To the Editor
Cutis laxa (CL) is a heterogeneous group of disorders characterized by loose, redundant, inelastic or prematurely wrinkled skin (Berk et al., 2012; Uitto et al., 2013). Several inherited forms of CL have been identified (Urban and Davis, 2013), with 9 causative genes known to date (ALDH18A1, ATP6V0A2, ATP7A, EFEMP2/FBLN4, ELN, FBLN5, LTBP4, PYCR1, RIN2). A shared feature of all types of inherited CL is a reduced or abnormal deposition of elastic fibers in the skin and other tissues (Berk et al., 2012). The consequences of this disorder for the biomechanics of the skin has received little attention (Grahame and Beighton, 1971). Such studies are essential for a fundamental understanding of the contribution of elastic fibers to the mechanical properties of the skin, and for an improved, objective diagnosis of cutis laxa.
In the present study, 118 participating controls and 17 CL patients were included with written informed consent. The same control cohort was used in a concurrent study on the mechanical properties of the skin in Williams-Beuren syndrome (Kozel et al., 2014). The IRB committees at Washington University School of Medicine and the University of Pittsburgh approved the studies. Age and sex were not significantly different between cases and controls (Table 1). The CL group comprised individuals diagnosed with cutis laxa based on physical examination and medical history. Eight individuals of the CL group were positive for mutations in known CL genes with LTBP4 mutations in 2, ELN mutations in 3 and ATP6V0A2 mutations in 3 patients (Table S1). In addition, we included 9 patients with unknown mutational status (Table S2): 3 individuals with congenital and 6 subjects with late-onset CL. As skin elasticity measurements did not show consistent differences between CL subgroups, we pooled all types of CL into one case group.
Table 1. Demography and elasticity parameters in the participants.
| Controls (n=118) | Patients (n=17) | p-value | |
|---|---|---|---|
| Age | 33.22 ± 1.58 | 29.21 ± 5.47 | 0.490* |
| Gender (male %) | 37.3% | 35.3% | 0.874** |
| E (MPa) | 11.61 ± 0.15 | 7.85 ± 0.60 | < 0.0001* |
| Retraction time (ms) | 622.82 ± 21.20 | 1152.82 ± 211.21 | 0.024* |
| VE (MPa) | 5.35 ± 0.14 | 2.51 ± 0.32 | < 0.0001* |
Abbreviations: E, elastic modulus; VE, viscoelastic modulus.
Continuous variables: mean ± standard error mean
independent t-test;
Chi-square test
Cases and controls underwent testing using a DermaLab® skin elasticity module, a suction cup device extensively validated in previous studies (Anthonissen et al., 2013; Gandanke et al., 2014; Grove et al., 2006; Pedersen et al., 2003). The device applies vacuum to a patch of skin and measures the pressure difference (ΔP) required to raise the skin to a height of 1.5 mm and the time required for the skin to return to the original position (retraction time, RT). The elastic modulus (E) was calculated from this pressure difference, assuming uniform skin thickness (1 mm). In addition, a viscoelastic modulus (VE) was computed from E and RT as variables (Supplementary Materials and Methods).
Cases had significantly lower E, higher RT, and lower VE (all p < 0.05) than controls (Figure S1, Table 1). In controls, RT and VE were significantly correlated with age, but E was not (Table S3). In cases, VE was marginally correlated with age (Table S3). Multivariate logistic regression analysis revealed that age (p=0.005, odds ratio (OR): 1.21, 95% confidence interval (CI): 1.06-1.37), VE (p=0.002, OR: 16.39, 95% CI: 2.85-95.18) and E (p=0.043, OR: 3.55, 95% CI: 1.04-12.15) were significant predictors of disease status. VE was the strongest predictor and one unit reduction in VE increased the odds of CL 16.39-fold.
We analyzed the receiver operating characteristic (ROC) to evaluate the utility of E, RT and VE as predictors. Of the three, VE performed best, with ROC area under curve (AUC) reaching 0.908 when applied to the entire study population (Figure 1a). Restricting the analysis to individuals younger than 47 years (bottom 75 percentile of the control group) yielded some improvement in the AUC (AUC = 0.964, Figure S2).
Figure 1. ROC analysis of biomechanical variables and viscoelastic modulus in relation to the causative gene mutation.
