Abstract
Parents of young children with type 1 diabetes (T1D) may experience poor sleep quality possibly impacting their confidence in T1D management. This study investigated sleep characteristics among parents of children with T1D and relationships amongst parents’ sleep quality, hypoglycemia worry, and diabetes self-efficacy. As part of baseline assessment for a randomized clinical trial (RCT) to promote parental management of T1D, 134 parents of children ≤ age 6 reported on demographics, parent sleep characteristics, hypoglycemia worry, and diabetes self-efficacy. Parents reported they slept less time than recommended by the National Sleep Foundation and endorsed greater global sleep problems than standardized norms of healthy adults; 1/3 of parents reported their overall sleep quality was “fairly bad” or “very bad.” Hypoglycemia worry and parents’ sleep quality were both significantly related to diabetes self-efficacy, but parents’ sleep quality did not mediate the relationship of hypoglycemia worry and diabetes self-efficacy. Many parents experience disrupted sleep that impacts their perceived ability to perform T1D management. Interventions designed to improve parental T1D self-efficacy should consider sleep and concerns about children’s hypoglycemia.
Keywords: type 1 diabetes, sleep, parents, young children
Introduction
Type 1 diabetes (T1D) is a common chronic illness that affects approximately 1 in every 400–600 youth in the United States under the age of 20 (SEARCH for Diabetes in Youth Study Group et al., 2006). The incidence of T1D among children under the age of 5 is rising, indicating that an increasing number of families are impacted by T1D diagnoses every year (Patterson, Dahlquist, Gyurus, Green, & Soltesz, 2009; Vehik et al., 2007). Parents of young children with T1D are responsible for the majority of their child’s T1D management, a complex and time consuming task that requires adherence to a T1D care regimen involving frequent blood glucose (BG) monitoring, insulin administration, and regulation of diet and physical activity (American Diabetes Association, 2013; Coffen & Dahlquist, 2009; Sullivan-Bolyai, Deatrick, Gruppuso, Tamborlane, & Grey, 2002). T1D management in young children is additionally challenging for physiological and developmental reasons: young children are more likely to experience BG fluctuations due, in part, to increased insulin sensitivity and unpredictable rates of carbohydrate intake and physical activity, making it difficult to establish T1D routines and recognize BG patterns (Golden, Russell, Ingersoll, Gray, & Hummer, 1985; Silverstein et al., 2005).
The impact of T1D care on parent functioning is extensive. Parents of both preschool and older age children with T1D regularly experience elevated levels of parenting stress, anxiety, and depression (Hansen, Weissbrod, Schwartz, & Taylor, 2012; Jaser, Whittemore, Ambrosino, Lindemann, & Grey, 2008; Monaghan, Hilliard, Cogen, & Streisand, 2011; Streisand et al., 2008; Streisand, Swift, Wickmark, Chen, & Holmes, 2005). One aspect of T1D care that may be particularly challenging is management of hypoglycemic episodes (low BG levels), during which children may experience confusion, disorientation, seizures, and/or loss of consciousness. Many parents of young children with T1D regularly express worry about hypoglycemia due to these potential acute symptoms, long-term neurocognitive effects, and the stress that may accompany treatment of hypoglycemic events (Bade-White & Obrzut, 2009). Among young children, detection and treatment of hypoglycemia is further complicated because children may be too young to verbally convey their physical symptoms and/or understand their medical cause (Sullivan-Bolyai, Deatrick, Gruppuso, Tamborlane, & Grey, 2002). Research suggests that parents of young children with T1D report high levels of hypoglycemia worry and may engage in hypoglycemia avoidance behaviors, such as intentionally maintaining higher than recommended BG levels (Barnard, Thomas, Royle, Noyes, & Waugh, 2010; Green, Wysocki, & Reineck, 1990; Patton, Dolan, Henry, & Powers, 2007, 2008).
Greater hypoglycemia worry is also related to greater parenting stress, which may contribute to parents’ varying degrees of diabetes self-efficacy; parents who experience elevated fear of hypoglycemia and parenting stress related to caring for children with T1D may perceive that they are less capable of managing their children’s T1D and consequently feel less confident in their T1D skills (Patton, Dolan, Smith, Thomas, & Powers, 2011; Streisand et al., 2005). Indeed, parents who report greater parenting stress and depressive symptoms tend to also report lower diabetes self-efficacy (Streisand et al., 2008; Streisand et al., 2005). There are mixed results regarding the impact of diabetes self-efficacy on diabetes management behaviors and glycemic control, with one study finding that maternal and youth diabetes self-efficacy were related to better glycemic control in adolescents due to a significant positive correlation with better disease management and another finding no relationship between self-efficacy and glycemic control (Herge et al., 2012; Marvicsin, 2008). Although additional research is needed to understand these associations, it is likely that the relationship among hypoglycemia worry, diabetes self-efficacy, and parent T1D management behaviors are intertwined.
In addition to stressors related to daytime T1D management and its concomitant effect on hypoglycemia worry, parents regularly engage in nighttime T1D management and report worry that children will experience a hypoglycemic episode while asleep; thus, many parents perform BG checks after their child is asleep, provide fast-acting carbohydrates before bedtime, or sleep with their children (Monaghan, Herbert, Cogen, & Streisand, 2012; Monaghan, Hilliard, Cogen, & Streisand, 2009; Patton et al., 2007; Sullivan-Bolyai, Deatrick, Gruppuso, Tamborlane, & Grey, 2003). Nighttime T1D activities regularly disrupt parent sleep, contribute to a shorter nightly sleep duration than recommended by the National Sleep Foundation, and are related to increased parenting stress and anxiety (McKnight-Eily et al., 2009; Monaghan et al., 2012; Monaghan et al., 2009).
