Abstract
Background
Optical coherence tomography (OCT) revealed that cells lining proximal convoluted tubules of living donor kidneys (LDKs) procured by laparoscopic procedures were very swollen in response to the brief period of ischemia experienced between the time of arterial vessel clamping and flushing the excised kidney with cold preservation solution. Damage to the tubules as a result of this cell swelling resulted in varying degrees of acute tubular necrosis (ATN) that slowed the recovery of the donor kidneys during the first 2 weeks after their transplantation.
Methods
To prevent this cell damage during LDK procurement, we changed the protocol for intravenous administration of mannitol (i.e., 12.5 or 25 g) to the donor. Specifically, we reduced the time of mannitol administration from 30 to 15 min or less before clamping the renal artery.
Result
OCT revealed that this change in the timing of mannitol administration protected the human donor proximal tubules from normothermic-induced cell swelling. An evaluation of posttransplant recovery of renal function showed that patients treated with this modified protocol returned to normal renal function significantly faster than those treated with mannitol 30 min or more before clamping the renal artery.
Conclusion
Because slow graft recovery in the first weeks after transplantation represents a risk factor for long-term graft function and survival, we believe that this change in pretreatment protocol will improve renal transplants in patients receiving LDK.
Keywords: Tubular Necrosis, Pathology, Imaging, Living donor transplantation
End-stage renal disease (ESRD) is associated with both high mortality rates and an enormous economic burden (1). The treatment of choice for ESRD that can extend patients’ lives and improve quality of life is kidney transplantation. Today, most kidneys for transplantation are obtained from either heart-beating cadavers (HBC) or living donors (LDK). Advantages to receiving a living donor kidney (LDK) over a deceased donor include decreased rejection rates and overall improved graft survival. A major reason that LDKs have improved survival is that they are subjected to a shorter period of cold storage preservation before their transplant (i.e., 30 min-2 hr), whereas HBC kidneys are cold stored for longer periods before their transplant (i.e., up to 36 hr). However, unlike most HBC kidneys, LDKs are subjected to an initial normothermic ischemic insult because of the time it takes between clamping the donor’s renal artery, excising the kidney from the donor, and finally flushing it with a cooled renal preservation solution. Although relatively short (minutes), in this investigation, we observed that the brief normothermic ischemia experience during laparoscopic procedures to procure the donor kidney is nevertheless enough to cause renal tubular damage and result in slow graft recovery.
In recent investigations, we have been using optical coherence tomography (OCT) to evaluate the histopathology of kidneys from living donors before and immediately after their transplantation (2). OCT is a rapidly emerging imaging modality that can function as a type of “optical biopsy,” providing noninvasive images of tissue morphology in situ and in real time (3, 4). As reported in this investigation, OCT revealed that immediately after their laparoscopic procurement (i.e., excision and subsequent flushing with preservation solution), the proximal convoluted from the kidneys of living donors are swollen and exhibit closed tubular lumens characteristic of acute tubular necrosis (ATN).
One of the compounds commonly administered to the donors of living kidneys before organ procurement is mannitol. Mannitol’s purported protective effects including increasing renal blood flow, decreasing intravascular cellular swelling, and free radical scavenging have been questioned (5). In most protocols, mannitol is infused 30 min or more before clamping the renal artery. However, previous studies by others and us using animal models have indicated that mannitol must be delivered 15 min before a normothermic ischemic insult in order to prevent ischemic-induced ATN (6, 7). In this study, we report that reducing the time interval between mannitol administration and clamping the renal artery to 15 min or less during laparoscopic procurement of LDK will protect living human donor kidneys from normothermic ischemic-induced cell swelling (i.e., ATN) and result in improved posttransplant renal function immediately after transplant. With accumulating evidence indicating that slow graft recovery in the first week after transplantation represents a risk factor for long-term graft function and survival (8–10), a procedure that minimizes normothermic insult during donor kidney procurement would improve LDK outcomes.
