Abstract
The rare inherited condition acrodermatitis enteropathica (AE) results from a defect in the absorption of dietary zinc. Recently, we used homozygosity mapping in consanguineous Middle Eastern kindreds to localize the AE gene to an ∼3.5-cM region on 8q24. In this article, we identify a gene, SLC39A4, located in the candidate region and, in patients with AE, document mutations that likely lead to the disease. The gene encodes a histidine-rich protein, which we refer to as “hZIP4,” which is a member of a large family of transmembrane proteins, some of which are known to serve as zinc-uptake proteins. We show that Slc39A4 is abundantly expressed in mouse enterocytes and that the protein resides in the apical membrane of these cells. These findings suggest that the hZIP4 transporter is responsible for intestinal absorption of zinc.
Introduction
Zinc is an essential and versatile element, utilized by proteins as diverse as metalloenzymes and transcription factors. Evidence for the manifold nature of zinc requirements comes from symptoms of nutritional zinc deficiency, including growth retardation, immune-system dysfunction, alopecia, severe dermatitis, diarrhea, and, occasionally, mental disorders. These symptoms are mirrored in a rare, autosomal recessively inherited human disease, acrodermatitis enteropathica (AE [MIM 201100]). Although AE is known to result from defective intestinal absorption of zinc (Barnes and Moynahan 1973; Lombeck et al. 1975; Weismann et al. 1979; Van den Hamer et al. 1985), the biochemical basis for this disorder is unknown.
Previously we had mapped the AE gene to a telomeric region of 3.5 cM on chromosome 8q24.3 (Wang et al. 2001). We now extend these studies to the discovery of the AE gene. Our findings show that mutations in patients with AE are likely to disrupt a zinc-uptake protein that lies on the luminal surface of the intestine.
Patients, Material, and Methods
Collection and Analysis of Material from Patients
Patients diagnosed as having AE contributed blood samples after informed consent had been given by either them or their parents. Whenever possible, lymphoblastoid cell lines were established by Epstein-Barr virus transformation. Clinical information for most of the patients has been presented elsewhere (Wang et al. 2001).
Genomic PCR was performed, by Platinum Taq DNA Polymerase High Fidelity (Life Technologies), on 1 μg of genomic DNA, with eight pairs of primers covering all coding regions and splicing sites of the human gene, SLC39A4 (see the HUGO Gene Nomenclature Committee Web site). Primer pairs were designed by inspection of genomic DNA sequence in GenBank (accession number AF205589 [see the National Center for Biotechnology Information Web site]), and these sequences are available on request from the corresponding author. In some areas, nesting was necessary to obtain enough PCR product for sequencing. PCR products were run on agarose gels, were purified by the QiaxII Gel Extraction kit (Qiagen), and were sequenced with the same primers that were used for amplification.
For Southern analysis, 20 μg of genomic DNA from each individual was digested with restriction enzymes overnight in a 200-ml reaction volume. Digested DNA was precipitated with ethanol, was separated on a 0.8% agarose gel by electrophoresis, and was transferred onto a nylon membrane. The blot was hybridized with [32P]-dCTP (Perkin-Elmer)–labeled probe made from human SLC39A4 PCR products, and this was followed by autoradiography.
For RT-PCR, total RNA was isolated from patient cell lines by Trizol (Life Technologies), and first-strand cDNA was obtained from 5 μg of RNA, by random hexamers and the SuperScript First-Strand Synthesis System (Life Technologies). First-step PCR was performed on the cDNA, with a pair of primers at the 5′ and 3′ ends of the SLC39A4 cDNA. Nesting PCRs were performed with two pairs of primers, each covering half of the cDNA. PCR products were purified as described above.
Northern Blot Hybridization
Human multiple-tissue northern (MTN) panels I and II and digestive-system northern blots (Clontech) were hybridized with [32P]-dCTP–labeled probe. The probe was generated from genomic PCR products covering exons 2, 6, and 10 of SLC39A4. Hybridization and autoradiography were performed according to the manufacturer’s instructions.
In Situ Hybridization
Colons from wild-type mice (strain C57BL/6J) were collected, fixed overnight in 4% paraformaldehyde, embedded in paraffin, and sectioned (8 μm), as described elsewhere (Kuo et al. 1997). Radiolabeled [33P]-UTP sense and antisense probes corresponding to a 600-bp region of murine Slc39A4 cDNA sequence (GenBank accession number AA396412) were hybridized to sections. Sections were subsequently washed and were exposed to a photographic emulsion (Kodak NBT3) for 14 d. The slides were developed, counterstained with toluidine blue (Sigma), and visualized by dark-field microscopy using a Nikon Eclipse E800 microscope.
