Abstract
The ventilatory CO2 chemoreflex is inherently low in inbred Brown Norway (BN) rats compared with other strains, including inbred Dahl salt-sensitive (SS) rats. Since the brain stem expression of various pH-sensitive ion channels may be determinants of the CO2 chemoreflex, we tested the hypothesis that there would be fewer pH-sensitive K+ channel-expressing cells in BN relative to SS rats within brain stem sites associated with respiratory chemoreception, such as the nucleus tractus solitarius (NTS), but not within the pre-Bötzinger complex region, nucleus ambiguus or the hypoglossal motor nucleus. Medullary sections (25 μm) from adult male and female BN and SS rats were stained with primary antibodies targeting TASK-1, Kv1.4, or Kir2.3 K+ channels, and the total (Nissl-stained) and K+ channel immunoreactive (-ir) cells counted. For both male and female rats, the numbers of K+ channel-ir cells within the NTS were reduced in the BN compared with SS rats (P < 0.05), despite equal numbers of total NTS cells. In contrast, we found few differences in the numbers of K+ channel-ir cells among the strains within the nucleus ambiguus, hypoglossal motor nucleus, or pre-Bötzinger complex regions in both male and female rats. However, there were no predicted functional mutations in each of the K+ channels studied comparing genomic sequences among these strains. Thus we conclude that the relatively selective reductions in pH-sensitive K+ channel-expressing cells in the NTS of male and female BN rats may contribute to their severely blunted ventilatory CO2 chemoreflex.
Keywords: CO2 chemoreception, control of breathing, K+ channels
resting membrane potential and overall membrane excitability in neurons depend on the compliment and activation state of multiple ion channels. It is thought that the leak or background potassium (K+) channels play a dominant role in this regard, driving membrane potential toward the K+ equilibrium potential. Different K+ channel families are distinguished by their sequence similarities and their gating properties contributing to diverse functions. The activity of some K+ channels is uniquely affected by changes in H+, with a known acidic dissociation constant (pKa) within or near the expected physiological pH range for the brain. Given the large role for K+ channels in determining membrane excitability, it has been postulated that the cellular expression of one or more pH-sensitive K+ channels might represent a mechanism by which specialized cells in the brain may serve as (central) respiratory chemoreceptors (34, 38).
In humans and other mammals, there is considerable variation within a species in the ventilatory response to increased inspired CO2. Different strains of rats, such as the Dahl salt-sensitive (SS) rat, increase pulmonary ventilation ∼200% after switching inspired gas from room air to a gas mixture with 7% CO2, whereas Brown Norway (BN; BN/NHsdMcwi) rats only increase pulmonary ventilation by 34% with the same stimulus (14, 15). This strain difference in the ventilatory chemoreflex is specific to hypercapnia, as BN and SS rats do not differ in room air breathing nor in acute ventilatory responses to hypoxia and moderate exercise (15). In addition, denervation of the peripheral chemoreceptors does not alter the hypercapnic ventilatory response, further indicating that the this large phenotypic difference in CO2 sensitivity among these strains is likely due to differences in central (brain) mechanisms of respiratory CO2 chemoreception (27).
We previously investigated whether there are differences between the highly CO2-sensitive SS and CO2-insensitive BN rats in the numbers of cells expressing three different pH-sensitive K+ channels [twik-related acid-sensitive (TASK-1), inwardly rectifying (Kir2.3), and voltage-activated (Kv1.4)] specifically in the medullary raphe nucleus (38). Consistent with the hypothesis that fewer cells expressing pH-sensitive K+ channels within a site with CO2 chemosensitivity might lead to lower CO2 sensitivity in the BN rat, we found ∼10% fewer K+ channel-expressing cells in male BN rats compared with SS rats. However, the BN female rats consistently showed 10% greater numbers of raphe K+ channel-expressing cells, which was counter to the hypothesis (38). Herein we utilized tissue from the same rats as studied previously to further test the hypothesis that there are strain-, sex-, and brain stem site-specific differences in expression of pH-sensitive K+ channels between male and female SS and BN rats within three additional brain stem sites, including: 1) the highly integrative and CO2 chemosensitive nucleus of the solitary tract (NTS); 2) a site critical for respiratory rhythm generation, the pre-Bötzinger complex (pre-BötC) region, which presumably has lower levels chemosensitivity (19); and 3) two sites containing respiratory-related motoneurons [the nucleus ambiguus (NA) and hypoglossal motor nucleus (XII)] known to express high levels of TASK-1 (44). We further tested the hypothesis that any strain-related differences in central CO2/pH chemosensitivity that arises from differences in these pH-sensitive K+ channels may arise from genetic polymorphisms and thus compared genomic sequences of these three K+ channel genes, along with upstream and downstream elements, among the SS and BN strains to identify sequence variants that may ultimately lead to altered membrane excitability.
METHODS
In-house male (n = 6) and female (n = 9) (SS; SS/JrHsdMcwi) rats, and male (n = 7) and female (n = 7) (BN; BN/HsDMcwi) rats were studied. The rats studied herein were the same as in the previous report on expression of K+ channels in medullary raphé cells (38); thus methods and procedures have been previously presented in detail. Both male and female rats were studied because of previously observed sex differences in some physiological phenotypes, including the control of breathing (11, 12, 33, 42). All rats were generated and continuously housed at the Medical College of Wisconsin Biomedical Resource Center Transgenic Barrier facility before use in experimental protocols. All animals were provided a diet of Purina Rat Chow and water ad libitum. None of the rats were used for any physiological studies. All aspects of the protocols were reviewed and approved by the MCW Institutional Animal Care and Use Committee.
Histology and immunohistochemistry.
At 70 days of age, all rats were euthanized with an overdose of pentobarbital (Nembutal; 150 mg/kg). The heart was exposed and the ascending aorta was cannulated via left ventricle puncture. The brain was then perfused with 0.2 M phosphate-buffered saline (PBS) followed by perfusion with 4% paraformaldehyde in PBS for 30 min. The medulla was then extracted, postperfusion fixed, dehydrated with sucrose solutions, and frozen at −80°C. Tissue was then cryostat sectioned at 25 μm in a transverse plane into four series, beginning 1 mm caudal to obex and continuing for 2 mm rostral to obex. The first series was stained with cresyl violet (Nissl) to profile the total number of cells within the caudal (cNTS) and rostral NTS (rNTS), NA, pre-BötC, and XII. The remaining three series were utilized for immunohistochemistry, as described previously (38), using polyclonal primary antibodies (1:100; Alomone Laboratories, Jerusalem, Israel) raised in rabbits targeting recombinant rat [Kv1.4 (amino acid residues 589–644; APC-007) or Kir2.3 (amino acid residues 418–437; APC-032)] or human [TASK-1 (amino acid residues 252–269; APC-024)] proteins. Specificity of each of these antibodies has been shown by the supplier (Western blots) and previous publications (22, 24–26). Confidence of specificity was also enhanced by the distinct subcellular localization of each antibody label, where TASK-1-immunoreactive (ir) was primarily cytosolic with no nuclear staining, Kir2.3-ir was found in a dense ring around and throughout the nucleus in addition to the cytoplasm, and Kv1.4 heavily stained the nucleus and to a lesser extent the cytosol (Fig. 1A). It is, however, worth noting that there is evidence suggesting that this particular antibody for TASK-1 may not be highly selective (1), although others have demonstrated reasonable selectivity (45). Moreover, all cell counts of K+ channel-ir cells were made in all regions from the same animals, mitigating any potential artifactual effects on the data. The slides were then washed in PBS and incubated with a secondary antibody (biotinylated anti-rabbit antibody for 3 h), rinsed, and reacted with diaminobenzidine for 10 min, rinsed with Tris buffer, dehydrated, and cover slipped.
