535 [DOI] [PubMed] [Google Scholar]
- Smith KD, Sack G, Beaty T, Bergin A, Naidu S, Moser A, Moser HW (1991) A genetic basis for the multiple phenotypes of X-linked adrenoleukodystrophy. Am J Hum Genet 49:165 [Google Scholar]
- Steinberg SJ, Corzo D, Gibson WT, Mithcell GA, Cox G, Cutting G, Boehm C, Tyson H, Watkins PA, Raymond GV, Moser AB, Moser HW (2001) Large 5′ deletions in the X-linked adrenoleukodystrophy gene, ABCD1, in two patients with a novel neonatal phenotype. Am J Hum Genet Suppl 69:A1823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg SJ, Kemp S, Braiterman LT, Watkins PA (1999a) Role of very-long-chain acyl-coenzyme A synthetase in X-linked adrenoleukodystrophy. Ann Neurol 46:409–412 [DOI] [PubMed] [Google Scholar]
- Steinberg SJ, Wang SJ, Kim DG, Mihalik SJ, Watkins PA (1999b) Human very-long-chain acyl-CoA synthetase: cloning, topography, and relevance to branched-chain fatty acid metabolism. Biochem Biophys Res Commun 257:615–621 [DOI] [PubMed] [Google Scholar]
- Tanaka K, West-Dull A, Hine DG, Lynn TB, Lowe T (1980) Gas-chromatographic method of analysis for urinary organic acids. II. Description of the procedure, and its application to diagnosis of patients with organic acidurias. Clin Chem 26:1847–1853 [PubMed] [Google Scholar]
- Wanders RJA, Barth PG, Heymans HSA (2001) Single peroxisomal enzyme deficiencies. In: Scriver CR, Beaudet AL, Sly WS, Valle D (eds) The metabolic and molecular bases of inherited diseases, 8th edition. McGraw-Hill, New York, pp 3219–3256 [Google Scholar]
- Wanders RJ, Kos M, Roest B, Meijer AJ, Schrakamp G, Heymans HS, Tegelaers WH, van den Bosch H, Schutgens RB, Tager JM (1984) Activity of peroxisomal enzymes and intracellular distribution of catalase in Zellweger syndrome. Biochem Biophys Res Commun 123:1054–1061 [DOI] [PubMed] [Google Scholar]
- Wanders RJ, van Roermund CW, van Wijland MJ, Schutgens RB, van den Bosch H, Schram AW, Tager JM (1988) Direct demonstration that the deficient oxidation of very long chain fatty acids in X-linked adrenoleukodystrophy is due to an impaired ability of peroxisomes to activate very long chain fatty acids. Biochem Biophys Res Commun 153:618–624 [DOI] [PubMed] [Google Scholar]
- Watkins PA, Gould SJ, Smith MA, Braiterman LT, Wei HM, Kok F, Moser AB, Moser HW, Smith KD (1995a) Altered expression of ALDP in X-linked adrenoleukodystrophy. Am J Hum Genet 57:292–301 [PMC free article] [PubMed] [Google Scholar]
- Watkins PA, McGuinness MC, Raymond GV, Hicks BA, Sisk JM, Moser AB, Moser HW (1995b) Distinction between peroxisomal bifunctional enzyme and acyl-CoA oxidase deficiencies. Ann Neurol 38:472–477 [DOI] [PubMed] [Google Scholar]
- Yamaguchi S, Iga M, Kimura M, Suzuki Y, Shimozawa N, Fukao T, Kondo N, Tazawa Y, Orii T (2001) Urinary organic acids in peroxisomal disorders: a simple screening method. J Chromatogr B Biomed Sci Appl 758:81–86 [DOI] [PubMed] [Google Scholar]
- Zenger-Hain J, Craft DA, Rizzo WB (1992) Diagnosis of inborn errors of phytanic acid oxidation using tritiated phytanic acid. In: Coates PM, Tanada K (eds) New developments in fatty acid oxidation, Wiley-Liss, New York, pp 339–407 [PubMed] [Google Scholar]