Immunohistochemistry
A colon from a 6-wk-old C57Bl/6J mouse was isolated, and fresh-frozen sections (8 μm) were cut and fixed for 20 min in 4% paraformaldehyde. A polyclonal rabbit anti-mouse Zip4 antibody was raised against a 15-mer peptide (AEETPELLNPETRRL) and was affinity purified with the peptide. The antibody was visualized by use of a goat AlexaFluor488 anti-rabbit IgG antibody (Molecular Probes). Nuclei were stained with 4′,6-diamidino-2-phenylindole, dihydrochloride (DAPI; Molecular Probes). Images were visualized, at 100× magnification, by a Nikon Eclipse E800 microscope.
Sequence Alignments and Phylogenetic Analysis
Multiple sequence alignments were constructed with ClustalX (Thomson et al. 1997; Jeanmougin et al. 1998). Alignment editing and shading were performed by the BioEdit Sequence Alignment Editor (Hall 1999). Phylogenetic analysis was performed by either ClustalX or Phylip 3.6 software (Felsenstein 1989), with the neighbor-joining method and (Saitou and Nei 1987) standard parameters. Tree representations were constructed by TreeView (Page 1996).
Results
On the basis of the map position of AE, we used GENSCAN (see the The New GENSCAN Web Server at MIT Web site; also see Burge and Karlin 1997) and the UCSC Human Genome Browser (see the UCSC Human Genome Project Working Draft Web site) to sift through genomic sequences from chromosome 8q24.3, for gene discovery. Of the >40 potential genes in the 3.5-cM candidate-gene region, one sequence appeared promising because it was predicted to produce a protein rich in histidines, ligands that are hallmarks of zinc binding.
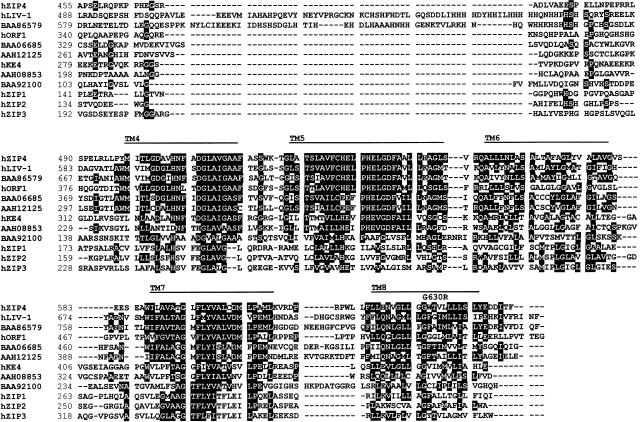
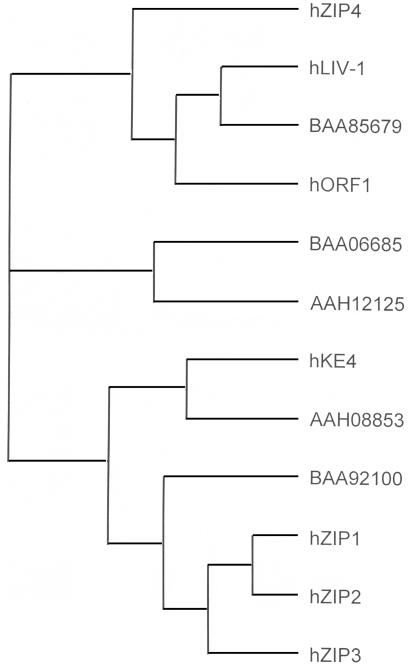
Further analysis of this candidate gene indicated that it encodes a novel transmembrane protein, related to a family of >100 sequences found in mammals, flies, worms, plants, and yeast. Figure 1 shows both the sequence of the predicted protein—here termed “hZIP4,” in relation to three other human proteins (hZIP1, hZIP2, and hZIP3), which have been shown to transport zinc into the cytoplasm (Gaither and Eide 2000, 2001a, 2001b), and sequences of eight other human proteins, whose function is unknown. The sequences are aligned according to their relatedness, which is shown in figure 2. All members of the protein family share membrane topologies, including eight transmembrane domains and a predicted cytoplasmic loop, typically rich in histidines, located between transmembrane domains 3 and 4 (reviewed by Guerinot [2000]). The similarity of hZIP4 to known zinc-uptake proteins made it a compelling candidate for AE.
