Key Points
EBV-CTLs resistant to calcineurin inhibitors mediate durable, potent antitumor responses despite immunosuppression in a murine model of PTLD.
This approach improves immunotherapy efficacy with EBV-CTLs for PTLD after SOT and obviates need for immunosuppression withdrawal.
Abstract
Epstein-Barr virus (EBV)-associated posttransplant lymphoma (PTLD) is a major cause of morbidity/mortality after hematopoietic stem cell (SCT) or solid organ (SOT) transplant. Adoptive immunotherapy with EBV-specific cytotoxic lymphocytes (CTLs), although effective in SCT, is less successful after SOT where lifelong immunosuppression therapy is necessary. We have genetically engineered EBV-CTLs to render them resistant to calcineurin (CN) inhibitor FK506 through retroviral transfer of a calcineurin A mutant (CNA12). Here we examined whether or not FK506-resistant EBV-CTLs control EBV-driven tumor progression in the presence of immunosuppression in a xenogeneic mouse model. NOD/SCID/IL2rγnull mice bearing human B-cell lymphoma were injected with autologous CTLs transduced with either CNA12 or eGFP in the presence/absence of FK506. Adoptive transfer of autologous CNA12-CTLs induced dramatic lymphoma regression despite the presence of FK506, whereas eGFP-CTLs did not. CNA12-CTLs persisted longer, homed to the tumor, and expanded more than eGFP-CTLs in mice treated with FK506. Mice receiving CNA12-CTLs and treated with FK506 survived significantly longer than control-treated animals. Our results demonstrate that CNA12-CTL induce regression of EBV-associated tumors in vivo despite ongoing immunosuppression. Clinical application of this novel approach may enhance the efficacy of adoptive transfer of EBV-CTL in SOT patients developing PTLD without the need for reduction in immunosuppressive therapy.
Introduction
Epstein-Barr virus (EBV) is a human γ-herpesvirus that infects and establishes latency in B lymphocytes in >90% of adults. In healthy individuals EBV-specific cytotoxic T lymphocytes (CTL) prevent the outgrowth of the EBV-transformed B cells1. In hematopoietic stem cell (SCT) or solid organ transplantation (SOT) recipients, this T-cell immune surveillance is compromised by the immunosuppressive medication used to prevent graft-versus-host disease/graft rejection. This can enable uncontrolled proliferation and malignant transformation of EBV-infected B cells, resulting in posttransplant lymphoproliferative diseases (PTLD). The prevalence of this complication in SOT can vary from 1% to 30%, depending on the organ transplanted, the patient’s age, and the intensity of immunosuppression.2
Therapies targeting EBV-infected B cells with monoclonal anti-CD20 antibodies (rituximab), reduction of immunosuppressive drugs, and chemotherapy are currently used3,4 but are often ineffective and have substantial toxicity. Rituximab as monotherapy is associated with a high rate of disease progression and relapse5; reduction of immunosuppression frequently results in graft rejection6 and, although chemotherapy results in better response rates, treatment-related mortality is high in this patient population.7 In the PTLD-1 trial8 combining rituximab with cyclophosphamide-hydroxydaunorubicin-oncovin-prednisone (CHOP) chemotherapy, 3-year progression-free survival was 54%. Thus, novel therapies are clearly needed.
Adoptive transfer of ex vivo–derived EBV-specific cytotoxic T cells (EBV-CTLs) to reconstitute immunity to EBV9-12 is a logical approach in the treatment of PTLD. However, the application of this approach for the treatment of PTLD in SOT patients, although feasible,10,13,14 has been challenging. This difference is likely to reflect the need for ongoing immunosuppression to prevent graft rejection post-SOT, which inhibits virus-specific T-cell responses.15,16 Although it is generally possible to withdraw other immunosuppressive medication (eg, mycophenolate mofetil [MMF]) to facilitate CTL function in SOT recipients developing PTLD, reduction in calcineurin (CN) inhibitors, the most critical immunosuppressive drugs used after SOT, frequently results in graft rejection. Indeed, in a major study, graft rejection was as common a cause of mortality in PTLD patients as was the disease itself.6
To address this problem, we have previously developed a strategy for genetically engineering EBV-CTLs to be resistant to the (CN) inhibitors, cyclosporin A (CsA) and tacrolimus (FK506).17 These drugs exert their immunosuppressive function by binding to cyclophilin (CyPA) and FK-binding protein 12 (FKBP-12), respectively. These complexes inhibit the calcium-sensitive phosphatase CN from binding to the transcription factor nuclear factor of activated T cells (NFAT), thus preventing activation of cytokine genes in T cells. To enable CTL to function in the presence of immunosuppression, EBV-CTLs have been genetically engineered to express CN mutations which inhibit docking of either or both FK506/FKBP12 and CsA/CyPA complexes, but do not affect the active site. The mutant used in our current experiments, CNA12 has 2 mutations T351E and L354A which disrupt the binding between CNA and the charged surface residues H87-P88 of FKBP12 to the CN heterodimer but do not affect NFAT dephosphorylation.
EBV-CTLs expressing such mutants maintain their ability to proliferate and secrete interferon (IFN)-γ in response to stimulation with EBV in vitro in the presence of therapeutic levels of FK506 and/or CsA.17
To evaluate the functionality of CN-resistant EBV-CTLs and determine whether they can mediate rejection of EBV+ B-cell lymphoma in the presence of immunosuppression with CN inhibitors in vivo, we assessed the efficacy of FK506-resistant EBV-CTLs in a xenograft tumor model in the immunodeficient mouse strain NOD/SCID/IL2rγnull (NSG). NSG mice were engrafted with human EBV lymphoblastic cells (LCL) and then treated with autologous EBV-CTLs transduced with the CN mutant CNA12 or control eGFP in the presence or absence of FK506. We demonstrate that EBV-CTLs resistant to CN inhibitors can mediate curative antitumor responses despite the presence of ongoing immunosuppression.
Materials and methods
Generation of LCLs and EBV CTLs
Peripheral blood was taken from healthy EBV-seropositive volunteers after their informed consent and peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll centrifugation (GE Healthcare, Amersham, UK). EBV-LCLs were generated by infection of PBMCs with B95.8 supernatant as previously described.15 EBV-CTLs were generated by repetitive stimulation of PBMCs with autologous irradiated LCLs as previously published.15
Generation of retrovirus and transduction
High-titer stable SFG retroviral supernatants that were either carrying the eGFP transgene alone or were expressed with the CNA12 mutant pseudotyped with the RD114 envelope were produced as described previously17 and were used to transduce EBV-CTLs. LCLs were transduced with an SFG retroviral vector encoding Firefly Luciferase (FLuc-eBFP) to facilitate imaging.
Phenotypical and functional analysis of transduced CTLs
CNA12eGFP or eGFP-EBV-CTLs were assessed for phenotypic analysis by flow cytometry and for functionality using 51Cr release, 3H proliferation, and IFN-γ enzyme-linked immunosorbent assays in the presence or absence of 10 ng/mL FK506, as detailed in the supplemental Methods available on the Blood Web site.
