Abstract
Organic chemists and metabolic engineers use largely orthogonal technologies to access small molecules like pharmaceuticals and commodity chemicals. As the use of biological catalysts and engineered organisms for chemical production grows, it is becoming increasingly evident that future efforts for chemical manufacture will benefit from the integration and unified expansion of these two fields. This review will discuss approaches that combine chemical and biological synthesis for small molecule production. We highlight recent advances in combining enzymatic and non-enzymatic catalysis in vitro, discuss the application of design principles from organic chemistry for engineering non-biological reactivity into enzymes, and describe the development of biocompatible chemistry that can be interfaced with microbial metabolism.
Introduction
Both organic chemists and metabolic engineers strive to efficiently access small molecule products that are essential components of our everyday lives. However, these two scientific disciplines rely on largely independent technologies to achieve this goal [1–5]. Over the course of almost two centuries, synthetic organic chemists have developed a vast array of reagents, catalysts, reaction conditions, and solvents to facilitate the conversion of one compound into another. By executing a series of these transformations in which the product of one reaction serves as the starting material for the subsequent reaction, chemists access final products of interest in a controlled, multi-step fashion. This stands in contrast to how organisms have evolved to synthesize small molecules. In a cellular setting, naturally occurring protein-based catalysts, enzymes, chemically modify small molecule metabolites in one reaction vessel (the cell) under highly constrained reaction conditions (ambient temperature in an aqueous environment). Metabolic engineers work within these in vivo parameters, using modern molecular biology techniques to both adjust and construct pathways within microorganisms to maximize production of a small molecule product of interest [6–8].
As the field of metabolic engineering continues to make rapid advances, the question arises as to which approach may be better suited for the production of a given target molecule. Perhaps the most important consideration is whether or not the synthetic tools offered by each approach possess the reactivity and selectivity needed to access the desired product. In the biological realm, enzymes offer unique advantages due to their exquisite substrate selectivity, high catalytic efficiency, and unparalleled levels of chemo-, regio-, and stereocontrol. Approaches like directed evolution enable access to enzymes tailored to accept non-natural substrates [9–11]. Additionally, it is now possible to transfer multiple enzymes, and even whole metabolic pathways, into host organisms for chemical production [12–14]. However, even with these advances, biological catalysts still offer a relatively limited reaction repertoire in comparison to synthetic organic chemistry. Having to employ further modification of metabolic engineering products using separate chemical synthesis steps to reach the final target is fairly common, especially in cases when the desired small molecule is not of natural origin [14]. While advances in enzyme engineering will undoubtedly expand the breadth of organisms’ synthetic capabilities, it seems entirely possible that they will never be able to match those of the organic chemist.
Beyond an initial assessment of feasibility, judging the success of a synthetic effort must also include the following factors: the overall yield of the product, the cost of its production, the environmental impact of the process, and its simplicity. The Nobel prize-winning chemist Sir John Cornforth described the ideal synthesis as “something to be carried out in a disused bathtub…, the product being collected continuously through the drain hole in 100% purity and yield” [15]. By avoiding multi-step reaction sequences and reducing environmental impact (e.g. using renewable feedstocks, minimizing hazardous waste), fermentation processes are quite attractive relative to traditional organic synthesis when applying these criteria.
Since the strengths and weaknesses of organic chemistry and metabolic engineering are largely complementary, the question should not be which approach is superior, but how can we realize opportunities to combine the beneficial aspects of each field (Figure 1) [16]. This review will discuss recent advances in interfaced organic and biological synthesis, focusing on examples that truly merge tools and design principles from synthetic chemistry with enzymes or living organisms for the purpose of small molecule production. We will not include semi-synthesis, sequential “one-flask” chemocatalytic-biocatalytic cascades, and bioorthogonal chemistry. We will include methodology that involves simultaneous use of non-enzymatic and enzymatic catalysis, enzymes engineered to display non-biological reactivity, and biocompatible reactions that can interface with the metabolism of living organisms. We will also highlight key experiments that seeded interest in each area and outline future challenges for this developing area of research.
Figure 1.
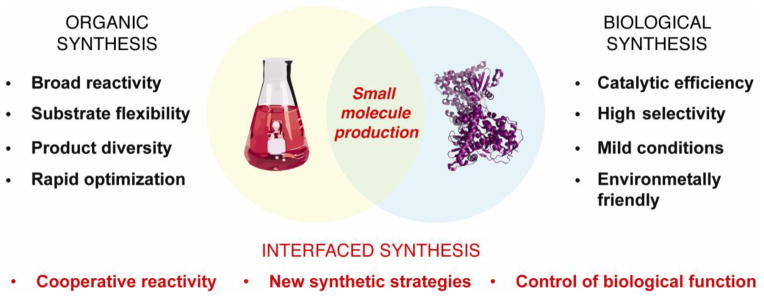
Opportunities for merging chemical and biological synthesis.
