Abstract
Mulberry leaves are an important ingredient in some traditional Chinese medicinal formulas and has been developed for use in functional food products. The antioxidant activity of mulberry leaf extract has been reported to have beneficial effects on diseases in vitro; however, it is not clear which components in mulberry leaf extracts have these functions. Furthermore, the mechanisms of action of these ingredients have not been extensively investigated. In this study, we extracted total mulberry leaf polyphenols (MLP) and identified its 13 phenolic monomers. Our results, using Caenorhabditis elegans as a model, indicated that MLPs delayed aging, improved oxidative stress resistance, and reduced fatty acid storage in vivo. Subsequent genetic screens and gene expression analyses demonstrated that the functions of MLP mainly depended on the germline signaling pathway, thus influencing the activities of downstream transcription factors (DAF-12, DAF-16, PHA-4, and NHR-80) as well as the expression levels of their target genes (fat-6, lipl-4, sod-3, unc-51, and fard-1). Our study determined that diverse modes of action on longevity were promoted by MLP exposure. These observations provide the first insight into MLP’s multifaceted functions on aging, fat accumulation, and reproduction in vivo and indicate a specific model for the mechanism of action of MLP. This is a significant finding that lends support to the hypotheses that mulberry leaf extracts can have an impact on human health.
Electronic supplementary material
The online version of this article (doi:10.1007/s11357-014-9719-z) contains supplementary material, which is available to authorized users.
Keywords: Mulberry leaf polyphenols, Caenorhabditis elegans, Germline signaling pathway, Transcription factors, Aging, Fat metabolism
Introduction
Mulberry (Morus alba L.) is native to eastern and central China and has been widely cultured in many countries, especially Asia, for centuries. Mulberry fruit, bark, roots, and leaves have been used as folk medicines in China. Mulberry leaf is an important ingredient in some traditional Chinese medicinal formulas and is considered to have high nutritional value and antioxidant activity, which could be developed for use in foods benefiting human health (Liang et al. 2012). Combined extracts from mulberry fruits and leaves was demonstrated to improve obesity-related inflammation and oxidative stress resistance in obese mice (Lim et al. 2013). Extracts from mulberry leaves were also reported to have beneficial effects on ameliorating type-2 diabetes (Mudra et al. 2007) as well as inhibiting the development of atherosclerosis in cholesterol-fed rabbits (Chan et al. 2013) and the growth of neuroblastoma cells (Park et al. 2012). Mulberry leaves have also been processed into commercial food products that are considered to have potential effects on weight loss. However, it is not clear which components in mulberry leaf extracts have these beneficial functions, and the mechanisms of action of these ingredients have not been extensively researched.
Mulberry leaves contains abundant varieties and quantities of polyphenols (Zou et al. 2012a, b). Each plant has a unique polyphenol profile, which makes their bioactivities and functions different from each other. Some studies have reported that mulberry leaf polyphenols (MLP) have extensive functions in antioxidant and free radical scavenging in vitro (Andallu and Varadacharyulu 2003; Choi et al. 21013; Katsube et al. 2009). However, these observations are insufficient to determine if all the reported beneficial MLP functions depend on its antioxidant activity. Polyphenols, the most commonly found chemical compounds in herbal beverages and foods, are reported to influence reactive oxygen species (ROS) regulation, confer neuroprotective effects, and impact cell signal transduction (Ebrahimi and Schluesener 2012). Natural polyphenols have been identified as important plant compounds with anti-aging effects in model animals, such as blueberry polyphenols (Wilson et al. 2006), black tea theaflavins (Peng et al. 2009), procyanidins from apples (Sunagawa et al. 2011), resveratrol (Bass et al. 2007), curcumin (Liao et al. 2011), and epigallocatechin gallate (Abbas and Wink 2010). To the best of our knowledge, the functions and mechanisms of MLP action in vivo have not been well studied, especially with regards to the effects of total MLP on aging.
The effects of edible and medicinal plant extracts on the aging of model animals have been widely tested (Honda et al. 2011; Sangha et al. 2012; Wu Z et al. 2002). In this study, we used Caenorhabditis elegans as a model to study the functions of MLP on aging. C. elegans is a microscopic (1.2 mm), self-fertilizing, free living nematode that is readily grown and maintained in the laboratory on simple agar petri dishes on a diet of Escherichia coli (Brenner 1974; Hulme and Whitesides 2011). The animal has a 3-day life cycle (from egg through four larval stages to reproducing adult) and a 20-day natural life span at 25 °C (Huang et al. 2004). C. elegans is a very popular and powerful tool for screening anti-aging compounds and for studying their mechanisms of action (Collins et al. 2006; Lithgow et al. 2005). We found that 25 mg/L MLP could extend the mean life span of C. elegans by up to about 25 %, improve stress resistance, reduce fatty acid storage, and influence germ cell development. In C. elegans, the germline signaling pathway modulates aging via partially overlapping effects and/or mechanisms including lipid metabolism (Hansen D et al. 2004; Lapierre and Hansen 2012). The glp-1(−) loss-of-function mutants were used to generate a germline loss model as described previously (Arantes-Oliveira et al. 2002). We found the life span-extension and fat-reduction effects of MLP were absent from glp-1(−) mutants. The germline signaling pathway modulates the downstream transduction factors DAF-16, DAF-12, NHR-80, and PHA-4 to regulate aging and lipid metabolism (Berman and Kenyon 2006; Gerisch et al. 2001; Yamawaki et al. 2010). Subsequent experiments demonstrated that MLP regulated the expression levels of target genes of these transcription factors. Furthermore, daf-12, daf-16, pha-4, or nhr-80 mutants could suppress the life span-extension function of MLP. This study indicated that MLP influenced aging, fat metabolism, and reproductive activity in C. elegans. Genetic analysis revealed that MLP regulated the activities of DAF-16, DAF-12, NHR-80, and PHA-4 by way of the germline signaling pathway.
Material and methods
C. elegans strains and reagents
Folin and Ciocalteu’s phenol reagent, Sudan Black B, and Paraquat dichloride were purchased from Sigma-Aldrich (St Louis, MO, USA). The 13 standard phenolic monomers were purchased from the National Institutes for Food and Drug Control (Beijing, China).
The white mulberry typical cultivar, which is widely grown in southern China, was used in this study. The mulberry plants for this study were cultivated in an experimental field in Guangzhou, which is managed by the Sericulture and Agri-food Research Institute of the Guangdong Academy of Agricultural Sciences. The fresh leaves were collected in early April, air dried, stored in plastic bags, and kept at 4 °C until extraction. The leaves were cut into small pieces using a blender before use.
All worm strains were provided by the Caenorhabditis Genetics Center, which is funded by NIH Office of Research Infrastructure Programs (P40 OD010440). Worm strains used in this study included: N2, wild-type Bristol; CF1038 [ daf-16(mu86)I]; AA86 [daf-12(rh61rh411)]; BX165 [nhr-80(tm1011)III]; SM190 [smg-1(cc546)I; pha-4(zu225)V]; VC199 [sir-2.1(ok434)IV]; CF1903 [glp-1(e2141)III]; EU1 [skn-1 (zu67)IV]; TK22 [mev-1(kn1)III]. All C. elegans strains were maintained at 20 °C as described previously (Brenner 1974), except that CF1903, the temperature-sensitive strain, was cultured under 25 °C when it was used during the life span tests.