Given that many parents of young children with T1D report hypoglycemia worry, stress about their child’s health at night, and T1D-related sleep disruption, it is likely that parents experience overall poor sleep quality, including shorter sleep duration, greater sleep disturbances, and sleep-related daytime dysfunction such as difficulty remaining awake and/or maintaining enthusiasm to complete tasks throughout the day. Poor sleep quality and related daytime dysfunction could impact parents’ competence as well as motivation to perform T1D care and follow a consistent schedule, their ability to make T1D decisions, and their perceptions of their diabetes self-efficacy, a phenomenon demonstrated among adults with type 2 diabetes (Chasens, Korytkowski, Sereika, & Burke, 2013; Chasens & Olshansky, 2008). Thus, one of the mechanisms by which hypoglycemia worry may decrease parents’ diabetes self-efficacy is that fear of nighttime hypoglycemia leads to poor sleep quality, and negatively impacts parents’ motivation and/or concentration to engage in optimal and consistent T1D care.
In order to understand the relationships among hypoglycemia worry, parent sleep, and diabetes self-efficacy, and to consider potential recommendations for clinicians and their patients, this study examined baseline data collected from parents of young children ages 1–6 years who participated in a randomized controlled trial (RCT) of a behavioral intervention designed to promote parental management of T1D in young children. The aims of the current study were to: 1) describe sleep characteristics among parents of young children with T1D, 2) investigate the relationship among hypoglycemia worry, parents’ sleep, and diabetes self-efficacy, and 3) determine if parents’ sleep quality mediates the relationship between hypoglycemia worry and diabetes self-efficacy. We hypothesized that parents of young children with T1D would report poorer sleep quality than standardized norms. We further hypothesized that parental hypoglycemia worry would be associated with overall parent sleep quality and diabetes self-efficacy, and. that the relationship between parent hypoglycemia worry and perception of diabetes self-efficacy would be mediated by parents’ sleep quality.
Methods
Participants
Parents of children with T1D were recruited from three tertiary care endocrinology services, two in the Mid-Atlantic region and one in the Midwest, to participate in a RCT investigating the impact of a behavioral intervention to promote parental management of T1D in young children. Primary caregivers of children between the ages of one and six years who had been diagnosed with diabetes for at least 6 months were eligible for participation. Parents who were not fluent in English or whose children were diagnosed with an additional major chronic illness or a developmental disorder were excluded from participation.
Two-hundred eighty-five parents were preliminarily identified as eligible and were mailed letters detailing the study. Of these parents, 66 were unable to be contacted, 16 did not meet inclusion criteria, and 36 declined to participate. One-hundred sixty-seven parents provided verbal consent to participate by phone. Thirty-three parents failed to complete baseline measures or written consent; thus, our final sample included 134 parents. There were no significant differences with respect to child age, sex, and illness duration (all ps > .05) between parents who agreed to participate (n=134) and parents who could not be reached, were not eligible, or did not agree to participate (n=151).
Parents had a mean age of 36.80 years (SD = 5.93) and were primarily female (90%), Caucasian (78%), and married (84%). Child mean age was 5.33 years (SD = 1.34) and 49% were female. Average hemoglobin A1c was 8.13% (SD = 0.88), which falls within the ADA recommendation that young children have a hemoglobin A1C of 8.50% or less. Most children (86%) were enrolled in school/daycare and, of these children, most attended a full day program (77%) five days a week (82%). Seventy-one percent of parents were employed either full time (45%) or part-time (26%). The ethnic diversity of our sample was comparable to US population estimates that indicate the highest rates of T1D are among non-Hispanic white youth (SEARCH for Diabetes in Youth Study Group et al., 2006). See Table 1 for additional demographic/medical information.
Table 1.
Demographic/Medical Information (n = 134)
| Percentage | M | SD | Range | |
|---|---|---|---|---|
| Child age (years) | 5.33 | 1.34 | 2.01–6.98 | |
| Child sex (% female) | 49.30 | |||
| Parent age (years) | 36.80 | 5.93 | 22.21–60.05 | |
| Parent sex (% female) | 89.50 | |||
| Parent ethnicity (% Caucasian) | 78.40 | |||
| Parent marital status (% married) | 83.50 | |||
| Household income (% ≥$50,000) | 76.10 | |||
| Illness duration (years) | 2.00 | 1.24 | 0.54–5.95 | |
| HbA1c | 8.13% | 0.88% | 6.40–11.00% | |
| Insulin regimen (% intensive) | 72.00 | |||
| Daily BG level (mg/dL)† | 180.42 | 54.63 | 67–336 | |
| Number of daily BG checks† | 5.78 | 2.39 | 2–14 |
Based on parent report on the 24-hour recall interview.
Procedure
Parents who met initial eligibility criteria were mailed a recruitment letter with details about the study and a postcard that could be returned if they did not wish to be contacted by the study team. Parents who did not return a postcard were contacted via phone by a trained research assistant approximately two weeks later, further screened for eligibility, provided additional study details, and completed verbal consent. Within one month of verbal consent, participants completed baseline questionnaires by phone, and within one month of baseline questionnaire completion, they met with a trained research assistant during their regularly scheduled clinic appointment to provide written consent, participate in an orientation to the overarching project, and allow for the attainment of glycemic control data. Participants were mailed a giftcard for completing baseline questionnaires. Each of the three recruitment sites completed eligibility screening and recruitment for patients at their site, met with parents for consent and the in-clinic orientation session, and accessed hemoglobin A1c and glucometer records. All baseline phone questionnaires were coordinated and completed by the primary study site. This study is based on baseline data only. This study was approved by each institution’s Institutional Review Board.
Measures
Demographic and medical questionnaire
The General and Medical Information Questionnaire, developed by the research team, assessed parent and child age, gender, race/ethnicity, parent education level, income level, marital status, child diabetes regimen, and illness duration.
Glycemic control
Hemoglobin A1C is the most widely accepted measure of average BG over the preceding 2–3 months (American Diabetes Association, 1994). Blood draws were performed by each site’s laboratory service phlebotomists as part of routine clinical care, using universal precautions as per hospital protocol. All assays were conducted with the DCA 2000 Analyzer, using high performance liquid chromatography to assure comparability between subjects (Tamborlane et al., 2005). All clinic appointments were less than one month after baseline questionnaire completion; thus, the measure of glycemic control chronologically aligned with baseline questionnaire data.
