Abstract
Familial autosomal dominant calcium pyrophosphate dihydrate (CPPD) chondrocalcinosis has previously been mapped to chromosome 5p15. We have identified a mutation in the ANKH gene that segregates with the disease in a family with this condition. ANKH encodes a putative transmembrane inorganic pyrophosphate (PPi) transport channel. We postulate that loss of function of ANKH causes elevated extracellular PPi levels, predisposing to CPPD crystal deposition.
Chondrocalcinosis is a common arthritic condition, affecting 3% of people aged 65–69 years (Felson et al. 1989), in which calcium-containing crystals form in articular cartilage. The crystals most commonly involved consist of either calcium pyrophosphate dihydrate (CPPD) or calcium hydroxyapatite. Although the heritability of the condition in the general community is unknown, many families with autosomal segregation of CPPD chondrocalcinosis have been described, implying a significant genetic effect. The condition is frequently asymptomatic but may cause acute flares of arthritis, termed “pseudogout,” or chronic degenerative joint disease. Recombination mapping in British, French, and Argentine families with autosomal dominant CPPD chondrocalcinosis has identified a region (CCAL2 [MIM 118600]) on chromosome 5p15 that is linked with the disease (Doherty et al. 1991; Andrew et al. 1999).
Loss of function of an inorganic pyrophosphate (PPi) transporter, the ANKH gene, has been demonstrated to cause excess calcium hydroxyapatite formation in the ank/ank mouse (Ho et al. 2000), resulting in calcium hydroxyapatite chondrocalcinosis and joint ankylosis. In humans, dysfunction of the ANKH gene has been demonstrated to cause a rare skeletal dysplasia, autosomal dominant craniometaphyseal dysplasia (CMD [MIM 123000]) (Nurnberg et al. 2001; Reichenberger et al. 2001). In this condition, increased bone density and skeletal dysplasia arise because of abnormal mineralization of both membranous and enchondral bone. It is postulated that lower extracellular levels of PPi due to failure of transport of PPi out of chondrocytes and/or osteoblasts permit excess calcium hydroxyapatite crystal formation in both CMD and the ank/ank mouse. The human homologue of the ANKH gene lies near the region identified by recombination mapping in the families with autosomal dominant CPPD chondrocalcinosis described above. Therefore, we have studied this gene to determine whether it is responsible for the CPPD deposition disease in the Argentine family of northern Italian descent with linkage to this region as reported elsewhere (Andrew et al. 1999). Mutation screening of the ANKH gene was performed by direct sequencing of 16 PCR fragments covering the 12 exons and their exon-intron boundaries and 500 bp of the promoter region, using combinations of primer pairs listed in table 1. Sequencing was performed using an ABI 377 automatic sequencer (Applied Biosystems). In all, six SNPs were identified over the 12 exons and their intronic boundaries, and two insertions in the promoter region up to 500 bp.
Table 1.
Primers for PCR Amplification of ANKH Gene Coding and Promoter Region Sequence
|
Primer(5′→3′) |
|||
| SegmentAmplified | FragmentLength(bp) | Forward | Reverse |
| Promoter 1 | 348 | caagcccccagaccgtccccg | ttctgctgacagcggctccat |
| Promoter 2 | 370 | cgcccgcccctgatttcctc | tctgctgccgcgaggggact |
| Promoter 3 | 267 | gagcagccgcgctcggagaa | gaagtcgatggctatgttgg |
| Exon 1 | 298 | agtcccctcgcggcagcaga | gctggaaaagtcacccttga |
| Exon 2 | 455 | accctataagctacttagtg | agaaacatttgataattagtg |
| Exon 3 | 407 | tgacccactctgggtagagg | cctgccattaagctgtacacac |
| Exon 4 | 354 | tgcagtccttcactccactg | cagttacacacgccagaagg |
| Exon 5 | 399 | cccctgtgcttctgtcagtc | ctgtctttccctgcagacatc |
| Exon 6 | 392 | ggcttgtgattaacatacgaagg | caccgaacgagatctttatgg |
