Abstract
Aims
While patients with diabetes mellitus (DM) have more extensive coronary disease and worse survival after acute myocardial infarction (AMI) than patients without DM, data on whether they experience more angina are conflicting.
Methods and Results
We examined angina prevalence over the year following AMI among 3367 patients, including 1080 (32%) with DM, from 24 US hospitals enrolled in the TRIUMPH registry from 2005–08. Patients with vs. without DM were more likely to be treated with antianginal medications both at discharge and over follow-up. Despite more aggressive angina therapy, patients with vs. without DM had higher prevalence and severity of angina prior to AMI (49% vs 43%, p=0.001) and at each follow-up assessment, although rates of angina declined in both groups over time. In a hierarchical, multivariable, repeated measures model that adjusted for multiple demographic and clinical factors including severity of coronary disease and in-hospital revascularization, DM was associated with a greater odds of angina over the 12 months of follow-up; this association increased in magnitude over time (12-month OR 1.18, 95% CI 1.01–1.37; DM*time pinteraction=0.008).
Conclusion
Contrary to conventional wisdom, angina is more prevalent and more severe among patients with DM, both prior to and following AMI. This effect is amplified over time and independent of patient and treatment factors, including the presence of multivessel disease and coronary revascularization. This increased burden of angina may be due to more diffuse nature of coronary disease, more rapid progression of coronary disease over time, or greater myocardial demand among DM patients.
Keywords: angina, diabetes mellitus, myocardial infarction
Type 2 diabetes mellitus (DM) is present in approximately one-third of patients hospitalized with an acute myocardial infarction (AMI)1 and, despite advances in care, continues to be associated with an increased risk of both short- and long-term mortality.2–3 While patients with DM have more extensive coronary disease4–5 and worse survival after AMI3, 6 than those without DM, the data on whether they experience more angina are conflicting. Several older studies suggested that patients with DM are more likely to have asymptomatic (or “silent”) ischemia7–9, with diabetic autonomic neuropathy posited as one potential explanation.7–8 However, more recent studies have challenged this assumption.10 One clinical trial of patients with stable coronary artery disease undergoing ambulatory electrocardiographic monitoring showed the proportion of patients with silent ischemia was similar among those with and without DM.11 Both an observational study and a large, multinational clinical trial suggested a higher burden of angina among patients with DM vs. without DM after an AMI.12–13 Collectively, these disparate findings underscore a need for greater clarity in regards to the angina burden among those with and without DM.
Angina after an AMI is a highly relevant condition affecting ~20% of patients14. Beyond the direct morbidity of each angina episode experienced by the patient, angina is associated with worse health-related quality of life and is a major driver of repeat hospitalizations and increased healthcare costs.15 As such, it would be valuable to identify patients at highest risk for residual angina after AMI as early as possible. These patients can then be targeted for early post-discharge follow up and more aggressive medical management with the goal of improving health status outcomes and potentially reducing repeat hospitalizations. Because patients with DM represent a particularly high-risk cohort, better understanding of the prevalence and predictors of angina in this group is warranted. Accordingly, we examined the association between DM and angina in a contemporary, population of post-AMI patients.
METHODS
Study Population and Protocol
Between June 2005 and December 2008, 4340 patients from 24 US hospitals were enrolled into the Translational Research Investigating Underlying disparities in acute Myocardial infarction Patients’ Health status (TRIUMPH) study. The full details of the sites, enrolment process, inclusion and exclusion criteria, and data collection protocols has been previously published.16 Briefly, patients were required to have biomarker evidence of myocardial necrosis and additional evidence supporting the clinical diagnosis of an AMI (e.g., prolonged ischemic signs/symptoms (≥20 minutes) or electrocardiographic ST changes) during the initial 24 hours of admission. Patients were enrolled within 24 to 72 hours following admission, at which time baseline data were obtained through chart abstraction and a structured interview by trained research staff. Prevalent DM was defined by chart abstraction based on medical history or the use of glucose-lowering medications at admission. Because the purpose of our study was to compare the burden of angina in patients with established DM versus those without DM, patients with newly-diagnosed DM (i.e., no known diagnosis of DM at admission and HbA1c ≥6.5%; n=245) were excluded from the analyses. Detailed follow-up telephone interviews were attempted on all survivors at 1, 6, and 12 months after AMI. In addition to an assessment of health status, participants were asked to read the names and doses of their medications from their prescription bottles. Each participating hospital obtained Institutional Research Board approval, and all patients provided written informed consent for baseline and follow-up assessments.