MLP extraction
The total MLP in the leaves was extracted using a previously published method (Zou S et al. 2012). In brief, 1 g of mulberry leaf was mixed with 40 mL of acidified 70 % ethanol solution (pH 4) and then extracted using low-power sonication for 4 h at 4 °C. The mixture was centrifuged at room temperature (8,000×g for 15 min) to obtain the phenolic-rich components in the supernatant. The supernatant was collected, and the ethanol in the solution was removed using a rotary vacuum evaporator over a water bath below 40 °C. The solution was reconstituted with distilled water to 40 mL, filtered using a 0.45 μm filter, and then stored at −20 °C until testing.
Determination of total MLP
Total MLP in the extract was determined using the Folin and Ciocalteu method with a slight modification (Zou S et al. 2012). A total of 1 mL of the MLP solution was mixed with 0.5 ml of Folin and Ciocalteu reagent and 2 ml of sodium carbonate (15 %). The volume was adjusted to 10 mL with distilled water. The mixture was incubated in the dark for 1 h at room temperature. Absorbance was measured at 760 nm after incubation at room temperature. Total MLP was expressed as milligrams per gram gallic acid equivalent. The results represent the mean data of three parallel determinations.
Identification of phenols
The 13 phenols in MLP were quantified using an Agilent 1200 series high-performance liquid chromatography (HPLC) system (Agilent Technologies, Karlsruhe, Germany). A total of 10 μL of MLP sample was injected into the system and separated in a reversed-phase Agilent Zorbax SB-C18 column (250 × 4.6 mm, 5 μm). The mobile phase contained 0.4 % acetic acid (MPA) and acetonitrile (MPB, pumped at 1 mL/min in a linear gradient mode) with 5–25 % MPB (0–40 min), 25–35 % MPB (40–45 min), 35–50 % MPB (45–50 min), and 50–55 % MPB (50–55 min). The flow rate was 1 mL/min, and the column was kept at 30 °C. The phenolic compounds were monitored at 280 nm, and their concentrations were calculated based on the standard curves constructed with authentic standards. Concentration was expressed as milligrams per gram.
Life span analysis
C. elegans is typically cultured on NGM plates spotted with live E. coli as a food source. However, this raises the possibility that a drug treatment could directly affect the E. coli and thus indirectly affect the worms (Liao et al. 2011). In addition, E. coli is pathogenic to aging worms. To exclude these issues, our experiments were conducted with dead E. coli that had been killed by heat incubation (30 min at 65 °C).
For life span analysis, worms of each strain were cultured on NGM plates containing MLP at 20 °C. The eggs were collected and synchronized at the L1 larval stage, transferred to NGM plates containing MLP, and then allowed to develop to young adults. The young adults were transferred to MLP NGM plates containing 40 μM FUDR, which could control the reproduction of worms. A total of 20–30 young adults were cultured on each NGM plate (50 mm, diameter), and five NGM plates were used for each life span test of one strain. Worms were counted and transferred to new NGM plates every other day, and worms that did not move when gently touched (with a platinum wire) were scored as dead. The day that L4 larvae worms or young adults were transferred to NGM plates was defined as day 0. All the life span assays were repeated at least three times. Survival curves and statistical analysis were carried out using SPSS software (SPSS, Chicago, IL, USA). P values were calculated using long rank test, Kaplan–Meier method. As for the CF1903 strain, the synchronized worms were cultured at 25 °C after developing to the early L4 larval stage.
Antioxidant stress
For resistance to hydrogen peroxide, we scored 5-h survival of adult N2 animals, which were cultured on MLP NGM plates for 6 days from adult day 1, in S-basal medium with the indicated concentrations of hydrogen peroxide.
For paraquat-induced oxidative stress assays, we incubated N2 worms at 20 °C as for the aging assays, except paraquat was added to the NGM medium to a 5 mM final concentration (Wilson et al. 2006).
Reproductive assay
The effects of MLP on reproductive ability were tested using N2 worms. The young adult N2 worms were transferred to NGM plates containing 25 mg/mL MLP. Each plate contained one worm, which was transferred to a new plate at the same time each day. The time from day zero to the last day of self-progeny production was referred to as the self-fertile reproductive span (Huang et al. 2004). The offspring yielded by each worm on each day were hatched at 25 °C and counted at the L4 or young adult stage. The number of N2 worms used in each experiment was more than 30.
Lipid staining
A previously published method was used for the lipid staining analysis (Singh et al. 2009). Worms were synchronized and treated with 25 mg/L MLP for 3 days on NGM plates. Subsequently, worms were washed in M9 buffer for 10 min and then fixed with 1 % paraformaldehyde in M9 buffer. The animals were then dehydrated through consecutive washes with 25, 50, and 70 % ethanol. Staining was performed overnight (approximately 16 h) in a 50 % saturated solution of Sudan Black B in 70 % ethanol. Following staining, worms were washed for 3 × 5 min with M9 buffer. The worms were photographed using a Ti microscope (Nikon, Tokyo, Japan). Total intensity of Sudan black B stained droplets was calculated using the Metamorph software package (Molecular Devices, Sunnyvale, CA, USA).
Gene expression
N2 worms were cultured on NGM plates with or without 25 mg/mL MLP for two generations. About 2,000 synchronized young adult N2 worms were transferred to five NGM plates (5 cm diameter) with or without MLP and culture at 20 °C for 24 h. Total RNA was extracted using RNAiso Plus (TaKaRa Bio, Shiga, Japan). Five micrograms of total RNA was used to synthesize cDNA using a High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, USA). The qRT-PCR reactions were performed using Power SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA, USA) and an ABI 7500 system. The relative expression levels of the genes were carried out using the 2-△△CT method and normalized to act-1 (Greer et al. 2007). The sequences of primers are available in the supporting information (Table S9).
Results
MLP extends life span of C. elegans
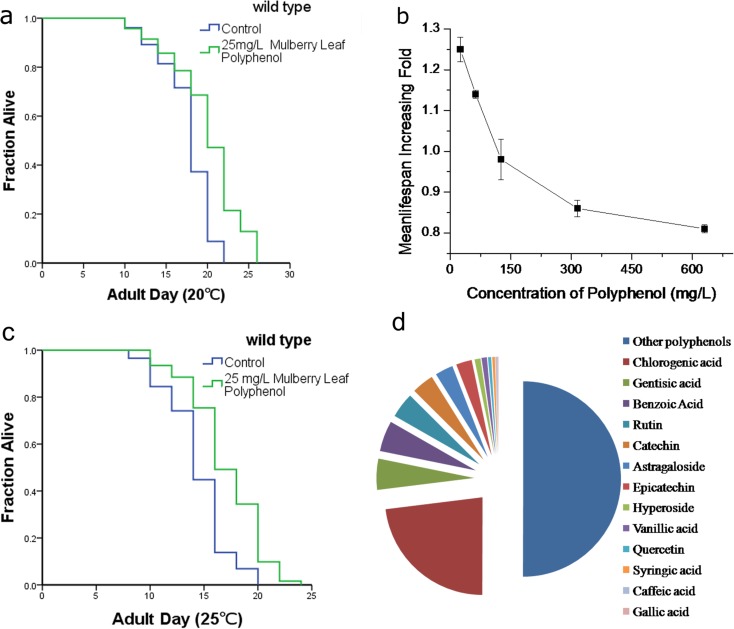
The total polyphenols extracted from mulberry leaves were applied to a C18 column and processed to remove the fructose, glucose, and organic acids. The quantity of the polyphenol fraction was determined using a modified Folin and Ciocalteu method. The total MLP in the extract was 41.2 ± 3.8 mg/g gallic acid equivalent, an average result of the three separate extractions. Thirteen common monomers were identified and calculated using the HPLC calibration curve method. Figure 1c illustrates the relative percentages of the identified polyphenols among the total MLP. Our analysis showed that the isolated MLP had a considerable variety of polyphenol content.