24-hour recall interview of diabetes management tasks
Parents provided detailed information regarding daily T1D-related management tasks by completing two 24-hour recall phone interviews. The 24-hour recall interview is a well-standardized tool for assessing diabetes management behaviors in children (Freund, 1991; Johnson, 1986), with excellent concordance rates between observers and youths, and has been successfully used solely with parents (Marvicsin, 2008). For the purposes of this study, two 24-hour recall interviews applicable for participants with children on any type of insulin regimen were conducted to assess glycemic variability (frequency of BG monitoring, BG excursions-i.e., frequency of BG values out of the recommended 70–200 mg/dL range, mean BG level), hypoglycemia (frequency of episodes of BG values < 70 mg/dL), and BG check contextual factors (time, who conducted the BG monitoring, if the child was asleep) via the BG monitoring module. The data from the two 24-hour recall interviews were comparable across interviews; thus, in order to preserve variability within the measure, data from the first 24-hour recall interview were used in analyses. However, if parents indicated the first interview day was atypical for their child, the second 24-hour recall interview was used instead.
Children’s mean daily BG level and average number of BG checks on the 24-hour recall interview were highly correlated with the 30 days of glucometer data that were downloaded at baseline for each participant: 180.42 mg/dL vs. 195.95 mg/dL, r(134) = .47, p < .001, 5.78 BG checks vs. 5.76 BG checks, r(134) = .74, p < .001; respectively. Parents were able to report on the 24-hour recall whether or not BG checks were conducted after their child had gone to sleep but these data were not obtainable from the 30-day glucometer downloads; thus, as this was information of primary importance to the study aims and these T1D management data from interviews and glucometers were highly correlated, 24-hour recall interview data were used for all analyses regarding children’s daily T1D management.
Pittsburgh Sleep Quality Index (PSQI)
Parent sleep was rated on the Pittsburgh Sleep Quality Index (Buysse, Reynolds, Monk, Burman, & Kupfer, 1989), a well-validated 19-item measure that assesses adult sleep disturbances during the past month. A global score assesses sleep quality using a range from “no difficulty” to “severe difficulty” and 7 subscales scores are generated. The first 4 items are open-ended questions that assess bed time, the amount of time it takes to fall asleep (sleep latency), wake time, and total hours of sleep. Subsequent items are rated on a 4-point Likert scale (0 = “not during the past month” to 3 = “three or more times a week”). The global scale ranges from 0–21 and subscale scores range from 0–3; higher scores reflect greater concerns with sleep habits and/or sleep quality. Validity and test-retest reliability estimates of the PSQI are good (Buysse et al., 1989). In the current sample, the PSQI achieved a Cronbach’s alpha of .67.
Six additional questions regarding sleep in the context of parenting a child with T1D were added in order to assess aspects of sleep that are specific to this sample. The first three questions asked parents to rate how often they had trouble sleeping due to attending to their child’s health care needs, stress related to their child’s health, and stress not related to their child’s health using the same 4-point Likert scale (Meltzer & Mindell, 2006). The final three additional questions asked parents, “How many nights in one week do you, on average, check your child’s BG level?” “Do BG checks disrupt your sleep? (yes/no),” and “Do BG checks disrupt your child’s sleep? (yes/no).”
Hypoglycemia Fear Survey-Parents of Young Children (HFS-PYC)
The Hypoglycemia Fear Survey-Parents of Young Children (Irvine & Saunders, 1989; Patton et al., 2007) is a 27-item measure that assesses parents’ fear about their child experiencing a low BG level, modified for young children from the original 21-item Hypoglycemia Fear Scale (Cox, Irvine, Gonder-Frederick, Nowacek, & Butterfield, 1987). The measure has two scales, a 16-item scale pertaining to worries about hypoglycemia and an 11-item scale assessing behaviors in which the parent engages in order to avoid hypoglycemia. Parents rate items on a 5-point Likert scale (1 = “never” to 5 = “always”). Scores are summed across each subscale with higher scores indicating greater hypoglycemia worries and avoidance behavior. Only the HFS-PYC Worry subscale was used in analyses for this study. In a sample of young children, Patton et al. (2007) reported acceptable internal consistency scores for the worry (α = .62) subscale and test-retest reliability has been strong, ranging from .73 to .91; internal consistency among this sample was good, α = .92.
Self-Efficacy for Diabetes Scale for Parents (SED-P)
Parents completed the Self-Efficacy for Diabetes Scale for Parents, a 22-item measure of parents’ confidence in their ability to manage their child’s T1D (Streisand et al., 2005), adapted from the original Self-Efficacy for Diabetes Scale (Grossman, Brink, & Hauser, 1987). Each item is rated on a 5-point Likert scale (1 = “very sure I can’t” to 5 = “very sure I can”), yielding a total score with a range of 22–110. Higher scores suggest parents have greater self-efficacy regarding their ability to care for their child’s T1D. Previous studies have found good internal consistency (α = .90), reliability, and construct validity estimates (Armstrong, Mackey, & Streisand, 2011; Grossman et al., 1987; Schilling et al., 2009; Streisand et al., 2005). In the current sample, the SED-P had an acceptable Cronbach’s alpha of .78.
Data Analysis Plan
All statistical analyses were conducted using SPSS (20th edition). Preliminary descriptive statistics were generated for parent and child demographic and medical characteristics, the 24-hour recall interview, and parents’ psychosocial functioning on the HFS-PYC and SED-P. To address Aim 1, descriptive statistics were generated for the frequency and range of parent sleep characteristics on the PSQI. T-tests were then conducted comparing this sample’s daytime dysfunction and global PSQI scores to standardized norms (Buysse aet al., 1989). Subsequently, we addressed Aim 2 by conducting bivariate correlational and intercorrelational analyses. Child and parent demographic and medical characteristics were correlated with parents’ sleep scores on the PSQI and T1D-related psychosocial functioning on the HFS-PYC and SED-P. (See Table 3.)
Table 3.