Health Status Assessment
Health status data were assessed by interview using the Seattle Angina Questionnaire (SAQ)17 and the Medical Outcomes Study 12-item Short Form (SF-12).18 The SAQ is a validated 19-item questionnaire comprised of 5 clinically important dimensions of health in patients with coronary artery disease: angina frequency, angina stability, disease-specific quality of life, physical limitations, and treatment satisfaction. The scores for all SAQ domains range from 0 to 100, with higher scores indicating less disease burden. The SAQ has a recall period of 4 weeks, and thus assessment at the time of AMI reflects the angina burden over the time period preceding the AMI. For this study, the primary outcome was the SAQ angina frequency, which was categorized as absent (score 100) or present (score <100). In addition, we categorized angina frequency as monthly (SAQ score=61–99), weekly (SAQ score=31–60), and daily (SAQ score=0–30).19 We also investigated the physical limitations and quality of life domains. The SF-12 is a reliable and valid measure of generic health status20 that provides summary component scales for overall physical and mental health using norm-based methods that standardize the scores to a mean of 50 and a standard deviation of 10 (higher scores indicate better health status).18
Statistical Analysis
Baseline characteristics of patients with and without DM present on admission for AMI were compared using t-tests for continuous variables and chi-square tests for categorical variables. Health status scores and mean number of antianginal medications prescribed were compared at baseline and 1, 6, and 12 months after AMI using t-tests, and Cohen’s d effect sizes were calculated for the differences in health status scores.21 In addition, the proportion of patients reporting angina (SAQ angina frequency score <100), the proportion of patients reporting different levels of angina (none, monthly, weekly, daily), and the proportions of patients taking each category of antianginal medication (beta blockers, calcium channel blockers, long-acting nitrates) were compared between patients with and without DM at each time point using chi-square tests. Hierarchical, multivariable, repeated measures regression models were used to evaluate the independent association of DM with angina over the 12 months of follow-up. Because the frequency of angina was >10%, we estimated relative rates (RR) directly using Poisson regression to avoid overestimation of effect sizes. The variables included in the multivariable model were selected a priori based on prior literature review and clinical judgment of factors that might impact anginal status: age, sex, race, hypertension, current smoking, depressive symptoms (as assessed with the 9-item Patient Health Questionnaire22[score ≥ 10]), prior bypass graft surgery, prior angioplasty, angioplasty during the acute AMI hospitalization, bypass graft surgery during the acute AMI hospitalization, presence of multivessel disease (≥70% stenosis in ≥2 major epicardial coronary arteries or ≥50% stenosis of the left main coronary artery), left ventricular systolic dysfunction (ejection fraction <40%), type of AMI (ST- or non-ST-elevation), and Global Registry of Acute Coronary Events (GRACE) score.23 Reference group is patients without diabetes. Interactions of DM with the other covariates (including time) were explored and were included when significant (defined a priori as pinteraction<0.10),24 and spline terms were considered for all continuous variables. Models were hierarchical, with hospital included as a random effect to adjust for patient clustering by site.
A second hierarchical, multivariable, repeated measures regression model was used to examine factors associated with angina among patients with DM. In addition to the above covariates, we examined the association of DM severity (by HbA1c measurement), DM duration, and discharge on insulin with angina over the 12 months of follow-up. Missing baseline covariate data were imputed using IVEware (Imputation and Variance Estimation Software; University of Michigan's Survey Research Center, Institute for Social Research, Ann Arbor, MI). All analyses were conducted using SAS v9.2 (SAS Institute, Inc., Cary, NC), and statistical significance was determined by a 2-sided p-value of <0.05.
RESULTS
Patient population
Of the 4340 patients enrolled into the TRIUMPH registry, 292 patients were excluded as they were newly-diagnosed as having DM during the AMI hospitalization and an additional 63 patients did not survive to 1 month after the AMI and thus had no opportunity for follow-up. Of the remaining 3985 patients who were eligible for analysis, 618 (15.5%) were excluded due to missing SAQ angina frequency data , yielding an analytic cohort of 3367, including 1080 (32%) with prevalent DM. While patients who were alive but missing follow-up health status assessments were more likely to report angina at baseline (missing vs. not: 49.1% vs. 44.4%, p=0.034; Supplemental Table 1), angina was not associated with missing follow-up in a multivariable logistic model (p=0.27).
There were several demographic and clinical differences between patients with and without DM (Table 1). Patients with DM were older, more frequently non-White, female, non-smokers, and had higher rates of prior coronary revascularization than patients without DM. During the acute AMI hospitalization, patients with DM (vs. without DM) were less likely to present with ST-elevations (33% vs. 49%, p<0.001), and more likely to have multivessel coronary disease (60% vs. 44%, p<0.001). Patients with DM were less likely to be invasively managed for their AMI (90% vs. 95%, p<0.001) and less likely to be treated with coronary revascularization (69% vs. 79%, p<0.001).
Table 1.