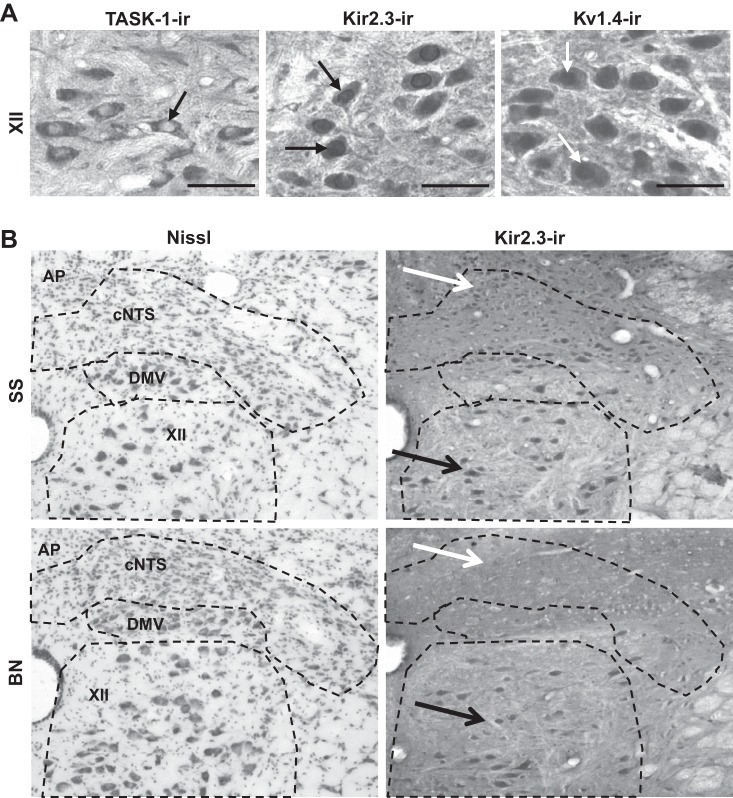
Fig. 1.
Twik-related acid-sensitive (TASK-1), inwardly rectifying (Kir) 2.3, and voltage-activated K+ channel (Kv) 1.4 stained brain stem tissues showed distinct subcellular patterns of immunoreactivity and strain-specific reductions in K+ channel-immunoreactive (ir) cells in Brown Norway (BN) rats. A: ×40 images of TASK-1-ir, Kir2.3-ir, and Kv1.4-ir cells within the hypoglossal nucleus (XII) of Dahl salt-sensitive (SS) rats. RGB color images of diaminobenzidine-labeled tissues were split, and the green channel is shown in gray scale. Bars in A are 25 μm, and arrows indicate distinct subcellular staining patterns for each antibody. B: gray-scale-converted ×10 images of Nissl-stained (left) and Kir2.3-ir (right) tissues, including the caudal nucleus of the solitary tract (cNTS), the dorsal motor nucleus of the vagus (DMV), and the hypoglossal nucleus (XII), and area postrema (AP) of male SS and BN rats. The black arrows point to regions containing positively labeled (Kir2.3-ir) cells within XII in both SS and BN rats, whereas the white arrows point to few Kir2.3-ir cells in the cNTS in BN rats. Note the paucity of Kir2.3 channel-ir cells in the NTS of the BN rat, which was qualitatively similar to TASK-1- and Kv1.4-stained tissues.
Data acquisition and statistical analysis.
The NTS and XII nuclei were manually counted on an upright microscope, where the NTS at its most caudal boundary was identified as dorsal to both the dorsal motor nucleus of the vagus and large motoneuron-containing XII, ventral to the gracile nucleus, and medioventral to the cuneate nucleus. NA was quantified from its position 0 to 1 mm rostral to obex (−13.3 to −12.3 bregma), where it was found at the caudal end to be dorsal to the pocket of the lateral reticular nucleus and ∼2 mm lateral from the midline, according to the rat brain atlas of Paxinos and Watson (31a). The pre-BötC area included a region 0 to 1.0 mm rostral to obex (−12.8 to −12.3 bregma) as being ventrolateral to NA and surrounded laterally by the spinal trigeminal nucleus. For quantification purposes, the pre-BötC area and NA were imaged using an upright microscope and charge-coupled device camera at ×10 magnification and defined by placing a 600-μm circle ventral to NA, with its center at the lateral edge of NA (13). Thus we counted cells within and near the pre-BötC along this rostrocaudal axis. Within each nucleus, cells included in counts were based on the following criteria: degree of staining, distinguishing (mainly neuronal) characteristics, and section quality. For each K+ channel antibody, the intensity of staining was variable, having a range of lightly stained cells (negative) and heavily stained cells (positive) compared with background levels, as demonstrated in Fig. 1A. Cells with clear distinguishing characteristics, such as an identifiable soma, nucleus, axonal processes, and/or dendrites (excluding glial cells), were considered immunoreactive based on the degree of staining. However, because we cannot be certain our antibodies do not also label other cell (glia, etc.) types, we refer to the counted objects as “cells” throughout. Furthermore, cells were not quantified in compromised tissue sections, i.e., folded, ripped, or damaged in any way. All cells were manually counted by one investigator blinded to sex and strain. Nissl-stained cells were used as an index of the total number of cells, and these were compared with cells immunostained for the K+ channels on serially juxtaposed sections. Although there are no differences in the ventilatory response to hypercapnia among males and females from these strains (15), statistical comparisons were made between strain and rostrocaudal distance within sex. For each sex (male or female), a two-way ANOVA was used to determine whether there were significant differences in number of cells between strains (BN vs. SS) with caudal-rostral distance within each nucleus (NTS, pre-BötC, NA or XII), or significant differences in the percentage of K+ channel-ir cells between strains and nuclei. A Bonferroni post hoc analysis was used to identify pairwise differences, with a threshold of P < 0.05.
Genomic sequence comparisons.
An analysis of single nucleotide variants (SNVs) was performed using an online resource and tool on the Rat Genome Database (RGD; http://rgd.mcw.edu/). The reference sequence for the rat genome is the BN strain from our home institution (BN/HsDMcwi). All comparisons made were using genomic sequence from the SS (SS/JrHsdMcwi), and using the RGD reference sequence version 3.4 (RGD_v3.4). Each gene was queried for SNVs by entering the gene of interest [Kcnj4 (Kir2.3), Kcnk3 (TASK-1), or Kcna4 (Kv1.4)] into the “Rat Genome” tool and selecting the “Strain Specific Variants (not validated, annotated)” track for display. SNV data for each gene were downloaded and displayed in MS Excel to determine chromosomal location, variant location within the gene, read depth, reference base and substitution, and predicted gene product function (synonymous vs. nonsynonymous). SNV data were also obtained within 5 kb up/downstream of each gene queried.
RESULTS
Body weights.
The body weight of the male SS (300.8 ± 10.3 g) rats was greater (P < 0.001) than that of the male BN (236.0 ± 4.5 g) rat strain. Similarly, the body weight of the female SS (200.2 ± 2.9 g) was greater (P < 0.001) than that of the female BN (156.0 ± 6.0 g) strain.