RESULTS
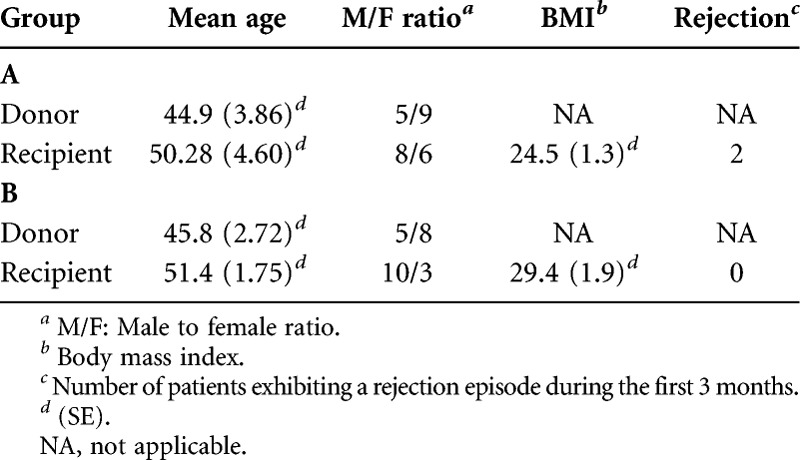
OCT imaging revealed a correlation between the presence of open proximal convoluted tubules in donor kidneys before transplantation, the timing of mannitol administration to the living donor, and posttransplant renal function. When the donor received mannitol (12.5–25 g iv,) 15 min or less before clamping the renal artery (i.e., Group A), the proximal convoluted tubules exhibited open lumens before transplant (Fig. 1A) with tubule diameters measuring ≈50 (±20) microns in diameter. The mean posttransplant renal function of individuals in Group A (i.e., 14 transplanted kidneys) was within the normal range (i.e., ≤1.4 mg/dL) within 2 days after transplant (Fig. 2). When, however, the donor received the same dose of mannitol 30 min or more before clamping the renal artery (i.e., Group B), cells lining the proximal convoluted tubules were swollen resulting in totally occluded tubule lumens immediately before their transplant (Fig. 1B). The appearance of the tubules was similar on both sides and both poles of the kidneys. Figure 1A and B are representative OCT images of the kidneys imaged in Groups A and B, respectively. The mean posttransplant renal function of individuals in Group B (i.e., 13 transplanted kidneys) did not return to within the normal range (i.e., ≤1.4 mg/dL) until 13 days after transplant (Fig. 2). The differences between patients in groups A versus B were significantly different on every day during the first week after transplant (i.e., P<0.05). Three-month data from the patients indicated a mean serum creatinine value of 1.13 mg/dL (± 0.06) for patients in Group A and versus 1.32 mg/dL (±0.11) for patients in Group B. Although the difference between the groups is not statistically different (i.e., P=0.16), five of the patients (i.e., 39%) in Groups B exhibited serum creatinine levels higher than normal (i.e., >1.4 mg/dL), whereas all the patients in Group A remained below 1.4 mg/dL at 3 months. Table 1 summarizes patient information for donors and recipients in Groups A and B. As noted in Table 1, the mean ages of the donors in both groups were nearly identical (i.e., 44.9 vs. 45.8 in Groups A and B, respectively), and the mean ages of the recipients were also nearly identical (i.e., 50.8 vs 51.4 in Groups A and B, respectively). Although the gender of the donors in Groups A versus B was similar, Group A had three more females receiving kidneys than Group B. The BMI (i.e., body mass indices) of patients in Groups B were higher than those in Group A (i.e., 24.5 vs. 29.4 for Groups A and B, respectively). Four patients in Group B were considered “obese” (i.e., BMI >30) versus only two in Group A. Nevertheless, only one of the patients in Group B that was categorized as “obese” was in the group with elevated serum creatinine values at the end of 3 months after transplantation. Therefore, obesity cannot be considered to be a significant factor affecting the long-term higher than normal serum creatinine levels of 4 of the 5 patients in Group B. No patients exhibited signs of rejection during the first 2 weeks after transplant. However, during the next two and a half months (i.e., up to 3 months after transplant), two patients in Group A exhibited signs of rejection that required additional therapy. There were no differences between Groups A versus B in the standard immunosuppression induction protocols after transplant that involved the use of alemtuzumab or basiliximab in combination with steroids.