Baseline Characteristics
| Diabetes n=1080 | No Diabetes n=2287 | P-Value | |
|---|---|---|---|
| Age (y) | 60.8 ± 11.3 | 58.6 ± 12.4 | <0.001 |
| White race | 60.6% | 75.6% | <0.001 |
| Male | 59.4% | 70.6% | <0.001 |
| High school or greater education | 75.3% | 82.4% | <0.001 |
| Insurance coverage for meds | 73.4% | 75.0% | 0.323 |
| Hypertension | 83.2% | 57.8% | <0.001 |
| Current smoking | 28.9% | 41.6% | <0.001 |
| Depressive symptoms | 20.8% | 13.6% | <0.001 |
| HbA1c (%) | 8.1 ± 2.0 | 5.6 ± 0.4 | <0.001 |
| History of angioplasty | 25.4% | 17.4% | <0.001 |
| History of bypass graft surgery | 18.5% | 7.9% | <0.001 |
| ST-elevations on arrival | 33.4% | 49.3% | <0.001 |
| Multivessel disease | 59.8% | 44.1% | <0.001 |
| Left ventricular dysfunction | 20.1% | 16.3% | 0.007 |
| GRACE discharge score | 106.8 ± 29.5 | 96.9 ± 28.6 | <0.001 |
| In-hospital angiogram | 90.0% | 95.0% | <0.001 |
| In-hospital revascularization | 68.7% | 78.9% | <0.001 |
| In-hospital angioplasty | 58.1% | 71.3% | <0.001 |
| In-hospital bypass graft surgery | 11.3% | 8.7% | 0.019 |
GRACE, Global Registry of Acute Coronary Events23
Angina management
Patients with DM required treatment with more antianginal medications (Table 2). As all patients were post-AMI, the use of beta blockers was high in both groups (~91% at discharge, 75–86% over follow-up). However, at discharge and at each follow-up time point, more patients with DM were taking calcium channel blockers (17–18%) and long-acting nitrates (14–19%) compared with those without DM (calcium channel blockers: 8–10%; long-acting nitrates: 6–8%; p<0.001 for comparisons between DM and non-DM for both medication groups and at all time points). Compared with patients without DM, nearly twice as many patients with DM required 2 or more antianginals at each time point.
Table 2.
Use of Antianginal Medications
| Diabetes n=1080 | No Diabetes n=2287 | P-Value | |
|---|---|---|---|
| Discharge | |||
| Mean number of antianginals | 1.29 ± 0.69 | 1.08 ± 0.49 | <0.001 |
| 2 or more antianginals | 27.5% | 12.9% | <0.001 |
| Beta-blocker | 90.7% | 91.2% | 0.655 |
| Calcium channel blocker | 17.5% | 8.1% | <0.001 |
| Long-acting nitrate | 18.5% | 8.0% | <0.001 |
| 1 Month | |||
| Mean number of antianginals | 1.23 ± 0.77 | 1.03 ± 0.55 | <0.001 |
| 2 or more antianginals | 27.8% | 12.4% | <0.001 |
| Beta-blocker | 84.0% | 85.8% | 0.264 |
| Calcium channel blocker | 18.4% | 8.2% | <0.001 |
| Long-acting nitrate | 16.2% | 6.4% | <0.001 |
| 6 Month | |||
| Mean number of antianginals | 1.20 ± 0.80 | 1.02 ± 0.60 | <0.001 |
| 2 or more antianginals | 28.2% | 14.1% | <0.001 |
| Beta-blocker | 79.5% | 82.5% | 0.089 |
| Calcium channel blocker | 18.0% | 10.2% | <0.001 |
| Long-acting nitrate | 18.8% | 7.5% | <0.001 |
| 12 Months | |||
| Mean number of antianginals | 1.09 ± 0.78 | 0.96 ± 0.60 | <0.001 |
| 2 or more antianginals | 24.8% | 12.4% | <0.001 |
| Beta-blocker | 75.0% | 79.5% | 0.02 |
| Calcium channel blocker | 16.8% | 9.4% | <0.001 |
| Long-acting nitrate | 14.3% | 6.0% | <0.001 |
Use of ranolazine was <1% in both groups at all time points, with no differences between groups
Angina and health status
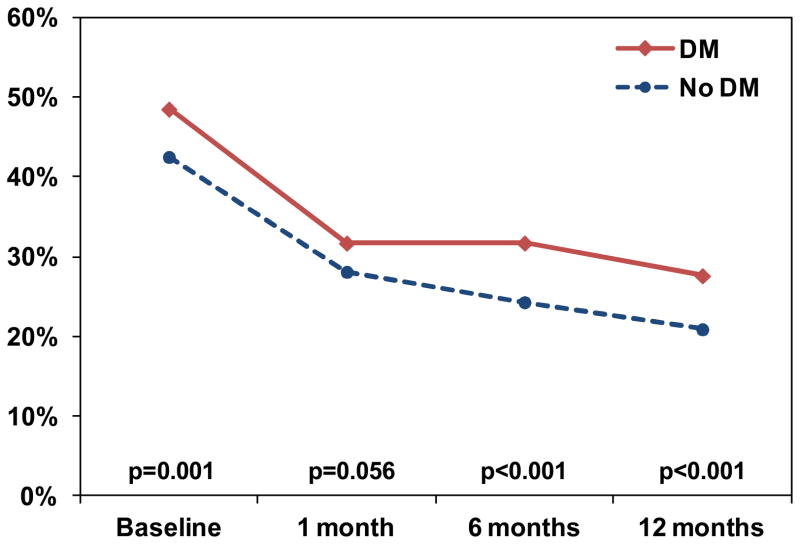
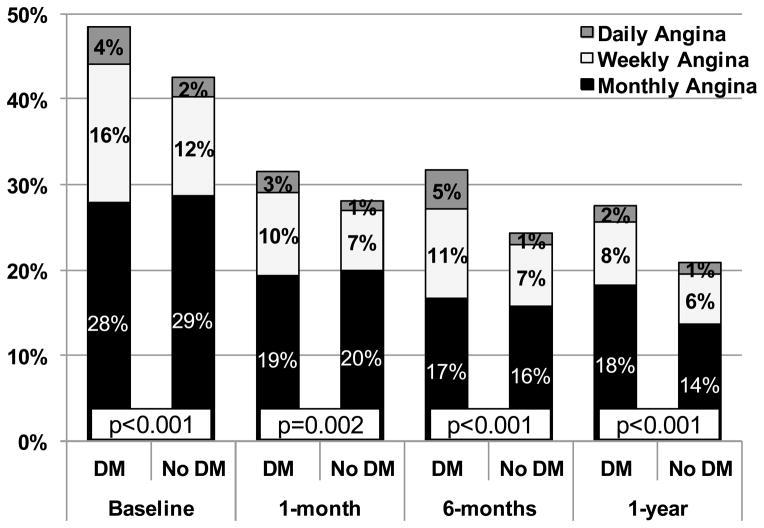