Total and K+ channel-ir cell counts in the NTS.
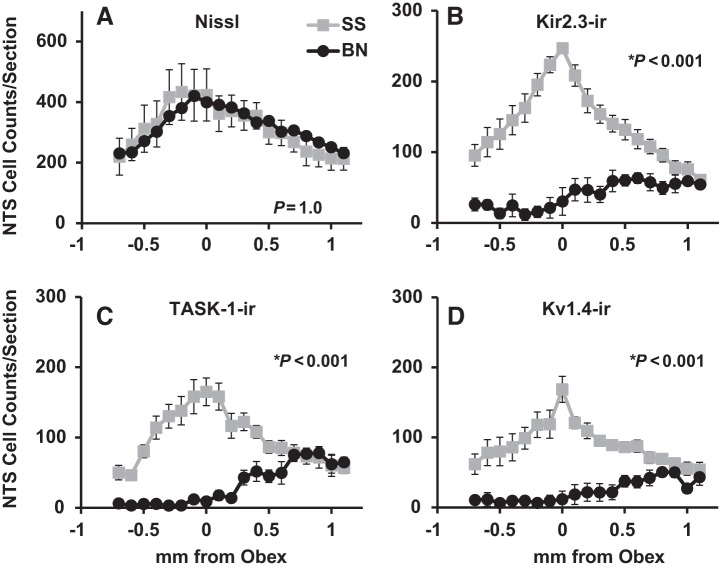
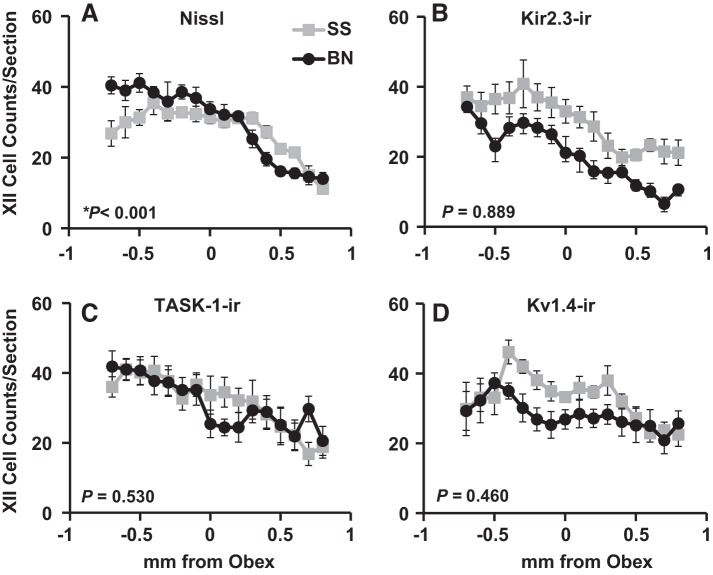
There were no gross differences in total cell numbers in the NTS of BN and SS male rats upon visual inspection of the Nissl-stained tissues (Fig. 1). This observation was consistent with manual counts of all cells in the NTS showing no differences among male BN and SS rats (P = 1.00; Fig. 2), similar to the comparison of total cells among female BN and SS rats within the NTS (P = 1.00; Table 1). In addition, the total cell counts and those for Kir2.3-ir, TASK-1-ir, and Kv1.4-ir cells significantly varied along the caudal-rostral distance measured in both male and female BN and SS rats (P ≤ 0.002; Fig. 2 and data not shown). In contrast to the total cell counts within the NTS, there were obviously fewer K+ channel-ir cells in BN male and females within the NTS. There were consistently fewer TASK-1-ir, Kir2.3-ir, and Kv1.4-ir cells throughout most of the rostrocaudal extent of the NTS in BN male and female rats compared with SS rats (P < 0.001; Fig. 2 and Table 1), although cell counts in the BN rats are similar to SS counts rostrally within the NTS.
Fig. 2.
Kir2.3-ir, TASK-1-ir, and Kv1.4-ir cell counts were markedly reduced in the nucleus of the solitary tract (NTS) of BN rats compared with SS rats. The average (±SE) total (Nissl; A) and Kir2.3-ir (B), TASK-1-ir (C), and Kv1.4-ir (D) cell counts from −0.7 to 1.1 mm caudal and rostral to obex, respectively, are shown for SS (n = 6) and BN (n = 7) male rats. Note that there are significantly fewer TASK-1-ir, Kir2.3-ir, and Kv1.4-ir cells in BN rats compared with SS rats in the NTS, despite equal numbers of total cells. P values included are for the interaction term of two-way ANOVA (strain × caudal-rostral distance).
Table 1.
Summary of cell counts for female BN and SS rats
| Region | Stain | Female SS | Female BN | P Value |
|---|---|---|---|---|
| NTS | Nissl | 314 ± 8.6 | 397 ± 9.8 | 1.00 |
| TASK-1 | 81 ± 2.6 | 55 ± 2.9 | <0.001* | |
| Kir2.3 | 119 ± 2.8 | 51 ± 3.2 | <0.001* | |
| Kv1.4 | 91 ± 2.1 | 46 ± 2.4 | <0.001* | |
| pre-BötC | Nissl | 115 ± 2.8 | 116 ± 3.2 | 0.656 |
| TASK-1 | 89 ± 2.0 | 97 ± 2.2 | 0.989 | |
| Kir2.3 | 103 ± 3.8 | 96 ± 4.3 | 0.999 | |
| Kv1.4 | 88 ± 3.0 | 98 ± 3.4 | 0.738 | |
| NA | Nissl | 13 ± 0.7 | 13 ± 0.8 | 0.999 |
| TASK-1 | 9 ± 0.4 | 10 ± 0.4 | 0.975 | |
| Kir2.3 | 11 ± 0.5 | 10 ± 0.6 | 0.919 | |
| Kv1.4 | 11 ± 0.4 | 11 ± 0.5 | 0.258 | |
| XII | Nissl | 31 ± 0.6 | 30 ± 0.7 | 0.565 |
| TASK-1 | 25 ± 0.6 | 24 ± 0.7 | 0.065 | |
| Kir2.3 | 23 ± 0.5 | 20 ± 0.5 | 0.242 | |
| Kv1.4 | 23 ± 0.6 | 24 ± 0.6 | 0.843 |
Values are means ± SE of total and K+ channel-immunoreactive cell counts in Dahl salt-sensitive (SS; n = 9) and Brown Norway (BN; n = 7) female rats within the nucleus of the solitary tract (NTS), pre-Botzinger complex (pre-BötC), nucleus ambiguous (NA) and hypoglossal motor nucleus (XII) nuclei. P values are included are for the interaction term of two-way ANOVA (strain x caudal-rostral distance).
Significant difference.
Total and K+ channel-ir cell counts in the pre-BötC region, NA, and XII.