Fig. 1.
MLP extends the lifespan of wild-type C. elegans. The mean life span of N2 worms treated with 25 mg/L MLP could be extended by up to 25 % at 20 °C (a) and 22.46 % at 25 °C (c). The figure shows the result of one experiment. Statistical details and repetitions are summarized in Table S1. c Dosage–response analysis of MLP. N2 animals were exposed to control, 25, 62.5 125, 312.5, or 625 mg/L MLP. The average percentage change in life span of three independent experiments was plotted as a function of dosage. Statistical details and repetitions of this experiment are summarized in Table S1 and S2. d The relative percentages of several polyphenol monomers in the gross MLP
The concentrated and purified total MLP was diluted into several working concentrations, which were applied to NGM plates to perform the life span tests. Our results showed that the effect of MLP on life span-extension of C. elegans was dose-dependent (Fig. 1b). Results from our dosage response analysis indicated that external 125 mg/L was the cut-off concentration (Fig. 1b). Worms cultured on NGM plates containing MLP concentrations >125 mg/L had a shortened life span compared with the control and vice versa. The 25 mg/L MLP displayed the largest life span extension (Fig. 1a). The mean life span of wild-type worms (N2) was extended by up to 25 % at 20 °C (Fig. 1a, Table S1) and 22.46 % at 25 °C (Fig. 1c, Table S1). The lower concentrations of MLP did not extend the life span further (Table S2).
MLP improves resistance to oxidative stress
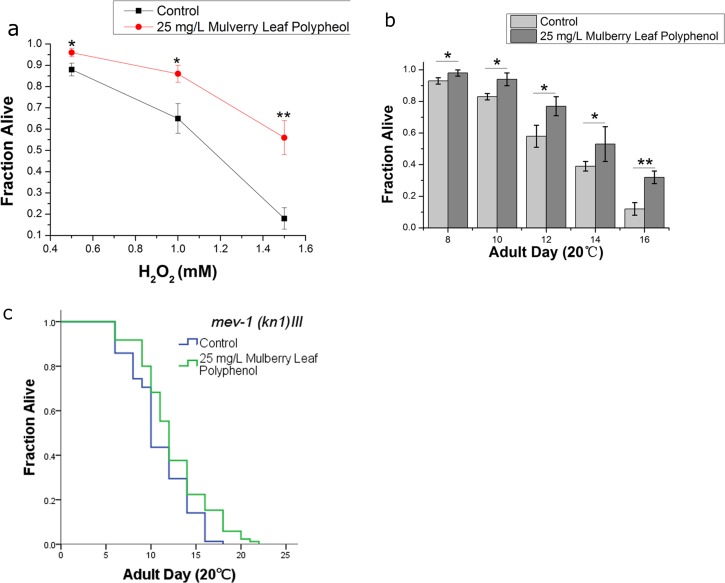
Prior studies have reported that mulberry leaf extract has antioxidant activity (Katsube et al. 2009; Lim et al. 2013); however, in these studies, the antioxidant activity was always tested using cell models or simple chemical reagent kits, such as a ferric reducing antioxidant power assay kit. Our results suggested that MLP extended the mean life span of C. elegans (Fig. 1a, Table S1). One possible explanation for this beneficial effect of MLP on aging in C. elegans is that MLP was able to increase organismal stress resistance. In several studies, increased longevity was closely associated with improving the resistance to heat or oxidative stress (Wilson et al. 2006). In this study, we used C. elegans to test the antioxidant activity of MLP in a whole living organism. Resistance to oxidative stress was examined by exposing worms to hydrogen peroxide or paraquat, an intracellular free-radical-generating compound. The results indicated that 25 mg/L MLP significantly improved the oxidative stress resistance of worms cultured in medium containing 5 mM paraquat (Fig. 2b, Table S4) or H2O2 (Fig. 2a, Table S3). In order to confirm that MLP could improve inner oxidant resistance, we also tested the effects of MLP on mev-1(kn1) mutants, which have a higher sensitivity to oxidative stress. The results indicated that MLP could significantly improve the survival of mev-1(kn1) mutants (Fig. 2c, Table S1).
Fig. 2.
MLP improves the resistance to oxidative stress. Survival on hydrogen peroxide (a) or paraquat (b) is significantly improved by 25 mg/L MLP. The error bars represent the standard deviation (SD). The figure shows the results of three independent trials. Statistical details and repetitions of this experiment are summarized in Tables S3 and S4. c The mean life span of mev-1 mutant worms was significantly extended by 25 mg/L MLP. The figure shows the result of one experiment. Statistical details and repetitions of this experiment are summarized in Table S1
Reproductive activity and fat accumulation of C. elegans are regulated byMLP
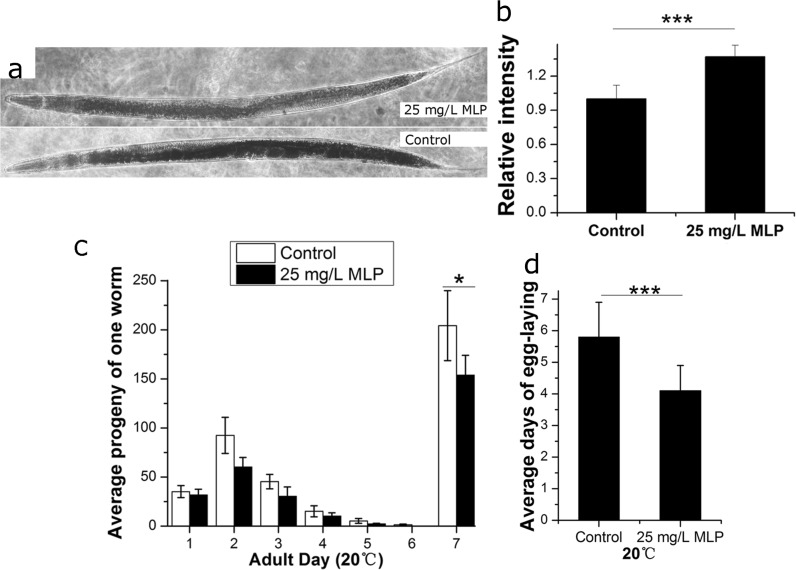
In the course of the MLP-treatment experiments, we found that the germline of MLP-treated N2 worms was less robust than those of the controls. Self-fertile hermaphrodites display an age-related decline in progeny production, and progeny number corresponds with the generation of oocytes that develop from germ cells. According to this observation, we speculated that MLP regulated the development of the germline system to delay aging of C. elegans. In order to confirm this speculation, we measured the number of progeny produced on each day of adulthood. The results showed that the self-fertile reproduction span of MLP-treated worms was significantly shorter than that of the control (Fig. 3d, Table S5) and the total progeny number was smaller than that of the control (Fig. 3c, Table S5). These results indicated that MLP had an effect on germ cell development and promoted germ cell loss.