In unadjusted comparisons, despite receiving more antianginal medications, patients with DM had more angina both preceding their AMI (4 week period prior to AMI, assessed with SAQ during hospitalization; 49% vs. 43%, p=0.001) and at every follow-up point after discharge through 12 months, compared with patients without DM (Figure 1). At 12 months after AMI, 28% of patients with DM reported having angina vs. 21% of patients without DM (p<0.001). In addition to an increased prevalence of angina, patients with DM reported more frequent angina compared with those without DM at each time point (p<0.01 for all time points; Figure 2). All other disease-specific and health status scores were lower (i.e., worse health status) for patients with DM than for those without DM at both baseline and 12 months, except for the SF-12 mental components summary scores, which were similar between groups at 12 months (Table 3). The largest differences between groups (according to effect sizes) were in the SAQ physical limitations scale and the SF-12 physical component summary score, whereas the differences between groups among the other measures were statistically significant but modest.
Figure 1. Percentage of Patients Reporting Angina Prior to and after Myocardial Infarction.
The red line indicates the percentage of patients with DM who reported any angina (SAQ angina frequency score <100), and the blue line represents the percentage of patients without DM who reported angina at each time point.
Figure 2. Percentage of Patients Reporting Different Levels of Angina Four weeks Prior to, and 1, 6, and 12 months after Myocardial Infarction.
As assessed with the Seattle Angina Questionnaire angina frequency domain. Scores 0–30 indicate daily angina; 31–60 indicate weekly angina; 61–99 indicate monthly angina; 100 indicates no angina. p<0.01 for comparisons between groups at all time points.
Table 3.
Health Status at Baseline and 12 Months after Myocardial Infarction
| Diabetes n=1080 | No Diabetes n=2287 | P-Value | Cohen’s d Effect Size* | |
|---|---|---|---|---|
| Health Status at Baseline† | ||||
| SAQ Angina Frequency | 83.6 ± 22.5 | 87.6 ± 19.2 | <0.001 | 0.19 |
| SAQ Quality of Life | 61.9 ± 24.5 | 64.5 ± 22.7 | 0.002 | 0.11 |
| SAQ Physical Limitation | 79.7 ± 25.5 | 89.2 ± 19.0 | <0.001 | 0.42 |
| SF-12 Physical Component | 38.2 ± 12.0 | 44.9 ± 11.7 | <0.001 | 0.57 |
| SF-12 Mental Component | 49.0 ± 12.0 | 50.3 ± 11.1 | 0.004 | 0.11 |
| Health Status at 12 months† | ||||
| SAQ Angina Frequency | 91.3 ± 17.7 | 93.6 ± 15.2 | <0.001 | 0.14 |
| SAQ Quality of Life | 80.2 ± 22.3 | 82.5 ± 20.1 | 0.008 | 0.11 |
| SAQ Physical Limitation | 89.8 ± 21.1 | 93.4 ± 16.2 | <0.001 | 0.19 |
| SF-12 Physical Component | 40.0 ± 12.2 | 45.1 ± 11.5 | <0.001 | 0.43 |
| SF-12 Mental Component | 51.7 ± 10.8 | 52.2 ± 10.2 | 0.31 | 0.05 |
SAQ, Seattle Angina Questionnaire
Effect size ~0.2 indicates small difference, ~0.5 indicates moderate difference, ~0.8 indicates large difference
Scores for the SAQ and SF-12 range from 0–100, with higher scores indicating less disease burden and better quality of life
In the repeated measures model that adjusted for multiple factors (demographics, clinical characteristics, acute AMI presentation, severity of coronary disease, in-hospital treatments [including coronary revascularization] and hospital site), DM was associated with a greater likelihood of angina over the 12 months of follow-up; and the magnitude of this association increased over time (interaction of DM*time p=0.008; Figure 3). At 1 month after AMI, after multivariable adjustment, there was no significant difference in the prevalence of angina based on DM status. However, at 12 months after AMI, patients with DM had 18% greater risk of having angina compared with patients without DM (RR 1.18, 95% CI 1.01–1.37; full model results in Supplemental Table 2).