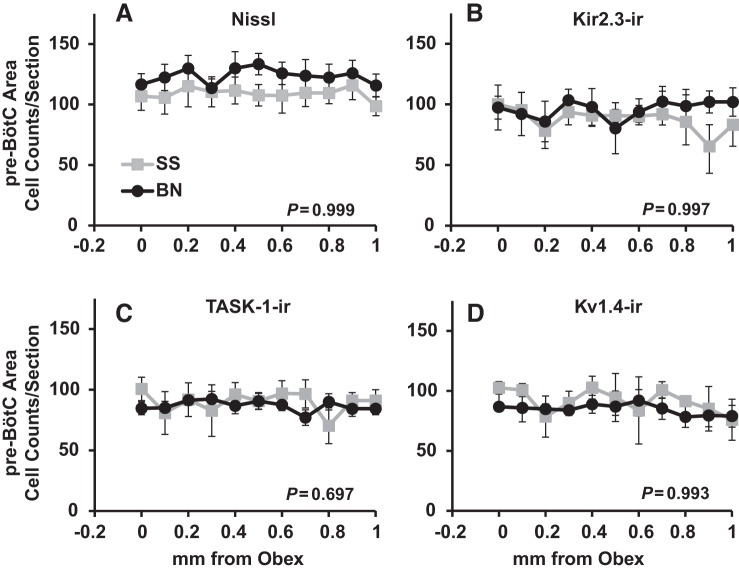
Total numbers of cells within the area of the pre-BötC consistently ranged from 90–130 cells in BN and SS rats (Fig. 3). Within this region, there were no strain differences in the total numbers of cells in male (P = 0.999; Fig. 3) or female (P = 0.656; Table 1) BN and SS rats. Similarly, counts of Kir2.3-ir, TASK-1-ir, Kv1.4-ir cells were equal among BN and SS male (P ≥ 0.697; Fig. 3) and female (P ≥ 0.738; Table 1) rats.
Fig. 3.
Total (Nissl; A), Kir2.3-ir (B), TASK-1-ir (C), and Kv1.4-ir (D) cell counts within the pre-Bötzinger complex (pre-BötC) region did not differ among BN and SS rats. The average (±SE) total, Kir2.3-ir, TASK-1-ir, and Kv1.4-ir cell counts from 0–1 mm rostral to obex are shown for SS (n = 6) and BN (n = 7) male rats. P values included are for the interaction term of two-way ANOVA (strain × caudal-rostral distance).
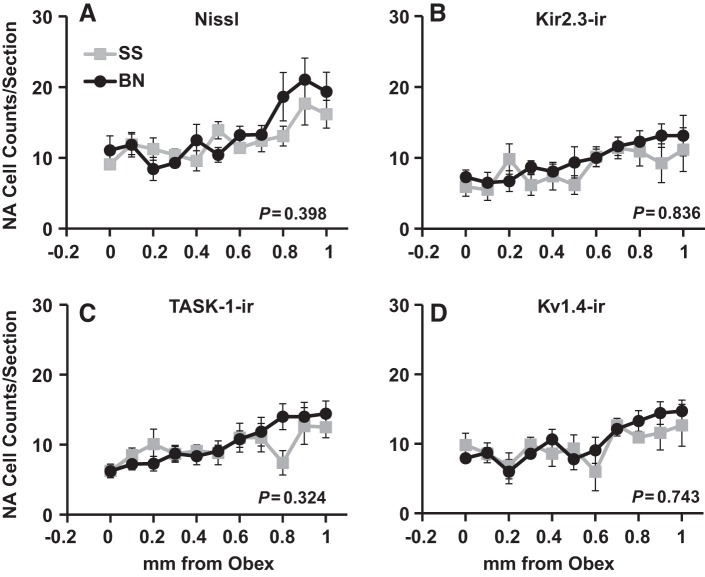
Within the NA, there was a significant trend of increasing total, TASK-1-ir, Kir2.3-ir, and Kv1.4-ir cell counts from obex to 1 mm rostral in male BN and SS rats (P > 0.05; Fig. 4), and a similar trend was found in female BN and SS rats (data not shown). However, we found no differences among BN and SS male rats in the total, TASK-1-ir, Kir2.3-ir, or Kv1.4-ir cells within the NA (P ≥ 0.324; Fig. 4). Similarly, there were no strain differences among female BN and SS rats in all cell counts (P ≥ 0.258; Table 1) within the NA.
Fig. 4.
Total (Nissl; A), Kir2.3-ir (B), TASK-1-ir (C), and Kv1.4-ir (D) cell counts within nucleus ambiguus (NA) did not differ among BN and SS rats. The average (±SE) total, Kir2.3-ir, TASK-1-ir, and Kv1.4-ir cell counts from 0–1 mm rostral to obex are shown for SS (n = 6) and BN (n = 7) male rats. P values included are for the interaction term of two-way ANOVA (strain × caudal-rostral distance).
All cell counts significantly decreased in the caudal-rostral direction within XII in male rats (P < 0.001; Fig. 5 and data not shown). In addition, we found a significant difference among male BN and SS rats in total cell counts within XII (P < 0.001; Fig. 5), where there were greater cell counts in BN rats caudally and fewer counts in BN rats rostrally. However, there were no differences in Kir2.3-ir, TASK-1-ir, or Kv1.4-ir cell counts within XII among male BN and SS rats (P ≥ 0.460; Fig. 5). Furthermore, there were no differences among female BN and SS rats in total, Kir2.3-ir, TASK-1-ir, or Kv1.4-ir cell counts in XII (P ≥ 0.242; Table 1).
Fig. 5.
Kir2.3-ir, TASK-1-ir, and Kv1.4-ir cell counts within XII did not differ among BN and SS rats. The average (±SE) total (A), Kir2.3-ir (B), TASK-1-ir (C), and Kv1.4-ir (D) cell counts from 0–1 mm rostral to obex are shown for SS (n = 6) and BN (n = 7) male rats. Note that there is a significant difference among the strains in total cell counts along the caudal-rostral distance within XII (P < 0.001), but not in all other K+ channel-ir cell counts. P values included are for the interaction term of two-way ANOVA (strain × caudal-rostral distance).
K+ channel-ir cell counts relative to the total in the NTS, NA, pre-BötC, and XII.
The counts for Kir2.3-ir, TASK-1-ir, and Kv1.4-ir were also expressed as a percentage of the total within each region (Fig. 6). Generally speaking, K+ channel-ir cell counts in male SS rats represented 30–60% of the total in the cNTS and rNTS. In contrast, >75% of cells within the pre-BötC region, NA, and XII were immunoreactive for all three K+ channels in both strains (Fig. 6). Similar to the results from absolute cell counts, we noted a significantly smaller percentage of cells that express Kir2.3-ir, TASK-1-ir, and Kv1.4-ir channels in the cNTS among BN compared with SS rats (P ≤ 0.002; Fig. 6), and a smaller percentage of Kir2.3-ir and Kv1.4-ir cells in the rNTS in BN compared with SS rats (P ≤ 0.038; Fig. 6). Similarly, there was a smaller percentage of Kir2.3-ir cells in XII in BN rats relative to male SS rats (P = 0.016; Fig. 6). Similar results were obtained in the female rats comparing strain and regions (data not shown).
Fig. 6.