Fig. 3.
MLP regulates lipid storage and reproduction. a Sudan Black B staining in control and 25 mg/L MLP-treated worms. b Quantification of fat staining based on total intensity of Sudan Black B-stained droplets. n = 20 animals in three independent experiments for each group. The error bars represent the SD. Statistical details are summarized in Table S6. The 25 mg/L MLP reduced the total progeny number (c) and shortened egg-laying time (d). n = 57 animals in three independent experiments for the control group; n = 69 animals in three independent experiments for the MLP-treatment group. The error bars represent the SD. Statistical details are summarized in Table S5
Because we noticed that the MLP-treated worms were less robust than the controls and that mulberry was reported to have effects on lipid accumulation (Andallu B. et al. 2001), we also analyzed the function of MLP on fat metabolism in C. elegans. C. elegans store fat in droplets in their intestinal and hypodermal cells. Because C. elegans have transparent bodies, these fat stores can be directly visualized in intact animals (Ashrafi 2007). We employed a classic method to stain fixed animals with the fat-soluble dye Sudan Black B. The result indicated that 25 mg/L MLP could significantly reduce the fat levels in intact living worms (Fig. 3a and b, Table S6).
Effects of MLP on aging depends on the germline signaling pathway
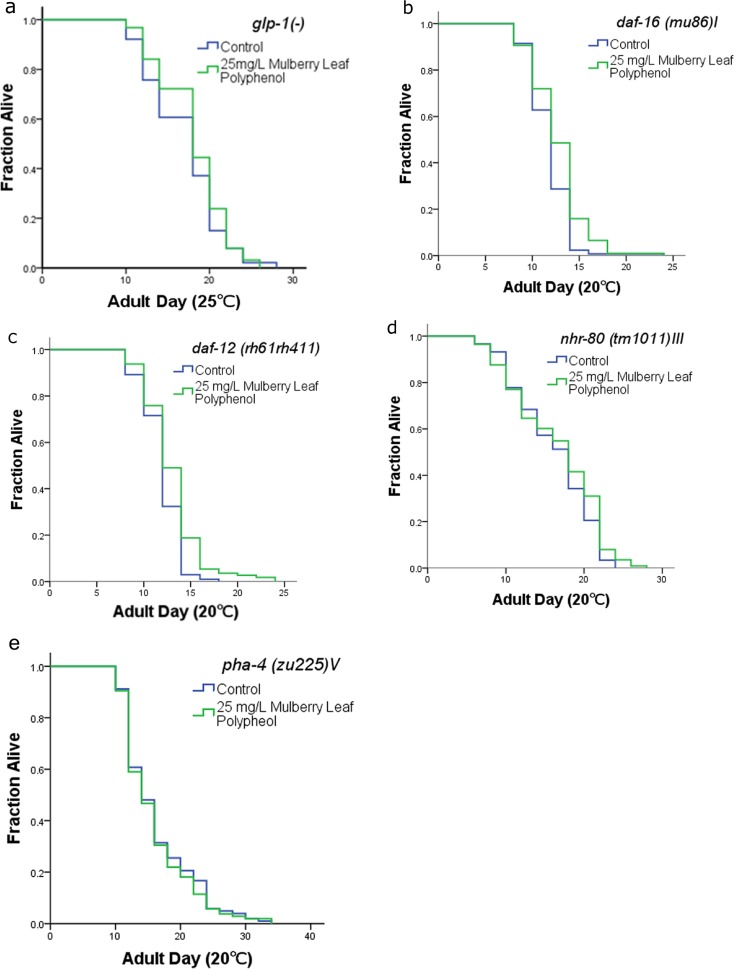
In C. elegans, the germline signaling pathway modulates aging through partially overlapping effects or mechanisms including lipid metabolism (Lapierre and Hansen 2012). Removal of the germline highlights a mechanistic connection between reproduction, fat metabolism, and life span (Hansen M et al. 2013). Our results confirmed that MLP extended the life span of C. elegans, reduced fat accumulation, and substantially reduced their reproductive ability. According to these observations, we speculated that the functions of MLP on C. elegans were dependent on the germline signaling pathway. In order to verify this hypothesis, we utilized strain CF1903, a glp-1(−) loss-of-function mutant, to analyze the relationship between MLP and the germline signaling pathway. CF1903 is a temperature-sensitive mutation and displays an absence of germline by shifting this mutant to the non-permissive temperature either during development or in early adulthood (Arantes-Oliveira et al. 2002). We found that the functions of MLP on life span-extension (Fig. 4a, Table S1) and fat reduction (Table S7) were absent in glp-1(−) mutants. The mean life span and fat accumulation of 25 mg/L MLP-treated glp-1(−) worms had no significant difference compared with that of controls (Fig. 4a, Table S1 and S7). However, the effect of MLP on oxidative stress was not totally suppressed by this mutation (data not show), which suggested that the antioxidant activity of MLP might also dependent on other mechanisms. However, these results were consistent with our previous speculation that the functions of MLP were mainly dependent on the germline signaling pathway.
Fig. 4.