Figure 3. Relative Risk of Angina among Patients with Diabetes over the Year Following Myocardial Infarction.
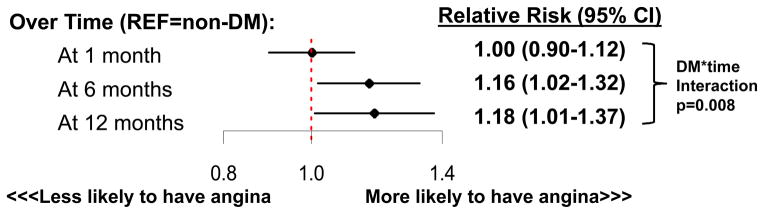
Reference group is patients without diabetes. Adjusted for age, sex, race, hypertension, smoking status, depressive symptoms, prior angioplasty, prior bypass graft surgery, in-hospital angioplasty, in-hospital bypass graft surgery, presence of multivessel disease, left ventricular dysfunction, ST-elevations on presentation, and GRACE discharge score
Predictors of angina among DM patients
In a second multivariable model that included only patients with DM, neither duration of DM nor long-term glucose control (estimated with HbA1c) were significantly associated with angina over the 12 months after AMI (p=0.31 and 0.52, respectively). However, compared with patients treated with oral hypoglycaemics or controlled with diet only, patients treated with insulin had a greater risk of angina over time (RR 1.18, 95% CI 1.04–1.34).
DISCUSSION
In a large, multicenter registry of AMI patients, we found that approximately 1 in 4 patients report angina at 12 months after an AMI, despite treatment with modern medical and interventional therapies. Contrary to conventional wisdom, patients with DM experienced greater angina burden than those without DM, both prior to and following AMI—an effect that was amplified over time. Patients with DM also had more physical limitations due to angina and worse quality of life. Of note, patients with DM received more antianginal medications, indicating that this association would likely be even stronger with similar intensity of medical treatment. Given the impact of angina on quality of life, repeat hospitalizations and resource utilization,15 continued efforts to reduce angina after an AMI are needed, particularly among patients with DM who have a higher burden of both coronary disease and angina.
Prior Studies
Whether patients with DM experience more angina than those without diabetes has been a subject of debate. Some older studies7–9 implied that patients with DM have more silent ischemia and experienced less angina. However, more recent investigations do not support this notion. A 2-center observational study of 1199 patients hospitalized with an acute coronary syndrome from 2001–02 showed that patients with DM had a higher angina burden 1 year after hospital discharge as compared with those without DM, with higher unadjusted rates of 1-year angina in both groups (37% and 27%, respectively) and the adjusted odds of angina were even higher than what we observed (Odds Ratio 1.36; 95% CI 1.01–1.38).12 Importantly, 43% of the patients in that study presented with unstable angina and 40% were medically managed, which could explain the greater difference in angina burden observed in that study. In a more recent study—the MERLIN-TIMI 36 multinational clinical trial of ranolazine after non-ST-elevation acute coronary syndrome—patients with DM had a greater incidence of recurrent ischemia over 1 year after hospitalization than those without DM.13 By demonstrating the greater burden of angina in patients with DM than those without DM using contemporary, multicenter, prospective real-world data and by finding this effect is amplified over time (as opposed to examining only 2 time points), our study substantially expands on these prior findings.
Potential Mechanisms
While more investigation is needed to determine the underlying reasons for this association, there is evidence to suggest that patients with DM have a more diffuse nature of coronary disease25 and greater progression of their coronary disease26 than those without DM. In addition, mechanisms unrelated to coronary anatomy, such as microvascular impairment27 and endothelial dysfunction28 or different metabolic demands29, may also play a role. Our finding of a significant association between DM and angina, independent of multivessel disease, suggests that it may be more than just an increased prevalence of epicardial disease that accounts for this increased burden of angina among patients with DM. Ultimately, recognizing the multiple macrovascular, microvascular, and metabolic differences between patients with and without DM, the reasons for increased angina burden among patients with DM are likely multifactorial.