FIGURE 1.
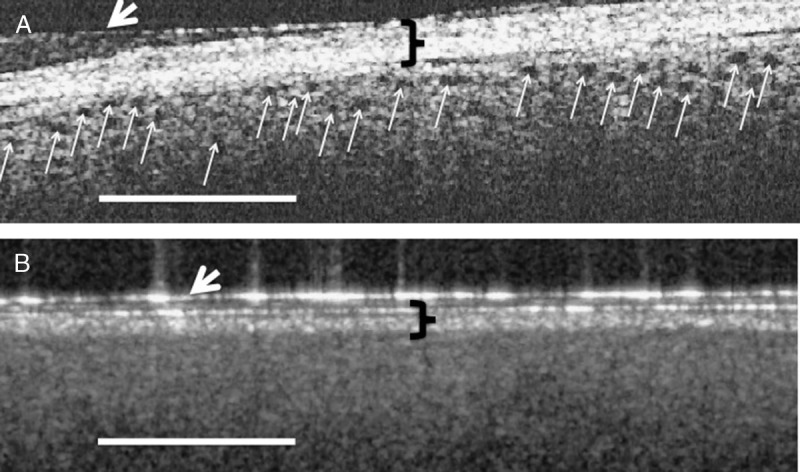
A, An OCT image of a donor kidney that received mannitol 15 min or less before clamping the renal artery. The black holes (indicated by the small white arrows) represent open tubules just under the kidney capsule. B, An OCT image of a donor kidney that received mannitol 30 min or more before clamping the renal artery. There are no open tubules in B because of occluding of the tubule lumens with swollen cells. The patient receiving the donor kidney seen in A exhibited a return to normal serum creatinine levels within 2 days, whereas the patient receiving the donor kidney seen in B did not recover to within normal serum creatinine values (i.e., <1.4 mg/dL) until nearly two weeks after transplant. The black brackets indicate the kidney connective tissue capsule, whereas the white arrows indicate a layer of Tegaderm. Bars= 500 μM.
FIGURE 2.
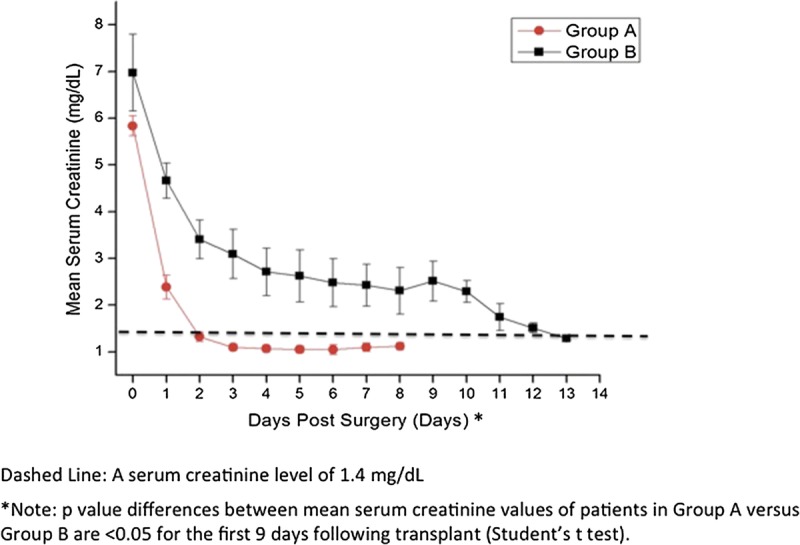
Graph comparing the mean posttransplant serum creatinine levels (±SEs) of individuals who received mannitol 15 min or less before clamping the renal artery (Group A, red ovals-red lines), with those of individuals who received mannitol 30 min or more before clamping the renal artery (Group B, black squares-black line). Individuals in Group A returned to within normal values (i.e., <1.4 mg/dL, indicated by dashed line) within 48 hours after transplant, whereas most in Group B remained elevated for 12 days after transplant.