Effects of MLP on life span-extension mainly depends on the germline signaling pathway. MLP has no effect on life span of glp-1(−) worms or worms harboring mutations in downstream transcription factors. The figure shows the result of one experiment. Statistical details and repetitions are summarized in Table S1
MLP regulates transcription factors and gene expression downstream of the germline signaling pathway
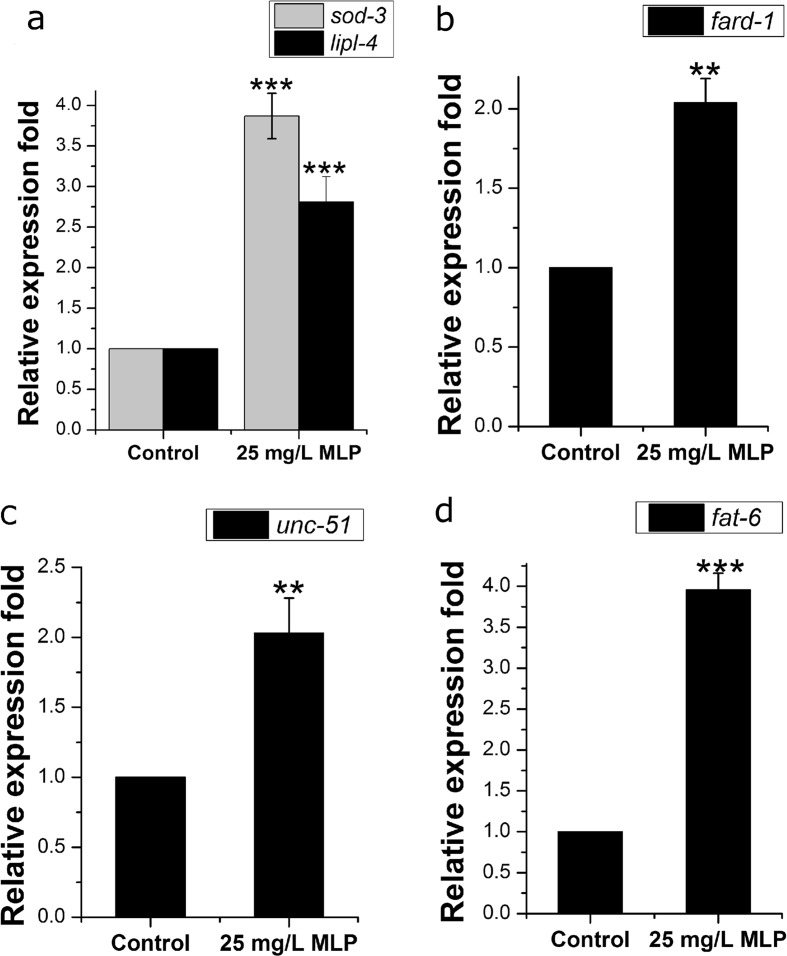
The germline signaling pathway regulates life span extension via four signaling mechanisms: reduced TOR signaling (which is reported to regulate autophagy), DAF-16/FOXO regulation, increased steroid signaling, and increased NHR-80/HNF-4 signaling (which enhances fatty-acid desaturation) (Lapierre and Hansen 2012). These four signaling mechanisms regulate reproduction, fat metabolism, and life span mainly through multiple transcription factors (DAF-12, DAF-16, PHA-4, and NHR-80 (Hansen M et al. 2013)), and mutations of any of these transcription factors can suppressed the function of the germline signal on aging (Berman and Kenyon 2006; Goudeau et al. 2011). In order to confirm that the effects of MLP were mainly dependent on the germline signaling pathway, we treated daf-12, daf-16, pha-4, or nhr-80 mutants with MLP. Our results confirmed that 25 mg/L MLP had no effect on the life span of these mutants (Fig. 4b–e, Table S1) and indicated that the function of MLP on C. elegans depends on the germline signaling pathway. In order to further confirm this conclusion, we tested the effect of MLP on expression of the target genes of DAF-12 (fard-1), DAF-16 (lipil-4, sod-3), PHA-4 (unc-51), and NHR-80 (fat-6). These genes affect fat metabolism and life span extension. Our results indicated that the expression levels of these genes following 25 mg/L MLP-treatment in N2 worms were all significantly upregulated (Fig. 5a–d, Table S8). All of these results further confirmed the speculation that the functions of MLP on life span-extension and fat metabolism mainly depend on the germline signaling pathway.
Fig. 5.
MLP affects the activity of transcription factors regulated by the germline signaling pathway. The target genes of DAF-16 (sod-3, lipl-4) (a), DAF-12 (fard-1) (b), NHR-80 (fat-6) (c), and PHA-4 (unc-51) (d) were significantly upregulated by 25 mg/L MLP. Figures show the results of three independent experiments. Data are averages of real-time PCR results ± SD. The error bars represent SD. P values were calculated using a t test, *P < 0.05; **P < 0.01; ***P < 0.001. Also see Table S8
Discussion
Mulberry fruit and leaves are processed into nutraceutical and functional foods. In China, mulberry leaves are also used in several traditional Chinese medicine formulas. Mulberry leaf and fruit extracts are reported to have beneficial effects on human health (Liang et al. 2012; Lim et al. 2013; Mudra et al. 2007); however, their material substances and mechanisms of action have not been well studied. In this study, we found that MLP could delay aging using the short-lived nematode C. elegans. We suggest that the total polyphenols extracted from mulberry leaves are the main compounds responsible for the health benefits of this plant. However, the antioxidant activity of MLP alone is likely insufficient to account for the many attributes of mulberry leaf extract in regard to human health.
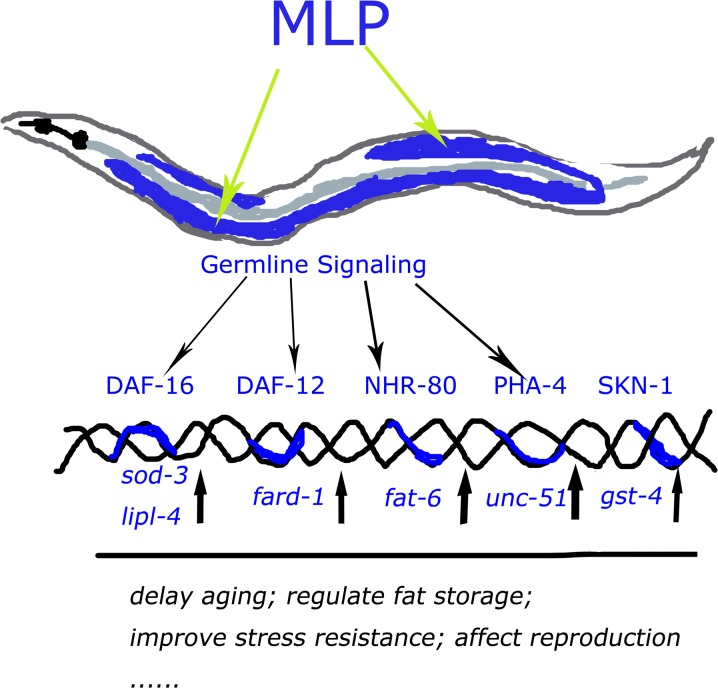
Prior studies regarding the mechanisms of action of MLP have been restricted to in vitro experiments. The relationship between the function of MLP and the related mechanisms of action has not been well elucidated. Our study confirmed that MLP can improve the resistance to oxidative stress in a whole living organism and indicates that MLP also has functions in lipid metabolism and germ cell development. The functions of MLP in vivo were further clarified by our observations that MLP could delay aging, reduce fat accumulation, and regulate reproductive ability in C. elegans. Our observations have led to a specific model for the mechanism of action of MLP on life span-extension, as shown in Fig. 6.
Fig. 6.
Model of mechanism of action of MLP
Total polyphenols extracted from mulberry leaves were used to test their anti-aging function. According to previously reported methods (Katsube et al. 2006) and our optimized protocols, a 60–70 % ethanol aqueous solution was determined to be the most effective extraction solvent; 70 % ethanol was also used for sample analysis. In order to retain the integrality and natural bioactivity of the phenols in the mulberry leaves, we utilized mild biomass extraction conditions: The air-dry mulberry leaves were incubated in 70 % ethanol and subsequently sonicated under a low power frequency at 4 °C.
We used the model animal C. elegans to study the effects of MLP in vivo. The C. elegans model has several distinct advantages, including a completely sequenced genome that shares extensive homology with that of mammals, ease of cultivation and storage, a relatively short life span, and advanced techniques for generating null and transgenic animals. Researchers have also combine drug or compound treatments with genetic analysis to uncover mechanisms of action, and C. elegans has been shown to be a powerful model for aging research, as functions and mechanisms of action for many anti-aging compounds and plant extracts have been studied using this organism (Abbas and Wink 2010; Bass et al. 2007; Liao et al. 2011; Peng et al. 2009; Sunagawa et al. 2011). The identification of chemical interventions on anti-aging signaling pathways can lead to a better understanding of the molecular mechanisms of natural compounds.