Correlations among demographic/medical variables and sleep, hypoglycemia worry, and self-efficacy subscales (n = 134)
| Child Age | Parent Age | Parent Marital Status | Family Income | HbA1c | Regimen | Illness Duration | Mean Daily BG Level | Number of Daily BG Checks | 1 | 2 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. PSQI-Total Score | −.13 | −.05 | .07 | .02 | .18* | .13 | .02 | .26** | .07 | 1.00 | |
| 2. HFS-Worry | −.06 | .03 | −.14 | .04 | −.08 | −.05 | −.05 | −.12 | .24** | .19* | 1.00 |
| 3. SED-Total Score | .20* | −.14 | .13 | −.14 | −.08 | −.12 | .06 | −.08 | −.10 | −20* | −.30** |
Note. Parent Marital Status: 0 = Not Married, 1 = Married; Regimen: 0 = Conventional, 1 = Intensive; BG = Blood Glucose.
p < .05.
p < .01.
p < .001.
Finally, to address Aim 3, a mediation model hypothesizing that hypoglycemia worry was associated with diabetes self-efficacy via their association with parents’ sleep quality was assessed. Baron and Kenny (1986) indicate that in order to establish full or partial mediation, there must be a significant association between 1) the predictor (HFS-PYC worry) and the outcome (SED-P), 2) the predictor and the mediator (PSQI global score), and 3) the mediator and the outcome. Furthermore, when the mediator is added to the regression model, Sobel’s test must indicate a decrease in the significance of the relationship between the predictor and the outcome. Child age was significantly correlated with parent ratings on the SED-P; thus, child age was included as a covariate in each regression. A power analysis for the final mediation regression model, using 3 predictor variables, a .05 significance level, and small effect size (f2 = .10) indicated that power in this sample was high (β = .98).
Results
Aim 1: Parent Sleep Characteristics
Parents reported a mean of 6.19 hours of nightly sleep (SD = 1.33, Range = 3–10 hours), and a mean sleep latency of 21.03 minutes (SD = 17.29, Range = 0–90 minutes) on the PSQI, which is less than the 7 to 9 hours of sleep recommended for adults by the National Sleep Foundation (McKnight-Eily et al., 2009). Parents reported a mean PSQI total score of 7.07 (SD = 3.34, Range = 1–15) (See Table 2 for PSQI subscale details.) Parents’ global PSQI scores and subjective sleep quality, sleep duration, habitual sleep efficiency, sleep disturbance, and daytime dysfunction subscale scores were higher than among healthy adults in the original publication (all t-test comparison ps < .05), indicating that many aspects of parents’ sleep may be impacted by T1D care (Buysse, Reynolds, Monk, Burman, & Kupfer, 1989). One-third of parents (36%) indicated their overall sleep quality was fairly bad or very bad. Many parents endorsed weekly sleep-related daytime dysfunction, including 36% who had difficulty maintaining enthusiasm to complete daily tasks and 11% who had difficulty staying awake during eating, driving, and/or social activities one or more times per week. Parent age, child hemoglobin A1c, and mean daily BG level were related to parent sleep. Parents of children who had a higher, or worse, hemoglobin A1c, and parents whose children had a higher mean daily BG level (which corresponds to higher hemoglobin A1c), had a higher PSQI global score, r(134) = .18, p < .05, r(134) = .26, p < .01; respectively. (See Table 3 for additional correlational analyses.)
Table 2.
Descriptive Information for Variables of Interest
| Variable | n | M | SD | Range |
|---|---|---|---|---|
| Pittsburgh Sleep Quality Index | ||||
| Subjective Sleep Quality | 134 | 1.31 | 0.84 | 0–3 |
| Sleep Latency | 134 | 0.66 | 0.77 | 0–3 |
| Sleep Duration | 134 | 1.70 | 1.09 | 0–3 |
| Habitual Sleep Efficiency | 132 | 0.75 | 0.96 | 0–3 |
| Sleep Disturbance | 134 | 1.21 | 0.51 | 0–3 |
| Sleep Medications | 133 | 0.48 | 1.00 | 0–3 |
| Daytime Dysfunction | 134 | 0.98 | 0.76 | 0–3 |
| Total Score | 134 | 7.07 | 3.34 | 1–15 |
|
| ||||
| Fear of Hypoglycemia-Parents of Young Children | ||||
| Worry | 134 | 23.86 | 11.64 | 0–56 |
|
| ||||
| Self-Efficacy for Diabetes Scale for Parents | ||||
| Total Score | 134 | 94.49 | 7.09 | 76–110 |
One-third of parents (34%) estimated they performed daily nighttime BG checks, and overall, parents reported a mean of 3.42 nighttime BG checks a week (SD = 2.98). The majority of the parents (58%) who performed nighttime BG checks indicated their sleep was disrupted; although only 10% reported this disrupted their child’s sleep as well. Parents’ self-report on the PSQI was corroborated by their self-report on the 24-hour recall. Parents’ reported a mean of 0.73 BG checks per night after children went to sleep (SD = 1.09). Most of these BG checks were routine checks (79%); other nighttime BG checks were due to a low BG level at bedtime (11%), child refusal to eat a bedtime snack (2%), and other reasons (9%). The frequency of nighttime BG checks was positively correlated with the number of BG level excursions over 200 mg/dL and below 70 mg/dL, r(134) = .32, p < .001, r(134) = .45, p < .001; respectively, suggesting that parents are more likely to complete nighttime BG checks when their child is at risk of hypoglycemia or hyperglycemia.
Aim 2: Hypoglycemia Worry, Diabetes Self-Efficacy, and Parent Sleep
Parents’ report of hypoglycemia worry on the HFS-PYC was lower than reports in previous studies with parents of young children with T1D, t(156) = 7.98, p < .01 (Patton et al., 2008; Patton et al., 2011); whereas, parent ratings of their confidence in their ability to manage their child’s T1D on the SED-P were higher than the mean reported by Streisand et al. (2005) among a sample of parents of children ages 9–17, t(266) = 14.85, p < .001. Hypoglycemia worry was negatively correlated with parents’ confidence in their ability to manage their child’s T1D, r(132) = −.30, p < .01. With respect to associations with demographic and medical characteristics, hypoglycemia worry was negatively correlated with child age, r(134) = −.21, p < .05, whereas diabetes self-efficacy was positively correlated with child age, r(134) = .20, p < .05, indicating that parents of younger children experienced greater hypoglycemia worry and less diabetes self-efficacy. Other correlations with demographic and medical variables were not significant. (See Table 3.)