TABLE 1.
Patient information
DISCUSSION
In this investigation, we studied the histopathologic and posttransplantation effects of administering intravenous mannitol within 15 min before clamping the renal artery during the laparoscopic procurement of kidneys from LKDs. Our findings indicate that this change will protect the kidney tubules from ATN and improve immediate posttransplant renal function. As noted previously, there is accumulating evidence that ATN during the first weeks after transplantation represents a significant risk for eventual graft and patient survival (8–10). The proximal convoluted tubules, which make up the vast majority of the renal cortex, swell rapidly in response to renal ischemia (11–13). In previous studies of rodent kidneys, we observed that when the renal artery is clamped, proximal convoluted lumens appeared occluded with swollen lining cells in approximately 30 sec (11). In human donor kidneys, the time elapsing between clamping the renal artery and flushing with a protective renal preservation solution is variable but exceeds 1 min. Once the tubules swell, flushing the kidneys with a renal preservation solution does not reverse the process. However, the preservation solutions will prevent further cell swelling and subsequent cell damage. This normothermic ischemic-induced swelling is a result of the highly metabolically active cells that make up the proximal tubules taking up water after an influx of small ions as a result of establishing a Gibbs-Donnan equilibrium (14). That is, the cells become increasing hypertonic because of an influx of small ions that together with impermeable larger intracellular colloids (mostly proteins and organic phosphates) renders the intracellular environment hypertonic. Water moves into the cells along an osmotic gradient resulting in cell swelling. As a result of this swelling, the tubule lumens diminish in size and eventually become entirely occluded with the swollen lining cells. With increasing ischemia, the cells can eventually rupture and the tubular lumens become filled with cytoplasmic debris. It is this ischemic induced swelling and damage to renal cells that results in postischemic ATN.
Why then were some proximal tubules protected from this brief, albeit very damaging renal ischemia, by changing the timing of mannitol infusion to the donor? Mannitol, like the major ingredients in renal preservation solutions, acts as an impermeant osmotic agent and prevents cell swelling associated with renal ischemia. Indeed, mannitol was the major protective impermeant osmotic agent in one of the first effective renal preservations solutions (i.e., Sacks solution) (15). The mechanism by which renal preservation solutions protect kidneys is by surrounding the uriniferous tubules with impermeant osmotic agents that will counter the build-up of small molecules within the ischemic cells (i.e., counter the osmotic driven movement of water into the cells by creating a more hypertonic environment surrounding the cells) (16). Previously, Collins et al. (6) and we in a later study (7) described the importance of timing in the ability of mannitol to provide protection against normothermic ischemia in animal models. Using a rabbit model of normothermic renal ischemia, Collins et al. (6) reported that if administered 30 min or more before normothermic ischemia, mannitol had no protective effects. However, when delivered within 15 min of normothermic ischemia, the protection is dramatically improved. We also reported the same time-dependent effect of mannitol in rat models of normothermic ischemia (7, 17).
Unlike living donor kidneys, kidneys from HBC are flushed with preservation solution with little or no interruption in blood flow. As a result, the uriniferous tubule lining cells are not subjected to normothermic ischemia, and their lumens remain patent (i.e., open) immediately after flushing. However, over time, the lining cells continue to swell, albeit slowly before their transplantation. We previously reported that this gradual swelling using a animal model is a good measure of posttransplant renal function (13). We have also noted similar swelling over time in human cadaver kidneys that were perfused with preservation solutions (unpublished observations).
In addition to immediate posttransplant function, we evaluated the donor kidneys 3 months after their transplant. Although the patients in Group A had a lower mean serum creatinine levels than patients in Group B (i.e., 1.13 vs. 1.32), this difference was not statistically significant. However, five patients in Group B had serum creatinine levels higher than normal, whereas all patients in Groups A had serum creatinine levels within the normal range (i.e., ≤1.4 mg/dL). Because obesity has been indicated to be a possible factor that can increase DRF (18), it is important to note that only one of the five patients in Group B with 3-month elevated serum creatinine levels had a BMI higher than 30 and could be considered obese. We do not have data on the patients beyond 3 months, but it is important to monitor these patients over time to determine their long-term renal outcomes.