Xenograft model and bioluminescence imaging
All animal studies were approved by the University College London Biological Services Ethical Review Committee and licensed under the Animals (Scientific Procedures) Act 1986 (Home Office, London, United Kingdom). NSG mice were obtained from Jackson Laboratory and raised under specific pathogen-free conditions. Mice were inoculated with 5 × 106 EBV-LCLs engineered to express firefly Luciferase (F-Luc) subcutaneously on the nape of the neck on day 0 (4 mice/cohort in 2 separate experiments). Expression of F-Luc allows for noninvasive monitoring of xenografted tumors in mice by luminescent imaging in vivo. To assess antitumor activity of control and genetically modified human EBV-CTLs in the presence of FK506 in vivo, mice received 5 × 106 autologous EBV-CTLs intravenously (IV) via the tail vein after 7 days. In addition, mice also received intraperitoneal (IP) injections of IL-2 (Proleukin; Chiron, Ratingen, Germany), 2500 U daily for 7 days post–EBV-CTL. 10 mg/kg body weight of FK506 (Prograf, Astellas Pharma US, Northbrook, IL) was administered IP 5 days per week from 3 to 4 days after LCL injection. Pilot dose titration experiments in which trough FK506 concentration levels in the whole blood were measured daily by mass spectrometry after varying doses of FK506 demonstrated that a dose of 10 mg/kg reliably resulted in blood levels within the human therapeutic range of 10 to 20 ng/mL.
Tumor growth was evaluated using the IVIS imaging system (Xenogen; Caliper Life Sciences, Hopkinton, MA). Animals were imaged before T-cell transfer and twice weekly thereafter. Photon emission from FLuc+ LCLs expressed in photon per second per cm2 per steradian (p/s/cm2/sr) was quantified using Living Image software (Xenogen) as previously described.18 In addition to monitoring with bioluminescence imaging, the size of LCL tumors was also measured twice weekly with calipers. Tumors were resected immediately after euthanasia for immunofluorescence staining.
Immunofluorescence
Double immunofluorescence was performed on formalin-fixed, paraffin-embedded tumor sections. Staining was carried out for B and T cells using a 1:100 dilution of primary rabbit monoclonal antibody specific for human CD3, and 1:20 dilution of primary mouse monoclonal antibody for human CD20 (Abcam, Cambridge, UK). This was followed by incubation with secondary antibodies, FITC anti-rabbit IgG, and TRITC anti-mouse IgG, respectively. Sections were viewed with epifluorescence using a Leica DM400B microscope and digitally captured using Metamorph software (Molecular Devices, Sunnyvale, CA).
Statistical analysis
Mean ± standard error of the mean (SEM) is depicted in all graphs. Statistical significance was determined by 2-way analysis of variance followed by Bonferroni posttest for longitudinal data. Two-tailed Student t test for unpaired samples was applied to evaluate persistence of transferred T cells, CNA12 mutant expression, proliferation, cytokine secretion, and specific cytolysis at single time points. Survival curves were plotted according to the Kaplan-Meier method, and treatment groups were compared using log-rank (Mantel-Cox) test analyses. Differences were considered significant for P values < .05.
Results
Characterization of EBV-CTLs before infusion
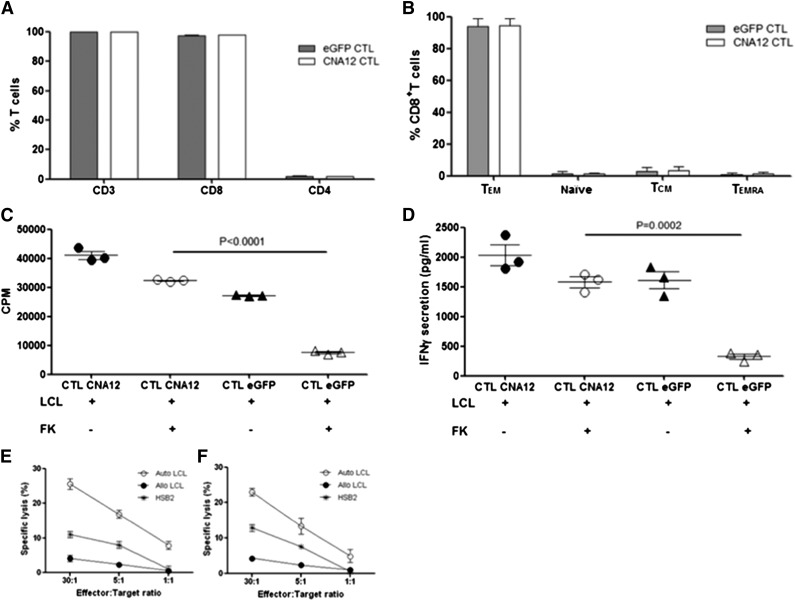
To investigate the ability of EBV-CTLs genetically engineered to be resistant to CN inhibitors to function in vivo despite ongoing immunosuppression, we first transduced human EBV-CTLs with retroviral vectors encoding CN mutant CNA12 or eGFP control. CTL transduction efficiency, as assessed by flow cytometry before infusion, was consistently high (mean 77.05% ± 0.6 for CNA12 and mean 89.5% ± 0.9 for eGFP). Before adoptive transfer of the EBV-CTLs into the mice, we characterized the phenotype of transduced CTLs. All CTLs expressed CD3: the majority (97.7% ± 0.1%; mean ± SD) were CD8+, whereas 2% ± 0.1% expressed CD4 (Figure 1A). Because differentiation status can influence their expansion potential and persistence of the adoptively transferred T cells, we also examined memory subsets. We found that the majority of CD8+ T cells (mean 88.7% ± 0.7%) showed an effector memory phenotype (CCR7– CD45RA–), as shown in Figure 1B. Only 2% to 3% of the CTLs were naïve (CCR7+CD45RA+), 5.3% to 5.7% were CCR7+CD45RA– central memory T cells, and 2% to 2.5% were terminally differentiated effector cells (CCR7–CD45RA+). Transduction of EBV-CTLs with either eGFP control vector or CNA12 did not change the phenotype compared with untransduced CTLs.
Figure 1.
Immunophenotypic and functional analysis of transduced EBV-specific CTLs before adoptive transfer. (A) CNA12- and eGFP-transduced EBV-CTLs were analyzed for T-cell marker expression by flow cytometry. Percentages of CD3+, CD4+, and CD8+ T cells are shown. (B) Distribution of memory subsets in CNA12- and eGFP-transduced EBV-CTLs. Effector memory (CCR7–CD45RA–), naïve (CCR7+CD45RA+), central memory (CCR7+CD45RA–), terminal-differentiated (TD) effector (CCR7–CD45RA+) T cells. (C) Proliferation ability of EBV-CTLs was evaluated after stimulation in vitro with autologous LCLs with or without therapeutic dose (10 ng/mL) of FK506 and was tested by 3H-thymidine uptake for 21 hours. Proliferation of eGFP-transduced EBV-CTLs after stimulation with LCLs in the presence of FK506 was significantly inhibited (P < .0001) compared with CNA12-transduced EBV-CTLs. (D) EBV-CTLs transduced with CNA12 were able to secrete IFN-γ in the presence of autologous LCLs plus 10 ng/mL of FK506 at comparable levels with those seen with CNA12-transduced T cells stimulated with LCLs alone. eGFP-transduced EBV-CTLs did not produce IFN-γ in the presence of LCLs plus 10 ng/mL FK506 compared with eGFP-transduced T cells stimulated with LCLs alone (P < .001). A standard 51Cr release cytotoxicity assay was performed to assess for the cytotoxicity of EBV-CTL lines against autologous mismatched LCL targets or the T-cell line HSB2 in the presence of 10 ng/mL of FK506. Cytolytic activity of eGFP- (E) and CNA12- (F) transduced cells at different effector-to-target ratios one week after fifth stimulation. The mean ± SEM of 2 CTL lines are shown.