Combining non-enzymatic and enzymatic catalysts in vitro
Conceptually, perhaps the simplest way to unite organic and biological chemistry is to combine synthetic tools from both approaches in vitro. This strategy is advantageous when the merging of enzymatic and non-enzymatic chemistry enables a synthetic transformation to proceed with a selectivity or efficiency not available for the corresponding sequential process. This phenomenon, known as cooperativity, was first achieved in the early 1970’s with Hafner and Wellner’s development of an amino acid stereoinversion reaction that utilized an amino acid oxidase enzyme with the non-enzymatic reagent sodium borohydride [17]. The combination of enzymatic and non-enzymatic catalysis has been particularly useful for dynamic kinetic resolutions, processes that combine an enantioselective enzymatic catalyst with a non-enzymatic reagent or catalyst that promotes the interconversion of starting material enantiomers [18–20].
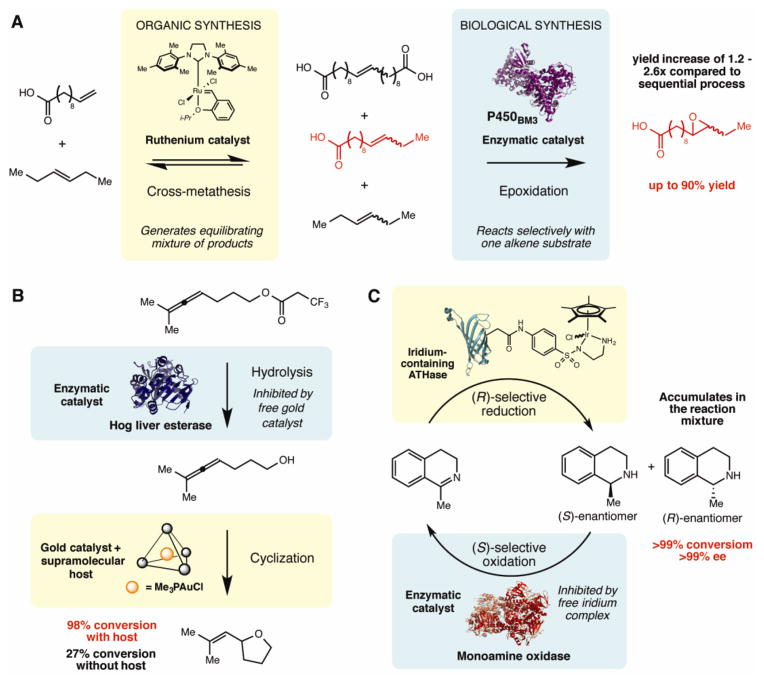
Recently the labs of Hartwig and Zhao reported an advance in tandem enzymatic/non-enzymatic catalysis: the first demonstration of cooperative catalysis involving an organometallic catalyst and a metalloenzyme (Figure 2A) [21••]. This transformation combined a ruthenium-catalyzed olefin cross-metathesis reaction with a cytochrome P450-catalyzed epoxidation. They envisioned cooperativity could arise from the selectivity of the P450 for only one alkene substrate, which would be generated via cross-metathesis as part of an equilibrating product mixture and continually replenished by the activity of the metathesis catalyst. In practice, this tandem one-pot reaction provided higher yields than would be obtainable using the corresponding two-step sequence. Conceptually this work represents a significant advance and should inspire efforts to incorporate a more diverse set of enzymatic and non-enzymatic reactions into tandem processes.
Figure 2.
Approaches for combining enzymatic and non-enzymatic catalysis in vitro. A. A cooperative cross metathesis-epoxidation reaction using a ruthenium(I) catalyst and a P450BM3 enzyme. B. A tandem hydrolysis-cyclization sequence using a a lipase/esterase enzyme and a encapsulated gold(I)-catalyst. C. A dynamic kinetic resolution of cyclic amines using an engineered monoamine oxidase and an artificial transfer hydrogenase (ATHase) consisting of biotin-conjugated iridium-catalyst bound to streptavidin.
Despite the promise of this approach, cooperative transformations combining chemical and biological catalysts have utilized a relatively small number of the reaction manifolds available to synthetic chemists. This is likely due to an inherent challenge faced in reaction development: mutual deactivation of the enzyme and the chemical catalyst when utilized together [22,23]. In the Hartwig and Zhao work, deactivation of the catalyst and enzyme was minimized by the use of a biphasic reaction system, but could not be completely circumvented. In the past year, two reports have offered alternative approaches to solving the deactivation problem, both of which rely on embedding a non-enzymatic transition metal catalyst in an environment that mimics an enzyme active site.
Bergman, Raymond, Toste, and co-workers utilized encapsulation by a supramolecular cluster to enable the simultaneous use of organometallic and enzymatic catalysis (Figure 2B) [24••]. This strategy stems from Breslow’s construction of “artificial enzymes” via encapsulation of a transition metal within a cyclodextrin [25]. In the context of individual reactions, this type of encapsulation design was known to improve lifetimes of organometallic catalysts, enhance their solubility in aqueous media, and prevent sensitive complexes from interacting with other reaction components [26,27]. Bergman, Raymond, Toste, and co-workers hypothesized that catalyst encapsulation could enable the use of an organometallic complex in combination with an enzyme. To test this idea, they designed tandem reaction sequences that coupled an enzymatic hydrolysis reaction with a cyclization catalyzed by a gold(I) species encapsulated within a tetrahedral Ga4L6 cluster (L= N, N′-bis(2,3-dihydroxybenzoyl)-1,5-diaminonapthalene). Using this strategy, increased reactivity and selectivity was achieved compared to reactions with the gold(I) catalyst alone. They also showed that the rate of the enzymatic reaction was dramatically reduced in the absence of the Ga4L6 cluster, indicating that the free gold(I) catalyst was detrimental to enzyme function and the host complex played a protective role in the tandem process [28]. Overall, this study demonstrated that supramolecular encapsulation is a viable strategy for combining an otherwise incompatible transition metal-catalyzed reaction with an enzyme.