In addition to the anatomic and physiological differences between patients with and without DM, differences in treatment and also differential responses to treatments may also play a role in the frequency and severity of angina after AMI. For example, revascularization was not only less common among patients with DM (likely resulting from coronary disease anatomy that does not lend itself well to feasible revascularization) but revascularization techniques also differed. Patients with DM were slightly more likely to undergo bypass graft surgery (11% vs. 9%), which has been shown in DM patients to be associated with small improvements in angina (SAQ angina frequency difference of 1.3 points at 2 years) compared with drug-eluting stents.30 While we did adjust for revascularization type in our multivariable analysis, this may be one potential target to narrow the gap in angina burden between patients with and without DM. In addition, antianginal therapies differed between groups, and it is unknown whether particular antianginal medications are more or less effective in patients with DM, although there is some data to support additional benefit of both nitrates31 and ranolazine13 in patients with DM. Further investigations as to the mechanisms underlying the observed association of DM with increased angina are necessary as they could provide important information both about potential targets for intervention as well as potential treatments to target angina among patients with DM.
Limitations
Our findings should be interpreted in the context of several potential limitations. First, within the DM patients, our assessments of the severity of DM or DM treatments were somewhat limited as we did not have detailed information about complications of DM, serial HbA1c measurements prior to AMI, or other measures of chronic severity of DM. More dedicated study among patients with DM with sensitive assessments of long-term DM control and complications could identify additional potential mediators of our findings. Second, the severity of anatomic coronary disease during the AMI hospitalization was defined based on chart review of the coronary angiogram and not by an angiographic core laboratory. As such, more sensitive definitions of coronary disease severity, such as SYNTAX score, could not be determined, which may have influenced our ability to fully adjust for the differences between patients based on DM status. However, given the number of clinical and treatment factors that we were able to adjust for, it is unlikely that this additional adjustment would have substantially altered our findings. Finally, patients with DM were less likely to undergo revascularization during the AMI compared with non-DM patients. While there was slightly less invasive management in the DM patients, this difference is more likely driven by anatomic differences (i.e., the ability to revascularize) between groups. While this could have impacted our unadjusted analyses, we did adjust for revascularization strategy in our multivariable model and, thus, this should not have affected our adjusted results.
Conclusion
In a large, multicenter AMI registry, angina prior to and after AMI was more prevalent and more severe among patients with DM than those without. This effect was present despite more aggressive angina treatment among patients with DM, was amplified over time, and was independent of patient and treatment factors, including the presence of multivessel disease. More attention to recognizing and treating angina after AMI, particularly among patients with DM, may improve the quality of life of these high-risk patients.
Supplementary Material
Acknowledgments
Funding: TRIUMPH was sponsored by a grant from the National Institutes of Health (National Heart, Lung, Blood Institute): SCCOR Grant #P50HL077113-01. This work was supported by an investigator-initiated research grant from Gilead Sciences, Foster City, CA. The funding organizations did not play a role in the design and conduct of the study or in the collection, management, analysis, and interpretation of the data.
Footnotes
Disclosures: SVA: Reseasch grants: Gilead, Genentech, Sanofi-Aventis; DKM: Consultant honoraria: Genentech; F Hoffmann LaRoche, Pfizer, Daiichi Sankyo, NovoNordisk, Sanofi Aventis, Regeneron, Tethys Bioscience. Clinical trial leadership honoraria: Boehringer Ingelheim, Takeda, Orexigen, AstraZeneca, Bristol Myers Squibb, Eli Lilly, Daiichi Sankyo, Merck Schering Plough. JAS: Research grants: NHLBI, AHA, ACCF, Gilead, Lilly, EvaHeart, Amorcyte. Consultation: United Healthcare, Genentech, Amgen. Ownership of the Seattle Angina Questionnaire. MK: Research grants: American Heart Association, Genetech, Sanofi-Aventis, Gilead, Medtronic Minimed, Glumetrics, Maquest, Eisai; Consultant honoraria: Genentech, Gilead, F Hoffmann LaRoche, Medtronic Minimed, AstraZeneca, Abbvie, Regeneron, Eli Lilly.
The other authors report no conflicts of interest.