Parents’ diabetes-related psychosocial functioning was associated with parent sleep quality as well. Greater hypoglycemia worry was associated with greater global PSQI scores, r(134) = .19, p < .05. Furthermore, parents who reported greater hypoglycemia worry reported greater sleep disruption as a result of attending to their child’s health needs, r(134) = .24, p < .01, and stress related to their child’s health, r(134) = .33, p < .001. Greater hypoglycemia worry was also significantly positively correlated with the number of BG checks parents reportedly completed in a week, r(133) = .19, p < .05.
Aim 3: Mediational Regression Analyses
A series of linear regressions, guided by Baron and Kenny’s (1986) criteria for mediation effect, and including child age as a covariate, was conducted to test the hypothesis that parents’ sleep quality mediates the relationship between hypoglycemia worry and diabetes self-efficacy. Parental hypoglycemia worry was significantly associated with parents’ sleep quality, F(2,131) = 3.47, p < .05; β = .19, p < .05, and diabetes self-efficacy, F(2,129) = 8.78, p < .001; β = −.29, p < .01, in separate analyses. Parents’ sleep quality was also significantly associated with diabetes self-efficacy, F(2,129) = 4.90, p < .01; β = −.18, p < .05. However, although the relationship between parental hypoglycemia worry and diabetes self-efficacy continued to be significant when parents’ sleep quality was included in the final model, F(3,128) = 6.75, p < .001, β = −.26, p < .01, the relationship between parents’ sleep quality and diabetes self-efficacy was no longer significant, β = −.13, p = .12. The relationship between parental hypoglycemia worry and diabetes self-efficacy was not mediated by parents’ sleep quality. (See Figure 1.)
Figure 1.
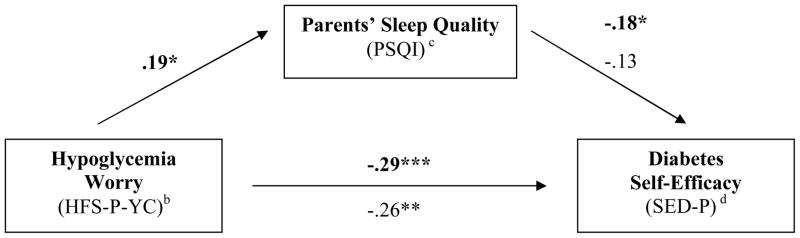
Parent Sleep Quality (PSQI) does not mediate the relationship between Hypoglycemia Worry (HFS-P-YC) and Diabetes Self-Efficacy (SED-P)a
*p ≤ .05 **p ≤ .01 *** p ≤ .001
a Figure illustrates that parents’ sleep quality si related to both hypoglycemia worry and diabetes self-efficacy, but does not mediate the relationship. Bold numbers are beta weights of the relationship between the individual constructs. The non-bolded numbers are beta weights for the entire model after hypoglycemia worry has been included.
b Hypoglycemia Fear Survey-Parents of Young Children
c Pittsburgh Sleep Quality Index
d Self-Efficacy for Diabetes Scale for Parents
Discussion
Parents of young children with T1D received, on average, one less hour of nightly sleep than the 7–9 hours recommended by the National Sleep Foundation. Additionally, one third of parents reported poor sleep quality. Overall, parents of young children with T1D reported greater sleep problems than standardized norms, thus, supporting our hypothesis that parents of children with T1D would report poor sleep quality. These results are consistent with literature demonstrating that parents of children with chronic illness experience sleep disruption (Cottrell & Khan, 2005; Meltzer & Moore, 2008). Many parents engaged in nightly diabetes management and experienced concomitant sleep disruption and sleep-related daytime dysfunction. Furthermore, parents of children who had a greater number of BG level excursions over 200 mg/dL and below 70 mg/dL were more likely to perform nighttime BG checks, but the causal nature of this relationship is not clear at this time and warrants further investigation. It is likely that parents whose children are prone to high or low BG levels worry more about nocturnal hypoglycemia and check their child’s BG level more frequently. It is also possible that parents who worry more about nocturnal hypoglycemia impact their child’s BG levels through daily T1D management behaviors, such as providing excessive nighttime carbohydrates.
Only 10% of parents who performed nighttime BG checks reported that this disrupted their child’s sleep. Notably, this estimation of child sleep disruption is based on parent report for a single question about sleep disruption due to BG checks, not comprehensive information about the impact of other nighttime T1D management such as eating a snack to treat a low BG level or objective data such as actigraphy. It is likely that parents underestimate the degree of sleep disruption experienced by their child and the impact this disruption may have on their child’s daytime functioning. Further examination of child sleep disruption is warranted and may have direct clinical implications for nighttime T1D management behaviors.
Our second hypothesis was supported as well. Parents who worried more about hypoglycemia experienced poorer overall sleep quality and lower diabetes self-efficacy. Additionally, parents’ with poorer sleep quality reported less diabetes self-efficacy. Parents who worry about hypoglycemia are likely to experience poorer sleep quality because they perform nighttime diabetes management and/or cannot sleep due to worry about nocturnal hypoglycemia. Subsequent sleep deprivation and fatigue may then impact parents’ alertness and focus, their ability to maintain enthusiasm to complete T1D-related tasks, and their self-assessment of their ability to successfully complete T1D management.