| Exon 7 | 305 | cgtgcttcgtcacctactgt | ccccaacgtcacattaacct |
| Exon 8 | 316 | gcccgagagacactcaacat | tgcccctttacaaaaaccaa |
| Exon 9 | 398 | agcaagcaggtggcagatctg | agtgaaattttacatttgtg |
| Exon 10 | 300 | accctggcaggaagatgag | cccaaactccctgacaacat |
| Exon 11 | 320 | gaagccagcagatggagaac | accatccaacctggtcagtg |
| Exon 12-1 | 351 | caaccaaactgcaaggaaca | gccgaagtgtcatcctgact |
| Exon 12-2 | 306 | agaatgaataaggcacgggacg | acaacgtcaaccgtgaggcag |
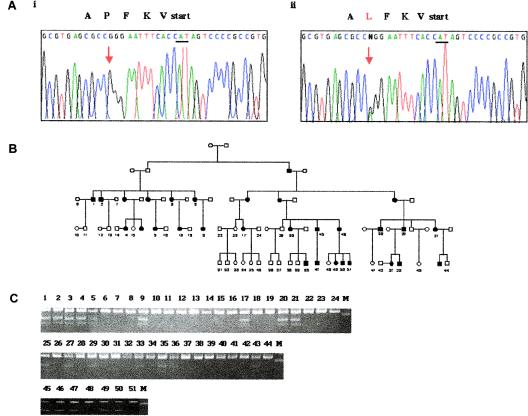
One variant, a C→T transition at 14 bp from the start codon, segregated with disease in the family (fig. 1). This sequence change is predicted to cause a proline-to-leucine substitution at amino acid position 5. This specific mutation was confirmed by PCR-RFLP assay using a BstNI (New England Biolab) restriction site if the mutation was found (fig. 1c). Individuals 24, 38, and 48 carry the disease haplotype and are unaffected clinically or radiographically, but they were too young to determine their affection status with certainty (ages 17–28 years; mean age at disease onset 29 years). Individuals 3 and 19 have a phenotype indistinguishable from that of the rest of the family but do not carry the disease haplotype. The family of the mother of individual 3 is also affected with chondrocalcinosis, but, as reported elsewhere, this is not linked with chromosome 5, which leads to the conclusion that individuals 3 and 19 represent phenocopies (Andrew et al. 1999). Individual 28 is also affected with chondrocalcinosis and carries a partial disease haplotype but not the P5L mutation. Extensive metabolic screening for other causes of chondrocalcinosis has been performed, and no abnormalities have been identified. Genotyping of multiple polymorphisms between D5S2114 and D5S416 revealed homozygosity at all sites, suggesting the presence of uniparental disomy or a microdeletion. Further studies with this individual are ongoing. We did not observe the P5L variant in 50 Northern Italian, 52 South American Hispanic, or 100 British white healthy controls, nor in 100 nonfamilial British white patients with CPPD chondrocalcinosis, strongly suggesting that it is a mutation unique to this family. Parametric linkage analysis using the MLINK option of Vitesse 2.0 was performed (O'Connell and Weeks 1995), assuming a disease allele frequency of 0.001, a phenocopy rate of 0.004, and penetrance of 0.9, and with an autosomal dominant disease model, as used in a previous analysis of this pedigree (Andrew et al. 1999). Strong linkage of the disease-causing variant was noted, with a maximum LOD score of 9.6 at recombination fraction (θ) 0.0.
Figure 1.
ANKH mutation changes a highly conserved hydrophobic protein. a, DNA sequence chromatograms (antisense sequence) showing heterozygous base substitution (red arrow) in affected (left chromatogram) and unaffected (right chromatogram) individuals. Protein translation of the first six amino acids of ANKH coding sequence is shown above sequence chromatograms; black arrows indicate direction of translation. b, Pedigree of CPPDD family described elsewhere (Andrew et al. 1999). c, Confirmation of sequence change in genomic DNA by restriction enzyme digestion (BstN1). Wild-type genotypes result in fragments of 24, 45, and 142 bp (the 24-bp fragment is too small to resolve). Carriers of the mutation display fragments of 24, 45, 69, and 142 bp.
Mutations in the ANKH gene have been reported in abstract form in other families with familial autosomal dominant CPPD chondrocalcinosis. These reports support our finding that this is a true disease-causing mutation (Johnson et al. 2001; Pendleton et al. 2001). Other families with CPPD chondrocalcinosis that is not linked to chromosome 5p have also been described, indicating the existence of locus heterogeneity (Baldwin et al. 1995).