References
- 1.Stolker JM, Sun D, Conaway DG, Jones PG, Masoudi FA, Peterson PN, Krumholz HM, Kosiborod M, Spertus JA. Importance of measuring glycosylated hemoglobin in patients with myocardial infarction and known diabetes mellitus. Am J Cardiol. 2010;105(8):1090–4. doi: 10.1016/j.amjcard.2009.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abi Khalil C, Roussel R, Mohammedi K, Danchin N, Marre M. Cause-specific mortality in diabetes: recent changes in trend mortality. Eur J Prev Cardiol. 2012;19(3):374–81. doi: 10.1177/1741826711409324. [DOI] [PubMed] [Google Scholar]
- 3.Donahoe SM, Stewart GC, McCabe CH, Mohanavelu S, Murphy SA, Cannon CP, Antman EM. Diabetes and mortality following acute coronary syndromes. JAMA. 2007;298(7):765–75. doi: 10.1001/jama.298.7.765. [DOI] [PubMed] [Google Scholar]
- 4.Duarte R, Castela S, Reis RP, Correia MJ, Ramos A, Pereira AP, Martins P, Correia JM. Acute coronary syndrome in a diabetic population--risk factors and clinical and angiographic characteristics. Rev Port Cardiol. 2003;22(9):1077–88. [PubMed] [Google Scholar]
- 5.Herlitz J, Wognsen GB, Emanuelsson H, Haglid M, Karlson BW, Karlsson T, Albertsson P, Westberg S. Mortality and morbidity in diabetic and nondiabetic patients during a 2-year period after coronary artery bypass grafting. Diabetes Care. 1996;19(7):698–703. doi: 10.2337/diacare.19.7.698. [DOI] [PubMed] [Google Scholar]
- 6.Haffner SM, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998;339(4):229–34. doi: 10.1056/NEJM199807233390404. [DOI] [PubMed] [Google Scholar]
- 7.Marchant B, Umachandran V, Stevenson R, Kopelman PG, Timmis AD. Silent myocardial ischemia: role of subclinical neuropathy in patients with and without diabetes. J Am Coll Cardiol. 1993;22(5):1433–7. doi: 10.1016/0735-1097(93)90554-e. [DOI] [PubMed] [Google Scholar]
- 8.Murray DP, O'Brien T, Mulrooney R, O'Sullivan DJ. Autonomic dysfunction and silent myocardial ischaemia on exercise testing in diabetes mellitus. Diabet Med. 1990;7(7):580–4. doi: 10.1111/j.1464-5491.1990.tb01452.x. [DOI] [PubMed] [Google Scholar]
- 9.Chiariello M, Indolfi C, Cotecchia MR, Sifola C, Romano M, Condorelli M. Asymptomatic transient ST changes during ambulatory ECG monitoring in diabetic patients. Am Heart J. 1985;110(3):529–34. doi: 10.1016/0002-8703(85)90070-5. [DOI] [PubMed] [Google Scholar]
- 10.May O, Arildsen H, Damsgaard EM, Mickley H. Prevalence and prediction of silent ischaemia in diabetes mellitus: a population-based study. Cardiovasc Res. 1997;34(1):241–7. doi: 10.1016/s0008-6363(97)00046-1. [DOI] [PubMed] [Google Scholar]
- 11.Caracciolo EA, Chaitman BR, Forman SA, Stone PH, Bourassa MG, Sopko G, Geller NL, Conti CR. Diabetics with coronary disease have a prevalence of asymptomatic ischemia during exercise treadmill testing and ambulatory ischemia monitoring similar to that of nondiabetic patients. An ACIP database study. ACIP Investigators. Asymptomatic Cardiac Ischemia Pilot Investigators Circulation. 1996;93(12):2097–105. doi: 10.1161/01.cir.93.12.2097. [DOI] [PubMed] [Google Scholar]
- 12.Peterson PN, Spertus JA, Magid DJ, Masoudi FA, Reid K, Hamman RF, Rumsfeld JS. The impact of diabetes on one-year health status outcomes following acute coronary syndromes. BMC Cardiovasc Disord. 2006;6:41. doi: 10.1186/1471-2261-6-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morrow DA, Scirica BM, Chaitman BR, McGuire DK, Murphy SA, Karwatowska-Prokopczuk E, McCabe CH, Braunwald E. Evaluation of the glycometabolic effects of ranolazine in patients with and without diabetes mellitus in the MERLIN-TIMI 36 randomized controlled trial. Circulation. 2009;119(15):2032–9. doi: 10.1161/CIRCULATIONAHA.107.763912. [DOI] [PubMed] [Google Scholar]
- 14.Maddox TM, Reid KJ, Spertus JA, Mittleman M, Krumholz HM, Parashar S, Ho PM, Rumsfeld JS. Angina at 1 year after myocardial infarction: prevalence and associated findings. Arch Intern Med. 2008;168(12):1310–6. doi: 10.1001/archinte.168.12.1310. [DOI] [PubMed] [Google Scholar]
- 15.Arnold SV, Morrow DA, Lei Y, Cohen DJ, Mahoney EM, Braunwald E, Chan PS. Economic impact of angina after an acute coronary syndrome: insights from the MERLIN-TIMI 36 trial. Circ Cardiovasc Qual Outcomes. 2009;2(4):344–53. doi: 10.1161/CIRCOUTCOMES.108.829523. [DOI] [PubMed] [Google Scholar]