The work of Hollman, Turner, Ward, and co-workers offers a complementary approach for facilitating the simultaneous use of non-enzymatic and enzymatic reactivity [29••]. By incorporating a biotin-conjugated iridium complex into the framework of the protein streptavidin, they generated an artificial transfer hydrogenase (ATHase) possessing reactivity associated with non-enzymatic, transition metal catalysts (Figure 2C). The ATHase could be used in tandem reactions employing a variety of enzymes, including a stereoselective deracemization of cyclic amines that utilized an engineered monoamine oxidase. In this reaction, the environment of the protein scaffold was critical for protecting the monoamine oxidase from deactivation; no enzymatic activity was observed in the presence of the unbound iridium complex. Another cascade process utilizing the ATHase was a coupled colorimetric assay that provided a direct readout of catalyst activity and was used to identify an improved ATHase variant. Coupled assays of this type could streamline efforts to engineer this class of organometallic hybrid catalysts via directed evolution.
These recent examples represent important advances in both the design and implementation of tandem enzymatic/non-enzymatic catalysis that should have a synergistic effect on future attempts to employ this approach in synthesis. Improved strategies for sequestering the two catalyst types will allow more flexibility in the types of reactivity incorporated into cascades. This will in turn enable the design of increasingly elegant and efficient cooperative reaction sequences that take full advantage of the unique aspects of organic and biological modes of catalysis.
Engineering enzymes to display non-biological reactivity in vitro and in vivo
Over the past decade, powerful advances in protein engineering technologies have fueled interest in extending the range of reactions amenable to biocatalysts to include those that have no natural antecedents [30,31]. As the development of ADHase illustrates, incorporating transition metals and transition metal complexes is a viable strategy for introducing non-enzymatic reactivity into protein scaffolds. This approach, which was originally pioneered by Whitesides [32], has been applied to a variety of protein scaffolds and transition metal complexes [33,34]. In many cases, the reactivity and selectivity of these hybrid catalysts do not surpass the results achieved by organic chemists. This may change with increasing efforts to engineer the protein scaffold for participation in the reaction mechanism [35–38].
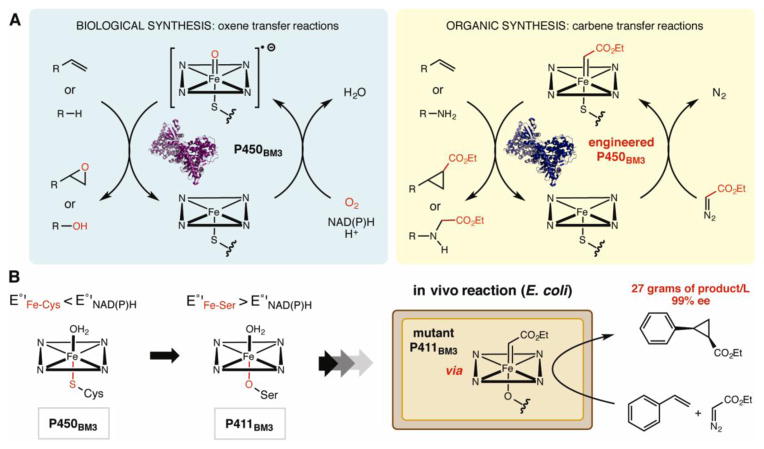
An alternative approach to generating non-biological reactivity using enzyme scaffolds utilizes design principles and reaction manifolds from organic chemistry to guide reengineering of natural metalloenzyme function. Several recent papers from the Arnold group demonstrating the use of engineered P450BM3 mutants for unnatural carbene and nitrene insertion reactions illustrate the potential of this strategy [39–42]. In each case, the design of the enzymatic transformation was inspired by the reactivity of iron porphyrins and related organometallic catalysts used in organic synthesis.
In their first report [39••], Arnold and co-workers hypothesized that cytochrome P450 enzymes, which catalyze a variety of reactions involving oxene transfer, might also be capable of generating and promoting transfer of carbene intermediates in the presence of suitable reagents (Figure 3A). After demonstrating that wild type P450BM3 could promote cyclopropanation of styrene in the presence of ethyl diazoacetate under anaerobic conditions, they screened a library of P450BM3 mutants to evaluate whether engineering the protein scaffold could impact the reactivity and selectivity. Mutations that increased cyclopropanation activity could be combined to generate P450BM3 variants exhibiting not only catalytic activity comparable to nativeP450-catalysed hydroxylation reactions, but also enhanced diastereo- and enantioselectivity. The Arnold group applied related logic to engineer P450s that promote carbene insertions into N–H bonds and to develop a C-H amination reaction using sulfonyl azide substrates [41•,42]. Most recently, this general approach has been extended beyond P450s with the report of C–N bond-forming reactions promoted by wild-type and engineered variants of the non-heme iron(II)-dependent halogenase SyrB2 in the presence of both azide and nitrite anions [43•].