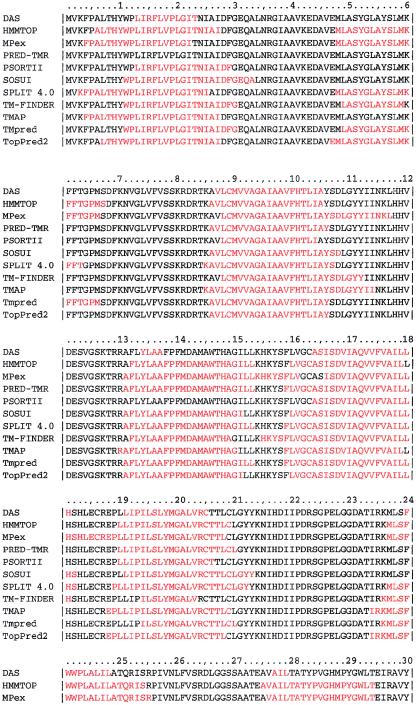
The entire sequence of the ANKH gene has been shown to be highly conserved, with 82% sequence identity among the human, mouse, Xenopus, and zebrafish sequences (Nurnberg et al. 2001). Proline at amino acid position 5 is conserved in all these species. The precise structure of the ANKH gene is unknown. Previous computer modeling based on the hydrophobicity pattern of the protein has suggested two alternate models with either 10 (Ho et al. 2000) or 12 (Nurnberg et al. 2001) transmembrane helices. The latter model suggests that the protein forms a transmembrane channel with a six-membered helical assembly of pairs of transmembrane domains, with alternate domains that either contribute to the internal channel or are exterior and interact with the membrane bilayer (Nurnberg et al. 2001). It has been proposed that the mutations in CMD and the ank/ank mouse cause loss of function of the channel, resulting in increased “leakiness” to PPi, elevating intracellular PPi, and reducing extracellular levels. We used 11 different structural prediction programs to investigate the ANKH protein (fig. 2). Although there are significant areas of agreement between the various programs, the level of similarity of predictions is not high, with the number of predicted transmembrane domains varying from 7 to 12. The TMpred program previously used to derive the proposed 12-transmembrane domain structure (Nurnberg et al. 2001) actually gives two predictions, one of which has only 10 transmembrane domains and has an inside/outside orientation opposite that of the 12-transmembrane domain model. Clearly, there is much yet to be determined about the structure of this protein.
Figure 2.
Predicted transmembrane domains from different structural prediction programs. Transmembrane domains are listed in red. The programs used were DAS, HMMTOP, MPEx, PRED-TMR, PSORTII, SOSUI, SPLIT 4.0, TM-Finder, TMAP, TMpred, and TopPred2.
CPPD chondrocalcinosis is associated with conditions favoring increased serum calcium or PPi (reviewed in Timms et al. [2002]). Patients with CPPD crystals and chondrocalcinosis have been shown to have high synovial fluid PPi levels (Russell et al. 1970; Doherty et al. 1996). Whether variation in ANKH is a cause of “sporadic” chondrocalcinosis remains to be determined. We postulate that the ANKH mutation present in this family causes a gain of ANKH function, thus increasing extracellular PPi, which combines with calcium to form CPPD crystals and causes CPPD chondrocalcinosis. This raises the possibility of treatment of CPPD chondrocalcinosis by interventions aimed at affecting rate of PPi transport by ANKH.
Acknowledgments
We thank all the family members who have assisted with this study. M.A.B., Y.Z., and A.T. are supported by the Arthritis Research Campaign, United Kingdom. C.J.W. and G.B. are supported by a National Institutes of Health/National Institute of Arthritis, Musculoskeletal, and Skin Diseases grant (to C.J.W., Principal Investigator).