However, the relationship between parent hypoglycemia worry and perception of diabetes self-efficacy was not mediated by parents’ sleep quality. This constellation of results indicates that sleep quality has an important role in parents’ perceptions regarding their ability to perform diabetes management tasks, but sleep quality is not the sole factor that impacts perception of diabetes self-efficacy. It is likely that other aspects of psychosocial functioning, such as parenting stress or perhaps depression, impact parents’ sleep quality. Although our mediation model was not significant, parent sleep among this sample was suboptimal and the cumulative effect of poor sleep quality has significant implications for parents’ abilities to cope with the demands of daily T1D management. It is possible that this series of events may become cyclical in nature: parents who believe they are unable to successfully manage their child’s T1D may worry more about hypoglycemia and modify their child’s T1D management, thereby increasing the frequency of their child’s day-time and nighttime BG excursions and unintentionally supporting their self-assessment of their ability to manage their child’s illness. Interventions that address parents’ diabetes self-efficacy may benefit from inclusion of components that target parent sleep and hypoglycemia concerns.
Strengths and Limitations
This study is one of the first to document poor sleep quality among parents of children with T1D and highlights the need to assess parent sleep during routine clinic appointments. Moreover, this sample is one of the largest known psychosocial samples of parents of young children with T1D and represents a broad demographic range; the sample is representative of the patients who receive care at these three endocrinology services and ethnically diverse for samples targeting T1D (SEARCH for Diabetes in Youth Study Group et al., 2006; Patton et al., 2008). An additional strength of this study is that both objective glucometer and hemoglobin A1c data and subjective parent report of T1D management behavior were collected. These data were highly correlated indicating parents’ report of daily T1D management behavior is a reliable indicator of daily T1D management.
However, study limitations should also be noted. Parents’ report of their sleep and potential child sleep disruption is retrospective and subjective; this study would be enhanced if sleep was measured using objective actigraphy data. Another limitation is that these data were collected only from primary caregivers. It is likely that the experiences of primary caregivers differ from secondary caregivers, as parents may divide T1D management responsibility according to time of day.
Future Directions
These results warrant longitudinal investigation of the impact of T1D care on parents’ sleep quality among multiple caregivers over the course of the child’s diagnosis. Although this study did not indicate an association of nighttime T1D management, parent sleep, and diagnosis duration, it is possible that there was little variability among this concentrated age range. The trajectory of nighttime T1D management and parent sleep may vary more according to diagnosis duration among older children. It is recommended that objective measures of sleep, such as actigraphy, be incorporated in study designs to further elucidate the complex relationship of sleep and T1D management. It will also be important to evaluate the impact of sleep quality and hypoglycemia worry on diabetes self-efficacy and the potential role they have on children’s glycemic control. The data from this study suggest that parents’ psychosocial functioning is related to children’s glycemic variability (i.e., more hypoglycemia worry was related to number of BG excursions), but these results are only preliminary.
In addition, investigating parent and child demographic variables, (e.g., parent employment, child school attendance) and child sleep variables that are related to parent sleep variables are recommended. The schedule demands of parent employment and school attendance may impact parent sleep and daily T1D management behaviors. Child sleep concerns have been shown to relate to parent sleep disruption, daytime sleepiness, and worse psychosocial functioning among healthy populations and chronic illness populations such as cerebral palsy (Boergers, Hart, Owens, Streisand, & Spirito, 2007; Wayte, McCaughey, Holley, Annaz, & Hill, 2012). Although the limited extant literature regarding sleep among young children with T1D suggests that children may experience typical sleep patterns, it also indicates that bedtime concerns and child insomnia are related to greater parent psychosocial functioning concerns, including stress, anxiety, and depression (Monaghan et al., 2012). Thus, it is likely the relationship between child sleep and parent sleep among chronic illness populations is multifaceted; further research regarding the factors involved in this relationship is needed.
There are clinical implications for these findings as well. Namely, parents would likely benefit from guidelines regarding nighttime T1D management. Although the American Diabetes Association recommends checking BG levels at bedtime, it does not provide additional information regarding nighttime T1D management or how nighttime T1D management needs may change over time (i.e., when changing regimens, schedules, etc.) (American Diabetes Association, 2013). As a result, some parents may engage in nighttime T1D management that is unnecessary. It is recommended that physicians provide their patients with more information about nighttime T1D management needs in order to help parents ensure their child is medically safe during the night while also getting adequate sleep.
With respect to the development of clinical interventions that address parents’ coping and diabetes self-efficacy, these results suggest that routine endocrinology appointments should include discussion of nighttime T1D management behavior, and provide specific guidelines on nocturnal blood glucose monitoring. Behavioral intervention components that target parent sleep and hypoglycemia concerns are crucial because they may also affect children’s glycemic control. Additionally, as sleep problems are frequently correlated with depression (Ford & Kamerow, 1989; Mellinger, Balter, & Uhlenhuth, 1985), future studies should further explicate the overlap between parental sleep concerns stress and nighttimedepression among this population, as this will inform the development of clinical interventions for these families. A T1D screening process that assesses parent sleep, stress, and depression may be an additional method to allocate intervention services as needed.
Conclusions
Many parents worry about hypoglycemia and experience T1D-related poor sleep quality, factors that impact their perception of their ability to perform diabetes management. The results of this study advocate for further investigation of the relationships among sleep quality and other psychosocial variables among parents of children with T1D, as well as the development of nighttime T1D management guidelines for parents. These results also support the need for increased awareness among health care providers about the challenges that families face after their children have gone to sleep. Interventions designed to improve diabetes self-efficacy should address parent sleep and hypoglycemia concerns, and additional research regarding the impact of sleep disturbance on children’s glycemic control is warranted.
Acknowledgments
Funding
This work was support by The National Institute of Diabetes and Digestive and Kidney Diseases, Grant Number R01DK080102, awarded to Randi Streisand, PhD.