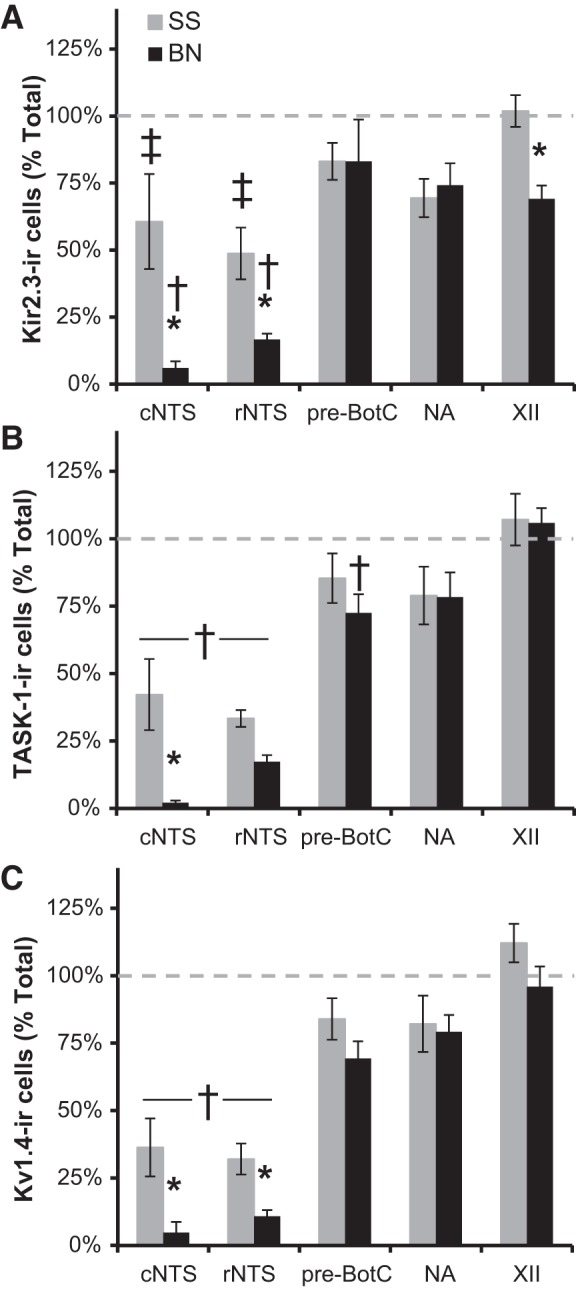
Regional and strain-related differences in K+ channel-ir cells in brain stem nuclei. The numbers of Kir2.3-ir (A), TASK-1-ir (B), and Kv1.4-ir (C) cells (expressed as a percentage of the total) within each region in male BN and SS rats are shown. There was generally a smaller percentage of K+ channel-ir cells within the NTS relative to other brain stem nuclei (P < 0.05), and a smaller percentage of Kir2.3-ir, TASK-1-ir, and Kv1.4-ir cells, particularly in the cNTS (P < 0.05), in BN compared with SS rats. †Percentage of K+ channel-ir cells is lower than that in pre-BötC, NA, and XII (P < 0.05). ‡Percentage of K+ channel-ir cells is lower than that in XII (P < 0.05). *Indicated strain difference within a region (P < 0.05).
Genomic sequence variant analyses among BN and SS strains.
Comparisons were made of the genomic sequences between the BN and SS strains for each of the K+ channels quantified herein to determine whether strain-related differences in K+ channel distribution and/or ventilatory CO2 sensitivity could be explained by SNVs. A summary of SNVs identified for Kir2.3 (Kcnj4), TASK-1 (Kcnk3), and Kv1.4 (Kcna4) are presented in Table 2. We found SNVs in the region of study in two of three (Kir2.3 and TASK-1) K+ channels among the strains, but found no variants between the strains in Kv1.4. Among the 48 SNVs located within or near the TASK-1 gene, 38 SNVs were located within the intronic portions. In contrast, among the 46 SNVs within or near Kir2.3, we noted only 23 SNVs within the gene. Importantly, one of the SNVs within Kir2.3 was within an exon, but this base substitution (reference = T to variant = C) is synonymous and codes for the same amino acid (glutamic acid). Thus, among the SNVs identified using this method, there was only one variant in a coding region, but it is not predicted to alter protein function. Therefore any differences in immunoreactivity among these strains are unlikely to result from SNVs within or near the Kir2.3, Kv1.4, or TASK-1 genes, but may instead result from altered transcriptional regulation in the BN strain.
Table 2.
Summary of single nucleotide variant analysis comparing the BN and SS genomic sequences for the Kcnj4 (Kir2.3), Kcnk3 (TASK-1), and Kcna4 (Kv1.4) genes
| Location and No. of SNVs |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Gene | Chromosome | Upstream | 5′-UTR | Intron | Exon | Downstream | BN Base/AA | SS Base/AA | Subtype |
| Kcnj4 (Kir2.3) | 7 | 19 | 12 | 10 | 1 | 4 | T/E | C/E | Synonymous |
| Kcnk3 (TASK-1) | 6 | 4 | 0 | 38 | 0 | 6 | |||
| Kcna4 (Kv1.4) | 3 | 0 | 0 | 0 | 0 | 0 | |||
Single nucleotide variants (SNVs) were queried by comparing genomic sequences (gene of interest plus 5 kb up/downstream) from the BN (reference sequence; version 3.4) and the SS strains using the publically available tools at RGD (www.rgd.mcw.edu). Listed are the gene name, chromosomal location, location of SNV, reference base and amino acid (AA) coded, and type of variant. UTR, untranslated region.
DISCUSSION
The major findings of the present study were that the numbers of cells with TASK-1, Kir2.3, and Kv1.4 channel immunoreactivity within the NTS were significantly less in CO2-insensitive BN rats compared with SS rats in both males and females, with few or no differences in total NTS cell counts. Furthermore, there were few or no differences among the BN and SS rats in total and K+ channel-ir cell counts in all other brain stem regions studied. The relative percentages of total cells expressing K+ channels were greater for pre-BötC, NA, and XII than for the NTS. Finally, among multiple SNVs within and near these K+ channel genes, we did not find any predicted to alter the function of the resulting protein, suggesting that the observed strain-related and site-specific differences in numbers of pH-sensitive K+ channel-ir cells likely results from altered expression levels rather than gene mutations.
Central CO2/H+ chemoreception and pH-sensitive ion channels.
Cells defined as central respiratory chemoreceptors are presumably intrinsically sensitive to CO2/H+, independent of inputs from other cells, and these cells are functionally connected to the respiratory neural control network (37). We and others postulate that the central chemoreceptors' intrinsic ability to respond to acidosis and/or alkalosis depends on the expression of one or multiple pH-sensitive ion channels, including one or more pH-sensitive Ca2+ (3, 12, 16), Ca2+-activated cation (36), transient receptor potential channels (8), and/or K+ channels (16, 34). There are several K+ channels that have biophysical properties that are modulated by pH. For example, Kir2.3 channels expressed in Xenopus oocytes are strongly inhibited by graded extracellular acidification (6) or intracellular acidification (35). Likewise, intracellular acidosis modulates Kv1.4 channels by shortening the N-type inactivation time constant (31), while extracellular acidosis slows the recovery time from N-type inactivation (4). Lastly, TASK channels are modulated by extracellular (not intracellular) acidosis and are robustly expressed within brain stem motoneurons and putative sites of central respiratory chemoreception (2, 9, 21). Ultimately, inhibition of these K+ channels via hypercapnic acidosis would theoretically lead to depolarization and increased membrane excitability/firing rates (34).