To assess the ability of CNA12-transduced CTLs to function in the presence of CN inhibitors in vitro before injection, we investigated both their ability to proliferate and to secrete IFN-γ in response to antigenic stimulation with autologous B-LCLs (Figure 1C-D) in the presence of therapeutic doses (10 ng/mL) of FK506. As previously described,17 CNA12-transduced CTLs were able to proliferate and secrete IFN-γ in vitro in the presence of FK506 at comparable levels as in its absence. Conversely, FK506 completely inhibited proliferation (P < .0001) and secretion of IFN-γ (P < .001) of eGFP-transduced CTLs. In cytotoxicity assays, as shown in Figure 1E-F, respectively, both CNA12 and eGFP-transduced CTLs showed equivalent specific cytotoxicity against autologous EBV-LCL targets (CNA12-transduced CTLs 25.3 ± 1.8% lysis, eGFP-transduced CTLs 22.9 ± 1.0% lysis at effector-target ratio of 30:1, n = 2). Cytotoxicity was MHC-restricted because no lysis of allogeneic LCLs was observed, and was not NK-mediated because significant cytotoxicity of the HSB2 cell line was not seen. Culture of either CNA12- or eGFP-CTLs with FK506 before or during the cytotoxicity assay had no effect on the cytotoxicity of EBV-CTLs against LCL targets (data not shown).
Taken together, these data show that the CNA12-transduced CTLs infused in murine experiments were able to secrete cytokines and proliferate in response to EBV, and were able to lyse EBV-infected targets in vitro despite the presence of FK506.
EBV-CTLs transduced with CN mutant lack alloreactivity
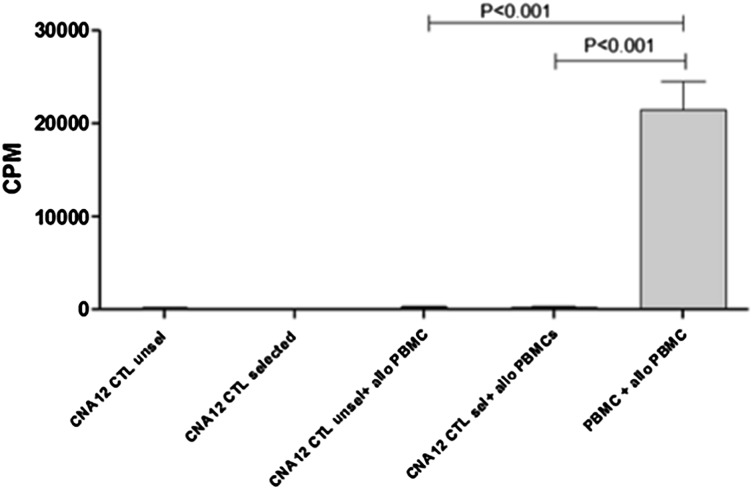
If tacrolimus-resistant CTLs are to be used clinically after organ transplant, it is critical that they are depleted of alloreactivity to avoid toxicity to the organ graft. We therefore assayed the proliferative responses of unselected and GFP+-sorted CNA12-CTLs to HLA-mismatched allogeneic PBMC stimulators, comparing this with the alloreactivity of unmanipulated PBMC from the same donor. As is shown in Figure 2, unmanipulated donor PBMCs proliferated strongly in response to stimulation with irradiated, allogeneic PBMCs (P < .001). In contrast, the response of both unselected and GFP+-sorted CNA12-CTLs to allogeneic PBMCs was negligible, indicating that the process of EBV-CTL generation, expansion, and transduction with CNA12 depletes alloreactivity.
Figure 2.
CNA12 EBV-CTLs lack alloreactivity versus HLA-mismatched donors. Alloreactive potential of unmanipulated PBMCs as well as CNA12-transduced EBV-CTLs from the same donor using a primary mixed lymphocyte reaction. Unmanipulated PBMCs proliferated significantly (P < .001) in response to allogeneic, irradiated PBMCs compared with the proliferation of CNA12-EBV-CTLs unselected or GFP+ selected. Data show the mean values and SEM of experiments from 4 donors.
Adoptive transfer of autologous CN-resistant EBV-CTLs induced rejection of EBV lymphoma in xenografted NSG mice in the presence of FK-506
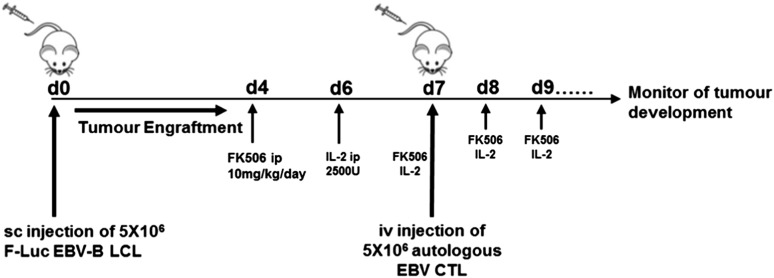
To determine whether CNA12-transduced CTLs could mediate rejection of EBV+ lymphoma in vivo despite the presence of immunosuppression, we adoptively transferred CNA12 or eGFP CTLs into NSG mice bearing autologous EBV+F-Luc+ LCL tumors in the presence or absence of FK506 (experimental schema shown in Figure 3).
Figure 3.
Experimental design of PTLD xenograft mice model. To perform analysis on the CN-resistant EBV-CTLs in vivo, we investigated their functionality as adoptive therapy in an NSG xenograft model. First, we began generating human EBV-LCLs by infecting PBMCs from EBV-seropositive donor cells with EBV-producing marmoset B-cell line (B95.8) in vitro. Once B-LCL was established, it was used to perform repetitive stimulation of autologous PBMCs to generate EBV-CTLs. For in vivo purposes, we transduced B-LCLs with a retroviral vector encoding FLuc to allow us to monitor tumor development in vivo. Mice were inoculated with 5 × 106 EBV-LCLs subcutaneously on the nape of the neck on day 0 (4 mice/cohort in 2 separate experiments). To assess antitumor activity of control and genetically modified human EBV-CTLs in the presence of FK506 in vivo, mice received 5 × 106 autologous EBV-CTLs IV via the tail vein after 7 days. In addition, mice also received IP injections of 2500 U of IL-2 daily for 7 days post–EBV-CTL. 10 mg/kg body weight of FK506 was also administered IP 5 days per week from 3 to 4 days after LCL injection. Tumor growth was evaluated using the IVIS imaging system. Photon emission from FLuc+ LCLs was quantified using Living Image software.