References
- American Diabetes Association. Self-monitoring of blood glucose. Diabetes Care. 1994;17:81–96. doi: 10.2337/diacare.17.1.81. [DOI] [PubMed] [Google Scholar]
- American Diabetes Association. Standards of medical care in diabetes-2013. Diabetes Care. 2013;36(1):S11–S66. doi: 10.2337/dc13-S011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong B, Mackey ER, Streisand R. Parenting behavior, child functioning, and health behaviors in preadolescents with type 1 diabetes. Journal of Pediatric Psychology. 2011;36(9):1052–1061. doi: 10.1093/jpepsy/jsr039. jsr039.10.1093/jpepsy/jsr039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bade-White PA, Obrzut JE. The neurocognitive effects of type 1 diabetes mellitus in children and young adults with and without hypoglycemia. Journal of Developmental and Physical Disabilities. 2009;21:425–440. doi: 10.1007/s10882-009-9151-y. [DOI] [Google Scholar]
- Barnard K, Thomas S, Royle P, Noyes K, Waugh N. Fear of hypoglycaemia in parents of young children with type 1 diabetes: A systematic review. BMC Pediatrics. 2010;10:50. doi: 10.1186/1471-2431-10-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. Journal of Personality and Social Psychology. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Boergers J, Hart C, Owens JA, Streisand R, Spirito A. Child sleep disorders: Associations with parental sleep duration and daytime sleepiness. Journal of Family Psychology. 2007;21(1):88–94. doi: 10.1037/0893-3200.21.1.88. [DOI] [PubMed] [Google Scholar]
- Buysse D, Reynolds CF, Monk TH, Burman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Research. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Chasens ER, Korytkowski M, Sereika SM, Burke LE. Effect of poor sleep quality and excessive daytime sleepiness on factors associated with diabetes self-management. The Diabetes Educator. 2013;39(1):74–82. doi: 10.1177/0145721712467683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chasens ER, Olshansky E. Daytime sleepiness, diabetes, and psychological well-being. Issues Ment Health Nurs. 2008;29(10):1134–1150. doi: 10.1080/01612840802319878. [DOI] [PubMed] [Google Scholar]
- Coffen R, Dahlquist L. Magnitude of type 1 diabetes self-management in youth: health care needs diabetes educators. Diabetes Educator. 2009;35(2):302–308. doi: 10.1177/0145721708327534. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. 2. Earlbaum, NJ: Hillsdale; 1988. [Google Scholar]
- Cottrell L, Khan A. Impact of childhood epilepsy on maternal sleep and socioemotional functioning. Clinical Pediatrics. 2005;44(7):613–616. doi: 10.1177/000992280504400709. [DOI] [PubMed] [Google Scholar]
- Cox DM, Irvine A, Gonder-Frederick LA, Nowacek G, Butterfield J. Fear of hypoglycemia: Quantification, validation and utilization. Diabetes Care. 1987;10(5):617–621. doi: 10.2337/diacare.10.5.617. [DOI] [PubMed] [Google Scholar]
- Ford D, Kamerow D. Epidemiologic study of sleep disturbances and psychiatric disorders. Journal of the American Medical Association. 1989;262:1479–1484. doi: 10.1001/jama.262.11.1479. [DOI] [PubMed] [Google Scholar]
- Freund A, Johnson SB, Silverman J, Thomas J. Assessing daily management of childhood diabetes using 24-hour recall interviews: Reliability and stability. Health Psychology. 1991;10:200–208. doi: 10.1037/0278-6133.10.3.200. [DOI] [PubMed] [Google Scholar]
- Golden MP, Russell BP, Ingersoll GM, Gray DL, Hummer KM. Management of diabetes mellitus in children younger than 5 years of age. American Journal of Diseases of Children. 1985;139(5):448–452. doi: 10.1001/archpedi.1985.02140070022019. [DOI] [PubMed] [Google Scholar]
- Green LB, Wysocki T, Reineck BM. Fear of hypoglycemia in children and adolescents with diabetes. Journal of Pediatric Psychology. 1990;15(5):633–641. doi: 10.1093/jpepsy/15.5.633. [DOI] [PubMed] [Google Scholar]
- Grossman H, Brink S, Hauser S. Self-efficacy in adolescent girls and boys with insulin-dependent diabetes mellitus. Diabetes Care. 1987;10(3):324–329. doi: 10.2337/diacare.10.3.324. [DOI] [PubMed] [Google Scholar]
- Hansen JA, Weissbrod C, Schwartz DD, Taylor WP. Paternal involvement in pediatric Type 1 diabetes: Fathers’ and mothers’ psychological functioning and disease management. Families, Systems, & Health. 2012;30(1):47–59. doi: 10.1037/a0027519. [DOI] [PubMed] [Google Scholar]
- Herge WM, Streisand R, Chen R, Holmes C, Kumar A, Mackey ER. Family and youth factors associated with health beliefs and health outcomes in youth with type 1 diabetes. Journal of Pediatric Psychology. 2012;37(9):980–989. doi: 10.1093/jpepsy/jss067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine A, Saunders T. Fear of hypoglycemia: Replication and validation. Diabetes. 1989;38(Suppl 2):109A. [Google Scholar]
- Jaser SS, Whittemore R, Ambrosino JM, Lindemann E, Grey M. Mediators of depressive symptoms in children with type 1 diabetes and their mothers. Journal of Pediatric Psychologyl. 2008;33(5):509–519. doi: 10.1093/jpepsy/jsm104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SB, Silverstein J, Rosenbloom A, Carter R, Cunningham W. Assessing daily management in childhood diabetes. Health Psychology. 1986;5:545–564. doi: 10.1037/0278-6133.5.6.545. [DOI] [PubMed] [Google Scholar]
- Marvicsin D. School-age children with diabetes: role of maternal self-efficacy, environment, and management behaviors. Diabetes Educator. 2008;34(3):477–483. doi: 10.1177/0145721708316944. [DOI] [PubMed] [Google Scholar]
- McKnight-Eily LR, Liu Y, Perry GS, Preseley-Cantrell LR, Strine TW, Lu H, Croft JB. Perceived insufficient rest or sleep among adults-United States, 2008. Journal of the American Medical Association. 2009;302:2532–2539. [Google Scholar]