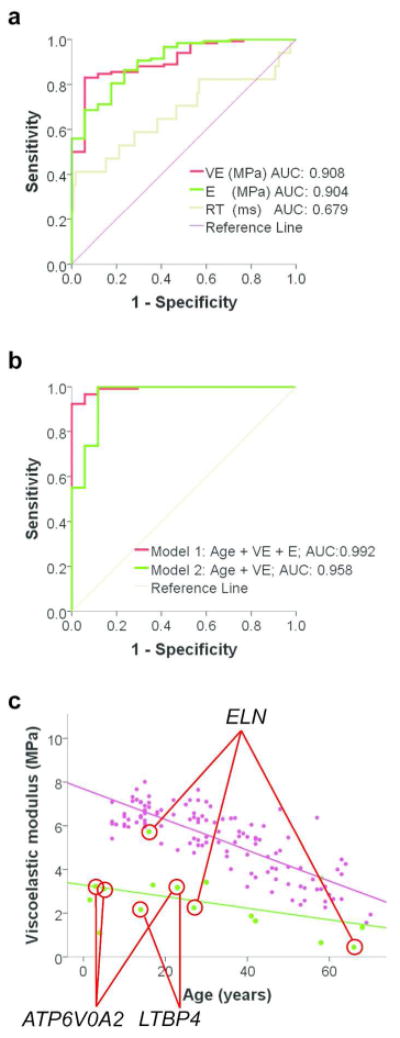
(a) Viscoelastic modulus is more effective than elastic modulus or retraction time in differentiating cases from controls as indicated by receiver operating characteristic (ROC) curves. (b) Composite variables under Model 1 (Age + VE + E) perform better than Model 2 (Age + VE). (c) VE values of individuals with known gene mutations are identified by red tie-lines. Controls are represented by magenta dots and CL cases by green dots. Linear regression lines are shown in each group (cases: green, controls: magenta). Note that one individual with ATP6V0A2-related CL had similar age and VE data to another participant with LTBP4-related CL, resulting in overlapping data points.
We next evaluated if an additional variables improved on VE only as a diagnostic measures in the entire study population. Two models were considered. Model 1 incorporated VE, age and E, as suggested by the logistic regression model described above. Model 2 included VE and age only. Model 1 (AUC=0.992) performed significantly (p=0.0026, ANOVA) better than Model 2 (AUC=0.958) in distinguishing cases from controls (Figure 1b).
The performance of a model on the same data that was used to fit that model can give an overly optimistic measure of performance. To test if such overfitting had occurred, cross-validation tests were performed. As the cross-validation, on average, resulted in only a modest decrease in AUC values (Table S4), overfitting was not a major issue in this analysis, and similar sensitivity and specificity values can be expected in future replication studies to our present results.
A diagnostic variable (D) can be calculated from the ROC analysis using the following device-specific formula: D = −27.570 + 0.187 × Age + 2.795 × VE + 1.267 × E, where Age is in years, VE is the viscoelastic modulus and E is the elastic modulus, both in MPa units. If an individual satisfies the inequality of D < 2.538, the probability of the individual having CL is 99.2%. This cutoff value of D distinguishes cases from controls with 100% specificity and 91.5% sensitivity, supporting the use of the DermaLab® device for the objective, specific and sensitive diagnosis of CL in future studies.
The skin was visually loose in individuals with cutis laxa and decreased VE values (Figure S3). Although there were insufficient number of individuals within each type of inherited cutis laxa to allow for subgroup analysis, individuals with autosomal dominant cutis laxa (ADCL) caused by ELN mutations showed the greatest degree of variation in VE (Figure 1b) compared to individuals with other mutations, consistent with previous reports of variable expression of the skin phenotype in ADCL patients (Szabo et al., 2006).
Our studies show significant reduction of the elastic (E) and viscoelastic moduli (VE) and significant increase of the retraction time (RT) of the skin of CL patients irrespective of the etiology of the disease. Individuals with acquired or late onset CL had similar reductions in VE compared to controls as individuals with ELN, LTBP4 or ATP6V0A2 mutations, suggesting that the disruption of elastic fibers leads to similar biomechanical alterations independent of the precise molecular disease mechanisms. VE showed significant inverse correlation with age in both CL and control individuals, but in controls the decline started from a higher level and was thus steeper. Therefore, VE appears to be a good measure of biomechanical aging of the skin, and our observations suggest that CL results in similar changes in skin mechanics to aging. VE also offers the best specificity and sensitivity in distinguishing cases from controls among the individual variables measured in our study.
To date, only one study investigated the mechanics of the skin in CL (Grahame and Beighton, 1971). As this early report had few cases and controls (6 each), and only measured E but not retraction, it did not find significant difference between cases and controls. In contrast, the present report demonstrates the utility of biomechanical measurements in CL.