Figure 1.
Sequence alignment of human ZIP4 and other human zinc transporters and related proteins. hZIP4 was used as a query to identify all human members of the ZIP family in public databases. All resulting human sequences are aligned according to their relatedness. Residues with ⩾50% identity are shaded black. Putative transmembrane (TM) domains are indicated by the horizontal lines, as is the putative signal-peptide sequence (predicted by the CBS SignalP V1.1 World Wide Web Prediction Server; also see Nielsen et al. 1997). A potential N-glycosylation site is indicated by an asterisk (*). Locations of amino acid changes found in patients with AE are noted above the hZIP4 sequence. Common polymorphisms, found in the process of sequencing DNA samples from patients and control subjects, include A272G (resulting in T58A), A440G (resulting in T114A), G1169A (resulting in A357T), and C2041T (resulting in F647F). Sequence records for the proteins shown in panel A—hZIP4 (accession number XP_035362); hLIV-1 (accession number XP_029402), in which hORF1 is derived from the nucleotide sequence (accession number AC023500); hKE4 (accession number CAA20238); hZIP1 (accession number XP_001483); hZIP2 (accession number XP_007499); and hZIP3 (accession number AAH05869)—are available at the National Center for Biotechnology Information Web site.
Figure 2.
Tree made by TreeView (Page 1996), demonstrating that hZIP4 falls within the LIV-1 subfamily of the human sequences.
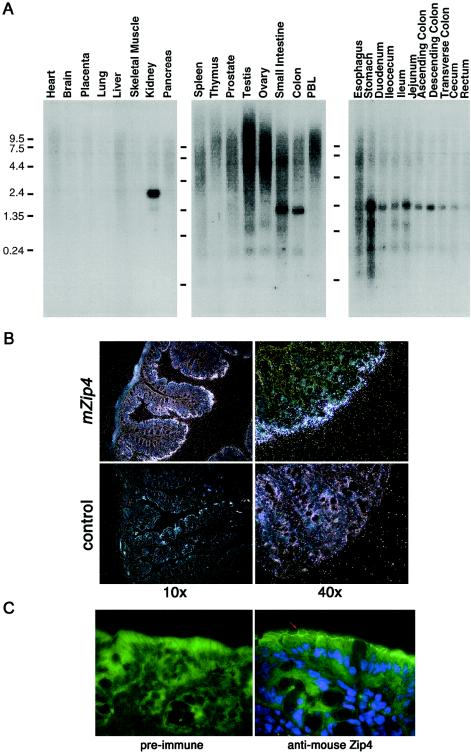
As befits a candidate for the AE gene, expression of the SLC39A4 gene was observed to be abundant in small intestine, stomach, and colon, as well as in kidney (fig. 3A). In situ hybridization to mouse colon confirmed that the gene is expressed in the intestinal villi (fig. 3B), and closer examination of the signals suggests that the mRNA is expressed mostly in the mature enterocytes, where zinc uptake has been predicted to occur (Steel and Cousins 1985). We were not able to confirm expression in the kidney by in situ hybridization in the mouse (data not shown). Immunohistochemistry using an anti-mouse Zip4 antibody demonstrated that the presumed zinc-uptake protein indeed resides on the apical surface of the enterocyte (fig. 3C).
Figure 3.
Tissue distribution of SLC39A4 expression. A, Hybridization of human SLC39A4 probes to Clontech human MTN blots and digestive-system northern blots. A 2.2-kb mRNA species was present at high levels in kidney and also in small intestine, colon, and most of the intestinal tract. The horizontal lines indicate the position of the molecular-weight markers in each blot. B, In situ hybridization of murine Slc39A4 antisense on adult mouse colon sections, at 10× and 40× magnification. Hybridization to the antisense probe is abundant in the enterocyte, whereas no hybridization is detected in the control (i.e., sense) probe. C, Immunohistochemical localization of mouse Zip4 to the apical membrane of the enterocyte in mouse colon. The red arrow indicates the apical surface, and the blue staining indicates nuclei stained with DAPI. Only nonspecific binding is seen with the preimmune sera. An identical result was obtained on paraffin-embedded tissue visualized with diamobenzidine (data not shown).