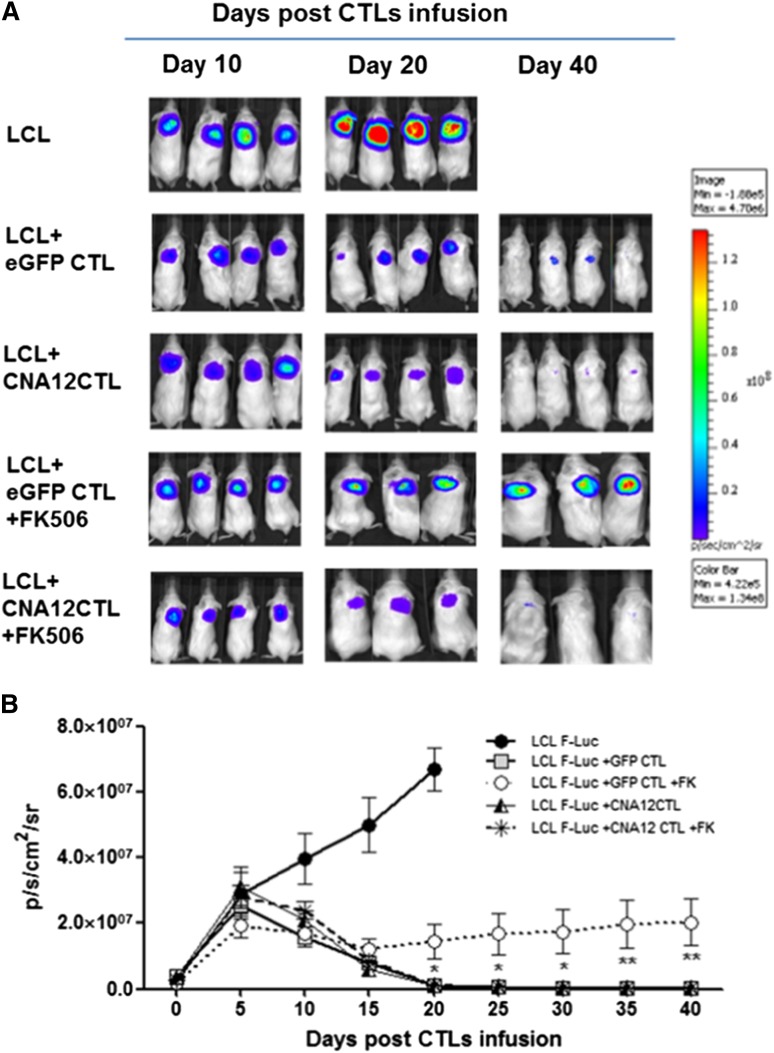
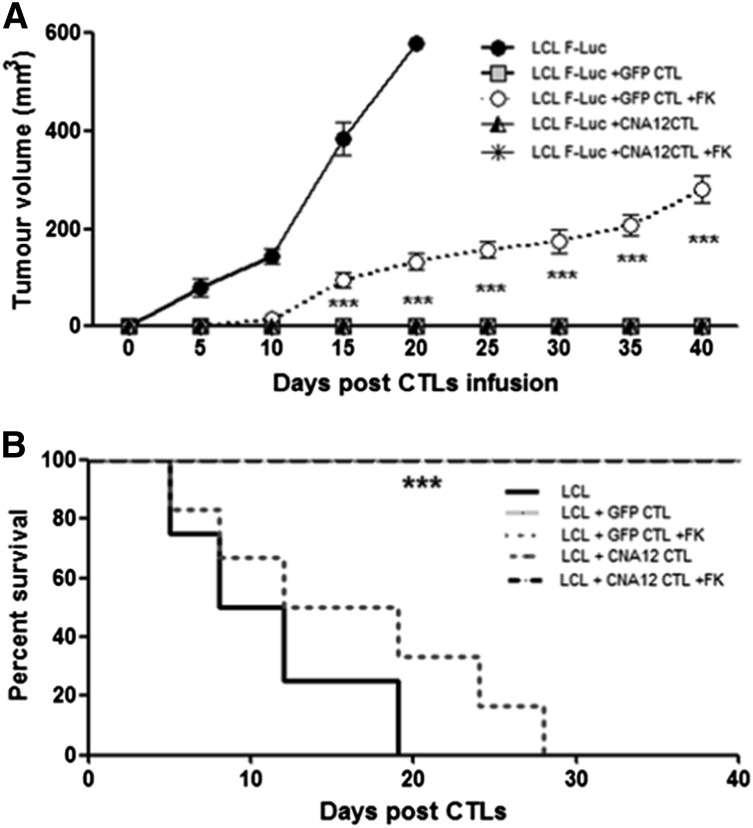
In vivo imaging showed tumor growth in the control mice treated with B-LCLs within 20 days, requiring mice to be sacrificed (Figure 4A-B). Mice treated with eGFP-CTLs or CNA12-CTLs without FK506 showed reduced tumor growth: mean bioluminescence values were 1.2 ± 0.3 × 105 p/s/cm2/sr (n = 8) and 1.1 ± 0.4 × 105 p/s/cm2/sr (n = 8), respectively, at day 40 compared with bioluminescence values of 6.7 × 107 ± 6.6 × 106 (n = 8) in untreated control mice, (P < .0001). Mice infused with resistant CNA12-CTLs showed tumor clearance from day 20 post–EBV-CTL injection despite FK506 treatment with bioluminescence values reducing from a mean of 2.6 ± 0.3 × 106 p/s/cm2/sr (n = 7) at day 0 to 1.3 ± 0.3 × 105 by day 40 (P < .05). In contrast, mice treated with control eGFP-CTLs in the presence of FK506 showed a progressive increase in tumor bioluminescence (mean 1.7 × 106 ± 0.2 p/s/cm2/sr, at day 0 to 2 ± 0.7 × 107 p/s/cm2/sr by day 40 (n = 7) (P < .01) (Figure 4B). Calcineurin inhibition per se led to minor tumor regression, as was previously observed in another model, consistent with the ability of these drugs to induce apoptosis of human lymphoma and leukemic cells.19
Figure 4.
Adoptive transfer of CN-resistant EBV-CTLs induces regression of EBV B-cell lymphoma in vivo in a xenogeneic mouse model despite the ongoing immunosuppression. (A) To evaluate in vivo antitumor activity, light emission by tumor cells was monitored as an indication of tumor growth using the IVIS in vivo imaging system in 4 NSG mice per group. Mice with the B-cell lymphoma cells (LCLs) showed tumor progression and were sacrificed after 20 days. Mice receiving autologous CNA12- or eGFP-CTLs without FK506 showed a decrease in tumor formation. CNA12-CTLs mediate tumor clearance despite the presence of FK506, as observed by reduction of F-Luc+ bioluminescence signal. On the contrary, mice receiving eGFP-CTLs showed tumor development in the presence of FK506. (B) The graph shows the kinetics of tumor growth. Photon emission from FLuc+ tumor cells was quantified and measured as maximum photon/sec/cm2/steradian (p/s/cm2/sr). The graph shows that tumor growth was significantly (P < .05) greater in mice receiving EBV-CTLs expressing eGFP than in mice receiving CNA12-CTLs in the presence of FK506. Lines represent cumulative results of light emission values ± SEM bioluminescence signal determined in 2 separate experiments (n = 8).
Tumor growth was also assessed by measuring the volume of the subcutaneous palpable tumor using calipers. In the absence of EBV-specific T-cell surveillance, challenge of NSG mice with EBV-LCLs induced the development of large tumors (mean volume 579.7 mm3 ± 2.9 SD, n = 8) at day 20. Mirroring our IVIS findings, adoptive transfer of either CNA12- or eGFP-CTLs led to complete tumor regression in the absence of FK506. Similarly, we did not observe tumor growth in the mice treated with CNA12-CTLs in the presence of FK506 (mean volume 0 mm3 at day 40, n = 7). In contrast, mice infused with control GFP-CTLs in the presence of FK506 showed progressive increase in tumor volume from day 15 post-CTL infusion (mean volume 132.6 mm3 ± 43.8 SD at day 20; P < .001; n = 8) (Figure 4A).