Figure 3.
Engineering enzymes to display non-biological reactivity using organic chemistry as inspiration. A. The mechanistic similarities between natural oxene transfer reactions of cytochrome P450 enzymes and carbene transfer reactions. B. A simple axial ligand mutation facilitated catalysis of cyclopropanation by P411BM3 in a whole-cell format.
Arnold and co-workers have also demonstrated that the P450BM3-mediated cyclopropanation reaction can be utilized in vivo for whole-cell biocatalysis (Figure 3B) [40••]. This advance was facilitated by the discovery that a simple C400S axial ligand mutation in the catalytic heme domain of a P450BM3 variant could raise the resting-state reduction potential of the enzyme, obviating the need for exogenous reducing agents and allowing reduction by NADH in vivo. These C440S mutants were named ‘P411BM3’s after the characteristic Fe(II)-CO band in their UV-visible spectra at 411 nm. In comparison to the purified enzymes, P411BM3 variants displayed increased activity in the whole-cell format. Most impressively, the whole-cell system was capable of generating cis-ethyl 2-phenylcyclopropane-1-carboxylate from ethyl diazoacetate and styrene in titres of 27 g L−1, 78% isolated yield (total turnovers (TTN) = 4.88×103), and in 99% ee. The success of this reaction on a large scale suggests that enzymes with non-biological reactivity may be powerful new tools for small molecule production.
Integrating organic chemistry with cellular metabolism
While the use of biocatalysis has become increasingly important in synthetic chemistry, the complementary possibility of incorporating non-enzymatic reagents and catalysts from organic chemistry into metabolic engineering efforts has been comparatively underexplored. To the best of our knowledge, the use of non-enzymatic chemistry to influence microbial metabolism originated with Neuberg’s bisulfite-steered glycerol fermentations during the latter stages of the First World War [44,45]. Substantial overproduction of glycerol from glucose in S. cerevisiae could be achieved through the addition of sodium bisulfite to the fermentation. It was later found that the bisulfite anion formed a stable adduct with the metabolite acetaldehyde. Formation of this type of adduct is used in organic synthesis as a strategy for protecting aldehydes [46]. The consequence of introducing this reactivity into the fermentation was inhibition of acetaldehyde reduction to ethanol (Figure 4A). This forced accumulating NADH to be re-oxidized to NAD+ via an alternate pathway that generated glycerol as the primary fermentation product. This technology contributed significantly to the German war effort by allowing the industrial manufacture of glycerol from sugar for use in the production of explosives. Since this achievement there have been only sporadic examples of systems that directly combine reagents from synthetic organic chemistry with metabolism. Mountfort and coworkers reported the hydrogenation of ethylene using hydrogen gas produced by the syntroph Syntrophomonas wolfei and super-stoichiometric amounts of a heterogeneous palladium catalyst, a transformation that disrupted the metabolic interaction between S. wolfei and the methanogen Methanospirillum hungatei [47]. Other researchers have used transition metal catalysts to hydrogenate membrane lipids in the cyanobacterium Synechocystis sp. PCC 6803, facilitating studies of the biological response to changes in membrane fluidity [48].
Figure 4.
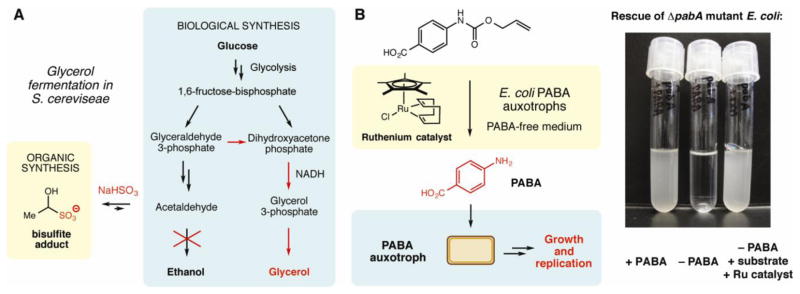
Integrating non-enzymatic chemistry with cellular metabolism. A. Neuberg’s bisulfite-steered glycerol fermentations. The chemical reagent sodium bisulfite was used to redirect the natural fermentation in S. cereviseae to form glycerol. NADH = nicotinamide adenine dinucleotide. B. Auxotroph rescue with non-enzymatic chemistry. A Ru-catalyzed deprotection reaction was used to support growth of an E. coli PABA auxotroph. PABA = para-aminobenzoic acid.