It should be noted that our studies were limited to living donor kidneys. The kidneys obtained from HBC are more at risk for both DGF and nonfunction due mainly to the greater ischemic insult that they are subjected to. It is our goal to study HBC in the future and determine the usefulness of OCT imaging in predicting the status of these kidneys.
In summary, we have provided evidence indicating that shortening the interval between mannitol administration to living donor kidneys and clamping the renal artery to 15 min or less will improve posttransplant renal function by protecting the kidney from normothermic ischemia. Because rapid recovery of renal function represents a risk factor for long term graft function and survival (8–10), this change in pretreatment protocol for the timing of mannitol administration can have a significant impact on improving renal transplants in patients receiving kidneys from living donors.
MATERIALS AND METHODS
Histopathologic Observations Using Optical Coherence Tomography
Living human donor kidneys were imaged using OCT while being stored in a sterile ice bath immediately after being flushed with cold preservation solution (i.e., UW solution). The study was approved by the IRB, and consents were obtained for all patients (IRB Protocol No. 2010-396). The schematics of the Fourier-domain OCT system used in our studies are shown in previous publications (2, 17). To maintain sterility, the hand-held OCT imaging probe and the six-foot length of cords connecting to the imaging system were covered with a sterile sleeve, similar to that used for ultrasound imaging. A 1.5 cm circular hole was cut in the end of the sleeve to permit passage of the laser signal. The circular hole in the sterile sleeve was then covered with sterile, adhesive, transparent “Tegaderm” film (3M Health Care, St. Paul MN). The Tegaderm film did not significantly impede the OCT beam, provided a barrier to moisture and a sterile interface between the OCT imaging probe and the kidney being imaged. Imaging the entire surface of the harvested donor kidney ex vivo (i.e., both sides and all poles) took between 2 and 4 min and provided a holistic evaluation of the pretransplant organ. All the donor kidneys were imaged, and the imaging data recorded and stored. The stored images were password protected and only available to the IRB approved members of the research team.
Living Donor Kidney Nephrectomies
All the living donor nephrectomies were performed using laparoscopic procedures. The laparoscopic procedures took between 2 and 3 hours to perform. Intra-abdominal pressures were maintained at less than 15 mmHg throughout all the procedures. There were no periods of intraoperative hypotension during kidney retrieval and the donors were all making urine before the cross-clamping.
Patient Population
There were no restrictions on the patient population other than that they are adults suffering from renal failure and in need of a kidney transplant. Thirteen patients received the original protocol and were designated as Group B, whereas 14 patients received the modified protocol and were designated as Group A. The demographics of the patient population including sex, age, body mass indices, and episodes of rejection are summarized in Table 1.
Mannitol Infusion
Original Protocol (Group B)
Intravenous mannitol (12.5–25 g) is infused once the patient is positioned before incision, then repeated 30 min before arterial clamping.
Modified Protocol (Group A)
Intravenous mannitol (12.5–25 g) is infused once the patient is positioned before incision, then repeated 5 to 15 min before arterial clamping.
In both protocols, the mean time between initial mannitol infusion and the repeated infusion before arterial clamping was approximately 2 hours (±30 min). In both protocols, furosemide (Lasix) was given concurrently with the mannitol at a dose of 20 to 40 mg.
Posttransplant Renal Function
Presurgical serum creatinine levels were recorded for all patients. Posttransplant serum creatinine levels were followed for 2 weeks or until they reached normal levels (i.e., ≤1.4 mg/dL). Patient data was again reviewed 3 months after transplant to evaluate renal function and signs of rejection.
Statistics
Student’s t test was used to determine significant differences between groups, with a P value of less than 0.05 being considered statistically significant. Values are presented as mean ± the standard error (SE).