To make our approach more feasible for clinical application, we developed a simpler, more rapid methodology for generation of tacrolimus-resistant EBV-specific T cells using IFN-γ selection after stimulation with immunodominant EBV peptides followed by transduction with a retroviral vector encoding the CNA12 mutant.20 As is shown in supplemental Figure 1, we observed that adoptive transfer of FK506-resistant EBV-CTLs generated with this methodology also led to complete tumor rejection of B-cell lymphoma in NSG mice treated with CNA12-CTLs in the presence of FK506, comparable with that seen with EBV-CTLs generated using conventional repetitive LCL stimulation.
Consistent with the control of tumor progression, injection of CNA12-CTLs in the presence of FK506 resulted in enhanced survival of these mice by day 40 post-CTLs injection (P < .0001) compared with mice receiving eGFP-CTLs with ongoing immunosuppression (Figure 5B). Similar results were observed in mice treated with CNA12-CTLs generated using IFN-γ selection, which were able to survive beyond 60 days after CTLs infusion (data not shown).
Figure 5.
Adoptive transfer of CN-resistant EBV-CTLs induces reduction in tumor growth and improves survival of the mice despite the presence of ongoing immunosuppression. (A) Tumor volume (mm3) was decreased in tumor-bearing NSG mice adoptively transferred with CNA12-CTLs, CNA12-CTL +FK506, and eGFP-CTLs compared with the mice receiving eGFP-CTL + FK506 (n = 8). (B) The Kaplan-Meier tumor-related survival curve showed increased long-term survival of mice adoptively transferred with CNA12-CTLs in the presence of FK506 (P < .0001) compared with mice receiving eGFP-CTLs in the presence of immunosuppression (n = 7).
Taken together, our results show that transfer of CNA12-CTLs into xenografted hosts was sufficient to induce complete regression of tumors despite ongoing immunosuppression, resulting in prolonged lymphoma-free survival. In contrast, in mice treated with control eGFP-CTLs, the immunosuppressive environment compromised the function of the adoptive transferred CTLs, leading to tumor progression.
Calcineurin-resistant EBV-CTLs show improved persistence and expansion
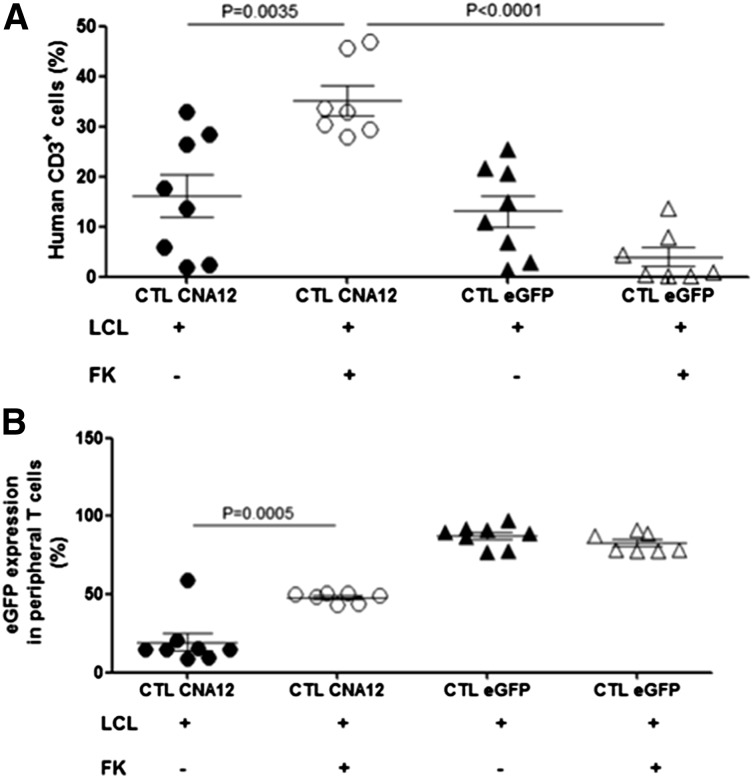
One of the major barriers to the development of effective adoptive T-cell therapy has been the inability of the transferred T cells to achieve prolonged persistence after infusion, which is required to mediate therapeutic efficacy and provide long-term tumor surveillance. In SOT patients, the immunosuppressed environment required to prevent graft rejection further limits the expansion and persistence of adoptive transferred T cells. We therefore examined whether the adoptively transferred CNA12-CTLs persisted in vivo despite the presence of FK506. To assess in vivo persistence, tail vein blood was collected from the mice the day of sacrifice (40 days after CTL infusion) and the percentage of human CD3+ cells was quantified. We observed that the adoptively transferred EBV-CTLs showed peripheral expansion and persistence. The level of human T-cell engraftment at 40 days after infusion was reproducibly higher in mice treated with FK506, which received CNA12-CTLs (35.2 ± 2.9%, mean ± SEM, n = 7) than mice receiving eGFP-CTLs and FK506 (3.9 ± 1.9%, n = 7) (P < .0001), as is shown in Figure 6A, correlating with the improved antitumor responses seen with CNA12-transduced CTLs. Further, mice receiving CNA12-CTLs showed improved expansion in the presence of FK506 (35.2 ± 2.9%, n = 7) than in its absence (16.2 ± 4.3%, n = 8) (P < .01), whereas proliferation of GFP-transduced CTLs was inhibited by FK506, resulting in a reduced percentage of circulating human T cells. These results demonstrate that CNA12-transduced CTLs have a growth advantage in the presence of FK506 in vivo compared with eGFP-CTLs, resulting in their preferential expansion in circulation.
Figure 6.
EBV-CTLs genetically engineered to express CNA12 showed improved expansion and persistence in vivo in the presence of FK506. (A) Persistence in vivo of genetically engineered human EBV-CTLs transduced with CNA12 or eGFP. Peripheral tail vein blood was collected from mice the day of sacrifice (40 days after CTL infusion) and quantified by flow cytometry for the persistence of human CD3+. Mean cell count ± SEM is shown (n = 8). (B) eGFP expression on human CD3+ T cells from the peripheral blood of treated mice measured by flow cytometry. Mean eGFP+ expression frequency ± SEM per group is shown (n = 8).
Next we looked at the percentage of circulating human T cells expressing eGFP to determine the fraction of persisting adoptive transferred EBV-CTLs expressing the transgene (Figure 6B). We observed that the transgene expression in the circulation 40 days after infusion was significantly higher (48.0 ± 2%, n = 7) in CNA12-CTLs from mice treated with FK506 compared with mice infused with CNA12-CTLs without FK506 (24.2 ± 11.6%, n = 8) (P < .001). These data indicate a selective enrichment in vivo of CNA12-transduced CTLs in the presence of FK506. In contrast, in mice receiving eGFP-CTLs, there was no selective advantage in the presence of FK506, so that the percentage of human T cells expressing eGFP was stable with (89.4 ± 4.1%, n = 8) or without (81.8 ± 3.5, n = 7) FK506, although the total number of circulating human T cells was reduced by FK506. The higher percentage of human T cells expressing GFP in mice receiving eGFP-CTLs compared with CNA12-CTLs reflects differential transduction efficiency.
Similar results were observed in mice treated with CNA12-CTLs generated using IFN-γ selection, which were able to persist in vivo beyond 60 days after adoptive transfer in the presence of FK506 (supplemental Figure 2A-B).