Despite the above rationale that support a role for these channels in cellular CO2/pH sensitivity, there are few data that directly demonstrate a functional role for any pH-sensitive K+ channels in determining cellular CO2/pH chemosensitivity, including those studied herein. TASK-1 and TASK-3 channels are expressed in raphe 5-HT cells, which are postulated to be intrinsically chemosensitive (5, 36). However, TASK-1 and TASK-3 are also expressed within pre-BötC inspiratory cells, which are thought to play prominent roles in respiratory rhythm generation and less of a role in central CO2 chemoreception (18). While genetic deletion of TASK-1, TASK-3, or in combination affected the cellular chemosensitivity of dorsal raphe 5-HT neurons, there was no effect on whole-animal ventilatory CO2 sensitivity in each TASK-1, TASK-3, or double knockouts (28). Like TASK channels, Kv1.4 channels are widely expressed throughout the brain and thus appear unlikely to mediate such a highly specific cellular property as intrinsic CO2/pH sensitivity. However, the relatively selective Kv1 channel family antagonist 4-aminopyridine significantly attenuates in vitro CO2 responses in chemosensitive locus coeruleus neurons and completely eliminates this response in the NTS (23), suggesting a functional and specific role in cellular chemosensitivity than previously recognized. The Kir2.3 isoform of heteromeric inwardly rectifying K+ channels confers pH sensitivity (29) and has high open probabilities at pH = 7.4, which is markedly reduced at pH = 7.0 (50). Other Kir channels, such as Kir4.1–5.1 heteromeric channels, may also be involved (17), particularly in pH-sensitive retrotrapezoid nucleus astrocytes via ATP release (49). However, based on the sum of the data, we favor the hypothesis that no single pH-sensitive channel confers intrinsic cellular chemosensitivity, but rather a combination of various pH-sensitive ion channels, including, but not limited to, those studied herein.
As others have suggested (34), we envision cellular CO2/pH sensitivity to be determined by multiple pH-sensitive ion channels in any individual chemoreceptor cell type. This model has advantages where activation or inactivation of pH-sensitive ion channels becomes progressive with greater changes in H+ concentration from the baseline (pH = 7.3), leading to greater changes in the cellular activity that encodes pH “sensing”. Expression of multiple pH-sensitive channels with slightly different pKa could underlie this property, and the pKa and/or conductance of each channel could be modulated (39–41). For example, the pKa of Kir2.3 is estimated to range between pH = 6.8 and 7.2 (6, 50), TASK-1 between 7.3 and 7.4 (10, 20), and Kv1.4 slightly in the alkaline range (pH = 7.5) (31). Other channels like TASK and the heteromeric Kir4.1./5.1 channel have a relatively high single-channel conductances and a broad estimated pKa (pH = 6.8–7.5) (7, 32), which make them intriguing candidates to play a dominant role in pH-dependent cellular activity (46). However, the expression of multiple pH-sensitive conductances within a given CO2-sensitive cell would allow for a higher degree of sensitivity, and the resulting changes in cellular responses varied among various chemoreceptor populations to optimize the ventilatory CO2 chemoreflex over a broad range of pH.
While the identities of pH-sensitive channels that functionally contribute to central respiratory chemoreception remain unclear, herein we examined the sequence similarities of pH-sensitive K+ channels among CO2-sensitive and -insensitive rat strains and quantified numbers of cells immunoreactive for various pH-sensitive K+ channels within four brain stem nuclei, including the NTS, NA, XII, and pre-BötC. Our conclusions based on the sequence data are that the basic function of each of these K+ channels within each strain are likely to be similar, and any difference in expression wherever found is not due to specific sequence variants within or 5 kb upstream or downstream of these genes. However, there may be sequence variation in regulatory elements for each of these genes we have not investigated that could lead to differences in detectable protein levels, or in factors unrelated to genomic sequence variation within the genes that ultimately alter the membrane content and/or modulation of channel function that vary among these strains. Nonetheless, the power in studying these specific rat strains is that they are inbred (isogenetic), their genomic sequences are complete, and they have large, inherent differences in ventilatory CO2 sensitivity without phenotypic differences in other aspects of ventilatory control (15). Thus these types of SNV analyses can be performed on all known genes for which there is adequate sequence coverage, including other hypothesized pH-sensitive ion channels not studied herein and previously (38).
Based on previous data, it has been suggested that the medullary raphe may be among the sites with the greatest cellular CO2/pH chemosensitivity (34, 47). We showed previously that the medullary raphe had as many as 300 cells per tissue section expressing K+ channels using the same quantification techniques and same tissues utilized herein (38). Using the same logic, a large contribution to chemosensitivity should also come from the NTS, which contains 100–200 cells per tissue section that express K+ channels. However, only a fraction of raphe and NTS neurons are chemosensitive (30, 48); there is a larger fraction of K+ channel expressing cells in the pre-BötC, XII, and NA relative to the NTS and raphe (see Fig. 6) (38); and there are data to suggest some level of cellular CO2/pH chemosensitivity within the pre-BötC (43) and XII (44). Thus, although there are many raphe and NTS cells with pH-sensitive K+ channel immunoreactivity, the CO2 chemoreflex and cellular CO2 sensitivity are likely to be determined by several additional factors. Instead, the major strain-related differences in K+ channel-ir cells in the NTS of BN compared with SS rats (which is true for both male and female rats) may have the effects on both cellular CO2 sensitivity and generalized levels of basal activity and/or responsiveness to synaptic inputs.
Accordingly, the sum of the observations herein support the overall hypothesis that the differences in CO2/H+ sensitivity inherent to BN and SS rat strains are due in part to differences in the numbers of cells staining positive for multiple pH-sensitive K+ channels in the chemosensitive NTS. A strength of our studies is that the data were obtained from the same tissues analyzed previously, validating direct comparisons between the raphé, NTS, NA, XII, and pre-BötC datasets (38). Nevertheless, no matter how objective, these comparisons only provide a correlative relationship between the relative expression levels of these specific pH-sensitive K+ channels and CO2 sensitivity in vivo, i.e., they do not demonstrate cause and effect. Another potential weakness is the quantification only of the numbers of cells that expressed each K+ channel, compared with providing a densitometric or other semiquantitative approach to measuring level of expression among the cells that express the channels. In other words, we have a snapshot of expression levels using a single threshold for detection, while there may actually be greater numbers of cells that express each channel. However, it remains possible that one or more of these differences in pH-sensitive K+ channels can account for some of the strain differences in CO2 sensitivity, although additional functional studies are needed to validate this possibility.
Lastly, the significance of the identified differences in K+ channel expression between four brain stem sites may provide a basis for future studies on factors determining membrane excitability and/or differences in electrophysiological “signature” and firing characteristics of neurons at these sites. While modified by pH and/or CO2, these channels also play major roles in homeostatic functions that are indirectly or not at all related to central respiratory chemoreception. Our data and other data characterizing the distribution and differences among strain, sex, and brain stem nucleus suggest major questions that, when addressed, should enhance understanding of the role of pH-dependent K+ channel cellular function. It is striking that, for both rat strains, a relatively small percentage of the total number of NTS cells express these K+ channels (Fig. 6), whereas, for the other three nuclei, there was a consistent large percentage of the total cells expressing K+ channels. In addition, it is also of interest that each of these K+ channels has a distinct subcellular localization, which could also be indicative of a more complex pH-sensing mechanism than absolute levels of plasma membrane expression alone. Together these findings would seem to have a yet to be determined significance for the role of these brain stem sites to regulation of physiological functions.
Summary and conclusions.