ACKNOWLEDGMENTS
The authors thank Dr. James Jiang and Alex Cable from Thorlabs, Inc., for technical support.
Footnotes
The authors declare no funding or conflicts of interest.
All authors participated in the performance of the research.
REFERENCES
- 1.United States Renal Data System Annual Data Report. 2009. [Google Scholar]
- 2. Andrews PM, Chen Y, Wierwille J, et al. Using OCT to predict post-transplant renal function. Proc SPIE 8565. 2013 Mar: 856511. [Google Scholar]
- 3. Fujimoto JG. Optical coherence tomography for ultrahigh resolution in vivo imaging. Nat Biotechnol 2003; 21: 1361. [DOI] [PubMed] [Google Scholar]
- 4. Hee MR, Izatt JA, Swanson EA, et al. Optical coherence tomography of the human retina. Arch Ophthalmol 1995; 113: 325. [DOI] [PubMed] [Google Scholar]
- 5. Power NE, Maschino AC, Sagage C, et al. Intraoperative mannitol use does not improve long-term renal function outcomes after minimally invasive partial nephrectomy. Urology 2012; 79: 821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Collins GM, Green RD, Boyer D, et al. Protection of kidneys from warm ischemic injury. Dosage and timing of mannitol administration. Transplantation 1980; 29: 83. [DOI] [PubMed] [Google Scholar]
- 7. Andrews PM, Bates SB. Improving Euro-Collins flushing solution’s ability to protect kidneys from normothermic ischemia. Miner Electrolyte Metab 1985; 11: 309. [PubMed] [Google Scholar]
- 8. Johnston O, O’Kelly P, Spencer S, et al. Reduced graft function (with or without dialysis) vs immediate graft function–a comparison of long-term renal allograft survival. Nephrol Dial Transplant 2006; 21: 2270. [DOI] [PubMed] [Google Scholar]
- 9. Gourishankar S, Jhangri GS, Cockfield SM, et al. Donor tissue characteristics influence cadaver transplant function and graft survival but not rejection. JASN 2003; 14: 493. [DOI] [PubMed] [Google Scholar]
- 10. Humar A, Johnson EM, Payne WD, et al. Effect of initial slow graft function on renal allograft rejection and survival. Clin Transplant 1997; 11: 623. [PubMed] [Google Scholar]
- 11. Andrews PM, Petroll WM, Cavanagh HD, et al. Tandem scanning confocal microscopy (TSCM) of normal and ischemic living kidneys. Am J Anat 1991; 191: 95. [DOI] [PubMed] [Google Scholar]
- 12. Andrews PM. Noninvasive vital microscopy to monitor tubular necrosis of cold-stored kidneys. Transplantation 1994; 57: 1143. [DOI] [PubMed] [Google Scholar]
- 13. Andrews PM, Khirabadi BS, Bengs BC. Using tandem scanning confocal microscopy to predict the status of donor kidneys. Nephron 2002; 91: 148. [DOI] [PubMed] [Google Scholar]
- 14. Leaf A. Maintenance of concentration gradients and regulation of cell volume. Ann N Y Acad Sci 1959; 72: 396. [DOI] [PubMed] [Google Scholar]
- 15. Sacks SA, Petritsch PH, Kaufman JJ. Canine kidney preservation using a new perfusate. Lancet 1973; 1: 1024. [DOI] [PubMed] [Google Scholar]
- 16. Andrews PM, Coffey AK. Factors that improve the preservation of nephron morphology during cold storage. Lab Invest 1982; 46: 100. [PubMed] [Google Scholar]
- 17. Wierwille J, Andrews PM, Onozato ML, et al. In vivo, label-free, three-dimensional quantitative imaging of kidney microcirculation using Doppler optical coherence tomography. Lab Invest 2011; 91: 1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cannon RM, Jones CM, Hughes MG, et al. The impact of recipient obesity on outcomes after renal transplantation. Ann Surg 2013; 257: 978. [DOI] [PubMed] [Google Scholar]