We believe that it is time to revisit the idea of merging reactivity from organic synthesis with cellular metabolism. Advances in protein and metabolic engineering have greatly expanded the types of reactions and pathways that can be introduced into living organisms. At the same time, organic chemists are pioneering new approaches for accelerating catalyst and reaction development [49,50]. Finally, there has been a dramatic increase in the diversity of reaction types employed in bioorthogonal chemistry for applications like labeling and imaging of macromolecules [51]. With this progress as a backdrop, we have begun to develop biocompatible chemistry: non-enzymatic reactions that influence metabolism by chemically modifying small molecules in the presence of living organisms. This approach is distinct from bioorthogonal chemistry, which utilizes non-enzymatic reactions designed to neither interact nor interfere with biological systems [52–54].
There are significant technical challenges associated with the implementation of biocompatible chemistry, most notably the perceived mismatch between the reaction conditions necessary for synthetic organic chemistry (organic solvents, extreme temperatures and pH) and the growth conditions necessary to support living organisms (aqueous media, ambient temperature, neutral pH). Other challenges include the complexity of the intra- and extracellular environments, the limited cell-permeability of non-biological reagents, and the low concentrations of cellular metabolites. Despite the many obstacles associated with their development, biocompatible reactions would offer scientists a unique toolkit for manipulating and augmenting biological functions that would complement existing approaches.
In the context of small molecule production, biocompatible chemical transformations could be combined with native or engineered metabolism in vivo. These hybrid pathways could harness the diverse reactivity associated with non-enzymatic reactions, providing access to chemical structures that could not be produced through the use of enzymatic chemistry alone. The metabolism of microbes could be employed to generate unstable or toxic chemical reagents directly in reaction mixtures, potentially obviating the need to chemically synthesize, transport, and store large quantities of these materials. Perhaps most importantly, biocompatible reactions would provide a means of manipulating metabolite structures and concentrations in vivo that would operate independently from the central dogma. As metabolites mediate many cellular processes, changing their chemical structures could serve as way to influence biological function. For metabolic engineering, this could mean new approaches for influencing pathway flux and regulation that would not require genetic manipulation.
A recent study from our laboratory has demonstrated the ability of biocompatible reactions to control biological function [55••]. We utilized non-enzymatic transition metal-catalyzed reactions to support the growth of auxotrophic microorganisms via the in vivo generation of essential nutrients from non-utilizable precursors (Figure 4B). Two different transformations were employed to rescue two distinct auxotrophies: a [Cp*Ru(cod)Cl]-catalyzed deprotection reaction that generated para-aminobenzoic acid (PABA) and an iron-catalyzed arene hydroxylation that formed para-hydroxybenzoic acid. In both cases, growth of the auxotroph was dependent on the success of the non-enzymatic reaction. While the efficiencies of both transformations were low, this work illustrates the dramatic influence that even a small change in metabolite levels can have on living organisms.
Overall, this study represents an initial step towards integrating reactions from organic chemistry with microbial metabolism; additional work will be required to overcome the substantial obstacles associated with this approach. Despite these challenges, we believe biocompatible chemistry will offer unique possibilities for small molecule production in the future.
Conclusions
The fields of organic chemistry and metabolic engineering have traditionally represented two independent solutions to the problem of small molecule synthesis. As recent work illustrates, the potential benefits of combining tools from both approaches are beginning to be realized. We believe that the time has come to explore in earnest the opportunities that exist at the intersection of these two areas of research, and we have chosen to discuss advances that we hope will inspire future research. We predict that more new strategies for small molecule production will emerge from continued research at the organic synthesis/biological synthesis interface.
Highlights.
Efforts to combine organic and biological synthesis are increasing.
Sequestering non-enzymatic and enzyme catalysts can overcome incompatibility.
Organic chemistry inspires engineering of non-biological reactivity into enzymes.
Non-enzymatic reactions can be integrated with cellular metabolism.