Before determining whether defects in hZIP4 lead to AE, we needed to define the exon/intron structure of the SLC39A4 gene, since only genomic DNA was available from the Middle Eastern kindreds in which AE had been mapped previously (Wang et al. 2001). By comparison of cDNA, EST, and genomic-sequence records, we determined that the gene consists of 12 exons and that two different transcripts are produced, one initiating at exon 1 and splicing into exon 2 and the other initiating upstream of exon 2. The former transcript appears to be the more prevalent and ubiquitously expressed of these two transcripts, on the basis of EST data, and the derived amino acid sequence of this transcript is presented in figure 1. Moreover, only this version of the sequence has been observed in the murine cDNA, EST, and genomic-sequence databases.
By direct sequence analysis of PCR-amplified SLC39A4 exons, we examined DNA samples from probands of six consanguineous Middle Eastern kindreds in whom AE had been previously mapped to chromosome 8q24.3 (Wang et al. 2001), and we discovered mutations in five of these kindreds. Distinct homozygous missense mutations were discovered in four kindreds (fig. 1 and table 1). Three of these mutations (G330D, L372P, and G630R) lead to substitutions of highly conserved amino acids in transmembrane domains and are very likely to be the cause of disease. None of these changes were observed either in the public databases or in 80–100 control chromosomes from an American population of mixed or unknown ethnicity. However, we cannot say with certainty that the P84L change found in Egyptian pedigree 2 leads to AE. Although this substitution is the only change that we found in this pedigree, it was also observed in 3 of 120 control chromosomes. In a fifth pedigree (pedigree 1 of Wang et al. 2001), we found a homozygous splice-acceptor mutation in which the AG preceding exon 7 is altered to GG. This mutation would be predicted to cause aberrant splicing. We were unable to discover a mutation in the remaining kindred (pedigree 9 of Wang et al. 2001), possibly because the mutation lies either within the exons 7–8-containing region that repeatedly failed to amplify adequately or in an intron or regulatory sequence that was not surveyed. The DNA from this proband proved to be of extremely poor quality and was insufficient for Southern analysis.
Table 1.
SLC39A4 Missense Mutations in Patients with AE and in Control Subjects[Note]
| Patient | Origin | Exon | Nucleotide Change (Amino Acid Change) | Frequency inControl Subjects |
| 2C | Egypt | 2 | C351T (P84L) | 3/120 |
| 1608a | France | 2 | C418A (N106K) | 0/120 |
| 1102 | Egypt | 6 | G1089A (G330D) | 0/80 |
| 303 | Egypt | 6 | T1215C (L372P) | 0/80 |
| 97025 | Jordan | 12 | G1988C (G630R) | 0/100 |
Note.— A homozygous splice acceptor mutation at exon 7 was found in patient III1 (who was of Egyptian origin).
Compound heterozygote for the mutation and a 2-kb upstream deletion.
Subsequent mutation analysis in a French patient with no family history of consanguinity or of AE uncovered a more complicated spectrum of SLC39A4 mutations. In this case, RT-PCR of SLC39A4 mRNA from a lymphoblastoid line from the patient revealed a missense mutation (N106K). However, although only the missense allele was apparent in the RT-PCR product, both the missense allele and the normal allele were observed in PCR of genomic DNA. Southern blot hybridization indicated, 5′ to the gene, a probable deletion of DNA (data not shown). This finding was confirmed by PCR and sequence analysis, identifying an ∼2-kb deletion of sequence located 3 kb upstream of the first exon in one allele (data not shown). These data suggest that the allele harboring the upstream deletion is not expressed in the French patient.
By sequence analysis of amplified genomic DNA, we found several additional missense mutations in patients with AE whose families had no history of consanguinity. One American patient of Hispanic descent was found to be homozygous both for the P84L change described above and for a mutation leading, at residue 309, to substitution of a highly conserved cysteine by tyrosine. Likewise, an Egyptian patient was found to be heterozygous for the P84L substitution but had, at residue 410 in the third transmembrane domain, an additional heterozygous amino acid change, of leucine to proline. We cannot discern from this analysis whether the patient is a compound heterozygote for these two changes. A heterozygous arginine-to-tryptophan substitution at position 251 was observed in a patient from the Netherlands. The implications of this substitution are hard to assess, since the sequence in this region is unique to hZIP4.