The antioxidant activity of polyphenols is their most prominently discussed effect (Pandey and Rizvi 2009). It is now widely known that oxidative stress plays a pivotal role in the pathophysiology of aging and age-related diseases (Ristow and Schmeisser 2011; Sohal and Orr 2012). Polyphenols exert their antioxidant effects through different mechanisms, such as interaction with age-regulated signaling pathways or by inducing expression of protective genes against ROS (Perron and Brumaghim 2009). In this study, the whole living organism was used to confirm the antioxidant effect of MLP in vivo. H2O2 is an external oxidant inducer, and paraquat is an intracellular free-radical-generating compound. The antioxidant activity of MLP was tested under both extrinsic and intrinsic stresses to the organism. Our studies also indicated that MLP could regulate fat accumulation in worms, and we speculated that all the observed effects of MLP were dependent on the germline signaling pathway. The intervention of MLP on germline signal transduction could have also influenced fat metabolism, but we could not exclude that the function of MLP on fat metabolism may be regulate by chlorogenic acid and anthocyanin present in our MLP preparations. Chlorogenic acid-enriched instant coffee appears to have a significant effect on the absorption and utilization of glucose from the diet (Thom 2007), and dietary intake of chlorogenic acid-enriched beverages and foods could reduce body mass and body fat. Anthocyanin also has been reported to suppress weight gain in mice receiving a high-fat diet (Wu T et al. 2013). However, we did not investigate or evaluate the functions of the phenolic monomers present in MLP on aging or other physiological phenotypes in this study. Furthermore, resveratrol has been reported to extend the life span of C. elegans via Sir-2, a NAD+-dependent protein deacetylase (Bass et al. 2007). Resveratrol did not extend the life span of sir-2.1 null mutant, indicating that sir-2.1 may be necessary for the effect of resveratrol. Resveratrol is also a component of MLP, but our results found that MLP could also extend the life span of sir-2.1 null mutant (Table S1). This result suggested that resveratrol might not be the compound in MLP that serves to extend the life span of C. elegans; alternatively, the quantity of resveratrol was not sufficient to influence the function of the SIR-2.1 enzyme. Because the largest effect of 18 % was observed with 1 mM resveratrol (Wood et al. 2004), the quantity of resveratrol in MLP in this study may be too low to see such effects. Even so, we could not conclude that resveratrol in MLP had no effect on the aging of C. elegans. The functions of MLP on aging, fat metabolism, and reproductive ability may be dependent on the interaction of all the phenolic monomers present in MLP. The role of individual phenols in total MLP and other confounding variables still need to be considered to make more accurate conclusions in this regard.
Our study indicated that MLP could regulate aging, fat metabolism, and fecundity in C. elegans. In C. elegans, the germline signaling pathway can regulate all of these physiological phenomenon (Hansen M et al. 2013; Lapierre and Hansen 2012). Furthermore, we performed a germline ablation test to determine the relationship between MLP and the germline signaling pathway. CF1903 is a temperature-sensitive mutation of glp-1, which is essential for the mitotic proliferation of germ cells and maintenance of germline cells (Arantes-Oliveira et al. 2002). CF1903 displays an absence of germline after a shift to the non-permissive temperature (25 °C) either during development or in early adulthood (Arantes-Oliveira et al. 2002). Our results indicated that MLP had no function on aging or fat accumulation in this glp-1 mutant. Germ cell loss suppressed the function of MLP, suggesting that the effects of MLP on C. elegans depended on the germline signaling pathway. Our current understanding of the underlying molecular mechanisms allowing ablation of the germline to increase life span and alter fat metabolism is mainly based on findings in C. elegans. Insulin/IGF-1-like, TOR, NHR, and steroid signaling pathways are required for the long life span of germline-loss animals (Hansen M et al. 2013). Germline signaling is associated with the regulation of a large number of transcription factors (namely daf-16/FOXO, daf-12/FXR, nhr-80/HNF4, and pha-4/FOXA) (Lapierre and Hansen 2012) and is known to modulate longevity. Mutants of these transcription factors have been reported to suppress the longevity and alter the regulation of fatty acid desaturation controlled by the germline signaling pathway (Berman and Kenyon 2006; Goudeau et al. 2011). Our results indicated that MLP had no effect on mutants of daf-12, daf-16, pha-4, or nhr-80. We also tested the expression profiles of target genes downstream of these transcription factors to further confirm our conclusions. These genes, including fat-6, lipl-4, sod-3, unc-51, and fard-1, affect fat metabolism and life span extension (Hansen M et al. 2013; Lapierre and Hansen 2012). Our results indicated that the expression levels of these genes were regulated by MLP. All of these observations confirmed the model that the action of MLP on C. elegans was dependent on the germline signaling pathway. However, the effect of MLP on oxidative stress was not absence in the glp-1 mutant worms. Our results indicated that MLP improved oxidative stress resistance mainly through the germline signaling pathway and that sod-3, a down-stream target of DAF-16 regulated by the germline pathway, could also enhance the antioxidant activity. However, we could not exclude the possibility that MLP might regulate other stress-related mechanisms. Antioxidant properties could be induced by oxidative stress, so MLP may also improve the antioxidant response through a stress-evoked signal. For example, we tested the effects of MLP on aging in skn-1 mutants (Table S1), and the results suggested MLP also had a function on SKN-1 and regulated the target gene (gst-4) of this transcription factor (Table S8). SKN-1 functions in the p38/MAPK pathway to regulate the oxidative stress response and works in parallel to DAF-16 in the IIS pathway to regulate adult life span in C. elegans (Tullet et al. 2008). The function of sod-3 in our experiments may be similar to that of the temporal requirements of heat shock factor-1 in regard to longevity (Volovik et al. 2012).
In conclusion, we provide evidence that total MLP is capable of extending life span, improving stress resistance to antioxidants, reducing body fat accumulation, and affecting reproductive ability in vivo using C. elegans. This is a significant finding that lends support to the hypotheses of the influence of mulberry leaf extract on human health. Our subsequent genetic tests and gene expression analysis demonstrates a model for the mechanism of MLP action in life span extension and fat regulation. The model, confirmed by our analysis, is that the functions of MLP mainly depend on the germline signaling pathway to influence the activities of transcription factors (DAF-12, DAF-16, PHA-4, and NHR-80) and to regulate the expression of downstream genes (fat-6, lipl-4, sod-3, unc-51, and fard-1) linking reproduction, fat metabolism, and life span. Our study has unraveled a diversity of modes of action in longevity promoted by MLP exposure. Our observations provide the first insights into MLP’s multifaceted functions on aging, fat accumulation, and reproduction in vivo, and raise a specific model for the mechanism of MLP action (Fig. 6).
Electronic supplementary material
(DOC 275 kb)
Acknowledgments
We thank the Caenorhabditis Genetics Center (CGC), which is funded by NIH Office of Research Infrastructure Programs (P40 OD010440), and Dr. Luo from Kunming Institute of Botany, Chinese Academy of Sciences, for providing all the worm strains. This work was supported by the Special Fund for Agro-scientific Research in the Public Interest (201403064) and the Nation Key Technology R&D Program (2012BAD36B07).