Supplementary Material
SupplementaryDiscussion
Supplementary Material and Methods
Supplementary References
Supplementary Table S1. CL cases with known mutations
Supplementary Table S2. CL cases with unknown mutations
Supplementary Table S3. Pearson's correlation (r) among age and dermal elasticity parameters
Supplementary Table S4. Results of the cross-validation study
Supplementary Figure S1. Mechanical properties of the skin in controls and individuals with CL.
Supplementary Figure S2. ROC analysis of biomechanical variables in individuals 47 years old or younger.
Supplementary Figure S3. Viscoelastic modulus in relation to visual skin laxity.
Acknowledgments
We thank the subjects of this study for their participation, and Dr. Daniel E. Weeks for statistical advice. This study was funded in part by NIH grants HL090648 (ZU) and UL1TR000005 (FCS, ZU). Funding was provided to Dr. Kozel by the Children's Discovery Institute of Washington University and St. Louis Children's Hospital. In addition, Dr. Kozel received funding for the study through her appointment as a scholar of the Child Health Research Center in Developmental Biology (NIH K12-HD01487) and the Genetic Basis of Inflammatory Airway Disease (NIH K12-HL089968).
Footnotes
Conflict of Interest Statement: The authors declare no conflict of interest.
References
- Anthonissen M, Daly D, Fieuws S, et al. Measurement of elasticity and transepidermal water loss rate of burn scars with the Dermalab((R)) Burns. 2013;39:420–8. doi: 10.1016/j.burns.2012.07.026. [DOI] [PubMed] [Google Scholar]
- Berk DR, Bentley DD, Bayliss SJ, et al. Cutis laxa: a review. J Am Acad Dermatol. 2012;66:842 e1–17. doi: 10.1016/j.jaad.2011.01.004. [DOI] [PubMed] [Google Scholar]
- Gandanke TU, Duke JM, Danielsen PL, et al. Reliability of scar assessments performed with an integrated skin testing device - the DermaLab Combo. Burns. 2014 doi: 10.1016/j.burns.2014.01.025. [DOI] [PubMed] [Google Scholar]
- Grahame R, Beighton P. The physical properties of skin in cutis laxa. The Br J Dermatol. 1971;84:326–9. doi: 10.1111/j.1365-2133.1971.tb14227.x. [DOI] [PubMed] [Google Scholar]
- Grove GL, Damia J, Grove MJ, et al. Suction chamber method for the measurement of skin mechanics: the DermaLab. In: Serup J, Jemec GBE, Grove GL, editors. Handbook of non-invasive methods and the skin. 2. Boca Raton, FL: CRC Press; 2006. pp. 593–9. [Google Scholar]
- Kozel BA, Bayliss SJ, Berk DR, et al. Skin findings in Williams-Beuren syndrome. Am J Med Genet A. 2014 doi: 10.1002/ajmg.a.36628. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen L, Hansen B, Jemec GB. Mechanical properties of the skin: a comparison between two suction cup methods. Skin Research and Technology. 2003;9:111–5. doi: 10.1034/j.1600-0846.2003.00021.x. [DOI] [PubMed] [Google Scholar]
- Szabo Z, Crepeau MW, Mitchell AL, et al. Aortic aneurysmal disease and cutis laxa caused by defects in the elastin gene. J Med Genet. 2006;43:255–8. doi: 10.1136/jmg.2005.034157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uitto J, Li Q, Urban Z. The complexity of elastic fibre biogenesis in the skin - a perspective to the clinical heterogeneity of cutis laxa. Exp Dermatol. 2013;22:88–92. doi: 10.1111/exd.12025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban Z, Davis EC. Cutis laxa: Intersection of elastic fiber biogenesis, TGFβ signaling, the secretory pathway and metabolism. Matrix Biol. 2014;33:16–22. doi: 10.1016/j.matbio.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SupplementaryDiscussion
Supplementary Material and Methods
Supplementary References
Supplementary Table S1. CL cases with known mutations
Supplementary Table S2. CL cases with unknown mutations
Supplementary Table S3. Pearson's correlation (r) among age and dermal elasticity parameters
Supplementary Table S4. Results of the cross-validation study
Supplementary Figure S1. Mechanical properties of the skin in controls and individuals with CL.
Supplementary Figure S2. ROC analysis of biomechanical variables in individuals 47 years old or younger.
Supplementary Figure S3. Viscoelastic modulus in relation to visual skin laxity.