- 16.Arnold SV, Chan PS, Jones PG, Decker C, Buchanan DM, Krumholz HM, Ho PM, Spertus JA. Translational Research Investigating Underlying Disparities in Acute Myocardial Infarction Patients' Health Status (TRIUMPH): Design and Rationale of a Prospective Multicenter Registry. Circ Cardiovasc Qual Outcomes. 2011;4(4):467–476. doi: 10.1161/CIRCOUTCOMES.110.960468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spertus JA, Winder JA, Dewhurst TA, Deyo RA, Prodzinski J, McDonell M, Fihn SD. Development and evaluation of the Seattle Angina Questionnaire: a new functional status measure for coronary artery disease. J Am Coll Cardiol. 1995;25(2):333–41. doi: 10.1016/0735-1097(94)00397-9. [DOI] [PubMed] [Google Scholar]
- 18.Ware J, Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220–33. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 19.Spertus JA, Salisbury AC, Jones PG, Conaway DG, Thompson RC. Predictors of quality-of-life benefit after percutaneous coronary intervention. Circulation. 2004;110(25):3789–94. doi: 10.1161/01.CIR.0000150392.70749.C7. [DOI] [PubMed] [Google Scholar]
- 20.Muller-Nordhorn J, Roll S, Willich SN. Comparison of the short form (SF)-12 health status instrument with the SF-36 in patients with coronary heart disease. Heart. 2004;90(5):523–7. doi: 10.1136/hrt.2003.013995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cohen J. Statistical power analysis for the behavioral sciences. 2. New Jersey: Lawrence Erlbaum; 1988. [Google Scholar]
- 22.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–13. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eagle KA, Lim MJ, Dabbous OH, Pieper KS, Goldberg RJ, Van de Werf F, Goodman SG, Granger CB, Steg PG, Gore JM, Budaj A, Avezum A, Flather MD, Fox KA. A validated prediction model for all forms of acute coronary syndrome: estimating the risk of 6-month postdischarge death in an international registry. JAMA. 2004;291(22):2727–33. doi: 10.1001/jama.291.22.2727. [DOI] [PubMed] [Google Scholar]
- 24.Fleiss JL, Levin BA, Paik MC. Statistical methods for rates and proportions. 3. Hoboken, N.J: Wiley; 2003. [Google Scholar]
- 25.Woodfield SL, Lundergan CF, Reiner JS, Greenhouse SW, Thompson MA, Rohrbeck SC, Deychak Y, Simoons ML, Califf RM, Topol EJ, Ross AM. Angiographic findings and outcome in diabetic patients treated with thrombolytic therapy for acute myocardial infarction: the GUSTO-I experience. J Am Coll Cardiol. 1996;28(7):1661–9. doi: 10.1016/s0735-1097(96)00397-x. [DOI] [PubMed] [Google Scholar]
- 26.Yeh RW, Normand SL, Wolf RE, Jones PG, Ho KK, Cohen DJ, Cutlip DE, Mauri L, Kugelmass AD, Amin AP, Spertus JA. Predicting the restenosis benefit of drug-eluting versus bare metal stents in percutaneous coronary intervention. Circulation. 2011;124(14):1557–64. doi: 10.1161/CIRCULATIONAHA.111.045229. [DOI] [PubMed] [Google Scholar]
- 27.Marciano C, Galderisi M, Gargiulo P, Acampa W, D'Amore C, Esposito R, Capasso E, Savarese G, Casaretti L, Lo Iudice F, Esposito G, Rengo G, Leosco D, Cuocolo A, Perrone-Filardi P. Effects of type 2 diabetes mellitus on coronary microvascular function and myocardial perfusion in patients without obstructive coronary artery disease. Eur J Nucl Med Mol Imaging. 2012;39(7):1199–206. doi: 10.1007/s00259-012-2117-9. [DOI] [PubMed] [Google Scholar]
- 28.Schalkwijk CG, Stehouwer CD. Vascular complications in diabetes mellitus: the role of endothelial dysfunction. Clin Sci (Lond) 2005;109(2):143–59. doi: 10.1042/CS20050025. [DOI] [PubMed] [Google Scholar]
- 29.Stanley WC, Lopaschuk GD, McCormack JG. Regulation of energy substrate metabolism in the diabetic heart. Cardiovasc Res. 1997;34(1):25–33. doi: 10.1016/s0008-6363(97)00047-3. [DOI] [PubMed] [Google Scholar]
- 30.Abdallah MS, Wang K, Magnuson EA, Spertus JA, Farkouh ME, Fuster V, Cohen DJ. Quality of life after PCI vs CABG among patients with diabetes and multivessel coronary artery disease: a randomized clinical trial. JAMA. 2013;310(15):1581–90. doi: 10.1001/jama.2013.279208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jansen R, Niemeyer MG, Cleophas TJ, Zwinderman AH. Factors influencing efficacy of nitrate therapy for stable angina pectoris: a multiple linear regression analysis. Angiology. 2000;51(12):1007–12. doi: 10.1177/000331970005101205. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.