Abbreviations
- MLP
Mulberry leaf polyphenols
- C. elegans
Caenorhabditis elegans
- DAF-16
Dauer formation protein 16
- DAF-12
Dauer formation protein 12
- FOXO
Forkhead box protein O
- PHA-4/FOXA
a FOXA transcription factor
- NHR-80
Nuclear hormone receptor 80
- IIS
Insulin/IGF-1 like signaling pathway
- SKN-1
Skinhead, a homolog of mammalian Nrf proteins
- WT=N2
Wild-type C. elegans
Contributor Information
Shanqing Zheng, Phone: +86-020-87596248, Email: nihaoshanqing@163.com.
Sentai Liao, Phone: +86-020-87596248, Email: liaost@163.com.
References
- Abbas S, Wink M. Epigallocatechin gallate inhibits beta amyloid oligomerization in Caenorhabditis elegans and affects the daf-2/insulin-like signaling pathway. Phytomedicine. 2010;17(11):902–909. doi: 10.1016/j.phymed.2010.03.008. [DOI] [PubMed] [Google Scholar]
- Andallu B, Varadacharyulu NC. Antioxidant role of mulberry (Morus indica L. cv. Anantha) leaves in streptozotocin-diabetic rats. Clin Chim Acta. 2003;338(1–2):3–10. doi: 10.1016/S0009-8981(03)00322-X. [DOI] [PubMed] [Google Scholar]
- Andallu B, Suryakantham V, Lakshmi Srikanthi B, Reddy GK. Effect of mulberry (Morus indica L.) therapy on plasma and erythrocyte membrane lipids in patients with type 2 diabetes. Clin Chim Acta. 2001;314(1–2):47–53. doi: 10.1016/S0009-8981(01)00632-5. [DOI] [PubMed] [Google Scholar]
- Arantes-Oliveira N, Apfeld J, Dillin A, Kenyon C. Regulation of life-span by germ-line stem cells in Caenorhabditis elegans. Science. 2002;295(5554):502–505. doi: 10.1126/science.1065768. [DOI] [PubMed] [Google Scholar]
- Ashrafi K (2007) Obesity and the regulation of fat metabolism. WormBook 1–20 [DOI] [PMC free article] [PubMed]
- Bass TM, Weinkove D, Houthoofd K, Gems D, Partridge L. Effects of resveratrol on lifespan in Drosophila melanogaster and Caenorhabditis elegans. Mech Ageing Dev. 2007;128(10):546–552. doi: 10.1016/j.mad.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Berman JR, Kenyon C. Germ-cell loss extends C. elegans life span through regulation of DAF-16 by kri-1 and lipophilic-hormone signaling. Cell. 2006;124(5):1055–1068. doi: 10.1016/j.cell.2006.01.039. [DOI] [PubMed] [Google Scholar]
- Brenner S (1974) The genetics of Caenorhabditis elegans. Genetics 77:71-94 [DOI] [PMC free article] [PubMed]
- Chan KC, Yang MY, Lin MC, Lee YJ, Chang WC, Wang CJ (2013) Mulberry leaf extract inhibits the development of atherosclerosis in cholesterol-fed rabbits and in cultured aortic vascular smooth muscle cells. J Agric Food Chem 61:2780-2788 [DOI] [PubMed]
- Choi J, Kang HJ, Kim SZ, Kwon TO, Jeong SI, Jang SI. Antioxidant effect of astragalin isolated from the leaves of Morus alba L. against free radical-induced oxidative hemolysis of human red blood cells. Arch Pharm Res. 2013;36(7):912–917. doi: 10.1007/s12272-013-0090-x. [DOI] [PubMed] [Google Scholar]
- Collins JJ, Evason K, Kornfeld K. Pharmacology of delayed aging and extended lifespan of Caenorhabditis elegans. Exp Gerontol. 2006;41(10):1032–1039. doi: 10.1016/j.exger.2006.06.038. [DOI] [PubMed] [Google Scholar]
- Ebrahimi A, Schluesener H. Natural polyphenols against neurodegenerative disorders: potentials and pitfalls. Ageing Res Rev. 2012;11(2):329–345. doi: 10.1016/j.arr.2012.01.006. [DOI] [PubMed] [Google Scholar]
- Gerisch B, Weitzel C, Kober-Eisermann C, Rottiers V, Antebi A. A hormonal signaling pathway influencing C. elegans metabolism, reproductive development, and life span. Dev Cell. 2001;1(6):841–851. doi: 10.1016/S1534-5807(01)00085-5. [DOI] [PubMed] [Google Scholar]
- Goudeau J, Bellemin S, Toselli-Mollereau E, Shamalnasab M, Chen Y, Aguilaniu H. Fatty acid desaturation links germ cell loss to longevity through NHR-80/HNF4 in C. elegans. PLoS Biol. 2011;9(3):e1000599. doi: 10.1371/journal.pbio.1000599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer EL, Dowlatshahi D, Banko MR, Villen J, Hoang K, Blanchard D, Gygi SP, Brunet A. An AMPK-FOXO pathway mediates longevity induced by a novel method of dietary restriction in C. elegans. Curr Biol. 2007;17(19):1646–1656. doi: 10.1016/j.cub.2007.08.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen D, Wilson-Berry L, Dang T, Schedl T. Control of the proliferation versus meiotic development decision in the C. elegans germline through regulation of GLD-1 protein accumulation. Development. 2004;131(1):93–104. doi: 10.1242/dev.00916. [DOI] [PubMed] [Google Scholar]
- Hansen M, Flatt T, Aguilaniu H. Reproduction, fat metabolism, and life span: what is the connection? Cell Metab. 2013;17(1):10–19. doi: 10.1016/j.cmet.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda Y, Fujita Y, Maruyama H, Araki Y, Ichihara K, Sato A, Kojima T, Tanaka M, Nozawa Y, Ito M, Honda S. Lifespan-extending effects of royal jelly and its related substances on the nematode Caenorhabditis elegans. PLoS One. 2011;6(8):e23527. doi: 10.1371/journal.pone.0023527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C, Xiong C, Kornfeld K. Measurements of age-related changes of physiological processes that predict lifespan of Caenorhabditis elegans. Proc Natl Acad Sci U S A. 2004;101(21):8084–8089. doi: 10.1073/pnas.0400848101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulme SE, Whitesides GM. Chemistry and the worm: Caenorhabditis elegans as a platform for integrating chemical and biological research. Angew Chem Int Ed Engl. 2011;50(21):4774–4807. doi: 10.1002/anie.201005461. [DOI] [PubMed] [Google Scholar]
- Katsube T, Imawaka N, Kawano Y, Yamazaki Y, Shiwaku K, Yamane Y. Antioxidant flavonol glycosides in mulberry (Morus alba L.) leaves isolated based on LDL antioxidant activity. Food Chem. 2006;97(1):25–31. doi: 10.1016/j.foodchem.2005.03.019. [DOI] [Google Scholar]
- Katsube T, Tsurunaga Y, Sugiyama M, Furuno T, Yamasaki Y. Effect of air-drying temperature on antioxidant capacity and stability of polyphenolic compounds in mulberry (Morus alba L.) leaves. Food Chem. 2009;113(4):964–969. doi: 10.1016/j.foodchem.2008.08.041. [DOI] [Google Scholar]