- Mellinger G, Balter M, Uhlenhuth E. Insomnia and its treatment: Prevalence and correlates. Archives of General Psychiatry. 1985;42(3):225–232. doi: 10.1001/archpsyc.1985.01790260019002. [DOI] [PubMed] [Google Scholar]
- Meltzer L, Mindell JA. Impact of a child’s chronic illness on maternal sleep and daytime functioning. Archives of Internal Medicine. 2006;166:1749–1755. doi: 10.1001/archinte.166.16.1749. [DOI] [PubMed] [Google Scholar]
- Meltzer L, Moore M. Sleep disruptions in parents of children and adolescents with chronic illnesses: Prevalence, causes, and consequences. Journal of Pediatric Psychology. 2008;33(3):279–291. doi: 10.1093/jpepsy/jsm118. [DOI] [PubMed] [Google Scholar]
- Monaghan M, Herbert LJ, Cogen FR, Streisand R. Sleep behaviors and parent functioning in young children with type 1 diabetes. Children’s Health Care. 2012;41:246–259. doi: 10.1080/02739615.2012.685385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaghan M, Hilliard M, Cogen F, Streisand R. Supporting parents of very young children with type 1 diabetes: results from a pilot study. Patient Education and Counseling. 2011;82(2):271–274. doi: 10.1016/j.pec.2010.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaghan M, Hilliard ME, Cogen FR, Streisand R. Nighttime caregiving behaviors among parents of young children with type 1 diabetes: Associations with illness characteristics and parent functioning. Families, Systems, & Health. 2009;27(1):28–38. doi: 10.1037/a0014770. [DOI] [PubMed] [Google Scholar]
- Patterson CC, Dahlquist GG, Gyurus E, Green A, Soltesz G. Incidence trends for childhood type 1 diabetes in Europe during 1989–2003 and predicted new cases 2005–20: a multicentre prospective registration study. Lancet. 2009;373(9680):2027–2033. doi: 10.1016/S0140-6736(09)60568-7. [DOI] [PubMed] [Google Scholar]
- Patton SR, Dolan LM, Henry R, Powers SW. Parental fear of hypoglycemia: young children treated with continuous subcutaneous insulin infusion. Pediatric Diabetes. 2007;8:362–368. doi: 10.1111/j.1399-5448.2007.00242.x. [DOI] [PubMed] [Google Scholar]
- Patton SR, Dolan LM, Henry R, Powers SW. Fear of hypoglycemia in parents of young children with type 1 diabetes mellitus. Journal of Clinical Psychology in Medical Settings. 2008;15(3):252–259. doi: 10.1007/s10880-008-9123-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton SR, Dolan LM, Smith LB, Thomas IH, Powers SW. Pediatric parenting stress and its relation to depressive symptoms and fear of hypoglycemia in parents of young children with type 1 diabetes mellitus. Journal of Clinical Psychology in Medical Settings. 2011;18(4):345–352. doi: 10.1007/s10880-011-9256-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilling L, Dixon J, Knafl K, Lynn M, Murphy K, Dumser S, Grey M. A new self-report measure of self-management of type 1 diabetes for adolescents. Nursing Research. 2009;58(4):228–236. doi: 10.1097/NNR.0b013e3181ac142a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liese A, D’Agostino RJ, Hamman R, Kilgo P, Lawrence J, Williams D SEARCH for Diabetes in Youth Study Group. The burden of diabetes mellitus among US youth: prevalence estimates from the SEARCH for Diabetes in Youth Study. Pediatrics. 2006;118(4):1510–1518. doi: 10.1542/peds.2006-0690. [DOI] [PubMed] [Google Scholar]
- Silverstein J, Klingensmith G, Copeland K, Plotnick LP, Kaufman F, Laffel L, Clark N. Care of children and adolescents with Type 1 Diabetes: A statement of the American Diabetes Association. Diabetes Care. 2005;28(1):186–212. doi: 10.2337/diacare.28.1.186. [DOI] [PubMed] [Google Scholar]
- Streisand R, Mackey E, Elliot B, Mednick L, Slaughter I, Turek J, Austin A. Parental anxiety and depression associated with caring for a child newly diagnosed with type 1 diabetes: opportunities for education and counseling. Patient Education & Counseling. 2008;73(2):333–338. doi: 10.1016/j.pec.2008.06.014. S0738-3991(08)00324-8. [DOI] [PubMed] [Google Scholar]
- Streisand R, Swift E, Wickmark T, Chen R, Holmes CS. Pediatric parenting stress among parents of children with Type 1 Diabetes: The role of self-efficacy, responsibility, and fear. Journal of Pediatric Psychology. 2005;30(6):513–521. doi: 10.1093/jpepsy/jsi076. [DOI] [PubMed] [Google Scholar]
- Sullivan-Bolyai S, Deatrick J, Gruppuso P, Tamborlane W, Grey M. Mothers’ experiences raising young children with type 1 diabetes. Journal for Specialists in Pediatric Nursing. 2002;7(3):93–103. doi: 10.1111/j.1744-6155.2002.tb00158.x. [DOI] [PubMed] [Google Scholar]
- Sullivan-Bolyai S, Deatrick J, Gruppuso P, Tamborlane W, Grey M. Constant vigilance: mothers’ work parenting young children with type 1 diabetes. Journal of Pediatric Nursing. 2003;18(1):21–29. doi: 10.1053/jpdn.2003.4. [DOI] [PubMed] [Google Scholar]
- Tamborlane W, Kollman C, Steffes M, Ruedy K, Dongyuan X, Beck R DirecNet Study Group. Comparison of fingerstick hemoglobin A1c levels assayed by DCA 2000 with the DCCT/EDIC central laboratory assay: results of a Diabetes Reseach in Children Network (DirecNet) study. Pediatric Diabetes. 2005;6(1):13–16. doi: 10.1111/j.1399-543X.2005.00088.x. [DOI] [PubMed] [Google Scholar]
- Vehik K, Hamman R, Lezotte D, Norris J, Klingensmith G, Bloch C, Dabelea D. Increasing incidence of type 1 diabetes in 0- to 17-year-old Colorado youth. Diabetes Care. 2007;30(3):503–509. doi: 10.2337/dc06-1837. [DOI] [PubMed] [Google Scholar]
- Wayte S, McCaughey E, Holley S, Annaz D, Hill CM. Sleep problems in children with cerebral palsy and their relationship with maternal sleep and depression. Acta Pediatrica. 2012;101:618–623. doi: 10.1111/j.1651-2227.2012.02603.x. [DOI] [PubMed] [Google Scholar]