Acknowledgments
This work was supported by the National Institutes of Health (DP2 GM105434). S. Wallace acknowledges receipt of a Marie Curie International Outgoing Fellowship from the European Commission.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Nicolaou KC. The emergence of the structure of the molecule and the art of its synthesis. Angew Chem Int Ed. 2013;52:131–146. doi: 10.1002/anie.201207081. [DOI] [PubMed] [Google Scholar]
- 2.Nicolaou KC, Hale CRH, Nilewski C, Ioannidou HA. Constructing molecular complexity and diversity: total synthesis of natural products of biological and medicinal importance. Chem Soc Rev. 2012;41:5185–5238. doi: 10.1039/c2cs35116a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schreiber S. Organic synthesis toward small-molecule probes and drugs. Proc Natl Acad Sci USA. 2011;108:6699–6702. doi: 10.1073/pnas.1103205108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Keasling J. Manufacturing molecules through metabolic engineering. Science. 2010;330:1355–1358. doi: 10.1126/science.1193990. [DOI] [PubMed] [Google Scholar]
- 5.Carothers JM, Goler JA, Keasling JD. Chemical synthesis using synthetic biology. Curr Opin Biotechnol. 2009;20:498–503. doi: 10.1016/j.copbio.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 6.Chen Y, Nielsen J. Advances in metabolic pathway and strain engineering paving the way for sustainable production of chemical building blocks. Curr Opin Biotechnol. 2013;24:965–972. doi: 10.1016/j.copbio.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 7.Rabinovitch-Deere CA, Oliver JWK, Rodriguez GM, Atsumi S. Synthetic biology and metabolic engineering approaches to produce biofuels. Chem Rev. 2013;113:4611–4632. doi: 10.1021/cr300361t. [DOI] [PubMed] [Google Scholar]
- 8.Na D, Park JH, Jang Y-S, Lee JW, Lee SY. Systems metabolic engineering of Escherichia coli for chemicals, materials, biofuels, and pharmaceuticals. In: Wittmann C, Lee SY, editors. Systems Metabolic Engineering. Springer; Netherlands: 2012. pp. 117–149. [Google Scholar]
- 9.Wang M, Si T, Zhao H. Biocatalyst development by directed evolution. Bioresour Technol. 2012;115:117–125. doi: 10.1016/j.biortech.2012.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bornscheuer UT, Huisman GW, Kazlauskas RJ, Lutz S, Moore JC, Robins K. Engineering the third wave of biocatalysis. Nature. 2012;485:185–194. doi: 10.1038/nature11117. [DOI] [PubMed] [Google Scholar]
- 11.Khersonsky O, Roodvelt C, Tawfik DS. Enzyme promiscuity: evolutionary and mechanistic aspects. Curr Opin Chem Biol. 2006;10:498–508. doi: 10.1016/j.cbpa.2006.08.011. [DOI] [PubMed] [Google Scholar]
- 12.Malpartida F, Hopwood DA. Molecular cloning of the whole biosynthetic pathway of a Streptomyces antibiotic and its expression in a heterologous host. Nature. 1984;309:462–464. doi: 10.1038/309462a0. [DOI] [PubMed] [Google Scholar]
- 13.Prather KLJ, Martin CH. De novo biosynthetic pathways: rational design of microbial chemical factories. Curr Opin Biotechnol. 2008;19:468–474. doi: 10.1016/j.copbio.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 14.Paddon CJ, Westfall PJ, Pitera DJ, Benjamin K, Fisher K, McPhee D, Leavell MD, Tai A, Main A, Eng D, et al. High-level semi-synthetic production of the potent antimalarial artemisinin. Nature. 2013;496:528–532. doi: 10.1038/nature12051. [DOI] [PubMed] [Google Scholar]
- 15.Conforth JW. Chem Br. 1975;11:342. (Talking Point) [Google Scholar]
- 16.Keasling JD, Mendoza A, Baran PS. Synthesis: a constructive debate. Nature. 2012;492:188–189. doi: 10.1038/492188a. [DOI] [PubMed] [Google Scholar]
- 17.Hafner EW, Wellner D. Demonstration of imino acids as products of the reactions catalyzed by D- and L-amino acid oxidases. Proc Nat Acad Sci USA. 1971;68:987–991. doi: 10.1073/pnas.68.5.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Allen JV, Williams JMJ. Dynamic kinetic resolution with enzyme and palladium combinations. Tetrahedron Lett. 1996;37:1859–1862. [Google Scholar]
- 19.Kim M-J, Ahn Y, Park J. Dynamic kinetic resolutions and asymmetric transformations by enzymes coupled with metal catalysis. Curr Opin Biotechnol. 2002;13:578–587. doi: 10.1016/s0958-1669(02)00347-6. [DOI] [PubMed] [Google Scholar]
- 20.Martin-Matute B, Backvall JE. Dynamic kinetic resolution catalyzed by enzymes and metals. Curr Opin Chem Biol. 2007;11:226–232. doi: 10.1016/j.cbpa.2007.01.724. [DOI] [PubMed] [Google Scholar]
- 21••.Denard CA, Huang H, Bartlett MJ, Lu L, Tan Y, Zhao H, Hartwig JF. Cooperative tandem catalysis by an organometallic complex and a metalloenzyme. Angew Chem Int Ed. 2013;52:465–469. doi: 10.1002/anie.201305778. A Ru(I)-catalyzed cross-metathesis reaction was combined with an enzymatic epoxidation reaction. A biphasic reaction set-up was used to avoid deactivation of the enzyme in the presence of the metal catalyst. Cooperativity was observed between the chemical and enzymatic catalysts resulting in higher overall product conversions relative to the two reactions being performed sequentially. [DOI] [PubMed] [Google Scholar]