Electronic-Database Information
Accession numbers and URLs for data presented herein are as follows:
- DAS Transmembrane Prediction server, http://www.sbc.su.se/~miklos/DAS/
- HMMTOP, http://www.enzim.hu/hmmtop/
- MPEx, http://blanco.biomol.uci.edu/mpex/
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for CCAL2 [MIM 118600] and CMD [MIM 123000])
- PRED-TMR, http://o2.db.uoa.gr/PRED-TMR/input.html
- PSORTII, http://psort.nibb.ac.jp/
- SOSUI, http://sosui.proteome.bio.tuat.ac.jp/cgi-bin/sosui.cgi?/sosui_submit.html
- SPLIT 4.0, http://pref.etfos.hr/split/
- TMAP, http://www.mbb.ki.se/tmap/index.html
- TM-Finder, http://www.bioinformatics-canada.org/TM/home.html
- TMpred, http://www.ch.embnet.org/software/TMPRED_form.html
- TopPred2, http://bioweb.pasteur.fr/seqanal/interfaces/toppred.html
References
- Andrew LJ, Brancolini V, de la Pena LS, Devoto M, Caeiro F, Marchegiani R, Reginato A, Gaucher A, Netter P, Gillet P, Loeuille D, Prockop DJ, Carr A, Wordsworth BF, Lathrop M, Butcher S, Considine E, Everts K, Nicod A, Walsh S, Williams CJ (1999) Refinement of the chromosome 5p locus for familial calcium pyrophosphate dihydrate deposition disease. Am J Hum Genet 64:136–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin CT, Farrer LA, Adair R, Dharmavaram R, Jimenez S, Anderson L (1995) Linkage of early-onset osteoarthritis and chondrocalcinosis to human chromosome 8q. Am J Hum Genet 56:692–697 [PMC free article] [PubMed] [Google Scholar]
- Doherty M, Belcher C, Regan M, Jones A, Ledingham J (1996) Association between synovial fluid levels of inorganic pyrophosphate and short term radiographic outcome of knee osteoarthritis. Ann Rheum Dis 55:432–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty M, Hamilton E, Henderson J, Misra H, Dixey J (1991) Familial chondrocalcinosis due to calcium pyrophosphate dihydrate crystal deposition in English families. Br J Rheumatol 30:10–15 [DOI] [PubMed] [Google Scholar]
- Felson DT, Anderson JJ, Naimark A, Kannel W, Meenan RF (1989) The prevalence of chondrocalcinosis in the elderly and its association with knee osteoarthritis: the Framingham Study. J Rheumatol 16:1241–1245 [PubMed] [Google Scholar]
- Ho AM, Johnson MD, Kingsley DM (2000) Role of the mouse ank gene in control of tissue calcification and arthritis. Science 289:265–270 [DOI] [PubMed] [Google Scholar]
- Johnson M, Ho A, McGrath R, Netter P, Loeuille D, Gillet P, Jonveaux P, Gaucher A, Reginato A, Gurley K, Kingsley D, Williams C (2001) Analysis of the ANK gene reveals a heterozygous missense mutation in a French family with calcium pyrophosphate deposition disease (CPPDD). Arthritis Rheum 44:S161 [Google Scholar]
- Nurnberg P, Thiele H, Chandler D, Hohne W, Cunningham ML, Ritter H, Leschik G, Uhlmann K, Mischung C, Harrop K, Goldblatt J, Borochowitz ZU, Kotzot D, Westermann F, Mundlos S, Braun HS, Laing N, Tinschert S (2001) Heterozygous mutations in ANKH, the human ortholog of the mouse progressive ankylosis gene, result in craniometaphyseal dysplasia. Nat Genet 28:37–41 [DOI] [PubMed] [Google Scholar]
- O'Connell JR, Weeks DE (1995) The VITESSE algorithm for rapid exact multilocus linkage analysis via genotype set-recoding and fuzzy inheritance. Nat Genet 11:402–408 [DOI] [PubMed] [Google Scholar]
- Pendleton A, Johnston M, Ho A, Gurley K, Kingsley D, Wright GD, Dixey J, Doherty M, Hughes AE (2001) A heterozygous mutation in the human ANK gene in a British family with adult onset chondrocalcinosis due to calcium pyrophosphate crystal deposition. Arthritis Rheum 44:S101 [Google Scholar]
- Reichenberger E, Tiziani V, Watanabe S, Park L, Ueki Y, Santanna C, Baur ST, Shiang R, Grange DK, Beighton P, Gardner J, Hamersma H, Sellars S, Ramesar R, Lidral AC, Sommer A, Raposo do Amaral CM, Gorlin RJ, Mulliken JB, Olsen BR (2001) Autosomal dominant craniometaphyseal dysplasia is caused by mutations in the transmembrane protein ANK. Am J Hum Genet 68:1321–1326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell RG, Bisaz S, Fleisch H, Currey HL, Rubinstein HM, Dietz AA, Boussina I, Micheli A, Fallet G (1970) Inorganic pyrophosphate in plasma, urine, and synovial fluid of patients with pyrophosphate arthropathy (chondrocalcinosis or pseudogout). Lancet 2:899–902 [DOI] [PubMed] [Google Scholar]
- Timms AE, Zhang Y, Russell RG, Brown MA (2002) Genetic studies of disorders of calcium crystal deposition. Rheumatology 41:725–729 [DOI] [PubMed] [Google Scholar]