Discussion
We describe the discovery of a gene, SLC39A4, located in chromosome 8q24.3, that encodes a novel member of a protein family that includes zinc-uptake proteins. The gene is highly expressed in the mouse enterocyte, and the protein is localized to the apical membrane of this cell. Eight missense mutations, one splicing defect, and one transcription-inactivating upstream deletion were discovered in this gene in patients with AE. Since AE is known to map to this region (Wang et al. 2001), and since it is a disease specifically of intestinal zinc uptake, these findings provide both compelling evidence that we have identified the AE gene and a molecular basis for understanding this disorder. Unpublished evidence, from our Australian colleagues, of a mutation in the orthologous gene in Angus cattle affected with AE further supports this discovery (I. Tammen, personal communication).
The similarity of hZIP4 to the other zinc-uptake proteins strongly implicates it in direct uptake of zinc into the enterocyte. Zinc uptake has already been demonstrated for hZIP1 and hZIP2 (Gaither and Eide 2000, 2001b). Although our attempts to complement a yeast zrt1 zrt2 mutant (defective in zinc uptake) with hZIP4 failed, similar complementation experiments with hZIP1 and hZIP2 also failed yet both proteins were ultimately shown to transport 65Zn into mammalian cultured cells (Gaither and Eide 2000, 2001b). Thus, we are confident that future zinc-transport studies will confirm the predicted role of hZIP4 in zinc uptake.
In contrast to the previously described hZIP proteins, hZIP4 harbors a large histidine-rich N-terminal sequence (fig. 1). This sequence is preceded by a hydrophobic segment that likely serves as a signal peptide, which is used for orienting the bulky amino-terminal sequence into the lumen of the endoplasmic reticulum and which then is released by cleavage (Martoglio and Dobberstein 1998). On the basis of our localization of murine Zip4 to the apical membrane of the enterocyte, this hypothesis would predict a large histidine-rich domain dangling into the intestinal lumen, where it might serve as a trap for dietary zinc.
Our findings foster speculation as to why zinc deficiency in patients with AE can be overcome by dietary supplementation of zinc (a 2- to 20-fold excess compared with the RDA). This clinical observation suggests that patients with AE retain some mechanism for intestinal zinc uptake. One possibility is that the mutations that we discovered in patients with AE—namely, missense mutations, a splicing defect, and a heterozygous deletion—may impede hZIP4 function but not completely destroy it. If so, the effects of such hypomorphic mutations might be overcome by increased zinc substrate. A second explanation may lie in the supplemental action of another zinc transporter, expressed in the intestine. For example, the divalent metal transporter DMT1 also resides on the apical surface of the enterocyte and can transport zinc in vitro (Gunshin et al. 1997). However, a more compelling candidate could lie in one of the putative zinc-uptake proteins depicted in figure 1. The sequence referred to provisionally as “hORF1” is particularly intriguing, since SAGE (Serial Analysis of Gene Expression [see the SAGEmap Web site]) data demonstrate that it is expressed primarily in the epithelium of the colon and in adenocarcinoma cells. Indeed, we might further speculate that mutations in this sequence could give rise to AE in patients in whom the disease does not map to chromosome 8q24.3.
Acknowledgments
We thank Martha Gunthorpe, Joe DeYoung, and Hernan Consengco for technical assistance, and we thank Dan Minor, Seymour Packman, and David Eide for helpful discussions. We are grateful to Drs. W. Mostafa, M. Al-aboosi, H. El-Shanti, and J. Van Wouwe and the many patients who contributed to this study. Part of this work was supported by a National Institute of Diabetes & Digestive & Kidney Diseases grant. J.G. is an Investigator with the Howard Hughes Medical Institute.