- 22.Poizat M, Arends IWCE, Hollmann F. On the nature of mutual inactivation between [Cp*Rh(bpy)(H2O)]2+ and enzymes – analysis and potential remedies. J Mol Catal B: Enzym. 2010;63:149–156. [Google Scholar]
- 23.Brooks RR, Watterson JR. Nobel Metals and Biological Systems. CRC Press; 1992. p. 180. [Google Scholar]
- 24••.Wang ZJ, Clary KN, Bergman RG, Raymond KN, Toste FD. A supramolecular approach to combining enzymatic and transition metal catalysis. Nat Chem. 2013;5:100–103. doi: 10.1038/nchem.1531. An Au(I) catalyst was encapsulated within a supramolecular cluster and used in a tandem enzymatic/non-enzymatic reaction sequence in vitro. Supramolecular encapsulation of the metal catalyst was shown to be essential in order to avoid deactivation of the enzyme. An encapsulated Ru(II) catalyst for the isomerization of allylic alcohols into aldehydes was also reported. [DOI] [PubMed] [Google Scholar]
- 25.Breslow R, Overman LE. An “artificial enzyme” combining a metal catalytic group and a hydrophobic binding cavity. J Am Chem Soc. 1970;92:1075–1077. doi: 10.1021/ja00707a062. [DOI] [PubMed] [Google Scholar]
- 26.Yoshizawa M, Klosterman JK, Fujita M. Functional molecular flasks: new properties and reactions within discrete, self-assembled hosts. Angew Chem Int Ed. 2009;48:3418–3438. doi: 10.1002/anie.200805340. [DOI] [PubMed] [Google Scholar]
- 27.Wiester MJ, Ulmann PA, Mirkin CA. Enzyme mimics based upon supramolecular coordination chemistry. Angew Chem Int Ed. 2010;50:114–137. doi: 10.1002/anie.201000380. [DOI] [PubMed] [Google Scholar]
- 28.Bhabak KP, Bhuyan BJ, Mugesh G. Bioinorganic and medicinal chemistry: aspects of gold(I)-protein complexes. Dalton Trans. 2011;10:2099–2111. doi: 10.1039/c0dt01057j. [DOI] [PubMed] [Google Scholar]
- 29••.Kohler V, Wilson YM, Durrenberger M, Ghislieri D, Churakova E, Quinto T, Knorr L, Haussinger D, Hollmann F, Turner NJ, Ward TR. Synthetic cascades are enabled by combining biocatalysts with artificial metalloenzymes. Nat Chem. 2013;5:93–99. doi: 10.1038/nchem.1498. Streptavidin-docking of a biotin-bound Ir catalyst forms an artificial transfer hydrogenase (ATHase) possessing non-enzymatic activity that was used in a range of tandem reaction cascades. The authors also report a colorimetric assay that provides a fluorescent readout as a direct function of enzyme activity. [DOI] [PubMed] [Google Scholar]
- 30.Khersonsky O, Tawfik DS. Enzyme promiscuity: a mechanistic and evolutionary perspective. Ann Rev Biochem. 2010;79:471–505. doi: 10.1146/annurev-biochem-030409-143718. [DOI] [PubMed] [Google Scholar]
- 31.Gatti-Lafranconi P, Hollfelder F. Flexibility and reactivity in promiscuous enzymes. Chem Bio Chem. 2013;14:285–292. doi: 10.1002/cbic.201200628. [DOI] [PubMed] [Google Scholar]
- 32.Wilson ME, Whitesides GM. Conversion of a protein to a homogeneous asymmetric hydrogenation catalyst by site specific modification with a diphosphinerhodium(I) moiety. J Am Chem Soc. 1978;100:306–307. [Google Scholar]
- 33.Ward TR. Artificial metalloenzymes based on the biotin–streptavidin technology: enantioselective catalysis and beyond. Acc Chem Res. 2011;44:47–57. doi: 10.1021/ar100099u. [DOI] [PubMed] [Google Scholar]
- 34.Lewis JC. Artificial metalloenzymes and metallopeptide catalysts for organic synthesis. ACS Catal. 2013;3:2954–2975. [Google Scholar]
- 35•.Hyster TK, Knorr L, Ward TR, Rovis T. Biotinylated Rh(III) complexes in engineered streptavidin for accelerated asymmetric C–H activation. Science. 2012;338:500–503. doi: 10.1126/science.1226132. A biotin-bound Rh-catalyst was anchored within an engineered streptavidin and shown to catalyze a C–H activation reaction with high stereocontrol. Introduction of a proximal glutamic acid or aspartic acid residue via site-directed mutagenesis was critical to product conversions and was shown to participate in the reaction. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zimbron JM, Heinisch, Schmid M, Hamels D, Nogueira ES, Schirmer T, Ward TR. A duel anchoring strategy for the localization and activation of artificial metalloenzymes based on the biotin-streptavidin technology. J Am Chem Soc. 2013;135:5384–5388. doi: 10.1021/ja309974s. [DOI] [PubMed] [Google Scholar]
- 37.Schwizer F, Kohler V, Durrenberger M, Knorr L, Ward TR. Genetic optimization of the catalytic efficiency of artificial imine reductases based on the biotin-streptavidin technology. ACS Catal. 2013;3:1752–1755. [Google Scholar]
- 38.Monnard FW, Nogueira ES, Heinisch T, Schirmer T, Ward TR. Human carbonic anhydrase II as host protein for the creation of artificial metalloenzymes: the asymmetric transfer hydrogenation of imines. Chem Sci. 2013;4:3269–3274. [Google Scholar]