The data demonstrate that there are fewer NTS cells that express pH-sensitive K+ channels in the CO2-insensitive BN rat compared with the CO2-sensitive SS rat. These findings were consistent in both male and female rats studied, unlike that previously observed in the medullary raphe (38). Furthermore, strain-related differences in the ventilatory CO2 chemoreflex are not likely due to sequence variation within the genes for these K+ channels, as we found no variants that would lead to changes in the amino acid sequences. Accordingly, we conclude that the inherent differences in CO2 sensitivity among BN and SS rat strains may be due to regional, differential expression of pH-sensitive K+ channels, including, but not limited to, TASK-1, Kv1.4, and/or Kir2.3 channels within chemosensitive brain stem nuclei.
GRANTS
This research was supported by National Heart, Lung, and Blood Institute Grants HL-066579 (H. V. Forster), HL-25739 (H. V. Forster) and HL-097033 (M. R. Hodges), and by the Department of Veterans Affairs (H. V. Forster).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: P.F.M., S.J.O., D.R., S.E.N., H.V.F., and M.R.H. conception and design of research; P.F.M., S.J.O., D.B., D.R., and S.E.N. performed experiments; P.F.M., S.J.O., D.B., and M.R.H. analyzed data; P.F.M., S.J.O., H.V.F., and M.R.H. interpreted results of experiments; P.F.M. and M.R.H. prepared figures; P.F.M., H.V.F., and M.R.H. drafted manuscript; P.F.M., S.E.N., H.V.F., and M.R.H. edited and revised manuscript; P.F.M., S.J.O., D.B., D.R., S.E.N., H.V.F., and M.R.H. approved final version of manuscript.
REFERENCES
- 1.Aller MI, Veale EL, Linden AM, Sandu C, Schwaninger M, Evans LJ, Korpi ER, Mathie A, Wisden W, Brickley SG. Modifying the subunit composition of TASK channels alters the modulation of a leak conductance in cerebellar granule neurons. J Neurosci 25: 11455–11467, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bayliss DA, Talley EM, Sirois JE, Lei Q. TASK-1 is a highly modulated pH-sensitive “leak” K(+) channel expressed in brainstem respiratory neurons. Respir Physiol 129: 159–174, 2001 [DOI] [PubMed] [Google Scholar]
- 3.Chen XH, Bezprozvanny I, Tsien RW. Molecular basis of proton block of L-type Ca2+ channels. J Gen Physiol 108: 363–374, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Claydon TW, Boyett MR, Sivaprasadarao A, Ishii K, Owen JM, O'Beirne HA, Leach R, Komukai K, Orchard CH. Inhibition of the K+ channel kv1.4 by acidosis: protonation of an extracellular histidine slows the recovery from N-type inactivation. J Physiol 526: 253–264, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Corcoran AE, Hodges MR, Wu Y, Wang W, Wylie CJ, Deneris ES, Richerson GB. Medullary serotonin neurons and central CO2 chemoreception. Respir Physiol Neurobiol 168: 49–58, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coulter KL, Perier F, Radeke CM, Vandenberg CA. Identification and molecular localization of a pH-sensing domain for the inward rectifier potassium channel HIR. Neuron 15: 1157–1168, 1995 [DOI] [PubMed] [Google Scholar]
- 7.Cui N, Giwa LR, Xu H, Rojas A, Abdulkadir L, Jiang C. Modulation of the heteromeric Kir4.1-Kir51 channels by Pco2 at physiological levels. J Cell Physiol 189: 229–236, 2001 [DOI] [PubMed] [Google Scholar]
- 8.Cui N, Zhang X, Tadepalli JS, Yu L, Gai H, Petit J, Pamulapati RT, Jin X, Jiang C. Involvement of TRP channels in the CO2 chemosensitivity of locus coeruleus neurons. J Neurophysiol 105: 2791–2801, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Duprat F, Lesage F, Fink M, Reyes R, Heurteaux C, Lazdunski M. TASK, a human background K+ channel to sense external pH variations near physiological pH. EMBO J 16: 5464–5471, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duprat F, Lesage F, Fink M, Reyes R, Heurteaux C, Lazdunski M. TASK, a human background K+ channel to sense external pH variations near physiological pH. EMBO J 16: 5464–5471, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dwinell MR, Forster HV, Petersen J, Rider A, Kunert MP, Cowley AW, Jr, Jacob HJ. Genetic determinants on rat chromosome 6 modulate variation in the hypercapnic ventilatory response using consomic strains. J Appl Physiol 98: 1630–1638, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Filosa JA, Putnam RW. Multiple targets of chemosensitive signaling in locus coeruleus neurons: role of K+ and Ca2+ channels. Am J Physiol Cell Physiol 284: C145–C155, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Gray PA, Janczewski WA, Mellen N, McCrimmon DR, Feldman JL. Normal breathing requires pre-Botzinger complex neurokinin-1 receptor-expressing neurons. Nat Neurosci 4: 927–930, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hodges MR, Echert AE, Puissant MM, Mouradian GC, Jr. Fluoxetine augments ventilatory CO sensitivity in Brown Norway but not Sprague Dawley rats. Respir Physiol Neurobiol 186: 221–228, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hodges MR, Forster HV, Papanek PE, Dwinell MR, Hogan GE. Ventilatory phenotypes among four strains of adult rats. J Appl Physiol 93: 974–983, 2002 [DOI] [PubMed] [Google Scholar]
- 16.Imber AN, Putnam RW. Postnatal development and activation of L-type Ca2+ currents in locus ceruleus neurons: implications for a role for Ca2+ in central chemosensitivity. J Appl Physiol 112: 1715–1726, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jiang C, Xu H, Cui N, Wu J. An alternative approach to the identification of respiratory central chemoreceptors in the brainstem. Respir Physiol 129: 141–157, 2001 [DOI] [PubMed] [Google Scholar]
- 18.Koizumi H, Smerin SE, Yamanishi T, Moorjani BR, Zhang R, Smith JC. TASK channels contribute to the K+-dominated leak current regulating respiratory rhythm generation in vitro. J Neurosci 30: 4273–4284, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krause KL, Forster HV, Davis SE, Kiner T, Bonis JM, Pan LG, Qian B. Focal acidosis in the pre-Botzinger complex area of awake goats induces a mild tachypnea. J Appl Physiol 106: 241–250, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leonoudakis D, Gray AT, Winegar BD, Kindler CH, Harada M, Taylor DM, Chavez RA, Forsayeth JR, Yost CS. An open rectifier potassium channel with two pore domains in tandem cloned from rat cerebellum. J Neurosci 18: 868–877, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lesage F, Lauritzen I, Duprat F, Reyes R, Fink M, Heurteaux C, Lazdunski M. The structure, function and distribution of the mouse TWIK-1 K+ channel. FEBS Lett 402: 28–32, 1997 [DOI] [PubMed] [Google Scholar]