These data suggest that EBV-CTLs genetically engineered to express CNA12 showed improved expansion and growth advantage and are able to persist in vivo in the presence of FK506 beyond 2 months post–adoptive transfer.
Calcineurin-resistant EBV-CTLs infiltrate the tumor in the presence of FK506
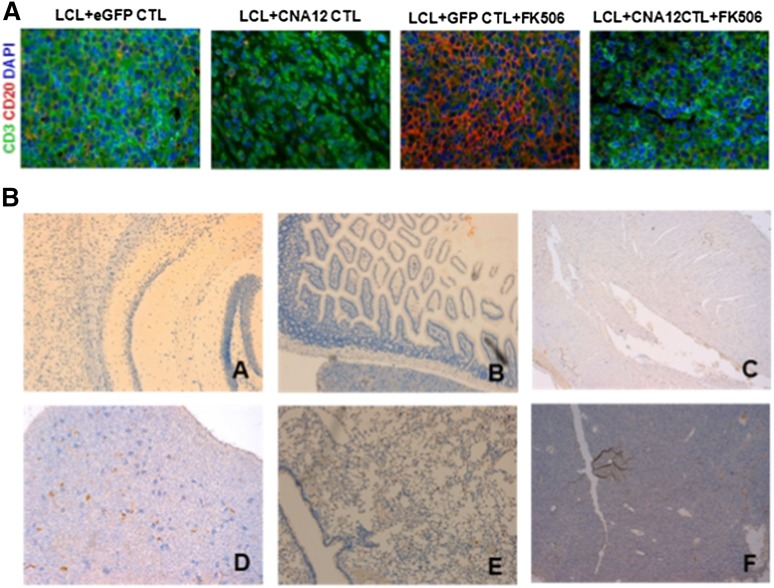
We next investigated the ability of transduced EBV-CTLs to home to LCL tumors in vivo using immunofluorescence analysis 40 days after CTL administration. In the absence of FK506, both CNA12- and eGFP-transduced CTLs homed well to the tumor, mediating regression (Figure 7A). Consistent with the increased persistence of CNA12-CTLs in the peripheral blood in the presence of FK506, we observed robust accumulation and infiltration of tumor-infiltrating T lymphocytes (human CD3+) in regressed B-cell lymphoma, whereas very few human CD3+ T cells were detected in tumors resected at the same time from mice that received eGFP-CTLs.
Figure 7.
EBV-CNA12-CTLs infiltrate B-cell lymphoma despite the presence of FK506. (A) Tumors were collected 40 days post-CTL infusion and stained by double immunofluorescence for human T-cell infiltration (CD3 expression, green) into B-cell lymphoma (CD20, red). Tumors treated with CNA12-CTLs show a substantial T-cell infiltration despite the presence of FK506. Representative sections are shown at 100× magnification. (B) CN-resistant EBV-CTLs do not infiltrate and do not induce toxicity in organs. Organs were collected 40 days post-CTL infusion and stained for human T-cell infiltration (CD3 expression). Organs from mice treated with CNA12-CTLs do not show any T-cell infiltration in the brain (A), intestine (B), heart (C), kidney (D), lungs (E), or liver (F). Representative sections are shown at ×20 magnification.
Calcineurin-resistant EBV-CTLs do not infiltrate and do not induce toxicity in organs
Histologic examination showed no human T-cell infiltration or histologic evidence of toxicity in brain, lungs, heart, kidneys, intestine, and liver of the mice sacrificed 40 days after injection of CNA12-CTLs (Figure 7B), demonstrating that FK506-resistant CTLs do not mediate off-target organ toxicity in this model.
Discussion
Adoptive immunotherapy with T cells directed against tumor-specific or tumor-associated antigens represents a novel strategy for the treatment of human malignancies with a great clinical potential.21-25 Adoptive transfer of ex vivo–derived EBV-specific CTLs to reconstitute EBV-specific T-cell immunity3,26 is a logical approach for patients in whom PTLD develops. This disease represents an ideal target for adoptive T-cell therapy because of the high level expression of immunogenic antigens from EBV presented by excellent antigen-presenting cells. The application of this approach has been shown to be extremely effective for PTLD arising after SCT, where immunosuppressive therapy can be reduced or suspended completely before EBV-CTLs administration.11,27,28 Conversely, in the SOT setting, although possible,10,13,14 the approach has been limited by the need for lifelong immunosuppressive therapy to avoid rejection of the transplanted organ, which inhibits the proliferation, expansion, and persistence of transferred EBV-CTLs.15,16 For example, in the study by Savoldo et al,10 where autologous EBV-CTLs were infused in 12 SOT patients with EBV viremia, only one patient cleared viremia, the in vivo expansion of infused EBV-CTLs was poor (1.5- to 4.8-fold), and the frequency of circulating EBV-CTLs returned to preinfusion levels in all patients within 2 months of infusion.
To overcome this problem, we have previously generated EBV-specific CTLs that are resistant to the CN inhibitors tacrolimus and cyclosporin and have demonstrated their ability to function in vitro despite the presence of immunosuppression.17
To translate novel T-cell therapy approaches from bench to bedside, in vivo modeling is critical. Here we have studied the antitumor effects of human CNA12-transduced EBV-CTLs against established EBV lymphoma in an immunodeficient NSG xenotransplant mouse model in the presence of immunosuppression. Previous studies in vivo have demonstrated that inoculation of EBV-transformed B-cell lines rapidly induces human EBV-induced B lymphoproliferation, with characteristics comparable with those observed in immunocompromised patients developing PTLD.29,30 In this study, we have demonstrated that adoptive transfer of human EBV-specific CTLs resistant to tacrolimus in NSG mice results in eradication of the lymphoma in vivo, even in the presence of immunosuppression with therapeutic levels of FK506. Our data show that FK506-resistant EBV-CTLs are able to home to the tumor site and persist in the circulation beyond 60 days after transfer, resulting in markedly improved survival of mice despite ongoing immunosuppressive treatment. We demonstrate that EBV-CTLs transduced with CNA12 are selectively enriched in vivo by immunosuppression with FK506, resulting in a growth advantage over untransduced EBV-CTLs. The potent antitumor effects of CNA12-transduced EBV-CTLs were durable even in the face of immunosuppression, and no off-target toxicity was observed. This approach may thus overcome the major barrier to application of chemotherapy with EBV-CTLs in the SOT setting.
An alternative strategy to rendering CTLs resistant to FK506 using small-interference RNA (siRNA) knockdown of native FKBP12 has been proposed by De Angelis et al.31 However, this methodology results in only partial resistance to FK506 in vitro. Similarly in vivo, mice receiving EBV-CTLs in which FKBP12 had been knocked down by siRNA, although showing delayed tumor progression, still had residual tumor detectable, whereas in our study CNA12-transduced EBV-CTLs resulted in complete tumor clearance even in the presence of FK506. This may reflect the fact that siRNA knockdown is relatively inefficient, and very high transgene expression is needed. Moreover, there is little experience of siRNA knockdown clinically, downregulating endogenous FKBP12 may have unpredictable sequelae on T-cell function and introduction of siRNA can potentially result in off-target suppression of other genes. Thus we believe our approach is both more effective in fully restoring CTL function in the presence of FK506 and more clinically feasible.