- 39••.Coelho PS, Brustard EM, Kannan A, Arnold FH. Olefin cyclopropanation via carbene transfer catalyzed by engineered cytochrome P450 enzymes. Science. 2013;339:307–309. doi: 10.1126/science.1231434. Engineering of a cytochrome-P450 enzyme produced mutants that could catalyze the highly efficient and stereoselective cyclopropanation of styrenes using ethyl diazoacetate in vitro. [DOI] [PubMed] [Google Scholar]
- 40••.Coelho PS, Wang ZJ, Ener ME, Baril SA, Kannan A, Arnold FH, Brustard EM. A serine-substituted P450 catalyzes highly efficient carbene transfer to olefins in vivo. Nat Chem Bio. 2013;9:485–487. doi: 10.1038/nchembio.1278. Expression of active cytochrome P411BM3 mutants in intact E. coli cells catalyzed the highly efficient cyclopropanation of styrene using ethyl diazoacetate in titres of up to 27 g/L. Axial ligand mutations in the heme domain of P450BM3 allowed for reduction-driven catalysis in vivo by exogenous NADPH. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41•.McIntosh JA, Coelho PS, Farwell CC, Wang ZJ, Lewis JC, Brown TR, Arnold FH. Enantioselective intramolecular C–H amination catalyzed by engineered cytochrome P450 enzymes in vitro and in vivo. Angew Chem Int Ed. 2013;52:9309–9312. doi: 10.1002/anie.201304401. Two mutations in the active site of a cytochrome P450BM3 produced a much more active catalyst for the intramolecular C-H amination of sulfonylazides. This finding demonstrates that engineered biocatalysts can be useful for reactions with no natural precedent. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang ZJ, Peck NE, Renata H, Arnold FH. Cytochrome P450-catalysed insertion of carbenoids into N–H bonds. Chem Sci. 2014;5:598–601. doi: 10.1039/C3SC52535J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43•.Matthews ML, Chang W, Layne AP, Miles LA, Krebs C, Bollinger JM., Jr Direct nitration and azidation of aliphatic carbons by an iron-dependent halogenase. Nat Chem Bio. 2014 doi: 10.1038/nchembio.1438. The wild-type halogenase (SyrB2) can bind azide and nitrate and catalyze the C–H nitration and azidation of un-activated substrates. The study presents a potentially general method for accessing other unnatural functional groups through the apparent promiscuity of the wild-type enzyme. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Neuberg C, Hirsch J. Uber den Verlauf der alkoholischen-Garung bei alkalischer Reaktion: II. Garung mit lebender Hefe in alkalischen Losungen. Biochem Z. 1919;96:175–202. [Google Scholar]
- 45.Neuberg C, Hirsch J. Die dritte Vergarungform des Zurkers. Biochem Z. 1919;100:304–322. [Google Scholar]
- 46.Wuts PGM, Greene TW. Greene’s Protective Groups In Organic Synthesis. 4. John Wiley & Sons, Inc; 2006. Sodium bisulfite adducts: RCH(OH)SO3Na. [Google Scholar]
- 47.Kaspar HF, Holland AJ, Mountfort DO. Simultaneous butyrate oxidation by Syntrophomonas wolfei and catalytic olefin reduction in absence of interspecies hydrogen transfer. Arch Microbiol. 1987;147:334–339. [Google Scholar]
- 48.Vigh L, Los DA, Horvath I, Murata N. The primary signal in the biological perception of temperature: Pd-catalyzed hydrogenation of membrane lipids stimulated the expression of the desA gene in Synechocystis PCC6803. Proc Natl Acad Sci USA. 1993;90:9090–9094. doi: 10.1073/pnas.90.19.9090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Robbins DW, Hartwig JF. A simple, multidimensional approach to high-throughput discovery of catalytic reactions. Science. 2011;333:1423–1427. doi: 10.1126/science.1207922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McNally A, Prier CK, MacMillan DW. Discovery of an α-amino C-H arylation reaction using the strategy of accelerated serendipity. Science. 2011;334:1114–1117. doi: 10.1126/science.1213920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lang K, Chin JW. Bioorthogonal reactions for labeling proteins. ACS Chem Biol. 2014;9:16–20. doi: 10.1021/cb4009292. [DOI] [PubMed] [Google Scholar]
- 52.Sletten EM, Bertozzi CR. Bioorthogonal chemistry: fishing for selectivity in a sea of functionality. Angew Chem Int Ed. 2009;48:6974–6998. doi: 10.1002/anie.200900942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Patterson DM, Nazarova LA, Prescher JA. Finding the right (bioorthogonal) chemistry. ACS Chem Biol. 2014 doi: 10.1021/cb400828a. [DOI] [PubMed] [Google Scholar]
- 54.Bertozzi CR. A decade of bioorthogonal chemistry. Acc Chem Res. 2011;44:651–653. doi: 10.1021/ar200193f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55••.Lee Y, Umeano A, Balskus EP. Rescuing auxotrophic microorganisms with nonenzymatic chemistry. Angew Chem Int Ed. 2013;52:11800–11803. doi: 10.1002/anie.201307033. Two metal-catalyzed biocompatible reactions were shown to support the growth of two auxotrophic strains of E. coli via the generation of essential nutrients in vivo. Growth of the auxotroph was shown to be dependent on the success of the non-enzymatic reaction in both cases. [DOI] [PMC free article] [PubMed] [Google Scholar]