Electronic-Database Information
Accession numbers and URLs for data presented herein are as follows:
- CBS SignalP V1.1 World Wide Web Prediction Server, http://www.cbs.dtu.dk/services/SignalP/
- HUGO Gene Nomenclature Committee, http://www.gene.ucl.ac.uk/nomenclature
- National Center for Biotechnology Information, http://www.ncbi.nlm.nih.gov/ (for hORF1 nucleotide record [accession numbers BAA86579 and AC023500], hKE4 [accession numbers BAA06685, AAH12125, and CAA20238], hLIV-1 [accession number XP_029402], hZIP1 [accession numbers AAH08853, AC023500, and XP_001483], hZIP2 [accession number XP_007499], hZIP3 [accession number AAH05869], and hZIP4a [accession number XP_035362])
- New GENSCAN Web Server at MIT, The, http://genes.mit.edu/GENSCAN.html
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for AE [MIM 201100])
- SAGEmap, http://www.ncbi.nlm.nih.gov/SAGE/
- UCSC Human Genome Project Working Draft, http://genome.ucsc.edu/index.html
References
- Barnes PM, Moynahan EJ (1973) Zinc deficiency in acrodermatitis enteropathica: multiple dietary intolerance treated with synthetic diet. Proc R Soc Med 66:327–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burge C, Karlin S (1997) Prediction of complete gene structures in human genomic DNA. J Mol Biol 268:78–94 [DOI] [PubMed] [Google Scholar]
- Felsenstein J (1989) PHYLIP—phylogeny inference package (version 3.2). Cladistics 5:164–166 [Google Scholar]
- Gaither LA, Eide D (2000) Functional expression of the human hZIP2 zinc transporter. J Biol Chem 275:5560–5564 [DOI] [PubMed] [Google Scholar]
- ——— (2001a) Eukaryotic zinc transporters and their regulation. Biometals 14:251–270 [DOI] [PubMed] [Google Scholar]
- ——— (2001b) The human ZIP1 transporter mediates zinc uptake in human K562 erythroleukemia cells. J Biol Chem 276:22258–22264 [DOI] [PubMed] [Google Scholar]
- Guerinot ML (2000) The ZIP family of metal tranpsorters. Biochim Biophys Acta 1465:190–198 [DOI] [PubMed] [Google Scholar]
- Gunshin H, Mackenzie B, Berger UV, Gunshin Y, Romero, MF, Boron, WF, Nussberger S, Gollan JL, Hediger MA (1997) Cloning and characterization of a mammalian proton-coupled metal-ion transporter. Nature 388:482–488 [DOI] [PubMed] [Google Scholar]
- Hall TA (1999) BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucl Acids Symp Ser 41:95–98 [Google Scholar]
- Jeanmougin F, Thompson JD, Gouy M, Higgins DG, Gibson TJ (1998) Multiple sequence alignment with Clustal X. Trends Biochem Sci 23:403–405 [DOI] [PubMed] [Google Scholar]
- Kuo YM, Gitschier J, Packman S (1997) Developmental expression of the mouse mottled and toxic milk genes suggests distinct functions for the Menkes and Wilson disease copper transporters. Hum Mol Genet 6:1043 [DOI] [PubMed] [Google Scholar]
- Lombeck T, Schnippering HG, Ritzl F, Feinendegen LE, Bremer HJ (1975) Absorption of zinc in acrodermatitis enteropathica. Lancet 1:855 [DOI] [PubMed] [Google Scholar]
- Martoglio B, Dobberstein B (1998) Signal sequences: more than just greasy peptides. Trends Cell Biol 8:410–415 [DOI] [PubMed] [Google Scholar]
- Nielsen H, Engelbrecht J, Brunak S, von Heijne G (1997) Identification of prokaryotic and eukaryotic signal peptides and prediction of their cleavage sites. Protein Eng 10:1–6 [DOI] [PubMed] [Google Scholar]
- Page RDM (1996) TreeView: an application to display phylogenetic trees on personal computers. Comput Appl Biosci 12:357–358 [DOI] [PubMed] [Google Scholar]
- Saitou N, Nei M (1987) The neighbour-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4:406–425 [DOI] [PubMed] [Google Scholar]
- Steel L, Cousins RJ (1985) Kinetics of zinc absorption by luminally and vascularly perfused rat intestine. Am J Physiol 248:G46–G53 [DOI] [PubMed] [Google Scholar]
- Thomson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG (1997) The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 25:4876–4882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Hamer CJA, Cornelisse C, Hoogenraad TU, Van Wouwe JP (1985) Use of 69mZn loading for monitoring of zinc malabsorption. In: Mills CF, Brenner I, Chesters JK (eds) Trace elements in man and animals—TEMA 5. Commonwealth Agricultural Bureaux, Slough UK, pp 689–691 [Google Scholar]
- Wang K, Pugh EW, Griffen S, Doheny KF, Mostafa WZ, Al-Aboosi MM, El-Shanti H, Gitschier J (2001) Homozygosity mapping places the acrodermatitis enteropathica gene on chromosome 8q24.3. Am J Hum Genet 68:1055–1060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weismann K, Hoe S, Knudsen L, Sorensen SS (1979) 65Zinc absorption in patients suffering from acrodermatitis enteropathica and in normal adults assessed by whole-body counting technique. Br J Dermatol 101:573–579 [DOI] [PubMed] [Google Scholar]