Reduction in immunosuppression is commonly used in SOT patients in whom PTLD develops, but this frequently results in graft rejection. Indeed, in the study reported by Webber et al, rejection was as common a cause of mortality in PTLD patients as was disease itself.6
Obviating the need for reduction in immunosuppression thus represents a major advance. After SOT, post-transplant immunosuppression with a variety of drugs including FK506, low-dose steroids, and MMF is used. Clearly, our strategy only confers resistance to CN inhibitors. However, this is the most critical component of post-SOT immunosuppression: MMF and steroids can generally be withdrawn without toxicity in the context of PTLD, but when FK506 is reduced or withdrawn, rejection is common. Therefore, our strategy overcomes the key issue of graft rejection by enabling clinicians to treat PTLD with EBV-CTLs while maintaining immunosuppression with FK506 at therapeutic levels.
One of the major barriers to broaden the application of this approach is also the complexity of generation of EBV-CTLs. We have recently simplified the generation of resistant CTLs by retroviral transduction of EBV-specific T cells selected on the basis of the secretion of IFN-γ in response to EBV immunodominant antigens.20 Further, we have shown that it is possible to generate FK506-resistant CTLs from SOT patients receiving immunosuppression therapy who have PTLD.20 Currently we are in the process of validating the production of FK506-resistant EBV-CTLs using this methodology under GMP conditions. We envision that resistant CTL could be used as adjuvant therapy after rituximab to improve response rates and prevent rejection without the need for chemotherapy, which is poorly tolerated in SOT patients. Importantly, such an approach would obviate the need for reduction in immunosuppression, reducing the risk of graft rejection, which is a major cause of mortality in PTLD patients. Given that the cost of generating transduced EBV-CTLs is approximately $15 000, such an approach could potentially have major health economic benefits by avoiding the need for prolonged admission for chemotherapy and reducing the risk of graft rejection. In terms of toxicity, we have shown that CNA12-transduced EBV-CTLs have negligible alloreactivity against HLA-mismatched targets, suggesting that they should have no detrimental effect on the organ graft. This is likely to reflect depletion of alloreactive T cells during the process of CTL culture, and our data suggest that selection of retrovirally transduced CTLs is not necessary to abrogate alloreactivity. Indeed, no graft toxicity was observed in previous clinical studies with either autologous or third-party CTLs.12,32 Because CTLs are autologously derived, they should not cause off-target toxicity to the host and, consistent with this, in our experiments no organ toxicity was observed in mice receiving FK506-resistant EBV-CTLs. Importantly, because transduced EBV-CTLs are terminally differentiated, retroviral gene transfer is extremely unlikely to result in leukemogenesis. As a safety strategy, in our model we have genetically modified EBV-CTLs with a CN mutant conferring resistance to tacrolimus only, allowing suppression of the infused T cells with cyclosporin in case of toxicity.
Overall, our findings provide strong support for the clinical application of CN inhibitor–resistant EBV-CTLs as adjuvant therapy for SOT patients in whom PTLD develops. Based on this work, we are now proceeding to a “double marking” clinical study in which such patients will be treated with both autologous CNA12-transduced and control EBV-CTLs generated using our simplified methodology20 without reduction in FK506 as adjuvant therapy after rituximab. The in vivo persistence/expansion of EBV-CTLs transduced with the 2 vectors will be compared. We hypothesize that CNA12-transduced CTLs will show preferential expansion and prolonged persistence compared with control CTLs because of ongoing FK506 therapy. The incidence of graft rejection and PTLD relapse will be compared with historical data on patients receiving rituximab alone. This study will determine whether this strategy improves the in vivo persistence and efficacy of adoptively transferred EBV-CTLs. Such an approach could potentially be of major clinical benefit to organ transplant patients in whom PTLD develops by avoiding the need for chemotherapy and reduction in immunosuppression. It remains possible, however, that other factors such as the absence of a lymphopenic environment resulting in reduced homeostatic expansion may contribute to the limited persistence and expansion of infused EBV-CTLs observed in previous studies in SOT patients, in which case, the addition of lymphodepleting chemotherapy before adoptive T-cell transfer may be required. More broadly, this represents a generic approach enabling T cells directed against other pathogens (eg, cytomegalovirus, Burkitt, and adenovirus) and tumor antigens to function in patients receiving immunosuppression therapy.
Acknowledgments
The authors thank Nick Davies and the staff at University College London Biological Services for assistance with animal handling.
This research was supported by a grant from the National Institute for Health Research and P.J.A. was is a recipient of an National Institute for Health Research Research Professorship.
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: I.R., P.J.A., and M.P. designed the work; I.R., M.P.B., and J.B. performed experiments and analyzed data; I.R., M.P., and P.J.A. wrote the manuscript; and all authors read and commented on the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Persis Amrolia, Bone Marrow Transplantation Department, Great Ormond Street Hospital, Great Ormond St, London WC1N 3JH, United Kingdom; e-mail: persis.amrolia@gosh.nhs.uk.
References
- 1.Murray RJ, Kurilla MG, Brooks JM, et al. Identification of target antigens for the human cytotoxic T cell response to Epstein-Barr virus (EBV): implications for the immune control of EBV-positive malignancies. J Exp Med. 1992;176(1):157–168. doi: 10.1084/jem.176.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bollard CM, Rooney CM, Heslop HE. T-cell therapy in the treatment of post-transplant lymphoproliferative disease. Nat Rev Clin Oncol. 2012;9(9):510–519. doi: 10.1038/nrclinonc.2012.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gottschalk S, Rooney CM, Heslop HE. Post-transplant lymphoproliferative disorders. Annu Rev Med. 2005;56:29–44. doi: 10.1146/annurev.med.56.082103.104727. [DOI] [PubMed] [Google Scholar]
- 4.Hislop AD, Taylor GS, Sauce D, Rickinson AB. Cellular responses to viral infection in humans: lessons from Epstein-Barr virus. Annu Rev Immunol. 2007;25:587–617. doi: 10.1146/annurev.immunol.25.022106.141553. [DOI] [PubMed] [Google Scholar]
- 5.Choquet S, Oertel S, LeBlond V, et al. Rituximab in the management of post-transplantation lymphoproliferative disorder after solid organ transplantation: proceed with caution. Ann Hematol. 2007;86(8):599–607. doi: 10.1007/s00277-007-0298-2. [DOI] [PubMed] [Google Scholar]
- 6.Webber SA, Naftel DC, Fricker FJ, et al. Pediatric Heart Transplant Study. Lymphoproliferative disorders after paediatric heart transplantation: a multi-institutional study. Lancet. 2006;367(9506):233–239. doi: 10.1016/S0140-6736(06)67933-6. [DOI] [PubMed] [Google Scholar]