- Lapierre LR, Hansen M. Lessons from C. elegans: signaling pathways for longevity. Trends Endocrinol Metab. 2012;23(12):637–644. doi: 10.1016/j.tem.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang L, Wu X, Zhu M, Zhao W, Li F, Zou Y, Yang L. Chemical composition, nutritional value, and antioxidant activities of eight mulberry cultivars from China. Pharmacogn Mag. 2012;8(31):215–224. doi: 10.4103/0973-1296.99287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao VH, Yu CW, Chu YJ, Li WH, Hsieh YC, Wang TT. Curcumin-mediated lifespan extension in Caenorhabditis elegans. Mech Ageing Dev. 2011;132(10):480–487. doi: 10.1016/j.mad.2011.07.008. [DOI] [PubMed] [Google Scholar]
- Lim HH, Yang SJ, Kim Y, Lee M, Lim Y. Combined treatment of mulberry leaf and fruit extract ameliorates obesity-related inflammation and oxidative stress in high fat diet-induced obese mice. J Med Food. 2013;16(8):673–680. doi: 10.1089/jmf.2012.2582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lithgow GJ, Gill MS, Olsen A, Sampayo JN. Pharmacological intervention in invertebrate aging. Age. 2005;27(3):213–223. doi: 10.1007/s11357-005-3625-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudra M, Ercan-Fang N, Zhong L, Furne J, Levitt M. Influence of mulberry leaf extract on the blood glucose and breath hydrogen response to ingestion of 75 g sucrose by type 2 diabetic and control subjects. Diabetes Care. 2007;30(5):1272–1274. doi: 10.2337/dc06-2120. [DOI] [PubMed] [Google Scholar]
- Pandey KB, Rizvi SI. Plant polyphenols as dietary antioxidants in human health and disease. Oxidative Med Cell Longev. 2009;2(5):270–278. doi: 10.4161/oxim.2.5.9498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S, Kim J, Kim Y. Mulberry leaf extract inhibits cancer cell stemness in neuroblastoma. Nutr Cancer. 2012;64(6):889–898. doi: 10.1080/01635581.2012.707280. [DOI] [PubMed] [Google Scholar]
- Peng C, Chan HY, Li YM, Huang Y, Chen ZY. Black tea theaflavins extend the lifespan of fruit flies. Exp Gerontol. 2009;44(12):773–783. doi: 10.1016/j.exger.2009.09.004. [DOI] [PubMed] [Google Scholar]
- Perron NR, Brumaghim JL. A review of the antioxidant mechanisms of polyphenol compounds related to iron binding. Cell Biochem Biophys. 2009;53(2):75–100. doi: 10.1007/s12013-009-9043-x. [DOI] [PubMed] [Google Scholar]
- Ristow M, Schmeisser S. Extending life span by increasing oxidative stress. Free Radic Biol Med. 2011;51(2):327–336. doi: 10.1016/j.freeradbiomed.2011.05.010. [DOI] [PubMed] [Google Scholar]
- Sangha JS, Sun X, Wally OS, Zhang K, Ji X, Wang Z, Wang Y, Zidichouski J, Prithiviraj B, Zhang J. Liuwei Dihuang (LWDH), a traditional Chinese medicinal formula, protects against beta-amyloid toxicity in transgenic Caenorhabditis elegans. PLoS One. 2012;7(8):e43990. doi: 10.1371/journal.pone.0043990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh SP, Niemczyk M, Zimniak L, Zimniak P. Fat accumulation in Caenorhabditis elegans triggered by the electrophilic lipid peroxidation product 4-hydroxynonenal (4-HNE) Aging (Albany NY) 2009;1(1):68–80. doi: 10.18632/aging.100005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohal RS, Orr WC. The redox stress hypothesis of aging. Free Radic Biol Med. 2012;52(3):539–555. doi: 10.1016/j.freeradbiomed.2011.10.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunagawa T, Shimizu T, Kanda T, Tagashira M, Sami M, Shirasawa T. Procyanidins from apples (Malus pumila Mill.) extend the lifespan of Caenorhabditis elegans. Planta Med. 2011;77(2):122–127. doi: 10.1055/s-0030-1250204. [DOI] [PubMed] [Google Scholar]
- Thom E. The effect of chlorogenic acid enriched coffee on glucose absorption in healthy volunteers and its effect on body mass when used long-term in overweight and obese people. J Int Med Res. 2007;35(6):900–908. doi: 10.1177/147323000703500620. [DOI] [PubMed] [Google Scholar]
- Tullet JM, Hertweck M, An JH, Baker J, Hwang JY, Liu S, Oliveira RP, Baumeister R, Blackwell TK. Direct inhibition of the longevity-promoting factor SKN-1 by insulin-like signaling in C. elegans. Cell. 2008;132(6):1025–1038. doi: 10.1016/j.cell.2008.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volovik Y, Maman M, Dubnikov T, Bejerano-Sagie M, Joyce D, Kapernick EA, Cohen E, Dillin A. Temporal requirements of heat shock factor-1 for longevity assurance. Aging Cell. 2012;11(3):491–499. doi: 10.1111/j.1474-9726.2012.00811.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson MA, Shukitt-Hale B, Kalt W, Ingram DK, Joseph JA, Wolkow CA. Blueberry polyphenols increase lifespan and thermotolerance in Caenorhabditis elegans. Aging Cell. 2006;5(1):59–68. doi: 10.1111/j.1474-9726.2006.00192.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood JG, Rogina B, Lavu S, Howitz K, Helfand SL, Tatar M, Sinclair D. Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature. 2004;430(7000):686–689. doi: 10.1038/nature02789. [DOI] [PubMed] [Google Scholar]
- Wu Z, Smith JV, Paramasivam V, Butko P, Khan I, Cypser JR, Luo Y. Ginkgo biloba extract EGb 761 increases stress resistance and extends life span of Caenorhabditis elegans. Cell Mol Biol (Noisy-le-grand) 2002;48(6):725–731. [PubMed] [Google Scholar]
- Wu T, Qi X, Liu Y, Guo J, Zhu R, Chen W, Zheng X, Yu T. Dietary supplementation with purified mulberry (Morus australis Poir) anthocyanins suppresses body weight gain in high-fat diet fed C57BL/6 mice. Food Chem. 2013;141(1):482–487. doi: 10.1016/j.foodchem.2013.03.046. [DOI] [PubMed] [Google Scholar]
- Yamawaki TM, Berman JR, Suchanek-Kavipurapu M, McCormick M, Gaglia MM, Lee SJ, Kenyon C (2010) The somatic reproductive tissues of C. elegans promote longevity through steroid hormone signaling. PLoS Biol 8(8) [DOI] [PMC free article] [PubMed]
- Zou S, Carey JR, Liedo P, Ingram DK, Yu B. Prolongevity effects of a botanical with oregano and cranberry extracts in Mexican fruit flies: examining interactions of diet restriction and age. Age (Dordr) 2012;34(2):269–279. doi: 10.1007/s11357-011-9230-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou Y, Liao S, Shen W, Liu F, Tang C, Chen CY, Sun Y. Phenolics and antioxidant activity of mulberry leaves depend on cultivar and harvest month in Southern China. Int J Mol Sci. 2012;13(12):16544–16553. doi: 10.3390/ijms131216544. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC 275 kb)