- 22.Li J, Blankenship ML, Baccei ML. Inward-rectifying potassium (Kir) channels regulate pacemaker activity in spinal nociceptive circuits during early life. J Neurosci 33: 3352–3362, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martino PF, Putnam RW. The effect of 4 aminopyradine (4AP) on the hypercapnic response of locus coeruleus (LC) neurons. Program No. 297.7/SS29. In: 2007 Neuroscience Meeting Planner. San Diego, CA: Society for Neuroscience, 2007 [Google Scholar]
- 24.Meiri N, Sun MK, Segal Z, Alkon DL. Memory and long-term potentiation (LTP) dissociated: normal spatial memory despite CA1 LTP elimination with Kv1.4 antisense. Proc Natl Acad Sci U S A 95: 15037–15042, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mienville JM, Maric I, Maric D, Clay JR. Loss of IA expression and increased excitability in postnatal rat Cajal-Retzius cells. J Neurophysiol 82: 1303–1310, 1999 [DOI] [PubMed] [Google Scholar]
- 26.Millar JA, Barratt L, Southan AP, Page KM, Fyffe RE, Robertson B, Mathie A. A functional role for the two-pore domain potassium channel TASK-1 in cerebellar granule neurons. Proc Natl Acad Sci U S A 97: 3614–3618, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mouradian GC, Forster HV, Hodges MR. Acute and chronic effects of carotid body denervation (CBD) on ventilation and chemoreflexes in three rat strains. J Physiol 590: 3335–3347, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mulkey DK, Talley EM, Stornetta RL, Siegel AR, West GH, Chen X, Sen N, Mistry AM, Guyenet PG, Bayliss DA. TASK channels determine pH sensitivity in select respiratory neurons but do not contribute to central respiratory chemosensitivity. J Neurosci 27: 14049–14058, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Munoz V, Vaidyanathan R, Tolkacheva EG, Dhamoon AS, Taffet SM, Anumonwo JM. Kir2.3 isoform confers pH sensitivity to heteromeric Kir2.1/Kir23 channels in HEK293 cells. Heart Rhythm 4: 487–496, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nichols NL, Hartzler LK, Conrad SC, Dean JB, Putnam RW. Intrinsic chemosensitivity of individual nucleus tractus solitarius (NTS) and locus coeruleus (LC) neurons from neonatal rats. Adv Exp Med Biol 605: 348–352, 2008 [DOI] [PubMed] [Google Scholar]
- 31.Padanilam BJ, Lu T, Hoshi T, Padanilam BA, Shibata EF, Lee HC. Molecular determinants of intracellular pH modulation of human Kv1.4 N-type inactivation. Mol Pharmacol 62: 127–134, 2002 [DOI] [PubMed] [Google Scholar]
- 31a.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates (6th ed.). San Diego, CA: Academic, 2009 [Google Scholar]
- 32.Pessia M, Imbrici P, D'Adamo MC, Salvatore L, Tucker SJ. Differential pH sensitivity of Kir4.1 and Kir42 potassium channels and their modulation by heteropolymerisation with Kir51. J Physiol 532: 359–367, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Polotsky VY, Wilson JA, Smaldone MC, Haines AS, Hurn PD, Tankersley CG, Smith PL, Schwartz AR, O'Donnell CP. Female gender exacerbates respiratory depression in leptin-deficient obesity. Am J Respir Crit Care Med 164: 1470–1475, 2001 [DOI] [PubMed] [Google Scholar]
- 34.Putnam RW, Filosa JA, Ritucci NA. Cellular mechanisms involved in CO2 and acid signaling in chemosensitive neurons. Am J Physiol Cell Physiol 287: C1493–C1526, 2004 [DOI] [PubMed] [Google Scholar]
- 35.Qu Z, Zhu G, Yang Z, Cui N, Li Y, Chanchevalap S, Sulaiman S, Haynie H, Jiang C. Identification of a critical motif responsible for gating of Kir2.3 channel by intracellular protons. J Biol Chem 274: 13783–13789, 1999 [DOI] [PubMed] [Google Scholar]
- 36.Richerson GB. Serotonergic neurons as carbon dioxide sensors that maintain pH homeostasis. Nat Rev Neurosci 5: 449–461, 2004 [DOI] [PubMed] [Google Scholar]
- 37.Richerson GB, Wang W, Hodges MR, Dohle CI, ez-Sampedro A. Homing in on the specific phenotype(s) of central respiratory chemoreceptors. Exp Physiol 90: 259–266, 2005 [DOI] [PubMed] [Google Scholar]
- 38.Riley D, Dwinell M, Qian B, Krause KL, Bonis JM, Neumueller S, Marshall BD, Hodges MR, Forster HV. Differences between three inbred rat strains in number of K+ channel-immunoreactive neurons in the medullary raphe nucleus. J Appl Physiol 108: 1003–1010, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rojas A, Cui N, Su J, Yang L, Muhumuza JP, Jiang C. Protein kinase C dependent inhibition of the heteromeric Kir4.1-Kir51 channel. Biochim Biophys Acta 1768: 2030–2042, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rojas A, Su J, Yang L, Lee M, Cui N, Zhang X, Fountain D, Jiang C. Modulation of the heteromeric Kir4.1-Kir51 channel by multiple neurotransmitters via Galphaq-coupled receptors. J Cell Physiol 214: 84–95, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rojas A, Wu J, Wang R, Jiang C. Gating of the ATP-sensitive K+ channel by a pore-lining phenylalanine residue. Biochim Biophys Acta 1768: 39–51, 2007 [DOI] [PubMed] [Google Scholar]
- 42.Schlenker EH, Hansen SN. Sex-specific densities of estrogen receptors alpha and beta in the subnuclei of the nucleus tractus solitarius, hypoglossal nucleus and dorsal vagal motor nucleus weanling rats. Brain Res 1123: 89–100, 2006 [DOI] [PubMed] [Google Scholar]
- 43.Solomon IC, Edelman NH, O'Neal MH, III. CO2/H+ chemoreception in the cat pre-Botzinger complex in vivo. J Appl Physiol 88: 1996–2007, 2000 [DOI] [PubMed] [Google Scholar]
- 44.Talley EM, Lei Q, Sirois JE, Bayliss DA. TASK-1, a two-pore domain K+ channel, is modulated by multiple neurotransmitters in motoneurons. Neuron 25: 399–410, 2000 [DOI] [PubMed] [Google Scholar]
- 45.Toyoda H, Saito M, Okazawa M, Hirao K, Sato H, Abe H, Takada K, Funabiki K, Takada M, Kaneko T, Kang Y. Protein kinase G dynamically modulates TASK1-mediated leak K+ currents in cholinergic neurons of the basal forebrain. J Neurosci 30: 5677–5689, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang S, Benamer N, Zanella S, Kumar NN, Shi Y, Bevengut M, Penton D, Guyenet PG, Lesage F, Gestreau C, Barhanin J, Bayliss DA. TASK-2 channels contribute to pH sensitivity of retrotrapezoid nucleus chemoreceptor neurons. J Neurosci 33: 16033–16044, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang W, Pizzonia JH, Richerson GB. Chemosensitivity of rat medullary raphe neurones in primary tissue culture. J Physiol 511: 433–450, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang W, Tiwari JK, Bradley SR, Zaykin RV, Richerson GB. Acidosis-stimulated neurons of the medullary raphe are serotonergic. J Neurophysiol 85: 2224–2235, 2001 [DOI] [PubMed] [Google Scholar]
- 49.Wenker IC, Kreneisz O, Nishiyama A, Mulkey DK. Astrocytes in the retrotrapezoid nucleus sense H+ by inhibition of a Kir4.1-Kir51-like current and may contribute to chemoreception by a purinergic mechanism. J Neurophysiol 104: 3042–3052, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhu G, Chanchevalap S, Cui N, Jiang C. Effects of intra- and extracellular acidifications on single channel Kir2.3 currents. J Physiol 516: 699–710, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]