- 7.Choquet S, Trappe R, Leblond V, Jäger U, Davi F, Oertel S. CHOP-21 for the treatment of post-transplant lymphoproliferative disorders (PTLD) following solid organ transplantation. Haematologica. 2007;92(2):273–274. doi: 10.3324/haematol.10595. [DOI] [PubMed] [Google Scholar]
- 8.Trappe R, Oertel S, Leblond V, et al. German PTLD Study Group; European PTLD Network. Sequential treatment with rituximab followed by CHOP chemotherapy in adult B-cell post-transplant lymphoproliferative disorder (PTLD): the prospective international multicentre phase 2 PTLD-1 trial. Lancet Oncol. 2012;13(2):196–206. doi: 10.1016/S1470-2045(11)70300-X. [DOI] [PubMed] [Google Scholar]
- 9.Rooney CM, Smith CA, Ng CY, et al. Infusion of cytotoxic T cells for the prevention and treatment of Epstein-Barr virus-induced lymphoma in allogeneic transplant recipients. Blood. 1998;92(5):1549–1555. [PubMed] [Google Scholar]
- 10.Savoldo B, Goss JA, Hammer MM, et al. Treatment of solid organ transplant recipients with autologous Epstein Barr virus-specific cytotoxic T lymphocytes (CTLs). Blood. 2006;108(9):2942–2949. doi: 10.1182/blood-2006-05-021782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heslop HE, Slobod KS, Pule MA, et al. Long-term outcome of EBV-specific T-cell infusions to prevent or treat EBV-related lymphoproliferative disease in transplant recipients. Blood. 2010;115(5):925–935. doi: 10.1182/blood-2009-08-239186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haque T, Wilkie GM, Jones MM, et al. Allogeneic cytotoxic T-cell therapy for EBV-positive posttransplantation lymphoproliferative disease: results of a phase 2 multicenter clinical trial. Blood. 2007;110(4):1123–1131. doi: 10.1182/blood-2006-12-063008. [DOI] [PubMed] [Google Scholar]
- 13.Khanna R, Bell S, Sherritt M, et al. Activation and adoptive transfer of Epstein-Barr virus-specific cytotoxic T cells in solid organ transplant patients with posttransplant lymphoproliferative disease. Proc Natl Acad Sci USA. 1999;96(18):10391–10396. doi: 10.1073/pnas.96.18.10391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Comoli P, Maccario R, Locatelli F, et al. Treatment of EBV-related post-renal transplant lymphoproliferative disease with a tailored regimen including EBV-specific T cells. Am J Transplant. 2005;5(6):1415–1422. doi: 10.1111/j.1600-6143.2005.00854.x. [DOI] [PubMed] [Google Scholar]
- 15.Savoldo B, Goss J, Liu Z, et al. Generation of autologous Epstein-Barr virus-specific cytotoxic T cells for adoptive immunotherapy in solid organ transplant recipients. Transplantation. 2001;72(6):1078–1086. doi: 10.1097/00007890-200109270-00017. [DOI] [PubMed] [Google Scholar]
- 16.Zhan X, Brown B, Slobod KS, Hurwitz JL. Inhibition of ex vivo-expanded cytotoxic T-lymphocyte function by high-dose cyclosporine. Transplantation. 2003;76(4):739–740. doi: 10.1097/01.TP.0000078623.64968.E5. [DOI] [PubMed] [Google Scholar]
- 17.Brewin J, Mancao C, Straathof K, et al. Generation of EBV-specific cytotoxic T cells that are resistant to calcineurin inhibitors for the treatment of posttransplantation lymphoproliferative disease. Blood. 2009;114(23):4792–4803. doi: 10.1182/blood-2009-07-228387. [DOI] [PubMed] [Google Scholar]
- 18.Savoldo B, Rooney CM, Di Stasi A, et al. Epstein Barr virus specific cytotoxic T lymphocytes expressing the anti-CD30zeta artificial chimeric T-cell receptor for immunotherapy of Hodgkin disease. Blood. 2007;110(7):2620–2630. doi: 10.1182/blood-2006-11-059139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Medyouf H, Alcalde H, Berthier C, et al. Targeting calcineurin activation as a therapeutic strategy for T-cell acute lymphoblastic leukemia. Nat Med. 2007;13(6):736–741. doi: 10.1038/nm1588. [DOI] [PubMed] [Google Scholar]
- 20.Ricciardelli I, Brewin J, Lugthart G, Albon SJ, Pule M, Amrolia PJ. Rapid generation of EBV-specific cytotoxic T lymphocytes resistant to calcineurin inhibitors for adoptive immunotherapy. Am J Transplant. 2013;13(12):3244–3252. doi: 10.1111/ajt.12475. [DOI] [PubMed] [Google Scholar]
- 21.Dudley ME, Wunderlich JR, Yang JC, et al. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J Clin Oncol. 2005;23(10):2346–2357. doi: 10.1200/JCO.2005.00.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yee C, Thompson JA, Byrd D, et al. Adoptive T cell therapy using antigen-specific CD8+ T cell clones for the treatment of patients with metastatic melanoma: in vivo persistence, migration, and antitumor effect of transferred T cells. Proc Natl Acad Sci USA. 2002;99(25):16168–16173. doi: 10.1073/pnas.242600099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dudley ME, Gross CA, Somerville RP, et al. Randomized selection design trial evaluating CD8+-enriched versus unselected tumor-infiltrating lymphocytes for adoptive cell therapy for patients with melanoma. J Clin Oncol. 2013;31(17):2152–2159. doi: 10.1200/JCO.2012.46.6441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosenberg SA, Restifo NP, Yang JC, Morgan RA, Dudley ME. Adoptive cell transfer: a clinical path to effective cancer immunotherapy. Nat Rev Cancer. 2008;8(4):299–308. doi: 10.1038/nrc2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morgan RA, Dudley ME, Wunderlich JR, et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314(5796):126–129. doi: 10.1126/science.1129003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khanna R, Moss D, Gandhi M. Technology insight: Applications of emerging immunotherapeutic strategies for Epstein-Barr virus-associated malignancies. Nat Clin Pract Oncol. 2005;2(3):138–149. doi: 10.1038/ncponc0107. [DOI] [PubMed] [Google Scholar]
- 27.Heslop HE, Ng CY, Li C, et al. Long-term restoration of immunity against Epstein-Barr virus infection by adoptive transfer of gene-modified virus-specific T lymphocytes. Nat Med. 1996;2(5):551–555. doi: 10.1038/nm0596-551. [DOI] [PubMed] [Google Scholar]
- 28.Gustafsson A, Levitsky V, Zou JZ, et al. Epstein-Barr virus (EBV) load in bone marrow transplant recipients at risk to develop posttransplant lymphoproliferative disease: prophylactic infusion of EBV-specific cytotoxic T cells. Blood. 2000;95(3):807–814. [PubMed] [Google Scholar]
- 29.Lacerda JF, Ladanyi M, Louie DC, Fernandez JM, Papadopoulos EB, O’Reilly RJ. Human Epstein-Barr virus (EBV)-specific cytotoxic T lymphocytes home preferentially to and induce selective regressions of autologous EBV-induced B cell lymphoproliferations in xenografted C.B-17 scid/scid mice. J Exp Med. 1996;183(3):1215–1228. doi: 10.1084/jem.183.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johannessen I, Bieleski L, Urquhart G, et al. Epstein-Barr virus, B cell lymphoproliferative disease, and SCID mice: modeling T cell immunotherapy in vivo. J Med Virol. 2011;83(9):1585–1596. doi: 10.1002/jmv.22164. [DOI] [PubMed] [Google Scholar]
- 31.De Angelis B, Dotti G, Quintarelli C, et al. Generation of Epstein-Barr virus-specific cytotoxic T lymphocytes resistant to the immunosuppressive drug tacrolimus (FK506). Blood. 2009;114(23):4784–4791. doi: 10.1182/blood-2009-07-230482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haque T, Wilkie GM, Taylor C, et al. Treatment of Epstein-Barr-virus-positive post-transplantation lymphoproliferative disease with partly HLA-matched allogeneic cytotoxic T cells. Lancet. 2002;360(9331):436–442. doi: 10.1016/S0140-6736(02)09672-1. [DOI] [PubMed] [Google Scholar]